Comparing Three Ways to Improve Quality of Life for Patients with Kidney Disease and Their Caregivers
Authors
Nasrollah Ghahramani, MD, MS, Jennifer L. Kraschnewski, MD, MPH, Eugene J. Lengerich, VMD, MS, Vernon M. Chinchilli, PhD, and Christopher N. Sciamanna, MD, MPH.Affiliations
Structured Abstract
Background:
Chronic kidney disease (CKD) is associated with substantial morbidity, mortality, and cost. CKD is associated with decreased patient quality of life (QOL) and increased caregiver burden. Peer mentoring (PM) improves activation of patients to participate in their own care and multiple outcomes in various chronic diseases. The effectiveness of a peer-led CKD intervention on patient QOL, patient activation, and caregiver burden has not been previously studied.
Objectives:
We conducted a randomized clinical trial to test the effectiveness of face-to-face (FTF) and online mentoring by trained peers, compared with usual care, on CKD patients' QOL, patient activation, and caregiver burden.
Methods:
We randomly assigned 155 patients with CKD and 86 caregivers to receive 6 months of intervention in 1 of 3 groups: (1) FTF PM, (2) online PM, and (3) textbook-only group who received an informational textbook about CKD. Peer mentors were patients with stage 4 or 5 CKD, ≥18 years of age, or caregivers of patients with CKD, ≥18 years of age. Candidates for PM received formal training through 16 hours of instructional sessions facilitated by patients, caregivers, and health care professionals. Upon successful completion of the training, each PM candidate was designated a certified peer mentor. Participants in all 3 groups received a textbook that contains detailed information about kidney disease. Participants assigned to FTF intervention groups received 6 months of PM. Participants assigned to the online intervention group also received 6 months of PM through a secure password-protected interactive online platform. At baseline, 12 months, and 18 months, the patients completed the Kidney Disease Quality of Life-36 (KDQOL-36) instrument and the Patient Activation Measure (PAM) survey. Caregivers completed the Zarit Burden Interview (ZBI). The primary outcomes were (1) improvement in QOL and (2) decreased caregiver burden. The secondary outcome was improved patient activation as measured by PAM. The primary analyses were by intention to treat (ITT). We applied repeated-measures analysis of variance with a linear mixed-effects model to estimate time-related changes in outcome measures for each of the groups over the study period. We conducted exploratory analyses including interaction terms in the statistical models to investigate heterogeneity of treatment effects (HTEs).
Results:
In our ITT analysis, we analyzed 52 patients in the FTF PM group, 52 patients in the online PM group, and 51 patients in the textbook-only group. Compared with baseline, online PM was associated with improved scores in Effects of Kidney Disease (EKD) (P = .01), Burden of Kidney Disease (BKD) (P = .01), Symptoms and Problems of Kidney Disease (P = .006), Short Form-12 (SF-12) Physical Composite Summary (PCS) (P = .001), and SF-12 Mental Composite Summary (MCS) (P = .0001). Compared with baseline, there were no statistically significant changes in KDQOL-36 domain scores in the FTF PM group and the textbook-only group.
For employed participants, exploratory subgroup analyses showed larger effects of FTF PM on increased EKD (P = .002) and BKD (P = .04) scores, and larger effects of online PM on increased EKD score (P = .02), all relative to the study population as a whole. We found a positive effect of “not employed” status on the association between online PM and increased PCS score (P = .01). We also found a positive effect of male sex on the association between online PM and increased MCS score (P < .0001).
Online PM was associated with increases in PAM score from baseline to 18 months (P = .0001). Compared with baseline, no statistically significant changes were found in PAM scores in the FTF group and the textbook-only group.
Exploratory analyses showed that male (P < .0001) and married (P = .003) participants had larger effects of online PM on increased PAM scores. Female (P = .01) and not married (P = .03) participants had larger effects of FTF PM on increased PAM scores, all relative to the study population as a whole.
In our ITT analysis, we analyzed 29 caregivers in the FTF PM group, 29 caregivers in the online PM group, and 28 caregivers In the textbook-only group. Compared with baseline, online PM was associated with a statistically significant decrease in the ZBI score (SE, −3.44; CI, −6.31 to −0.57; P = .002) with no HTE from any of the demographic variables. Compared with baseline, there were no statistically significant changes in ZBI score in the FTF group and the textbook-only group.
Conclusions:
Compared with baseline, online PM was associated with increased scores in 4 domains of the KDQOL-36 and PAM among patients with CKD. We detected HTE response for the effects of employment, sex, and marital status. Compared with baseline, online PM was associated with decreased burden of care among caregivers of patients with CKD. No statistically significant changes from baseline were found in domain scores of KDQOL-36, patient activation scores, or caregiver burden scores among the FTF PM and textbook-only groups.
Limitations:
The study was limited to English-speaking participants with computer literacy and internet access.
Background
Chronic kidney disease (CKD) and end-stage renal disease (ESRD) are significant global health problems. In 2016, the incidence of ESRD in the United States was 124 675 people, and the prevalence reached 726 331, among the highest rates in the world.1 In addition to substantial mortality, morbidity, and cost, CKD has significant negative effects on quality of life (QOL)2,3 that is, in turn, correlated with mortality among CKD patients.4
CKD is a general term for a variety of disorders that affect the structure and function of the kidneys. CKD is characterized by kidney damage, as defined by either abnormal urinary albumin excretion or decreased kidney function, or both, for >3 months. Based on severity of reduction in kidney function, CKD is classified into 5 stages. The complications and chance of progression to the need for kidney replacement therapy (dialysis or transplant) are more likely in severe CKD (stages 4 and 5). Patients with stage 5 CKD, who require kidney replacement therapy for continued survival, are defined as having ESRD.5,6 Patients with stage 1 or 2 CKD progress to more advanced stages at approximately 0.5% per year, and patients with stage 3 or 4 disease progress to stage 5 or ESRD at a rate of 1.5% per year.7
Patient Information Needs
Providing CKD patients with information results in patient-centered care, alleviating anxiety, enabling the individual to cope, facilitating awareness, and improving self-management adherence.8,9 Educating patients with CKD is associated with improved outcomes and is recommended as an area of priority in systematic reviews, professional guidelines, and consensus conferences involving professional societies, providers, patients, and stakeholders.10-18 In response to these recommendations, education programs have been developed.
Despite these educational programs, however, studies have highlighted suboptimal understanding of CKD and its treatment options by patients,19,20 and significant gaps have been identified in desired and provided information.21 Standard CKD eeducational programs are based on information perceived by professionals as important. Information is presented late and often immediately before treatment initiation,22 not allowing time for understanding, engagement, and informed decision-making by patients and caregivers.23,24
A systematic review explored the evidence on the information needs of patients with CKD.25 Some areas that were identified included function of kidneys and manifestations of kidney failure26-28; how progression of kidney disease can be prevented28; unbiased information about the advantages and disadvantages of different treatment options9,27-33; questions about lifestyle, social relationships, networks, activities, and commitments31,32,34-37; information about diet, medications, and their purposes and adverse effects8,27-29,32-34,36,38; rationale for tests, significance of abnormal tests, their relationship with symptoms, and what patients can do when they are informed about abnormal values28,29; coping with consequences of a failed transplant32; how to identify additional support36,38; potential impact of CKD and its treatments on physical appearance29,32,34,37; information about the possibility of death as a result of refusing treatment37; and the realistic chances of survival.21,27,32,37
Findings from another systematic review indicated that patients with CKD value knowledge that allows them to regain some control in their lives, to cope with illness, and to alleviate anxiety stemming from uncertainty.39 Well-informed patients are better able to be engaged in their care; lack of knowledge is a source of anxiety and stress. Another important finding from this systematic review was that patients place value on relationships and being part of social networks of family, friends, health care staff, and other patients. These networks provide strength and support.39
Family members of patients with CKD, in addition to the practical aspects of being caregivers (eg, transportation), often provide psychological and cognitive support during the decision-making process. Caregivers are likely to broaden the range of considerations influencing decisions about kidney replacement therapy.40,41 CKD and its treatment influences the patients' role and function within the family and affects the entire family.32,34 In their role as caregivers, family members often experience stress, depression, and poor QOL. Yet their needs are often neglected and underprioritized, despite the obvious impact they may have on patient-centered care.
Important Factors in Educational Programs and Peer Support
Educational programs addressing factors that patients with CKD and their families consider important are likely to facilitate choice of treatment congruent with patients' and families' wishes and values.40 Such education will improve the family members' understanding of the risks and benefits of various treatments, facilitating discussions about expectations and goals of care. Knowledge about goals of care will help prepare family members for the practical aspects of the burden related to various treatment options, engaging them in the patient's care. Patients have also expressed concern about the devastating impact of CKD on caregivers and have suggested assessment of caregiver burden and improvement of support for caregivers among research priorities.42
Interactive educational interventions that involve individuals and groups may improve knowledge, activation to participate in care decisions, and patient outcomes.14 Peers with experience in managing their CKD may be in a unique position to communicate knowledge and confidence to a recently diagnosed patient in a more personalized manner. Patients with CKD have indicated the desire to meet other patients.21,30,32,36,38 They are interested in the experiences of others, not as a source of medical information27 but to discuss ideas on how to cope.30 Comparing themselves with their peers will reassure them of their own situation and will reduce their sense of isolation.31,43 There are at least 2 differences between a patient navigator program and peer mentoring (PM): First, a patient navigator is not necessarily a peer with a similar set of experiences; second, the primary goal of patient navigator programs is to overcome barriers for access to care, whereas PM has multiple additional goals, including sharing experiences, discussing coping mechanisms, and communicating knowledge and confidence.
Heisler proposed several models of peer support.44 These models include professional-led group visits; peer-led self-management training; peer coaches; community health workers; support groups; telephone-based peer support; and web- and email-based programs. The effectiveness of PM has been attributed to the nonhierarchical reciprocal relationship created by sharing similar experiences.45 Relationship-centered mentoring by trained peers is an effective strategy that provides individualized patient-centered information. PM has been shown to lead to improvements in activation of patients to participate in their own care, confidence in care, and multiple outcomes in various chronic diseases.46-53
Patient and Family Partner Program
Since 2004, the Kidney Foundation of Central Pennsylvania (KFCP) has operated a comprehensive patient engagement and empowerment program (Patient and Family Partner Program [PFPP]). Through the PFPP, trained patients with CKD and their family partners serve as volunteer peer mentors to other patients with CKD and their caregivers to help them engage successfully in their own care.54 Information about the impact of trained mentors on the care of patients with CKD is lacking. More specifically, a peer-led CKD intervention has not been assessed for its impact on patient activation, patient QOL, and caregiver burden.
