Abstract
INTRODUCTION:
In alcoholic hepatitis (AH), high interleukin (IL)-22 production is associated with disease improvement, purportedly through enhanced infection resistance and liver regeneration. IL-22 binding protein (BP) binds and antagonizes IL-22 bioactivity, but data on IL-22BP in liver disease suggest a complex interplay. Despite the scarcity of human data, IL-22 is in clinical trial as treatment of AH. We, therefore, in patients with AH, described the IL-22 system focusing on IL-22BP and associations with disease course, and mechanistically pursued the human associations in vitro.
METHODS:
We prospectively studied 41 consecutive patients with AH at diagnosis, days 7 and 90, and followed them for up to 1 year. We measured IL-22 pathway proteins in liver biopsies and blood and investigated IL-22BP effects on IL-22 in hepatocyte cultures.
RESULTS:
IL-22BP was produced in the gut and was identifiable in the patients with AH' livers. Plasma IL-22BP was only 50% of controls and the IL-22/IL-22BP ratio thus elevated. Consistently, IL-22-inducible genes were upregulated in AH livers at diagnosis. Low plasma IL-22BP was closely associated with high 1-year mortality. In vitro, IL-22 stimulation reduced IL-22 receptor (R) expression, but coincubation with IL-22BP sustained IL-22R expression. In the AH livers, IL-22R mRNA expression was similar to healthy livers, although IL-22R liver protein was higher at diagnosis.
DISCUSSION:
Plasma IL-22BP was associated with an adverse disease course, possibly because its low level reduces IL-22R expression so that IL-22 bioactivity was reduced. This suggests the IL-BP interplay to be central in AH pathogenesis, and in future treatment trials (see Visual abstract, Supplementary Digital Content 5, http://links.lww.com/CTG/A338).
INTRODUCTION
Alcoholic hepatitis (AH) carries a high mortality (1). The current, but controversial treatment with corticosteroids, has uncertain effects on survival, but increases the risk of infections (2–4). New treatment strategies are urgently needed, and interleukin (IL)-22 is a promising candidate.
The bioactivity of IL-22 is regulated by its binding protein (BP) IL-22BP, which has an off-rate 1,000× that of the surface bound receptor (5). Production of IL-22BP is detected in dendritic cells, eosinophils, and T cells in the gut and can be inhibited by components of the inflammasome, which is highly activated in AH (6–9). IL-22BP is proposed to act as a rheostat to tightly regulate IL-22 functions (10). However, data on the role of IL-22BP in liver diseases are few and conflicting. Gene variants of IL-22BP associated with high IL-22BP expression are related to the development of severe fibrosis in schistosomiasis and hepatitis C, whereas IL-22BP knock-out mice are more susceptible to acute liver injury (11,12).
Regarding AH, a high number of IL-22-producing T cells is associated with disease improvement, which may be related to both improved resistance to infection and repair of the liver (13,14). Based on animal studies, the effects are proposed to be IL-22-dependent production of antimicrobial peptides such as lipocalin-2 and mitogenic and antiapoptotic gene induction (15–18). Whether these are also the mechanisms of action of IL-22 in human beings and particularly in AH has not been explored.
IL-22 signals via its surface-bound IL-22 receptor (R)-IL10R2 complex, which is almost exclusively expressed on epithelial-derived cells such as hepatocytes (17). This activates the signal transducer and activator of transcription (STAT)3 and its inhibitor suppressor of cytokine signaling (SOCS)3 (19).
Given this background, we conducted a series of human and in vitro experiments: the former to describe the components of the IL-22 system and associations to clinical outcomes and the latter to mechanistically explore the human findings. We hypothesized that low IL-22BP and high IL-22 were associated with a favorable outcome, that IL-22 pathway proteins were induced and that IL-22BP would block IL-22 mediated effects.
METHODS
Study design and population
In this prospective cohort study, the patients with AH were diagnosed at and consecutively recruited from the Department of Hepatology and Gastroenterology, Aarhus University Hospital, Denmark between March 2013 and December 2017. Diagnosis was based on the following criteria: a history of excessive alcohol consumption for men >50 g/d and women >40 g/d (mean intake men: 120 g/d [SD 60] and women: 121 g/d [SD 82]) with a period of abstinence of less than 4 weeks leading up to disease presentation, acute jaundice with presentation within the previous 2 weeks and with serum bilirubin > 80 μmol/L, and age between 18 and 75 years. Liver biopsy was performed in 25 cases (see Supplementary Digital Content 1, http://links.lww.com/CTG/A325). Exclusion criteria were other underlying liver disease, hepatocellular carcinoma, gallstones, uncontrolled infection, upper gastrointestinal bleeding, or immunomodulatory therapy within the past 8 weeks. None of the patients had psoriasis or inflammatory bowel disease. Infection screening was performed on admission and included clinical examination, chest x-ray, analyses of urine, culture of blood and ascites (when present), and other individual tests if indicated. If infection was diagnosed, patients were treated with antibiotics and included once infection was controlled. According to local clinical guidelines at the time of study, patients were treated medically with either Pentoxifylline (before 2015) or prednisolone when their Glasgow Alcoholic Hepatitis Score (GAHS) was 9 or above. Blood was sampled from patients with AH on the day of diagnosis, on days 7 and on day 90 after diagnosis, and the patients were followed for 1 year.
