Abstract
Antibodies having light (L) chains encoded by the κII-A2 variable region gene segment predominate in the human response to the Haemophilus influenzae type b polysaccharide (Hib PS). To determine whether the closely related homologue of the A2 gene, the κII-A18 gene, has the potential to contribute to the repertoire, we examined Hib PS binding to a series of recombinant Fab fragments having either A2 or A18 L chains isolated from a Hib PS-vaccinated adult. The ability to bind Hib PS resided exclusively with those Fab fragments having A2 and containing an insertional arginine at the variable-joining junction. Thus, despite the sequence similarity between A2 and A18, only A2 contributes to the canonical Hib PS paratope.
Variable (V) regions of human immunoglobulin kappa light (L) chains are encoded by two homologous clusters of gene segments on chromosome 2. The cluster most proximal to the κ-constant locus contains 40 genes with all but 2 in the same transcriptional orientation as the κ-constant locus. The distal cluster consists of 36 genes in the opposite transcriptional orientation. This second cluster of κ-variable (Vκ) genes apparently arose through duplication of most of the proximal region (5). The two clusters are separated by 850 kb, and together they contain about 41 functional κ L-chain genes (reviewed in references 3 and 8).
As a result of the origin and organization of the Vκ locus, most Vκ genes exist as homologous pairs. The κII A2c/A18b V gene homologues differ at three bases within the coding region and can therefore be differentiated at the level of expression. This difference offers a unique opportunity to examine the contribution made by two recently divergent V genes to a protective human antibody response. Antibodies specific for the capsular polysaccharide (PS) of the human pathogen Haemophilus influenzae type b (Hib) commonly utilize V regions encoded by alleles of the A2 gene. These antibodies express an L-chain-associated idiotype known as HibId-1 (13) and are the predominant species in a majority of individuals following vaccination. Anti-Hib PS antibodies utilizing the A2 L chain have been isolated from serum (21) and hybridomas (1) and by phage display (18). In the present study, we sought to determine if, following vaccination, an individual known to express both A2 and A18 gene products utilizes A18 in the generation of antibodies specific for Hib PS.
Both A2 and A18 L-chain gene products express the HibId-1 idiotype. HibId-1 expression is independent of kappa joining region (Jκ) usage and heavy (H)-chain association. In a previous study, human B cells expressing A2 and A18 L chains were isolated from an adult immunized 5 days previously with Hib PS, and RNA from these cells was used to construct an expression library in pComb 3. A HibId-1+, Hib PS-specific Fab fragment designated Sol10 was isolated from this library and has been described previously (18). To generate the backcross library utilized in the present study, the H-chain fragment from Sol10 was ligated into an L-chain library containing both A2 and A18 L chains isolated from the same immunized donor. Forty clones were selected, screened for Fab fragment production by a capture enzyme-linked immunosorbent assay (ELISA), and assayed for Hib PS binding by a modified Farr assay (18). The sequence of the L-chain insert was then determined. The data are summarized in Table 1. The 40 clones analyzed in this study (representing 26 unique sequences) are all products of either the A2c or A18b Vκ gene. Hib PS binding clearly segregates with those Fab fragments utilizing A2c. Furthermore, Hib PS binding within the A2c population is restricted to those Fab fragments whose L chains express a nontemplated insertional arginine residue at the variable-joining (V-J) junction. All but one Hib PS-binding A2 rearrangement utilize Jκ1. The frequencies of mutations for the Hib PS-binding and nonbinding groups were similar.
TABLE 1.
