Abstract
Background
Polymorphisms in ATP2B4 coding for PMCA4b, the primary regulator of erythrocyte calcium concentration, have been shown by GWAS and cross-sectional studies to protect against severe malaria but the mechanism remains unknown.
Methods
Using a recall-by-genotype design, we investigated the impact of a common haplotype variant in ATP2B4 using in vitro assays that model erythrocyte stage malaria pathogenesis. Ninety-six donors representing homozygotes (carriers of the minor alleles, T/T (variant), heterozygote T/C and wildtype C/C (ancestral)) carriers of the tagging SNP rs1541252 were selected from a cohort of over 12,000 participants in the Keneba Biobank.
Results
Red blood cells (RBCs) from homozygotes showed reduced PMCA4b protein expression (mean fluorescence intensities (MFI = 2428 ± 124, 3544 ± 159 and 4261 ± 283], for homozygotes, heterozygotes and wildtypes respectively, p < 0.0001) and slower rates of calcium expulsion (calcium t½ ± SD = 4.7 ± 0.5, 1.8 ± 0.3 and 1.9 ± 0.4 min, p < 0.0001). Growth of a Plasmodium falciparum laboratory strain (FCR3) and two Gambian field isolates was decreased in RBCs from homozygotes compared to heterozygotes and wildtypes (p < 0.01). Genotype group did not affect parasite adhesion in vitro or var-gene expression in malaria-infected RBCs. Parasite growth was inhibited by a known inhibitor of PMCA4b, aurintricarboxylic acid (IC50 = 122uM CI: 110–134) confirming its sensitivity to calcium channel blockade.
Conclusion
The data support the hypothesis that this ATP2B4 genotype, common in The Gambia and other malaria-endemic areas, protects against severe malaria through the suppression of parasitaemia during an infection. Reduction in parasite density plays a pivotal role in disease outcome by minimizing all aspects of malaria pathogenesis. Follow up studies are needed to further elucidate the mechanism of protection and to determine if this ATP2B4 genotype carries a fitness cost or increases susceptibility to other human disease.
Background
Globally, Plasmodium falciparum malaria remains one of the most common infectious diseases, infecting about 241 million people worldwide in 2020 [1]. The majority of people infected with malaria recover without developing life-threatening complications and develop short- and mid-term immunity against future infections and serious sequelae [2]. Host genetic factors are estimated to account for one-quarter of the total variability in malaria severity [3]. Recent genome-wide association (GWAS) and cross-sectional studies have confirmed the protection afforded by known traits such as haemoglobinopathies, the ABO blood group system, and glucose-6-phosphate dehydrogenase (G6PD) deficiency, and have identified new polymorphisms associated with resistance to severe malaria [4–7]. Polymorphisms with some of the strongest associations are in the ATP2B4 gene and the glycophorin gene cluster. Polymorphisms on the glycophorins appear to be spatially limited to Eastern Africa [6–8]. ATP2B4 polymorphisms are widely distributed in malaria endemic regions and have been reproducibly linked to altered susceptibility to malaria in multiple African populations [9–11].
ATP2B4 codes for a plasma calcium membrane ATPase 4 (PMCA4) which is part of the plasma membrane calcium ATPase (PMCA) pump family. PMCAs are ATP dependent pumps that actively remove calcium from the cytoplasm [12]. ATP2B4 codes for PMCA4b, the primary regulator of RBC calcium concentration. PMCAs are found ubiquitously in eukaryotic cells and are responsible for the expulsion of calcium from the cytosol [13]. There are four isoforms of the PMCAs: PMCA1, PMCA2, PMCA3 and PMCA4. Each of these isoform transcripts can undergo alternative splicing. PMCA4b is a splice variant of PMCA4, expressed in RBCs and coded by ATP2B4 [13]. PMCA4b is not specific to the RBC, it is ubiquitously expressed in all other cell types tested to date.
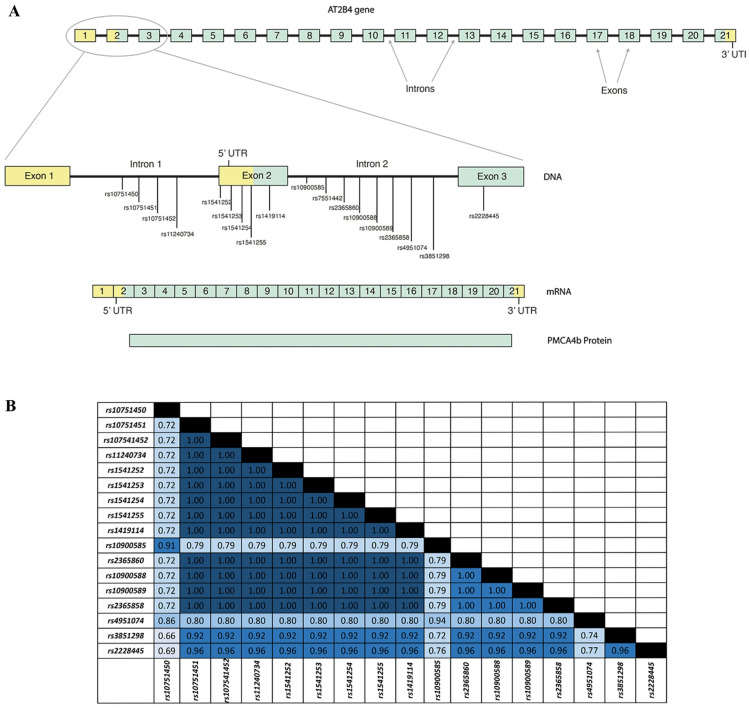
The variant haplotype in the ATP2B4 gene described here will likely impact only RBCs and not the expression of PMCA4b in other cell types, since several of the polymorphisms described are located in the erythroid enhancer region of the gene (Fig. 1).
Fig. 1.
A Relative positions of SNPs on ATP2B4 gene. Schematic view of the ATP2B4 gene, mRNA and protein showing the genomic location of all SNPs associated with malaria altered susceptibility and one SNP (rs7551442) associated with altered erythrocytic MCHC. Boxes represent coding region (exons). Boxes in yellow represent the exons that form the 5’ and 3’ untranslated regions. Boxes in green represent the exons that code for the translated protein. B Linkage disequilibrium analysis of ATP2B4 SNPs associated with malaria protection and PMCA4b function. LD analysis was done using LDlink on the 1000 genome data of Gambian population. Matrix was created to describe the relationship between the SNPs as a function of R2 presented. R2 between each pair of SNPs are color coded according to strength of the LD (R2 = 1 to 0.9, dark blue or ‘strong’; R2 = 0.8 to 0.7, medium blue or ‘moderate’ and R2 = 0.6, light blue for ‘weak’
Previous work has found decreased expression and function of PMCA4b in erythrocytes from individuals with ATP2B4 polymorphisms [14]. Deletion of ATP2B4 alters RBC homeostasis which presents a hostile environment for the parasite [15].
