Abstract
The unique apical hook in dicotyledonous plants protects the shoot apical meristem and cotyledons when seedlings emerge through the soil. Its formation involves differential cell growth under the coordinated control of plant hormones, especially ethylene and auxin. Microtubules are essential players in plant cell growth that are regulated by multiple microtubule-associated proteins (MAPs). However, the role and underlying mechanisms of MAP-microtubule modules in differential cell growth are poorly understood. In this study, we found that the previously uncharacterized Arabidopsis MAP WAVE-DAMPENED2-LIKE4 (WDL4) protein plays a positive role in apical hook opening. WDL4 exhibits a temporal expression pattern during hook development in dark-grown seedlings that is directly regulated by ethylene signaling. WDL4 mutants showed a delayed hook opening phenotype while overexpression of WDL4 resulted in enhanced hook opening. In particular, wdl4-1 mutants exhibited stronger auxin accumulation in the concave side of the apical hook. Furthermore, the regulation of the auxin maxima and trafficking of the auxin efflux carriers PIN-FORMED1 (PIN1) and PIN7 in the hook region is critical for WDL4-mediated hook opening. Together, our study demonstrates that WDL4 positively regulates apical hook opening by modulating auxin distribution, thus unraveling a mechanism for MAP-mediated differential plant cell growth.
Microtubule-associated protein WDL4 promotes apical hook opening by regulating the auxin maxima and differential cell growth.
Introduction
The apical hook structure is believed to protect the shoot apical meristem and cotyledons from damage by soil particles during while dark-grown dicotyledonous seedlings travel through the soil to reach the surface (Harpham et al., 1991; Shen et al., 2016). The formation of the apical hook is the result of different cell elongation rates between the inner and outer sides of the hook (Erickson, 1978; Raz and Koornneef, 2001; Li et al., 2004; Mazzella et al., 2014). Studies have indicated that the development of the apical hook follows three phases: formation, maintenance, and opening. The time course of hook development in Arabidopsis (Arabidopsis thaliana) has been described in detail (Zadnikova et al., 2010). Generally, the formation phase takes place ∼24 h after seed germination, with hook curvature reaching ∼180°. The maintenance phase goes on for 24–30 h, and hook opening is completed after 68–88 h when seedlings are grown in the dark (Vandenbussche et al., 2010; Zadnikova et al., 2010). Studies have demonstrated that these phases are regulated by diverse factors, such as CLATHRIN LIGHT CHAINs CLC2 and CLC3, the auxin transport-regulatory protein kinase WAG2 and cell wall components, which participate in hook formation and opening (Willige et al., 2012; Yu et al., 2016; Aryal et al., 2020; Jonsson et al., 2021). Identification of a new regulator will improve our understanding of the underlying mechanisms of apical hook development, especially differential cell growth under diverse conditions.
Differential cell growth in the apical hook region is mediated by diverse plant hormones, such as ethylene and auxin, which has been extensively investigated (Guzman and Ecker, 1990; Ecker and Theologis, 1994; Lehman et al., 1996). The exaggerated curvature of the apical hook was observed in dark-grown (etiolated) Arabidopsis seedlings treated with ethylene or its biosynthetic precursor 1-aminocyclopropane-1-carboxylic acid (ACC) and in ethylene overproduction (ethylene overproducer, eto) and constitutive ethylene-responsive (constitutive triple response1) mutants (Guzman and Ecker, 1990; Ecker, 1995; Woeste et al., 1999). Notably, the sensitivity to ethylene during hook development is restricted to a time window of 2–3 days after germination (Raz and Ecker, 1999). These data suggest that ethylene plays a crucial role in prolonging the hook formation phase while also suppressing the maintenance phase. The asymmetric distribution of auxin in the apical region results in differential growth and, consequently, hook development. Mutants accumulating high levels of free auxin and auxin signaling pathway mutants (e.g. superroot1 and nonphototropic hypocotyl4) exhibit abnormal hook curvature (Boerjan et al., 1995; Harper et al., 2000). In addition, mutations in auxin influx carriers (e.g. AUXIN-RESISTANT1), auxin efflux carriers (e.g. PIN-FORMED1 [PIN1] and PIN7), or applied polar auxin transport (PAT) inhibitors affect hook development (Lehman et al., 1996; Yang et al., 2006; Vandenbussche et al., 2010; Zadnikova et al., 2010). Various evidence has demonstrated the crosstalk between ethylene and auxin signaling in regulating multiple aspects of seedling growth and development (Muday et al., 2012; Hu et al., 2017; Zemlyanskaya et al., 2018). During apical hook formation, auxin modulates the effect of ethylene, while ethylene regulates auxin biosynthesis, transport, and signaling. HOOKLESS1 (HLS1) has been identified to be a key player in this crosstalk (Lehman et al., 1996; Li et al., 2004). HLS1 is upregulated by ETHYLENE-INSENSITIVE3 (EIN3) and in turn inhibits AUXIN RESPONSE FACTOR2 levels, thus modulating auxin distribution and promoting apical hook formation (Lehman et al., 1996; Shen et al., 2016). In the later stage of apical hook development, ethylene signaling needs to be restricted to prevent exaggeration of the apical hook and the decrease of auxin signaling is required for growth at the inner side for hook opening, which is tightly controlled to avoid unwanted damage and ensure seedling survival (Beziat and Kleine-Vehn, 2018).
Microtubules are essential regulators of plant growth. Multiple studies have shown that diverse microtubule-associated proteins (MAPs) regulate cortical microtubules to mediate plant growth in response to multiple developmental and environmental cues (Li et al., 2012; Wang et al., 2012; Chen et al., 2014; Wang and Mao, 2019; Lian et al., 2021). For example, the phytohormone brassinosteroids (BRs) upregulate the expression of MICROTUBULE DESTABILIZING PROTEIN40 via the key transcription factor BRASSINAZOLE-RESISTANT1 in the BR signaling pathway to promote hypocotyl elongation in etiolated Arabidopsis seedlings (Wang et al., 2012). Auxin also induces cortical microtubule reorientation in the cells from hypocotyls and roots (Le et al., 2005; Chen et al., 2014). Surprisingly, this reorientation of microtubules was unrelated to short-term auxin-induced cell elongation (Baskin, 2015). In addition, a reverse mechanism was revealed, wherein microtubules influence PAT. The MAP CLIP-ASSOCIATED PROTEIN (CLASP) modulates PIN2 levels by tethering SORTING NEXIN1 (SNX1) endosomes to cortical microtubules, which in turn regulate the auxin maxima at the root apical meristem (Ambrose et al., 2013). Thus, the relationship between auxin and the MAP-microtubule module in plant cell growth is complicated. Thus far, the canonical mechanism by which the MAP-microtubule module mediates plant growth is through orienting newly deposited cellulose microfibrils. This process has been well investigated in both roots and hypocotyls (Li et al., 2012; Chen et al., 2014). Plant structures in nature, such as the apical hook, are formed due to the result of differential cell growth. Recently, Baral et al. (2021) have reported that the dynamic rearrangement of cortical microtubules and its effect on cellulose deposition are critical for mechanically induced hook formation, through TRANSMEMBRANE KINASE-mediated auxin signaling and thus regulation of tissue folding (Baral et al., 2021). However, the function and underlying mechanisms behind the MAP-microtubule module during hook maintenance and opening process, especially their relationship to ethylene and auxin signaling, remain unknown.
In this study, we demonstrated that the microtubule-stabilizing protein WAVE-DAMPENED2 (WVD2)-LIKE4 (WDL4) promotes apical hook opening. WDL4 expression in the hook region is regulated by ethylene signaling in the dark. Moreover, altering the auxin maxima in the hook region is critical for WDL4-mediated hook opening. Thus, we propose a mechanism of MAP-based plant differential growth through the regulation of auxin distribution.
Results
WDL4 is a positive regulator of apical hook opening in Arabidopsis seedlings
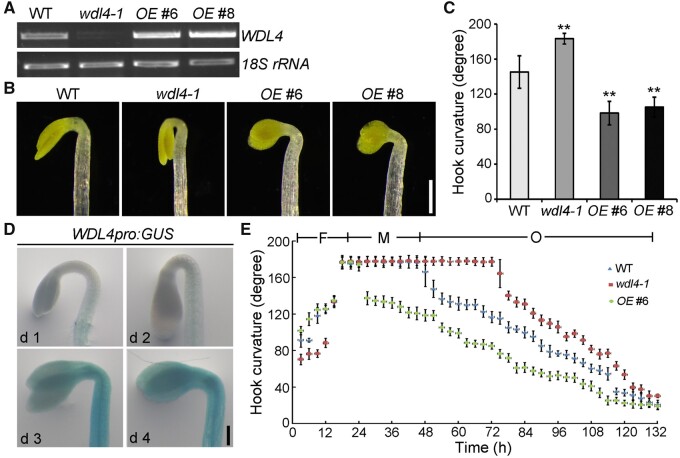
The formation of the apical hook structure is orchestrated by the differential elongation rate of cells in the outer and inner edge of the hook (Erickson, 1978). To investigate the roles of microtubules and MAPs in this process, we screened mutants of known Arabidopsis MAPs for hook phenotypes. We discovered that hook curvature in a WDL4 T-DNA insertion mutant (wdl4-1) was more exaggerated compared to that of wild-type (WT) Wassilewskija (Ws) seedlings when grown in the dark for 3 days (Figure 1, A–C). This phenotype was complemented by the introduction of a WDL4pro:WDL4-GFP transgene (Supplemental Figure S1). In addition, we generated WDL4-overexpressing (OE) seedlings and determined that hook curvature was significantly reduced in 3-day-old etiolated OEWDL4-OE seedlings (Figure 1, A–C; Supplemental Figure S2). To validate this phenotype, we generated another mutant allele in WDL4 (wdl4-2) using Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and the CRISPR-associated nuclease Cas9 in the Columbia-0 (Col-0) background (Wang et al., 2015). This mutant exhibited a similar delayed hook opening phenotype as wdl4-1 that was also complemented by the introduction of a WDL4pro:WDL4-GFP transgene (Supplemental Figure S3, A–D). These results demonstrate that WDL4 participates in apical hook development.
Figure 1.