L-chain CDR3 region and Hib PS binding of Fab clones
| Germ line | Clone | VLa | Residue at position:
|
Jκ | Hib PS bindingc | Mutations (M, R)d | ||
|---|---|---|---|---|---|---|---|---|
| 95ab | 96 | 97 | ||||||
| A18b | M Q G I H L P | |||||||
| BC1 | - - - - - - - | P | T | Jκ4 | − (142) | 1, 1 | ||
| BC2 | - - - - - - - | P | Y | I | Jκ2 | − (170) | 3, 1 | |
| BC6 | - - - - - - - | L | T | Jκ4 | − (90) | 1, 0 | ||
| BC13 | - - S - - - | I | T | Jκ5 | − (71) | 2, 1 | ||
| BC16 | - - - - - - - | I | T | Jκ3 | − (90) | 4, 2 | ||
| BC17 | - - - - F P - | L | T | Jκ4 | − (130) | 10, 5 | ||
| BC19 | - - - - - - - | W | T | Jκ1 | − (90) | 11, 6 | ||
| BC26 | - - - - - - | L | T | Jκ4 | − (142) | 2, 1 | ||
| BC30 | - - - - Y - - | P | T | Jκ4 | − (118) | 5, 2 | ||
| BC32 | - - - - - - - | I | T | Jκ3 | − (99) | 2, 1 | ||
| BC36 | - - - - - P - | Y | T | Jκ2 | − (217) | 3, 1 | ||
| A2c | M Q S I Q L P | |||||||
| BC7 | - - - - - P - | L | T | Jκ4 | − (130 | 1, 1 | ||
| BC9 | - - - - - - - | Y | T | Jκ2 | − (65) | 2, 1 | ||
| BC29 | - - - V - F - | R | T | Jκ1 | − (217) | 7, 4 | ||
| BC31 | - - - - - - - | L | T | Jκ4 | − (118) | 0, 0 | ||
| BC3 | - - - - - F - | R | W | T | Jκ1 | + | 7, 5 | |
| BC5 | - - - - - - - | R | W | T | Jκ1 | + | 3, 0 | |
| BC8 | - - - - - H - | R | W | T | Jκ1 | + | 4, 2 | |
| BC10 | - - - - - - - | R | W | T | Jκ1 | + | 7, 3 | |
| BC12 | - - - - - - - | R | W | T | Jκ1 | + | 6, 2 | |
| BC14 | - - T - - - - | R | F | T | Jκ3 | + | 3, 1 | |
| BC15 | - - - - - - - | R | W | T | Jκ1 | + | 5, 1 | |
| BC24 | - - - - - - - | R | W | T | Jκ1 | + | 4, 0 | |
| BC33 | - - - - - F - | R | W | T | Jκ1 | + | 9, 5 | |
| BC34 | - - - - - - - | R | W | T | Jκ1 | + | 9, 4 | |
| BC35 | - - - - - - - | R | W | T | Jκ1 | + | 6, 0 | |
Amino acid residues 89 to 95 of VL. Residues 89 to 95 of the respective germ line-encoded proteins are shown for comparison. A dash indicates identity with the germ line.
Junctional residue not encoded by either the 3′ Vκ gene segment or the 5′ Jκ segment.
All clones listed as negative bound ≤20% of the added counts per minute under polyvalent conditions at the maximum concentration tested (shown in micrograms per milliliter).
Total number of base changes (M) and amino acid replacements (R) compared to the germ line-encoded sequence over the entire V region. Purified phagemid DNA from each clone was sequenced directly with Sequenase version 2.0 (U.S. Biochemical Corp., Cleveland, Ohio).
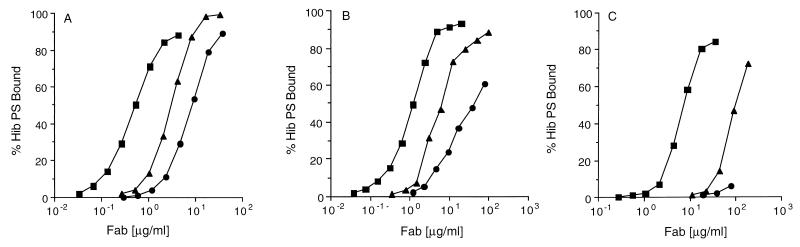
Since the binding potential of the A2 L chain is highly biased towards those rearrangements containing an insertional arginine and Jκ1, the question arose as to whether an A18 gene product would, if present with the same CDR-3 configuration, produce an L chain with the ability to bind Hib PS. To address this question, a Fab fragment whose A18 L chain had an arginine insertional residue was constructed by mutagenesis with unique-site elimination (4). The template plasmid encoded an A18b/proline/Jκ1 L chain isolated in this laboratory. This L chain was paired with the Sol10 H-chain fragment described above, and the ability of the resultant Fab fragment (designated A18R) to bind Hib PS was determined. Figure 1 shows the relative abilities of BC35 (A2/R/Jκ1), BC14 (A2/R/Jκ3), and A18R (A18/R/Jκ1) to bind radiolabeled Hib PS. Under permissive binding conditions, where the Fab fragments were polymerized with a polyclonal anti-kappa chain antibody (Fig. 1A), all three Fab fragments bound Hib PS, but with marked differences in avidity. BC35 required an approximately 6-fold-lower concentration of Fab fragments to achieve 50% binding than did the naturally occurring BC14 and about a 20-fold-lower concentration than did A18R. When Fab fragment dimers were produced by using a monoclonal anti-kappa chain antibody (Fig. 1B), the relative ranking of BC35 and BC14 was maintained, but the A18R Fab fragment required ≈35-fold more antibody than did BC35 to achieve 50% binding. Under monovalent conditions (i.e., no facilitating antibody [Fig. 1C]) avidity differences between BC35 and BC14 were more apparent (about 14-fold), and binding by A18R was only slightly above the background level at the maximum concentration tested.
