Diabetes in America is in the public domain of the United States. You may use the work without restriction in the United States.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lawrence JM, Casagrande SS, Herman WH, et al., editors. Diabetes in America [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); 2023-.
Summary
Interventions to address obesity are recommended as treatments for type 2 diabetes. In this article, nationally representative data are used to describe behavioral, pharmacologic, and surgical interventions employed by adults with diabetes for obesity treatment. Literature focused on youth with type 2 diabetes is also reviewed.
Data from the National Health and Nutrition Examination Survey (NHANES) 2015–2018 showed that only 2.3% of U.S. adults with diagnosed diabetes had Healthy Eating Index (HEI) scores ≥80 indicating a “good” diet, while 44.6% had HEI scores <50 indicating a “poor” diet. Data from the NHANES 2015–2020 showed that only 47.0% of U.S. adults with diabetes reported meeting physical activity standards, 15.6% achieved less than the recommended amount of physical activity, and 37.4% reported no physical activity. Between 2015 and 2020, NHANES data showed that 36.7% of U.S. adults with diagnosed diabetes reported attempting to lose weight in the past year. In order of decreasing frequency, the following weight reduction strategies supported by scientific evidence were reported: eating less, eating healthier, exercising, eating special diets, joining weight loss programs, taking prescription medications, and undergoing weight loss surgery.
The American Diabetes Association recommends that providers and patients consider the impact of antihyperglycemic medications on weight. Data from the NHANES 2015–2020 showed that metformin, which is associated with modest weight loss, was the most frequently prescribed antihyperglycemic agent (43.8%). Sulfonylureas and insulins, which are associated with weight gain, were the next most frequently prescribed classes of antihyperglycemic medications (23.2% and 22.0%, respectively). The frequency of prescribing glucagon-like peptide-1 (GLP-1) receptor agonists (RAs), which are associated with weight loss, was 5.0%. Rates of GLP-1 RA use among commercially insured adult patients with type 2 diabetes increased substantially between 2015 and 2019 (3.2% to 10.7%) and have increased more in recent years but remain lower among Asian, Black, and Hispanic patients, those with lower incomes, and among adult patients with atherosclerotic cardiovascular disease, who are known to benefit from this class of medications.
Data from the Healthcare Cost and Utilization Project 2019 showed that metabolic (bariatric) surgery was infrequently performed as an inpatient (~1.4 per 100 per year) or outpatient (~2.0 per 100 per year) procedure among U.S. adults with diagnosed type 2 diabetes despite an increase in the total number of metabolic surgery procedures performed between 2015 and 2019 (n=163,072 to 202,804). The American Diabetes Association recommends considering metabolic surgery for adolescents with type 2 diabetes and body mass index (BMI) ≥35 kg/m2. Anti-obesity medications appear to be effective but require further study in youth.
Introduction
Insulin resistance and progressive pancreatic beta cell dysfunction are hallmarks of type 2 diabetes mellitus (1). Obesity contributes to the development of hyperglycemia as excess body fat contributes to elevations in circulating free fatty acids and adipocyte-derived cytokines that mediate insulin resistance through inflammatory pathways (2,3).
In patients with obesity and type 2 diabetes, intensive lifestyle interventions including low- or very low-energy diets, pharmacologic therapies, including some classes of antihyperglycemic agents approved for diabetes and others specifically approved for weight loss, and metabolic surgery can improve glycemia and induce remission in type 2 diabetes (4,5,6,7,8,9). Weight loss-induced remissions of type 2 diabetes are most likely to occur early in the natural history of type 2 diabetes when obesity-associated insulin resistance has caused reversible beta cell dysfunction but insulin secretory capacity remains relatively preserved (10,11).
The goal of this article is to provide national data describing dietary, physical activity, and other weight loss strategies, including pharmacologic and surgical interventions for obesity, as treatments for adults with type 2 diabetes. These analyses are supplemented by clinical trial and observational evidence when no or scant evidence exists from the population data. Published literature on obesity treatment among youth with type 2 diabetes is summarized at the end of this article.
Sources and Limitations of National Data on Obesity Management in Diabetes
National Health and Nutrition Examination Survey
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional, national probability sample that has been conducted periodically since 1971 and continuously since 1999. This article utilizes NHANES data collected from individuals for the period 2015–2020 to assess dietary intake, physical activity, and other strategies to lose weight and antihyperglycemic medication use. Participants self-reported diagnosed diabetes status based on the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Analysis of antihyperglycemic medication use excluded adults with probable type 1 diabetes, defined as current insulin use alone and continuous insulin use within 1 year of diagnosis. An advantage of the NHANES is that the survey includes a health exam in a mobile examination center where height and weight were measured by a trained examiner to determine body mass index (BMI, kg/m2). The Healthy Eating Index (HEI) (12) was utilized to assess how dietary patterns aligned with federal dietary guidelines; a score of 100 indicates 100% alignment with the guidelines. Participants self-reported dietary intake (24-hour dietary recalls), physical activity (minutes of moderate and vigorous work-related and leisure time activity), strategies to lose weight among those who reported trying to lose weight in the past year, and use of prescription antihyperglycemic medications. Participants were asked to bring their prescription medication bottles to the interview for verification. Due to the small sample size, data from youth with diabetes could not be evaluated in the NHANES.
Healthcare Cost and Utilization Project (National [Nationwide] Inpatient Sample and Nationwide Ambulatory Surgery Sample)
The Healthcare Cost and Utilization Project (HCUP) National (Nationwide) Inpatient Sample (NIS) is the largest publicly available all-payer inpatient health care database designed to produce U.S. national estimates of in-patient utilization, access, cost, quality, and outcomes. The HCUP-NIS estimates around 35 million hospitalizations nationally. Data from the HCUP-NIS 2019 were used to estimate inpatient metabolic surgery discharges among adults with type 2 diabetes.
The HCUP Nationwide Ambulatory Surgery Sample (NASS) is the largest all-payer ambulatory surgery database in the United States that produces national estimates of major ambulatory surgery encounters in hospital-owned facilities. Data from the HCUP-NASS 2019 were used to estimate ambulatory metabolic surgery discharges among adults with type 2 diabetes.
Statistical Methods
All results from national surveys were statistically weighted to produce estimates that are representative of the noninstitutionalized U.S. population. Weighted standard errors for the estimates are shown in the tables. In cases where the sample size is small and the results may not be representative of the population, the relative standard error (RSE = [SE/estimate]*100) is provided in the tables. If the RSEs were >50%, the results are not shown.
Diet, Physical Activity, and Other Weight Loss Strategies
Diet and physical activity to achieve and maintain ≥5% weight loss are recommended for most people with overweight or obesity and type 2 diabetes (13,14). Weight loss ≥15% has been associated with remission of type 2 diabetes in individuals with overweight or obesity and relatively recent onset (≤5 years) type 2 diabetes (15). Effective lifestyle interventions generally involve frequent contact (≥16 sessions in 6 months), behavioral counseling to achieve a 500–750 kcal energy deficit per day, and 150–180 minutes of moderate-intensity physical activity per week (13,14). Long-term weight loss maintenance programs are recommended to provide ongoing monitoring of body weight and behavioral support and to encourage increased moderate-intensity physical activity (200–300 minutes per week) (13,14).
Diet
Table 1 shows scores based on the 2015 version of the HEI for U.S. adults with and without diagnosed diabetes between 2015 and 2018 by age, sex, race and ethnicity, and BMI. HEI scores range from 0 to 100, with higher scores indicating better diet quality (independent of quantity) and, therefore, better alignment with U.S. Department of Agriculture dietary guidelines (16). A score of 100 indicates perfect alignment with federal dietary guidelines. HEI scores ≥80 indicate a “good” diet, scores ranging from 50 to 79 reflect a diet that “needs improvement,” and HEI scores <50 imply a “poor” diet (17).
TABLE 1.
