NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Neutropenic Sepsis: Prevention and Management of Neutropenic Sepsis in Cancer Patients. London: National Institute for Health and Clinical Excellence (NICE); 2012 Sep. (NICE Clinical Guidelines, No. 151.)
15. Changing primary empiric treatment in patients with unresponsive fever. (Topic E6)
Guideline subgroup members for this question
Wendy King (lead), Anton Kruger, Jeanette Hawkins, Bob Phillips and Rosemary Barnes.
Review question
What is the optimal time to change the primary empiric treatment in unresponsive fever?
Rationale
Some patients admitted to hospital with neutropenic sepsis may continue to have unresponsive fever beyond 48 hours, despite been treated with primary empiric antibiotics. It is also possible that these patients will not have a focus for their infection.
What is the evidence that antibiotic therapy should be changed and is there any evidence to advise when this change should be made e.g. 24, 48, or 96 hours or later post admission? What are the risks to the patient if antibiotics are not changed at a given time? A review of the literature may help to resolve these clinical questions as at present there are different practices occurring. It is possible that continuing empiric antibiotics could result in increased length of stay, critical care admission or death.
Question in PICO format
| Patients/population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Modification to empiric therapy (report subgroups by time). | Continuing with primary empiric treatment |
|
| Antibacterial Antifungal Antiviral |
METHODS
Information sources and eligibility criteria
The information specialist (SB) searched the following electronic databases: Medline, Premedline, Embase, Cochrane Library, Cinahl, BNI, Psychinfo, Web of Science (SCI & SSCI), ISI proceedings and Biomed Central. The search was restricted to published randomised trials and systematic reviews of randomised trials. The final search was done on 7th of November 2011.
Selection of studies
The information specialist (SB) conducted the first screen of the literature search results. One reviewer (KF) then selected potentially eligible studies by comparing their title and abstract to the inclusion criteria set out by the PICO question. Full text articles were obtained for studies identified as potentially relevant. These were read and checked against the inclusion criteria.
RESULTS
Results of the literature searches
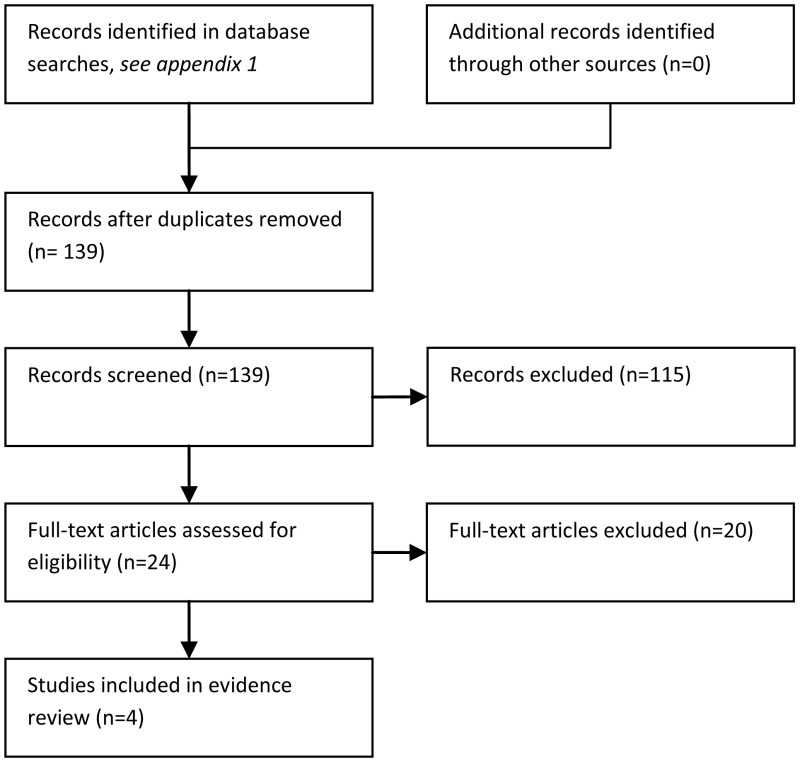
The literature searches identified 139 potentially relevant studies of which four were included as evidence. The structure of this question is such that it can only be properly answered by randomised studies comparing neutropenic patients with persistent fever, despite having been treated with an empiric antibiotic, to either stay on the empiric therapy or have some sort of treatment modification i.e. a different drug to replace or add to the empiric antibiotic. However, the overwhelming majority of papers identified in the literature search described studies in which patients had stopped empiric antibiotics before being randomised to one or two second line drugs. These studies would not answer this question.
The evidence base is very poor, consisting of four randomised studies, two of which are more than twenty years out of date. Patients (N=461 patients in total) with low granulocyte counts and persistent fever were randomised to either remain on the empiric antibiotic (alone or with an added placebo) or to primary treatment with the addition of another agent. The point at which these studies were initiated i.e. the number of days of persistent fever, varied between two and seven days. The length of stay and the incidence of over-treatment were not specifically addressed and nor was the patients' quality of life. None of the studies dealt adequately with the methods of randomisation, allocation or blinding and, although some authors stated that appropriate statistics had been used for data analysis, the details were sometimes scant or absent and very few outcomes had more than a P (probability) value reported. For these reasons, all four studies have been classified by GRADE as being of ‘low’ or ‘very low’ quality. The variability of data and study design precluded pooling.
Evidence summary
Generally, none of the studies demonstrated a significant difference between patients kept on empiric antibiotics and those given an additional drug or drugs (Table 15.1). The general consensus seemed to be that patients seemed to respond to the initial antibiotic treatment eventually and that glycopeptides in particular could potentially be of more harm than benefit if the infectious agent did not warrant such treatment. Bearing in mind the age of these studies, these points may no longer be of relevance.
Pizzo et al (1981) reported on fifty patients who, having received empiric antibiotics for fever and granulocytopenia of unknown infectious aetiology, had failed to respond to treatment after seven days. These patients were randomised to stop empiric antibiotics (group 1), continue with empiric antibiotics (group 2) or continue empiric antibiotics with the addition of amphotericin B (group 3). Six patients in group 1 experienced shock compared with no patients in the other two groups (P<0.001). The incidence of infectious complications was significantly higher (N=9) in group 1 compared with group 3 (N=2) (P=0.013) but not between group 1 and group 2 (N=6) i.e. for patients stopping versus continuing antibiotics. Although no statistical analyses were presented, the low patients numbers, low event rates and wide ranges of data suggest that there was no significant difference between time to the resolution of granulocytopenia or to defervescence between the three groups. The number of non-infectious complications did not differ significantly. The numbers of fatalities appeared to be similar between groups: N=5 in groups 1 and 2 versus N=3 in group 3. There were more patients with fungal infections in group 2 (N=5) compared with the other two groups but this might be a random effect but no evidence was offered to suggest causality.
A study by the EORTC antimicrobial therapy co-operative group (1989) compiled results from two consecutive trials on the use of empiric antibodies in patients with fever and granulocytopenia. One hundred and thirty-two patients who were unresponsive to treatment after four days, were randomised to continue antibiotics with (group 1) or without (group 2) amphotericin B. Clinical response was assessed five days after randomisation and considered a failure if the patient remained febrile. Under this criterion, 47/68 (69%) of patients in group 1 versus 34/64 (53%) of patients in group 2 experienced treatment success (P=0.09). More patients with a clinically documented infection at day 4, had a positive clinical response with combined treatment than with antibiotics alone (P=0.03). Similarly, patients that had not received prior anti-fungal prophylaxis had a better response to the combined treatment regime than antibiotics only (P=0.04) but other sub-group comparisons were not statistically significant. Fewer patients in group 1 had died by day 30 (11 versus 14 (P=0.039) but most deaths were described as being due to ‘other causes’ rather than being specifically treatment related. More patients in group 2 developed fungal disease than in group 1 but the difference was not significant. All sub-group analyses were of very low patient number.