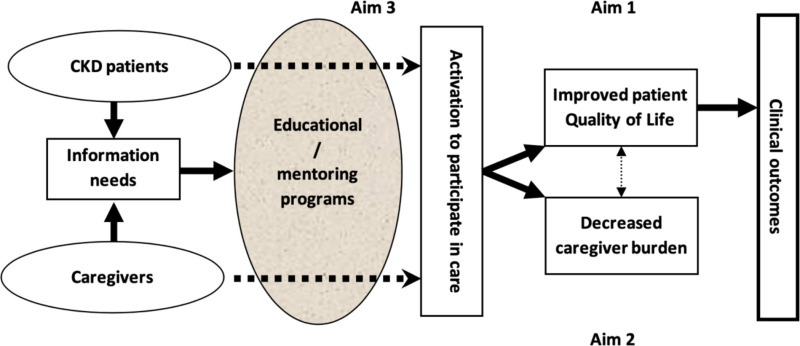
To address this issue, we examined the impact of a formal PM program on patient QOL, patient activation, and caregiver burden among patients with CKD and their caregivers. Knowledge gained from this randomized clinical trial (RCT) can potentially be extrapolated to other chronic disease states. We hypothesized, as depicted in Figure 1, that a formal structured mentoring program, based on information needs of patients and caregivers, would lead to improved patient QOL (aim 1) and decreased caregiver burden (aim 2). We also hypothesized that such educational programs would lead to activation of patients to participate in their own care (aim 3).
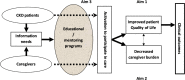
Figure 1
Conceptual Model of the Effect of a PM Program for Patients with CKD and Their Caregivers.
The specific aims of this RCT were as follows:
- To determine whether PM leads to improved QOL among patients with CKD.
- To determine whether PM leads to improved caregiver burden among caregivers of patients with CKD.
- To determine whether PM leads to improved patient activation among patients with CKD.
Patient and Stakeholder Engagement
Patients, caregivers, and other stakeholders were involved in various stages of the study. The PFPP, initially named Patient-Partner Program (PPP), was envisioned by a kidney transplant recipient who was a registered nurse and a member of the KFCP board of directors.55 She partnered with a renal social worker and a transplant coordinator to develop a PM program for patients with ESRD. Following a thorough literature and internet search, as well as personal contacts, the founding group developed a preliminary curriculum for the PPP. Based on input from renal physicians and social workers, the curriculum and the program has undergone periodic modification.55 Acknowledging the vital role of caregivers and family members in the care of patients with CKD, the founding group, consisting of a patient, a renal social worker, and a transplant coordinator, changed the name of the program to PFPP in 2011.
By 2011, approximately 80 mentors and >200 mentoring partnerships had been established. Review of mentor and mentee evaluations pointed toward relative success of the program. Mentored patients noted improved adherence with treatment and overall satisfaction with the improved adherence; mentors reported satisfaction with their roles; and both groups perceived the bonding experience as a very positive influence.
To assess the performance of the program and to discuss its future direction, we convened a focus group in August 2011. Participants in the focus group included the 3 original founders (a patient, a renal social worker, and a transplant coordinator), a dialysis patient, spouse of a transplanted patient, a social worker, director of the KFCP, the program coordinator, and the medical director of the PFPP. The current study is congruent with the 2 main themes identified in the focus group as the proposed goals of expansion of the PFPP: (1) Creating an online version of the program was proposed as a possible strategy to expand the PFPP to include rural areas; and (2) expanding the PFPP to include patients with pre-ESRD was deemed essential to the overall success of the program.
Figure 2 presents the governance of the research team. For this study, the research team organized 3 advisory groups and engaged 3 individual advisors (see the Acknowledgments section) as follows:
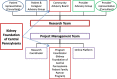
Figure 2
Governance of Research Team.
- Patient and Caregiver Advisory Group consisted of 12 members (7 patients and 5 caregivers) and provided the patients' and caregivers' perspectives about the content of the interventions, strategies for recruiting participants, and interpreting and disseminating findings. We recruited Patient and Caregiver Advisory Group members from among previously trained PFPP mentors, through a general call for volunteers on the KFCP website, and nomination by the KFCP and PFPP program coordinator.
- Provider Advisory Group consisted of 12 members and included nephrologists (2), nephrology advanced practice providers (1), primary care providers (1), dialysis social workers (2), transplant social workers (2), dialysis nurses (2), and transplant coordinators (2). The research team identified members of the Provider Advisory Group through professional networks in the community.
- Community Advisory Board consisted of the 15-member volunteer board of directors of the KFCP. The membership of the board of directors included patients (2), caregivers (2), community nephrologist (1), academic nephrologist (1), transplant surgeon (1), senior medical director of a major health care network and a primary care physician (1), vice president of another health system (1), dialysis social workers (2), members of the community (3), and an attorney (1). Some board members have been trained as mentors. The Community Advisory Board provided insights for developing the proposed research and oversight during the study. The principal investigator, a member of the board of directors since 2011, presented updates on the project at the quarterly meetings to seek input on recruitment, study flow, evaluation, and dissemination of findings. The Community Advisory Board's partnership with the KFCP board provided an opportunity to present preliminary results at the KFCP annual symposium on kidney disease.
- Individual advisors included an experienced renal social worker who was one of the original founders of the PFPP. She is highly regarded in the renal social work community of central Pennsylvania and, to improve recruitment, assisted in the description of the PFPP for potential participants and health care providers. The 2 patient advisors had previously completed training as mentors in the PFPP. Collectively, the 3 advisors provided insight into creating interventions; they particularly assisted in developing the content of the online PM intervention.
In addition to their roles within the advisory groups, patients, caregivers, and stakeholders played key roles in implementing the study. During training of peer mentors, stakeholders delivered parts of the content. The interventions (face-to-face [FTF] and online PM) were carried out entirely by patients and caregivers. Each year, a panel of patients and caregivers who were involved in the study presented updates and their personal experiences at the KFCP annual symposium on kidney disease. During the second year of the study, the Community Engagement Studio, organized by the Penn State Clinical and Translational Science Institute Comparative Effectiveness Research Center, was convened to present results and to explore options for public dissemination.
Methods
Study Overview
We conducted an RCT to test the effectiveness of mentoring by trained peers on QOL for patients with CKD as measured by the Kidney Disease Quality of Life-36 (KDQOL-36) instrument (specific aim 1), caregiver burden as measured by the Zarit Burden Interview (ZBI) (specific aim 2), and patient activation as measured by the Patient Activation Measure (PAM) (specific aim 3). We randomly assigned patients with CKD and their caregivers into 3 groups: (1) FTF PM plus informational textbook about CKD; (2) online PM plus informational textbook about CKD; and (3) textbook only (participants received the informational textbook about CKD but no PM). We used the PFPP, described earlier, as the mentoring program for the PM intervention groups.
Patients with CKD and their caregivers assigned to FTF intervention groups received 6 months of PM and the informational textbook of the PFPP. Participants assigned to the online intervention group also received 6 months of PM through an interactive online platform and the informational textbook of the PFPP. The textbook-only group received the informational textbook of the PFPP to review independently.
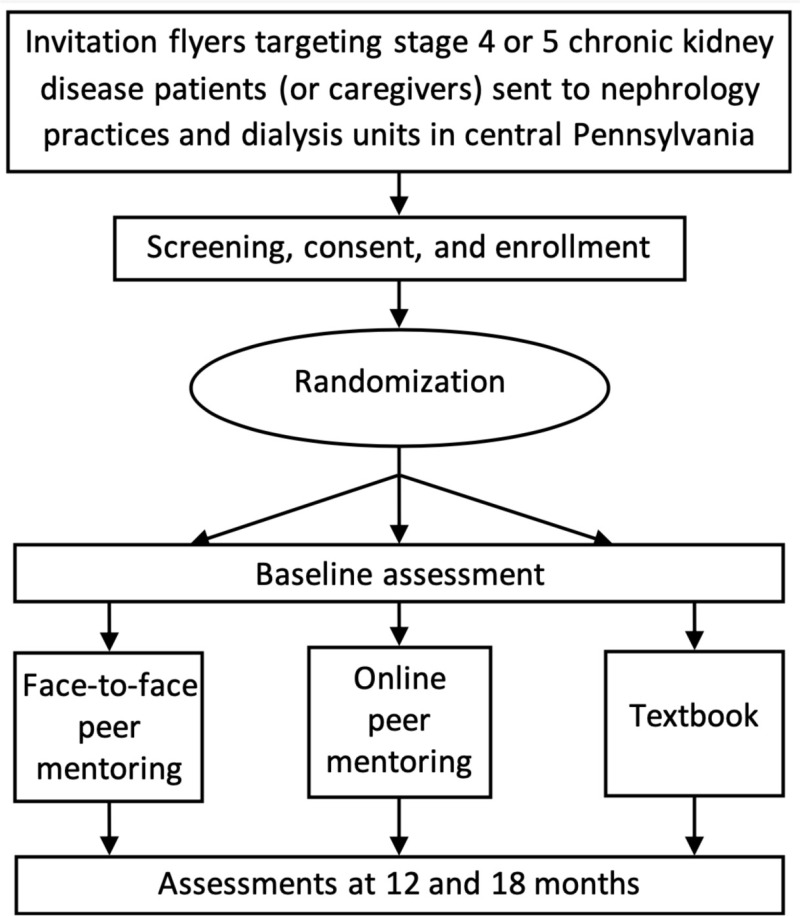
At baseline, 12 months, and 18 months, the patients completed the KDQOL-36 instrument and the PAM survey; the caregivers completed the ZBI. Figure 3 presents the study design.
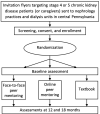
Figure 3
Design of the Trial: Recruitment, Enrollment, Interventions, and Assessments.
Study Setting
We conducted the trial among patients with CKD and their caregivers residing in 1 of the 28 counties served by the KFCP. The wide recruitment area included urban and rural areas, all racial and ethnic categories, and all socioeconomic levels. The Pennsylvania State University College of Medicine IRB approved the research protocol. We registered the protocol with ClinicalTrials.gov (identifier NCT02429115).
Participants
The study population came from dialysis units and nephrology practices in central Pennsylvania. We recruited participants from among patients with stage 4 or 5 CKD, or caregivers of patients with CKD. Inclusion criteria included the following: (1) patients diagnosed with stage 4 or 5 CKD by a physician, or caregivers of a patient with CKD; (2) at least 18 years of age; (3) able to read and write in English at the eighth-grade level; (4) having access to a computer with internet and email capability; and (5) willing to participate. Exclusion criteria were the following: (1) participation in previous PM for CKD; (2) inability to provide consent; (3) currently an inmate; and (4) physical condition preventing active participation in mentoring program as determined by primary nephrologist.
We sent information packets about the program to dialysis units and nephrology practices asking renal professionals to distribute them among patients and caregivers. The principal contact person in each practice was the social worker. The packets included an introduction to the program and an application form. A substantial number of the renal professionals, particularly renal social workers within the area served by the KFCP, have been familiar with the PFPP since its establishment. The program coordinator contacted all the nephrology practices and dialysis units every quarter to provide them with information about the program. The program coordinator, research coordinator, or both traveled to the dialysis units and provided information about PFPP and recruitment flyers to be distributed among patients and their caregivers. The flyers encouraged patients and their caregivers who might be interested in exploring being matched with a mentor to contact their social worker, the program coordinator, or the research coordinator.
Following informed consent, candidates underwent permuted block randomization with 1:1:1 allocation into “FTF PM,” “online PM,” and “textbook only.” The investigators did not have access to the identity of the participant assignment until they confirmed that the participant was eligible for enrollment.
Recruitment occurred continuously until we achieved the target sample sizes. In the initial screening questionnaire, we asked the potential candidates who declined to participate to indicate reasons for declining. To improve retention, we compensated participants with a $50 stipend for the baseline assessment and each of the follow-up assessments (ie, 12 months and 18 months) for a total of $150 per participant.