Healthy Eating Index Among Adults With and Without Diagnosed Diabetes, by Age, Sex, Race and Ethnicity, and BMI, U.S., 2015–2018
The mean HEI score was 53.1 among the 24,816,593 U.S. adults with diabetes: only 2.3% (n=571,868) had HEI scores ≥80, 53.1% (n=13,172,903) had HEI scores 50–79, and 44.6% (n=11,071,822) had HEI scores <50. In general, mean HEI scores increased with age. There was little difference between men and women. HEI scores were substantially higher among non-Hispanic Asian people compared to non-Hispanic White, non-Hispanic Black, and Hispanic people. Mean HEI scores did not differ substantially among non-Asian people by BMI category but were highest among those with BMIs between 25.0 and 34.9 kg/m2. HEI scores were highest among Asian people in the BMI 23.0–27.4 kg/m2 category. The percentage of the population with HEI scores ≥80 was highest among Asian people.
HEI scores and patterns were similar among U.S. adults with and without diagnosed diabetes.
Physical Activity
Table 2 shows self-reported physical activity among U.S. adults with and without diagnosed diabetes between 2015 and 2020 by age, sex, race and ethnicity, and BMI. Physical activity is categorized as meeting physical activity standards, less than recommended amount of physical activity, and no physical activity. Physical activity standards are defined as ≥150 minutes of self-reported moderate or ≥75 minutes of self-reported vigorous leisure-time or work-related physical activity per week (18).
TABLE 2.
Physical Activity Among Adults With and Without Diagnosed Diabetes, by Age, Sex, Race and Ethnicity, and BMI, U.S., 2015–2020
Between 2015 and 2020, 47.0% of U.S. adults with diabetes reported meeting physical activity standards (n=12,773,838), 15.6% reported achieving less than the recommended amount of physical activity (n=4,242,905), and 37.4% reported no physical activity (n=10,174,670). When adherence to physical activity standards is assessed by self-report, it is substantially higher than objectively measured physical activity assessed using accelerometers (19). The percentage of Americans with diabetes meeting physical activity standards decreased with age, and the percentage reporting no physical activity increased with age. Men with diabetes were more likely than women with diabetes to meet physical activity standards, and men were less likely than women to report no physical activity.
Non-Hispanic White and Hispanic people with diabetes were more likely to meet physical activity standards than non-Hispanic Black people with diabetes. Non-Hispanic Asian people with diabetes were least likely to meet physical activity standards. Similar trends were observed when the population with diabetes was stratified by sex and race and ethnicity. Within each race and ethnicity subgroup, men were more likely to meet physical activity standards than women, and women were more likely to report no physical activity than men. The groups most likely to report no physical activity were non-Hispanic Asian women (55.7%) and Hispanic women (51.5%) with diabetes. Among non-Asian people, those with the highest BMI (≥40.0 kg/m2) were least likely to meet physical activity standards and most likely to report no physical activity. Among Asian people, those with the highest BMI (≥27.5 kg/m2) were most likely to meet physical activity standards and least likely to report no physical activity.
A smaller percentage of U.S. adults with diagnosed diabetes reported meeting physical activity standards than U.S. adults without diagnosed diabetes (47.0% vs. 62.1%). Whether stratified by age group, sex, or race and ethnicity, U.S. adults with diagnosed diabetes were less likely to report meeting physical activity standards than U.S. adults without diagnosed diabetes.
Other Weight Loss Strategies
Table 3 shows the percentages and numbers of U.S. adults with diagnosed diabetes who reported attempting to lose weight in the past year by age, sex, race and ethnicity, and BMI and the strategies they reported using to lose weight. Between 2015 and 2020, 36.7% of adults with diagnosed diabetes reported attempting to lose weight in the past year. In order of decreasing frequency, people with diagnosed diabetes who were attempting to lose weight reported using the following weight reduction strategies supported by scientific evidence: eating less, eating healthier, exercising, eating special diets, joining weight loss programs, and taking prescription medications; the prevalence of undergoing weight loss surgery was too small to estimate. In order of decreasing frequency, individuals also reported engaging in the following strategies that lack evidence or are not recommended for weight loss: skipping meals, taking nonprescription supplements, and taking laxatives, vomiting, or smoking.
TABLE 3.
Strategies to Lose Weight Among Adults With Diagnosed Diabetes Who Reported Attempting to Lose Weight, by Age, Sex, Race and Ethnicity, and BMI, U.S., 2015–2020
Of the 10,000,691 adults with diabetes who reported attempting to lose weight in the past year, 96.0% (n=9,598,120) reported eating less food, switching to foods with lower calories, changing eating habits, eating more fruits, vegetables, and salads, and drinking a lot of water. Of adults with diabetes who reported attempting to lose weight, 72.2% (n=7,218,276) reported eating healthier, including eating less fat, fewer carbohydrates, less sugar, candy, and sweets, and eating less junk food or fast food. In addition, 46.8% (n=4,680,650) reported exercising to lose weight; 22.1% (n=2,210,120) reported eating special diets (diet foods or products, using a liquid diet formula, such as Slimfast or Optifast, or following a special diet); 4.6% (n=458,830) reported joining a weight loss program, such as Weight Watchers, Jenny Craig, TOPS, or Overeaters Anonymous; 1.5% (n=149,374) reported taking diet pills prescribed by a doctor; and fewer than 1% (n=6,863) reported having weight loss surgery. Finally, 23.4% (n=2,343,137) of adults with diabetes reported skipping meals to lose weight; 3.5% (n=348,717) reported taking other pills, medicines, herbs, or supplements not needing a prescription; and 1.4% (n=143,686) reported taking laxatives or vomiting or starting to smoke or beginning to smoke again to lose weight.
Evidence-Based Strategies to Lose Weight by Age, Sex, Race and Ethnicity, and BMI
Among those with diabetes who reported using evidence-based strategies to lose weight, there was little difference by age or sex among those who reported eating less or reducing calories to lose weight. Non-Hispanic White and Hispanic people were more likely to report eating less or reducing calories to lose weight compared to non-Hispanic Black and non-Hispanic Asian people. Among non-Asian people with diabetes, those with BMIs 18.0–24.9 and ≥30 kg/m2 were more likely to report eating less and restricting calories. Adults ages 20–44 and ≥65 years were more likely to report eating healthier to lose weight compared to those age 45–64 years. Men with diabetes were more likely to report eating healthier than women, and non-Hispanic White, non-Hispanic Black, and Hispanic people were all more likely to report eating healthier to lose weight than non-Hispanic Asian people. Non-Asian people with BMIs <30.0 and ≥40.0 kg/m2 were likely to report eating healthier than individuals with BMI 30.0–39.9 kg/m2. Individuals age 20–44 years and women were more likely to report eating special diets to lose weight, and women were more likely to report having joined a weight loss program than men.
U.S. adults age 20–64 years, men, and non-Hispanic Black and non-Hispanic Asian people were more likely to report exercising to lose weight. Non-Asian people with lower BMIs were more likely to report exercising to lose weight than non-Asian people with higher BMIs.
Non-Evidence-Based Strategies to Lose Weight by Age, Sex, Race and Ethnicity, and BMI
Among those with diabetes who reported using non-evidence-based strategies to lose weight, younger people and men were more likely to report skipping meals to lose weight. Non-Hispanic Black people were most likely to report skipping meals, and Non-Hispanic Asian people were least likely to report skipping meals to lose weight. Non-Asian people with higher BMIs were more likely to report skipping meals to lose weight. Use of nonprescription supplements was more frequently reported by younger people and women. Taking laxatives, vomiting, and smoking (particularly harmful behaviors) among individuals trying to lose weight were infrequently reported, and there were no clear trends by age, sex, race and ethnicity, or BMI.