Cometta et al. (2003) reported the results of a prospective double blinded trial in which one hundred and sixty-five patients who had persistent fever after 48-60 hours, were randomised to receive an empiric broad spectrum antibiotic plus vancomycin (group 1) or with saccharose solution (group 2). The main outcome of interest was the rate of fever resolution at three days post randomisation, which was not significantly different between study arms: 82/86 (95%) in group 1 versus 73/79 (92%) in group 2. More than half of the patients in both groups had their therapy modified, either by adding a glycopeptide to vancomycin or by stopping the placebo and giving amphotericin B. There was no significant difference in the time to fever resolution between groups, regardless of whether the treatment regime had been modified or not. Fewer (N=4) patients died in group 1 compared with group 2 (N=8) but the differences are unlikely to have been statistically significant. More patients in group 1 (N=9) experienced treatment related side effects compared with group 2 (N=3). The study had very low patient numbers and event rates and was underpowered to have detected a clinically meaningful difference between comparators for the main outcome. The study was closed for this reason.
Erjavec et al. (2000) conducted a randomised double blinded placebo-controlled study of one hundred and fourteen patients with febrile neutropenia who had persistent fever after three to four days of treatment with an empiric antibiotic. Group 1 continued with imipenum and added teicoplanin whilst group 2 had imipenum with a placebo. The primary outcome was the rate of treatment response after 72 hours. There was no significant difference between study arms: 25/56 (45%) in group 1 versus 27/58 (47%). The number of deaths throughout the study was 6 in group 1 and 4 in group 2. Many of the patients had received anti-bacterial prophylaxis and some had also been given G-CSF. The numbers of patients and event rates were low.
Evidence Statements
Mortality
There was very low quality evidence from 4 studies about when to change empiric antibiotics in patients with unresponsive fever (Table 7.1). No study compared changing empiric therapy at two different time points. Patients (N=461) with persistent fever were randomised to either remain on the empiric antibiotic or to primary treatment with the addition of another agent. No study detected a significant difference between the short term mortality of those who changed treatment and those who remained on the initial empiric treatment.
Critical care, quality of life and length of stay
The included studies did not report these outcomes.
Duration of fever
There was very low quality evidence about this outcome and none of the studies reported the influence of time of treatment change. Pizzo, et al., (1982) and Cometta, et al., (2003) reported shorter median time to defervesence in patients whose empiric therapy was changed (8 versus 6 days and 4.3 versus 3.5 days respectively), but there was no statistically significant difference. Erjavec, et al., (2000) reported similar rates of defervesence within 72 hours in patients who did or did not change empiric treatment.
EVIDENCE TABLES
Download PDF (340K)
REFERENCES
- Cometta A, Kern WV, De Bock R, Paesmans M, Vandenbergh M, Crokaert F, Engelhard D, Marchetti O, Akan H, Skoutelis A, Korten V, Vandercam M, Gaya H, Padmos A, Klastersky J, Zinner S, Glauser MP, Calandra T, Viscoli C., The International Antimicrobial Therapy Group of the European Organization for Research Treatment of Cancer. Vancomycin versus placebo for treating persistent fever in patients with neutropenic cancer receiving piperacillin-tazobactam monotherapy. Clin Infect Dis. 2003;37(3):382–389. [PubMed: 12884163]
- EORTC International Antimicrobial Therapy Cooperative Group. Empiric antifungal therapy in febrile granulocytopenic patients. Am J Med. 1989 Jun;86(6 Pt 1):668–72. 1989. [PubMed: 2658574]
- Erjavec Z, de Vries-Hospers HG, Laseur M, Halie RM, Daenen S. A prospective, randomized, double-blinded, placebo-controlled trial of empirical teicoplanin in febrile neutropenia with persistent fever after imipenem monotherapy. J. Antimicrob. Chemother. 2000;45(6):843–849. [PubMed: 10837439]
- Pizzo PA, Robichaud KJ, Gill FA, Witebsky FG. Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia. Am. J. Med. 1982;72:101–111. [PubMed: 7058815]
16. Switching from intravenous to oral antibiotic therapy. (Topic E5)
Guideline subgroup members for this question
Anton Kruger (lead), Wendy King, Barbara Crosse, Bob Phillips and Rosemary Barnes.
Review question
When is the optimal time to switch (step down) from intravenous to oral antibiotic therapy?
Rationale
Empiric antibiotic therapy for patients with neutropenic sepsis is, by definition, given without a microbiological diagnosis. If an organism is identified subsequently, the treatment regimen and duration can be adjusted appropriately. However, for a substantial proportion of patients, ongoing therapy remains empiric. These individuals may have an undetected bacterial infection or could be unwell for other reasons. The standard approach to treatment is to continue with empiric antibiotics for a predetermined length of time after resolution of the fever or symptoms or neutrophil recovery.
The outcome of any episode of neutropenic sepsis will depend on a number of patient specific factors, on the anti infective treatment received, the environment and on the nature of the infective organism. Patient specific factors would include the underlying illness, chemotherapy regimen, presence of indwelling intravenous catheters or other devices and co- morbidities. The sensitivities and prevalence of local microbiological flora may also play a part. Depending on these factors, it is possible to devise strategies that allow for “step-down” from empiric intravenous to empiric oral antibiotics based on specific criteria or pre treatment risk scores or depending on response to the current treatment episode. Alternatively, a blanket policy of step-down therapy may be possible for all patients who are on empiric treatments in a particular setting.
Almost all currently recommended empiric antibiotic regimens comprise one or two intravenous drugs with a broad microbiological spectrum given in multiple daily doses. Treatment is most likely to have to be administered in hospital or, even if ambulatory care is possible, will be heavily dependent on resources such as nursing time. The advantages for a step down approach to care are therefore obvious. Hospital stays may be shortened since oral treatments allow for ambulatory care, patients can be freed of intravenous cannulae which are in themselves an infective risk and specific side effects of the intravenous agents may be avoided. On the other hand there are risks of failure of this treatment strategy and risks particular to oral antibiotics, such as diarrhoea and infection with Clostridium difficile.
This research question seeks to find evidence that defines suitable patient groups and the optimum time to switch from empiric intravenous antibiotic to oral therapy in neutropenic sepsis.
Question in PICO format
| Patients/population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with neutropenic sepsis on intravenous antibiotics | Switch to oral antibiotics (intervention subgrouped by time) Ciprofloxacin Levofloxacin Augmentin (Co-amoxiclav) Amoxycillin | Remain on intravenous antibiotics |
|
METHODS
Information sources and eligibility criteria
The full search strategy is available in appendix X. We restricted the search to published randomised trials and systematic reviews of such trials. The search was done on the 23rd of November 2010 and updated on 2nd November 2011.
Selection of studies and data synthesis
The information specialist (SA) performed an initial screening of the literature search results. One reviewer (MSH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO question.
It was anticipated that evidence would come from trials comparing different times for switching to intravenous to oral antibiotics. However, in the absence of such studies, subgroup analyses was done (according to time-of-switch) in trials which compared switching from intravenous to oral antibiotics with continued intravenous antibiotics.