Interventions and Comparators or Controls
The description of the intervention follows the Template for Intervention Description and Replication (TIDieR) checklist developed by Hoffmann et al.56 The existing training of peer mentors in the PFPP involved the following: Mentors were patients with stage 4 or 5CKD, ≥18 years of age, or caregivers of patients with CKD, ≥18 years of age. Candidates for becoming a mentor received formal training through 16 hours of instructional sessions facilitated by patients, caregivers, and health care professionals. Based on feedback from participants and the board of directors of the KFCP, the curriculum committee revised the training program to include new topics, such as understanding mentees' choices, special needs of older adults, special needs of children and adolescents, and the effect of CKD on the family. The final curriculum is shown in Appendix A.
Upon successful completion of the training, each peer mentor received a certificate from the KFCP as a certified mentor. Key features of a successful PM program include sufficient training and ongoing support for peer mentors, regular opportunities for mentors to receive additional training, and appropriate recognition for their efforts.44 Recognizing the importance of continuing education following the initial training, we conducted refresher courses on a quarterly basis. These sessions, which the program coordinator facilitated, featured discussions among mentors about their actual experiences and a focused review of communication skills. Each mentor was required to attend at least 1 refresher course per year to maintain certification.
The 3 interventions were (1) FTF PM, (2) online PM, and (3) textbook only. Participants in all 3 groups received a copy of the PFPP textbook that contains detailed information about kidney disease and was prepared targeting an eighth-grade reading level. The choice of interventions emerged following a review of mentor and mentee evaluations and a focus group discussion involving dialysis and transplant patients, renal social workers, the director of the KFCP, the PFPP program coordinator, and the PFPP medical director.
We mounted the online intervention based on the focus group discussions and evidence that web-based peer support is a potential alternative to FTF mentoring, particularly for populations with limited ability to travel and for regions with limited resources. Online peer support groups have enhanced the care of diabetes.57,58 For example, Zrebiec evaluated a web-based educational and emotional resource for patients with diabetes and their family members at Joslin Diabetes Center in Boston, Massachusetts. Over 74 months, 74% of all respondents rated participation in the discussion board as having a positive effect on coping with diabetes, and 71% rated participation as helping them to feel more hopeful.59 Additional evidence supports the effectiveness of online chronic disease self-management.60
FTF PM Group
Individual mentees had the choice of meeting their mentor at a location preferred by the mentee. If the pair decided on a lunch meeting, KFCP provided reimbursement. The frequency of contact by a mentor was weekly by telephone and monthly for an FTF visit. At the end of each month, the mentor presented the log of the meetings to the program coordinator. The partnership was maintained for 6 months. The program coordinator maintained records of adherence by mentor-mentee pairs.
Online PM Group
Mentors and mentees communicated using a secure password-protected interactive online platform, which consisted of a web-based bulletin board designed specifically for this study. We developed the contents of the online PM program according to the themes that emerged from qualitative interviews and discussions with experienced peer mentors, patients, caregivers, and health care professionals. Each enrolled participant assigned to the online PM group was matched with a mentor based on treatment modality (for caregivers, this was the treatment modality of the patient for whom they were the caregiver). All communications between mentors and mentees were online.
Following initial introductions, the mentors initiated the discussion thread by presenting an overview of the program. The mentors and mentees also reviewed mutual goals and expectations at the time of initial communication. Mentors encouraged mentees to familiarize themselves with the textbook and to post any questions or update their status using written statements or mood and symptom icons. The mentors responded to questions or posted a query about the updated status. The mentors sent a weekly reminder email to the mentees to log on to the website to review posted contents and to post a weekly action plan, as well as any problems they wished to discuss with their individual mentors. The frequency of contact by mentors was weekly or more frequently as initiated by the mentees. The program coordinator maintained regular contact with the mentors and mentees and closely monitored the frequency and content of the interactions to ensure appropriate communication, to correct inaccurate information posted by participants, and to direct the mentees to their care providers in situations requiring medical intervention. The partnership was maintained for 6 months. The program coordinator maintained records of adherence by mentor-mentee pairs.
Textbook-Only Group
This group received the textbook with instructions to ask questions of the care providers. The textbook contains information about the structure and function of the kidneys, causes of kidney failure, dietary considerations in kidney disease, and modes of treatment for CKD. Adherence to the review of material was by self-report.
Study Outcomes
Primary Outcomes
The primary outcome for specific aim 1 was improvement in health-related QOL (HRQOL) as measured by the KDQOL-36 instrument from the Rand Corporation. This 36-item instrument was developed as a self-reported HRQOL tool designed specifically for patients with CKD.61 The KDQOL-36 combines the SF-12 and 24 domains specifically targeted to kidney disease. The SF-12 yields the Physical Composite Summary (PCS) and the Mental Composite Summary (MCS). The domains specifically targeted to kidney disease comprise 3 scales: the 4-item Burden of Kidney Disease (BKD), the 12-item Symptoms and Problems of Kidney Disease (SPKD), and the 8-item Effects of Kidney Disease (EKD). The instruments are scored on a scale of 0 to 100, with higher numbers indicating better HRQOL.62
The KDQOL-36 has been validated among patients with CKD with predictive value for several outcomes, including survival; it is the most widely used measure of HRQOL in the nephrology literature.62-71 A single KDQOL-36 Summary Score was recently proposed by Peipert et al.73 In this report, we present the results in the form of separate component summaries of the KDQOL-36.
The primary outcome for specific aim 2 was caregiver burden as measured by the ZBI. Despite the increasing awareness of the burden and adverse effects of CKD on caregivers, high-quality evidence is lacking about the effect of information or support interventions on the psychosocial well-being of caregivers.74 The ZBI is a self-administered questionnaire of 22 items that measures the impact of caregiving in psychological, physical, and social domains. The items are rated on a 5-point Likert scale.75 The total score is calculated from 0 to 88; the higher the score, the heavier the burden.76 The ZBI has been used to measure caregiver burden among caregivers of patients undergoing dialysis77 and for numerous other chronic disease states.78-84 Higher ZBI scores are associated with depression and anxiety.85
Secondary Outcome
The secondary outcome was improvement in patient activation (specific aim 3) as measured by the PAM, a valid and reliable instrument to measure activation of patients to participate in their own care. The PAM was developed based on qualitative research, Rasch analysis, and classical test theory.86 The instrument is a participant-completed questionnaire that yields a continuous activation score ranging from 0 to 100, representing patients' positions along a multistage hierarchical process of activation. Activated patients identified by higher PAM scores are more likely to benefit from interventions designed to improve their health-related knowledge, skills, motivation, and behaviors.
The PAM is a well-validated tool for assessing patient activation and the impact of such activation on outcomes among individuals with various chronic diseases, such as inflammatory bowel disease87 and multiple sclerosis.88 The PAM was also validated as a measure of patient activation and adherence to physical therapy for individuals undergoing elective spine surgery, as reflected in attendance and engagement.89 The high reliability estimate of PAM (Cronbach α = .91) maintains precision across sex and age groups90 and geographic locations.91-93 Higher PAM scores are associated with better self-management health behaviors such as adherence to medication regimens and other health behaviors, higher satisfaction, better QOL, and improved physical and mental functioning.90,94,95 Initially developed as a 22-item instrument, the 13-item short version of the PAM was shown to have similar validity to the original version.91,96
Sample Size Calculations and Power
Sample Size for Patients
The primary outcome for specific aim 1 was improvement in KDQOL-36 score. A 10-point change in the components of the KDQOL-36 was associated with significant changes in clinically relevant outcomes.72 Considering a minimally clinically important difference (MCID) of 10, a mean of 63, an SD of 13 (Mapes et al72), an α of .05, and a comparison of 3 means, we expected that a sample size of 39 patients per group would yield a statistical power of 0.8. Assuming a dropout rate of 15%,60 the total target sample size was 132 patients (44 for each of the 3 study groups).
Sample Size for Caregivers
Change in ZBI score was the primary outcome for specific aim 2. We computed the number of participants needed using data from a study assessing burden among caregivers of patients undergoing peritoneal dialysis, in which the mean combined caregiver burden score (±SD) was 12.5 ± 8.7 (Shimoyama et al77). For our study, we selected 1 SD as the MCID. Assuming an effect size of 1 SD, an α of .05, and a comparison of 3 means, we expected that a sample size of 23 would yield a statistical power of 0.8. Our target total sample size was 84 caregivers (28 for each of the 3 study groups) to adjust for a dropout rate of 15%.
Time Frame for the Trial
According to individual studies,98,99 a systematic review of 15 studies,100 a qualitative synthesis of 25 studies,101 and a review of 11 studies,102 the effective duration of PM for chronic diseases has ranged from 6 weeks to 2 years. Based on these reports, we selected 6 months as the length of intervention. Sustainability of any QOL effect has ranged from 3 months103 to 18 months.104 We chose 12 months postenrollment (6 months after completion of intervention) for quantitative follow-up assessments. To evaluate any sustained intervention effect, we repeated assessments at 18 months.
Data Collection and Sources
Quantitative data for this RCT included responses to self-administered survey instruments delivered by regular mail. We transferred electronically coded data into a secure database that was constantly password protected with no linkage to any personal identifiers in this file. We used statistical alterations to ensure that individuals could not be identified through any study data set.
To maximize follow-up rates, the program coordinator used email messages and telephone calls to remind the participants to complete the surveys. Participants received monetary compensation for their time. The program coordinator contacted participants and their respective social workers to determine reasons for withdrawal or loss to follow-up.
Analytical and Statistical Approaches
The independent variable was allocation to the FTF PM, online PM, or textbook-only groups. Our 3 dependent variables (outcomes) were the following: scores on the KDQOL-36 (aim 1), ZBI (aim 2), and PAM (aim 3). The outcome variables were continuous and measured at 3 time points (baseline, 12 months, and 18 months). Covariates included demographic measures (age, sex, race/ethnicity, marital status, attended or completed college, employment status, and rural vs urban location). We used repeated-measures analysis of variance within the context of a linear mixed-effects model to estimate time-related changes in scores. The analyses included values of all 3 measurements (baseline and subsequent follow-ups) of the outcome variables.
The data source for covariates other than rural/urban location was predominantly participant self-report. The data source for rural vs urban location was based on the address and rural-urban commuting area code.105 We used type 3 fixed effects to test the statistical significance of each of the demographic variables.
To minimize the limitations of self-report data, we ensured that the questions were clearly worded and that we collected data at time of enrollment and at each follow-up visit (Appendices B and C). Analyses were by intention to treat (ITT). We computed descriptive statistics for all variables. We assumed that missing data would occur at random. None of the variables had >10% missing data. The statistical analyses based on restricted maximum likelihood estimation for the linear mixed-effects model allowed for valid and unbiased conclusions in the presence of data that are missing at random.
We had insufficient evidence to support a priori hypotheses about heterogeneity of treatment effects (HTEs), and thus we planned no hypothesis-driven HTE analyses. To investigate HTEs, however, we conducted exploratory analyses by including interaction terms, such as employment status, in the statistical models. We also pursued exploratory subgroup analyses (age, sex, race/ethnicity, education, employment, and rural/urban residence). We investigated reasons for participants' dropout. We used SAS, v.9.4 (SAS Institute, Inc) for data analysis.
