This work was produced by Curry et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Curry N, Davenport R, Thomas H, et al. Early high-dose cryoprecipitate to reduce mortality in adult patients with traumatic haemorrhage: the CRYOSTAT-2 RCT with cost-effectiveness analysis. Southampton (UK): National Institute for Health and Care Research; 2024 Nov. (Health Technology Assessment, No. 28.76.)
Recruitment and baseline results
The first patient was enrolled on 23 August 2017 and the last patient was enrolled on 2 November 2021. Recruitment was stopped temporarily between 6 April 2020 and 1 May 2020 due to the COVID-19 pandemic. Trial-specific data collection continued until 1 December 2021 in the UK and until 5 July 2022 in the USA. Central data linkage for mortality continued until 27 May 2022 in the UK.
The original planned sample size was 1568, later amended to 1600. A planned sample size re-estimation was conducted after 300 participants had been randomised and repeated after 750 participants had been randomised following a DMC request. In both cases, the committee recommended that the study continue with the original sample size. The final sample size was subsequently increased to 1600 after the TMG reviewed lost to follow-up rates. Two pre-planned interim analyses for harm or benefit after the recruitment of 500 and 1000 participants were conducted using data on 578 and 1027 participants, respectively. In both cases, the test statistic did not cross the O’Brien–Fleming early stopping boundary, and the DMC recommended that the trial continue to the full sample size. Due to delayed randomisation notifications, recruitment closed after the recruitment of 1604 participants. In total, 1555 participants were recruited across 25 UK centres, and 49 participants were recruited in one US centre (see Appendix 2, Table 17).
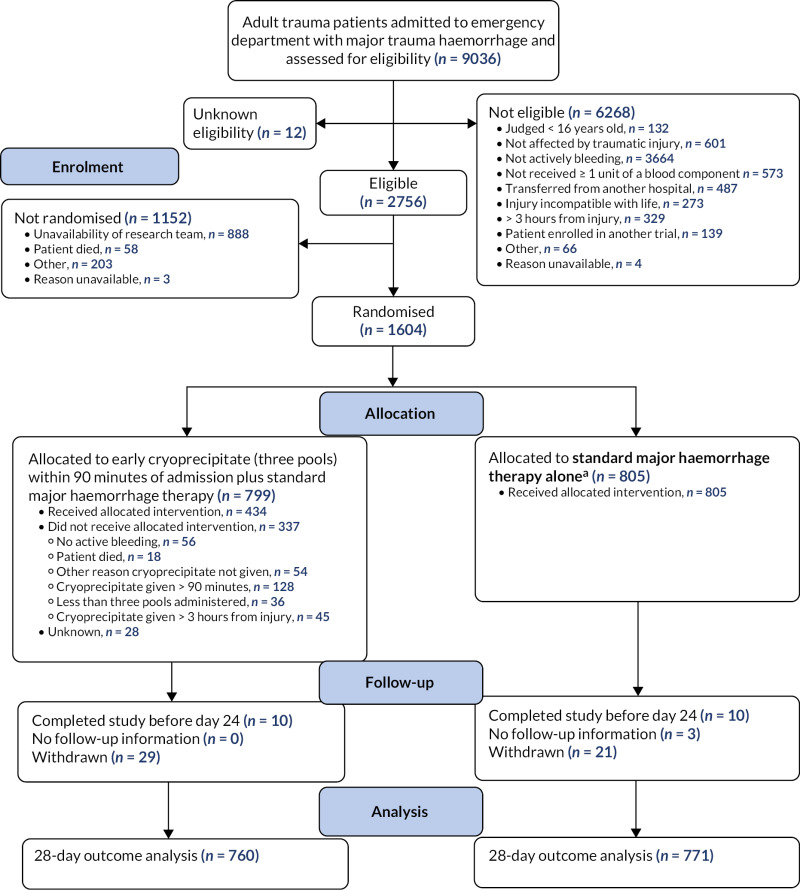
The Consolidated Standards of Reporting Trials diagram is shown in Figure 2. A total of 9036 patients were assessed for eligibility, of whom 2756 (31%) were found to be eligible for the study; 1604 (58%) of these 2756 patients were randomised. The most common reason for not randomising eligible patients was that the research team was unavailable for patients presenting at sites out of hours.