RESULTS
Results of the literature searces
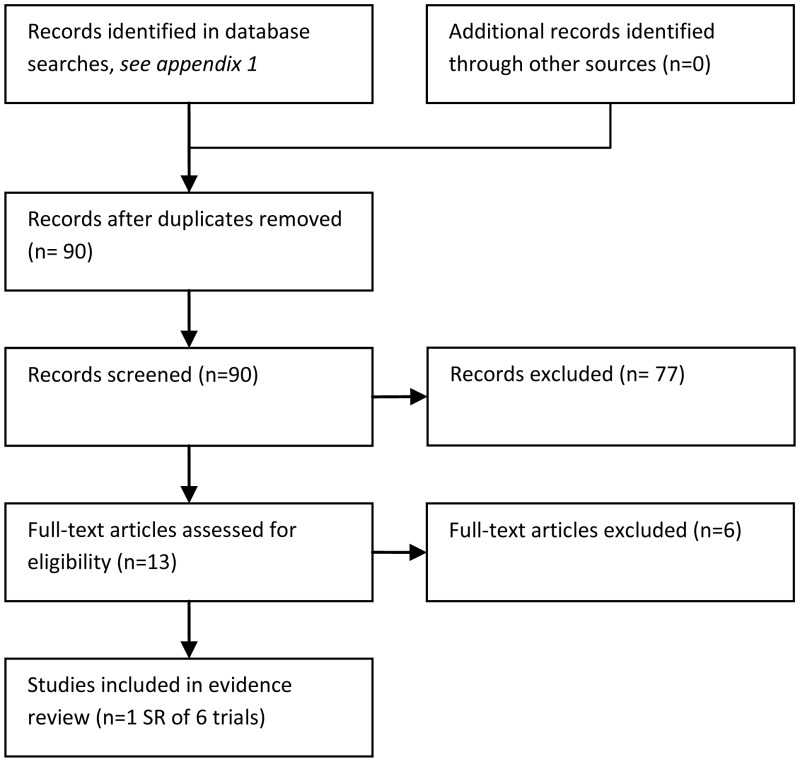
90 studies were identified in the literature searches. Of these, 83 were excluded because they were narrative reviews (N = 6), not in PICO (N = 59), not RCT (N = 16), reporting data already included (N = 1) or a comment (N = 1).
One Cochrane review (Vidal et al., 2004) was indentified for inclusion. The review included 6 RCTs (Flaherty et al., 1989; Giamarellou et al., 2000; Mullen et al., 1999; Paganini et al., 2000, 2003; Shenep et al., 2001). These 6 trials were also checked individually for outcomes not reported in the Cochrane review.
Detailed information about the populations, interventions, outcomes and overall risk of bias in the included trials is given in the Evidence and Grade Tables below.
Evidence Statements
Death or critical care
Very low quality evidence from a Cochrane review (Vidal, et al., 2004, Table 7.2) suggested uncertainty about the relative effectiveness of the two treatment strategies for IV-to-oral versus IV-only the relative risk of short term mortality was 1.14 (95%C.I. 0.48 to 2.73). Critical care was not included as an outcome in any of the included studies, although one study (Paganini, et al.,, 2003) did report that none of their patients required admission to the intensive care unit.
Overtreatment, Length of stay and Quality of life
These outcomes were not reported in any of the included studies.
Duration of fever / Treatment failure
Duration of fever was not reported in the systematic review (Vidal, et al., 2004). Three of the included trials reported this outcome but none of these reported a statistically significant difference in the duration of fever between treatment groups.
Vidal, et al., (2004) reported treatment failure as a composite outcome comprising one or more of the following: death; persistence, recurrence or worsening of clinical signs or symptoms of presenting infection; any addition to or modification of the assigned intervention Low quality evidence suggested no significant difference in the rate of treatment failure in the IV-to-oral group compared to the IV only group, RR 1.07 (0.9 to 1.27).
Table 16.1GRADE evidence profile, Switching from intravenous to oral antibiotic therapy
| Quality assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of patients | Effect | Quality | |||||||||
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | IV-to-oral antibiotics at any time | IV antibiotics | Relative (95% CI) | Absolute | |
| Death | |||||||||||
| 6 | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none | 11/442 (2.5%) | 8/422 (1.9%) | RR 1.14 (0.48 to 2.73) | 3 more per 1000 (from 10 fewer to 33 more) | VERY LOW |
| Treatment failure (composite measure3) | |||||||||||
| 6 | randomised trials | serious1 | no serious inconsistency | no serious indirectness | Serious4 | none | 158/482 (32.8%) | 137/464 (29.5%) | RR 1.07 (0.9 to 1.27) | 21 more per 1000 (from 30 fewer to 80 more) | LOW |
- 1
Two of the trials observed a number of deaths whereas no deaths were observed in the remaining 4 trials.
- 2
The number of events was very low, with no events observed in 4/6 trials. This clearly suggests that the trials were not powered to detect this outcome.
- 3
Treatment failure defined as a composite end-point comprising one or more of the following: death; persistence, recurrence or worsening of clinical signs or symptoms of presenting infection; any addition to or modification of the assigned intervention.
- 4
Relatively low number of events.
| Quality assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of patients | Effect | Quality | |||||||||
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | IV-to-oral antibiotics after 72 hours of IV antibiotics and response to IV antibiotics | IV antibiotics | Relative (95% CI) | Absolute | |
| Death | |||||||||||
| 2 | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none | 11/173 (6.4%) | 8/152 (5.3%) | RR 1.14 (0.48 to 2.73) | 7 more per 1000 (from 27 fewer to 91 more) | VERY LOW |
| Treatment failure (Composite outcome3) | |||||||||||
| 2 | randomised trials | serious1 | no serious inconsistency | no serious indirectness | Serious4 | none | 98/180 (54.4%) | 87/162 (53.7%) | RR 1.01 (0.83 to 1.23) | 5 more per 1000 (from 91 fewer to 124 more) | LOW |
- 1
The designs of the included trials were both compromised either by providing no information about the method of randomisation and about whether allocation concealment or blinding was used or by not using intention to treat analysis.
- 2
The number of events was very low. This clearly suggests that the trials were not powered to detect this outcome.
- 3
Treatment failure defined as a composite end-point comprising one or more of the following: death; persistence, recurrence or worsening of clinical signs or symptoms of presenting infection; any addition to or modification of the assigned intervention.
- 4
The number of events was < 300
| Quality assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of patients | Effect | Quality | |||||||||
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | IV-to-oral antibiotics after 48-72 hours of IV antibiotics | IV antibiotics | Relative (95% CI) | Absolute | |
| Death | |||||||||||
| 2 | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious2 | none | 0/174 (0%) | 0/180 (0%) | Not estimable | - | VERY LOW |
| Treatment failure (Composite outcome3) | |||||||||||
| 2 | randomised trials | serious1 | no serious inconsistency | no serious indirectness | very serious5 | none | 29/174 (16.7%) | 29/180 (16.1%) | RR 1 (0.64 to 1.56) | 0 fewer per 1000 (from 58 fewer to 90 more) | VERY LOW |
- 1
The design of one of the included trials was compromised by providing no or inadequate information about whether allocation concealment or blinding was used and by not using intention to treat analysis.
- 2
There were no events in either trial which indicates that these trials were not powered for this outcome.