Changes to the Original Study Protocol
We requested a 10-month no-cost extension to allow for assessment of all outcomes at 18 months; this let us perform analyses beyond the initial 12-month assessment in our original milestones. The extension facilitated evaluating persistence of the effects of the interventions on the outcomes.
In the original protocol, we had included 3 primary outcomes. We subsequently changed the patient activation to a secondary outcome. We added “participation in previous peer mentoring for CKD” and “physical condition preventing active participation in mentoring program” to the exclusion criteria.
Results
Patient Populations and Demographics
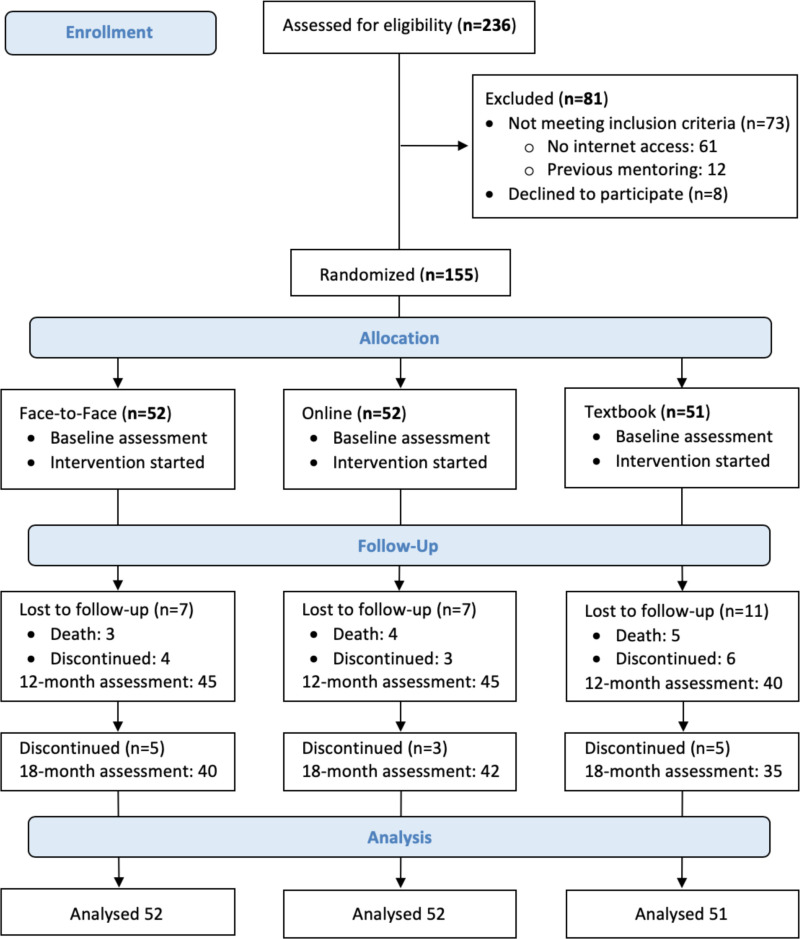
The CONSORT diagram (Figure 4) describes the flow of patient participants through the study interventions. We assessed 236 patients with stage 4 or 5 CKD for eligibility. We excluded 81 patients. Of these, 73 did not meet inclusion criteria (no internet access, n = 61; previous mentoring for CKD, n = 12), and 8 eligible patients declined to participate after receiving more information about the study because of the perceived burden of participating in the study.
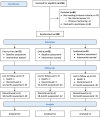
Figure 4
CONSORT Diagram for Flow of Patients Through the Trial.
Using permuted block randomization with 1:1:1 allocation, we randomly assigned 155 patients to 3 groups. All patients began participation in the interventions and completed the baseline assessment. Of these, 117 patients completed the interventions and the 18-month assessment. Assessments consisted of completion of the KDQOL-36 instrument (specific aim 1) and PAM (specific aim 3).
Table 1 presents the baseline characteristics of the patients. Baseline demographic characteristics did not significantly differ statistically among the 3 groups (Appendix D documents the details of patients' demographic characteristics).
Table 1
Baseline Characteristics of Patients by Intervention Group.
Among the FTF group, 45 patients completed the allocated intervention and the 12-month assessment; 40 patients completed the 18-month assessment. Among the online PM group, 45 patients completed the allocated intervention and the 12-month assessment; 42 patients completed the 18-month assessment. Among the textbook-only group, 40 patients completed the allocated intervention and the 12-month assessment; 35 patients completed the 18-month assessment. We included 117 patients in the final analyses.
Patient Outcomes: Aims 1 and 3
The key outcomes for aim 1 and aim 3 were KDQOL-36 score and PAM score, respectively.
Aim 1: KDQOL-36 scores: main analyses
In Table 2, we document the unadjusted mean scores of the EKD, BKD, SPKD, and SF-12 PCS and MCS of the KDQOL-36 in the 3 groups at baseline, at 12 months, and at 18 months.
Table 2
Mean Unadjusted Domain Scores of the KDQOL-36 Throughout the Study Period by Intervention Group (ITT Analysis).
Table 3 shows the changes in mean (∆) scores from the KDQOL-36 over the study period. We note the following findings:
Table 3
Unadjusted Changes in Mean Scores of Domains of the KDQOL-36 Throughout the Study Period by Intervention Group (ITT Analysis).
- Online PM was associated with a statistically significant increase in 4 mean scores at 12 months:
- BKD score (∆, 11.7; 95% CI, 0.4-23.0 [P = .04])
- SPKD score (∆, 9.0; 95% CI, 1.9-16.1 [P = .01])
- SF-12 PCS score (∆, 7.8; 95% CI, 4.1-11.6 [P = .0001])
- SF-12 MCS score (∆, 6.5; 95% CI, 2.4-10.6 [P = .002])
- Online PM was associated with a statistically significant increase in 5 mean scores at 18 months:
- EKD score (∆, 9.8; 95% CI, 0.2-19.4 [P = .04]).
- BKD score (∆, 13.4; 95% CI, 1.0-25.8 [P = .03]).
- SPKD score (∆, 8.0; 95% CI, 0.8-15.2 [P = .03]).
- SF-12 PCS score (∆, 5.8; 95% CI, 1.8-9.8 [P = .005]).
- SF-12 MCS score (∆, 7.7; 95% CI, 3.7-11.7 [P = .0002]).
- FTF PM was associated with a statistically significant increase in 2 mean scores at 12 months:
- SPKD score (∆, 8.3; 95% CI, 1.5-15.1 [P = .01]).
- SF-12 MCS score (∆, 6.2; 95% CI, 1.8-10.6 [P = .006]).
FTF PM was not associated with any significant change in the mean scores of any of the KDQOL-36 domains at 18 months.
Among the textbook-only group, no significant changes were found in the mean scores of any of the KDQOL-36 domains at 12 months or 18 months.
Table 4 shows changes in the scores from the KDQOL-36 over the study period, adjusted for demographic variables (race/ethnicity, sex, age quartile, marital status, attended or completed college, employment status, and rural/urban location), using ITT analysis. The slope estimates represent change in scores in standard points over 18 months.
Table 4
Changes in Scores on the KDQOL-36 Instrument Throughout the Study Period Among Groups (ITT Analysis).
Online PM was associated with a statistically significant increase in 5 slope scores:
- EKD score (slope estimate, 4.13; 95% CI, 0.87-7.4 [P = .01]).
- BKD score (slope estimate, 5.44; 95% CI, 1.24-9.64 [P = .01]).
- SPKD score (slope estimate, 6.00; 95% CI, 3.09-8.91 [P = .006]).
- SF-12 PCS score (slope estimate, 2.50; 95% CI, 0.95-4.06 [P = .001]).
- SF-12 MCS score (slope estimate, 3.46; 95% CI, 1.78-5.13 [P = .0001]).
Compared with textbook only, online PM was associated with a statistically significant increase in 4 comparisons of slope scores:
- In the BKD domain (6.43; 95% CI, 0.27-12.58 [P = .04]).
- In the SPKD domain (8.71; 95% CI, 5.35-12.07 [P = .003]).
- In the PCS domain (3.20; 95% CI, 0.95-5.48 [P = .01]).
Compared with textbook only, FTF PM was associated with a statistically significant increase in the slope score for the SPKD domain (6.12; CI, 2.62-9.38 [P = .04]).
Aim 1: KDQOL-36 Scores: Subgroup Analyses
We performed exploratory analyses to investigate HTEs. We used type 3 fixed-effects models to identify statistically significance (P ≤ .05) [demographic variable × KDQOL domain score] to include in the multivariable model. Results appear in Table 5.
Table 5
Type 3 Tests of Fixed Effects for Demographic Variables and Scores of KDQOL-36 Domains.
Exploratory subgroup analyses displayed in Tables 6 and 7 (for EKD and BKD, respectively) showed heterogeneity for the effects of being employed on the association between FTF PM and increased EKD (P value of slope estimate = .002) and BKD (P value of slope estimate = .04) scores and the association between online PM and increased EKD score (P value of slope estimate = .02). When comparing FTF PM with textbook only, employment had a positive effect on the difference between the slopes of change of EKD (P value of slope estimate = .02) (Table 6) and BKD (P value of slope estimate = .03) (Table 7) in the FTF group vs the textbook-only group. All analyses were by ITT.
Table 6
Change in EKD Scores Among the Groups by Employment Status (ITT Analysis).
Table 7
Change in BKD Scores Among the Groups by Employment Status (ITT Analysis).
In exploratory subgroup analyses shown in Table 8, “not employed” status had a positive effect on the association between online PM and increased SF-12 PCS score (P = .01). We also detected a positive effect of “not employed” status on the difference between slopes of change in SF-12 PCS score in the online PM group vs the textbook-only group (P = .04). All analyses were by ITT.
Table 8
Change in SF-12 PCS Score Among Groups by Employment Status (ITT Analysis).
Being male (shown in Table 9) had a positive effect on the association between online PM and increased SF-12 MCS score (P < .0001). We also saw a positive effect of male sex on the difference between slopes of change in the SF-12 MCS score in the FTF PM group vs the online PM group (P = .02) and between slopes of change in SF-12 MCS score in the online PM group vs the textbook-only group (P = .02).
Table 9
Change in SF-12 MCS Score Among Groups by Sex (ITT Analysis).
Aim 3: PAM scores: main analyses
Patient activation highlights patients' preparedness and ability to take independent actions to manage their health and care and is a component of patient engagement. An improvement of 4 points in the PAM score is considered an MCID.97 The mean PAM scores in the 3 groups at baseline, 12 months, and 18 months are shown in Table 10. The mean baseline scores were not statistically different among the 3 groups.
Table 10
Mean PAM Scores Throughout the Study Period by Intervention Group (ITT Analysis).
Table 11 shows the changes in mean PAM scores from baseline to 12 months and 18 months. Online PM was associated with a statistically significant increase in the mean PAM score at 12 months (∆, 10.5; 95% CI, 4.5-16.6 [P = .0009]).
Table 11
Changes in Mean PAM Scores Throughout the Study Period by Intervention Group (ITT Analysis).
Online PM was also associated with a statistically significant increase in mean PAM score at 18 months (∆, 11.0; 95% CI, 4.7-17.3 [P = .0008]).
FTF PM and textbook only were not associated with statistically significant changes in mean PAM scores at 12 months or at 18 months.