The 1604 patients were randomly assigned to receive either early cryoprecipitate in addition to the standard MHP (n = 799, 49.8%) or the standard MHP alone (n = 805, 50.2%).
Sixty-nine participants were randomised in error (intervention arm, n = 29; standard care arm, n = 40), having found to be ineligible for the study after randomisation, most commonly because they had not received at least one unit of any blood component prior to randomisation. All patients randomised in error were included in the ITT analysis if they had primary outcome data.
A total of 88 patients (intervention arm, n = 50; standard care arm, n = 38) withdrew consent after randomisation, but, of these, primary outcome data were missing for only 50 (intervention arm, n = 29; standard care arm, n = 21). For the remaining 38 patients, primary outcome data were available due to either the timing of withdrawal or the participants’ agreement to continued data collection after withdrawal.
Primary outcome data were missing for a further 23 participants (intervention arm, n = 10; standard care arm, n = 13) for whom no follow-up data were available at all, or available only beyond day 24 (as the 28-day follow-up form had a reporting window of ± 4 days patients reported alive at day 24 were assumed alive at day 28 as specified in the SAP).
Baseline characteristics were similar in both arms (Table 3). Seventy-nine per cent of participants were male and the median age was 39 years (IQR 26–55 years). Sixty-four per cent of patients sustained a blunt injury, 26% had a head AIS ≥ 4 and the median ISS was 29 (IQR 18–43). Forty-three per cent were administered a blood component and 79% were administered tranexamic acid prior to hospital arrival.
As expected in the absence of a placebo, protocol deviations were more common in the intervention arm. One patient was transfused the wrong component in error and a further 337 of the 799 patients (42%) in the intervention arm did not receive the full intervention per protocol. Of those 337 patients, 128 did not receive any early cryoprecipitate (16% of those randomised to early cryoprecipitate) because the patient had died, no active bleeding was identified at the time, or it was reported that haemostasis or correction of coagulopathy had been achieved. Other reasons for protocol deviations were administering cryoprecipitate more than 90 minutes after admission (e.g. due to delayed activation of the MHP), administering fewer than three pools of cryoprecipitate or administering cryoprecipitate more than 3 hours after injury.
Four patients in the standard care arm had protocol deviations. Two were randomised when recruitment at the site was paused due to COVID-19-related pressures, one was randomised when human error resulted in production of the randomisation envelope that showed the incorrect treatment, and one patient was randomised twice [in the emergency department and intensive care unit (ICU), randomised to the same arm, only one set of data was collected and analysed].
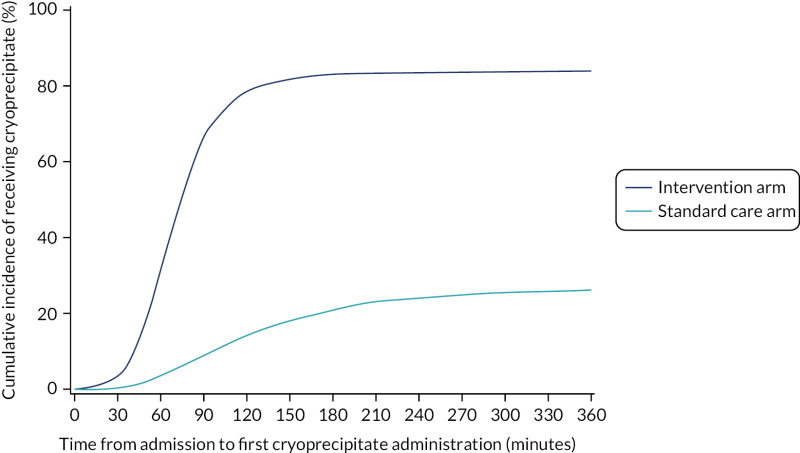
Eighty-five per cent of those in the intervention arm and 32% of those in the standard care arm received cryoprecipitate within the first 24 hours of arrival at hospital. Sixty-eight per cent of participants in the intervention arm received it within 90 minutes of arrival at hospital, compared with 9% in the standard care arm (p < 0.0001; Figure 3). Median (IQR) time to first cryoprecipitate (among those who received it) was 68 (53–85) minutes in the intervention arm and 120 (79–184) minutes in the standard care arm.