- 3
Treatment failure defined as a composite end-point comprising one or more of the following: death; persistence, recurrence or worsening of clinical signs or symptoms of presenting infection; any addition to or modification of the assigned intervention.
- 5
The number of events was very low.
EVIDENCE TABLES
Download PDF (380K)
REFERENCES
- Vidal L, Ben dor I, Paul M, Pokroy E, Soares-Weiser K, Leibovici L. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database of Systematic Reviews. 2004;(4) Art. No.: CD003992. [PubMed: 15495074] [CrossRef]
17. Duration of inpatient care. (Topic E8)
Guideline subgroup members for this question
Wendy King (lead), Anton Kruger, Jeanette Hawkins, Bob Phillips and Rosemary Barnes.
Review question
What is the optimal duration of inpatient care for patients receiving empiric treatment for neutropenic sepsis?
Rationale
The risk and pattern of infection in patients with cancer and/or neutropenia depends on the primary diagnosis and the type, duration and intensity of the treatment.
Fever in the neutropenic patient requires prompt investigation and treatment with intravenous antibiotics, selected at first empirically in the light of known possible pathogens and the clinical circumstances. The most frequent pathogens are: Staph. Epidermidis, various Streps, Gram-negative rods and staph aureus. The most rapidly lethal are E. Coli, Klebsiella and Pseudomonas aeruginosa.
Any patient with neutropenic sepsis is unable to mount a response to infection. They are therefore at risk of an overwhelming infection and in particular a gram negative sepsis, which could ultimately result in a critical care admission or death. There is no way of telling which febrile neutropenic patients have potentially life-threatening infection.
Patients with neutropenic sepsis are usually admitted to hospital and commenced on empiric intravenous antibiotic treatment. However, there appears to be little evidence to support what the optimal duration of inpatient care should be. Currently there are different practices across the country with paediatric areas discharging low risk patients after 48 hours (if they have negative blood cultures, neutrophils above 0.1 and are clinically well) and adult units keeping patients in hospital until they are afebrile for 48 hours. A review of the evidence might help to standardise practice and provide evidence to improve outcomes.
Question in PICO format
| Patients/population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Inpatients receiving empiric treatment for neutropenic sepsis | Early discharge | Continued inpatient care until antibiotics discontinued for at least 24 hours |
|
Information sources and eligibility criteria
The information specialist (SB) searched the following electronic databases: Medline, Premedline, Embase, Cochrane Library, Cinahl, BNI, Psychinfo, Web of Science (SCI & SSCI), ISI proceedings and Biomed Central. The full strategy is available in appendix 1 to the evidence review
Study selection
The information specialist (SB) did the first screen of the literature search results. Two reviewers (CL and NB) subsequently selected potentially eligible studies by comparing titles and abstracts to the inclusion criteria presented in the PICO question. Full text articles were obtained for all studies identified as being potentially eligible. These articles were checked against the inclusion criteria. Data were extracted by one reviewer (CL) and checked by another (NB).
RESULTS
Results of the literature searches
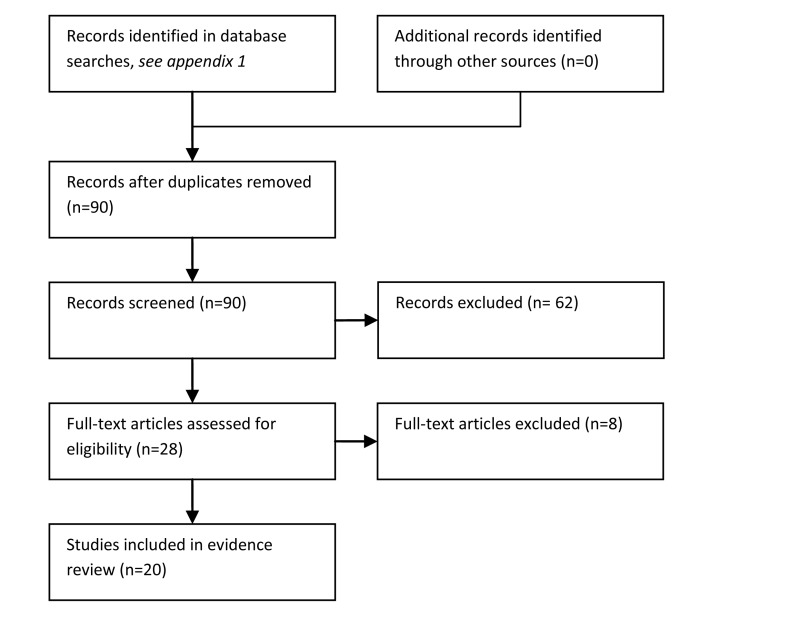
Two Randomised Controlled Trials (RCTs) evaluating duration of inpatient care in the management of suspected bacterial infection (Innes et al 2003 and Santolaya et al 2004) were identified. One non-randomised comparative feasibility study was identified (Lau et al 1994). Eight prospective observational studies (Cherif et al 2006; Girmenia et al 2007; Klastersky 2006; Nijhuis et al 2005; Dommett 2009; Lehrnbecher 2002 and Bash 1994) and seven retrospective observational studies (Tordecilla et al 1994; Aquino 1997; Mullen et al 1990; Griffin et al 1992; Wacker et al 1997; Hodgson-Viden et al 2005 and Tomiak et al 1994) evaluated optimal inpatient duration. We identified one survey of the management of febrile neutropenia. One systematic review published 11 years ago was also identified (Castagnola et al 2000).
Studies in adult patients
We identified one RCT that addressed the question of inpatient duration in the management of suspected bacterial infection in adult patients (Innes et al 2003). Detailed criteria for stratifying patients according to risk of complications were presented based on those proposed by Talcott et al (1988). Patients were randomised to oral or IV antibiotic therapy. Although the duration of inpatient care was shorter for the oral group, both groups were eligible for discharge irrespective of ANC.
Three prospective consecutive case series considered duration of inpatient treatment for febrile neutropenia (FN) in adults (Cerif et al 2006, Girmenia et al 2007, Klatersky et al 2006). Two of the three studies considered only patients with FN subsequent to chemotherapy for haematologic malignancies. The other study considered FN following chemotherapy for both haematologic and solid malignancies. The MASCC criteria for stratifying FN patients according to risk of complications was used in all three studies. All used a cut off score of ≥ 21 to indicate low risk. In each study patients were discharged early with oral antibiotics. One study posed the requirement that patients were afebrile for 24 hours (Cherif et al. 2006); one required patients to be afebrile for 48 hours; and the other study (Girmenia et al 2007) indicated a requirement for patients to be hospitalised for a minimum of 24 hours. One prospective case series considered adult and paediatric patients (Nijhuis 2005). This study did not use the MASCC. A range of criteria were used, including the necessity of being afebrile for 12 hours. One retrospective case series of adult patients was identified (Tomiak 1994). This study gave negative blood cultures as the only criteria for early discharge.
Studies in paediatric patients
We identified one RCT that addressed the question of inpatient duration in the management of suspected bacterial infection in paediatric patients (Santolaya 2004). This study randomised low risk patients to ambulatory or hospital-based antibiotic treatment after 24 to 36 hours of hospitalisation. We identified one non-randomised feasibility study, which switched low risk patients from IV to oral antibiotics, subsequently treating the first 12 patients as inpatients, and the next 11 as outpatients.