Controls
For the measurement of soluble proteins, control groups were healthy individuals (n = 10) and patients with stable alcoholic cirrhosis (n = 15) from the department's biobank. In addition, patients with nonalcoholic fatty liver (n = 12) and nonalcoholic steatohepatitis (n = 13) were included (20). Moreover, a cohort of patients with cirrhosis (n = 29) undergoing transjugular intrahepatic portosystemic shunt was acquired from Sapienza University of Rome for comparison of peripheral vein and portal vein plasma. Healthy liver tissue for RNA sequencing was obtained from the healthy rim of liver resectates during operations for metastatic colon cancer at the Department of Abdominal Surgery, Aarhus University Hospital, and for immunohistochemistry, from the pathologist biobank at Aarhus University Hospital. Written informed consent was obtained before inclusion in the study, and the study was conducted in accordance with the Helsinki declaration. The study was approved by the Central Denmark Region Ethical Committee (j.no. 1-10-72-40-13) and the Danish Data Protection Agency and registered at clinicaltrials.gov (NCT01918462).
ELISA of IL-22, IL-22BP, and lipocalin-2
Commercially available ELISA kits (IL-22 [ThermoFisher Scientific], IL-22BP [Raybio, Norcross], and lipocalin-2 [R&D, Abingdon, UK] were tested and optimized to avoid the influence of heterophilic antibodies (21). Spiking the IL-22 and IL-22BP ELISAs with recombinant IL-22BP and IL-22, respectively, did not change the measured protein concentration, suggesting that we were in fact measuring total IL-22 and IL-22BP. We therefore report the calculated IL-22/IL-22BP ratio as a measure of free IL-22.
Histology and immunohistochemistry
Liver biopsies (n = 25) were scored by an experienced liver pathologist according to the Alcoholic Hepatitis Histologic Score (22). Immunohistochemistry was performed using the fully automated VENTANA BenchMark ULTRA staining system (Ventana Medical Systems, Tucson, AZ) with antigen retrieval at high pH using ULTRA Cell Conditioning (Ventana Medical Systems). Incubation was performed with optimized concentrations of the following antibodies IL-22BP (Clone #214518, R&D Systems, Minneapolis, MN, 1:500), IL-22R (HPA042399, Sigma-Aldrich, St. Louis, MO, 1:500), and lipocalin-2 (HPA002695, Sigma-Aldrich, 1:200) for 32 minutes, followed by peroxidase staining and detection with Optiview DAB (Ventana Medical Systems). A range of IL-22 antibodies were tested without successful results.
RNA sequencing of liver biopsies
RNA was extracted from snap-frozen liver biopsies (n = 21) and stored at −80 °C until processing. RNA was isolated using the NucleoSpin kit (MACHEREY-NAGEL, Düren, Germany), and purified RNA from each sample was used to generate a copy DNA library using the NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA). Copy DNA libraries were then sequenced on a NextSeq 500 using NextSeq 500/550 High Output Kit V2 (Illumina, San Diego, CA). The sequencing data were aligned to the human genome from the Ensembl database using the Spliced Transcripts Alignment to a Reference software (PMID: 23104886).
In vitro set up and qPCR
HepG2 cells were cultured in Dulbecco's Modified Eagle's Medium, low glucose (ThermoFisher Scientific) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (ThermoFisher Scientific), and 10% heat-inactivated, sterile-filtered human AB serum for at least 1 week before stimulation. Primary human hepatocytes (pooled, 5 donors) were purchased from ThemoFischer Scientific and cultured according to the suppliers' instructions. Before the day of stimulation, the hepatocytes were seeded in 12- or 24-well plates at a concentration of 600.000–700.000 hepatocytes/mL. Confluency of greater than 70% was verified before stimulation with rIL22 BP (11025-H08H, Sino Biological Inc, Wayne, PA) and/or rIL-22 (200-22-10, PeproTech, TriChem ApS, Skanderborg, Denmark). Hepatocytes were harvested, and RNA extracted using the E.N.Z.A. Total RNA kit (Omega Bio-Tek, Norcross, GA). Copy DNA was synthesized using OligDT primers and the Thermo Scientific Hybaid PCR Express (ThermoFisher Scientific). Quantitative polymerase chain reaction was performed on the StepOnePlus Real-Time PCR system (Applied Biosystems, ThermoFisher Scientific) using SYB(R) (Synergy Brands) Green detection with optimized primers and Glyceraldehyde 3-phospahte dehydrogenase as reference gene. Gene expression is reported as fold change relative to unstimulated hepatocytes. All stimulations were run in duplicates or triplicates and repeated at least twice (for additional methods see, Supplementary Digital Content 2, http://links.lww.com/CTG/A326).
Statistics
Data were log-transformed to obtain normal distribution and among groups comparisons performed by a T-test (2 groups) or analysis of variance (>2 groups). A Kaplan-Meier survival analysis was used to assess the impact of plasma IL-22BP levels at diagnosis on mortality. A cutoff of 225 ng/mL was chosen because it defined the lower limit of the SD for the healthy controls. For correlation analyses, the Pearson correlation coefficient was calculated. Statistics for differential gene expression was calculated with the R-package DESeq2 (PMID: 25516281). Expression values are reported as reads per kilobase of transcript per million reads mapped. A P-value of below 0.05 was considered statistical significant.