Primary outcome
We obtained primary outcome data for 760 patients in the intervention arm (95%) and 771 participants (96%) in the standard care arm. Patients discharged from hospital prior to 28 days were assumed alive at 28 days, together with anyone reported alive at day 24 or later.
Table 4 presents the ITT analysis of the primary outcome. In the intervention arm, 25.3% died within 28 days of admission compared with 26.1% in the standard care arm. The OR was 0.96 (95% CI 0.75 to 1.23) for 28-day mortality for early cryoprecipitate versus standard MHP, with a p-value of 0.7406 for the difference between the arms. The relative risk was 0.97 (95% CI 0.81 to 1.17).
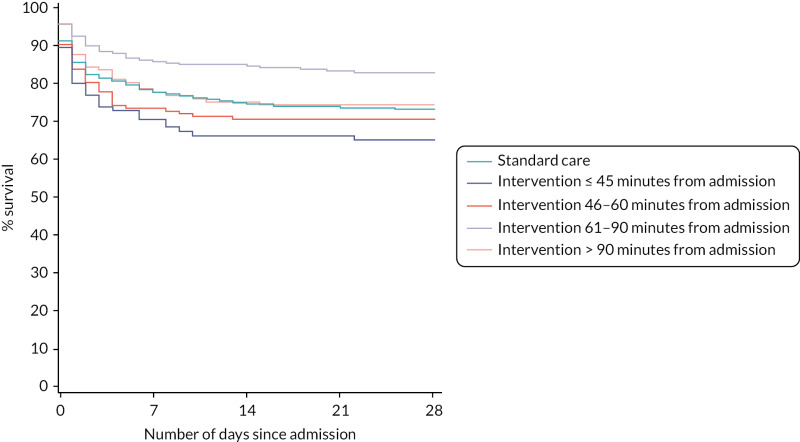
After risk adjustment for statistically significant patient factors (see Appendix 2, Table 20, Figures 11 and 12, for the details of this model), the OR for mortality was 1.15 (95% CI 0.93 to 1.42). Figure 4 presents an unadjusted Kaplan–Meier plot of survival to 28 days by treatment arm.
Similar results were obtained in a per-protocol analysis (those excluded from the per-protocol analysis are summarised in Appendix 2, Table 21). In the per-protocol analysis, 23.1% of those in the intervention arm and 22.5% in the standard care arm died within 28 days of admission (OR 1.03, 95% CI 0.77 to 1.37; p = 0.8272). The relative risk was 1.02 (95% CI 0.82 to 1.27). After adjustment for significant participant factors (as per ITT analysis), the OR was 1.24 (95% CI 1.00 to 1.55).
Similar results were obtained from other sensitivity analyses, including an ITT analysis unadjusted for centre (OR 0.96, 95% CI 0.76 to 1.21) and a sensitivity analysis that assumed that 2% of those discharged died before day 28 (OR 0.95, 95% CI 0.75 to 1.21). A forest plot is presented in Appendix 2, Figure 13.