Pharmacologic Therapies
The American Diabetes Association (ADA) recommends a patient-centered approach to choosing pharmacologic therapies for patients with type 2 diabetes (20). The approach considers the impact of antihyperglycemic medications on weight. Accordingly, the ADA has classified the various classes of antihyperglycemic medications by their impact on weight. GLP-1 RAs, the dual GLP-1 RA/glucose-dependent insulinotropic polypeptide (GIP) co-agonist, and sodium-glucose cotransporter-2 (SGLT2) inhibitors are classified as being associated with weight loss; metformin is classified as being associated with the potential for modest weight loss; dipeptidyl peptidase-4 (DPP-4) inhibitors are classified as being weight neutral; and sulfonylureas, thiazolidinediones, and insulin are classified as being associated with weight gain. Although alpha-glucosidase inhibitors and amylin analogues have been associated with varying degrees of weight loss, their utilization was too infrequent to report herein. Because of their substantial impact on body weight, a number of GLP-1 RAs and one GLP-1 RA/GIP co-agonist, tirzepatide, have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of obesity. Other classes of medications, including glucocorticoids, hormonal contraceptives containing progestins, beta-blockers, atypical antipsychotics, some antidepressants, and some anticonvulsant medications, have been shown to contribute to weight gain. Guidelines published by the Endocrine Society in 2015 caution clinicians about prescribing medications associated with weight gain, when possible, in patients with obesity (21).
Table 4 shows the use of antihyperglycemic medications between 2015 and 2020 by the estimated 25,791,754 adults with diagnosed type 2 diabetes in the United States by age, sex, race and ethnicity, and BMI. The columns are ordered according to the agents’ impact on weight, with medications associated with weight loss in the left columns, weight-neutral medications in the middle columns, and medications associated with weight gain in the right columns.
TABLE 4.
Antihyperglycemic Medication Use Among Adults With Diagnosed Type 2 Diabetes, by Age, Sex, Race and Ethnicity, and BMI, U.S., 2015–2020
Consistent with the recommendation of the ADA that metformin be considered as the first-line pharmacologic therapy for most patients with type 2 diabetes, metformin is the most commonly prescribed antihyperglycemic agent (43.8%) and is used by 11,286,827 adults with type 2 diabetes. Sulfonylureas are the second most commonly prescribed oral antihyperglycemic medications (23.2%, n=5,972,183). GLP-1 RAs are prescribed only for 5.0% (n=1,278,816) of adults with type 2 diabetes, but their use has increased dramatically in recent years (22,23,24). DPP-4 inhibitors are prescribed as monotherapy for 1.9% (n=486,919) and SGLT2 inhibitors are prescribed as monotherapy for 1.3% (n=329,251) of adults with type 2 diabetes. Thiazolidinediones are prescribed for 4.1% (n=1,047,432), and insulin is prescribed for 22.0% (n=5,673,892). SGLT2 inhibitors are commonly prescribed in combination with DPP-4 inhibitors or metformin, and DPP-4 inhibitors are commonly prescribed in combination with metformin or thiazolidinediones.
GLP-1 RA use is somewhat more common in middle-aged patients (45–64 years) with type 2 diabetes than in younger and older patients. Men and non-Hispanic White people with type 2 diabetes are more likely to use GLP-1 RAs. GLP-1 RA use is higher in non-Asian people with BMI ≥35.0 kg/m2. SGLT2 inhibitor use is also more common in middle-aged patients and among men with type 2 diabetes. SGLT2 inhibitor use tends to be highest among non-Hispanic White and Hispanic people.
Anti-obesity Medications
Anti-obesity medications (AOMs) are indicated for the treatment of overweight and obesity among persons with BMI 27–29.9 kg/m2 in the presence of complications related to excess body fat or when BMI is ≥30 kg/m2 (25). AOM treatment is used as an adjunct to lifestyle therapy, including modification of dietary intake and increased physical activity (25). The impact of most AOMs in the treatment plan is improvement in the ability of the individual to implement a reduction in calorie intake due to the AOM effect on counterregulatory responses to reductions in energy intake and enhancement of gut-brain signaling (21). Enhanced gut-brain signaling may include early satiety, decreased hunger and food cravings, diminished attentiveness to food stimuli, and reduced hedonic responses to highly palatable foods (26). This effect leads to an improved treatment response, resulting in a greater proportion of individuals who achieve clinically meaningful weight loss, a larger magnitude of weight loss, and a sustained weight reduction.
In the United States, several AOM options are approved by the FDA for the treatment of overweight and obesity in persons with and without type 2 diabetes. These agents have a number of different mechanisms of action. The earliest FDA-approved AOMs that are still used today include the sympathomimetic monoamines, such as phentermine and diethylpropion. These anorexiants decrease energy intake by increasing norepinephrine (and to a lesser extent, dopamine and serotonin) in several hypothalamic nuclei, resulting in appetite inhibition (27,28). Orlistat is a lipase inhibitor that leads to malabsorption of dietary fat; this drug is ideally used when patients are attempting to implement a low-fat dietary pattern (29). In the early 2000s, two combination AOMs were introduced to the market. Phentermine/topiramate extended release combines the aforementioned sympathomimetic amine with the antiepileptic medication topiramate. The mechanism of action by which topiramate affects appetite is unclear, but presumably it acts through gamma-aminobutyric acid (GABA) type A receptors (30). The other combination medication features the dopamine and norepinephrine reuptake inhibitor, bupropion, combined with naltrexone—a blocker of opioid receptors—to target proopiomelanocortin neurons (31).
The first incretin mimetic approved for obesity treatment was liraglutide 3.0 mg, a daily injectable GLP-1 RA. Liraglutide has some effects in slowing gastric emptying but appears to decrease energy intake largely through agonism of GLP-1 receptors in appetite centers in the brain (32). Subsequent to liraglutide, a once-weekly GLP-1 RA, semaglutide 2.4 mg, was approved for obesity treatment. The once-weekly GLP-1 RA has higher potency in terms of binding with central GLP-1 receptors and a longer half-life that allows for weekly dosing (33). In the Phase 3 STEP 2 trial, semaglutide 2.4 mg once a week in adults with BMI ≥27 kg/m2 and type 2 diabetes resulted in a change in mean body weight from baseline to week 68 of -9.6% compared to -3.4% with placebo (6). In the five SUSTAIN trials that compared once-weekly semaglutide to either DPP-4 inhibitors or other GLP-1 RAs in patients with type 2 diabetes, there was significantly greater weight loss with semaglutide compared to DPP-4 inhibitors (mean difference -3.19 kg, 95% confidence interval [CI] -3.66 to -2.72) and other GLP-1 RAs (mean difference -2.50 kg, 95% CI -3.91 to -1.09) (34).
The GLP-1 RA/GIP co-agonist, tirzepatide, is approved by the FDA for lowering glucose in those with type 2 diabetes and for the treatment of obesity. It has been shown to facilitate a mean 13.4% and 15.7% weight loss in adults with type 2 diabetes taking 10 mg and 15 mg doses, respectively, compared to placebo (3.3%). Tirzepatide has also been associated with marked improvement in glycemic control, with 55.3% and 50.2% of participants taking 10 mg and 15 mg doses, respectively, achieving glycated hemoglobin (A1C) <5.7% (<39 mmol/mol) compared to 2.8% with placebo (35). In a head-to-head trial comparing tirzepatide to semaglutide, tirzepatide resulted in a greater reduction in A1C with a mean change from baseline of -2.01, -2.24, and -2.30 percentage points with 5 mg, 10 mg, and 15 mg of tirzepatide, respectively, compared to -1.86 percentage points with semaglutide 1 mg (36). Other clinical trials are underway with promising double and triple incretin therapies that include GLP-1 RAs in combination with GIP, glucagon, peptide YY (PYY), and amylin analogues.
The number of prescriptions for GLP-1 RAs for weight loss has increased substantially since 2019 (22,23); however, some insurers are rolling back coverage for GLP-1 RAs and GLP-1 RA/GIP co-agonists because of cost (37). While the GLP-1 RAs are approved for management of type 2 diabetes, none of the medications whose indication is to manage obesity are covered by Medicare (38). In contrast, a few state Medicaid programs cover weight loss drugs (39).