This study is the first time the effect of ATP2B4 polymorphisms on erythrocytes and on malaria pathogenesis from people living in a malaria endemic region has been investigated Here the effects of three common ATP2B4 genotype groups defined by the tagging SNP rs1541252 on in vitro P. falciparum growth, adhesion, invasion are reported.
Methods
Study population: Kiang West Longitudinal Population Study (KWLPS) cohort and the Keneba Biobank
The Keneba Biobank contains samples from over 12,000 individuals. The Keneba Biobank, in conjunction with the Keneba Electronic Medical Records System and Kiang West Demographic Surveillance System, forms a substantial research platform, which supports health care provision and research within the Kiang West Longitudinal Population Study [16]. The Keneba Biobank also contains a genetic database which is currently comprised of Illumina HumanExome array data on 80,000 directly genotyped putative functional variants from over 3000 participants. The KWDSS forms the sampling framework for all data collection in this study.
Ethics statement
The study proposal was reviewed and approved by the Scientific Coordinating Committee of the Medical Research Council Unit The Gambia at London School of Hygiene and Tropical Medicine (MRCG at LSHTM) and ethical approval was granted by the Joint Gambia Government/Medical Research Council Ethics committee (SCC number 1421). The study was conducted according to Good Clinical Practice standards. The study procedures were explained to the donors orally and in writing. Individuals donated 5 mls of whole blood to the study only after the written informed consent was provided.
DNA extraction
200 uL of the donated blood was aliquoted for DNA extraction to confirm the genotypes obtained from their Keneba Biobank DNA. DNA was extracted using the QIAamp DNA mini kit (Qiagen cat. 51,306) as per manufacturer’s instructions.
Taqman genotyping
Taqman assay was used to determine SNPs from DNA samples for targets rs2228445, rs2365858, rs54951074 and rs1541252. Taqman SNP assay mix (ThermoFisher Scientific cat. 4,351,379) was used for each target and assay was carried out as per manufacturer’s instructions.
PMCA4b protein expression
A previously described method with few modifications was used [14]. In brief, erythrocytes were permeabilized with paraformaldehyde to create ‘ghost’ erythrocytes which will allow measurement of PMCA4b expression. To distinguish RBC ‘ghost’ from debris, this was incubated with a lectin, Wheat Germ Agglutinin Alexa Fluor 647 conjugate (Life Technologies) at 1.6 µg/mL. This was simultaneously incubated with a primary antibody that binds to the PMCA4b protein on the membrane of the erythrocyte ‘ghosts’ followed by a secondary fluorescent antibody. Primary antibody, Anti-PMCA4b clone JA3 (Merck Millipore cat.) concentration was optimized at 4 µg/mL and the secondary antibody, Alexa Fluor 488 goat labelled anti-mouse (H + L) (Life Technologies) at 10 µg/mL. For each sample, a negative test was prepared without the primary antibody to assess the background stain of Alexa Fluor 488 on erythrocytes and to control for a failed run. Fluorescence was then measured with BD Accuri™ C6 Plus flow cytometer (BD Biosciences) and only WGA expressing cells (FL4 channel) were gated for PMCA4b expression which was assessed by mean fluorescent intensity (MFI) on (FL1 channel). Flow data were analysed with on FlowJo® (Version 10.1) software.
Calcium extrusion assay
The activity of the PMCA4b was assessed as previously described [14, 17]. In brief, RBCs were initially loaded with Ca2+ using an ionophore, Ionomycin (Molecular Probes/Invitrogen cat. 12,422) in a calcium rich buffer. Calcium loss from the cells was then measured by detecting emitted fluorescence from Fluo-4-AM (Molecular Probes/Invitrogen cat. F14201), a calcium dye indicator that fluoresces when bound to calcium. The dynamics of calcium loss from the RBCs was assessed continuously for 15 min using BD Accuri™ C6 Plus flow cytometer (BD Biosciences).
Plasmodium falciparum culture lines and maintenance
Plasmodium falciparum strain FCR3-FMG (MR4, MRA-736) strain was used for most of the in vitro malaria assays. Parasites were maintained under standard conditions using RBCs from healthy donors. Schizonts stage infected RBCs were purified using a magnetic-activated cell sorting system (MACS, Miltenyi Biotec, Inc) for all assays. Parasite strains 952 and 998 were isolated from patients presenting with symptomatic malaria infections at the outpatient clinic at MRC Fajara. Isolates were collected as part of a larger study during the annual malaria transmission seasons (September–January) from 2005 to 2011, as described in [18]. FCR3- VAR2CSA parasite isolates were gifted by Benoit Gamain and colleagues from the Institute National de la Santé et de la Recherche Médicale (INSERM).
Plasmodium falciparum growth assay
Plasmodium falciparum growth assay was carried out as previously described [19]. In brief, MACS purified schizonts were added to the WBC depleted erythrocytes at 1% parasitaemia and 2% haematocrit, plated in triplicates and incubated at 37 °C with 5% CO2, 5% O2 and 90% N. Parasitaemia at culture initiation (0 h) and after 72 h of culture was confirmed using a BD Accuri™ C6 flow cytometer as described [19]. Parasite growth rates were determined using formula: (final parasitaemia—initial parasitaemia)/ initial parasitaemia.
Barcoded invasion assay
This was performed as described previously [19]. Donor RBCS were stained with three different concentrations of CellTrace Far Red DDAO dye (Life technologies cat. C34553): 1 uM, 0.5 uM and 0.1 uM. Erythrocytes were then pooled and seeded with MACS purified schizonts at 1% parasitaemia and incubated for 24 h. Parasitaemia was then assessed by flow cytometry as above.
RT-qPCR for var gene transcripts
500 ul pellet of each parasite culture was dissolved in Trizol (Life Technologies). RNA was extracted using phenol–chloroform and with RNeasy mini extraction kit (Qiagen) as described in [20]. Quantification of var transcripts was done as previously described [21] with the following minor modifications: cDNA was synthesized using Multiscribe reverse transcription kits as per manufacturer’s instructions (Invitrogen). qPCR was done on CFX96 Touch Real-Time PCR detection system (BIO-RAD) using SYBR green supermix (BIO-RAD). The var gene transcription profiles were performed using the same set of primers, targeting the IT4/FCR-3 var repertoire as previously described [21].