Table 12 shows changes in PAM scores from baseline to 18 months adjusted for demographic variables (race/ethnicity, sex, age quartile, marital status, attended or completed college, employment status, and rural/urban location). The online PM group experienced a significant increase in the PAM score in the study period (slope estimate, 5.66; 95% CI, 2.79-8.52 [P = .0001]). The difference between the slopes of the FTF PM PAM score and the online PM PAM score was also statistically significant (SE, −4.70 ± 2.07; 95% CI, −8.79 to −0.62 [P = .02]). The difference between the slopes of the online PM PAM score and textbook-only PAM score was also statistically significant (SE, −5.63 ± 2.12; 95% CI, 1.44-9.83 [P = .01]).
Table 12
Change in PAM Scores Throughout the Study Period Among Groups (ITT Analysis).
Subgroup analyses: PAM scores: aim 3
We performed exploratory analyses to investigate HTEs. We used type 3 fixed-effects models to identify statistical significance (P ≤ .05) [demographic variable × PAM score] to include in the multivariable model (Table 13).
Table 13
Type 3 Tests of Fixed Effects for Demographic Variables and PAM Scores.
In exploratory subgroup analyses documented in Table 14, we detected a positive effect of male sex on the association between online PM and increased PAM score (P < .0001). Female sex had a positive effect on the association between FTF PM and increased PAM score (P < .01). With respect to the comparisons, being male had a positive effect on the difference between slopes of change in PAM score for the FTF PM group vs the online PM group (P < .0001) and between the online PM group and the textbook-only group (P < .0001).
Table 14
Change in PAM Among Groups by Sex (ITT Analysis).
In other exploratory subgroup analyses of marital status (Table 15), being married had a positive effect on the association between online PM and PAM score (P = .003). In contrast, “not married” status had a positive effect on the association between FTF PM and increased PAM score (P = .03). “Not married” status also had a positive effect on the association between online PM and increased PAM score (P = .008). In addition, we found a positive effect of “married” status on the differences in slopes of change for PAM score in the FTF PM group vs the online PM group (P = .0008) and in the online PM group vs the textbook-only group (P = .02).
Table 15
Change in PAM Among Groups by Marital Status (ITT Analysis).
Aim 2: Caregiver Outcomes
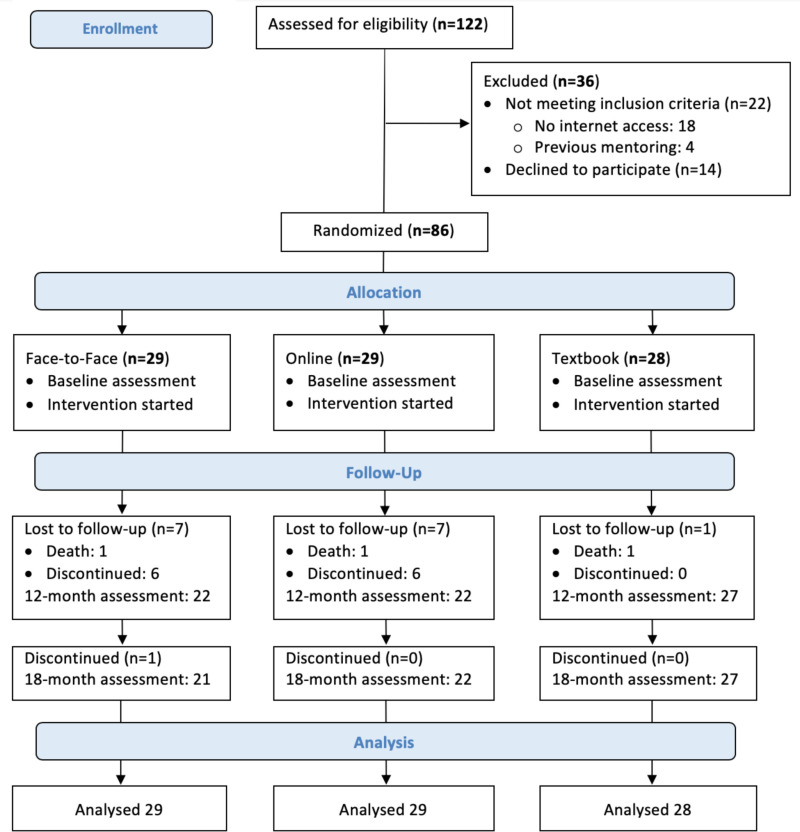
The CONSORT diagram in Figure 5 describes the flow of caregiver participants through the study interventions. This focus involves specific aim 2 and completion of the ZBI. We assessed 122 caregivers of patients with stage 4 or 5 CKD for the caregivers' eligibility. We excluded 36 caregivers: 22 did not meet inclusion criteria (no internet access, n = 18; previous mentoring experience, n = 4), and 14 eligible caregivers declined to participate after receiving more information about the study owing to the perceived burden.
Figure 5
CONSORT Diagram for Flow of Caregivers Through the Trial.
We randomly assigned 86 caregivers to the 3 groups. All caregivers completed the baseline assessment and started participation in the interventions. Seventy caregivers completed the interventions and the 18-month assessment. Fifteen caregivers were lost to follow-up before the 12-month assessment, and 1 caregiver withdrew from the trial after completing the 12-month assessment.
Table 16 documents the primary baseline characteristics of the caregivers. Appendix E presents details of caregiver demographics. Baseline demographic characteristics did not significantly differ statistically across the 3 groups.
Table 16
Baseline Characteristics of Caregivers According to Intervention Group.
Aim 2: Caregiver burden scores
The mean ZBI scores in the 3 groups at baseline, 12 months, and 18 months are shown in Table 17. The mean baseline scores did not statistically differ among the 3 groups.
Table 17
Unadjusted Mean ZBI Scores Throughout the Study Period Among Intervention Groups (ITT Analysis).
Table 18 shows the changes in mean scores from the ZBI over the study period.
Table 18
Changes in Unadjusted Mean ZBI Scores Throughout the Study Period by Intervention Group (ITT Analysis).
FTF PM was associated with a statistically significant decrease (improvement) in the mean ZBI score at 12 months (∆, −5.1; 95% CI, −10.0 to −0.2 [P = .04]). FTF PM was also associated with a statistically significant improvement in the ZBI score at 18 months (∆, −7.1; 95% CI, −12.2 to −2.1 [P = .007]). Online PM was associated with a statistically significant improvement in the mean ZBI score at 12 months (∆, −7.1; 95% CI, −13.3 to −0.9 [P = .03]). Online PM was also associated with a statistically significant improvement in the ZBI score at 18 months (∆, −8.4; 95% CI, −14.9 to −1.9 [P = .01]).
Table 19 shows changes in caregivers' ZBI scores over the study period, adjusted for demographic variables (race/ethnicity, sex, age quartile, marital status, education, employment status, and rural/urban location). ZBI scores decreased significantly in the study period in the online PM group (SE, −3.44; 95% CI, −6.31 to −0.57 [P = .002]). ZBI scores did not significantly change among the FTF PM group or the textbook-only group.
Table 19
Change in Adjusted ZBI Score Throughout the Study Period Among Groups (ITT Analysis).
No demographic variables had a significant independent effect on the change in ZBI scores among groups.
Discussion
Key Findings
The findings from this clinical trial support the hypothesis that PM improved some QOL and patient activation scores among patients with advanced (stages 4 and 5) CKD. PM was also associated with improvements in caregiver burden score.
Compared with textbook-only and FTF PM, online PM was associated with improvements in scores for the EKD, the BKD, the SPKD, the SF-12 PCS and the MCS of the KDQOL-36, and the PAM. Online PM was also associated with improved caregiver burden (ie, improved ZBI scores).
Compared with textbook only, FTF PM was associated with improved SPKD scores. FTF PM was associated with improved outcomes after exploratory subgroup analyses, with a positive effect in EKD and BKD scores among employed participants and a positive effect of female sex and unmarried status on PAM scores (see the Treatment Response Heterogeneity section).
Our findings are consistent with studies that have shown effectiveness of online social support networks in improving patient-related outcomes. The use of professionally moderated internet discussion groups and peer-led online interventions has been proposed as a strategy for patient engagement, self-management, information exchange, and support for patients with chronic disease.106-108 Lorig et al109,110 found that an internet-based, peer-led chronic disease self-management program (CDSMP) reduced health care use and improved health-related behavior change, self-efficacy, and satisfaction with the health care system. The effects were similar to results for small-group CDSMP. Other forms of internet-based interventions associated with improved chronic disease management include discussion boards, computer-tailored feedback, and behavioral e-counseling.111,112
Despite the findings just noted, uncertainty persists about the effectiveness of such interventions on long-term outcomes of clinical significance. Eysenbach et al113 cited lack of sufficient evidence to support use of peers for online programs. They suggested that research is required to evaluate specific conditions for which the effect of online PM might be maximized. A Cochrane review concluded that peer-led self-management programs may lead to small short-term improvements; it reported no evidence to suggest that these programs lead to significant long-term improvements in health-related outcomes.114
General conclusions about the effectiveness of PM based on review of clinical trials are limited for several reasons. These include the substantial variation in online mentoring programs, ambiguity about differentiation between mentoring programs and support groups, the variety of disease states, the severity of illness, and the variety of studied outcomes.
In contrast, our study was unique in several aspects and addressed some concerns about the effect of PM. For example, the peer mentors received formal training. We monitored the delivery of the mentoring program that proved relatively consistent. The disease state and severity of illness were well defined. Finally, the outcomes were distinct and measurable over a period of 18 months. Possible reasons for the superiority of online PM compared with FTF PM include convenient access without the restrictions of arranging for a meeting and flexibility of time of communication with less concern about imposing on the mentor's time. Furthermore, online communication allows for questions and answers to be presented in a less pressured setting, permitting time to think, as well as mutually more thoughtful communication.
Implications for Application to Other Chronic Diseases
Although certain aspects of the study are unique to CKD, and although the study benefited considerably from the already existing infrastructure of the KFCP and the PFPP, the protocol and results may be generalizable to other chronic diseases. Engaging patients, caregivers, and other stakeholders during the early phases is likely to produce important benefits to any patient-centered research study. Furthermore, and more specifically, engaging patients and caregivers by formally training them to be peer mentors is likely to improve patient-related outcomes.
The success of such programs will depend on cooperation among renal professionals, patients, caregivers, and other stakeholders, particularly patient advocacy organizations. In our study, we benefited considerably from collaboration with renal social workers for recruitment. Such programs and studies have their own unique challenges that need to be mitigated. For example, medical professionals may be concerned that the program will interfere with the patient-provider relationship.
Treatment Response Heterogeneity
We performed exploratory analyses to investigate HTEs with these results:
- Among the online PM group, “not employed” status had a positive effect on the increased SF-12 PCS score, and being male had a positive effect on the increased SF-12 MCS score.
- Among the FTF group, employed status had a positive effect on EKD and BKD scores.
- Among the online PM group, married status and male sex had a positive effect on PAM scores.
- Among the FTF PM group, unmarried status and female sex had positive effects on PAM scores.
Strengths and Limitations
The major strength of the study was the engagement of patients, caregivers, and other stakeholders throughout various stages. Stakeholders were involved in designing the study, recruiting participants, delivering the interventions, and disseminating the interim results to the community. Another strength was the relatively strong adherence to protocol and completion of the trial, although patients with stage 4 or 5 CKD have multiple highly severe comorbidities that might prevent them from participating in clinical trials lasting more than a few months.