Eleven case series of paediatric patients were identified (Dommett 2009; Lehrnbecher 2002; Bash 1994; Tordecilla et al 1994; Aquino 1997; Mullen et al 1990; Griffin et al 1992; Wacker et al 1997; Hodgson-Viden et al 2005 and Tomiak et al 1994). There were no set criteria for determining eligibility for early discharge in studies of paediatric patients. The requirement of being afebrile for at least 24 hours, and having negative blood cultures were common. Many studies also added the subjective criteria of the patient “appearing well”.
Evidence statements
The evidence is summarized in Tables 17.1 and 17.2.
Early discharge rates
In four observational studies the percentage of adult patients meeting the criteria for early hospital discharge ranged from 38% to 90% (Cherif. et al., 2006; Girmenia, et al., 2007; Klastersky, et al., 2006 and Tomiak, et al., 1994). In order to be discharged early, low risk patients were required to meet additional criteria including ability to tolerate oral antibiotics, no history of poor compliance and ability to read a thermometer. The percentage of patients who were actually discharged early ranged from 13% to 69% (Cherif, et al., 2006; Girmenia, et al., 2007; Klastersky. et al., 2006 and Tomiak. et al., 1994).
In eleven observational studies the percentage of paediatric patients meeting the criteria for early hospital discharge ranged from 27% to 63% (Lau, et al., 1994; Dommett, et al., 2009; Lehrnbecher, et al., 2002; Bash, et al., 1994; Tordecilla, et al., 1994; Aquino, et al., 1997; Mullen, et al., 1990; Griffin, et al., 1992; Wakcker, et al., 1997; Hodgson-Veiden, et a.,l 2005 and Santos-Muchado, et al., 1999). Most of these studies were retrospective and patients were not prospectively assigned to high/low risk groups. These studies reported the outcomes of those who were actually discharged early, which ranged from 19% to 68%.
Hospital readmission
In the Innes, et al., (2003) randomised trial, 5% of patients discharged early required hospital readmission
In four observational studies the rate of hospital re-admission for adult patients discharged early ranged from 0% - 13%. Re-admission rates ranged from 0% to 9% in eleven observational studies of paediatric patients.
Short term mortality
Patients selected for early discharge were at low risk of adverse events thus mortality data were sparse: in the Innes, et al., (2003) trial there were no deaths during follow-up. The reported short term (within 30 days of follow up) mortality rate was 0% for patients discharged early from hospital in all but one study of adult patients (Tomiak, 1994). This study reported one death (a mortality rate of 3%). This was the only study of adult patients that did not use the MASCC criteria to stratify patients according to risk.
The reported short term mortality rate was 0% for patients discharged early from hospital in all studies of paediatric patients.
Quality of life and overtreatment
These outcomes were not reported by any of the identified studies of adult or paediatric patients
REFERENCES
- Innes HE, Smith DB, O'Reilly SM, Clark PI, Kelly V, Marshall E. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: a prospective randomised controlled single centre study. British Journal of Cancer. 2003;89:43–49. [PMC free article: PMC2394220] [PubMed: 12838298]
- Santolaya ME, Alvarez AM, Aviles CL, Becker A, Cofre J, Cumsille MA, et al. Early hospital discharge followed by outpatient management versus continued hospitalization of children with cancer, fever, and neutropenia at low risk for invasive bacterial infection. Journal of Clinical Oncology. 2004;22:3784–3789. [PubMed: 15365075]
- Lau RC, Doyle JJ, Freedman MH, King SM, Richardson SE. Early discharge of pediatric febrile neutropenic cancer patients by substitution of oral for intravenous antibiotics. Pediatric Hematology & Oncology. 1994;11:417–421. [PubMed: 7947014]
- Bash RO, Katz JA, Cash JV, Buchanan GR. Safety and cost effectiveness of early hospital discharge of lower risk children with cancer admitted for fever and neutropenia. Cancer. 1994;74:189–196. [PubMed: 8004575]
- Cherif H, Johansson E, Bjorkholm M, Kalin M. The feasibility of early hospital discharge with oral antimicrobial therapy in low risk patients with febrile neutropenia following chemotherapy for hematologic malignancies. Haematologica. 2006;91:215–222. [PubMed: 16461306]
- Girmenia C, Russo E, Carmosino I, Breccia M, Dragoni F, Latagliata R, et al. Early hospital discharge with oral antimicrobial therapy in patients with hematologic malignancies and low-risk febrile neutropenia. Annals of Hematology. 2007;86:263–270. [PubMed: 17225113]
- Klastersky J, Paesmans M, Georgala A, Muanza F, Plehiers B, Dubreucq L, et al. Outpatient oral antibiotics for febrile neutropenic cancer patients using a score predictive for complications. Journal of Clinical Oncology. 2006;24(25):4129–4134. [PubMed: 16943529]
- Tomiak AT, Yau JC, Huan SD, Cripps MC, Goel R, Perrault DJ, et al. Duration of intravenous antibiotics for patients with neutropenic fever. Annals of Oncology. 1994;5:441–445. [PubMed: 8075051]
- Lehrnbecher T, Stanescu A, Kuhl J. Short courses of intravenous empirical antibiotic treatment in selected febrile neutropenic children with cancer. Infection. 2002;30(1):17–21. [PubMed: 11876510]
- Dommett R, Geary J, Freeman S, Hartley J, Sharland M, Davidson A, et al. Successful introduction and audit of a step-down oral antibiotic strategy for low risk paediatric febrile neutropaenia in a UK, multicentre, shared care setting. European Journal of Cancer. 2009;45:2843–2849. [PubMed: 19616427]
- Tordocilla CJ, Campbell BM, Joannon SP, Rodriguez RN. Neutropenia and fever. Revista Chilena de Pediatria. 1994;65(5):260–263.
- Aquino VM, Buchanan GR, Tkaczewski I, Mustafa MM. Safety of early hospital discharge of selected febrile children and adolescents with cancer with prolonged neutropenia. Medical and Pediatric Oncology. 1997;28(3):191–195. [PubMed: 9024515]
- Mullen CA, Buchanan GR. Early hospital discharge of children with cancer treated for fever and neutropenia: identification and management of the low-risk patient. Journal of Clinical Oncology. 1990;8:1998–2004. [PubMed: 2230891]
- Wacker P, Halperin DS, Wyss M, Humbert J. Early hospital discharge of children with fever and neutropenia: a prospective study. Journal of Pediatric Hematology/Oncology. 1997;19:208–211. [PubMed: 9201142]
- Hodgson-Viden H, Grundy PE, Robinson JL. Early discontinuation of intravenous antimicrobial therapy in pediatric oncology patients with febrile neutropenia. BMC Pediatrics. 2005;5:10. [PMC free article: PMC1156908] [PubMed: 15904510]
- Griffin TC, Buchanan GR. Hematologic predictors of bone marrow recovery in neutropenic patients hospitalized for fever: implications for discontinuation of antibiotics and early discharge from the hospital. Journal of Paediatrics. 1992;121:28–33. [PubMed: 1625089]
- Nijhuis CO, Kamps WA, Daenen SM, Gietema JA, van der Graaf WT, Groen HJ, et al. Feasibility of withholding antibiotics in selected febrile neutropenic cancer patients. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(30) [PubMed: 16234511]
- Santos-Machado TM, De Aquino MZ, Almeida MTA, Bakhit S, Cristofani LM, Maluf PT, et al. Short-term intravenous antibiotic therapy and early discharge of febrile neutropenic patients. International Journal of Pediatric Hematology/Oncology. 99 A.D.;6(1):33–38.