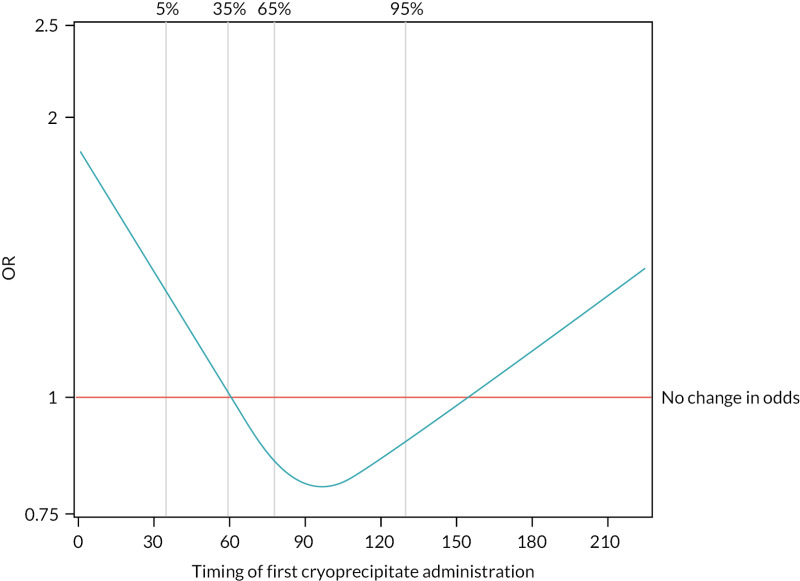
The 28-day mortality rate ranged between 16.5% and 34.4% according to when early cryoprecipitate was given (see Table 5 and Figure 5). Those given early cryoprecipitate 61–90 minutes after admission had significantly lower 28-day mortality than all those in the standard arm (p = 0.0093). Differences between the standard care arm and the other categories of cryoprecipitate timing were non-significant. Figure 6 presents a plot showing the effect of the timing of first cryoprecipitate administration on the odds of 28-day mortality after adjustment for patient factors described in Appendix 2, Table 20. The timing term was fitted as a restricted cubic spline as there was evidence of non-linearity. The plot demonstrates the U-shaped relationship also seen in Table 5.
In a post hoc analysis, the baseline characteristics of participants who were given cryoprecipitate at different times were examined (see Appendix 2, Table 18), and those given cryoprecipitate early were found to be more severely injured and shocked on admission. As there was no placebo, there is no equivalent ‘timing’ in the standard arm, and so a comparison can only be made against the whole standard MHP group, rather than with a similarly severely injured subgroup.
Appendix 2, Table 22 presents the 28-day mortality rate for those in the standard care arm compared with those in the intervention arm who did or did not receive early cryoprecipitate. The 28-day mortality rate was 31.7% among those in the intervention arm who did not receive early cryoprecipitate and 24.0% among those who did. There were no statistically significant differences compared with the standard care arm. However, Appendix 2, Figure 14, demonstrates that in the group who did not receive early cryoprecipitate there were many very early deaths, indicating that the higher mortality rate may be because participants died before cryoprecipitate could be administered. This was confirmed when the analysis was repeated on the per-protocol cohort, from which early deaths were excluded (23.3% mortality among those in the intervention arm who did not receive early cryoprecipitate and 23.1% among those who did).
Table 6 presents the cause of death for the 393 participants who died within 28 days. The primary cause of death in both arms was traumatic brain injury (37%), followed by uncontrolled bleeding (22%).
Subgroup analysis
There was no evidence of a differential effect of early cryoprecipitate on 28-day mortality for four of the five prespecified subgroup analyses: UK versus non-UK participants, head AIS < 4 versus ≥ 4, participant sex, and participant age < 70 versus ≥ 70 years (see Appendix 2, Tables 23–26 and Figures 15–18). However, there was evidence of a differential effect according to whether the participant’s injury was blunt or penetrating (see Table 7 and Figure 7). For penetrating injuries, early cryoprecipitate increased the odds of death compared with those who received only the standard MHP (OR 1.74, 95% CI 1.20 to 2.51). For blunt injuries, early cryoprecipitate decreased the odds of death, but this was not statistically significant (OR 0.82, 95% CI 0.62 to 1.09). There were no apparent differences in baseline characteristics between the two subgroups (see Appendix 2, Table 19). Head AIS < 4 versus ≥ 4 also did not have a differential effect on 6- or 24-hour mortality. Subgroup analysis results are also presented in the forest plot in Appendix 2, Figure 13.