Although the overall loss to follow-up was higher than expected, to minimize potential bias resulting from the effect of loss to follow-up, we used ITT analysis. The highest loss to follow-up occurred in the textbook-only (control) group, reducing concern about burden of intervention as the main reason for dropout. Loss to follow-up rates were similar between the 2 active intervention groups (ie, FTF PM and online PM).
Our study also had several limitations. First, we limited the participants to English-speaking individuals with computer literacy and internet access. Second, adherence to review of material by the textbook-only group was by self-report without monitoring by the study team. Third, although the program coordinator monitored adherence to the PFPP protocol, we did not collect information as part of the research, and we did not include frequency of contact with the interventions in the analyses.
Fourth, although the total number of participants included in the final analyses was sufficient per our power calculations, the number of participants belonging to certain subpopulations, such as rural and Hispanic patients, was limited, not allowing for exploratory subgroup analyses in those populations. Fifth, we conducted the trial in central Pennsylvania, limiting geographic generalizability. Sixth, because of the nature of the disease and its treatment, patients with CKD, particularly those undergoing hemodialysis, already belong to a community and are likely more willing than patients with other chronic diseases to engage actively in peer-related activities. Finally, the small number of patients using modalities of treatment other than hemodialysis did not allow for accounting for the effect of hemodialysis on outcome measures.
Future Research
As suggested by our findings, larger studies drawing participants from diverse backgrounds and geographic locales, as well as a larger number of patients with CKD not undergoing hemodialysis, will allow for more robust subgroup analyses. We concur with the recommendations from the Cochrane review114 of peer-led programs that future research should focus on assessing longer-term outcomes of interest to patients, such as repeated hospitalizations and satisfaction with treatment decisions. Other potential areas of research include assessing outcomes such as fatigue and cognition; examining relationships between health literacy and outcomes; and evaluating the role and end results of PM among children and adolescents, among patients with earlier stages of CKD, and among patients with other chronic disease states.
Because we used the existing infrastructure of our partnering organization, the KFCP, to deliver the interventions, our costs were limited to developing and maintaining the online platform and covering research staff salaries and participant stipends. Therefore, detailed cost-effectiveness studies of interventions that include PM are needed to help clarify the feasibility of such interventions in settings where a new infrastructure might be required. Finally, although we do not believe that the interventions in this trial were complex, using process evaluation to test these interventions is a potential area for future research.115,116
Conclusions
The fundamental aim of this RCT was to evaluate the effect of a PM program on patient QOL and patient activation among patients with CKD and on burden of care among their caregivers. We compared 2 forms of PM, 1 delivered in FTF meetings and the other by online contact only, with a control group whose only intervention was access to an informational textbook on kidney disease. Online PM was associated with increased QOL as measured by the KDQOL-36 and patient activation as measured by the PAM among patients with CKD. Online PM was also associated with decreased burden of care among caregivers of patients with CKD as measured by the ZBI. In contrast, FTF PM was associated with improvement in fewer outcomes than online PM and only in subgroups identified in exploratory analyses. We conclude that online PM is an effective strategy for patient activation that leads to improved QOL and reduced caregiver burden with potential for use in other chronic disease states.
References
- 1.
- Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73(3 Suppl 1):A7-A8. [PMC free article: PMC6620109] [PubMed: 30798791]
- 2.
- Kalender B, Ozdemir AC, Dervisoglu E, Ozdemir O. Quality of life in chronic kidney disease: effects of treatment modality, depression, malnutrition and inflammation. Int J Clin Pract. 2007;61(4):569-576. [PubMed: 17263698]
- 3.
- Walters BAJ, Hays RD, Spritzer KL, Fridman M, Carter WB. Health-related quality of life, depressive symptoms, anemia, and malnutrition at hemodialysis initiation. Am J Kidney Dis. 2002;40(6):1185-1194. [PubMed: 12460037]
- 4.
- Tsai YC, Hung CC, Hwang SJ, et al. Quality of life predicts risks of end-stage renal disease and mortality in patients with chronic kidney disease. Nephrol Dial Transplant. 2010;25(5):1621-1626. [PubMed: 20037172]
- 5.
- Levey AS, Eckardt K-U, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney int. 2005;67(6):2089-2100. [PubMed: 15882252]
- 6.
- Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. [PubMed: 21840587]
- 7.
- Hsu C-y, Vittinghoff E, Lin F, Shlipak MG. The Incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med. 2004;141(2):95-101. [PubMed: 15262664]
- 8.
- DeCuir JR. Praying for the power of patience (POP)—life is great, even with kidney failure! Adv Ren Replace Ther. 1998;5(3):252-253. [PubMed: 9686636]
- 9.
- Hedman H. Patient compliance in renal replacement therapy: whose problem is it? The patient's perspective. EDTNA ERCA J. 1998;24(4):15-16. [PubMed: 10222907]
- 10.
- Waterman AD, Robbins ML, Peipert JD. Educating prospective kidney transplant recipients and living donors about living donation: practical and theoretical recommendations for increasing living donation rates. Curr Transplant Rep. 2016;3(1):1-9. [PMC free article: PMC4918088] [PubMed: 27347475]
- 11.
- Waterman AD, Morgievich M, Cohen DJ, et al. Living donor kidney transplantation: improving education outside of transplant centers about live donor transplantation—recommendations from a consensus conference. Clin J Am Soc Nephrol. 2015;10(9):1659-1669. [PMC free article: PMC4559502] [PubMed: 26116651]
- 12.
- LaPointe Rudow D, Hays R, Baliga P, et al. Consensus conference on best practices in live kidney donation: recommendations to optimize education, access, and care. Am J Transplant. 2015;15(4):914-922. [PMC free article: PMC4516059] [PubMed: 25648884]
- 13.
- Cassidy BP, Getchell LE, Harwood L, Hemmett J, Moist LM. Barriers to education and shared decision making in the chronic kidney disease population: a narrative review. Can J Kidney Health Dis. 2018;5:2054358118803322. https://www
.ncbi.nlm .nih.gov/pmc/articles/PMC6236635/ [PMC free article: PMC6236635] [PubMed: 30542621] - 14.
- Lopez-Vargas PA, Tong A, Howell M, Craig JC. Educational interventions for patients with CKD: a systematic review. Am J Kidney Dis. 2016;68(3):353-370. [PubMed: 27020884]
- 15.
- Hsu C-K, Lee C-C, Chen Y-T, et al. Multidisciplinary predialysis education reduces incidence of peritonitis and subsequent death in peritoneal dialysis patients: 5-year cohort study. PLoS One. 2018;13(8):e0202781. doi:10.1371/journal.pone.0202781 [PMC free article: PMC6107258] [PubMed: 30138478] [CrossRef]
- 16.
- Moss AH. Ethical principles and processes guiding dialysis decision-making. Clin J Am Soc Nephrol. 2011;6(9):2313. https://cjasn
.asnjournals .org/content/6/9/2313 [PubMed: 21896833] - 17.
- Tuso P. Choosing wisely and beyond: shared decision making and chronic kidney disease. Perm J. 2013;17(4):75-78. [PMC free article: PMC3854813] [PubMed: 24361024]
- 18.
- Covic A, Bammens B, Lobbedez T, et al. Educating end-stage renal disease patients on dialysis modality selection. NDT Plus. 2010;3(3):225-233. [PMC free article: PMC5477971] [PubMed: 28657058]
- 19.
- Finkelstein FO, Story K, Firanek C, et al. Perceived knowledge among patients cared for by nephrologists about chronic kidney disease and end-stage renal disease therapies. Kidney Int. 2008;74(9):1178-1184. [PubMed: 18668024]
- 20.
- Whaley-Connell A, Shlipak MG, Inker LA, et al. Awareness of kidney disease and relationship to end-stage renal disease and mortality. Am J Med. 2012;125(7):661-669. [PMC free article: PMC3383388] [PubMed: 22626510]
- 21.
- Iles-Smith H. Perceptions and experiences of pre-dialysis patients. EDTNA ERCA J. 2005;31(3):130-133. [PubMed: 16363411]
- 22.
- Ameling JM, Auguste P, Ephraim PL, et al. Development of a decision aid to inform patients' and families' renal replacement therapy selection decisions. BMC Med Inform Decis Mak. 2012;12:140. [PMC free article: PMC3560257] [PubMed: 23198793]
- 23.
- Morton RL, Howard K, Webster AC, Snelling P. Patient Information about Options for Treatment (PINOT): a prospective national study of information given to incident CKD stage 5 patients. Nephrol Dial Transplant. 2011;26(4):1266-1274. [PubMed: 20819955]
- 24.
- McIntyre NJ, Fluck R, McIntyre C, Taal M. Treatment needs and diagnosis awareness in primary care patients with chronic kidney disease. Br J Gen Pract. 2012;62(597):e227-232. doi:10.3399/bjgp12X636047 [PMC free article: PMC3310028] [PubMed: 22520909] [CrossRef]
- 25.
- Ormandy P. Information topics important to chronic kidney disease patients: a systematic review. J Ren Care. 2008;34(1):19-27. [PubMed: 18336519]
- 26.
- O'Donnell A, Tucker L. Pre-dialysis education. A change in clinical practice. How effective is it? EDTNA ERCA J. 1999;25(2):29-32. [PubMed: 10531879]
- 27.
- Juhnke J, Curtin RB. New study identifies ESRD patient education needs. Nephrol News Issues. 2000;14(6):38-39. [PubMed: 11249457]
- 28.
- Schatell D, Ellstrom-Calder A, Alt PS, Garland JS. Survey of CKD patients reveals significant gaps in knowledge about kidney disease. Part 2. Nephrol News Issues. 2003;17(6):17-19. [PubMed: 12778612]
- 29.
- Coupe D. Making decisions about dialysis options: an audit of patients' views. EDTNA ERCA J. 1998;24(1):25-26, 31. [PubMed: 9873281]
- 30.
- Klang B, Bjorvell H, Clyne N. Predialysis education helps patients choose dialysis modality and increases disease-specific knowledge. J Adv Nurs. 1999;29(4):869-876. [PubMed: 10215978]
- 31.
- Tweed AE, Ceaser K. Renal replacement therapy choices for pre-dialysis renal patients. Br J Nurs. 2005;14(12):659-664. [PubMed: 16010217]
- 32.
- Groome PA, Hutchinson TA, Tousignant P. Content of a decision analysis for treatment choice in end-stage renal disease: who should be consulted? Med Decis Making. 1994;14(1):91-97. [PubMed: 8152362]
- 33.
- Niccum KJ, Perez D. Wheels within wheels: creating a circle of knowledge through communication. Adv Ren Replace Ther. 2000;7(4 Suppl 1):S56-S64. [PubMed: 11053588]
- 34.
- Bass EB, Jenckes MW, Fink NE, et al. Use of focus groups to identify concerns about dialysis. Choice study. Med Decis Making. 1999;19(3):287-295. [PubMed: 10424835]
- 35.
- Whittaker AA, Albee BJ. Factors influencing patient selection of dialysis treatment modality. ANNA J. 1996;23(4):369-375; discussion 376-367. [PubMed: 8900682]
- 36.
- Wilkinson H. Pre-dialysis information: how effective is it? EDTNA ERCA J. 1998;24(1):32-34. [PubMed: 9873284]
- 37.