WDL4 is required for apical hook opening. A, RT-PCR analysis of WDL4 transcript levels in the apical hook of 3-day-old etiolated seedlings of WT, wdl4-1 mutant, and two independent GFP-WDL4 OE transgenic lines. 18S rRNA was used as a control. B, Apical hook phenotypes of 3-day-old etiolated seedlings of WT, wdl4-1 mutant, and two independent OE lines. Scale bar = 0.5 mm. C, Quantification of the apical hook curvature angles, as shown in (B). Values represent mean ± sd (n > 30, **P < 0.01). D, Representative images of WDL4 expression pattern in the apical hook region from WDL4pro:GUS transgenic etiolated seedlings maintained in the dark for the indicated number of days. Scale bar = 0.2 mm. E, Kinematic analyses of apical hook development in etiolated seedlings of WT, wdl4-1 mutant, and OE lines. Compared to WT, the wdl4-1 mutant shows a defect at the hook opening stage.
Hook development can be divided into formation, maintenance, and opening phases (Raz and Ecker, 1999). To investigate which process WDL4 was involved in, we examined the expression pattern of WDL4 during hook development using transgenic lines expressing the β-GLUCURONIDASE (GUS) reporter gene driven by the native WDL4 promoter (WDL4pro:GUS). GUS staining showed that WDL4 was rarely expressed in the hook region when grown in the dark for 1 or 2 days but rose significantly when seedling growth was extended to 3 or 4 days in the dark, indicating that WDL4 might function during the maintenance and opening phases (Figure 1D). Furthermore, we analyzed hook curvature in WT, wdl4-1 mutant, and OEWDL4-OE etiolated seedlings at the indicated time points after germination. Kinematic analysis of hook development showed that the formation phase and the maintenance phase in WT seedlings lasted ∼21 and 24 h under our experimental conditions, respectively, which is consistent with previous reports (Vandenbussche et al., 2010; Zadnikova et al., 2010). By contrast, the maintenance stage in the wdl4-1 mutant was much longer compared to that of WT seedlings; the hook opening stage was also delayed. Conversely, the maintenance stage in WDL4-OE seedlings was shorter and the hook opening stage was much earlier (Figure 1E). Taken together, these results demonstrate that WDL4 positively regulates apical hook opening.
WDL4 expression is negatively regulated by ethylene signaling through EIN3 during hook development
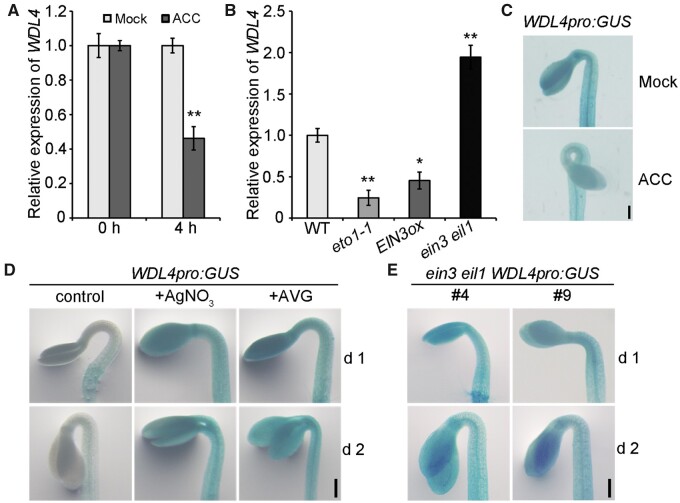
Previous studies indicated that ethylene mainly functions in the phases of hook formation and maintenance (Raz and Ecker, 1999; Zadnikova et al., 2010). The temporal expression pattern of WDL4 led us to test whether ethylene regulated WDL4 expression through its signaling pathway. Real-time quantitative PCR (RT-qPCR) analysis indicated that treatment with the ethylene precursor ACC resulted in the decrease of WDL4 expression in 3-day-old dark-grown seedlings (Figure 2A). Moreover, the expression of WDL4 in the eto1-1 mutant and EIN3-overexpressing (EIN3ox) seedlings was also significantly decreased compared to that of WT seedlings, while it increased in the ein3 eil1 mutant (Figure 2B), indicating that ethylene signaling downregulates WDL4 expression. Furthermore, we performed GUS staining experiments to confirm the regulation of WDL4 expression in the apical hook region. Consistent with previous results, treatment with ACC resulted in the decrease of GUS staining in the hook region of WDL4pro:GUS transgenic seedlings grown in the dark for 3 days (Figure 2C). In contrast, silver cations (Ag+, an ethylene receptor antagonist) or the ethylene biosynthesis inhibitor aminoethoxyvinylglycine (AVG) caused the accumulation of GUS staining in the hook region of seedlings grown in the dark for 1 or 2 days (Figure 2D). We observed a similar accumulation of GUS staining in ein3 eil1 WDL4pro:GUS seedlings (Figure 2E). Taken together, these results demonstrate that ethylene signaling downregulates WDL4 expression during apical hook development.
Figure 2.
Ethylene signaling negatively regulates WDL4 expression during hook development. A, RT-qPCR analysis of WDL4 transcript levels in 3-day-old etiolated WT seedlings grown on half-strength MS medium alone (mock) or treated with 100 µM ACC for 4 h. Values represent mean ± sd for three individual biological repeats (**P < 0.01). B, RT-qPCR analysis of WDL4 transcript levels in 3-day-old etiolated seedlings of WT, the eto1-1 mutant, the EIN3ox transgenic line, and the ein3 eil1 mutant. Values represent mean ± sd for three individual biological repeats (*P < 0.05, **P < 0.01). C, Representative images of GUS staining in the hook region of WDL4pro:GUS transgenic seedlings in medium (mock) or with 10 µM ACC treatment. Seedlings were stained and imaged after growth in the dark for 3 days. D, Representative images of GUS staining in the hook region of WDL4pro:GUS transgenic seedlings in medium without (control) or with the indicated chemicals, the ethylene receptor antagonist AgNO3 and the ethylene biosynthesis inhibitor AVG. Seedlings were stained and imaged after growth in the dark for 1 or 2 days. E, Representative images of GUS staining in the hook region of WDL4pro:GUS transgenic ein3 eil1 seedlings. Two independent lines were checked after seedling growth in the dark for 1 or 2 days. Scale bar = 0.2 mm.
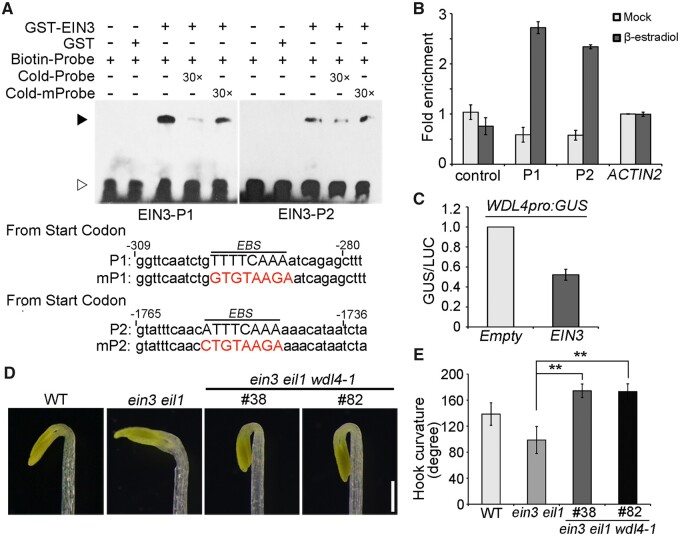
To explore the regulatory mechanism of WDL4 expression in detail, we analyzed the sequence of the WDL4 promoter: we identified two putative EIN3-binding sites (EBSs) in the P1 and P2 regions (Figure 3A, lower). Electrophoretic mobility shift assays (EMSAs) showed that recombinant GST-EIN3 directly binds to the P1 and P2 regions of the WDL4 promoter, but this binding was abolished by the addition of unlabeled P1 and P2 probes. When we mutated the putative EBSs in the competitive unlabeled probes, they lost their ability to abolish the binding between GST-EIN3 and biotin-labeled probes (Figure 3A; upper). Furthermore, chromatin immunoprecipitation (ChIP) followed by qPCR using seedlings with β-estradiol-induced expression of EIN3-3×FLAG showed that the P1 and P2 fragments of the WDL4 promoter are enriched in chromatin immunoprecipitated with the anti-FLAG antibody (Figure 3B) (An et al., 2012; Shi et al., 2012). These results indicated that WDL4 is a direct target gene of EIN3. We further investigated the direct effect of EIN3 on WDL4 expression using transient GUS activity assay in Nicotiana benthamiana leaves. GUS was placed under the control of the WDL4 promoter and luciferase (LUC) under the control of the Super promoter as an internal control for infiltration efficiency. We then co-infiltrated the reporter constructs with the effector Super:EIN3 or empty vector and determined their effect on the WDL4 promoter. Relative GUS activity significantly decreased when EIN3 was co-infiltrated, demonstrating the negative effect of EIN3 on WDL4 expression (Figure 3C). Moreover, a genetic analysis showed that loss of WDL4 function attenuates the abnormal hook-opening phenotype of the ein3 eil1 double mutant (Figures 3, D and E) (An et al., 2012). Based on these findings, WDL4 appears to be negatively regulated by ethylene signaling through EIN3 during hook development.
Figure 3.
WDL4 is an EIN3 target gene. A, EMSA assay for EIN3 binding to the WDL4 promoter. Each biotin-labeled DNA fragment was incubated with recombinant GST-EIN3. Free GST was used as a control. Competition for the labeled promoter fragments was performed by adding an excess (30×) of unlabeled WT or mutated probes. Two putative EBSs (P1 and P2) in the WDL4 promoter were tested. White arrowhead indicates free probe and black arrowhead indicates bound complex. B, ChIP assay for EIN3 binding to the WDL4 promoter. Chromatin was immunoprecipitated from etiolated seedlings expressing EIN3-3 × FLAG under the β-estradiol-inducible promoter using an anti-FLAG antibody. Seedlings treated with mock buffer (half-strength MS medium with the same volume of DMSO used for β-estradiol treatment) were used as a control. The amount of indicated DNA in the immune complex was determined by qPCR. Values represent mean ± sd from three individual biological repeats. C, Transient expression of WDL4pro:GUS with or without EIN3 in N. benthamiana leaves. LUC was expressed from the Super promoter as an internal control. GUS and LUC activity was quantified, and the GUS/LUC ratio used to estimate the binding activity of EIN3 to the WDL4 promoter. At least three independent experiments were performed with similar results. D and E, Representative images and quantification of the hook curvature in the ein3 eil1 wdl4-1 triple mutant compared to that in WT and the ein3 eil1 double mutant. Two independent ein3 eil1 wdl4-1 triple mutant lines were used for analysis. Values represent mean ± sd (n > 43 independent seedlings; **P < 0.01). Scale bar = 0.5 mm.