Plasmodium falciparum iRBC chondroitin sulphate spot binding
Plasmodium falciparum FCR3-CSA strain infected RBCs were used in binding assays as previously described [22]. In brief, ‘knobs’ expressing infected erythrocytes were bound on spots of coated Chondroitin Sulphate A Sodium salt (CSA) from bovine trachea (Millipore Sigma) at 100 ug/mL on petri dish (FALCON). Infected erythrocytes per mm2 was calculated using the formula: Total counted infected erythrocytes in 4 fields/ 0.1735 (area field 40X).
Aurintricarboxylic acid (ATA) inhibition assay
Plasmodium falciparum iRBC’s susceptibility to ATA (Sigma Aldrich cat. A1895-5G) was tested using conventional IC50 drug assay as described in [23]. Experiments were carried out in duplicate for three biological repeats. Parasite’s response to ATA was determined using a nonlinear regression dose–response analysis (with ATA concentration in logarithm) to obtain IC50 value.
Statistical analysis
Minor Allelic Frequency (MAF) in the population of 175 was determined from the PCR genotyping and LD analysis of the PCR genotypes was conducted in R (package “Genetics”) and LDlink (NIH, NCBI). All results obtained from the flow cytometer (BD Accuri C6) were analysed with FlowJo® software (version 10.1) to determine MFI and parasitaemia. Data analysis and figure generation were carried out using GraphPad Prism (version 9). ANOVA and Dunn’s multiple comparisons were performed to compare the calcium half-lives values, protein expression MFI, growth rate, percentage invasion of wild type, heterozygote and mutant samples obtained. In all analyses, a p-value < 0.05 was considered significant.
Results
ATP2B4 SNPs associated with resistance to severe malaria are in strong linkage disequilibrium (LD)
ATP2B4 SNPs associated with altered resistance to malaria by GWAS are numerous and found in the region from the first to the third exon spanning a 16.5 kilobase (KB) region (Fig. 1A). Most of the ATP2B4 SNPs are found in non-coding (intronic) and regulatory regions [e.g., the 5’ untranslated region (5’UTR)] of the ATP2B4 gene]. Previous analysis of four of these SNPs (rs1541252, rs1541253, ra1419114 and rs2228445) demonstrated that they were in complete LD [13]. To extend this observation, an LD analysis for all 17 SNPs in ATP2B4 previously linked to malaria susceptibility using LDLink [24] and 1000 genome data on Gambians in Western Division (GWD) was conducted. Overall, a strong pairwise linkage between all SNPs with R2 range of 0.66 to 1 was found and characterized the haplotype block (Fig. 1B).
Recall-by-genotype recruitment
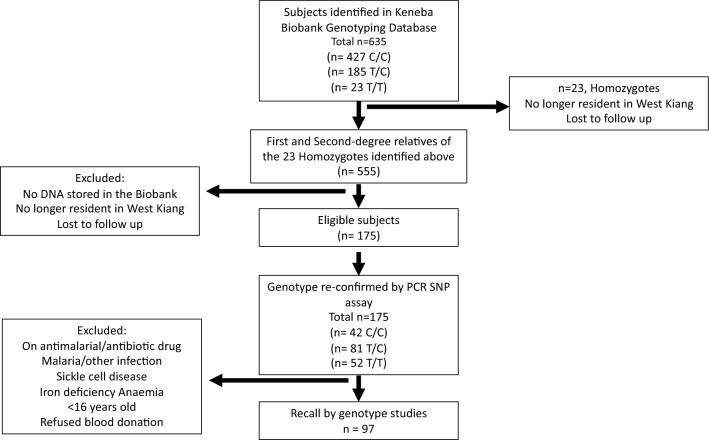
Using a Recall-By-Genotype design, RBC donors were selected from a pre-genotyped cohort of 635 individuals in the Keneba Biobank (MRCG at LSHTM) [16]. Among those, 23 individuals who were homozygous carriers of the minor rs1541252 allele (T/T), were lost to follow up due to migration out of the study area. Nevertheless, 175 ungenotyped first- and second-degree relatives of these 23 donors that met study inclusion criteria were identified and typed for rs1541252 by Taqman PCR. Individuals were further screened and excluded if they no longer lived in the study area or had any of the following: haemoglobin S (heterozygous and homozygous), haemoglobin < 11 g/dL, history of malaria in the past 3 months, known history of any chronic illness or current use of anti-malarials or antibiotics. A total of 96 RBC donors were recruited based on three genotype groups (31 homozygotes for the minor allele (T/T), 35 heterozygotes (T/C) and 30 wildtypes (C/C)) (Fig. 2).
Fig. 2.
RBC donor identification and selection. Flow diagram
PMCA4b protein expression in RBCs from donors with ATP2B4 variant genotype
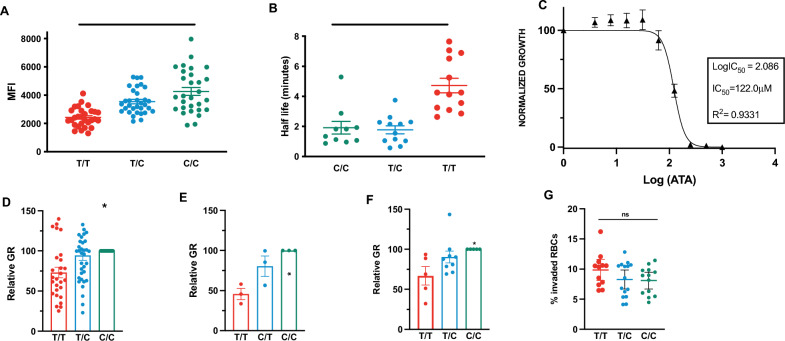
First, the effect of homozygous carriage of the ATP2B4 minor allele on the expression of PMCA4b protein in the donor RBCs was investigated. ‘Ghost’ RBCs were stained with an antibody directed against PMCA4b followed by fluorescent secondary antibody to determine the relative levels of protein expression. The mean fluorescent intensity (MFI) was compared between genotype groups (Fig. 3A). RBCs from homozygote carriers had significantly decreased protein expression (MFI ± SD, 2427.7 ± 124) compared to RBCs from heterozygotes (3544.4 ± 158.7) and wildtypes (4261.3 ± 283) (p < 0.0001 ANOVA). The difference in protein expression between RBCs from homozygotes versus wildtypes was significant (p < 0.0001, Dunn’s multiple comparison test), but not between RBCs from the heterozygotes and wildtypes (p > 0.05, Dunn’s multiple comparison test).
Fig. 3.