RESULTS
IL-22BP is present in the human liver
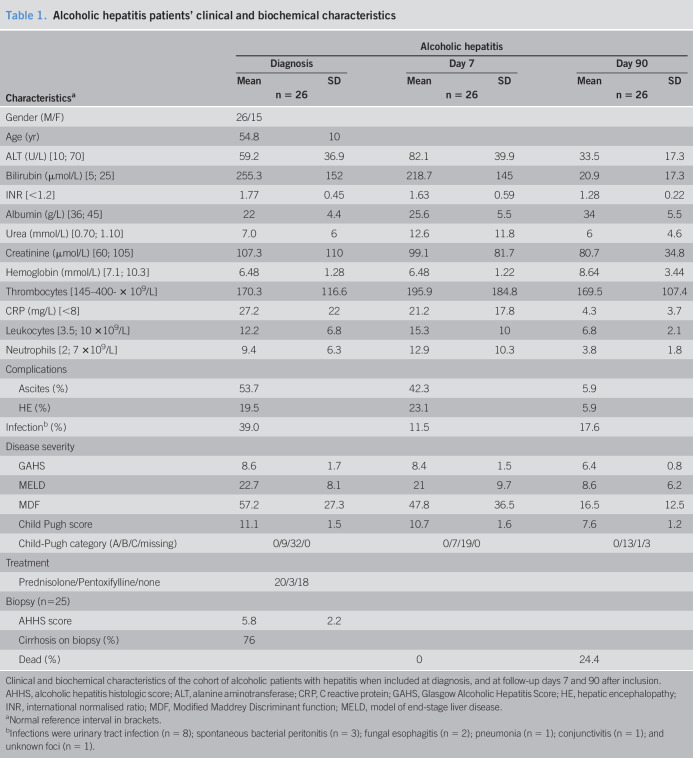
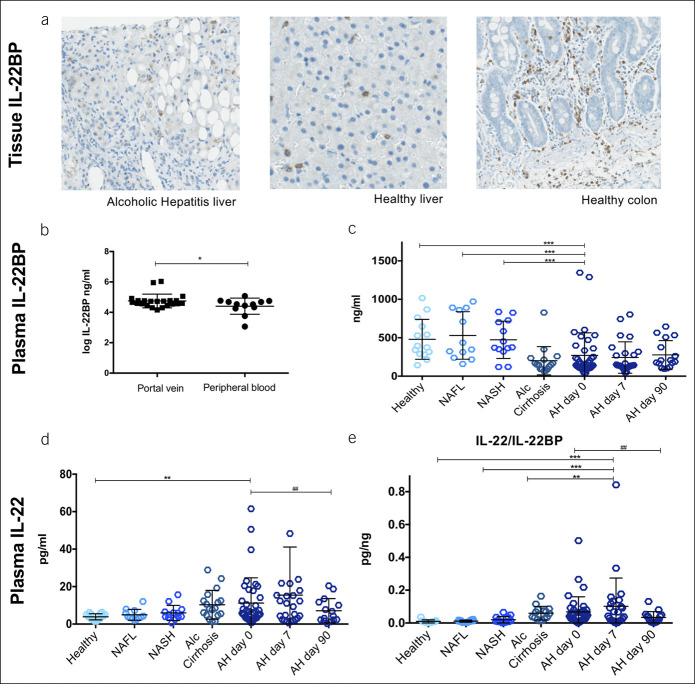
Clinical and biochemical characteristics of the patients with AH are presented in Table 1. By immunohistochemical staining, IL-22BP was found in livers, both from patients with AH and from healthy controls. Positive signals were found in nonhepatocytes that corresponded to leukocytes (Figure 1a). IL-22BP mRNA was not detected in patient nor control livers (Table 2).
Table 1.
Alcoholic hepatitis patients' clinical and biochemical characteristics
| Characteristicsa | Alcoholic hepatitis | |||||
| Diagnosis | Day 7 | Day 90 | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| n = 26 | n = 26 | n = 26 | ||||
| Gender (M/F) | 26/15 | |||||
| Age (yr) | 54.8 | 10 | ||||
| ALT (U/L) [10; 70] | 59.2 | 36.9 | 82.1 | 39.9 | 33.5 | 17.3 |
| Bilirubin (μmol/L) [5; 25] | 255.3 | 152 | 218.7 | 145 | 20.9 | 17.3 |
| INR [<1.2] | 1.77 | 0.45 | 1.63 | 0.59 | 1.28 | 0.22 |
| Albumin (g/L) [36; 45] | 22 | 4.4 | 25.6 | 5.5 | 34 | 5.5 |
| Urea (mmol/L) [0.70; 1.10] | 7.0 | 6 | 12.6 | 11.8 | 6 | 4.6 |
| Creatinine (μmol/L) [60; 105] | 107.3 | 110 | 99.1 | 81.7 | 80.7 | 34.8 |
| Hemoglobin (mmol/L) [7.1; 10.3] | 6.48 | 1.28 | 6.48 | 1.22 | 8.64 | 3.44 |
| Thrombocytes [145–400- × 109/L] | 170.3 | 116.6 | 195.9 | 184.8 | 169.5 | 107.4 |
| CRP (mg/L) [<8] | 27.2 | 22 | 21.2 | 17.8 | 4.3 | 3.7 |
| Leukocytes [3.5; 10 ×109/L] | 12.2 | 6.8 | 15.3 | 10 | 6.8 | 2.1 |
| Neutrophils [2; 7 ×109/L] | 9.4 | 6.3 | 12.9 | 10.3 | 3.8 | 1.8 |
| Complications | ||||||
| Ascites (%) | 53.7 | 42.3 | 5.9 | |||
| HE (%) | 19.5 | 23.1 | 5.9 | |||
| Infectionb (%) | 39.0 | 11.5 | 17.6 | |||
| Disease severity | ||||||
| GAHS | 8.6 | 1.7 | 8.4 | 1.5 | 6.4 | 0.8 |
| MELD | 22.7 | 8.1 | 21 | 9.7 | 8.6 | 6.2 |
| MDF | 57.2 | 27.3 | 47.8 | 36.5 | 16.5 | 12.5 |
| Child Pugh score | 11.1 | 1.5 | 10.7 | 1.6 | 7.6 | 1.2 |
| Child-Pugh category (A/B/C/missing) | 0/9/32/0 | 0/7/19/0 | 0/13/1/3 | |||
| Treatment | ||||||
| Prednisolone/Pentoxifylline/none | 20/3/18 | |||||
| Biopsy (n=25) | ||||||
| AHHS score | 5.8 | 2.2 | ||||
| Cirrhosis on biopsy (%) | 76 | |||||
| Dead (%) | 0 | 24.4 | ||||
Clinical and biochemical characteristics of the cohort of alcoholic patients with hepatitis when included at diagnosis, and at follow-up days 7 and 90 after inclusion.