Secondary outcomes
Tables and figures relating to secondary outcomes are presented in Appendix 2 or below. All results relate to the ITT analysis. Per-protocol analysis of certain secondary outcomes was planned in the protocol, and for these outcomes the results were very consistent with those of the ITT analysis.
Mortality data
There were no statistically significant differences between arms in all-cause mortality and deaths from bleeding at 6 and 24 hours (see Appendix 2, Table 27). The median (IQR) time to death from bleeding among those who bled was 191 (81–445) and 86 (40–205) minutes in the intervention and standard care arms, respectively.
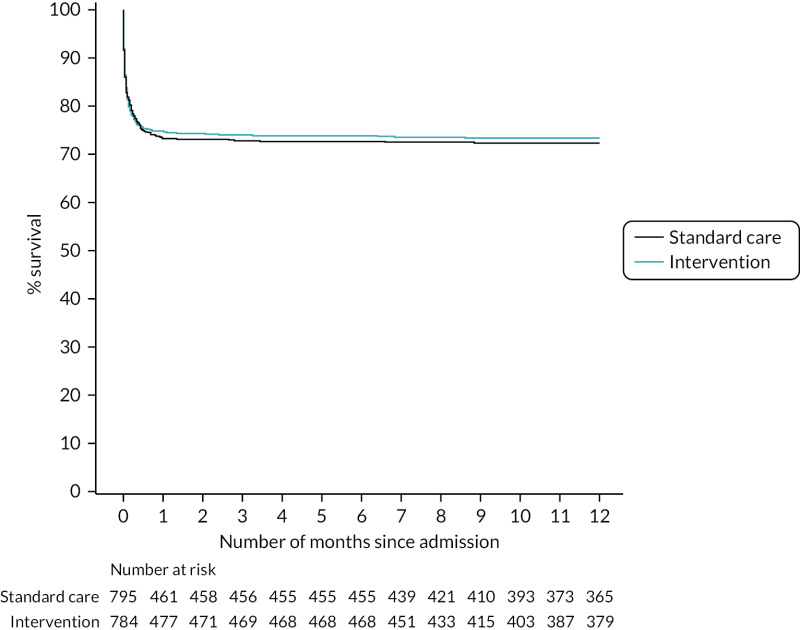
Figure 8 presents an unadjusted Kaplan–Meier plot of survival to 12 months by treatment arm. The estimated mortality rate at 6 months (using the Kaplan–Meier method) was 26.1% for the intervention arm and 27.3% for the standard care arm (see Appendix 2, Table 31). This gave a hazard ratio of 0.96 (95% CI 0.79 to 1.17) for early cryoprecipitate compared with standard MHP and a p-value of 0.6748 for the difference between the arms. After risk adjustment for statistically significant patient factors (Glasgow Coma Scale score, ISS, age, systolic blood pressure and sex), the hazard ratio was 1.08 (95% CI 0.89 to 1.32).
The estimated mortality rate at 12 months was 26.6% for the intervention arm and 27.7% for the standard care arm (see Appendix 2, Table 31). The primary analysis gave the same hazard ratio as at 6 months, with a p-value of 0.7120 for the difference between arms. After risk adjustment for the same statistically significant patient factors, the hazard ratio was 1.09 (95% CI 0.90 to 1.32).
Transfusion requirements
Participants in the intervention arm received more cryoprecipitate per hour from injury to 24 hours (p < 0.0001), but the use of other blood components was similar in the two arms (see Appendix 2, Table 28 and Figures 19 and 20).
Quality of life
Data completeness for the EQ-5D-5L was poor at discharge (59%) and at 6 months (11%). The median index value at discharge was 0.50 in the standard care arm and 0.51 in the intervention arm, and the median self-evaluated health score at discharge was 60 in the standard care arm and 50 in the intervention arm; the difference in medians was not statistically significant for either measure. There was also no statistically significant difference in GOS at discharge between the two arms. At 6 months, the median index value was 0.66 in the standard care arm and 0.76 in the intervention arm and the median self-evaluated health score was 65 in the standard care arm and 75 in the intervention arm (see Appendix 2, Table 29).