Role of variant ATP2B4 genotype group on PMCA4b expression, function and P. falciparum erythrocytic stage growth. Individuals from homozygous (T/T) genotype (red), heterozygous (T/C) genotype (blue) and wild type (C/C) genotype (green) for the ATP2B4 variant haplotype A Expression of PMCA4b protein in RBCs from homozygous (T/T) donors (n = 29) compared to heterozygous (T/C) donors (n = 29) and wild type (C/C) donors (n = 30). ANOVA followed by Dunn’s multiple comparison testing was used (**** = p > 0.001). B Comparison of calcium expulsion from RBCs indicated by calcium half-life in RBCs from homozygous (T/T), (n = 13), heterozygous (T/C) donors (n = 13) and wild type (C/C) donors (n = 13). ANOVA followed by Dunn’s multiple comparison testing was used (**** = p > 0.001). C Inhibition of parasite growth by PMCA4b inhibitor aurintricarboxylic acid (ATA) in RBCs (n = 3 from wild type (C/C) donors). Inhibitory concentration at 50% (IC50) was calculated using a non-linear regression dose–response model. D Growth of P. falciparum strain FCR3-FMG in vitro in RBCs from homozygous (T/T) donors (n = 27), heterozygous (T/C) donors (n = 35) and wild type (C/C) (n = 30) E Growth of P. falciparum field isolates in vitro in RBCs from homozygous (T/T) donors (n = 3), heterozygous (T/C) donors (n = 3) and wild type (C/C) donors (n = 3) were infected with P. falciparum clinical strain 998. F Growth of P. falciparum field isolates in vitro in RBCs from homozygous (T/T) donors (n = 5), heterozygous (T/C) donors (n = 9) and wild type (C/C) donors (n = 5) were infected with P. falciparum clinical strain 952. Values for all panels (D) (E) and (F) are presented relative to growth in RBCs from wild type (C/C) donors ANOVA (*p < 0.5). Each dot represents the mean result of triplicate growth assays from each donor and the error bars represent SEM. G P. falciparum strain FCR3-FMG invasion in RBCs from homozygous (T/T) donors (n = 12), heterozygous (T/C) donors (n = 15) and wild type (C/C) donors (n = 13), Means were compared using one-way ANOVA (ns)
Rate of calcium expulsion from RBCs with ATP2B4 variant genotype
RBCs from donors in all three genotype groups were incubated with fluorescently-labelled Ca2+ to assess the efflux efficiency of the PMCA4b using flow cytometry. An exponential decay curve model was used to determine calcium half-life in the RBCs. Figure 3B shows a difference in Ca2+ half-life between RBCs from homozygotes compared to those from heterozygotes and wildtypes. The median half-life (4.7 ± 0.5 min) for Ca2+ efflux was significantly longer in donor RBCs from homozygote than in RBCs from heterozygote (1.8 ± 0.3 min) and wildtype (1.9 ± 0.4 min) donors (p < 0.0001, ANOVA). Calcium extrusion rates did not differ between wild type and heterozygote individuals (p > 0.05, Dunn’s multiple comparison test).
Plasmodium falciparum growth in RBCs from individuals with the ATP2B4 variant genotype
To assess whether the PMCA4b could influence P. falciparum growth, we incubated the RBCs from wildtype donors with a specific PMCA4b inhibitor aurintricarboxylic acid (ATA) (Sigma Aldrich cat. A1895-5G)) [25]. ATA inhibited the iRBCs growth (Fig. 3C) at an IC50 of 112.2 (SD ± 14.2 mM), confirming the crucial role of PMCA4b in P. falciparum growth.
In addition, the intraerythrocytic growth of P. falciparum (laboratory strain FCR3-FMG) was investigated in donated RBCs from all three genotype groups. A decrease in the parasite growth rate (GR) in RBCs from donors in homozygotes was found compared to heterozygotes and wildtypes (p < 0.05 ANOVA) (Fig. 3D). Growth rates of parasites in RBCs from heterozygotes and wildtypes did not differ (p > 0.05; Dunn’s multiple comparisons). To verify that this phenotype was not an artefact of the laboratory adaptation of the FCR3-FMG strain, in vitro growth of two Gambian clinical isolates of P falciparum (isolates 998 and 952) was assessed. These isolates were collected as part of a larger study during the annual malaria transmission seasons (September–January) from 2005 to 2011, as described elsewhere [18]. Both field isolates, 998 (Fig. 3E) and 952 (Fig. 3F) also showed decreased growth rates (GR ± SEM, 45.8% ± 7.01 and 66.9% ± 7.42) in RBCs from homozygous carriers of the minor allele.
Plasmodium falciparum merozoite invasion of RBCs from individuals with the ATP2B4 variant genotype
Using a barcoded RBC flow cytometry, assay invasion by merozoites in RBCs from individuals with the three ATP2B4 haplotypes was assessed. The mean percentage of invaded RBCs did not differ between the RBCs from homozygous (9.9 ± 0.8%), heterozygous (8.3 ± 0.7%) or wild-type individuals (8.1 ± 0.6%) (p > 0.05, ANOVA) (Fig. 3G).
Influence of ATP2B4 variant haplotype on parasite adhesion in vitro
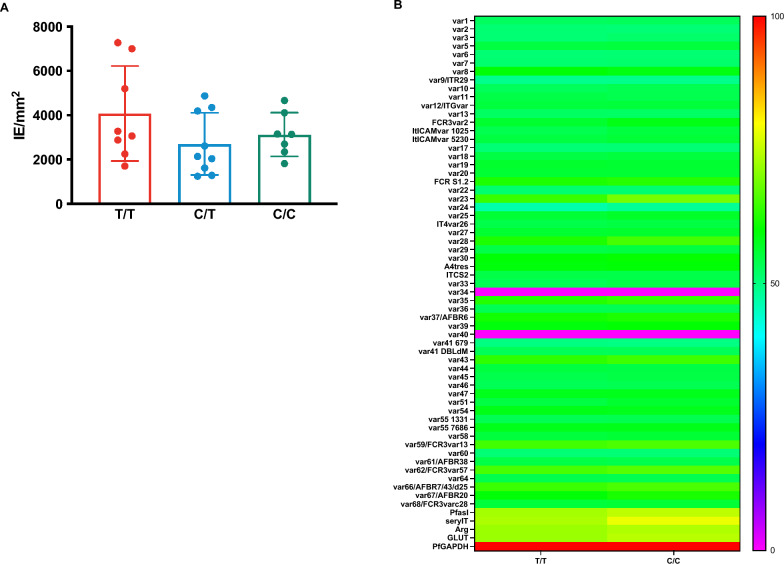
Finally, the adhesive properties of malaria-infected RBCs from donors with the three different genotypes was examined using two approaches. First, an in vitro system designed to mimic the adhesion of malaria-infected RBCs to the placenta was used. RBCs were infected with a P. falciparum line expressing VAR2CSA (FCR3-VAR2CSA), a gene that codes for a parasite ligand known to bind chondroitin sulphate (CSA) expressed in the placenta. The var2CSA system was chosen because of the relative ease of the assay and our ability to perform it under field conditions. Furthermore, VAR2CSA is the only var gene product known to bind CSA and it is unusually well conserved between parasite isolates [21, 26] The number of parasite-infected RBCs bound per mm2 of a CSA-coated plastic surface was assessed. No differences were observed in the binding of parasite-infected RBCs from the homozygous donors (infected RBCs /mm2 ± SD, 4079 ± 756) heterozygous (2704 ± 545) or wildtypes (3130 ± 373) (p > 0.05, ANOVA) (Fig. 4A). Second, the expression of other var genes using RT-PCR in parasites grown in the RBCs from participants in homozygotes and wildtypes was assessed. No difference in expression of any of the var genes tested were detected (Fig. 4B).