The WDL4-mediated mechanism contributes to apical hook opening beyond conventional microtubule-guided plant cell growth
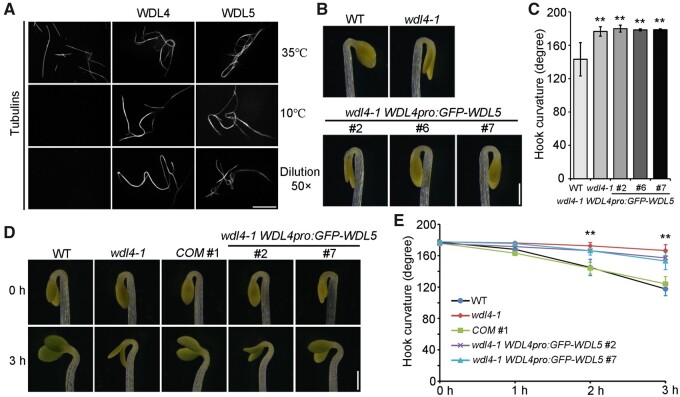
WDL4 belongs to the microtubule-associated protein WVD2/WDL family (Yuen et al., 2003; Perrin et al., 2007). Like other members, WDL4 directly binds to microtubules in vitro and colocalizes with cortical microtubules in vivo (Supplemental Figure S4, A–C). It was previously reported that WDL5, a close homolog of WDL4, is involved in ethylene-regulated etiolated hypocotyl cell elongation by modulating the organization and stability of cortical microtubules (Sun et al., 2015). Surprisingly, although WDL5 showed constant expression in the hook region, we observed no obvious altered phenotypes in the apical hook development in either wdl5-1 mutants or WDL5-OE seedlings (Supplemental Figure S5, A and B). We analyzed the properties of WDL4 using in vitro low temperature and dilution treatments and discovered that WDL4 and WDL5 exhibit similar activity in the bundling and stabilizing of microtubules (Figure 4A). However, WDL5 did not complement the delayed hook opening phenotype of the wdl4-1 mutant when driven by the WDL4 promoter (Figure 4, B and C; Supplemental Figure S6). These results suggest that the mechanism of WDL4-mediated hook development is different from that of WDL5-mediated hypocotyl elongation (Sun et al., 2015).
Figure 4.
WDL5 driven by the WDL4 promoter does not rescue the delayed hook opening phenotype of wdl4-1 mutant. A, Representative images of microtubules incubated with WDL4 or WDL5 using in vitro assays under low temperature and dilution treatments. Scale bar = 10 μm. B and C, Representative images and quantification of hook curvature in wdl4-1 WDL4pro:GFP-WDL5 complementation lines. Three independent lines were used for analysis. Values in (C) represent mean ± sd (n > 46 independent seedlings; **P < 0.01). Scale bar = 0.5 mm. D and E, Light-triggered rapid hook opening process in WT, wdl4-1, wdl4-1 WDL4pro:WDL4-GFP complementation line (COM #1) and two independent wdl4-1 WDL4pro:GFP-WDL5 lines. Seedlings were germinated and grown vertically in the dark for 42 h, followed by short-term white light treatment as indicated. Representative photographs were taken, and hook curvature was quantified at different time points as labeled. Values in (E) represent mean ± sd (n > 5 independent experiments each containing >10 seedlings; **P < 0.01). Scale bar = 0.5 mm.
Light exposure induces the apical hook to open quickly (Liscum and Hangarter, 1993; Wang et al., 2009; Yu et al., 2016; Beziat et al., 2017; Zhang et al., 2018). To further verify our hypothesis, we utilized the system of light-induced hook opening to determine the function of WDL4 during this rapid process in the following experiments. To make sure that WT and wdl4-1 seedlings are at the same developmental stage, we chose seedlings growing in the dark for 42 h as a starting point, when both genotypes have closed hooks, as shown in Figure 1E, and when WDL4 is expressed at a low level (Supplemental Figure S7A). We exposed seedlings to white light for 1–3 h before measuring hook curvature. Light exposure induced rapid hook opening in WT seedlings as previously reported, and did not change the expression level of WDL4 (Figures 4, D and E; Supplemental Figure S7B) (Yu et al., 2016; Zhang et al., 2018). In contrast, wdl4-1 mutant seedlings displayed significantly reduced apical hook opening under the same conditions (Figure 4, D and E). This phenotype was complemented by the introduction of a WDL4pro:WDL4-GFP transgene but not by WDL4pro:GFP-WDL5. These results are consistent with the previously demonstrated wdl4-1 phenotype in the dark and confirmed that the function of WDL4 in promoting apical hook opening might not be achieved by the same mechanisms used for microtubule-based hypocotyl cell elongation, which relies on microtubule-guided cellulose fibril deposition and usually requires a relatively long time (Li et al., 2012; Baskin, 2015; Schopfer and Palme, 2016).
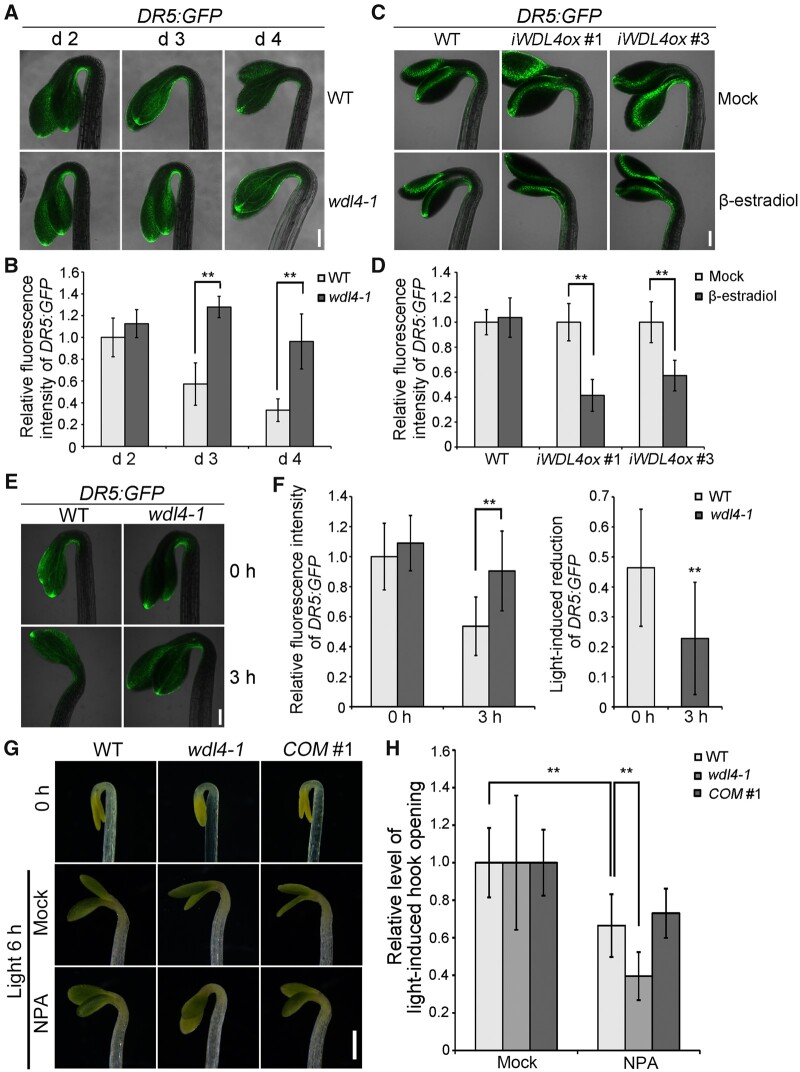
WDL4 regulates the auxin maxima during hook development
Previous studies demonstrated that the auxin maxima in the apical hook established by auxin influx and efflux is critical for hook development (Vandenbussche et al., 2010; Zadnikova et al., 2010). To gain insight into the cellular mechanism by which WDL4 participates in apical hook development, we crossed the auxin reporter DR5:GFP with the wdl4-1 mutant (Benkova et al., 2003). Compared to WT seedlings, the wdl4-1 mutant exhibited stronger DR5:GFP signals in the concave side of the apical hook on d 3 and 4 (Figure 5, A and B; Supplemental Figure S8). We generated inducible WDL4 overexpression transgenic plants (iWDL4ox) and crossed them with the DR5:GFP reporter line, to directly analyze the function of WDL4 on auxin distribution over a short growth period. To specifically assess the effect of WDL4 on auxin accumulation during hook opening, we treated 2-day-old etiolated seedlings with β-estradiol to induce the overexpression of WDL4. DR5:GFP signals in the concave side of the apical hook were significantly weaker in transgenic seedlings treated with β-estradiol relative to seedlings treated with mock buffer (Figure 5, C and D). As a control, DR5:GFP signals in WT seedlings showed no obvious differences when treated with β-estradiol or with mock buffer. These results indicate a role for WDL4 in modulating the auxin maxima during hook opening. We further examined the auxin maxima of WT and wdl4-1 seedlings during light-induced hook opening. DR5:GFP signals in the hook concave side of the wdl4-1 mutant were similar to that of WT seedlings at the starting point of the experiment (Figure 5, E and F). After illumination, we observed a significant reduction of the auxin maxima in the hook concave side of WT seedlings, which is consistent with previous reports (Yu et al., 2016; Beziat et al., 2017). However, the light-induced reduction of DR5:GFP was significantly attenuated in the wdl4-1 mutant, suggesting that WDL4 is required for the regulation of the auxin maxima during light-induced hook opening (Figure 5, E and F).
Figure 5.
WDL4 mutation alters auxin maxima and impairs sensitivity to auxin efflux inhibitor during hook development. A and B, Representative images of DR5:GFP expression patterns and quantified fluorescence intensity in the hook region of dark-grown WT and wdl4-1 mutants at different time points. Values in (B) represent mean ± sd (n > 12 independent seedlings; **P < 0.01). Scale bar = 0.2 mm. C and D, Representative images of DR5:GFP expression patterns and quantified fluorescence intensity in the hook region of etiolated seedlings with induced overexpression of WDL4 (iWDL4ox). Seedlings were germinated and grown vertically in the dark for 48 h, and transferred to new plates with or without β-estradiol for another 24 h in the dark. Values in (D) represent mean ± sd (n > 12 independent seedlings; **P < 0.01). Scale bar = 0.2 mm. E and F, Representative images of DR5:GFP expression patterns and quantified fluorescence intensity during light-induced hook opening. WT and wdl4-1 seedlings were germinated and grown vertically in the dark for 42 h, and transferred to white light for another 3 h. Values in (F) represent mean ± sd (n > 15 independent seedlings; **P < 0.01). Scale bar = 0.2 mm. G and H, Representative images and quantified analysis of NPA effect on light-induced hook opening. WT, wdl4-1, and wdl4-1 WDL4pro:WDL4-GFP (COM #1) seedlings were germinated and grown vertically in the dark for 42 h, and transferred to white light for another 6 h with or without NPA treatment. Values in (F) represent mean ± sd (n > 17 independent seedlings; **P < 0.01). Scale bar = 0.5 mm.