FIG. 1.
Binding of radiolabeled Hib PS by BC35 (■), BC14 (▴), and A18R (•) under polyvalent (A), bivalent (B), and monovalent (C) conditions.
The availability of an anti-HibId-1 reagent allows A2 utilization to be examined in large numbers of samples. This reagent has been extensively utilized in characterizing the Hib PS-specific repertoire that develops in response to different vaccine formulations and in different age groups (6, 11, 12, 15). The sequence originally reported for A18a, the A2 homologue, contained a point mutation in the codon for residue 88 (where a conserved cysteine is located) that converted it to a stop codon (9). Since this L chain is not expressed, it cannot contribute to the HibId-1+ anti-Hib PS response. Atkinson et al. and Juul et al. (2, 7) have, however, recently described other alleles of A18 which lack this mutation and are therefore potentially functional genes. We have demonstrated that A18b is functionally expressed in peripheral blood and is recognized by the HibId-1-specific monoclonal antibody LuC9 (17). The A18b and A2c gene products differ at 1 residue in CDR2 (L2) and 2 residues in CDR3 (L3). The functional A18b allele has been found in all populations examined so far, although its prevalence varies in different ethnic groups (2, 7). Since A18b gene products are also HibId-1+, the idiotypic analysis of Hib PS-specific antibodies described above cannot discern between A2 and A18 use.
This report demonstrates that, at least in the individual we studied, the VκII A18b gene does not pair with the canonical VH26 VH to contribute to the Hib PS repertoire. Since multiple nonbinding A18b rearrangements were isolated, it is unlikely that our methodology is biased in favor of A2. It is possible that our sample size was not large enough to detect all A18 rearrangements. However, 73% of the A2 rearrangements bound Hib PS, and based on Jκ and junctional arginine codon use, this represents at least four independent rearrangement events. In addition, the nonbinding A2 and A18 isolates contain examples of all possible Jκ regions. These findings suggest that the A2c/A18b repertoire isolated from this individual constitutes a representative sample. We tested only a single VH region and cannot, therefore, discount the possibility that the A18b L chains described here would bind Hib PS if they were paired with some other VH region. If they would, this should manifest itself as a bias in the observed A18 rearrangements, such as the clear bias towards A2/R/Jκ1 rearrangements that we observed in the A2 isolates. No such bias towards a particular A18 rearrangement was found. Further, when the original expression library made from this individual was affinity selected on Hib PS, only Fab fragments utilizing the A2 L chain were isolated (18). It is possible that A18 could contribute to the Hib PS repertoire in individuals expressing allelic variants of A18 or in individuals lacking A2. The frequency of individuals homozygous for the deletion of the distal portion of the kappa locus (i.e., haplotype 11) has been estimated to be about 0.6% of the general population (19), and these individuals would, by definition, lack the A2 gene. One such individual has been analyzed, and HibId-1+ antibodies specific for Hib PS were not detected (20). It is not known whether this individual expressed A18.
By analogy to A2, the most likely A18 rearrangement to participate in Hib PS binding would be A18 paired with Jκ1 with an arginine residue inserted at the V-J junction. We constructed such an L chain and showed that under certain conditions of polymerization it forms a Hib PS-binding Fab fragment when paired with the Sol10 H chain. It is not known whether the affinity of this binding site for Hib PS is sufficient for B-cell triggering when it is present as the antigen-specific receptor on a B cell. Although the BC14 (A2c/R/Jκ3) clone isolated in this study binds Hib PS with a lower affinity than do A2c/R/Jκ1 isolates, it is assumed to have arisen as a result of immunization. Therefore, the affinity of BC14 places a limit on the receptor affinity required for signaling. As can be seen in Fig. 1, A18R bound Hib PS less efficiently than did BC14 under valency conditions most similar to those present on the cell surface. There remains the possibility, as mentioned before, that A18R, when paired with another H chain, would bind Hib PS with the same affinity as the A2c/R/Jκ1 clones. The fact that no A18b L chain with a junctional arginine was isolated from this vaccinated individual leads us to believe that this is not the case.
