By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.

Immunobiology: The Immune System in Health and Disease. 5th edition.
Show detailsThe ability of high-affinity antibodies to neutralize toxins, viruses, or bacteria can protect against infection but does not, on its own, solve the problem of how to remove the pathogens and their products from the body. Moreover, many pathogens cannot be neutralized by antibody and must be destroyed by other means. Many pathogen-specific antibodies do not bind to neutralizing targets on pathogen surfaces and thus need to be linked to other effector mechanisms in order to play their part in host defense. We have already seen how antibody binding to antigen can activate complement. Another important defense mechanism is the activation of a variety of accessory effector cells bearing receptors called Fc receptors because they are specific for the Fc portion of antibodies of a particular isotype. Through these receptors, accessory cells dispose of neutralized microorganisms and attack resistant extracellular pathogens. This mechanism maximizes the effectiveness of all antibodies regardless of where they bind. Accessory cells include the phagocytic cells (macrophages and neutrophils), which ingest antibodycoated bacteria and kill them, and other cells—natural killer (NK) cells, eosinophils, basophils, and mast cells (see Fig. 1.4)—which are triggered to secrete stored mediators when their Fc receptors are engaged. Accessory cells are activated when their Fc receptors are aggregated by binding to the multiple Fc regions of antibody molecules coating a pathogen. They can also be activated by soluble mediators, which include products of the complement cascade, which can itself be activated by antibody.
9-19. The Fc receptors of accessory cells are signaling receptors specific for immunoglobulins of different isotypes
The Fc receptors are a family of cell-surface molecules that bind the Fc portion of immunoglobulins. Each member of the family recognizes immunoglobulin of one isotype or a few closely related isotypes through a recognition domain on the α chain of the Fc receptor. Fc receptors are themselves members of the immunoglobulin superfamily. Different accessory cells bear Fc receptors for antibodies of different isotypes, and the isotype of the antibody thus determines which accessory cell will be engaged in a given response. The different Fc receptors, the cells that express them, and their isotype specificity are shown in Fig. 9.30.
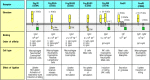
Figure 9.30
Distinct receptors for the Fc region of the different immunoglobulin isotypes are expressed on different accessory cells. The subunit structure and binding properties of these receptors and the cell types expressing them are shown. The complete multimolecular (more...)
Most Fc receptors function as part of a multisubunit complex. Only the α chain is required for specific recognition; the other chains are required for transport to the cell surface and for signal transduction when an Fc region is bound. Signal transduction by many of these Fc receptors is mediated by the γ chain, which is closely related to the ζ chain of the T-cell receptor complex. Some Fcγ receptors, the Fcα receptor, and the high-affinity receptor for IgE use a γ chain for signaling; an exception is human FcγRII-A, a single-chain receptor in which the cytoplasmic domain of the α chain replaces the function of the γ chain. FcγRII-B1 and FcγRII-B2 are also single-chain receptors but function as inhibitory receptors as they contain an ITIM that engages the inositol 5′-phosphatase SHIP (see Section 6-14). Although the most prominent function of Fc receptors is the activation of accessory cells to attack pathogens, they can also contribute in other ways to immune responses. For example, the FcγRII-B receptor negatively regulates B cells, mast cells, macrophages, and neutrophils by adjusting the threshold at which immune complexes will activate these cells. Fc receptors expressed by dendritic cells enable them to ingest antigen:antibody complexes and present antigenic peptides to T cells.
9-20. Fc receptors on phagocytes are activated by antibodies bound to the surface of pathogens and enable the phagocytes to ingest and destroy pathogens
Phagocytes are activated by IgG antibodies, especially IgG1 and IgG3, that bind to specific Fcγ receptors on the phagocyte surface (see Fig. 9.30). As phagocyte activation can initiate an inflammatory response and cause tissue damage, it is essential that the Fc receptors on phagocytes are able to distinguish antibody molecules bound to a pathogen from the much larger number of free antibody molecules that are not bound to anything. This distinction is made possible by the aggregation or multimerization of antibodies that occurs when they bind to multimeric antigens or to multivalent antigenic particles such as viruses and bacteria. Fc receptors on the surface of an accessory cell bind antibody-coated particles with higher avidity than immunoglobulin monomers, and this is probably the principal mechanism by which bound antibodies are distinguished from free immunoglobulin (Fig. 9.31). The result is that Fc receptors enable accessory cells to detect pathogens through bound antibody molecules. Thus, specific antibody together with Fc receptors gives accessory cells that lack intrinsic specificity the ability to identify and remove pathogens and their products from the extracellular spaces.