- Orsino A, Cameron JI, Seidl M, Mendelssohn D, Stewart DE. Medical decision-making and information needs in end-stage renal disease patients. Gen Hosp Psychiatry. 2003;25(5):324-331. [PubMed: 12972223]
- 38.
- Harwood L, Locking-Cusolito H, Spittal J, Wilson B, White S. Preparing for hemodialysis: patient stressors and responses. Nephrol Nurs J. 2005;32(3):295-302; quiz 303. [PubMed: 16035471]
- 39.
- Burns T, Fernandez R, Stephens M. The experiences of adults who are on dialysis and waiting for a renal transplant from a deceased donor: a systematic review. JBI Database System Rev Implement Rep. 2015;13(2):169-211. [PubMed: 26447040]
- 40.
- DePasquale N, Ephraim PL, Ameling J, et al. Selecting renal replacement therapies: what do African American and non-African American patients and their families think others should know? A mixed methods study. BMC Nephrol. 2013;14:9. doi:10.1186/1471-2369-14-9 [PMC free article: PMC3565884] [PubMed: 23317336] [CrossRef]
- 41.
- Wuerth DB, Finkelstein SH, Schwetz O, Carey H, Kliger AS, Finkelstein FO. Patients' descriptions of specific factors leading to modality selection of chronic peritoneal dialysis or hemodialysis. Perit Dial Int. 2002;22(2):184-190. [PubMed: 11990402]
- 42.
- Tong A, Sainsbury P, Carter SM, et al. Patients' priorities for health research: focus group study of patients with chronic kidney disease. Nephrol Dial Transplant. 2008;23(10):3206-3214. [PubMed: 18445638]
- 43.
- Bath J, Tonks S, Edwards P. Psychological care of the haemodialysis patient. EDTNA ERCA J. 2003;29(2):85-88. [PubMed: 14598951]
- 44.
- Heisler M. Different models to mobilize peer support to improve diabetes self-management and clinical outcomes: evidence, logistics, evaluation considerations and needs for future research. Fam Pract. 2010;27:I23-I32. [PMC free article: PMC2902359] [PubMed: 19293400]
- 45.
- Dennis C-L. Peer support within a health care context: a concept analysis. Int J Nurs Stud. 2003;40(3):321-332. [PubMed: 12605954]
- 46.
- Lucksted A, McNulty K, Brayboy L, Forbes C. Initial evaluation of the peer-to-peer program. Psychiatr Serv. 2009;60(2):250-253. [PubMed: 19176421]
- 47.
- Wright L, Pennington JJ, Abbey S, Young E, Haines J, Ross H. Evaluation of a mentorship program for heart transplant patients. J Heart Lung Transplant. 2001;20(9):1030-1033. [PubMed: 11557200]
- 48.
- Mandalia PK, Stone MA, Davies MJ, Khunti K, Carey ME. Diabetes self-management education: acceptability of using trained lay educators. Postgrad Med J. 2014;90(1069):638-642. [PubMed: 25258417]
- 49.
- Jerson B, D'Urso C, Arnon R, et al. Adolescent transplant recipients as peer mentors: a program to improve self-management and health-related quality of life. Pediatr Transplant. 2013;17(7):612-620. [PubMed: 23905874]
- 50.
- Hibbard MR, Cantor J, Charatz H, et al. Peer support in the community: initial findings of a mentoring program for individuals with traumatic brain injury and their families. J Head Trauma Rehabil. 2002;17(2):112-131. [PubMed: 11909510]
- 51.
- Ahola Kohut S, Stinson JN, Ruskin D, et al. iPeer2Peer program: a pilot feasibility study in adolescents with chronic pain. Pain. 2016;157(5):1146-1155. [PubMed: 26808145]
- 52.
- Doyle M. Peer support and mentorship in a US rare disease community: findings from the Cystinosis in Emerging Adulthood study. Patient. 2015;8(1):65-73. [PubMed: 25231828]
- 53.
- Perry E, Swartz J, Brown S, Smith D, Kelly G, Swartz R. Peer mentoring: a culturally sensitive approach to end-of-life planning for long-term dialysis patients. Am J Kidney Dis. 2005;46(1):111-119. [PubMed: 15983964]
- 54.
- Ghahramani N, Asick R, Hodge JM. Patient and Family Partner Program. Accessed December 27, 2013. http://www.kfcp.org/WhatWeDo/PatientandFamilyPartnerProgram.aspx
- 55.
- Pierce P, Asick R, Weaver M. Trained ESRD mentors: an important resource for total patient care. Nephrol News Issues. 2009;23(3):36, 38, 40 passim. [PubMed: 19353975]
- 56.
- Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi:10.1136/bmj.g1687 [PubMed: 24609605] [CrossRef]
- 57.
- McKay HG, King D, Eakin EG, Seeley JR, Glasgow RE. The diabetes network internet-based physical activity intervention: a randomized pilot study. Diabetes Care. 2001;24(8):1328-1334. [PubMed: 11473065]
- 58.
- Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA. 2003;289(14):1833-1836. [PubMed: 12684363]
- 59.
- Zrebiec JF. Internet communities: do they improve coping with diabetes? Diabetes Educ. 2005;31(6):825-836. [PubMed: 16288090]
- 60.
- Lorig KR. Internet-based chronic disease self-management: a randomized trial. Med Care. 2006;44(11):964-971. [PubMed: 17063127]
- 61.
- Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3(5):329-338. [PubMed: 7841967]
- 62.
- Peipert JD, Bentler PM, Klicko K, Hays RD. Psychometric properties of the Kidney Disease Quality of Life 36-Item Short-Form Survey (KDQOL-36) in the United States. Am J Kidney Dis. 2018;71(4):461-468. [PubMed: 29128411]
- 63.
- Rao S, Carter WB, Mapes DL, et al. Development of subscales from the symptoms/problems and effects of kidney disease scales of the Kidney Disease Quality of Life instrument. Clin Ther. 2000;22(9):1099-1111. [PubMed: 11048907]
- 64.
- Korevaar JC, Merkus MP, Jansen MA, Dekker FW, Boeschoten EW, Krediet RT. Validation of the KDQOL-SF: a dialysis-targeted health measure. Qual Life Res. 2002;11(5):437-447. [PubMed: 12113391]
- 65.
- Fukuhara S, Lopes AA, Bragg-Gresham JL, et al. Health-related quality of life among dialysis patients on three continents: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;64(5):1903-1910. [PubMed: 14531826]
- 66.
- Wight JP, Edwards L, Brazier J, Walters S, Payne JN, Brown CB. The SF36 as an outcome measure of services for end stage renal failure. Qual Health Care. 1998;7(4):209-221. [PMC free article: PMC2483621] [PubMed: 10339023]
- 67.
- Mapes DL, Bragg-Gresham JL, Bommer J, et al. Health-related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004;44(5 Suppl 2):54-60. [PubMed: 15486875]
- 68.
- Bakewell AB, Higgins RM, Edmunds ME. Quality of life in peritoneal dialysis patients: decline over time and association with clinical outcomes. Kidney Int. 2002;61(1):239-248. [PubMed: 11786106]
- 69.
- Vazquez I, Valderrabano F, Fort J, et al. Psychosocial factors and health-related quality of life in hemodialysis patients. Qual Life Res. 2005;14(1):179-190. [PubMed: 15789952]
- 70.
- Manns B, Johnson JA, Taub K, Mortis G, Ghali WA, Donaldson C. Quality of life in patients treated with hemodialysis or peritoneal dialysis: what are the important determinants? Clin Nephrol. 2003;60(5):341-351. [PubMed: 14640240]
- 71.
- Lopes AA, Bragg-Gresham JL, Satayathum S, et al. Health-related quality of life and associated outcomes among hemodialysis patients of different ethnicities in the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2003;41(3):605-615. [PubMed: 12612984]
- 72.
- Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int. 2003;64(1):339-349. [PubMed: 12787427]
- 73.
- Peipert JD, Nair D, Klicko K, Schatell DR, Hays RD. Kidney Disease Quality of Life 36-Item Short Form Survey (KDQOL-36) normative values for the United States dialysis population and new single summary score. J Am Soc Nephrol. 2019;30(4):654-663. [PMC free article: PMC6442334] [PubMed: 30898868]
- 74.
- Tong A, Sainsbury P, Craig JC. Support interventions for caregivers of people with chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2008;23(12):3960-3965. [PubMed: 18658178]
- 75.
- Zarit SH, Toseland RW. Current and future direction in family caregiving research. Gerontologist. 1989;29(4):481-483. [PubMed: 2521107]
- 76.
- Bocquet H, Pous J, Charlet JP, Grand A. Measuring the burden for carers of dependent elderly with the Zarit inventory [in French]. Rev Epidemiol Sante Publique. 1996;44(1):57-65. [PubMed: 8851943]
- 77.
- Shimoyama S, Hirakawa O, Yahiro K, Mizumachi T, Schreiner A, Kakuma T. Health-related quality of life and caregiver burden among peritoneal dialysis patients and their family caregivers in Japan. Perit Dial Int. 2003;23(Suppl 2):S200-S205. [PubMed: 17986549]
- 78.
- Yoo R, Yeom J, Kim GH, et al. A multicenter, randomized clinical trial to assess the efficacy of a therapeutic intervention program for caregivers of people with dementia. J Clin Neurol. 2019;15(2):235-242. [PMC free article: PMC6444149] [PubMed: 30938110]
- 79.
- Gratão ACM, Brigola AG, Ottaviani AC, et al. Brief version of Zarit Burden Interview (ZBI) for burden assessment in older caregivers. Dement Neuropsychol. 2019;13(1):122-129. [PMC free article: PMC6497029] [PubMed: 31073389]
- 80.
- Gutiérrez-Maldonado J, Caqueo-Urízar A. Effectiveness of a psycho-educational intervention for reducing burden in Latin American families of patients with schizophrenia. Qual Life Res. 2007;16(5):739-747. [PubMed: 17286192]
- 81.
- Sharif F, Shaygan M, Mani A. Effect of a psycho-educational intervention for family members on caregiver burdens and psychiatric symptoms in patients with schizophrenia in Shiraz, Iran. BMC Psychiatry. 2012;12:48. doi:10.1186/1471-244X-12-48 [PMC free article: PMC3441201] [PubMed: 22632135] [CrossRef]
- 82.
- Yu Y, Liu Z-W, Zhou W, et al. Assessment of burden among family caregivers of schizophrenia: psychometric testing for short-form Zarit Burden Interviews. Front Psychol. 2018;9:2539. doi:10.3389/fpsyg.2018.02539 [PMC free article: PMC6305706] [PubMed: 30618960] [CrossRef]
- 83.
- Hu X, Peng X, Su Y, Huang W. Caregiver burden among Chinese family caregivers of patients with lung cancer: a cross-sectional survey. Eur J Oncol Nurs. 2018;37:74-80. [PubMed: 30473054]
- 84.
- Carrilho PEM, Rodrigues MA, de Oliveira BCR, et al. Profile of caregivers of Parkinson's disease patients and burden measured by Zarit Scale Analysis of potential burden-generating factors and their correlation with disease severity. Dement Neuropsychol. 2018;12(3):299-305. [PMC free article: PMC6200152] [PubMed: 30425794]
- 85.