Hospital resource use and destination of participant at time of discharge from hospital
There was no statistically significant difference between arms in median ventilator-days, critical care stay or hospital stay (see Appendix 2, Table 30). Forty-nine per cent of participants in each arm were discharged before the end of the study, and the discharge destinations of participants in the two arms were very similar.
Adverse events
The numbers of thromboembolic events and arterial thromboembolic events were similar in the two treatment arms (Table 8). There were three serious transfusion-related adverse events, which all occurred in the intervention arm. Two were anaphylaxis (one reported as unlikely to be related to the intervention and the other reported to be possibly related) and one serious adverse event was reported as potential transfusion-associated circulatory overload.
Figures
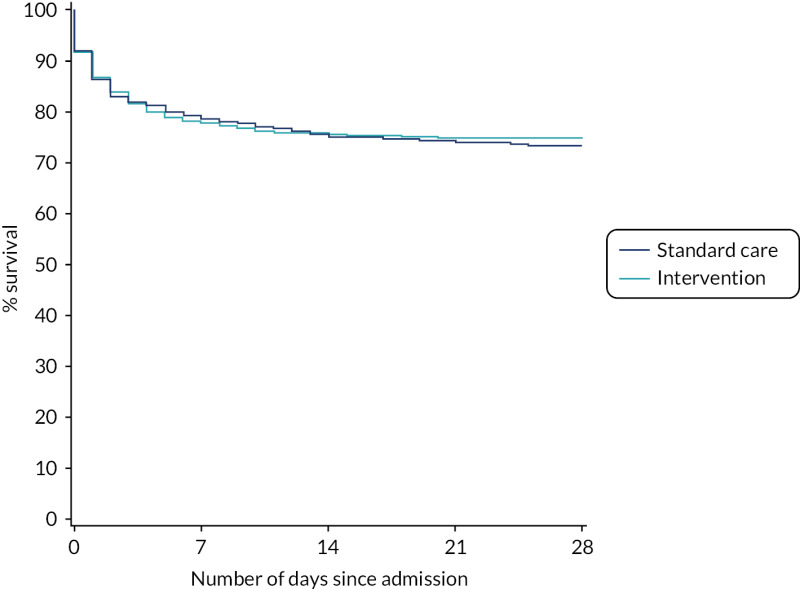
FIGURE 4
Kaplan–Meier survival plot up to 28 days from admission by treatment arm.
FIGURE 6
Effect of timing of first cryoprecipitate administration on 28-day mortality, relative to a baseline participant with first cryoprecipitate administration at 60 minutes, after adjustment for Glasgow Coma Scale score, ISS, participant age and systolic blood pressure (vertical lines indicate the distribution of the data and where the knots were applied for the restricted cubic spline).
Tables
TABLE 3
Baseline characteristics: data are number/total number (%) for categorical variables and median (IQR) for continuous variables
| Characteristic | Standard care arm (n = 805) | Intervention arm (n = 799) | Overall (n = 1604) |
|---|---|---|---|
| Male | 633/796 (80) | 618/785 (79) | 1251/1581 (79) |
| Age (years) | 40 (26–55) | 38 (25–55) | 39 (26–55) |
| Time from injury to admission to emergency department (minutes) | 77 (55–100) | 75 (55–99) | 76 (55–100) |
| Time from admission to randomisation (minutes) | 14 (7–27) | 15 (7–28) | 15 (7–27) |
| Injuries and physiology at admission to emergency department | |||
| Blunt injury | 519/796 (65) | 495/785 (63) | 1014/1581 (64) |
| ISS | 29 (18–43) | 29 (17–43) | 29 (18–43) |
| Head AIS ≥ 4 | 191/664 (29) | 157/665 (24) | 348/1329 (26) |
| Systolic blood pressure (mmHg) | 103 (83–126) | 102 (84–124) | 103 (83–125) |
| Systolic blood pressure < 90 mmHg | 250/738 (34) | 230/724 (32) | 480/1462 (33) |
| Heart rate (per minute) | 108 (88–127) | 108 (88–126) | 108 (88–127) |
| In cardiac arrest | 17/735 (2) | 12/717 (2) | 29/1452 (2) |
| Glasgow Coma Scale score | 13 (3–15) | 14 (3–15) | 14 (3–15) |
| Pre hospital | |||
| Administered any blood components | 348/795 (44) | 323/783 (41) | 671/1578 (43) |
| RBC (units) | 0 (0–2) | 0 (0–2) | 0 (0–2) |
| FFP (units) | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| Crystalloids (ml) | 0 (0–250) | 0 (0–250) | 0 (0–250) |
| Colloids (ml) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| TXA administered | 639/796 (80) | 615/783 (79) | 1254/1579 (79) |
TXA, tranexamic acid.