Fig. 4.
Role of variant ATP2B4 genotype group in cytoadherence and var gene expression of parasitized RBCs. Donor RBCs were infected with a P. falciparum strain expressing var2CSA (FCR3-var2CSA). The number of infected RBC binding to CSA per mm2 was quantified for RBCs from individuals in homozygous (T/T) donors (n = 8), heterozygous (T/C) donors (n = 11) and wild type (C/C) donors (n = 8). Adhesion was measured and compared between the three haplotypes using one-way ANOVA, ns). B RNA was extracted from donor RBCs infected with P. falciparum strain FCR3-var2CSA. RT-PCR was conducted to detect four variants of var2CSA which are known to be expressed in this parasite line. Heat map showing the relative expression profile of the entire var gene repertoire (n = 63) excluding var2CSA of FCR3 strain. Parasites were grown in homozygous (T/T) donors (n = 8) and wild type (C/C) donors (n = 8) RBCs and RT-PCR performed following RNA extraction. No significant difference in the expression of any of the var genes tested between parasites grown in the homozygous (T/T) and wild type (C/C) donors infected RBCs
Discussion
The strength of the study presented here includes the parsimonious recruitment of participants by means of the recall by genotype design and the existence of a field laboratory permitting assays on freshly drawn blood. We present evidence that a variant haplotype of ATP2B4, composed of 17 SNPs in tight LD and represented here by the tagging SNP rs1541252, decreases PMCA4b protein expression and its function of calcium expulsion and affects P. falciparum growth in RBCs. The variant ATP2B4 genotype did not affect iRBC adhesion to the host receptor CSA, nor did it alter var gene expression. Decrease of the growth of the P. falciparum FCR3-FMG laboratory strain in RBCs from homozygous subjects were also confirmed in clinical isolates from The Gambia. The entire study was performed in a field laboratory in rural Gambia using freshly collected RBCs and as such, has multiple limitations. Most importantly, attempts to directly measure intracellular calcium in the RBCs were unsucessful. In addition, mRNA for PMCA4b was not measured. There was neither a fluorescent microscope at the field station nor a camera attached to the AccuriC6 flow cytometer, so visualization of the protein expression and localization of PMCA4b on the RBC membranes was not done.
The observations on the relationship between several polymorphisms in ATP2B4 and risk of malaria was first demonstrated in Ghanaian and Gambian populations [4] and was subsequently replicated in other African [7], Asian and Oceanic populations [9]. The average minor allele frequency is 0.32 in Kenyan [7], 0.4 in Senegalese [27] and 0.36 in all African populations. Recent studies have shown that PMCA4b expression in RBCs is controlled by an erythroid-specific transcription enhancer site and that there is a specific transcript of ATP2B4 found only in RBCs [10, 14]. All 17 of the SNPS within the ATP2B4 haplotype variant defined here are near or in this erythroid-specific transcription enhancer (Fig. 1). One of the SNPs (rs10751451) disrupts a GATA motif and the same SNP has previously been shown to associate with ATP2B4 expression levels and calcium concentration in RBCs [14]. Due to the very strong LD across this region of the ATP2B4 gene, this work is unable to address the possibility that rs10751451 may be the single causal variant in ATP2B4 that mediates protection from malaria as previously proposed [10]
The normal blood plasma calcium level is 1.8 mM [28], yet RBCs are able to maintain an intracellular calcium concentration between 30 to 60 nM. Excessively high intracellular Ca2+ concentrations lead to numerous types of erythrocyte dysfunction [21–23]. Most importantly, Ca2+ plays a role in the regulation of other ions, including Na+ and K+. An increase in erythrocytic Ca2+ concentration activates the Gardos channel which leads to erythrocyte dehydration and shrinkage similar to that observed in sickle cell disease [28, 29].
The source of calcium for post-invasion parasite development in RBCs is not fully understood, but increasing levels of Ca2+ are observed as the parasite grows from rings to schizonts and depletion of calcium would result in an arrest of growth [30–32]. Shortly before release of merozoites from the RBCs, Ca2+ increases sharply which results in swelling and degradation of the parasitophorous vacuole and the RBC membrane [31–35]. Several calcium-dependent enzymes are involved in this process including, sub-like protease 1 (SUB1), a Pf-perforin-like protein and CDPK5 [33, 36, 37]. Additional roles of calcium during the RBCs invasion have also been established. It is however still unclear whether the Ca2+ origin is exclusively extracellular or has an intracellular erythrocytic source as well [38].
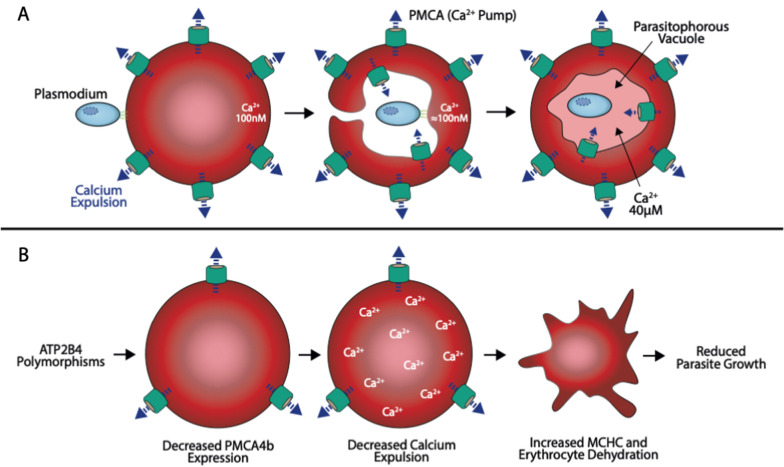
There are two possible models which could explain the mechanism by which the alterations in PMCA4b function could protect against severe falciparum malaria.