To determine whether WDL4 regulates the auxin maxima through modulating auxin efflux during hook opening, we analyzed the effects of the auxin efflux inhibitor 1-N-naphthylphthalamic acid (NPA) on light-induced hook opening in WT and wdl4-1 seedlings. Etiolated seedlings grown for 42-h dark were illuminated with white light for 6 h with or without NPA. In WT seedlings, NPA treatment resulted in the inhibition of light-induced hook opening, as shown in Figure 5, G and H, confirming that auxin efflux is required for light-induced hook opening. Interestingly, the inhibitory effect of NPA on hook opening was significantly stronger in the wdl4-1 mutant compared to WT seedlings, indicating additional inhibition of auxin efflux in wdl4-1 seedlings (Figure 5, G and 5). This phenotype was complemented by the introduction of a WDL4pro:WDL4-GFP transgene into the mutant background. Accordingly, the inhibitory effect of NPA on hook opening was weaker when WDL4 overexpression was induced with β-estradiol (Supplemental Figure S9, A and B). Together, these results reveal the significant contribution of WDL4 to auxin efflux-dependent hook development and light-induced hook opening.
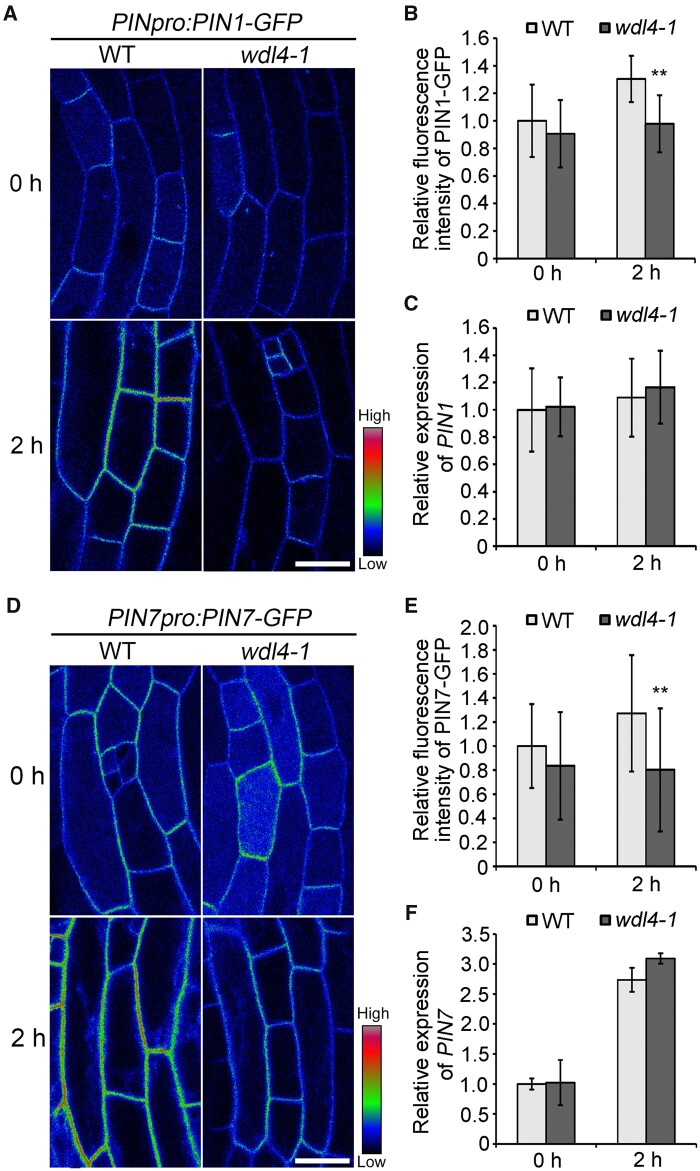
WDL4 regulates the trafficking of PIN transporters during hook opening
Previous studies demonstrated that proper trafficking and distribution of the auxin efflux transporter PINs are critical for hook development (Petrasek et al., 2006; Blakeslee et al., 2007; Zadnikova et al., 2010; Wang et al., 2017). In particular, PIN1, PIN4, and PIN7 have been reported to function in the regulation of the transition from the maintenance stage to the opening stage (Zadnikova et al., 2010; Baral et al., 2021; Jonsson et al., 2021). Combined with the temporal expression pattern of WDL4 and the phenotype of wdl4 mutants with a delayed transition to the opening stage, our results indicated a possible coupling between WDL4 and PIN proteins. To obtain additional information on the functions of WDL4 in the hook opening stage, we determined the cellular localization and protein trafficking of PIN1-GFP and PIN7-GFP in the hook region of WT and wdl4-1 mutant seedlings using the light-induced hook opening system. PIN1-GFP was previously reported to be asymmetrically distributed in the hook region from the maintaining stage, exclusively on the concave side (Zadnikova et al., 2010). We observed a similar distribution for PIN1-GFP in the middle view of apical hooks in both WT and wdl4-1 mutant seedlings, which were grown in the dark for 42 h and then illuminated with white light for the following observation (Supplemental Figure S10). With white light illumination for 2 h, we detected a higher PIN1-GFP signal intensity at the plasma membrane (PM) in epidermal cells of WT hook regions. In contrast, PM-localized PIN1-GFP maintained a similar level over the course of the illumination period in the epidermis of the wdl4-1 mutant (Figure 6, A and B). RT-qPCR analysis indicated a similar expression level for PIN1 in the hook region of both WT and wdl4-1 seedlings with or without illumination, demonstrating that the observed difference in PM-localized PIN1–GFP between WT and wdl4-1 mutant seedlings is not due to differences in PIN1 transcript levels (Figure 6C). We observed a similar phenomenon with PIN7–GFP in epidermal cells of WT and wdl4-1 seedlings (Figure 6, D and E). Notably, although PIN7 expression was rapidly induced by white light illumination, the expression level of PIN7 was similar in WT and the wdl4-1 mutant in the hook region (Figure 6F). These results indicate a role for WDL4 in regulating the accumulation of PIN proteins at the PM.
Figure 6.
WDL4 mutation attenuates the enhanced distribution of PIN1–GFP and PIN7–GFP at the PM during light-induced hook opening. A and B, Representative images shown as pseudocolors (red-green-blue palette) and relative fluorescence intensity of PM-localized PIN1–GFP in hook epidermal cells in WT and wdl4-1 seedlings expressing PIN1pro:PIN1-GFP. Seedlings were germinated and grown vertically in the dark for 42 h, and transferred to white light for another 2 h. Values represent mean ± sd (n > 14 independent seedlings; **P < 0.01). Scale bar = 20 μm. C, Relative expression of PIN1 in the apical hook of the seedlings used in A. One to two millimeter of the apical hook regions from seedlings were used for RNA extraction. Values represent mean ± SD for three individual biological repeats. D and E, Representative images shown as pseudocolors and relative fluorescence intensity of PM-localized PIN7–GFP in hook epidermal cells in WT and wdl4-1 seedlings expressing PIN7pro:PIN7–GFP. Seedlings were grown and treated the same way as in A. Values represent mean ± sd (n > 21 independent seedlings; **P < 0.01). Scale bar = 20 μm. F, Relative expression of PIN7 in the apical hook of the seedlings used in D. One to two millimeter of the apical hook regions from seedlings were used for RNA extraction. Values represent mean ± sd for three individual biological repeats.
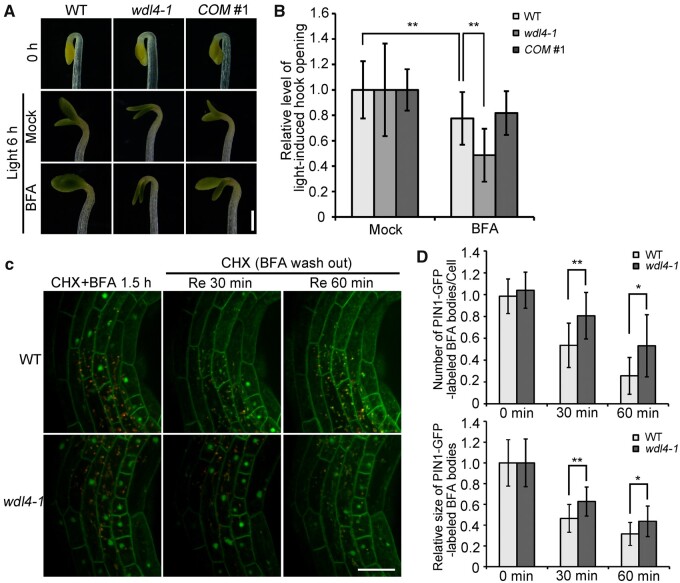
PIN proteins undergo internalization and recycling back to the PM (Kleine-Vehn et al., 2011; Adamowski and Friml, 2015; Sauer and Kleine-Vehn, 2019). Thus, we further explored whether WDL4 participates in PIN protein trafficking using the well-studied PIN1–GFP fusion protein. PIN1 vesicle trafficking has been reported to be mediated by a brefeldin A (BFA)-sensitive pathway that results in the aggregation of the trans-Golgi network/early endosomes in plant cells (Kleine-Vehn et al., 2006). Therefore, we first examined the effect of BFA treatment on light-induced hook opening in WT and wdl4-1 seedlings. We illuminated etiolated seedlings grown for 42 h with white light for 6 h with or without BFA. In WT seedlings, BFA treatment inhibited light-induced hook opening. Interestingly, the inhibitory effect of BFA on hook opening was strongly enhanced in the wdl4-1 mutant compared to WT seedlings, but was significantly weaker when WDL4 overexpression was induced with β-estradiol (Figure 7, A and B; Supplemental Figure S9, C and D). We further examined the trafficking of PIN1–GFP in hook epidermal cells from WT and wdl4-1 mutant seedlings. We illuminated etiolated seedlings grown for 42 h with white light and pre-treated them with cycloheximide (CHX) for 1 h to inhibit de novo protein biosynthesis, followed by treatment with CHX together with BFA for 30 min (Yu et al., 2016). Endocytosed PIN1–GFP was trapped in the BFA bodies inside the cells. We observed no significant difference between WT and wdl4-1 seedlings, indicating that the endocytosis of PIN1-GFP is not affected in the wdl4-1 mutant during light-induced hook opening (Supplemental Figure S11). To examine whether the recycling of PIN1 is affected by the loss of WDL4, we illuminated etiolated seedlings grown for 42 h with white light and pre-treated with CHX for 30 min, followed by BFA + CHX treatment for 1.5 h and the removal of BFA while maintaining CHX. We observed that the trapped signals from PIN1-GFP decreased after BFA washout for 30–60 min in epidermal cells of WT hooks, indicating the recycling of PIN1–GFP (Figure 7C). In contrast, the recovery of PIN1-GFP from the BFA bodies in the wdl4-1 mutant cells was much slower than that in WT cells, indicating impaired recycling of PIN1–GFP in mutant hook epidermal cells (Figure 7C). To quantify the effect of WDL4 deficiency on PIN1–GFP recycling, we measured the number and relative size of PIN1–GFP-labeled BFA bodies (Figure 7D). The results further confirmed that the recycling of PIN1–GFP signals was significantly reduced in the wdl4-1 mutant compared to that in WT seedlings. Taken together, our observations suggest that WDL4 participates in apical hook opening through the regulation of the auxin maxima and PIN protein recycling.