Note
Summary of missing data: data on all characteristics were missing for 23 participants. In addition, ISS, cardiac arrest and blood pressure were missing for 246, 129 and 119 participants, respectively. There was a small number of missing data for other items.
TABLE 4
Primary outcome
| Outcome | Standard care arm (n = 805) | Intervention arm (n = 799) | Overall (n = 1604) | p-value |
|---|---|---|---|---|
| Participants who died on or before day 28 from admission, n/N (%) | 201/771 (26.1) | 192/760 (25.3) | 393/1531 (25.7) | |
| Relative riska (95% CI) | 0.97 (0.81 to 1.17) | |||
| ORb (95% CI) | 0.96 (0.75 to 1.23) | 0.7406 | ||
| OR also adjusted for participant factorsc (95% CI) | 1.15 (0.93 to 1.42) | |||
| Participants for whom 28-day vital status was not available from any source, n/N (%) | 34/805 (4.2) | 39/799 (4.9) | 73/1604 (4.6) | |
- a
Intervention arm relative to standard care arm, adjusted for centre.
- b
Intervention arm relative to standard care arm, adjusted for centre; p-value for treatment term in mixed logistic regression model.
- c
Intervention arm relative to standard care arm adjusted for centre and significant participant factors.
Notes
Participants for whom 28-day vital status was not available were not included in this analysis. No participants were excluded for other reasons.
Shaded cells are used where the metric is not relevant or where a formal hypothesis test was not planned in the SAP and therefore a p-value is not presented.
TABLE 5
All-cause mortality at 28 days in the standard arm and in the early cryoprecipitate arm by timing of first cryoprecipitate dose
| Cryoprecipitate administered at | Mortality rate, n/N (%) | Relative risk (95% CI)a | OR (95% CI)a | p-valueb |
|---|---|---|---|---|
| Standard care arm | 201/771 (26.1) | 1.00 | 1.00 | |
| Intervention arm ≤ 45 minutes from admission | 33/96 (34.4) | 1.29 (0.94 to 1.77) | 1.45 (0.91 to 2.31) | 0.1195 |
| Intervention arm 46–60 minutes from admission | 42/144 (29.2) | 1.11 (0.84 to 1.48) | 1.16 (0.78 to 1.73) | 0.4550 |
| Intervention arm 61–90 minutes from admission | 44/267 (16.5) | 0.65 (0.46 to 0.91) | 0.57 (0.38 to 0.87) | 0.0093 |
| Intervention arm > 90 minutes from admission | 31/123 (25.2) | 1.00 (0.71 to 1.41) | 1.00 (0.62 to 1.60) | 0.9870 |
- a
Cryoprecipitate administered in the time period relative to standard arm overall, adjusted for centre.
- b
Wald test p-value from logistic regression model, adjusted for centre.
Note
Participants for whom 28-day vital status was not available were not included in this analysis, in addition to 130 participants excluded due to missing timing of cryoprecipitate.