AHHS, alcoholic hepatitis histologic score; ALT, alanine aminotransferase; CRP, C reactive protein; GAHS, Glasgow Alcoholic Hepatitis Score; HE, hepatic encephalopathy; INR, international normalised ratio; MDF, Modified Maddrey Discriminant function; MELD, model of end-stage liver disease.
Normal reference interval in brackets.
Infections were urinary tract infection (n = 8); spontaneous bacterial peritonitis (n = 3); fungal esophagitis (n = 2); pneumonia (n = 1); conjunctivitis (n = 1); and unknown foci (n = 1).
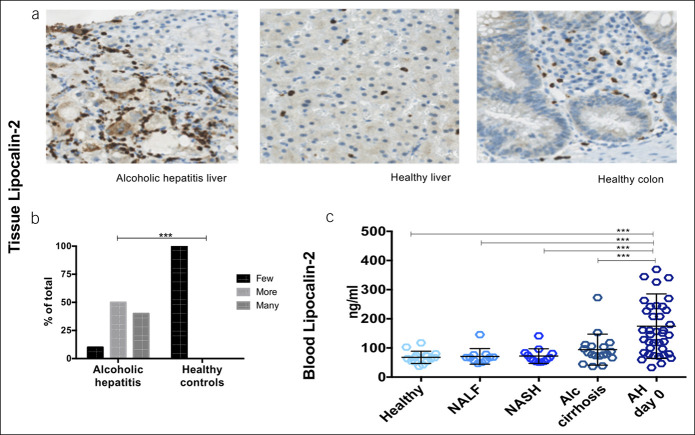
Figure 1.
IL-22BP is present in the liver and produced in the gut, and plasma levels are markedly reduced. (a) Immunohistochemical staining for IL-22BP in paraffin-embedded sections of liver from patients with alcoholic hepatitis and healthy liver and colon. Representative sections displayed. (b) Plasma IL-22BP by ELISA from the portal vein and peripheral blood in a cohort of patients with cirrhosis and in (c–e) peripheral plasma from patients with alcoholic hepatitis (AH) and controls; nonalcoholic fatty liver (NAFL) and steatohepatitis (NASH) and stable alcoholic (Alc) cirrhosis (analysis of variance, T-test, *P < 0.05,**P < 0.01, ***P < 0.001, bars represent means and SDs).
Table 2.
RNA sequencing of liver biopsies
| Gene | HL | AH | AH vs HL | P |
| Median (IQR)a | Median (IQR) | Log2-Expression (fold change) | ||
| Signaling | ||||
| IL10RB | 4.66(4.63; 6.63) | 12.38(10.10; 14.36) | 1.223 | <0.001 |
| SOCS3 | 18.06(2.87; 57.13) | 5.94(4.7; 7.47) | −2.537 | <0.001 |
| STAT3 | 18.55(15.49; 19.86) | 22.62(20.16; 27.36) | 0.472 | <0.05 |
| Antimicrobial peptides | ||||
| LCN2 | 0.87(0.78; 1.01) | 86.55(38.47; 121.88) | 6.394 | <0.001 |
| SAA1 | 410.29(180.56; 875.51) | 2080.21(628.32; 3,665.59) | 1.99 | <0.01 |
| SAA2 | 61.06(20.62; 72.66) | 198.84(72.09; 369.72) | 2.107 | <0.001 |
| Regeneration | ||||
| BCL2 | 0.43(0.28; 0.52) | 1.12(0.88; 1.27) | 1.369 | <0.001 |
| CCND1 | 19.56(8.62; 35.82) | 46.35(29.46; 51.81) | 0.811 | <0.05 |
| IL22RA2 | Not detectable | Not detectable | ||
| IL22 | Not detectable | Not detectable | ||
Total RNA was isolated from liver tissue from the healthy residual liver (HL) resectates from patients with metastatic colon cancer and from liver biopsies from patients with alcoholic hepatitis (AH) and RNA sequencing was performed.
Expression level in reads per kilobase of transcript per million reads mapped (RPKM) (median [IQR]).
The gut produces IL-22BP
Previous studies identified leukocyte subsets in the gut as the main IL-22BP producers. We, therefore, studied a cohort of patients with cirrhosis undergoing transjugular intrahepatic portosystemic shunt and verified a plasma IL-22BP concentration downgradient with higher IL-22BP in portal blood than in the blood from the systemic circulation (Figure 1b). Opposite, no gradient for IL-22 was present (data not shown).