Figure 7.
Inactivation of WDL4 impairs trafficking of PIN1–GFP during light-induced hook opening. A and B, Representative images and quantified analysis of BFA effects on light-induced hook opening. WT, wdl4-1, and wdl4-1 WDL4pro:WDL4-GFP (COM #1) seedlings were germinated and grown vertically in the dark for 42 h, and transferred to white light for another 6 h with or without BFA treatment. Values in B represent mean ± sd (n > 36 independent seedlings; **P < 0.01). Scale bar = 0.5 mm. C, WT and wdl4-1 seedlings expressing PIN1pro:PIN1–GFP were germinated and grown vertically in the dark for 42 h, and transferred to white light with CHX pre-treatment (50 μM) for 30 min, followed by CHX + BFA (100 μM) for 1.5 h to induce the formation of BFA bodies. BFA was removed and seedlings were incubated in the presence of CHX and white light for another 30 or 60 min. The images of PIN1–GFP were taken at different time points. The red channel shows auto-fluorescence signals. Scale bar = 20 μm. D, Number of PIN1–GFP-labeled BFA bodies per cell and their relative size. Values represent mean ± sd (n > 14 independent seedlings; *P < 0.05, **P < 0.01).
Discussion
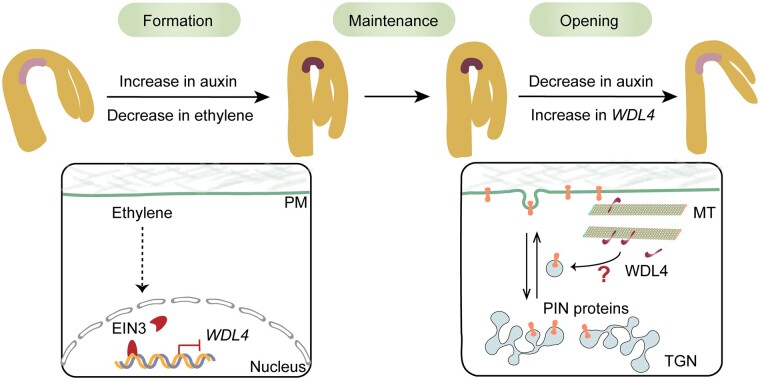
The apical hook is essential in dicotyledonous plants by providing mechanical protection to the shoot apical meristem and cotyledons when seedlings travel through the soil to the surface, and its development process is tightly controlled (Harpham et al., 1991; Shen et al., 2016; Jonsson et al., 2021). Hook curvature is achieved by growth inhibition along with asymmetric auxin accumulation at the concave side, while hook opening is realized by growth promotion and a decrease in the auxin maxima (Beziat and Kleine-Vehn, 2018). In this study, we demonstrated that a previously uncharacterized MAP (WDL4) is a positive regulator of apical hook opening. We elucidated the temporal regulation of WDL4 in the apical hook of Arabidopsis and propose a working model for WDL4 function during apical hook development (Figure 8).
Figure 8.
A hypothetical model for WDL4 function in apical hook development. The formation of hook curvature in etiolated seedlings is achieved by growth inhibition along with asymmetric auxin accumulation at the concave side, while hook opening is achieved by growth promotion and a decrease in the auxin maxima. During the early stage of hook formation, the level of ethylene is high, and the key transcription factor EIN3 accumulates. EIN3 binds to the promoter region of WDL4 and suppresses WDL4 expression. During hook opening, when ethylene levels are relatively low, EIN3 is degraded, and the inhibition of WDL4 expression is released. WDL4 accumulates and promotes hook opening by influencing the auxin maxima and PIN protein distribution and trafficking. The question mark indicates the yet-unknown mechanism by which WDL4 regulates auxin distribution and the trafficking of auxin efflux carriers during hook opening.
In particular, we provided several lines of evidence supporting the notion that altering auxin distribution is critical for WDL4-promoted hook opening. We provided additional information on how a MAP participates in differential plant cell growth, in addition to the conventional function of the MAP-microtubule module in plant cell growth by guiding the orientation of cellulose microfibrils deposition and regulating cell expansion (Li et al., 2012; Baskin, 2015). First, knockout or overexpression of WDL5, the close homolog of WDL4, did not exhibit the same effects on hook opening as with wdl4 mutants, although WDL5 is expressed in the hook region. Interestingly, although WDL4 and WDL5 have similar activities on bundling and stabilizing microtubules, WDL5 failed to complement the phenotype of the wdl4-1 mutant when driven by the WDL4 promoter. This result indicates that WDL4 plays an additional function during hook opening beyond bundling and stabilizing microtubules, which might be achieved by potentially specific interaction partners. Second, we employed the well-established system of rapid hook opening in response to light for phenotypic analysis and investigation of WDL4 function on auxin distribution and trafficking of PIN proteins (Yu et al., 2016; Beziat et al., 2017; Zhang et al., 2018). We specifically examined the effect of WDL4 on the opening process of hook curvature, and showed that the activities linking WDL4 and differential cell growth in the apical hook are relatively fast, which is in agreement with the idea that auxin regulates cell growth rapidly in the short-term (Schopfer and Palme, 2016). Accordingly, light-triggered elimination of the auxin maxima in the concave side and enhanced PIN1–GFP at the PM was significantly affected by the loss of WDL4. These results strongly support the working model of WDL4-mediated apical hook opening through modulating PIN proteins and auxin distribution.
It is noteworthy that WDL4pro:GUS expression in the hook region did not obviously change with white light illumination, suggesting that WDL4 may not specifically respond to light signaling and might function more generally during both slow hook opening in the dark and fast hook opening triggered by light. Indeed, we observed a similar phenotype of delayed hook opening and impaired auxin maxima in wdl4 mutants in both conditions. Interestingly, the phenotype of the wdl4-1 mutant and WDL4-OE transgenic seedlings was relatively strong compared to the mild phenotypes seen in pin1 and pin7 mutants, which also exhibit shortened maintenance phases (Zadnikova et al., 2010). Previous studies showed that PIN proteins function redundantly during hook development, and that hook formation is completely abolished in the pin1 pin3 pin4 pin7 quadruple mutant (Zadnikova et al., 2010; Baral et al., 2021; Jonsson et al., 2021). This observation suggests that multiple PIN proteins might be regulated by WDL4 during the late maintenance and opening stages of apical hook development. Consistent with this hypothesis, we found that the loss of WDL4 impaired PIN1 and PIN7 abundance at the PM of hook epidermal cells in a similar manner. However, in this study, we assessed the effect of WDL4 on the trafficking of PIN1 and PIN7 during light-triggered hook opening instead of the slow hook opening in the dark. Previous reports have illustrated the complex feedback regulation between auxin and PIN proteins (Adamowski and Friml, 2015). Auxin accumulation strongly upregulates PIN expression (Vieten et al., 2005). Moreover, auxin may further regulate PIN proteins at the protein level. Short-term auxin treatment inhibits the endocytosis of PIN proteins while extended treatment with auxin promotes the degradation of PIN proteins (Paciorek et al., 2005; Abas et al., 2006; Adamowski and Friml, 2015). Therefore, the contribution of different mechanisms to PIN protein abundance and trafficking would be rather complicated over a relatively long time during slow hook opening in the dark. In this light-triggered hook opening system, we dissected in greater detail the effect of WDL4 over a shorter time period and minimized the influence of auxin itself.
Although the dynamic reorientation of cortical microtubules has been demonstrated to be important for plant cell growth in both roots and hypocotyls, its relation to auxin-induced cell growth is rather complicated (Adamowski et al., 2019). Our findings are in agreement with previous studies in which auxin-regulated cell growth rapidly in the short-term (Baskin, 2015). In the wdl4-1 mutant, we indeed observed abnormal accumulation of the auxin reporter DR5:GFP in the concave side of the hook region and impaired sensitivity to short-term treatment with the auxin efflux inhibitor NPA, demonstrating that WDL4 is involved in apical hook opening by modulating proper auxin transport and distribution. High auxin levels are known to be inhibitory in the concave side of the apical hook (Beziat and Kleine-Vehn, 2018; Cao et al., 2019). Our observation of high auxin accumulation in wdl4-1 seedlings is consistent with the phenotype of delayed hook opening. Notably, we observed impaired PIN1 and PIN7 distribution, as well as attenuated hook opening induced by BFA treatment in the wdl4-1 mutant, suggesting that WDL4 may be actively involved in cellular activities during hook development via the regulation of protein trafficking. It is well established that proper trafficking and distribution of multiple PM-localized auxin transporters, such as auxin efflux carriers from the PIN family and influx carrier of the AUX/LAX family, are critical for the establishment of the auxin maxima and for hook development (Petrasek et al., 2006; Blakeslee et al., 2007; Zadnikova et al., 2010; Boutte et al., 2013; Jonsson et al., 2017; Wang et al., 2017). Thus we hypothesize that a potential molecular linker might exist between microtubules and the vesicles carrying auxin transporters during hook opening. The MAP CLASP has been shown to function together with the retromer component SNX1 to link microtubules and PIN2-positive endosomes in root cells (Ambrose et al., 2013). Indeed, WDL4 was previously identified in a proteomics study as a protein binding to SOLUBLE N-ETHYLMALEIMIDE-SENSITIVE FACTOR ATTACHMENT PROTEIN RECEPTOR (SNARE) proteins on secretory vesicles (Fujiwara et al., 2014). Our own yeast-two hybrid assay also supported an interaction between WDL4, but not WDL5, and the cytosolic domain of SYNTAXIN OF PLANTS121 (SYP121) (Supplemental Figure S12) (Grefen et al., 2010). Besides the direct effects of the microtubule-MAP module on PIN protein localization by regulating vesicle trafficking, another possibility has been recently reported whereby cortical microtubules affected PIN behavior depending on cellulose microfibrils (Baral et al., 2021). Thus, future study will further unravel these candidate mechanisms and their potential crosstalk for their contribution to differential cell growth in plants.