TABLE 6
Causes of death for all-cause mortality at 28 days: n/N (% of those who died)
| Cause of death | Standard care arm, n/N (% of those who died) (N = 805) | Intervention arm, n/N (% of those who died) (N = 799) | Overall, n/N (% of those who died) (N = 1604) |
|---|---|---|---|
| Total deaths within 28 days, n/N (% of those randomised) | 201/771 (26) | 192/760 (25) | 393/1531 (26) |
| Multiorgan failure | 26/201 (13) | 21/192 (11) | 47/393 (12) |
| Multiple injury | 38/201 (19) | 31/192 (16) | 69/393 (18) |
| Myocardial infarction | 0/201 (0) | 3/192 (2) | 3/393 (1) |
| Pulmonary embolism | 1/201 (0) | 0/192 (0) | 1/393 (0) |
| Sepsis | 4/201 (2) | 0/192 (0) | 4/393 (1) |
| Stroke | 0/201 (0) | 5/192 (3) | 5/393 (1) |
| Traumatic brain injury | 78/201 (39) | 67/192 (35) | 145/393 (37) |
| Uncontrolled bleeding | 41/201 (20) | 46/192 (24) | 87/393 (22) |
| Other | 13/201 (6) | 19/192 (10) | 32/393 (8) |
| All | 201/201 (100) | 192/192 (100) | 393/393 (100) |
TABLE 7
All-cause mortality at 28 days by treatment arm: blunt vs. penetrating injury type
| Outcome | Blunt | Penetrating | ||
|---|---|---|---|---|
| Standard care arm (N = 519) | Intervention arm (N = 495) | Standard care arm (N = 277) | Intervention arm (N = 290) | |
| Participants who died on or before day 28 from admission, n/N (%) | 174/500 (34.8) | 147/483 (30.4) | 27/271 (10.0) | 45/277 (16.2) |
| Relative riska (95% CI) | 0.88 (0.72 to 1.06) | 1.62 (1.17 to 2.23) | ||
| ORa (95% CI) | 0.82 (0.62 to 1.09) | 1.74 (1.20 to 2.51) | ||
| p-value for subgroupa | 0.1634 | 0.0058 | ||
| p-value for interaction termb | 0.0040 | |||
| Participants for whom 28-day vital status was not available from any source, n/N (%) | 19/519 (3.7) | 12/495 (2.4) | 6/277 (2.2) | 13/290 (4.5) |
- a
Intervention arm relative to standard care arm, adjusted for centre.
- b
p-value for interaction, adjusted for centre and blunt vs. penetrating injury.
Note
Participants for whom 28-day vital status was not available were not included in this analysis. No participants were excluded for other reasons.
TABLE 8
Safety outcomes
| Outcome | Standard care arm (N = 805) | Intervention arm (N = 799) | Overall (n = 1604) | p-value |
|---|---|---|---|---|
| Thrombotic events, n | 85 | 85 | 170 | |
| Venous thromboembolism | 59 | 58 | 117 | |
| Pulmonary embolus | 36 | 38 | 74 | |
| Deep-vein thrombosis | 23 | 20 | 43 | |
| Participants affected, n/N (%) | 57/805 (7.1) | 55/799 (6.9) | 112/1604 (7.0) | |
| Arterial thrombotic events | 26 | 27 | 53 | |
| Myocardial infarction | 4 | 4 | 8 | |
| Stroke | 11 | 11 | 22 | |
| Other occlusion of any other artery | 11 | 12 | 23 | |
| Participants affected, n/N (%) | 26/805 (3.2) | 26/799 (3.3) | 52/1604 (3.2) | |
| Cumulative incidence of thrombotic events at day 28, % (95% CI)a | 12.9 (10.2 to 15.8) | 12.7 (10.1 to 15.6) | 12.8 (10.9 to 14.8) | 0.8852 |
| Serious transfusion-related adverse events, n/N (%)b | 0/805 (0) | 3/799 (0.4) | 3/1604 (0.2) | 0.1234 |
- a
p-value from Fine and Gray model.
- b
p-value for Fisher’s exact test.