Low plasma IL-22BP and high IL-22/IL-22BP ratio
Plasma IL-22BP was markedly lower in patients with AH compared with healthy controls, nonalcoholic fatty liver, and nonalcoholic patients with steatohepatitis (Figure 1c). In the patients with AH, plasma IL-22BP remained low on day 90 after entry (Figure 1c). In the livers, we found no IL-22 by immunohistochemistry and no IL-22 transcripts by RNA sequencing. The patients with AH had higher entry plasma IL-22 than healthy controls, but this difference leveled out by day 90 (Figure 1d). Consequently, the plasma IL-22/IL-22BP ratio was increased in AH, and although the ratio decreased significantly from entry to day 90, it remained higher than in healthy controls (Figure 1e).
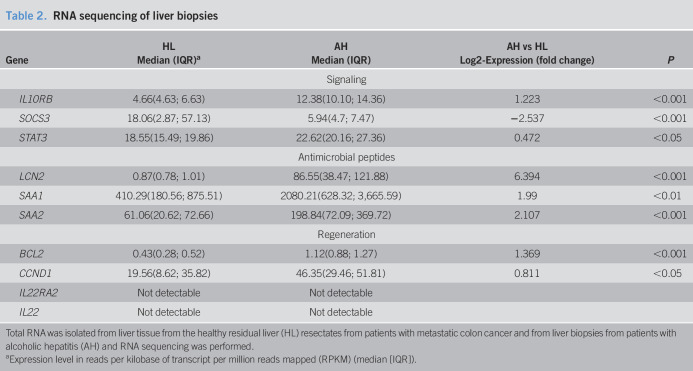
Low IL-22BP was associated with higher mortality and more severe disease
A low-study-entry plasma IL-22BP was associated with markedly poorer 1-year survival (Figure 2a). Similarly, patients with AH who died before day 90 had significantly lower IL-22BP at day 7 than those still alive (Figure 2b). Low entry IL-22BP was associated with higher entry disease severity as measured by the model of end-stage liver disease (MELD) (r = −0.35, P < 0.05) and GAHS (r = −0.32, P < 0.05) but lower circulating leucocyte counts (r = 0.35, P < 0.05) and IL-8 (r = 0.33, P = 0.06). Patients with low IL-22BP had lower study-entry thrombocyte counts (132.5 × 10^−9/L [71.4] vs 247.2 × 10^−9/L [41.9], P = 0.0043) higher international normalised ratio (1.90 [0.39] vs 1.52 [0.35], P = 0.005), and lower hemoglobin (6.06 [1.16] vs 7.23 [0.36], P = 0.01). In addition, the IL-22BP gene's fibrosis-related (the nucleotide bases) guanine-guanine genotype in single nucleotide polymorphisms (rs6570136) was associated with MELD and GAHS (P < 0.05). Neither IL-22 nor the IL-22/IL-22BP ratio was related to mortality, disease severity, liver leukocyte infiltration, or proinflammatory cytokines in plasma. There was no association between gene variants in the IL-22BP gene and plasma IL-22BP that could explain the low IL-22BP (data not shown).
Figure 2.
Low IL-22BP at diagnosis is associated with 1-year mortality. Plasma IL-22BP by ELISA. (a) Kaplan-Meier survival curve of all patients with alcoholic hepatitis stratified by plasma IL-22BP at diagnosis above or below 225 ng/mL (Log-rank test: P = 0.037). (b) The plasma IL-22BP at day 7 was compared between alcoholic hepatitis patients, who had died by day 90 and those still alive (T-test, bars display mean and SD, *P < 0.05). BP, binding protein; IL, interleukin.
Expression of IL-22-inducible liver genes in AH
In accordance with the high IL-22/IL-22BP ratio, STAT3 expression was upregulated in the livers of the patients with AH and the inhibitor SOCS3 was downregulated (Table 2). In addition, the IL-22-inducible antimicrobial genes LCN2 and SAAs and regenerative genes BCL2 and CCND1 were upregulated as evidence of active IL-22 signaling. We validated these RNA sequencing data for lipocalin-2 and showed increased protein in the AH livers (Figure 3a, b P < 0.001) and elevated protein in blood compared with all control groups at study entry (Figure 3c). In addition, the predominant fraction of lipocalin-2 in blood was the IL-22-inducible, epithelial-derived monomeric form (see Supplementary Digital Content 3, http://links.lww.com/CTG/A327).
Figure 3.
Upregulation of lipocalin-2 in liver and blood. (a and b) Immunohistochemical staining for lipocalin-2 in livers of patients with alcoholic hepatitis (AH) compared with healthy controls (Fisher exact test, P < 0.001). (c) Blood lipocalin-2 by ELISA in patients with alcoholic hepatitis, healthy controls, nonalcoholic fatty liver (NAFL) and steatohepatitis (NASH) and from stable alcoholic (Alc) cirrhosis patients (T-test, bars represent means and SDs, *P < 0.05, **P < 0.01).