Materials and methods
Plant growth conditions and materials
Seeds were surface-sterilized, plated on half-strength Murashige and Skoog (MS) medium (2.2 g/L MS salt, 10 g/L sucrose, pH 5.8, and 8 g/L agar), and stratified in the dark at 4°C for 2 days. Germination was induced by placing the plates for 18 h in white light (116 μmol m−2 s−1) at 22°C. Subsequently, seedlings were grown vertically in the dark at 22°C until the designated time points. To exclude the possibility that loss of WDL4 may affect seed germination and thereby causes a delay in hook opening, we examined the germination rate of WT and wdl4 mutant seeds. There was no significant difference between WT and wdl4 mutant seeds.
The eto1-1 (Guzman and Ecker, 1990), EIN3ox (Chao et al., 1997), ein3 eil1 mutants (Alonso et al., 2003), and EIN3-3 × FLAG transgenic ein3 eil1 (iE ein3 eil1) plants (An et al., 2012) used for the ChIP assays were described previously and were kindly provided by Prof. Shuhua Yang of China Agricultural University. The DR5:GFP (Ottenschläger et al., 2003), PIN1pro:PIN1-GFP (Benkova et al., 2003), and PIN7pro:PIN7-GFP (Blilou et al., 2005) lines were described previously and were kindly provided by Prof. Jing Zhang of China Agricultural University. The wdl5-1 mutant, WDL5-OE, and WDL5pro:GUS transgenic lines were used as previously reported (Sun et al., 2015).
The Arabidopsis (A. thaliana) accessions Ws and Col-0 were used as WT in this study. The wdl4-1 (FLAG_414D12) mutant was obtained from the Arabidopsis Biological Resource Center and is in the Ws background with a T-DNA insertion in the second exon of At2g35880. The wdl4-2 mutant line was generated using CRISPR/Cas9 technology as described previously (Wang et al., 2015) and is in the Col-0 background. Two target sites were selected from the second exon of At2g35880. Of the 30 T1 plants obtained, line 5 carried a deletion in the second exon and was used for further phenotypic characterization.
To generate WDL4-OE transgenic lines, the WDL4-coding sequence (CDS) in frame with GFP or mCherry was cloned into the pCAMBIA1300 vector under the control of the Super promoter (Invitrogen), and introduced into Ws plants. Two independent WDL4 transgenic lines were used for further analysis. The WDL4 CDS was cloned into the inducible gene expression vector pER8, then introduced into the DR5:GFP background. Two independent lines were used for further analysis.
To complement the hook phenotype of the wdl4-1 and wdl4-2 mutants, a genomic DNA fragment of WDL4 (1,915-bp upstream of the initiation codon ATG to the stop codon TGA) in frame with GFP was cloned into the pCAMBIA1390 vector, then introduced into the wdl4-1 and wdl4-2 mutants. Two independent lines per mutant were used for further analysis.
To generate transgenic wdl4-1 WDL4pro:GFP-WDL5 plants, the native WDL4 promoter (1,915-bp upstream of the initiation codon ATG) and WDL5 CDS in frame with GFP were PCR-amplified and ligated into the pCAMBIA1390 vector. The construct was introduced into the wdl4-1 mutant. Twelve transgenic lines were obtained, but none complemented the defect in hook opening of the wdl4-1 mutant. Lines 2, 6, and 7 were used for further analysis. The WDL4pro:GUS transgenic lines were generated by amplifying the 1,915-bp promoter of WDL4 from genomic DNA, which was then cloned into the pCAMBIA1391 vector and introduced into WT Col-0. Line 10 was used for histochemical GUS staining analysis. All plant transformations were performed by floral dipping with Agrobacterium (Agrobacterium tumefaciens) strain GV3101 pMP90. Homozygous T3 transgenic lines were used for further analyses.
The ein3 eil1 double mutant, DR5:GFP, PIN1pro:PIN1-GFP, and PIN7pro:PIN7-GFP transgenic lines were in the Col-0 background. To generate the ein3 eil1 wdl4-1 triple mutant, wdl4-1 DR5:GFP, and wdl4-1 PIN1pro:PIN1-GFP lines, the wdl4-1 in the Ws background was backcrossed at least three times to Col-0 and the F2 population was characterized by PCR-based genotyping each time. Genetic crosses generated mCherry-WDL4 GFP-TUA6, ein3 eil1 WDL4pro:GUS, wdl4-1 DR5:GFP, wdl4-1 PIN1pro:PIN1-GFP, and wdl4-1 PIN7pro:PIN7-GFP with the genotypes verified using PCR- or GFP-based assays. Four independent lines of ein3 eil1 WDL4pro:GUS were obtained, and all produced similar results. Two of these lines were used in this study. Thirty-six independent wdl4-1 DR5:GFP lines were obtained, and at least twenty lines were characterized. The observed lines showed similar results, and line 16 is presented in this study. Twelve independent wdl4-1 PIN1pro:PIN1-GFP lines and five independent wdl4-1 PIN7pro:PIN7-GFP lines were obtained, and three lines were observed. The observed lines showed similar results. Line 9 (wdl4-1 PIN1pro:PIN1-GFP) and line 48 (wdl4-1 PIN7pro:PIN7-GFP) presented in this study.
The ein3 eil1 wdl4-1 triple mutant was generated by crossing wdl4-1 and ein3 eil1, followed PCR-based genotyping of the F2 population. Two independent ein3 eil1 wdl4-1 lines were used for further analysis.
Phenotypic analysis
Representative images of hook curvature were taken using an Olympus SEX16 (10X objective, 2X magnification). The angles between cotyledons and hypocotyl growth direction were measured using ImageJ software, as described previously (Schindelin et al., 2012; Yu et al., 2016).
For kinematic analyses of apical hook development, images were captured every 3 h after seed germination. In this experiment, plates containing uniform seedlings were grown vertically in the dark at 22°C. One plate was removed from the dark at each time point and photographed to record apical hook angles. Hook curvature was measured using ImageJ software.
For light-triggered rapid hook opening assays, seedlings were grown vertically in the dark for 42 h after germination, followed by short-term white light exposure (116 μmol m−2 s−1) at 22°C for 1–3 h. The phenotype was recorded at different time points. Hook curvature was measured to calculate hook angle relative to change after illumination.
For NPA (Sigma-Aldrich, St Louis, MO, USA; 10 mM stock in DMSO) and BFA (Sigma-Aldrich, 50 mM stock in DMSO) treatments, seedlings were germinated and grown vertically in the dark for 42 h after germination, and then transferred to plates supplemented with DMSO, β-estradiol (10 μM), NPA (5 μM), or BFA (5 μM). The opening curvature phenotype was recorded and measured using ImageJ after growth for 3–6 h in white light (116 μmol m−2 s−1) at 22°C.
GUS histochemical staining analysis
Seedlings were vertically grown in the dark for 1 or 2 days on half-strength MS plates with or without 5 μM AgNO3 or AVG (Sigma-Aldrich, 1 mM stock in DMSO) and collected after growth for different time points. For ACC (Sigma-Aldrich, 25 mM stock in H2O) treatment, seedlings were grown vertically in the dark for 3 days on half-strength MS plates with or without ACC (10 μM). Collected seedlings were subjected to GUS staining using staining buffer (10 mM EDTA, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 0.1% Triton X-100, and 1 mg/mL X-gluc in 0.1 M phosphate buffer, pH 7.0) for 4 h at 37°C in the dark, as previously described (Wang et al., 2007). GUS-stained seedlings were photographed using an Olympus SEX16 dissecting microscope (10X objective, 4X magnification).
GUS/LUC assay
GUS and LUC assays were performed as previously described (Zhao et al., 2016). Agrobacterium GV3101 pMP90 cultures harboring WDL4pro:GUS together with Super:EIN3 or Super empty vector (pCAMBIA1300) were co-infiltrated into 4- to 6-week-old N. benthamiana leaves using syringes. Super:LUC was added as an internal control. GUS and LUC activities in the infiltrated leaves were quantified after 2 days, and the GUS/LUC ratio was used to measure the binding activity of EIN3 to the WDL4 promoter.
RNA extraction and RT-PCR analysis
Total RNA was extracted with an RNA purification kit (Bio Teke, Beijing, China). First-strand cDNAs were synthesized from total RNA (3 µg) using oligo dT primers and M-MLV reverse transcriptase (TaKaRa, Shiga, Japan). Semi-quantitative RT-PCR analysis was performed to detect WDL4 expression. RT-qPCR was performed to determine WDL4, WDL5, PIN1, and PIN7 relative transcript levels using an ABI 7500 RT PCR detection system with SYBR Premix ExTaq Mix (TaKaRa), according to the manufacturer’s instructions. For expression of PIN1 and PIN7, total RNA was extracted from 1 to 2 mm of apical hypocotyl (excluding cotyledons). In the experiment of ACC transient treatment of WT seedlings to detect WDL4 transcript levels, total RNA was extracted from whole etiolated seedlings. Primers used for RT-qPCR are listed in Supplemental Table S1.
ChIP assay
ChIP analysis was performed using transgenic lines expressing EIN3-3×FLAG under the control of an β-estradiol-inducible promoter, as previously described (Huang et al., 2015). Three-day-old dark-grown seedlings were treated with 10 µM β-estradiol or DMSO (vehicle) for 4 h. After incubation, seedlings were subjected to crosslinking, nuclei isolation, and sonication. After induction, EIN3-3 × FLAG was immunoprecipitated by Protein A agarose (Merck-Millipore, Burlington, MA, USA) with an anti-FLAG monoclonal antibody (Sigma-Aldrich). The enrichment of DNA fragments was analyzed by qPCR using an ABI 7500 RT PCR detection system. The primers used to detect EIN3-targeted WDL4 promoter fragments (P1 and P2) are listed in Supplemental Table S1. P3 was used as a negative control. ACTIN2 was used as a control.