In the NHANES 2015–2020, 686,314 of 25,791,754 adults with type 2 diabetes in the United States (2.5% [0.4 SE]) reported using GLP-1 RAs for weight loss. At that time, the only GLP-1 RA approved for obesity was liraglutide 3.0 mg daily. The use of anorexiants (phentermine, phentermine with topiramate, phendimetrazine, and benzphetamine), lipase inhibitors (orlistat), and bupropion plus naltrexone were too infrequent to reliably estimate their use among adults with type 2 diabetes.
Metabolic Surgery
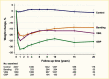
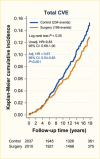
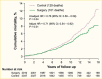
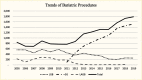
Surgical procedures to treat obesity, often referred to as bariatric or metabolic surgery, can promote substantial weight loss and improve glycemia in persons with type 2 diabetes (8,40,41,42). Given the magnitude and rapidity of the improvement in glucose homeostasis, these procedures have been recommended as treatments for diabetes (43) even in the absence of severe obesity. Some organizations have recommended lowering the BMI criterion for metabolic surgery from 35 kg/m2 to 30 kg/m2 (27.5 kg/m2 for Asian Americans) for adults with type 2 diabetes who have not achieved sufficient weight loss or improved comorbidities with the appropriate use of nonsurgical treatments. Studies have documented diabetes remission at 1–5 years in 30%–80% of patients following Roux-en-Y gastric bypass (RYGB) or vertical sleeve gastrectomy (VSG) (40,41,44,45). In addition to improving glycemia, metabolic surgery was associated with reduction in long-term cardiovascular events and improved survival in the observational Swedish Obese Subjects study (Figures 1, 2, and 3) (46,47,48). Metabolic surgery also improves health-related quality of life (49).
In 2019, the most frequently performed metabolic surgery procedures in adults with type 2 diabetes in the United States were RYGB and VSG. Both procedures can be performed laparoscopically or as open surgical procedures and in an inpatient or outpatient setting. In RYGB, a small pouch is created from the stomach and the pouch is connected to the more distal small intestine. The remainder of the stomach and the top part of the small intestine are thus bypassed. In VSG, a large part of the stomach is removed so that the remaining stomach forms a narrow tube called a sleeve. The sleeve remains connected to the small intestine as it was before surgery.
Table 5 shows the incidence of inpatient metabolic surgeries in U.S. adults with type 2 diabetes by age, sex, race and ethnicity, and BMI. In 2019, these procedures were infrequently performed, with only 1.3% (n=100,765) of U.S. adults with type 2 diabetes undergoing RYGB, 0.1% (n=10,110) undergoing VSG, and <0.01% (n=70) undergoing gastric banding. Although VSG is by far the most frequently performed metabolic surgery procedure in the U.S. population (50), RYGB was more frequently performed among adults with type 2 diabetes, perhaps related to the better outcomes observed with RYGB compared to VSG in some studies of patients with type 2 diabetes (8,48). In general, inpatient RYBG was most frequently performed in patients age 45–64 years with type 2 diabetes and in women, White, Black, and Native American people, and in non-Asian individuals with higher BMIs.
TABLE 5.
Incidence of Inpatient Metabolic Surgery Discharges Among Adults With Type 2 Diabetes, by Age, Sex, Race and Ethnicity, and BMI, U.S., 2019
The Alliance of Randomized Trials of Medicine versus Metabolic Surgery in Type 2 Diabetes (ARMMS-T2D) is a composite analysis of four independent clinical trials, STAMPEDE (Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently), TRIABETES (Randomized Trial to Compare Surgical and Medical Treatments for Type 2 Diabetes), SLIMM-T2D (Surgery or Lifestyle With Intensive Medical Management in the Treatment of Type 2 Diabetes), and CROSSROADS (Calorie Reduction or Surgery: Seeking to Reduce Obesity and Diabetes Study). ARMMS-T2D published 7- and 12-year outcomes of participants randomized to medical/lifestyle intervention or metabolic surgery for management of type 2 diabetes (51). The trials were conducted between 2007 and 2013 with observational follow-up through 2022. Of 355 patients randomized in the four original trials, 262 (74%) were available for study. Participants were middle-aged (although slightly younger in the surgical groups), primarily female and White, with average BMI 36 kg/m2, and duration of type 2 diabetes of 9±5 years. At 7 years, A1C decreased by 0.2% (95% CI -0.5% to 0.2%) from a baseline of 8.2% (66 mmol/mol) in the medical/lifestyle group (n=96) and by 1.6% (95% CI -1.8% to -1.3%) from a baseline of 8.7% (72 mmol/mol) in the metabolic surgery group (n=89 RYGB; n=41 VSG; n=36 adjustable gastric banding). The between-group difference was -1.4% (95% CI -1.8% to -1.0%, p<0.001) at 7 years and -1.1% (95% CI -1.7% to -0.5%, p=0.002) at 12 years. Diabetes remission, defined as A1C <6.5% (<48 mmol/mol) without diabetes medications for at least 3 months, was greater after metabolic surgery at 7 years (6.2% in the medical/lifestyle group vs. 18.2% in the metabolic surgery group, p=0.02) and 12 years (0.0% in the medical/lifestyle group vs. 12.7% in the metabolic surgery group, p<0.001).
In interpreting these results, it is important to recognize that the medical/lifestyle intervention was provided only for one year in two of the studies (SLIMM-T2D (52,53) and CROSSROADS (54)), for 1 year followed by 4 years of a lower-level lifestyle intervention provided to both the medical/lifestyle and metabolic surgery groups in one of the studies (TRIABETES (55,56,57)), and for 2 years followed by 3 years of a lower-level lifestyle intervention that involved only two visits per year with an endocrinologist in the last study (STAMPEDE (58)). In addition, in ARMMS-T2D, diabetes remission was defined on the basis of A1C and not taking diabetes medications, which were an essential part of medical therapy in each of the four clinical trials. Although medical therapy/lifestyle intervention reflected the state of the art at the time the studies were performed, the only study that specifically reported use of GLP-1 RAs in the medical therapy arm (SLIMM-T2D) reported that 11% of participants were prescribed GLP-1 RAs at baseline in 2010–2011 (52). The only GLP-1 RAs approved by the FDA for the treatment of diabetes before 2017 were exenatide and liraglutide, and the only GLP-1 RA approved for the treatment of obesity before 2021 was liraglutide (approved in 2014). Semaglutide was first approved by the FDA for the treatment of type 2 diabetes in 2017 and for obesity in 2021, and tirzepatide was first approved for the treatment of type 2 diabetes in 2022 and for the treatment of obesity in late 2023. Finally, there was substantial treatment cross-over in the ARMMS-T2D study. At 12 years, 25% of participants randomized to medical/lifestyle intervention had undergone metabolic surgery and approximately 30% of those randomized to metabolic surgery were taking incretin medications (51). For all of these reasons, the results of long-term observational studies that compare the outcomes of short-term clinical trials of medical/lifestyle interventions and metabolic surgery must be interpreted with caution.
Table 6 shows the incidence of ambulatory metabolic surgery in 2019 in U.S. adults with type 2 diabetes by age, sex, race and ethnicity, and BMI. Substantially more procedures were performed on an inpatient compared to an ambulatory basis (RYGB, n=100,765 for inpatient vs. n=19,743 for ambulatory visits, see Table 5 vs. Table 6). Younger patients, women, and Black, White, Hispanic, and Native American people were more likely to have ambulatory RYGB procedures. Rates of ambulatory RYGB procedures by BMI were similar to rates of inpatient RYGB surgeries by BMI.
TABLE 6.