IL-22 reduces the expression of the IL-22 receptor, and IL-22BP reverts this reduction
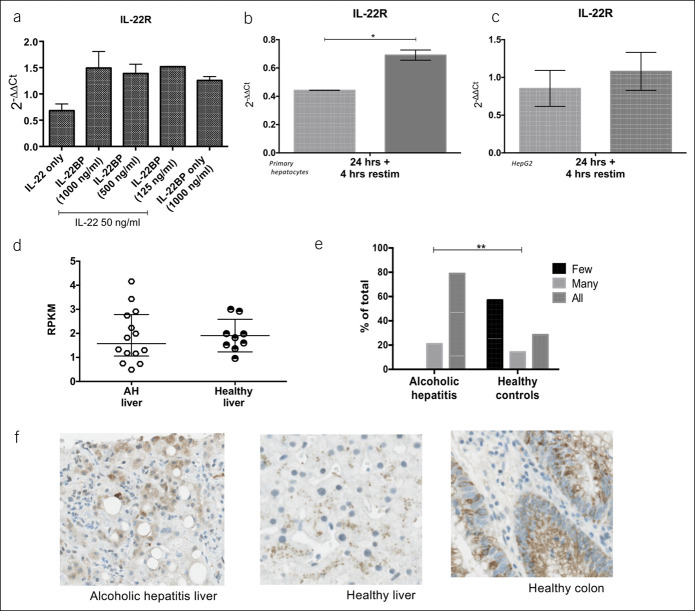
To directly approach the effects of low IL-22BP, we coincubated HepG2 cells with IL-22 and varying concentrations of IL-22BP for 4 and 24 h. In vitro, IL-22BP levels comparable with those detected in vivo effectively blocked IL-22 mediated induction of LCN2, SAA1-2, BCL2, STAT3, and SOCS3 at both time points (see Supplementary Digital Content 4, http://links.lww.com/CTG/A328). In the HepG2 cells, IL-22 decreased the transcription of its own IL-22R gene, and IL-22BP supplementation blocked this (Figure 4a). To mimic in vivo conditions, we stimulated with either IL-22 alone or IL-22 in combination with IL-22BP for 24 hours and repeated IL-22 stimulation for 4 hours in both HepG2 cells and primary human hepatocytes. Again, in both cell types, IL-22R transcription was higher in the IL-22 + IL-22BP pretreated cells than in those pretreated with IL-22 alone (Figure 4b, c). In the patients with AH, IL-22R gene expression in the liver tissue was not different from that of controls at diagnosis (Figure 4d), although immunohistochemical staining for IL-22R protein was increased compared with healthy livers (Figure 4e, f). This suggests that the high IL-22/IL-22BP ratio at diagnosis may downregulate IL-22R expression through the course of AH.
Figure 4.
Presence of IL-22BP sustains IL-22 receptor expression. (a) HepG2 cells stimulated with 50 ng/mL recombinant IL-22 alone or in combination with varying concentrations of recombinant IL-22BP for 4 hours. (b and c) primary human hepatocytes and HepG2 cells stimulated with 50 ng/mL recombinant IL-22 alone or in combination with 250 ng/mL recombinant IL-22BP for 24 h and followed by repeat IL-22 stimulation for 4 hours. IL-22 receptor (R) expression by quantitative polymerase chain reaction relative to GAPDH and an unstimulated control. (d) IL-22R expression by RNA sequencing and (e and f) immunohistochemistry of livers from alcoholic hepatitis patients compared with healthy liver. BP, binding protien; GAPDH, Glyceraldehyde 3-phospahte dehydrogenase; IL, Interleukin.
DISCUSSION
This study suggests that IL-22BP is of decisive importance for the bioactivity of the IL-22 system so that low plasma IL-22BP in patients with AH predicts a poor outcome. Despite the longstanding interest in IL-22 in this disease, this study is the first to investigate in detail the complete IL-22 system including its BP and receptor (23). The first clinical trial of IL-22 for the treatment of AH is coming to an end, and expectably, there will be a need to consider which factors may influence the treatment outcome; our study suggests IL-22BP to be such a factor.
In our cohort of patients with AH, the study-entry plasma IL-22BP was very low. This parallels the onset of other acute inflammatory diseases where IL-22BP is low and IL-22 elevated (6,8). However, in our patients, IL-22BP remained low 90 days later when IL-22 had normalized at variance with these diseases. The depression of IL-22BP, therefore, seems not only to be related to the acute phase of AH but may also be a related to a more long-lasting immune activation. In analogy with this, we observed low IL-22BP in the patients with cirrhosis and also that IL-22BP was associated clinical factors related to liver cirrhosis such as thrombocyte count depression and international normalised ratio elevation. The only available data on IL-22BP in liver disease stem from mice studies of acute liver injury, and here, the concentration was unchanged, inherently with the limitation that mice express only 1 of the 3 IL-22BP isotypes of humans (11,24). Our finding is thus the first observation of a chronic lowering of plasma IL-22BP in human disease to our knowledge. One explanation for this may be the long-term induction of the inflammasome, which is present both in AH and in stable cirrhosis (6,25). This seems to be a reasonable explanation because bacteria penetrating the highly permeable intestinal barrier in AH have this effect (26).
In the AH livers, the scarce immunohistochemistry staining of IL-22BP could not be distinguished from the similarly low staining in our control livers, but it was markedly lower than in our controls' colon biopsies. This is in line with the higher IL-22BP in portal than in systemic blood, which points to the gut being the predominant IL-22BP producer in alcohol-related liver disease, as is the case in health (6,9). This is further supported by no detection of IL-22BP mRNA in the livers of the patients with AH. Conceptually, this is plausible because bacterial translocation from the gut is a codriver of alcohol-related liver disease and there is experimental evidence for a role of IL-22 in mitigating this translocation (27).