EMSA
GST and GST-EIN3 truncated fusion proteins (amino acids 141–352, containing the DNA binding domain) were produced in Escherichia coli and purified as described (Shi et al., 2012). EMSA was performed using the LightShift Chemiluminescent EMSA Kit (Thermo Fisher Scientific, Waltham, MA, USA). Labeled probes (10 nM) were incubated in 1× binding buffer, 2.5% glycerol, 50 mM KCl, 5 mM MgCl2, and 10 mM EDTA with or without proteins at room temperature for 25 min. For nonlabeled probe competition, the amount of P1 or mP1 and P2 or mP2 competitors was 30-fold more than that of the labeled probe. The oligonucleotide probes, which were labeled at the 5′-end with biotin, are listed in Supplemental Table S1.
Microtubule co-sedimentation assay
Porcine brain tubulin was purified and tubulin assembly and co-sedimentation of microtubules with MBP–WDL4–His fusion protein were performed as previously described (Mao et al., 2005; Li et al., 2011). The purified proteins were centrifuged at 100,000g for 15 min at 4°C before use. Pre-polymerized, paclitaxel-stabilized microtubules (4 μM) were incubated with WDL4 fusion protein (6 μM) in PEMT buffer (10 μM paclitaxel, 1 mM MgCl2, 1 mM EGTA, and 100 mM PIPES-KOH, pH 6.9) at room temperature for 20 min. The samples were centrifuged at 75,000g for 20 min. The supernatant (S) and pellet (P) fractions were subjected to SDS–PAGE.
Low-temperature and dilution assays
Rhodamine-labeled tubulin (20 μM) and MBP-WDL4-His protein/MBP-WDL5-His protein (3 μM) were added to PEM buffer (1 mM MgCl2, 1 mM EGTA, and 100 mM PIPES-KOH, pH 6.9) containing 1 mM GTP. The samples were incubated at 35°C for 40 min for tubulin assembly. For low-temperature experiments, samples were immediately transferred to 10°C for 30 min. For dilution treatments, samples were diluted 50-fold with pre-warmed PEM buffer and incubated for 1 h at 35°C. Samples were fixed with 1% glutaraldehyde for observation and imaging using a spinning disk confocal microscope (Yokogawa, Tokyo, Japan) using an Olympus IX81 microscope with a 100× oil objective.
Ballistics-mediated transient expression in leaf epidermal cells
Ballistics-mediated transient expression of 35Spro:WDL4-GFP was performed to visualize the cellular localization of WDL4-GFP in Col-0 leaf epidermal cells using previously published methods (Fu et al., 2002). We used 2.5 μg plasmid (35Spro:WDL4-GFP) for particle bombardment. GFP signal was observed after incubation for 6–8 h using confocal microscopy (Carl Zeiss LSM710, 40× objective). To confirm the localization of WDL4-GFP, leaf epidermal cells were treated with 10 μM oryzalin (Sigma-Aldrich, 10 mM in DMSO) and 200 nM latrunculin A (Sigma-Aldrich, 1 mM in DMSO) for 10 min.
BFA treatment
To monitor the internalization of PIN1–GFP, seedlings expressing PIN1pro:PIN1-GFP were grown in the dark for 42 h after germination and pretreated with half-strength MS liquid medium containing 50 μM CHX (Sigma-Aldrich, 10 mM in DMSO) for 60 min and then treated with 100 μM BFA in the presence of 50 μM CHX for another 30 min in white light.
For BFA washout assays, seedlings expressing PIN1pro:PIN1-GFP were grown in the dark for 42 h after germination and pretreated with half-strength MS liquid medium containing 50 μM CHX for 30 min, and then 100 μM BFA in the presence of 50 μM CHX for 1.5 h to induce the formation of BFA bodies. Treated seedlings were washed three times and then transferred to half-strength MS liquid medium containing 50 μM CHX for another 30 or 60 min. All treatments were performed under white light.
PIN1–GFP-labeled BFA bodies in the hook region were captured using spinning disk confocal microscopy (40× objective). The auto-fluorescence signals were collected in a separate channel. The images for WT and wdl4-1 mutants expressing PIN1pro:PIN1-GFP were obtained using the same acquisition parameters. The number of PIN1–GFP-labeled BFA bodies per cell and their size was quantified using ImageJ software. The relative values were calculated as a percentage of measured values in each seedling over time.
Live-cell imaging
The DR5:GFP expression pattern in the apical hook region of the dark-grown seedlings was recorded using confocal microscopy (Carl Zeiss LSM710, 10× objective). Seedlings were grown in the dark for different number of days prior to imaging. Identical acquisition parameters were used for WT and wdl4-1 mutants at different time points. DR5:GFP fluorescence intensity at the concave side of the apical hook was measured using ImageJ software. For the effect of induced WDL4 expression on auxin distribution, seedlings were grown vertically in the dark for 48 h after germination and then transferred to half-strength MS liquid medium with DMSO, or β-estradiol in the dark. After incubation for 24 h in the dark, the relative fluorescence intensity of DR5:GFP in seedlings before and after treatment was recorded and measured using ImageJ software. For light treatment, seedlings were grown in the dark for 42 h after germination and were exposed to white light at 22°C for 3 h.
The distribution and cellular localization of PIN1–GFP or PIN7–GFP in the hook region of WT and wdl4-1 mutant plants were visualized using the same acquisition parameters by confocal microscopy (Carl Zeiss LSM880, 40× objective). The fluorescence signals of PIN1–GFP and PIN7–GFP at the PM were measured using ImageJ software.
Yeast two-hybrid assay
To test for the interaction between WDL4 and SYP121, a yeast two-hybrid assay was performed. The CDS corresponding to the cytosolic fragment of the Qa-SNAREs SYP121 (named as SYP121ΔC: amino acids 1–283) was cloned and inserted into the pGBKT7 vector (containing the GAL4 DNA binding domain sequence) as bait (Grefen et al., 2010). WDL4 and WDL5 CDS were separately cloned into the pGADT7 vector (containing the GAL4 activation domain sequence) as prey. Yeast transformation was performed according to the manufacturer’s protocol (Clontech, Mountain View, CA, USA). Transformed yeast cells (strain AH109) were separately spread onto synthetic dropout medium (–Trp –Leu) and selective medium (–Leu –Trp –Ade –His), and incubated at 28°C for 4 days.
Statistical analysis
Student’s t tests were used to compare groups in the indicated experiments. P <0.05 was taken as statistical significance. In all comparisons, *P <0.05 and **P <0.01.
Accession numbers
Sequence data of genes described in this article can be found in the Arabidopsis Genome Initiative under the following accession numbers: WDL4 (At2g35880); WDL5 (At4g32330); EIN3 (At3g20770); PIN1 (At1g73590); PIN7 (At1g23080).
Supplemental data
The following supplemental materials are available in the online version of this article.
Supplemental Figure S1. The phenotype of wdl4-1 is complemented by the expression of WDL4pro:WDL4-GFP.
Supplemental Figure S2. Characterization of GFP-WDL4-OE in the apical hook.
Supplemental Figure S3. The phenotype of wdl4-2 is consistent with wdl4-1 and complemented by the expression of WDL4pro:WDL4-GFP.
Supplemental Figure S4. WDL4 colocalizes with cortical microtubules in vivo and directly binds to microtubules in vitro.
Supplemental Figure S5. WDL5 overexpression has no obvious effect on hook development.
Supplemental Figure S6. Expression and localization of GFP-WDL5 in wdl4-1 WDL4pro:GFP-WDL5 lines.
Supplemental Figure S7. WDL4 expression pattern before hook opening in the dark and during light-induced hook opening.
Supplemental Figure S8. Quantification of DR5:GFP signal in the hook region.
Supplemental Figure S9. Overexpression of WDL4 attenuates sensitivity to NPA and BFA treatment during light-induced hook opening.
Supplemental Figure S10. Asymmetric distribution of PIN1-GFP in apical hooks.
Supplemental Figure S11. WDL4 mutation does not affect the endocytosis of PIN1-GFP during light-induced hook opening.
Supplemental Figure S12. WDL4 interacts with the cytosolic domain of SYP121.
Supplemental Table S1. List of primers used in this study.
Supplemental Table S2. Genetics module.
Supplemental Data Set S1. Statistical analyses.
Supplementary Material
Acknowledgments
The authors thank Prof. Ming Yuan and Prof. Jing Zhang (China Agricultural University) for critical comments on the study.
Funding
This work was supported by grants from the National Science Fund for Distinguished Young Scholars (31625005 to T.M.) and the Natural Science Foundation of China (31872821 to T.M. and 31872644 to X.W.).
Conflict of interest statement. The authors declare no conflict of interests.
X.W. and T.M. designed the experiments; J.D., X.W., and Z.L. performed the experiments; J.D., X.W., and T.M. analyzed the data and wrote the article.
The author responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is Tonglin Mao (maotl2005@cau.edu.cn).