Model 1: Malaria parasite is starved of calcium: During the invasion of the merozoite into the RBC, there is an invagination of the erythrocyte plasma membrane such that the usually exterior RBC membrane, along with its receptors and transporters, faces into the parasitophorous vacuole (PV) and supplies the parasites’ calcium needs (Fig. 5A). Decreased calcium in the parasitophorous vacuole would decrease parasite viability.
Fig. 5.
A proposed model of how ATP2B4 variant haplotype could protect from malaria: A Illustration shows invagination of erythrocyte membrane during invasion and formation of the parasitized vacuole with the inverted PMCA4b. B A second proposed model of how ATP2B4 variant haplotype may protect from malaria: RBCs with variant ATP2B4 variant haplotype will have increased concentration of Ca2+ resulting to dehydration
Model 2 (proposed by Lessard et al. [15]): RBCs are dehydrated and at increased risk of lysis: The impaired PMCA4b expression and activity results in increased intraerythrocytic Ca2+ concentration which disrupts ion homeostasis of the RBC and results in its dehydration. Dehydration causes the cell to shrink and leads to premature lysis and clearance of malaria infected RBCs [28] (Fig. 5B). Either or a combination of these pathways might be responsible for the impairment of P. falciparum growth that we observed in RBCs with ATP2B4 variant haplotype.
The finding that P. falciparum growth in RBCs from homozygotes for the ATP2B4 variant, with decreased levels of PMCA4b is inconsistent with a recent publication by Villegas-Mendez et al. [39]. These authors show that if the ATP2B4 gene is ablated in a mouse model, there is no difference in the growth of Plasmodium berghei, Plasmodium yoelii or Plasmodium chabaudi following an in vivo malaria infection between wildtype mice and those without the ablated ATP2B4 gene. These finding presented here are consistent, however with a recently human study on P. falciparum published [40] that showed a decrease in parasite densities in malaria infected children with the protective ATP2B4 gene polymorphisms.
A key aim of malaria GWAS studies is to identify possible new targets for anti-malarial drug development [41]. As part of this effort and to provide proof of principle, we tested the PMCA4b inhibitor, aurintricarboxylic acid (ATA), which is under investigation as a blood pressure modulator [42]. These experiments reveal that the IC50 value for parasite inhibition was over 100 × higher than common drugs such as chloroquine and artemisinin. Because of PMCA4b’s central role in calcium homeostasis in multiple cell types [43–46], future work could be focused on the identification of an anti-malarial compound to block the erythrocyte-specific enhancer in ATP2B4 rather than the calcium transporter itself.
Conclusion
Taken together, these data are consistent with the conclusion that polymorphisms in ATP2B4 common in African populations and inherited as a large haplotype block, protect against severe malaria by controlling parasite density. Reduction in parasite density plays a pivotal role in disease outcome as it can ameliorate all of the multifaceted complications that lead to severe outcomes (including cerebral malaria, respiratory distress and severe anaemia) [48,49].
Although cytoadherence is critical to some of these complications, host immune response to the presence of the malaria parasite also plays an important role. For example, the reaction against the parasite pigment released during RBC lysis is the cause of the cyclic febrile episodes in malaria disease [49]. Additionally, the malaria pigment and hypoxia can contribute to the activation of cytokines which has a role in cerebral malaria and respiratory distress [26, 50]. Severe anaemia in malaria disease is due to loss of lysed cells by high levels of clearance of infected RBCs by the host immune system correlated with parasite density. Therefore, the reduction of parasite growth mediated by the variant ATP2B4 haplotype will likely diminish each of these risks and thus explain its association with severe malaria.
Acknowledgements
We thank the study donors and their families. We also thank the field and data teams at the Keneba field station, including Bakary Sonko, Foday Bah and Lamin Jatta for their support during the study.
Abbreviations
- ATA
Aurintricarboxylic acid
- CSA
Chondroitin sulphate
- DFID
UK Department for International Development
- GWAS
Genome-wide association study
- G6PD deficiency
Glucose-6-phosphate dehydrogenase deficiency
- LD
Linkage disequilibrium
- MRCG at LSHTM
Medical Research Council Unit The Gambia at London School of Hygiene and Tropical Medicine
- MFI
Mean fluorescence intensities
- RBCs
Red blood cells
- PMCA4
Plasma calcium membrane ATPase 4
- PMCA
Plasma membrane calcium ATPase
- UK MRC
UK Medical Research Council
Author contributions
FJ contributed to the conception of the project, design of experiments, development of the protocols, field and laboratory data collection, development of the data analysis plan, conducting the data analysis and drafting of the manuscript. EH and AJ contributed to the laboratory analyses and field data collection. AC contributed to the laboratory analyses. JC contributed to the data analysis. MA trained FJ in the adhesion and var2 expression assays and contributed to reagents. AMP contributed to the conception of the project, data analysis and the drafting of the manuscript. CC contributed to the conception of the study and obtained the funding. She was responsible for the overall development of the protocols, conducting the data analysis and the drafting of the manuscript. All authors reviewed the final manuscript prior to submission.
Funding
This work was supported by the Medical Research Council Unit The Gambia at London School of Hygiene and Tropical Medicine (MRCG at LSHTM) PhD fellowship. We acknowledge core funding to the MRC International Nutrition Group through MCA760-5QX00 from the UK Medical Research Council (UK MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement. The funding agency had no role in the design and conduct of the study, nor in the collection, management, analyses or interpretation of the data or in the preparation, review, or approval of the manuscript.
Availability of data and materials
All data will be made available to researchers upon reasonable request to the study PI and clearance by the MRCG Scientific Coordinating and Ethics Committees.
Declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/25/2023
A Correction to this paper has been published: 10.1186/s12936-023-04565-8
References
- 1.WHO. World malaria report 2020: 20 years of global progress and challenges. Geneva, World Health Organization; 2020; https://apps.who.int/iris/bitstream/handle/10665/337660/9789240015791-eng.pdf
- 2.Band G, Le QS, Jostins L, Pirinen M, Kivinen K, Jallow M, et al. Imputation-based meta-analysis of severe malaria in three African populations. PLoS Genet. 2013;9:e1003509. doi: 10.1371/journal.pgen.1003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackinnon MJ, Mwangi TW, Snow RW, Marsh K, Williams TN. Heritability of malaria in Africa. PLoS Med. 2005;2:e340. doi: 10.1371/journal.pmed.0020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timmann C, Thye T, Vens M, Evans J, May J, Ehmen C, et al. Genome-wide association study indicates two novel resistance loci for severe malaria. Nature. 2012;489:443–446. doi: 10.1038/nature11334. [DOI] [PubMed] [Google Scholar]
- 5.Network MGE, Network MGE. Reappraisal of known malaria resistance loci in a large multicenter study. Nat Genet. 2014;46:1197–1204. doi: 10.1038/ng.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Band G, Rockett KA, Spencer CCA, Kwiatkowski DP, Malaria Genomic Epidemiology Network A novel locus of resistance to severe malaria in a region of ancient balancing selection. Nature. 2015;526:253–7. doi: 10.1038/nature15390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ndila CM, Uyoga S, Macharia AW, Nyutu G, Peshu N, Ojal J, et al. Human candidate gene polymorphisms and risk of severe malaria in children in Kilifi, Kenya: a case-control association study. Lancet Haematol. 2018;5:e333–e345. doi: 10.1016/S2352-3026(18)30107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leffler EM, Band G, Busby GBJ, Kivinen K, Le QS, Clarke GM, Bojang KA, Conway DJ, Jallow M, Sisay-Joof F, Bougouma EC, Mangano VD, Modiano D, Sirima SB, Achidi E, Apinjoh TO, Marsh K, Ndila CM, Peshu N, Williams TN, Drakeley C, Manjurano A, Reyburn H, Riley E, Kachala D, Molyneux M, Nyirongo V, Taylor T, Thornton N, Tilley L, Grimsley S, Drury E, Stalker J, Cornelius V, Hubbart C, Jeffreys AE, Rowlands K, Rockett KA, Spencer CCA, Kwiatkowski DP, Malaria Genomic Epidemiology Network Resistance to malaria through structural variation of red blood cell invasion receptors. Science. 2017 doi: 10.1126/science.aam6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gouveia MH, Bergen AW, Borda V, Nunes K, Leal TP, Ogwang MD, et al. Genetic signatures of gene flow and malaria-driven natural selection in sub-Saharan populations of the “endemic Burkitt Lymphoma belt”. PLoS Genet. 2019;15:e1008027. doi: 10.1371/journal.pgen.1008027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Network MGE. Insights into malaria susceptibility using genome-wide data on 17,000 individuals from Africa Asia and Oceania. Nat Commun. 2019;10:5732. doi: 10.1038/s41467-019-13480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedu–Addo G, Meese S, Mockenhaupt FP. An ATP2B4 polymorphism protects against malaria in pregnancy. J Infect Dis. 2013 May 15;207(10):1600-3. 10.1093/infdis/jit070. Epub 2013 Feb 26. PMID: 23444010. [DOI] [PubMed]
- 12.Calì T, Brini M, Carafoli E. Regulation of cell calcium and role of plasma membrane calcium ATPases. Int Rev Cell Mol Biol. 2017;332:259–296. doi: 10.1016/bs.ircmb.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 14.Zámbó B, Várady G, Padányi R, Szabó E, Németh A, Langó T, et al. Decreased calcium pump expression in human erythrocytes is connected to a minor haplotype in the ATP2B4 gene. Cell Calcium. 2017;65:73–79. doi: 10.1016/j.ceca.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Lessard S, Gatof ES, Beaudoin M, Schupp PG, Sher F, Ali A, et al. An erythroid-specific ATP2B4 enhancer mediates red blood cell hydration and malaria susceptibility. J Clin Invest. 2017;127:3065–3074. doi: 10.1172/JCI94378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennig BJ, Unger SA, Dondeh BL, Hassan J, Hawkesworth S, Jarjou L, et al. Cohort profile: the Kiang West Longitudinal Population Study (KWLPS)-a platform for integrated research and health care provision in rural Gambia. Int J Epidemiol. 2017;46:e13. doi: 10.1093/ije/dyv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong K, Kuypers FA. Flow cytometric determination of PMCA-mediated Ca2+-extrusion in individual red blood cells. Cytometry A. 2007;71:693–699. doi: 10.1002/cyto.a.20429. [DOI] [PubMed] [Google Scholar]
- 18.Mwesigwa J, Okebe J, Affara M, Di Tanna GL, Nwakanma D, Janha O, et al. On-going malaria transmission in The Gambia despite high coverage of control interventions: a nationwide cross-sectional survey. Malar J. 2015;14:314. doi: 10.1186/s12936-015-0829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goheen MM, Bah A, Wegmüller R, Verhoef H, Darboe B, Danso E, et al. Host iron status and erythropoietic response to iron supplementation determines susceptibility to the RBC stage of falciparum malaria during pregnancy. Sci Rep. 2017;7:17674. doi: 10.1038/s41598-017-16896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moussa EM, Huang H, Thézénas ML, Fischer R, Ramaprasad A, Sisay-Joof F, et al. Proteomic profiling of the plasma of Gambian children with cerebral malaria. Malar J. 2018;17:337. doi: 10.1186/s12936-018-2487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janes JH, Wang CP, Levin-Edens E, Vigan-Womas I, Guillotte M, Melcher M, et al. Investigating the host binding signature on the Plasmodium falciparum PfEMP1 protein family. PLoS Pathog. 2011;7:e1002032. doi: 10.1371/journal.ppat.1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avril M, Cartwright MM, Hathaway MJ, Smith JD. Induction of strain-transcendent antibodies to placental-type isolates with VAR2CSA DBL3 or DBL5 recombinant proteins. Malar J. 2011;10:36. doi: 10.1186/1475-2875-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amambua-Ngwa A, Okebe J, Mbye H, Ceesay S, El-Fatouri F, Joof F, et al. Sustained ex vivo susceptibility of Plasmodium falciparum to artemisinin derivatives but increasing tolerance to artemisinin combination therapy partner quinolines in The Gambia. Antimicrob Agents Chemother. 2017;61:e00759–e817. doi: 10.1128/AAC.00759-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–3557. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamed TMA, Abou-Leisa R, Stafford N, Maqsood A, Zi M, Prehar S, et al. The plasma membrane calcium ATPase 4 signalling in cardiac fibroblasts mediates cardiomyocyte hypertrophy. Nat Commun. 