Our data is of clinical importance because low IL-22BP was associated with more severe disease, a higher 1-year mortality, and distinguished between short-term survivors and fatalities. Together, these observations suggest IL-22BP to exert protective functions in the course of AH. This possibility was supported by a murine study of IL-22BP in liver injury (11). The finding is, however, seemingly paradoxical because low IL-22BP increases the fraction of free IL-22 and thereby increases supposedly beneficial IL-22 gene products. Indeed, in our patients with AH, where entry IL-22 was elevated, we detected upregulation of the same IL-22-inducible genes, as has been reported beneficial in rodent models of alcohol-mediated liver injury (16,28,29). The paradox seems to be explained by the low IL-22BP we measured, which may fail to sustain adequate IL-22R expression through the course of AH as indicated by our in vitro and in vivo data. In our hepatocyte cultures, IL-22 exerted negative feedback on expression of its IL-22R, as earlier described in keratinocytes via miR-197 and also for other cytokines (30–32). Addition of IL-22BP effectively inhibited this negative feedback. In our patients with AH, plasma IL-22 was increased at study entry, but IL-22BP stayed low. Accordingly, we see evidence of the same negative feedback as indicated by the not increased liver IL-22R mRNA despite the high IL-22R protein i.e. a decrease in the mRNA/protein ratio. This is one possible interpretation of our data. Alternatively, low IL-22BP may be associated with poor prognosis because it functions as a chaperone and donor of IL-22, which at adequately high levels will potentiate the IL-22 signal, as actually described and mathematically modeled by others (33). Regardless of these basic mechanistics, a balanced IL-22 and IL-22BP response seems necessary for appropriate IL-22 signaling. If so, the disturbance in AH of the normal balance between IL-22 and IL-22BP may well explain why low IL-22BP is associated with inappropriate IL-22 signaling and hence a poor prognosis.
An alternative interpretation of the association between low IL-22BP and high mortality could be more radical, namely that IL-22 contrary to belief is not protective but harmful in AH. This has actually been proposed in models of chronic liver injury and is thought to be attributable to proinflammatory damaging effects from high ‘uncontrolled’ IL-22 i.e. elevation without a concomitant increase in IL-22BP in these models (11,34). We found no support for this line of thought in our patients: there was no correlation between L-22 and proinflammatory markers, and low IL-22BP conversely was associated with lower circulating leucocytes and IL-8. Likewise, ascending-dose human trials of recombinant IL-22 report no systemic or liver-related inflammatory consequence (35,36).
The noninterventional nature of this study restricts our results to be of associative character. However, the combination of liver—gut—blood data with in vitro assays all pointing in the same direction suggests IL-22BP be taken into primary consideration in future IL-22 studies in AH. The patients should probably be stratified according to their entry IL-22BP to control the response modification by IL-22BP. It may also be worth considering if trials with IL-22BP as active agent are justified in AH because supplementation with IL-22BP in itself could allow sufficient endogenous IL-22 signaling without a need to administer exogenous IL-22. Alternatively, in the stratum with low IL-22BP, it may be worth considering IL-22BP as an add-on to IL-22.
In conclusion, this study of the IL-22 system points at a pivotal role for IL-22BP in human AH, best and directly illustrated by the protein's strong association with the disease course. This prompts an expanded understanding of the IL-22 system where the ILs interplay with its BP and receptor may be at least as important as the interleukin itself.
CONFLICTS OF INTEREST
Guarantor of the article: Sidsel Støy, MD.
Specific author contributions: Planning the study: S.S., B.D., H.V., and T.D.S. Designed the research studies: S.S., T.L.L., E.G., and T.D.S. Included the patients: S.S., T.L.L., E.G., E.T.D., N.E.M., S.S.V., and K.R. Conducted the experiments and acquired the data: S.S., T.L.L., E.T.D., N.E.M., S.S.V., K.R., and S.H.D. Analyzed the data: P.L.E., F.M., and R.O. Provided samples and reagents: S.S., T.L.L., B.D., H.V., and T.D.S. Wrote the manuscript: All authors have approved the final manuscript and authorship list.
Financial support: Aarhus University, Aase og Ejnar Danielsens Fond, Kong Christian den Tiendes Fond.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ IL-22 ameliorates alcohol-induced liver injury in mice.
✓ IL-22BP binds and antagonizes IL-22.
✓ IL-22 is in clinical trial as treatment of AH.
WHAT IS NEW HERE
✓ IL-22BP is present in the liver and produced in the gut in AH.
✓ Plasma IL-22/IL-22BP ratio is high and the IL-22 pathway active in the liver.
✓ Low IL-22BP is associated with high mortality in AH.
✓ IL-22 reduces hepatocyte expression of the IL-22 receptor in vitro, and IL-22BP reverts this.
TRANSLATIONAL IMPACT
✓ Low IL-22BP reduces IL-22 signaling through lowered IL-22 receptor expression through the course of AH.
✓ Patient IL-22BP levels may modify response to IL-22 treatment.