Incidence of Ambulatory Metabolic Surgery Discharges Among Adults With Diagnosed Type 2 Diabetes, by Age, Sex, Race and Ethnicity, and BMI, U.S., 2019
Endoscopic and Endoluminal Therapies
Endoscopic and endoluminal therapies for obesity management include space-occupying devices (either fluid or gas-filled intragastric balloons), an oral capsule that contains superabsorbent hydrogel particles that expands in the stomach and decreases the volume of the stomach, and the transpyloric shuttle, a device that causes intermittent obstruction to gastric outflow and delays gastric emptying. Other endoscopic procedures reduce the volume of stomach by plication (i.e., folding) or suturing. Duodenal mucosal resurfacing involves circumferential hydrothermal ablation of the duodenal mucosa to elicit beneficial metabolic effects. In general, uptake of all of these procedures has been low, and no studies have focused explicitly on individuals with obesity and type 2 diabetes. Accordingly, no population-based data are available on the uptake or outcomes of these procedures in individuals with type 2 diabetes.
Type 2 Diabetes and Obesity Among Youth
Between 2001 and 2017, the prevalence of type 2 diabetes in youth had a 95% relative increase (0.34 to 0.67 per 1,000 youths) (59). This increase in youth-onset type 2 diabetes has been attributed to the increase in childhood obesity. In 2020, more than 20% of U.S. children age 2–19 years were estimated to have obesity. Rates were similar among boys and girls (60). Evidence suggests that youth-onset type 2 diabetes differs from the adult-onset condition—youth with type 2 diabetes have beta cell hyperresponsiveness, lower insulin sensitivity, and aggressive, rapid deterioration of beta cell function compared to adults with newly diagnosed type 2 diabetes (61,62,63,64).
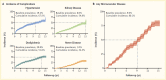
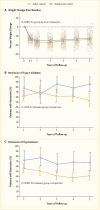
Initial results from the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study showed that 51.7% of pediatric patients with type 2 diabetes and overweight/obesity failed to maintain glycemic control on metformin monotherapy (65). Median time to treatment failure was just under one year. The addition of a lifestyle intervention to metformin therapy did not significantly improve glycemic control, despite achieving a clinically meaningful weight loss. Most TODAY participants transitioned into a long-term observational cohort and have reached young adulthood (mean age 26.4 years in 2020) (66). BMI has remained elevated in the class II obesity range, and 34% of participants have poor glycemic control (A1C ≥10% [≥86 mmol/mol]). Of particular concern, individuals with youth-onset type 2 diabetes are at risk for early complications, including hypertension, dyslipidemia, retinopathy, nephropathy, and neuropathy (Figure 4) (66,67). In the TODAY study cohort of individuals with youth-onset type 2 diabetes, the prevalence of retinal disease was 51%, and the cumulative incidence of diabetic kidney disease was 55%, nerve disease was 32%, hypertension was 68%, and dyslipidemia was 52% at approximately 13 years after diagnosis (Figure 4) (66).
The difference between youth- and adult-onset type 2 diabetes was further illustrated by the Restoring Insulin Secretion (RISE) study. Among adults with impaired glucose tolerance or early type 2 diabetes, the decline in beta cell function was slowed with liraglutide and metformin (68). However, adolescents enrolled in RISE, who received either metformin monotherapy or treatment with glargine followed by metformin, had beta cell function decline from baseline with no significant differences between groups.
Improving glycemic outcomes and altering the course of beta cell decline among adolescents with type 2 diabetes may require large-magnitude weight reduction (69,70). Indeed, the ADA recommends considering metabolic surgery for adolescents with type 2 diabetes and BMI ≥35 kg/m2 (71). Analysis of the NIS 2005–2019 database identified more than 16,000 hospitalizations for metabolic surgery among patients age <20 years (72). The annual number of procedures increased from 839 in 2005 to 1,785 in 2019. Patients were predominantly female (76%) and White (56%). Over time, there was a significant shift away from laparoscopic RYGB and laparoscopic adjustable gastric band (LAGB) to laparoscopic VSG procedures. In 2005, 67% of procedures were RYGB, 27% were LAGB, and 0% were VSG; in 2019, 85% were VSG and 14% were RYGB (Figure 5) (72).
A secondary analysis was performed using data collected by the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study and the TODAY study to compare 2-year outcomes in adolescents with severe obesity and type 2 diabetes who underwent metabolic surgery (n=30) or medical therapy (n=63) with metformin alone or in combination with either rosiglitazone or an intensive lifestyle intervention (69). Insulin was used if there was progression of disease. Among Teen-LABS participants, 77% underwent RYGB, 20% VSG, and 3% LAGB. Over 2 years, BMI decreased by 29% (95% CI 24%–34%) in Teen-LABS and increased by 4% (95% CI 1%–7%) in TODAY. A1C decreased from 6.8% (51 mmol/mol; 95% CI 6.4%–7.3%) to 5.5% (37 mmol/mol; 95% CI 4.7%–6.3%) in Teen-LABS and increased from 6.4% (46 mmol/mol; 95% CI 6.1%–6.7%) to 7.8% (62 mmol/mol; 95% CI 7.2%–8.3%) in TODAY. During the 2-year follow-up, 7 of 30 individuals in the Teen-LABS cohort (23%) experienced complications that required readmission and/or surgery related to their prior metabolic surgery. In comparison, only 2 of 63 TODAY participants (3%) required hospital admission during the 2-year follow-up period (69).
Three-year follow-up of the entire Teen-LABS cohort has reported additional outcomes (73). Among the 242 adolescents who underwent weight loss surgery (161/242 RYGB, 67/242 VSG) at five U.S. centers between 2007 and 2023, mean ± SD age was 17±2 years, mean BMI was 53 kg/m2, 75% were female, and 72% were White. At 3 years, mean weight loss was 27% (95% CI 25%–29%) with no significant difference between procedures. Remission of type 2 diabetes occurred in 19 of the 20 (95%) adolescents who had type 2 diabetes at baseline. There were also improvements in blood pressure, lipids, and weight-related quality of life. Iron deficiency was common (57%), and 13% of participants required one or more additional intraabdominal procedures.
The health effects of RYGB were compared in a cohort of adolescents (161 patients enrolled from 2006 through 2012) and a cohort of adults (396 patients enrolled from 2006 through 2009) who were enrolled in two related but independent studies (Figure 6) (74). There was no significant difference in percentage weight change between adolescents (-26%, 95% CI -29% to -23%) and adults (-29%, 95% CI -31% to -27%) 5 years after surgery (p=0.08). After surgery, adolescents were significantly more likely than adults to have remission of type 2 diabetes (86% vs. 53%, risk ratio 1.27, 95% CI 1.03–1.57) and of hypertension (68% vs. 41%, risk ratio 1.51, 95% CI 1.21–1.88). The rate of abdominal reoperations was significantly higher among adolescents than among adults (19 vs. 10 reoperations per 500 person-years, p=0.003). More adolescents than adults had low ferritin levels (72 of 132 patients [48%] vs. 54 of 179 patients [29%], p=0.004). Three adolescents (1.9%) and seven adults (1.8%) died in the 5 years after surgery (74).
While these initial results demonstrate a beneficial effect of metabolic surgery on weight and glycemia in youth, most results reported to date have evaluated RYGB, which is associated with more complications and is now rarely performed. In addition, no studies have compared VSG to modern medical therapy in youth with type 2 diabetes. Accordingly, the National Institutes of Health has funded a prospective, open-label clinical trial, Surgical or Medical Treatment for Pediatric T2D (ST2OMP) (75). The study is comparing the impact of VSG and lifestyle intervention and multidrug therapy, including GLP-1 RAs and SGLT2 inhibitors, on weight and glycemia.
Semaglutide is approved by the FDA for the treatment of obesity in children age ≥12 years. In a randomized controlled trial of 201 adolescents age 12–17 years with overweight or obesity and at least one associated condition, once-weekly semaglutide 2.4 mg resulted in mean BMI reduction of 16.1% at 68 weeks (76). A1C decreased by 0.4% among adolescents without diabetes in the semaglutide group; however, few participants had type 2 diabetes in the trial (n=8; 4% of the total sample).