- Innes H, Lim SL, Hall A, Chan SY, Bhalla N, Marshall E. Management of febrile neutropenia in solid tumours and lymphomas using the Multinational Association for Supportive Care in Cancer (MASCC) risk index: feasibility and safety in routine clinical practice. Supportive Care in Cancer. 2008;16:485–491. [PubMed: 17899215]
- Castagnola E, Paola D, Giacchino R, Viscoli C. Clinical and laboratory features predicting a favorable outcome and allowing early discharge in cancer patients with low-risk febrile neutropenia: a literature review. Journal of Hematotherapy & Stem Cell Research. 2000;9:645–649. [PubMed: 11091488]
EVIDENCE TABLES
Download PDF (843K)
18. Duration of empiric antibiotic therapy. (Topic E7)
Guideline group members for this question
Rosemary Barnes (lead), Wendy King, Anton Kruger, Jeanette Hawkins, and Bob Phillips.
Review question
What is the optimal duration of empiric antibiotic therapy in patients with neutropenic sepsis?
Rationale
The risk and pattern of infection in patients with cancer and/or neutropenia depends on the primary diagnosis and the type, duration and intensity of the treatment.
There is no way of telling which febrile neutropenic patients have potentially life-threatening infection. For this reason, the assessment and treatment of febrile neutropenia is always a medical emergency. Signs of infection and CXR changes may be minimal or absent in the presence of neutropenia. The type and risk of infection is influenced by the following:
- Duration and severity of neutropenia
- Associated gut toxicity, due to cytotoxic drugs and/or total body irradiation (TBI)
- Previous radiotherapy, particularly TBI or whole neuraxis irradiation
- Long term immunosuppressive treatment, as in continuing maintenance therapy for ALL
- Presence of indwelling intravenous access device
Fever in the neutropenic patient requires prompt investigation and treatment with intravenous antibiotics, selected at first empirically in the light of known possible pathogens and the clinical circumstances. The most frequent pathogens are: Staph. Epidermidis, various Streps, Gram-negative rods and staph aureus. The most rapidly lethal are E. Coli, Klebsiella and Pseudomonas aeruginosa.
Currently patients admitted with neutropenic sepsis receive empiric antibiotic therapy for a certain period of time. This can range from 48 hours to 14 days with different criteria been applied to determine when the empiric antibiotic therapy should be discontinued. A review of the evidence might help to standardise practice. It is important to know whether stopping empiric antibiotics early will have a negative impact on clinical outcomes and wheat other influences impact of the decision to stop empiric antibiotics early
Question in PICO format
| Patients/population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with neutropenic sepsis receiving empiric antibiotic therapy | Stop empiric antibiotics early | Continuing empiric antibiotics until afebrile with recovered neutrophil count | Overtreatment Death/critical care Length of stay Duration of fever Quality of life |
METHODS
Information sources and eligibility criteria
The search strategy will be available in the full guideline.
We restricted the search to published randomised trials and systematic reviews of such trials. The search was done on the 9th of May 2011 and updated on 7th November 2011.
Selection of studies and data synthesis
The information specialist (SB) performed an initial screening of the literature search results. One reviewer (MSH) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO question.
It was anticipated that results from studies which compare early stopping with continuing empiric antibiotics until afebrile with a recovered neutrophil count would be pooled with the potential of doing subgroup analyses to compare the different stopping criteria for empiric antibiotics (e.g., neutrophil count) used by the included randomised trials.
RESULTS
Description of included studies
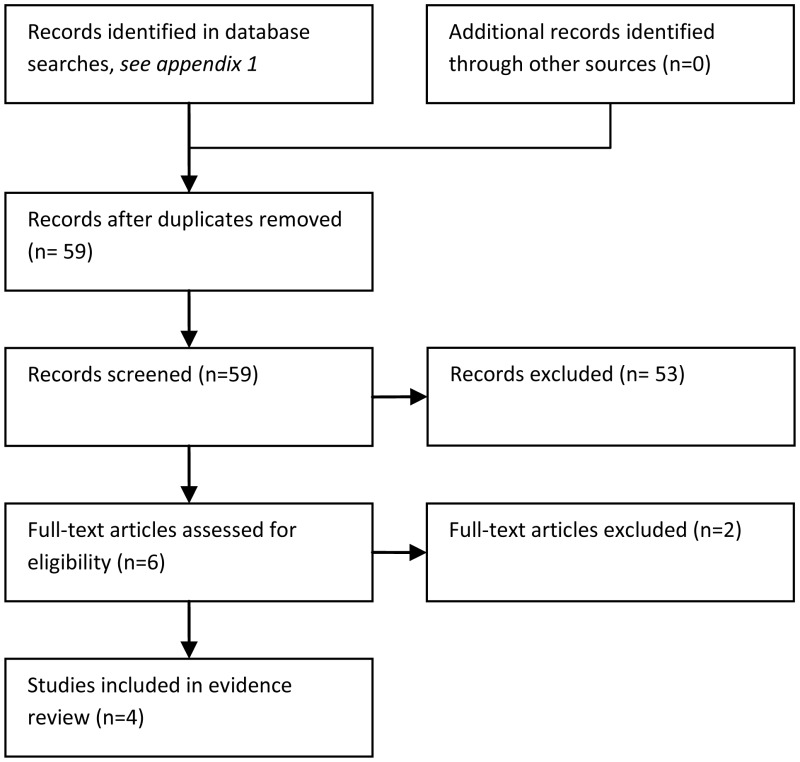
59 studies were identified in the literature searches. Of these, 55 were excluded because they were narrative reviews (N = 9), not in PICO (N = 36), not RCT (N = 9), or a protocol (N = 1).
Four RCTs were indentified for inclusion (Bjornsson, 1977; Klaassen, 2000; Pizzo, 1979; Santolaya, 1997). Two of these studies were conducted in children (Klaassen, 2000; Santolaya, 1997), one was conducted in adults (Bjornsson, 1977) and one study was conducted in a mixed population of children and adults (Pizzo, 1979). These four studies examined a variety of different antibiotic regimens. Detailed information about the populations, interventions, outcomes and overall risk of bias in the included trials is given in the Evidence and GRADE profile (Table 18.1) below.
Evidence statements
Death (short term mortality)
Very low quality evidence from four randomised trials suggested an increased odds of short term mortality in patients whose empirical antibiotics were stopped early compared with those who continued treatment, OR = 5.18 (95% C. I. 0.95 to 28.16). In two studies (Klaassen, 2000; Santolaya, 1997) there were no deaths while in the other two studies seven deaths occurred within 30 days (Bjornsson, 1977 Pizzo, 1979). The two studies in which deaths occurred were both from the 1970s and used first generation empiric antibiotic treatment.
Overtreatment,critical care and quality of life
These outcomes were not reported by any of the included trials.
Length of stay
One paediatric study (Santolaya, 1997) reported this outcome. There was low quality evidence that stopping antibiotics before resolution of neutropenia and fever had uncertain benefit in terms of length of stay. The mean length of stay was 0.7 days less in those who stopped empricial antibiotics early (95% C.I. 5.54 less to 4.41 more).
Duration of fever
One paediatric study (Santolaya, 1997) reported this outcome. There was low quality evidence that stopping antibiotics before resolution of neutropenia and fever had uncertain benefit in terms of duration of fever. The mean duration of fever was 0.8 days less in those who stopped empirical antibiotics early (95% C.I. 2.08 days less to 0.48 more).