2016;7:11074. doi: 10.1038/ncomms11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JD, Rowe JA, Higgins MK, Lavstsen T. Malaria’s deadly grip: cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2013;15:1976–1983. doi: 10.1111/cmi.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nisar S, Torres M, Thiam A, Pouvelle B, Rosier F, Gallardo F, et al. Identification of ATP2B4 regulatory element containing functional genetic variants associated with severe malaria. Int J Mol Sci. 2022;23:4849. doi: 10.3390/ijms23094849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogdanova A, Makhro A, Wang J, Lipp P, Kaestner L. Calcium in red blood cells-a perilous balance. Int J Mol Sci. 2013;14:9848–9872. doi: 10.3390/ijms14059848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas SLY, Bouyer G, Cueff A, Egée S, Glogowska E, Ollivaux C. Ion channels in human red blood cell membrane: actors or relics? Blood Cells Mol Dis. 2011;46:261–265. doi: 10.1016/j.bcmd.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Glushakova S, Lizunov V, Blank PS, Melikov K, Humphrey G, Zimmerberg J. Cytoplasmic free Ca2+ is essential for multiple steps in malaria parasite egress from infected erythrocytes. Malar J. 2013;12:41. doi: 10.1186/1475-2875-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adovelande J, Bastide B, Deleze J, Schrevel J. Cytosolic free calcium in Plasmodium falciparum-infected erythrocytes and the effect of verapamil: a cytofluorometric study. Exp Parasitol. 1993;76:247–258. doi: 10.1006/expr.1993.1030. [DOI] [PubMed] [Google Scholar]
- 32.Kramer R, Ginsburg H. Calcium transport and compartment analysis of free and exchangeable calcium in Plasmodium falciparum-infected red blood cells. J Protozool. 1991;38:594–601. [PubMed] [Google Scholar]
- 33.Withers-Martinez C, Strath M, Hackett F, Haire LF, Howell SA, Walker PA, et al. The malaria parasite egress protease SUB1 is a calcium-dependent redox switch subtilisin. Nat Commun. 2014;5:3726. doi: 10.1038/ncomms4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanabe K, Mikkelsen RB, Wallach DF. Calcium transport of Plasmodium chabaudi-infected erythrocytes. J Cell Biol. 1982;93:680–684. doi: 10.1083/jcb.93.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zipprer EM, Neggers M, Kushwaha A, Rayavara K, Desai SA. A kinetic fluorescence assay reveals unusual features of Ca++ uptake in Plasmodium falciparum-infected erythrocytes. Malar J. 2014;13:184. doi: 10.1186/1475-2875-13-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal S, Singh MK, Garg S, Chitnis CE, Singh S. Ca(2+) -mediated exocytosis of subtilisin-like protease 1: a key step in egress of Plasmodium falciparum merozoites. Cell Microbiol. 2013;15:910–921. doi: 10.1111/cmi.12086. [DOI] [PubMed] [Google Scholar]
- 37.Garg S, Agarwal S, Kumar S, Yazdani SS, Chitnis CE, Singh S. Calcium-dependent permeabilization of erythrocytes by a perforin-like protein during egress of malaria parasites. Nat Commun. 2013;4:1736. doi: 10.1038/ncomms2725. [DOI] [PubMed] [Google Scholar]
- 38.Brochet M, Billker O. Calcium signalling in malaria parasites. Mol Microbiol. 2016;100:397–408. doi: 10.1111/mmi.13324. [DOI] [PubMed] [Google Scholar]
- 39.Villegas-Mendez A, Stafford N, Haley MJ, Pravitasari NE, Baudoin F, Ali A, et al. The plasma membrane calcium ATPase 4 does not influence parasite levels but partially promotes experimental cerebral malaria during murine blood stage malaria. Malar J. 2021;20:297. doi: 10.1186/s12936-021-03832-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uyoga S, Watson JA, Wanjiku P, Rop JC, Makale J, Macharia AW, et al. The impact of malaria-protective red blood cell polymorphisms on parasite biomass in children with severe Plasmodium falciparum malaria. Nat Commun. 2022;13:3307. doi: 10.1038/s41467-022-30990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damena D, Denis A, Golassa L, Chimusa ER. Genome-wide association studies of severe P. falciparum malaria susceptibility: progress, pitfalls and prospects. BMC Med Genomics. 2019;12:120. doi: 10.1186/s12920-019-0564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis S, Little R, Baudoin F, Prehar S, Neyses L, Cartwright EJ, et al. Acute inhibition of PMCA4, but not global ablation, reduces blood pressure and arterial contractility via a nNOS-dependent mechanism. J Cell Mol Med. 2018;22:861–872. doi: 10.1111/jcmm.13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohamed TMA, Oceandy D, Zi M, Prehar S, Alatwi N, Wang Y, et al. Plasma membrane calcium pump (PMCA4)-neuronal nitric-oxide synthase complex regulates cardiac contractility through modulation of a compartmentalized cyclic nucleotide microdomain. J Biol Chem. 2011;286:41520–41529. doi: 10.1074/jbc.M111.290411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M, Ho PWL, Pang SYY, Tse ZHM, Kung MHW, Sham PC, et al. PMCA4 (ATP2B4) mutation in familial spastic paraplegia. PLoS One. 2014;9:e104790. doi: 10.1371/journal.pone.0104790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berrocal M, Marcos D, Sepúlveda MR, Pérez M, Avila J, Mata AM. Altered Ca2+ dependence of synaptosomal plasma membrane Ca2+-ATPase in human brain affected by Alzheimer’s disease. FASEB J. 2009;23:1826–1834. doi: 10.1096/fj.08-121459. [DOI] [PubMed] [Google Scholar]
- 46.Varga K, Pászty K, Padányi R, Hegedűs L, Brouland J-P, Papp B, et al. Histone deacetylase inhibitor- and PMA-induced upregulation of PMCA4b enhances Ca2+ clearance from MCF-7 breast cancer cells. Cell Calcium. 2014;55:78–92. doi: 10.1016/j.ceca.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Cserti-Gazdewich CM, Dhabangi A, Musoke C, Ssewanyana I, Ddungu H, Nakiboneka-Ssenabulya D, et al. Inter-relationships of cardinal features and outcomes of symptomatic pediatric Plasmodium falciparum malaria in 1,933 children in Kampala. Uganda Am J Trop Med Hyg. 2013;88:747–756. doi: 10.4269/ajtmh.12-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sypniewska P, Duda JF, Locatelli I, Althaus CR, Althaus F, Genton B. Clinical and laboratory predictors of death in African children with features of severe malaria: a systematic review and meta-analysis. BMC Med. 2017;15:147. doi: 10.1186/s12916-017-0906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newton CR, Hien TT, White N. Cerebral malaria. J Neurol Neurosurg Psychiatry. 2000;69:433–441. doi: 10.1136/jnnp.69.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olivier M, Van Den Ham K, Shio MT, Kassa FA, Fougeray S. Malarial pigment hemozoin and the innate inflammatory response. Front Immunol. 2014;5:25. doi: 10.3389/fimmu.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be made available to researchers upon reasonable request to the study PI and clearance by the MRCG Scientific Coordinating and Ethics Committees.