- Yu Y, Liu Z-W, Zhou W, et al. Cutoff of the Zarit Burden Interview in predicting depression and anxiety. Qual Life Res. 2019;28(9):2525-2533. [PubMed: 31089989]
- 86.
- Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4 Pt 1):1005-1026. [PMC free article: PMC1361049] [PubMed: 15230939]
- 87.
- Munson GW, Wallston KA, Dittus RS, Speroff T, Roumie CL. Activation and perceived expectancies: correlations with health outcomes among veterans with inflammatory bowel disease. J Gen Intern Med. 2009;24(7):809-815. [PMC free article: PMC2695514] [PubMed: 19444526]
- 88.
- Stepleman L, Rutter MC, Hibbard J, Johns L, Wright D, Hughes M. Validation of the patient activation measure in a multiple sclerosis clinic sample and implications for care. Disabil Rehabil. 2010;32(19):1558-1567. [PubMed: 20590506]
- 89.
- Skolasky RL, Mackenzie EJ, Wegener ST, Riley LH III. Patient activation and adherence to physical therapy in persons undergoing spine surgery. Spine (Phila Pa 1976). 2008;33(21):E784-E791. https://www
.ncbi.nlm .nih.gov/pmc/articles/PMC6153437/ [PMC free article: PMC6153437] [PubMed: 18827683] - 90.
- Skolasky RL, Green AF, Scharfstein D, Boult C, Reider L, Wegener ST. Psychometric properties of the patient activation measure among multimorbid older adults. Health Serv Res. 2011;46(2):457-478. [PMC free article: PMC3064914] [PubMed: 21091470]
- 91.
- Hung M, Carter M, Hayden C, et al. Psychometric assessment of the patient activation measure short form (PAM-13) in rural settings. Qual Life Res. 2013;22(3):521-529. [PubMed: 22466721]
- 92.
- Rademakers J, Nijman J, van der Hoek L, Heijmans M, Rijken M. Measuring patient activation in The Netherlands: translation and validation of the American short form Patient Activation Measure (PAM13). BMC Public Health. 2012;12:577. doi:10.1186/1471-2458-12-577 [PMC free article: PMC3490810] [PubMed: 22849664] [CrossRef]
- 93.
- Steinsbekk A. Patient Activation Measure [in Swedish]. Tidsskr Nor Laegeforen. 2008;128(20):2316-2318. [PubMed: 19096487]
- 94.
- Hibbard JH, Mahoney ER, Stock R, Tusler M. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42(4):1443-1463. [PMC free article: PMC1955271] [PubMed: 17610432]
- 95.
- Mosen DM, Schmittdiel J, Hibbard J, Sobel D, Remmers C, Bellows J. Is patient activation associated with outcomes of care for adults with chronic conditions? J Ambul Care Manage. 2007;30(1):21-29. [PubMed: 17170635]
- 96.
- Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40(6 Pt 1):1918-1930. [PMC free article: PMC1361231] [PubMed: 16336556]
- 97.
- Anderson JK, Wallace LM. Evaluation of uptake and effect on patient-reported outcomes of a clinician and patient co-led chronic musculoskeletal pain self-management programme provided by the UK National Health Service. Br J Pain. 2018;12(2):104-112. [PMC free article: PMC5958511] [PubMed: 29796262]
- 98.
- Knox L, Huff J, Graham D, et al. What peer mentoring adds to already good patient care: implementing the Carpeta Roja Peer Mentoring Program in a well-resourced health care system. Ann Fam Med. 2015;13(suppl 1):S59-S65. [PMC free article: PMC4648130] [PubMed: 26304973]
- 99.
- Sandhu S, Veinot P, Embuldeniya G, et al. Peer-to-peer mentoring for individuals with early inflammatory arthritis: feasibility pilot. BMJ Open. 2013;3(3):e002267. doi:10.1136/bmjopen-2012-002267 [PMC free article: PMC3612764] [PubMed: 23457326] [CrossRef]
- 100.
- Maslow GR, Chung RJ. Systematic review of positive youth development programs for adolescents with chronic illness. Pediatrics. 2013;131(5):e1605-18. doi:10.1542/peds.2012-1615 [PubMed: 23610201] [CrossRef]
- 101.
- Embuldeniya G, Veinot P, Bell E, et al. The experience and impact of chronic disease peer support interventions: a qualitative synthesis. Patient Educ Couns. 2013;92(1):3-12. [PubMed: 23453850]
- 102.
- Enriquez M, Conn VS. Peers as facilitators of medication adherence interventions: a review. J Prim Care Community Health. 2016;7(1):44-55. [PMC free article: PMC5695224] [PubMed: 26303976]
- 103.
- Joshi A, Arutagi V, Nahar N, Tiwari S, Singh D, Sethia S. Diabetes ongoing sustainable care and treatment (DOST): a strategy for informational deliverance through visual dynamic modules sustained by near peer mentoring. J Family Med Prim Care. 2016;5(2):270-275. [PMC free article: PMC5084546] [PubMed: 27843826]
- 104.
- Wenzel L, Huang HQ, Monk BJ, Rose PG, Cella D. Quality-of-life comparisons in a randomized trial of interval secondary cytoreduction in advanced ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2005;23(24):5605-5612. [PMC free article: PMC4920482] [PubMed: 16110020]
- 105.
- WWAMI. RUCA data: using RUCA data. University of Washington. Accessed July 11, 2014. http://depts
.washington .edu/uwruca/ruca-uses.php - 106.
- Zrebiec JF, Jacobson AM. What attracts patients with diabetes to an internet support group? A 21-month longitudinal website study. Diabet Med. 2001;18(2):154-158. [PubMed: 11251681]
- 107.
- Zrebiec JF. Internet communities. Diabetes Educ. 2005;31(6):825-836. [PubMed: 16288090]
- 108.
- McKay HG, King D, Eakin EG, Seeley JR, Glasgow RE. The Diabetes Network internet-based physical activity intervention. Diabetes Care. 2001;24(8):1328. doi:10.2337/diacare.24.8.1328 [PubMed: 11473065] [CrossRef]
- 109.
- Lorig KR, Ritter PL, Laurent DD, Plant K. Internet-based chronic disease self-management: a randomized trial. Med Care. 2006;44(11):964-971. [PubMed: 17063127]
- 110.
- Lorig KR, Ritter PL, Dost A, Plant K, Laurent DD, McNeil I. The Expert Patients Programme online, a 1-year study of an Internet-based self-management programme for people with long-term conditions. Chronic Illn. 2008;4(4):247-256. [PubMed: 19091933]
- 111.
- Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an internet weight loss program. JAMA Int Med. 2006;166(15):1620-1625. [PubMed: 16908795]
- 112.
- Tate DF, Jackvony EH, Wing RR. Effects of internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA. 2003;289(14):1833-1836. [PubMed: 12684363]
- 113.
- Eysenbach G, Powell J, Englesakis M, Rizo C, Stern A. Health related virtual communities and electronic support groups: systematic review of the effects of online peer to peer interactions. BMJ. 2004;328(7449):1166. doi:10.1136/bmj.328.7449.1166 [PMC free article: PMC411092] [PubMed: 15142921] [CrossRef]
- 114.
- Foster G, Taylor SJC, Eldridge S, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev. 2007;(4):CD005108. doi:10.1002/14651858.CD005108.pub2 [PubMed: 17943839] [CrossRef]
- 115.
- Guise J-M, Butler ME, Chang C, Viswanathan M, Pigott T, Tugwell P. AHRQ series on complex intervention systematic reviews—paper 6: PRISMA-CI extension statement and checklist. J Clin Epidemiol. 2017;90:43-50. [PubMed: 28720516]
- 116.
- Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ. 2015;350:h1258. doi:10.1136/bmj.h1258 [PMC free article: PMC4366184] [PubMed: 25791983] [CrossRef]
Related Publication
- Cukor D, Cohen LM, Cope EL, et al. Patient and other stakeholder engagement in Patient-Centered Outcomes Research Institute funded studies of patients with kidney diseases. Clin J Am Soc Nephrol. 2016;11(9):1703-1712. [PMC free article: PMC5012486] [PubMed: 27197911]
Acknowledgments
We wish to acknowledge the following individuals for their assistance in various stages of the study:
- All patients and caregivers who participated in the study as peer mentors or as study participants. Without their active and enthusiastic participation, this study would not have been possible.
- The KFCP and members of its board of directors who served as members of the Community Advisory Board (Larry Carroll, MD; Luann Cartwright; John Eyster; Carole Fair; Cheryl Green, PA-C; Nancy J. Hammonds, FHFMA; Karen Lehman; Tammie Loper; Nina Myers; Seth Narins, MD; Nicole J. Radziewicz, Esq; M. Kris Srinivasan, MD, MBA, MHSE; Barbara Terry; and Kristy Washinger, CRNP). The Community Advisory Board played a major role in development of the protocol, implementation of the study, dissemination of interim results to the community, and engagement of the patients and other stakeholders in the study at various stages. In addition, the KFCP board members provided continuous supervision for the PFPP. We extend special thanks to patient and caregiver members of the KFCP board of directors for providing patient/caregiver perspectives.
- Members of the advisory groups, particularly for assistance in recruitment and retention.
- –
Patient and Caregiver Advisory Group (Raymond Thorn, Anthony Baltimore, Dennis Adams, Kenneth Ellison, Pamela Jester, Francine Citrone, Sally Anderson, David Deibler, Tim Derrick, Donald Hartzel, Mark Maines, and Cynthia Godanis)
- –
Provider Advisory Group (Teresa Bruno, MSW; William Curry, MD; Umar Farooq, MD; Gail Flannery, MSW; Phyllis Hicks, RN, BSN; Julia Hix, RN; Hillary Hoover, MSW; Rajiv Sharma, MD; Elizabeth Snyder, PA-C; Cynthia Royer, MSW; Sandra Ryan, RN, MSN; and Eileen Swartz, RN, BSN)
- Robin Asick (consultant) for her guidance in recruitment and for oversight of the mentoring program. Her leadership role as a highly regarded renal social worker in central Pennsylvania played a critical role in advocating for participation in the study.
- Patient representatives Michelle Bentley Mullikin and Mark Barnhardt for providing the patient perspective.
- Research coordinator Tara Sofia Liaghat for her invaluable assistance in recruitment, delivery of assessments, facilitating focus group discussions, and data entry.
- PFPP coordinator Tabitha Semancik for her dedication to the mentorship program and for regular monitoring of the online platform. We extend special thanks for her role in dissemination of the interim results to the community and for organizing and facilitating the panel discussions by the study participants at the annual KFCP conferences.
Research reported in this report was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (#CDR-1310-07055). Further information available at: [https://www.pcori.org/research-results/2014/comparing-three-ways-improve-quality-life-patients-kidney-disease-and-their]
Appendices
Appendix A.
Curriculum of the PFPP (PDF, 110K)
Appendix B.
Data Collection Form for Patients (PDF, 95K)
Appendix C.
Data Collection Form for Caregivers (PDF, 95K)
Appendix D.
Appendix E.
Suggested citation:
Ghahramani N, Kraschnewski JL, Lengerich EJ, Chinchilli VM, Sciamanna CN. (2020). Comparing Three Ways to Improve Quality for Life for Patients with Kidney Disease and Their Caregivers. Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/10.2020.CDR.131007055
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/