EVIDENCE TABLES
Download PDF (396K)
Tables
Table 15.1GRADE evidence profile for optimal time to change the primary empiric treatment in unresponsive fever
| Quality assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of patients | Effect | Quality | |||||||||
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | No empiric antibiotic | Empiric antibiotic ± placebo | Antibiotic & additional drug | Relative RR (95%CI) P value | Absolute effect | |
| Mortality Pizzo, et al., (1982) | |||||||||||
| 1 | randomised trial | v. serious limitations1 | N/A | no serious indirectness | serious imprecision2 | 5 | 5 | 2 | - | - | VERY LOW |
| Median time to defervescence (range). Pizzo, et al., (1982) | |||||||||||
| 1 | randomised trial | v. serious limitations1 | N/A | no serious indirectness | serious imprecision2 | 11 days (3-22 days) | 8 days (3-23 days) | 6 days (2-20 days) | - | - | VERY LOW |
| Mortality (within 30 days). EORTC International anti-microbial therapy co-operative group (1989) | |||||||||||
| 1 | randomised trial | serious limitations3 | N/A | no serious indirectness | serious imprecision4 | - | 14 | 11 | P=0.04 | - | VERY LOW |
| Median time to defervescence (95%CI). Cometta, et al., (2003) | |||||||||||
| 1 | randomised trial | no serious limitations | N/A | no serious indirectness | serious imprecision5 | - | 4.3 days (3.5-5.1 days) | 3.5 days (2.4-4.4 days) | P=0.75 | - | LOW |
| Mortality between days 14 and 31. Cometta, et al., (2003) | |||||||||||
| 1 | randomised trial | no serious limitations | N/A | no serious indirectness | serious imprecision5 | - | 8/79 | 4/86 | RR=0.46 (0.15-1.38) P=0.29 | - | LOW |
| Defervescence within 72 hours. Erjavec, et al., (2000) | |||||||||||
| 1 | randomised trial | serious limitations6 | N/A | no serious indirectness | serious imprecision4 | - | 27/58 | 25/56 | RR=0.96 (0.64-1.43) P=0.98 | - | VERY LOW |
| Mortality whilst aplastic. Erjavec, et al., (2000) | |||||||||||
| 1 | randomised trial | serious limitations6 | N/A | no serious indirectness | serious imprecision4 | - | 4/58 | 6/56 | RR=1.55 (0.49-4.98) P=0.70 | - | VERY LOW |
- 1
No mention of allocation concealment; randomisation method not discussed; blinding not apparent.
- 2
Very low patient numbers and/or event rates.
- 3
No mention of allocation concealment; randomisation method not discussed; blinding of assessment may have occurred but not of treatment.
- 4
Low patient numbers and/or event rates.
- 5
Low patient numbers and /or event rates. Trial terminated early.
- 6
No mention of allocation concealment, scant details of randomisation of treatment.
Table 17.1GRADE evidence profile for early discharge with continued inpatient care
| Quality assessment | No of patients | Effect | Quality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | Early discharge | Continued inpatient care | Relative (95% CI) | Absolute | |
| Short term mortality in paediatric observational studies | |||||||||||
| 11 | observational studies1 | no serious limitations | no serious inconsistency | no serious indirectness | serious2 | none | 0/934 (0%) | - | - | - | VERY LOW |
| Hospital readmission in paediatric observational studies | |||||||||||
| 9 | observational studies1 | no serious limitations | no serious inconsistency | no serious indirectness | serious3 | none | 42/889 (4.7%) | - | - | - | VERY LOW |
| Short term mortality in adult case series using MASCC ≥ 21 as criteria for early discharge | |||||||||||
| 3 | observational studies | no serious limitations | no serious inconsistency | no serious indirectness | very serious3 | none | 0/215 (0%) | - | - | - | VERY LOW |
| Hospital readmission in adult case series using MASCC ≥ 21 as criteria for early discharge | |||||||||||
| 3 | observational studies1 | no serious limitations | no serious inconsistency | no serious indirectness | serious3 | none | 8/215 (3.7%) | - | - | - | VERY LOW |
| Short term mortality in paediatric RCT (Santolaya, et al., 2004) | |||||||||||
| 1 | randomised trials | serious4 | no serious inconsistency | no serious indirectness | serious3 | none | 0/78 (0%) | 1/71 (1.4%) | - | - | LOW |
| Short term mortality in adult RCT (Innes, et al., 2003) | |||||||||||
| 1 | randomised trials | serious4 | no serious inconsistency | no serious indirectness | serious3 | none | 0/66 | 0/60 | - | - | LOW |
| Overtreatment - not reported | |||||||||||
| 0 | - | - | - | - | - | none | - | - | - | - | |
| Quality of life - not reported | |||||||||||
| 0 | - | - | - | - | - | none | - | - | - | - | |
| Adverse events - not reported | |||||||||||
| 0 | - | - | - | - | - | none | - | - | - | - | |
- 1
Case series
- 2
Case series
- 3
Low number of events
- 4
Method of randomisation was unclear. No blinding (but this was unlikely to affect outcome
Table 17.2Early discharge criteria and rates
| Study ID | Population | Cancer | Study period | Episodes febrile neutropenia | Number of patients | Criteria for discharge | No. meeting basic criteria for early discharge | No. discharged early | Hospital re-admission in early discharge group | Death in early discharge group |
|---|---|---|---|---|---|---|---|---|---|---|
| Randomised Controlled trials | ||||||||||
| 1. Innes 2003 | Adult | Haematologic and solid malignancies | 1997-2000 | 102 | 126 | Anticipated duration of neutropenia < 7 days Haemodynamically stable No signs or symptoms that required IV fluid support Adequate renal function Ability to maintain satisfactory oral intake Living with responsible adult prepared to act as a carer Patient or carer able to read a thermometer | N.A. | N.A. | 5 (13%) | 0 (0%) |
| 2. Santolaya 2004 | Paediatric | Haematologic and solid malignancies | 2000 -2003 | 390 | 390 | Serum C-reactive protein (CRP) levels lower 90 mg/L No hypotension No relapse of leukaemia as cancer type Platelet count of more than 50,000/μL No recent (≤ 7 days) chemotherapy | N.A. | N.A. | Not reported | 0 (0%) |
| Open un-randomised comparative study | ||||||||||
| 3. Lau 1994 | Paediatric | 1990-1991 | 21 | 23 | Negative blood culture | N.A. | 11 (only considered low risk patients) | Not reported by group | 0 (0%) | |
| Prospective case series | ||||||||||
| 4. Cherif 2006 | Adult | Haematologic malignancies | 2003-2005 | 279 | 191 | MASCC (score ≥ 21) Afebrile for 24 hours Discharged with oral antibiotics | 105 (38%) | 67 (24%) | 3 (4%) | 0 (0%) |
| 5. Girmenia 2007 | Adult | Haematologic malignancies | 2001-2002 | 100 | 87 | MASCC (score ≥ 21) Afebrile for 48 hours Discharged with oral antibiotics | 90 (90%) | 69 (69%) | 2 (3%) | 0 (0%) |
| 6. Klastersky 2006 | Adult | Haematologic and solid malignancies | 1999-2003 | 611 | 441 | MASCC (score ≥ 21) Hospitalised for minimum of 24 hours Discharged with oral antibiotics | 383 (63%) | 79 (13%) | 3 (4%) | 0 (0%) |