Conclusions
Although weight loss is recommended as a treatment for most patients with type 2 diabetes complicated by overweight or obesity, nationally representative data about healthy eating, physical activity, and other strategies to lose weight are limited among U.S. adults with diabetes. Based on the NHANES, only 2.3% of U.S. adults with diagnosed diabetes have HEI scores >80, indicating that most Americans with diabetes consume diets that are not aligned with U.S. Department of Agriculture Dietary Guidelines. NHANES data also reveal that fewer than one-half of U.S. adults with diabetes report meeting physical activity standards and 37% report no physical activity, a figure that is likely an upper estimate given that self-reported physical activity overestimates actual physical activity. Although ~37% of U.S. adults with diagnosed diabetes report attempting to lose weight, very few report using the proven-effective, evidence-based strategies recommended by the U.S. Preventive Services Task Force for weight loss, and some report engaging in maladaptive strategies, including skipping meals, taking nonprescription supplements, and taking laxatives, vomiting, or smoking (77).
Although the ADA has recommended that providers and patients consider the impact of antihyperglycemic medications on weight, the classes of antihyperglycemic medications judged as being associated with weight loss are still infrequently used, likely due to their cost. This discrepancy has contributed to health disparities such that lower income, underinsured, and uninsured patients appear less likely to report using these medications. Similarly, although metabolic surgery has been shown to be safe and effective for obesity management in type 2 diabetes, uptake remains low.
List of Abbreviations
- A1C
glycated hemoglobin
- ADA
American Diabetes Association
- AOM
anti-obesity medication
- ARMMS-T2D
Alliance of Randomized Trials of Medicine versus Metabolic Surgery in Type 2 Diabetes
- BMI
body mass index
- CI
confidence interval
- CROSSROADS
Calorie Reduction or Surgery: Seeking to Reduce Obesity and Diabetes Study
- DPP-4
dipeptidyl peptidase-4
- FDA
U.S. Food and Drug Administration
- GIP
glucagon-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide-1
- GLP-1 RA
glucagon-like peptide-1 receptor agonist
- HCUP
Healthcare Cost and Utilization Project
- HEI
Healthy Eating Index
- LAGB
laparoscopic adjustable gastric band
- NHANES
National Health and Nutrition Examination Survey
- NIS
National (Nationwide) Inpatient Sample
- RISE
Restoring Insulin Secretion study
- RYGB
Roux-en-Y gastric bypass
- SD
standard deviation
- SE
standard error
- SGLT2
sodium-glucose cotransporter-2
- SLIMM-T2D
Surgery or Lifestyle With Intensive Medical Management in the Treatment of Type 2 Diabetes study
- STAMPEDE
Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently study
- Teen-LABS
Teen-Longitudinal Assessment of Bariatric Surgery
- TODAY
Treatment Options for Type 2 Diabetes in Adolescents and Youth study
- TRIABETES
Randomized Trial to Compare Surgical and Medical Treatments for Type 2 Diabetes
- VSG
vertical sleeve gastrectomy
Conversions
A1C: (% x 10.93) - 23.50 = mmol/mol
Article History
Received in final form on March 8, 2024.
References
- 1.
- DeFronzo RA, Abdul-Ghani MA. Preservation of β-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96(8):2354-2366. doi:10.1210/jc.2011-0246 [PubMed: 21697254] [CrossRef]
- 2.
- Després JP, Lemieux I, Prud’homme D. Treatment of obesity: need to focus on high risk abdominally obese patients. BMJ. 2001;322(7288):716-720. doi:10.1136/bmj.322.7288.716 [PMC free article: PMC1119905] [PubMed: 11264213] [CrossRef]
- 3.
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852-871. doi:10.1016/j.cell.2012.02.017 [PMC free article: PMC3294420] [PubMed: 22385956] [CrossRef]
- 4.
- Rothberg AE, McEwen LN, Kraftson AT, Fowler CE, Herman WH. Very-low-energy diet for type 2 diabetes: an underutilized therapy? J Diabetes Complications. 2014;28(4):506-510. doi:10.1016/j.jdiacomp.2014.03.014 [PMC free article: PMC4350259] [PubMed: 24849710] [CrossRef]
- 5.
- Taheri S, Zaghloul H, Chagoury O, et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): an open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2020;8(6):477-489. doi:10.1016/S2213-8587(20)30117-0 [PubMed: 32445735] [CrossRef]
- 6.
- Davies M, Faerch L, Jeppesen OK, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971-984. doi:10.1016/S0140-6736(21)00213-0 [PubMed: 33667417] [CrossRef]
- 7.
- Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143-155. doi:10.1016/S0140-6736(21)01324-6 [PubMed: 34186022] [CrossRef]
- 8.
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641-651. doi:10.1056/NEJMoa1600869 [PMC free article: PMC5451258] [PubMed: 28199805] [CrossRef]
- 9.
- Murphy R, Plank LD, Clarke MG, et al. Effect of banded Roux-en-Y gastric bypass versus sleeve gastrectomy on diabetes remission at 5 years among patients with obesity and type 2 diabetes: a blinded randomized clinical trial. Diabetes Care. 2022;45(7):1503-1511. doi:10.2337/dc21-2498 [PMC free article: PMC9274222] [PubMed: 35554515] [CrossRef]
- 10.
- Rothberg AE, Herman WH, Wu C, et al. Weight loss improves β-cell function in people with severe obesity and impaired fasting glucose: a window of opportunity. J Clin Endocrinol Metab. 2020;105(4):e1621-e1630. doi:10.1210/clinem/dgz189 [PMC free article: PMC7059991] [PubMed: 31720686] [CrossRef]
- 11.
- White MG, Shaw JA, Taylor R. Type 2 diabetes: the pathologic basis of reversible β-cell dysfunction. Diabetes Care. 2016;39(11):2080-2088. doi:10.2337/dc16-0619 [PubMed: 27926891] [CrossRef]
- 12.
- Kirkpatrick SI, Reedy J, Krebs-Smith SM, et al. Applications of the Healthy Eating Index for surveillance, epidemiology, and intervention research: considerations and caveats. J Acad Nutr Diet. 2018;118(9):1603-1621. doi:10.1016/j.jand.2018.05.020 [PMC free article: PMC6730554] [PubMed: 30146072] [CrossRef]
- 13.
- LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320(11):1172-1191. doi:10.1001/jama.2018.7777 [PubMed: 30326501] [CrossRef]
- 14.
- ElSayed NA, Aleppo G, Aroda VR, et al. 1. Improving Care and Promoting Health in Populations: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S10-S18. doi:10.2337/dc23-S001 [PMC free article: PMC9810463] [PubMed: 36507639] [CrossRef]
- 15.
- Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2022;399(10322):394-405. doi:10.1016/S0140-6736(21)01919-X [PubMed: 34600604] [CrossRef]
- 16.
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition. December 2015. Updated August 24, 2021. Accessed March 13, 2023. https://health
.gov/our-work /food-nutrition /previous-dietary-guidelines/2015 - 17.
- Hurley KM, Oberlander SE, Merry BC, Wrobleski MM, Klassen AC, Black MM. The Healthy Eating Index and Youth Healthy Eating Index are unique, nonredundant measures of diet quality among low-income, African American adolescents. J Nutr. 2009;139(2):359-364. doi:10.3945/jn.108.097113 [PMC free article: PMC2646206] [PubMed: 19074210] [CrossRef]
- 18.
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd Edition. U.S. Department of Health and Human Services; 2018. https://health
.gov/our-work /nutrition-physical-activity /physical-activity-guidelines /current-guidelines - 19.
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188. doi:10.1249/mss.0b013e31815a51b3 [PubMed: 18091006] [CrossRef]
- 20.
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140-S157. doi:10.2337/dc23-S009 [PMC free article: PMC9810476] [PubMed: 36507650] [CrossRef]
- 21.
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342-362. doi:10.1210/jc.2014-3415 [PubMed: 25590212] [CrossRef]
- 22.
- Khan T, Kim C. Prescriptions for Trendy Diabetes and Weight-Loss Drugs Increased Over 2,000% Since 2019. Komodo Health Insights. February 17, 2023. Accessed September 12, 2023. https://www
.komodohealth .com/insights/prescriptions-for-trendy-diabetes-and-weight-loss-drugs-increased-over-2000-since-2019 - 23.