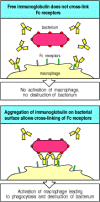
Figure 9.31
Bound antibody is distinguishable from free immunoglobulin by its state of aggregation. Free immunoglobulin molecules bind most Fc receptors with very low affinity and can not cross-link Fc receptors. Antigen-bound immunoglobulin, however, can bind effectively (more...)
The most important accessory cells in humoral immune responses are the phagocytic cells of the monocytic and myelocytic lineages, particularly macrophages and neutrophils (see Chapter 2). Many bacteria are directly recognized, ingested, and destroyed by phagocytes, and these bacteria are not pathogenic in normal individuals (see Chapter 2). Bacterial pathogens, however, often have polysaccharide capsules that allow them to resist direct engulfment by phagocytes. These bacteria become susceptible to phagocytosis, however, when they are coated with antibody and complement that engages the Fcγ or Fcα receptors and CR1 on phagocytic cells, triggering bacterial uptake (Fig. 9.32). Phagocytosis by binding to complement receptors is particularly important early in the immune response, before isotypeswitched antibodies have been made. Capsular polysaccharides belong to the TI-2 class of thymus-independent antigens (see Section 9-11) and therefore can stimulate the early production of IgM antibodies. IgM is not an opsonizing antibody in itself, as there are no Fc receptors for IgM, but it is effective at activating the complement system. IgM binding to encapsulated bacteria thus triggers opsonization of these bacteria by complement and their prompt ingestion and destruction by phagocytes bearing complement receptors.
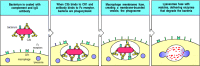
Figure 9.32
Fc and complement receptors on phagocytes trigger the uptake and degradation of antibody-coated bacteria. Many bacteria resist phagocytosis by macrophages and neutrophils. Antibodies bound to these bacteria, however, enable them to be ingested and degraded (more...)
Both the internalization and destruction of microorganisms are greatly enhanced by interactions between the molecules coating an opsonized microorganism and their receptors on the phagocyte surface. When an antibody-coated pathogen binds to Fcγ receptors on the surface of a phagocyte, for example, the cell surface extends around the surface of the particle through successive binding of Fcγ receptors to the antibody Fc regions bound to the pathogen surface. This is an active process triggered by the stimulation of Fcγ receptors. Endocytosis leads to enclosure of the particle in an acidified cytoplasmic vesicle called a phagosome. The phagosome then fuses with one or more lysosomes to generate a phagolysosome, releasing the lysosomal enzymes into the phagosome interior where they destroy the bacterium (see Fig. 9.32). The process of bacterial destruction in the phagolysosome was described in detail in Section 2-3.
Some particles are too large for a phagocyte to ingest; parasitic worms are one example. In this case, the phagocyte attaches to the surface of the antibody-coated parasite via its Fcγ, Fcα, or Fcε receptors, and the lysosomes fuse with the attached surface membrane. This reaction discharges the contents of the lysosome onto the surface of the parasite, damaging it directly in the extracellular space. While the principal phagocytes in the destruction of bacteria are macrophages and neutrophils, large parasites such as helminths are more usually attacked by eosinophils (Fig. 9.33). Thus, Fcγ and Fcα receptors can trigger the internalization of external particles by phagocytosis, or the externalization of internal vesicles by exocytosis. Cross-linking of IgE bound to the high-affinity FcεRI usually results in exocytosis. We will see in the next three sections that natural killer cells and mast cells also release mediators stored in their vesicles when their Fc receptors are aggregated.

Figure 9.33
Eosinophils attacking a schistosome larva in the presence of serum from an infected patient. Large parasites, such as worms, cannot be ingested by phagocytes; however, when the worm is coated with antibody, especially IgE, eosinophils can attack it through (more...)
9-21. Fc receptors activate natural killer cells to destroy antibody-coated targets
Infected cells are usually destroyed by T cells alerted by foreign peptides bound to cell-surface MHC molecules. However, virus-infected cells can also signal the presence of intracellular infection by expressing on their surfaces viral proteins that can be recognized by antibodies. Cells bound by such antibodies can then be killed by a specialized non-T, non-B lymphoid cell called a natural killer cell (NK cell), which we met earlier in Chapter 2. NK cells are large lymphoid cells with prominent intracellular granules; they make up a small fraction of peripheral blood lymphoid cells. They bear no known antigen-specific receptors but are able to recognize and kill a limited range of abnormal cells. They were first discovered because of their ability to kill some tumor cells but are now known to have an important role in innate immunity.