| 7. Nijhuis 2005 | Adult and Paediatric | Haematologic and solid malignancies | 1999-2002 | 196 | 128 | No local bacterial infection / abnormal vital signs (systolic blood pressure less than 99 mmHg, or both heart rate higher than 100/min in adults or less than -2SD for age in children and respiratory rate higher than 20/min in adults or both heart and respiratory rate higher than +2 SD for age in children suggesting sepsis. Interleukin 8 level below cut off value of 60 ng/L Antibiotics completely withheld. Afebrile for 12 hours | 36 (18%) | 36 (18%) | 0 (0%) | 0 (0%) |
| 8. Dommett 2009 | Paediatric | Haematologic and solid malignancies | 2004-2005 | 762 | 368 | Excluded from low risk protocol if: Age<1 year, shock/ compensated shock, haemorrhage, dehydration, metabolic instability, altered mental status, pneumonitis, mucositis, respiratory distress/ compromise, peri-rectal / other soft tissue abscess, rigors, irritability/meningism, organ failure, acute lymphoblastic leukaemia at diagnosis/relapse <28 d, acute lymphoblastic leukaemia not in remission >28 d acute myeloid leukaemia, infant acute lymphoblastic leukaemia, intensive B-NHL protocols, haemopoietic stem cell transplant, sequential high dose chemotherapy with PBSC rescue, intensive care admission during last FN episode, non adherence (social concerns or patient), inability to tolerate oral antibiotics, positive blood culture result at 48 h, ANC < 0.1 · 109/L at 48 h, child not clinically well at 48 h (clinician judgement). Hospitalised for minimum of 48 hours Discharged with oral antibiotics | 212 (27%) | 143 (19%) | 8 (6%) | 0 (0%) |
| 9. Lehrnbecher 2002 | Paediatric | Haematologic and solid malignancies | 1994-1996 | 106 | 56 | Patients were not formally categorised as high / low risk. Were eligible for discharge when following criteria met: good clinical condition, negative blood culture results, no infectious focus, absence of fever for at least 24 hrs. Early discharge only allowed in cases of fever of unknown origin Hospitalised for minimum of 72 hours. Afebrile for 24 hours. Antibiotics ceased before discharge | N/A | 24 (23%) | 0 (0%) | 0 (0%) |
| 10. Bash 1994 | Paediatric | Haematologic and solid malignancies | 1989-1990 | 131 | 74 | Appeared clinically well Negative blood cultures Exhibited control of local infection Hematologic evidence of bone marrow recovery Antibiotics ceased before discharge | 82 (63%) | 78 (58%) | 7 (9%)* *these patients said to violate the early discharge protocol | 0 (0%) |
| Retrospective case series | ||||||||||
| 11. Tordecilla 1994 | Paediatric | Solid malignancies | 1992-1993 | 84 | 50 | *retrospective analysis, patients were not prospectively assigned to low/high risk groups Appeared well Negative blood cultures Normal chest x-ray Afebrile Discharged with/ without antibiotics | N.A. | 30 (35.7%) | 0 (0%) | 0 (0%) |
| 12. Aquino 1997 | Paediatric | Haematologic and solid malignancies | 1992 -1995 | 580 | 253 | *retrospective analysis, patients were not prospectively assigned to low/high risk groups Clinically well appearance Sterility of all blood cultures Control of local infection with antibiotic therapy (defined as reduction or resolution of local signs of inflammation such as erythema, induration and tenderness) Evidence of bone marrow recovery (defined as any sustained increase in platelet count and ANC or APC) Afebrile for 24 hours Discharged with/without oral antibiotics | N.A. | 330 (57%) | 21 (6%) | 0 (0%) |
| 13. Mullen 1990 | Paediatric | Haematologic and solid malignancies | 1988-1999 | 114 | 61 | *retrospective analysis, patients were not prospectively assigned to low/high risk groups Negative blood cultures (Usually) some evidence of bone-marrow recovery Afebrile for 1-2 days | N.A. | 77 (68%) | 3 (3.9%) | 0 (0%) |
| 14. Griffin 1992 | Paediatric | Haematologic and solid malignancies | 1989 | 107 | 64 | Initial blood cultures were sterile after 48 hours Appeared well Any identified infection is under control Afebrile for 24 hours | N.A | 70 (65%) | 1/70 (1%) | 0 (0%) |
| 15. Wacker 1997 | Paediatric | Haematologic and solid malignancies | 1992-1995 | 88 | 30 | *retrospective analysis, patients were not prospectively assigned to low/high risk groups No documented infection (no pathogenic organisms identified on cultures) throughout hospital course Normal physical exam Afebrile for 24 hours | 44 (50%) | 25 (28%) | 2 (8%) | 0 (0%) |
| 16. Hodgson-Viden 2005 | Paediatric | Haematologic and solid malignancies | 1997 -2002 | 276 | 127 | *retrospective analysis, patients were not prospectively assigned to low/high risk groups No formal criteria for early discharge. Decision based solely on clinician's judgement. Patients were discharged on the day intravenous antimicrobial therapy (IVAMT) was ceased. Early discharge was defined as discontinuation of IVAMT with an ANC ≤ 500/mm3. | N.A. | 112 (41%) | 0 (0%) | 0 (0%) |
| 17. Tomiak 1994 | Adult | Haematologic and solid malignancies | 1991-1993 | 134 | 134 | *retrospective analysis, patients were not prospectively assigned to low/high risk groups Negative blood cultures Afebrile and clinically stable for 48 hours | N.A. | 37 (28%) | 2 (5%) | 1 (3%) |
| 18. Santos-Muchado 1999 | Paediatric | Haematologic and solid malignancies | 1996 | 79 | 46 | Negative blood cultures Afebrile for 24 hours Discharged with oral antibiotics (in most cases) | N.A. | 34 (43%) | Not reported | 0 (0%) |
Table 18.1GRADE evidence profile for duration of empiric antibiotic therapy
| Quality assessment | No of patients | Effect | Quality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Shorter duration empiric antibiotics | Longer duration empiric antibiotics | Relative (95% CI) | Absolute | |
| Death (within 30 days) | |||||||||||
| 4 | randomised trials | very serious1 | no serious inconsistency | serious2 | very serious3 | none | 5/95 (5.3%) | 2/103 (1.9%) | OR 5.18 (0.95 to 28.16) | 74 more per 1000 (from 1 fewer to 339 more) | VERY LOW |
| Length of stay (Better indicated by lower values) | |||||||||||
| 1 | randomised trials | serious | no serious inconsistency | no serious indirectness | serious3 | none | 36 | 39 | - | mean 0.7 days lower (5.54 lower to 4.41 higher) | LOW |
| Duration of fever (Better indicated by lower values) | |||||||||||
| 1 | randomised trials | serious4 | no serious inconsistency | no serious indirectness | serious5 | none | 36 | 39 | - | mean 0.8 days lower (2.08 lower to 0.48 higher) | LOW |
- 1
3 of the 4 studies were not placebo-controlled and reported no detail about the method of randomisation employed, whether there was allocation concealment and no power analysis.
- 2
2 of the 4 studies were from the 1970s and used first generation antibiotic agents and all the deaths occurred in these two older trials.
- 3
Very low event rate.
- 4
Unclear allocation concealment, insufficient details about randomisation and not placebo controlled
- 5
Uncertainty in the estimate of effect, the confidence interval spans both appreciable benefit and harm.