References
- Abas L, Benjamins R, Malenica N, Paciorek T, Wisniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C (2006) Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 8: 249–256 [DOI] [PubMed] [Google Scholar]
- Adamowski M, Friml J (2015) PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 27: 20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamowski M, Li L, Friml J (2019) Reorientation of cortical microtubule arrays in the hypocotyl of Arabidopsis thaliana is induced by the cell growth process and independent of auxin signaling. Int J Mol Sci 20: 3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR (2003) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA 100: 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C, Ruan Y, Gardiner J, Tamblyn LM, Catching A, Kirik V, Marc J, Overall R, Wasteneys GO (2013) CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana. Dev Cell 24: 649–659 [DOI] [PubMed] [Google Scholar]
- An F, Zhang X, Zhu Z, Ji Y, He W, Jiang Z, Li M, Guo H (2012) Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res 22: 915–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal B, Jonsson K, Baral A, Sancho-Andres G, Routier-Kierzkowska AL, Kierzkowski D, Bhalerao RP (2020) Interplay between cell wall and auxin mediates the control of differential cell elongation during apical hook development. Curr Biol 30: 1733–1739 e1733 [DOI] [PubMed] [Google Scholar]
- Baral A, Aryal B, Jonsson K, Morris E, Demes E, Takatani S, Verger S, Xu T, Bennett M, Hamant O. et al. (2021) External mechanical cues reveal a Katanin-independent mechanism behind auxin-mediated tissue bending in plants. Dev Cell 56: 67–80 e63 [DOI] [PubMed] [Google Scholar]
- Baskin TI (2015) Auxin inhibits expansion rate independently of cortical microtubules. Trends Plant Sci 20: 471–472 [DOI] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Beziat C, Kleine-Vehn J (2018) The road to auxin-dependent growth repression and promotion in apical hooks. Curr Biol 28: R519–R525 [DOI] [PubMed] [Google Scholar]
- Beziat C,, Barbez E, Feraru MI, Lucyshyn D, Kleine-Vehn J (2017) Light triggers PILS-dependent reduction in nuclear auxin signalling for growth transition. Nat Plants 3: 17105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, Cheng Y, Bouchard R, Adamec J, et al. (2007) Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19: 131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inze D (1995) Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7: 1405–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutte Y, Jonsson K, McFarlane HE, Johnson E, Gendre D, Swarup R, Friml J, Samuels L, Robert S, Bhalerao RP (2013) ECHIDNA-mediated post-Golgi trafficking of auxin carriers for differential cell elongation. Proc Natl Acad Sci USA 110: 16259–16264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Chen R, Li P, Yu Y, Zheng R, Ge D, Zheng W, Wang X, Gu Y, Gelova Z, et al. (2019) TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature 568: 240–243 [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144. [DOI] [PubMed] [Google Scholar]
- Chen X, Grandont L, Li H, Hauschild R, Paque S, Abuzeineh A, Rakusova H, Benkova E, Perrot-Rechenmann C, Friml J (2014) Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules. Nature 516: 90–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JR (1995) The ethylene signal transduction pathway in plants. Science 268: 667–675 [DOI] [PubMed] [Google Scholar]
- Ecker JR, Theologis A (1994) Ethylene: A Unique Plant Signaling Molecule. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 485–521. [Google Scholar]
- Erickson WKSRO (1978) Kinematics of hypocotyl curvature. Am J Bot 65: 310–319 [Google Scholar]
- Fu Y, Li H, Yang Z (2002) The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14: 777–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, Uemura T, Ebine K, Nishimori Y, Ueda T, Nakano A, Sato MH, Fukao Y (2014) Interactomics of Qa-SNARE in Arabidopsis thaliana. Plant Cell Physiol 55: 781–789 [DOI] [PubMed] [Google Scholar]
- Grefen C, Chen Z, Honsbein A, Donald N, Hills A, Blatt MR (2010) A novel motif essential for SNARE interaction with the K(+) channel KC1 and channel gating in Arabidopsis. Plant Cell 22: 3076–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpham NVJ, Berry AW, Knee EM, Roveda-Hoyos G, Raskin I, Sanders IO, Smith AR, Wood CK, Hall MA (1991) The effect of ethylene on the growth and development of wild-type and mutant Arabidopsis thaliana (L.) Heynh. Ann Bot 68: 55–61 [Google Scholar]
- Hu Y, Vandenbussche F, Van Der Straeten D (2017) Regulation of seedling growth by ethylene and the ethylene-auxin crosstalk. Planta 245: 467–489 [DOI] [PubMed] [Google Scholar]
- Huang M, Hu Y, Liu X, Li Y, Hou X (2015) Arabidopsis LEAFY COTYLEDON1 mediates postembryonic development via interacting with PHYTOCHROME-INTERACTING FACTOR4. Plant Cell 27: 3099–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson K, Boutte Y, Singh RK, Gendre D, Bhalerao RP (2017) Ethylene regulates differential growth via BIG ARF-GEF-dependent post-Golgi secretory trafficking in Arabidopsis. Plant Cell 29: 1039–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson K, Lathe RS, Kierzkowski D, Routier-Kierzkowska AL, Hamant O, Bhalerao RP. (2021) Mechanochemical feedback mediates tissue bending required for seedling emergence. Curr Biol 31: 1–11 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Swarup R, Bennett M, Friml J (2006) Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell 18: 3171–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Wabnik K, Martiniere A, Langowski L, Willig K, Naramoto S, Leitner J, Tanaka H, Jakobs S, Robert S, et al. (2011) Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Mol Syst Biol 7: 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Vandenbussche F, De Cnodder T, Van Der Straeten D, Verbelen J (2005) Cell elongation and microtubule behaviour in the Arabidopsis hypocotyl: responses to ethylene and auxin. J Plant Growth Regul 24: 166–178 [Google Scholar]
- Lehman A, Black R, Ecker JR (1996) HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85: 183–194 [DOI] [PubMed] [Google Scholar]
- Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR (2004) Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev Cell 7: 193–204 [DOI] [PubMed] [Google Scholar]
- Li J, Wang X, Qin T, Zhang Y, Liu X, Sun J, Zhou Y, Zhu L, Zhang Z, Yuan M, et al. (2011) MDP25, a novel calcium regulatory protein, mediates hypocotyl cell elongation by destabilizing cortical microtubules in Arabidopsis. Plant Cell 23: 4411–4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Lei L, Somerville CR, Gu Y (2012) Cellulose synthase interactive protein 1 (CSI1) links microtubules and cellulose synthase complexes. Proc Natl Acad Sci USA 109: 185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian N, Wang X, Jing Y, Lin J (2021) Regulation of cytoskeleton-associated protein activities: linking cellular signals to plant cytoskeletal function. J Integr Plant Biol 63: 241–250 [DOI] [PubMed] [Google Scholar]
- Liscum E, Hangarter RP (1993) Light-stimulated apical hook opening in wild-type Arabidopsis thaliana seedlings. Plant Physiol 101: 567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T, Jin L, Li H, Liu B, Yuan M (2005) Two microtubule-associated proteins of the Arabidopsis MAP65 family function differently on microtubules. Plant Physiol 138: 654–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzella MA, Casal JJ, Muschietti JP, Fox AR (2014) Hormonal networks involved in apical hook development in darkness and their response to light. Front Plant Sci 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17: 181–195 [DOI] [PubMed] [Google Scholar]
- Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100: 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Zazimalova E, Ruthardt N, Petrasek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jurgens G, Geldner N, et al. (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Perrin RM, Wang Y, Yuen CY, Will J, Masson PH (2007) WVD2 is a novel microtubule-associated protein in Arabidopsis thaliana. Plant J 49: 961–971 [DOI] [PubMed] [Google Scholar]
- Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Wisniewska J, Tadele Z, Kubes M, Covanova M, et al. (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312: 914–918 [DOI] [PubMed] [Google Scholar]
- Raz V, Ecker JR (1999) Regulation of differential growth in the apical hook of Arabidopsis. Development 126: 3661–3668 [DOI] [PubMed] [Google Scholar]
- Raz V, Koornneef M (2001) Cell division activity during apical hook development. Plant Physiol 125: 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Kleine-Vehn J (2019) PIN-FORMED and PIN-LIKES auxin transport facilitators. Development 146: dev168088. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P, Palme K (2016) Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules? A critical comment. Plant Physiol 170: 23–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Li Y, Pan Y, Zhong S (2016) Activation of HLS1 by mechanical stress via ethylene-stabilized EIN3 is crucial for seedling soil emergence. Front Plant Sci 7: 1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Tian S, Hou L, Huang X, Zhang X, Guo H, Yang S (2012) Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 24: 2578–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ma Q, Mao T (2015) Ethylene regulates the Arabidopsis microtubule-associated protein WAVE-DAMPENED2-LIKE5 in etiolated hypocotyl elongation. Plant Physiol 169: 325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Petrasek J, Zadnikova P, Hoyerova K, Pesek B, Raz V, Swarup R, Bennett M, Zazimalova E, Benkova E, et al. (2010) The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 137: 597–606 [DOI] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wisniewska J, Benkova E, Benjamins R, Beeckman T, Luschnig C, Friml J (2005) Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132: 4521–4531 [DOI] [PubMed] [Google Scholar]
- Wang L, Uilecan IV, Assadi AH, Kozmik CA, Spalding EP (2009) HYPOTrace: image analysis software for measuring hypocotyl growth and shape demonstrated on Arabidopsis seedlings undergoing photomorphogenesis. Plant Physiol 149: 1632–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mao T (2019) Understanding the functions and mechanisms of plant cytoskeleton in response to environmental signals. Curr Opin Plant Biol 52: 86–96 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang J, Yuan M, Ehrhardt DW, Wang Z, Mao T (2012) Arabidopsis microtubule destabilizing protein40 is involved in brassinosteroid regulation of hypocotyl elongation. Plant Cell 24: 4012–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhu L, Liu B, Wang C, Jin L, Zhao Q, Yuan M (2007) Arabidopsis MICROTUBULE-ASSOCIATED PROTEIN18 functions in directional cell growth by destabilizing cortical microtubules. Plant Cell 19: 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang L, Tang Y, Tang R, Jing Y, Zhang C, Zhang B, Li X, Cui Y, Zhang C. et al. (2017) Arabidopsis choline transporter-like 1 (CTL1) regulates secretory trafficking of auxin transporters to control seedling growth. PLoS Biol 15: e2004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZP, Xing HL, Dong L, Zhang HY, Han CY, Wang XC, Chen QJ (2015) Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol 16: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige BC, Ogiso-Tanaka E, Zourelidou M, Schwechheimer C (2012) WAG2 represses apical hook opening downstream from gibberellin and PHYTOCHROME INTERACTING FACTOR 5. Development 139: 4020–4028 [DOI] [PubMed] [Google Scholar]
- Woeste KE, Ye C, Kieber JJ (1999) Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol 119: 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E (2006) High-affinity auxin transport by the AUX1 influx carrier protein. Curr Biol 16: 1123–1127 [DOI] [PubMed] [Google Scholar]
- Yu Q, Zhang Y, Wang J, Yan X, Wang C, Xu J, Pan J (2016) Clathrin-mediated auxin efflux and maxima regulate hypocotyl hook formation and light-stimulated hook opening in Arabidopsis. Mol Plant 9: 101–112 [DOI] [PubMed] [Google Scholar]
- Yuen CY, Pearlman RS, Silo-Suh L, Hilson P, Carroll KL, Masson PH (2003) WVD2 and WDL1 modulate helical organ growth and anisotropic cell expansion in Arabidopsis. Plant Physiol 131: 493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadnikova P, Petrasek J, Marhavy P, Raz V, Vandenbussche F, Ding Z, Schwarzerova K, Morita MT, Tasaka M, Hejatko J, et al. (2010) Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 137: 607–617 [DOI] [PubMed] [Google Scholar]
- Zemlyanskaya EV, Omelyanchuk NA, Ubogoeva EV, Mironova VV (2018) Deciphering auxin-ethylene crosstalk at a systems level. Int J Mol Sci 19: 4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ji Y, Xue C, Ma H, Xi Y, Huang P, Wang H, An F, Li B, Wang Y. et al. (2018) Integrated regulation of apical hook development by transcriptional coupling of EIN3/EIL1 and PIFs in Arabidopsis. Plant Cell 30: 1971–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Zhang ML, Ma TL, Wang Y (2016) Phosphorylation of ARF2 relieves its repression of transcription of the K+ transporter gene HAK5 in response to low potassium stress. Plant Cell 28: 3005–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.