In this study we have also demonstrated the strong concordance of an arginine at the V-J junction of A2c rearrangements with Hib PS binding. This residue has been reported previously to be present in both A2 and non-A2 (10, 16) L chains of Hib PS-specific antibodies. We have recently shown that there is no absolute structural requirement for an arginine at this position (14). Its dominance may result from selection pressure shaping the Hib PS-specific repertoire of adults. It may be that the increased length of L3 is more predictive of binding potential than is the residue at position 95a. The predominance of Jκ1 in the Hib PS-binding clones suggests that the tryptophan at position 96 is important, as it is the only residue that varies between the Jκ segments within the CDR. Whether the poor binding of BC14 to Hib PS is due to its utilization of Jκ3, its deviation from germ line at residue 91, or a combination of the two is unknown.
Taken together, the apparent requirement for an insertional arginine, the bias towards Jκ1 use, and the fact that two of the three amino acid differences between A2c and A18b are in L3 suggest that the ability of the VH26-A2 canonical pair to bind Hib PS is highly dependent on the primary sequence of the L-chain CDR3. Mutation and modeling studies currently under way will further define the role of the junctional arginine in the binding of these Fab fragments to Hib PS. Our findings also suggest that the formation of the Hib PS-specific paratope most commonly utilized by both infants and adults depends on a rearrangement event involving the distal region of the kappa locus.
Nucleotide sequence accession numbers.
The sequences referred to in this report have been deposited in GenBank with the following accession numbers: U70027 (BC1), U70028 (BC2), AF049691 (BC3), AF049692 (BC5), AF049682 (BC6), AF049687 (BC7), AF049693 (BC8), AF049688 (BC9), AF049694 (BC10), AF049695 (BC12), AF049683 (BC13), AF049696 (BC14), AF049697 (BC15), U70029 (BC16), AF049684 (BC17), AF049685 (BC19), AF049698 (BC24), U70031 (BC26), AF049689 (BC29), U70032 (BC30), AF049690 (BC31), AF049686 (BC32), AF049699 (BC33), AF049700 (BC34), AF049701 (BC35), and U70030 (BC36).
Acknowledgments
This work was supported by Public Health Service grant AI-25008 from The National Institute of Allergy and Infectious Diseases.
We thank Carlos F. Barbas III and the Scripps Research Institute for providing us with the pComb3 and pComb 3H vectors.
REFERENCES
- 1.Adderson E E, Shackelford P G, Insel R A, Quinn A, Wilson P M, Carroll W L. Immunoglobulin light chain variable region gene sequences for human antibodies to Haemophilus influenzae type b capsular polysaccharide are dominated by a limited number of V kappa and V lambda segments and VJ combinations. J Clin Investig. 1992;89:729–738. doi: 10.1172/JCI115649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson M J, Cowan M J, Feeney A J. New alleles of IGKV genes A2 and A18 suggest significant human IGKV locus polymorphism. Immunogenetics. 1996;44:115–120. [PubMed] [Google Scholar]
- 3.Cox J P, Tomlinson I M, Winter G. A directory of human germ-line V kappa segments reveals a strong bias in their usage. Eur J Immunol. 1994;24:827–836. doi: 10.1002/eji.1830240409. [DOI] [PubMed] [Google Scholar]
- 4.Deng W P, Nickoloff J A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992;200:81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- 5.Ermert K, Mitlohner H, Schempp W, Zachau H G. The immunoglobulin kappa locus of primates. Genomics. 1995;25:623–629. doi: 10.1016/0888-7543(95)80003-5. [DOI] [PubMed] [Google Scholar]
- 6.Granoff D M, Shackelford P G, Holmes S J, Lucas A H The Collaborative Vaccine Study Group. Variable region expression in the antibody responses of infants vaccinated with Haemophilus influenzae type b polysaccharide-protein conjugates. Description of a new lambda light chain-associated idiotype and the relation between idiotype expression, avidity, and vaccine formulation. J Clin Investig. 1993;91:788–796. doi: 10.1172/JCI116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juul L, Hougs L, Andersen V, Garred P, Ryder L, Svejgaard A, Hogh B, Lamm L, Graugaard B, Barington T. Population studies of the human V kappa A18 gene polymorphism in Caucasians, blacks and Eskimos. New functional alleles and evidence for evolutionary selection of a more restricted antibody repertoire. Tissue Antigens. 1997;49:595–604. doi: 10.1111/j.1399-0039.1997.tb02807.x. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Jaenichen R, Zachau H G. Expressed human immunoglobulin kappa genes and their hypermutation. Eur J Immunol. 1993;23:3248–3262. doi: 10.1002/eji.1830231231. [DOI] [PubMed] [Google Scholar]