Supplementary Material
Acknowledgments
We thank Rikke Charlotte Andersen, Mette Mejlbye Hansen, Karin Skovgaard, and Anne-Kathrine Nissen Pedersen for expert assistance in sample collection and experiments; Kristina Bang Christensen for help with immunohistochemistry; Aarhus University, Aase og Ejnar Danielsens Fond, Kong Christian den Tiendes Fond for funding the study.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at https://links.lww.com/CTG/A325, https://links.lww.com/CTG/A326, https://links.lww.com/CTG/A327, https://links.lww.com/CTG/A328, https://links.lww.com/CTG/A338
References
- 1.Sandahl TD, Jepsen P, Thomsen KL, et al. Incidence and mortality of alcoholic hepatitis in Denmark 1999–2008: A nationwide population based cohort study. J Hepatol 2011;54(4):760–4. [DOI] [PubMed] [Google Scholar]
- 2.Thursz MR, Richardson P, Allison M, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372(17):1619–28. [DOI] [PubMed] [Google Scholar]
- 3.Vergis N, Atkinson SR, Knapp S, et al. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology 2017;152(5):1068–77 e1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orntoft NW, Sandahl TD, Jepsen P, et al. Short-term and long-term causes of death in patients with alcoholic hepatitis in Denmark. Clin Gastroenterol Hepatol. 2014;12(10):1739–44 e1731. [DOI] [PubMed] [Google Scholar]
- 5.Jones BC, Logsdon NJ, Walter MR. Structure of IL-22 bound to its high-affinity IL-22R1 chain. Structure 2008;16(9):1333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber S, Gagliani N, Zenewicz LA, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012;491(7423):259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng Y, French BA, Tillman B, et al. The inflammasome in alcoholic hepatitis: Its relationship with Mallory-Denk body formation. Exp Mol Pathol 2014;97(2):305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelczar P, Witkowski M, Perez LG, et al. A pathogenic role for T cell-derived IL-22BP in inflammatory bowel disease. Science 2016;354(6310):358–62. [DOI] [PubMed] [Google Scholar]
- 9.Martin JC, Beriou G, Heslan M, et al. Interleukin-22 binding protein (IL-22BP) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid. Mucosal Immunol 2014;7(1):101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim C, Hong M, Savan R. Human IL-22 binding protein isoforms act as a rheostat for IL-22 signaling. Sci Signal 2016;9(447):ra95. [DOI] [PubMed] [Google Scholar]
- 11.Kleinschmidt D, Giannou AD, McGee HM, et al. A protective function of IL-22BP in ischemia reperfusion and acetaminophen-induced liver injury. J Immunol 2017;199(12):4078–90. [DOI] [PubMed] [Google Scholar]
- 12.Sertorio M, Hou X, Carmo RF, et al. IL-22 and IL-22 binding protein (IL-22BP) regulate fibrosis and cirrhosis in hepatitis C virus and schistosome infections. Hepatology 2015;61(4):1321–31. [DOI] [PubMed] [Google Scholar]
- 13.Stoy S, Sandahl TD, Dige AK, et al. Highest frequencies of interleukin-22-producing T helper cells in alcoholic hepatitis patients with a favourable short-term course. PLoS One 2013;8(1):e55101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoy S, Dige A, Sandahl TD, et al. Cytotoxic T lymphocytes and natural killer cells display impaired cytotoxic functions and reduced activation in patients with alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol 2015;308(4):G269–276. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Chen Y, Wei H, et al. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res 2008;14(20):6432–9. [DOI] [PubMed] [Google Scholar]
- 16.Park O, Wang H, Weng H, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011;54(1):252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolk K, Kunz S, Witte E, et al. IL-22 increases the innate immunity of tissues. Immunity 2004;21(2):241–54. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen L, Masouminia M, Mendoza A, et al. Alcoholic hepatitis versus non-alcoholic steatohepatitis: Levels of expression of some proteins involved in tumorigenesis. Exp Mol Pathol 2018;104(1):45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brand S, Dambacher J, Beigel F, et al. IL-22-mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro. Am J Physiol Gastrointest Liver Physiol 2007;292(4):G1019–28. [DOI] [PubMed] [Google Scholar]
- 20.Eriksen PL, Soerensen M, Gronbaek H, et al. Non-alcoholic fatty liver disease causes dissociated changes in metabolic liver functions. J Hepatol 2019;70:E302–E303. [DOI] [PubMed] [Google Scholar]
- 21.Kragstrup TW, Vorup-Jensen T, Deleuran B, et al. A simple set of validation steps identifies and removes false results in a sandwich enzyme-linked immunosorbent assay caused by anti-animal IgG antibodies in plasma from arthritis patients. Springerplus 2013;2(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146(5):1231–9 e1231-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong X, Feng D, Mathews S, et al. Hepatoprotective and anti-fibrotic functions of interleukin-22: Therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol 2013;28(Suppl 1):56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang X, Feng D, Hwang S, et al. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming of impaired regeneration pathways in mice. J Hepatol 2020;72:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma X, Zheng X, Pan L, et al. NLRP3 inflammasome activation in liver cirrhotic patients. Biochem Biophys Res Commun 2018;505(1):40–4. [DOI] [PubMed] [Google Scholar]
- 26.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012;482(7384):179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartmann P, Chen P, Wang HJ, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology 2013;58(1):108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: Role of signal transducer and activator of transcription 3. Hepatology 2010;52(4):1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong X, Feng D, Wang H, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012;56(3):1150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Busse D, de la Rosa M, Hobiger K, et al. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci USA 2010;107(7):3058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer J, Lengyel G, Bauer TM, et al. Regulation of interleukin-6 receptor expression in human monocytes and hepatocytes. FEBS Lett 1989;249(1):27–30. [DOI] [PubMed] [Google Scholar]
- 32.Lerman G, Sharon M, Leibowitz-Amit R, et al. The crosstalk between IL-22 signaling and miR-197 in human keratinocytes. PLoS One 2014;9(9):e107467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shulgin B, Helmlinger G, Kosinsky Y. A generic mechanism for enhanced cytokine signaling via cytokine-neutralizing antibodies. PLoS One 2016;11(2):e0149154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng D, Wang Y, Wang H, et al. Acute and chronic effects of IL-22 on acetaminophen-induced liver injury. J Immunol 2014;193(5):2512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang KY, Lickliter J, Huang ZH, et al. Safety, pharmacokinetics, and biomarkers of F-652, a recombinant human interleukin-22 dimer, in healthy subjects. Cell Mol Immunol. 2019;16(5):473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arab JP, Sehrawat T, Simonetto DA, et al. An open label, cohort dose escalation study to assess the safety and efficacy of IL-22 agonist F-652 in patients with alcoholic hepatitis. Hepatology 2018;68(6):1454a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.