The destruction of antibody-coated target cells by NK cells is called antibody-dependent cell-mediated cytotoxicity (ADCC) and is triggered when antibody bound to the surface of a cell interacts with Fc receptors on the NK cell (Fig. 9.34). NK cells express the receptor FcγRIII (CD16), which recognizes the IgG1 and IgG3 subclasses and triggers cytotoxic attack by the NK cell on antibodycoated target cells. The mechanism of attack is exactly analogous to that of cytotoxic T cells, involving the release of cytoplasmic granules containing perforin and granzymes (see Section 8-22). The importance of ADCC in defense against infection with bacteria or viruses has not yet been fully established. However, ADCC represents yet another mechanism by which, through engaging an Fc receptor, antibodies can direct an antigen-specific attack by an effector cell that itself lacks specificity for antigen.

Figure 9.34
Antibody-coated target cells can be killed by NK cells in antibody-dependent cell-mediated cytotoxicity (ADCC). NK cells (see Chapter 2) are large granular non-T, non-B lymphoid cells that have FcγRIII (CD16) on their surface. When these cells (more...)
9-22. Mast cells, basophils, and activated eosinophils bind IgE antibody via the high-affinity Fcε receptor
When pathogens cross epithelial barriers and establish a local focus of infection, the host must mobilize its defenses and direct them to the site of pathogen growth. One mechanism by which this is achieved is to activate a specialized cell type known as a mast cell. Mast cells are large cells containing distinctive cytoplasmic granules that contain a mixture of chemical mediators, including histamine, that act rapidly to make local blood vessels more permeable. Mast cells have a distinctive appearance after staining with the dye toluidine blue that makes them readily identifiable in tissues (see Fig. 1.4). They are found in particularly high concentrations in vascularized connective tissues just beneath body epithelial surfaces, including the submucosal tissues of the gastrointestinal and respiratory tracts and the dermis that lies just below the surface of the skin.
Mast cells can be activated to release their granules, and to secrete lipid inflammatory mediators and cytokines, via antibody bound to Fc receptors specific for IgE (FcεRI) and IgG (FcγRIII). We have seen earlier that most Fc receptors bind stably to the Fc region of antibodies only when these are bound to antigen. By contrast, FcεRI binds monomeric IgE antibodies with a very high affinity, measured at approximately 1010 M-1. Thus, even at the low levels of IgE found circulating in normal individuals, a substantial portion of the total IgE is bound to the FcεRI on mast cells and on circulating basophilic granulocytes or basophils. Eosinophils can also express Fc receptors, but only express FcεRI when activated and recruited to an inflammatory site.
Although mast cells are usually stably associated with bound IgE, they are not activated simply by the binding of monomeric antigens to this IgE. Mast-cell activation only occurs when the bound IgE is cross-linked by multivalent antigen. This signal activates the mast cell to release the contents of its granules, which occurs in seconds (Fig. 9.35), and to synthesize and release lipid mediators such as prostaglandin D2 and leukotriene C4, and to secrete cytokines such as TNF-α, thereby initiating a local inflammatory response. Degranulation releases the stored histamine, causing a local increase in blood flow and vascular permeability that quickly leads to accumulation of fluid and blood proteins, including antibodies, in the surrounding tissue. Shortly afterwards, there is an influx of blood-borne cells such as polymorphonuclear leukocytes and later macrophages, eosinophils, and effector lymphocytes. This influx can last a few minutes to a few hours and produces an inflammatory response at the site of infection. Thus, mast cells are part of the front-line host defenses against pathogens that enter the body across epithelial barriers.
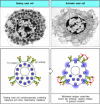
Figure 9.35
IgE antibody cross-linking on mast-cell surfaces leads to a rapid release of inflammatory mediators. Mast cells are large cells found in connective tissue that can be distinguished by secretory granules containing many inflammatory mediators. They bind (more...)
9-23. IgE-mediated activation of accessory cells has an important role in resistance to parasite infection
Mast cells are thought to serve at least three important functions in host defense. First, their location near body surfaces allows them to recruit both specific and nonspecific effector elements to sites where infectious agents are most likely to enter the internal milieu. Second, they also increase the flow of lymph from sites of antigen deposition to the regional lymph nodes, where naive lymphocytes are first activated. Third, their ability to trigger muscular contraction can contribute to the physical expulsion of pathogens from the lungs or the gut. Mast cells respond rapidly to the binding of antigen to surfacebound IgE antibodies, and their activation leads to the recruitment and activation of basophils and eosinophils, which contribute further to the IgE-mediated response. There is increasing evidence that such IgE-mediated responses are crucial to defense against parasite infestation.