- 9.Lautner R A, Huber C, Meindl A, Pargent W, Schäble K F, Thiebe R, Zocher I, Zachau H G. The human immunoglobulin kappa locus. Characterization of the duplicated A regions. Eur J Immunol. 1992;22:1023–1029. doi: 10.1002/eji.1830220422. [DOI] [PubMed] [Google Scholar]
- 10.Lucas A H. Genetic basis and somatic evolution of a human polysaccharide-specific antibody repertoire. In: Zouali M, editor. Human B cell superantigens. Heidelberg, Germany: Springer-Verlag; 1996. pp. 121–143. [Google Scholar]
- 11.Lucas A H, Azmi F H, Mink C M, Granoff D M. Age-dependent V region expression in the human antibody response to the Haemophilus influenzae type b polysaccharide. J Immunol. 1993;150:2056–2061. [PubMed] [Google Scholar]
- 12.Lucas A H, Granoff D M. A major crossreactive idiotype associated with human antibodies to the Haemophilus influenzae b polysaccharide. Expression in relation to age and immunoglobulin G subclass. J Clin Investig. 1990;85:1158–1166. doi: 10.1172/JCI114548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas A H, Langley R J, Granoff D M, Nahm M H, Kitamura M Y, Scott M G. An idiotypic marker associated with a germ-line encoded kappa light chain variable region that predominates the vaccine-induced human antibody response to the Haemophilus influenzae b polysaccharide. J Clin Investig. 1991;88:1811–1818. doi: 10.1172/JCI115502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas A H, Moulton K D, Reason D C. Role of κII-A2 light chain CDR-3 junctional residues in human antibody binding to the Haemophilus influenzae type b polysaccharide. J Immunol. 1998;161:3776–3780. [PubMed] [Google Scholar]
- 15.Lucas A H, Reason D C. Aging and the immune response to the Haemophilus influenzae type b capsular polysaccharide: retention of the dominant idiotype and antibody function in the elderly. Infect Immun. 1998;66:1752–1754. doi: 10.1128/iai.66.4.1752-1754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reason D C, Lucas A H. Content and dynamics of the human antibody variable region repertoire to the Haemophilus influenzae type b polysaccharide. Springer Semin Immunopathol. 1993;15:119–137. doi: 10.1007/BF00201096. [DOI] [PubMed] [Google Scholar]
- 17.Reason D C, Lucas A H. Functional expression of the IGKV A18b gene and its idiotypic cross-reactivity with the A2 variable region. Immunogenetics. 1997;45:343–344. doi: 10.1007/s002510050214. [DOI] [PubMed] [Google Scholar]
- 18.Reason D C, Wagner T C, Lucas A H. Human Fab fragments specific for the Haemophilus influenzae b polysaccharide isolated from a bacteriophage combinatorial library use variable region gene combinations and express an idiotype that mirrors in vivo expression. Infect Immun. 1997;65:261–266. doi: 10.1128/iai.65.1.261-266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaible G, Rappold G A, Pargent W, Zachau H G. The immunoglobulin kappa locus: polymorphism and haplotypes of Caucasoid and non-Caucasoid individuals. Hum Genet. 1993;91:261–267. doi: 10.1007/BF00218268. [DOI] [PubMed] [Google Scholar]
- 20.Scott M G, Crimmins D L, McCourt D W, Chung G, Schable K F, Thiebe R, Quenzel E M, Zachau H G, Nahm M H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. IV. The less frequently expressed VL are heterogeneous. J Immunol. 1991;147:4007–4013. [PubMed] [Google Scholar]
- 21.Scott M G, Crimmins D L, McCourt D W, Zocher I, Thiebe R, Zachau H G, Nahm M H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. III. A single VκII gene and one of several Jκ genes are joined by an invariant arginine to form the most common L chain V region. J Immunol. 1989;143:4110–4116. [PubMed] [Google Scholar]