- Monostra M. More Adults With Type 2 Diabetes Now Using GLP-1s Instead of DPP-IV Inhibitors. Healio; September 1, 2023. Accessed September 12, 2023. https://www
.healio.com /news/endocrinology /20230831/more-adults-with-type-2-diabetes-now-using-glp1s-instead-of-dppiv-inhibitors - 24.
- Eberly LA, Yang L, Essien UR, et al. Racial, ethnic, and socioeconomic inequities in glucagon-like peptide-1 receptor agonist use among patients with diabetes in the US. JAMA Health Forum. 2021;2(12):e214182. doi:10.1001/jamahealthforum.2021.4182 [PMC free article: PMC8796881] [PubMed: 35977298] [CrossRef]
- 25.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102-S138. doi:10.1161/01.cir.0000437739.71477.ee [PMC free article: PMC5819889] [PubMed: 24222017] [CrossRef]
- 26.
- Kumar RB, Srivastava G, Reid TJ, Aronne LJ. Understanding the pathophysiologic pathways that underlie obesity and options for treatment. Expert Rev Endocrinol Metab. 2021;16(6):321-338. doi:10.1080/17446651.2021.1991310 [PubMed: 34904501] [CrossRef]
- 27.
- Bray GA, Greenway FL. Pharmacological treatment of the overweight patient. Pharmacol Rev. 2007;59(2):151-184. doi:10.1124/pr.59.2.2 [PubMed: 17540905] [CrossRef]
- 28.
- Patel D. Pharmacotherapy for the management of obesity. Metabolism. 2015;64(11):1376-1385. doi:10.1016/j.metabol.2015.08.001 [PubMed: 26342499] [CrossRef]
- 29.
- Harp JB. Orlistat for the long-term treatment of obesity. Drugs Today (Barc). 1999;35(2):139-145. doi:10.1358/dot.1999.35.2.527969 [PubMed: 12973416] [CrossRef]
- 30.
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330 [PMC free article: PMC3270297] [PubMed: 22051941] [CrossRef]
- 31.
- Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21(5):935-943. doi:10.1002/oby.20309 [PMC free article: PMC3739931] [PubMed: 23408728] [CrossRef]
- 32.
- Knudsen LB. Inventing liraglutide, a glucagon-like peptide-1 analogue, for the treatment of diabetes and obesity. ACS Pharmacol Transl Sci. 2019;2(6):468-484. doi:10.1021/acsptsci.9b00048 [PMC free article: PMC7088919] [PubMed: 32259078] [CrossRef]
- 33.
- Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242-1251. doi:10.1111/dom.12932 [PMC free article: PMC5573908] [PubMed: 28266779] [CrossRef]
- 34.
- Mishriky BM, Cummings DM, Powell JR, Sewell KA, Tanenberg RJ. Comparing once-weekly semaglutide to incretin-based therapies in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 2019;45(2):102-109. doi:10.1016/j.diabet.2018.09.002 [PubMed: 30243806] [CrossRef]
- 35.
- Garvey WT, Frias JP, Jastreboff AM, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402(10402):613-626. doi:10.1016/S0140-6736(23)01200-X [PubMed: 37385275] [CrossRef]
- 36.
- Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515. doi:10.1056/NEJMoa2107519 [PubMed: 34170647] [CrossRef]
- 37.
- Robbins R. Buried in Wegovy Costs, North Carolina Will Stop Paying for Obesity Drugs. New York Times. January 26, 2024. Accessed February 13, 2024. https://www
.nytimes.com /2024/01/26/business /obesity-drugs-insurance-north-carolina.html - 38.
- Baig K, Dusetzina SB, Kim DD, Leech AA. Medicare Part D coverage of antiobesity medications—challenges and uncertainty ahead. N Engl J Med. 2023;388(11):961-963. doi:10.1056/NEJMp2300516 [PubMed: 36912541] [CrossRef]
- 39.
- Court E, Langreth R. Good Luck Paying for Those $10,000 Obesity Drugs Everyone’s Talking About. Businessweek. April 27, 2023. Accessed September 12, 2023. https://www
.bloomberg .com/news/features/2023-04-27 /ozempic-s-high-cost-limits-access-to-weight-loss-drug - 40.
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248-256.e5. doi:10.1016/j.amjmed.2008.09.041 [PubMed: 19272486] [CrossRef]
- 41.
- McTigue KM, Wellman R, Nauman E, et al. Comparing the 5-year diabetes outcomes of sleeve gastrectomy and gastric bypass: the National Patient-Centered Clinical Research Network (PCORNet) Bariatric Study. JAMA Surg. 2020;155(5):e200087. doi:10.1001/jamasurg.2020.0087 [PMC free article: PMC7057171] [PubMed: 32129809] [CrossRef]
- 42.
- Arterburn D, Wellman R, Emiliano A, et al. Comparative effectiveness and safety of bariatric procedures for weight loss: a PCORnet cohort study. Ann Intern Med. 2018;169(11):741-750. doi:10.7326/M17-2786 [PMC free article: PMC6652193] [PubMed: 30383139] [CrossRef]
- 43.
- Eisenberg D, Shikora SA, Aarts E, et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): indications for metabolic and bariatric surgery. Surg Obes Relat Dis. 2022;18(12):1345-1356. doi:10.1016/j.soard.2022.08.013 [PubMed: 36280539] [CrossRef]
- 44.
- Parikh M, Issa R, Vieira D, et al. Role of bariatric surgery as treatment for type 2 diabetes in patients who do not meet current NIH criteria: a systematic review and meta-analysis. J Am Coll Surg. 2013;217(3):527-532. doi:10.1016/j.jamcollsurg.2013.04.023 [PubMed: 23890843] [CrossRef]
- 45.
- Puzziferri N, Roshek TB, 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934-942. doi:10.1001/jama.2014.10706 [PMC free article: PMC4409000] [PubMed: 25182102] [CrossRef]
- 46.
- Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56-65. doi:10.1001/jama.2011.1914 [PubMed: 22215166] [CrossRef]
- 47.
- Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish Obese Subjects. N Engl J Med. 2007;357(8):741-752. doi:10.1056/NEJMoa066254 [PubMed: 17715408] [CrossRef]
- 48.
- Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219-234. doi:10.1111/joim.12012 [PubMed: 23163728] [CrossRef]
- 49.
- Adams TD, Pendleton RC, Strong MB, et al. Health outcomes of gastric bypass patients compared to nonsurgical, nonintervened severely obese. Obesity (Silver Spring). 2010;18(1):121-130. doi:10.1038/oby.2009.178 [PMC free article: PMC2864142] [PubMed: 19498344] [CrossRef]
- 50.
- American Society for Metabolic and Bariatric Surgery. Estimate of Bariatric Surgery Numbers, 2011–2021. Accessed September 12, 2023. https://asmbs
.org/resources /estimate-of-bariatric-surgery-numbers - 51.
- Courcoulas AP, Patti ME, Hu B, et al. Long-term outcomes of medical management vs bariatric surgery in type 2 diabetes. JAMA. 2024;331(8):654-664. doi:10.1001/jama.2024.0318 [PMC free article: PMC10900968] [PubMed: 38411644] [CrossRef]
- 52.
- Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149(7):716-726. doi:10.1001/jamasurg.2014.514 [PMC free article: PMC4274782] [PubMed: 24899464] [CrossRef]
- 53.
- Ding SA, Simonson DC, Wewalka M, et al. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab. 2015;100(7):2546-2556. doi:10.1210/jc.2015-1443 [PMC free article: PMC4490302] [PubMed: 25909333] [CrossRef]
- 54.
- Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia. 2016;59(5):945-953. doi:10.1007/s00125-016-3903-x [PMC free article: PMC4826815] [PubMed: 26983924] [CrossRef]
- 55.
- Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149(7):707-715. doi:10.1001/jamasurg.2014.467 [PMC free article: PMC4106661] [PubMed: 24899268] [CrossRef]
- 56.
- Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg. 2015;150(10):931-940. doi:10.1001/jamasurg.2015.1534 [PMC free article: PMC4905566] [PubMed: 26132586] [CrossRef]
- 57.
- Courcoulas AP, Gallagher JW, Neiberg RH, et al. Bariatric surgery vs lifestyle intervention for diabetes treatment: 5-year outcomes from a randomized trial. J Clin Endocrinol Metab. 2020;105(3):866-876. doi:10.1210/clinem/dgaa006 [PMC free article: PMC7032894] [PubMed: 31917447] [CrossRef]
- 58.
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567-1576. doi:10.1056/NEJMoa1200225 [PMC free article: PMC3372918] [PubMed: 22449319] [CrossRef]
- 59.
- Lawrence JM, Divers J, Isom S, et al. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001–2017. JAMA. 2021;326(8):717-727. doi:10.1001/jama.2021.11165 [PMC free article: PMC8385600] [PubMed: 34427600] [CrossRef]
- 60.
- Hu K, Staiano AE. Trends in obesity prevalence among children and adolescents aged 2 to 19 years in the US from 2011 to 2020. JAMA Pediatr. 2022;176(10):1037-1039. doi:10.1001/jamapediatrics.2022.2052 [PMC free article: PMC9315946] [PubMed: 35877133] [CrossRef]
- 61.
- TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care. 2013;36(6):1749-1757. doi:10.2337/dc12-2393 [PMC free article: PMC3661836] [PubMed: 23704674] [CrossRef]
- 62.
- Sam S, Edelstein SL, Arslanian SA, et al. Baseline predictors of glycemic worsening in youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes in the Restoring Insulin Secretion (RISE) Study. Diabetes Care. 2021;44(9):1938-1947. doi:10.2337/dc21-0027 [PMC free article: PMC8740917] [PubMed: 34131048] [CrossRef]
- 63.
- Kahn SE, Mather KJ, Arslanian SA, et al. Hyperglucagonemia does not explain the β-cell hyperresponsiveness and insulin resistance in dysglycemic youth compared with adults: lessons from the RISE Study. Diabetes Care. 2021;44(9):1961-1969. doi:10.2337/dc21-0460 [PMC free article: PMC8740916] [PubMed: 34131047] [CrossRef]
- 64.
- Kahn SE, Edelstein SL, Arslanian SA, et al. Effect of medical and surgical interventions on α-cell function in dysglycemic youth and adults in the RISE Study. Diabetes Care. 2021;44(9):1948-1960. doi:10.2337/dc21-0461 [PMC free article: PMC8740921] [PubMed: 34135015] [CrossRef]
- 65.
- TODAY Study Group, Zeitler P, Hirst K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247-2256. doi:10.1056/NEJMoa1109333 [PMC free article: PMC3478667] [PubMed: 22540912] [CrossRef]
- 66.
- TODAY Study Group, Bjornstad P, Drews KL, et al. Long-term complications in youth-onset type 2 diabetes. N Engl J Med. 2021;385(5):416-426. doi:10.1056/NEJMoa2100165 [PMC free article: PMC8697255] [PubMed: 34320286] [CrossRef]
- 67.
- Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317(8):825-835. doi:10.1001/jama.2017.0686 [PMC free article: PMC5483855] [PubMed: 28245334] [CrossRef]
- 68.
- RISE Consortium. Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care. 2018;41(8):1717-1725. doi:10.2337/dc18-0787 [PMC free article: PMC6054504] [PubMed: 29941500] [CrossRef]
- 69.
- Inge TH, Laffel LM, Jenkins TM, et al. Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr. 2018;172(5):452-460. doi:10.1001/jamapediatrics.2017.5763 [PMC free article: PMC5875354] [PubMed: 29532078] [CrossRef]
- 70.
- Abrams P, Levitt Katz LE, Moore RH, et al. Threshold for improvement in insulin sensitivity with adolescent weight loss. J Pediatr. 2013;163(3):785-790. doi:10.1016/j.jpeds.2013.04.003 [PMC free article: PMC3817268] [PubMed: 23706362] [CrossRef]
- 71.
- American Diabetes Association Professional Practice Committee. 14. Children and Adolescents: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S208-S231. doi:10.2337/dc22-S014 [PubMed: 34964865] [CrossRef]
- 72.
- Salimi-Jazi F, Chkhikvadze T, Shi J, et al. Trends in adolescent bariatric procedures: a 15-year analysis of the National Inpatient Survey. Obes Surg. 2022;32(11):3658-3665. doi:10.1007/s11695-022-06265-9 [PubMed: 36103080] [CrossRef]
- 73.
- Inge TH, Courcoulas AP, Jenkins TM, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113-123. doi:10.1056/NEJMoa1506699 [PMC free article: PMC4810437] [PubMed: 26544725] [CrossRef]
- 74.
- Inge TH, Courcoulas AP, Jenkins TM, et al. Five-year outcomes of gastric bypass in adolescents as compared with adults. N Engl J Med. 2019;380(22):2136-2145. doi:10.1056/NEJMoa1813909 [PMC free article: PMC7345847] [PubMed: 31116917] [CrossRef]
- 75.
- Shah AS, Nadeau KJ, Helmrath MA, Inge TH, Xanthakos SA, Kelsey MM. Metabolic outcomes of surgery in youth with type 2 diabetes. Semin Pediatr Surg. 2020;29(1):150893. doi:10.1016/j.sempedsurg.2020.150893 [PMC free article: PMC7125189] [PubMed: 32238292] [CrossRef]
- 76.
- Weghuber D, Barrett T, Barrientos-Perez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. 2022;387(24):2245-2257. doi:10.1056/NEJMoa2208601 [PMC free article: PMC9997064] [PubMed: 36322838] [CrossRef]
- 77.
- US Preventive Services Task Force, Curry SJ, Krist AH, et al. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(11):1163-1171. doi:10.1001/jama.2018.13022 [PubMed: 30326502] [CrossRef]
Drs. Rothberg, Ard, Gudzune, and Herman reported no conflicts of interest.
- Summary
- Introduction
- Sources and Limitations of National Data on Obesity Management in Diabetes
- Diet, Physical Activity, and Other Weight Loss Strategies
- Pharmacologic Therapies
- Metabolic Surgery
- Endoscopic and Endoluminal Therapies
- Type 2 Diabetes and Obesity Among Youth
- Conclusions
- List of Abbreviations
- Conversions
- Article History
- References
- Review Obesity Management in Adults: A Review.[JAMA. 2023]Review Obesity Management in Adults: A Review.Elmaleh-Sachs A, Schwartz JL, Bramante CT, Nicklas JM, Gudzune KA, Jay M. JAMA. 2023 Nov 28; 330(20):2000-2015.
- Bariatric surgery: an evidence-based analysis.[Ont Health Technol Assess Ser....]Bariatric surgery: an evidence-based analysis.Medical Advisory Secretariat. Ont Health Technol Assess Ser. 2005; 5(1):1-148. Epub 2005 Jan 1.
- Review Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force[ 2018]Review Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task ForceLeBlanc EL, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. 2018 Sep
- Bariatric Surgery for Adults With Class I Obesity and Difficult-to-Manage Type 2 Diabetes: A Health Technology Assessment.[Ont Health Technol Assess Ser....]Bariatric Surgery for Adults With Class I Obesity and Difficult-to-Manage Type 2 Diabetes: A Health Technology Assessment.Ontario Health (Quality). Ont Health Technol Assess Ser. 2023; 23(8):1-151. Epub 2023 Dec 5.
- Review Screening and Interventions for Childhood Overweight[ 2005]Review Screening and Interventions for Childhood OverweightWhitlock EP, Williams SB, Gold R, Smith P, Shipman S. 2005 Jul
- Obesity Management for the Treatment of Type 2 Diabetes - Diabetes in AmericaObesity Management for the Treatment of Type 2 Diabetes - Diabetes in America
Your browsing activity is empty.
Activity recording is turned off.
See more...

 1
1