A role for mast cells in the clearance of parasites is suggested by accumulation of mast cells in the intestine, known as mastocytosis, that accompanies helminth infection, and by observations in W/WV mutant mice, which have a profound mast-cell deficiency caused by mutation of the gene c-kit. These mutant mice show impaired clearance of the intestinal nematodes Trichinella spiralis and Strongyloides species. Clearance of Strongyloides is even more impaired in W/WV mice that lack IL-3 and therefore, in addition to lacking mast cells, fail to produce basophils. Thus both mast cells and basophils seem to contribute to defense against these helminth parasites. Other evidence also points to the importance of IgE antibodies and eosinophils in defense against parasites. Infections by certain classes of parasite, particularly helminths, are strongly associated with the production of IgE antibodies and the presence of an abnormally large number of eosinophils (eosinophilia) in blood and tissues. Furthermore, experiments in mice show that depletion of eosinophils by using polyclonal anti-eosinophil antisera increases the severity of infection by the parasitic helminth Schistosoma mansoni. Eosinophils seem to be directly responsible for helminth destruction; examination of infected tissues shows degranulated eosinophils adhering to helminths, and experiments in vitro have shown that eosinophils can kill Schistosoma mansoni in the presence of specific IgE (see Fig. 9.33), IgG, or IgA anti-schistosome antibodies.
The role of IgE, mast cells, basophils, and eosinophils can also be seen in resistance to the feeding of blood-sucking ixodid ticks. Normal skin at the site of a tick bite shows degranulated mast cells, and an accumulation of basophils and eosinophils that are degranulated, an indicator of recent activation. Resistance to subsequent feeding by these ticks develops after the first exposure, suggesting a specific immunological mechanism. Mast-cell deficient mice show no such acquired resistance to tick species, and in guinea pigs the depletion of either basophils or eosinophils by specific polyclonal antibodies also reduces resistance to tick feeding. Finally, recent experiments have shown that resistance to ticks in mice is mediated by specific IgE antibody.
Thus, many clinical studies and experiments support a role for this system of IgE binding to the high-affinity FcεRI in host resistance to pathogens that enter across epithelia. We will see later, in Chapter 12, that the same system accounts for many of the symptoms in allergic diseases such as asthma, hayfever, and the life-threatening response known as systemic anaphylaxis.
Summary
Antibody-coated pathogens are recognized by accessory effector cells through Fc receptors that bind to the multiple constant regions (Fc portions) provided by the bound antibodies. Binding activates the accessory cell and triggers destruction of the pathogen. Fc receptors comprise a family of proteins, each of which recognizes immunoglobulins of particular isotypes. Fc receptors on macrophages and neutrophils recognize the constant regions of IgG or IgA antibodies bound to a pathogen and trigger the engulfment and destruction of IgG- or IgA-coated bacteria. Binding to the Fc receptor also induces the production of microbicidal agents in the intracellular vesicles of the phagocyte. Eosinophils are important in the elimination of parasites too large to be engulfed; they bear Fc receptors specific for the constant region of IgG, as well as high-affinity receptors for IgE; aggregation of these receptors triggers the release of toxic substances onto the surface of the parasite. NK cells, tissue mast cells, and blood basophils also release their granule contents when their Fc receptors are engaged. The high-affinity receptor for IgE is expressed constitutively by mast cells and basophils, and is induced in activated eosinophils. It differs from other Fc receptors in that it can bind free monomeric antibody, thus enabling an immediate response to pathogens at their site of first entry into the tissues. When IgE bound to the surface of a mast cell is aggregated by binding to antigen, it triggers the release of histamine and many other mediators that increase the blood flow to sites of infection; it thereby recruits antibodies and effector cells to these sites. Mast cells are found principally below epithelial surfaces of the skin and the digestive and respiratory tracts, and their activation by innocuous substances is responsible for many of the symptoms of acute allergic reactions, as will be described in Chapter 12.
- The Fc receptors of accessory cells are signaling receptors specific for immunoglobulins of different isotypes
- Fc receptors on phagocytes are activated by antibodies bound to the surface of pathogens and enable the phagocytes to ingest and destroy pathogens
- Fc receptors activate natural killer cells to destroy antibody-coated targets
- Mast cells, basophils, and activated eosinophils bind IgE antibody via the high-affinity Fcε receptor
- IgE-mediated activation of accessory cells has an important role in resistance to parasite infection
- Summary
- The destruction of antibody-coated pathogens via Fc receptors - ImmunobiologyThe destruction of antibody-coated pathogens via Fc receptors - Immunobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...