Summary
HUWE1 is a HECT-domain ubiquitin E3 ligase expressed in various tissues. Although HUWE1 is known to promote degradation of the tumor suppressor p53, given a growing list of its substrates, in vivo functions of HUWE1 remain elusive. Here, we investigated the role of HUWE1 in the female reproductive system. Homozygous deletion of Huwe1 in mouse oocytes of primary follicles caused oocyte death and female infertility, whereas acute depletion of HUWE1 protein by Trim-Away technology did not impact oocytes from antral follicles. Interestingly, oocytes from Huwe1 heterozygous females matured and fertilized normally, but the majority of embryos that lacked maternal Huwe1 were arrested at the morula stage after fertilization. Consequently, Huwe1 heterozygous females only produced wild-type pups. Concomitant knockout of p53 did not recover fertility of the Huwe1 knockout females. These findings make HUWE1 a unique and critical maternal factor indispensable for maintaining the quality of oocytes and embryos.
Subject Areas: Cell Biology, Developmental Biology
Graphical Abstract
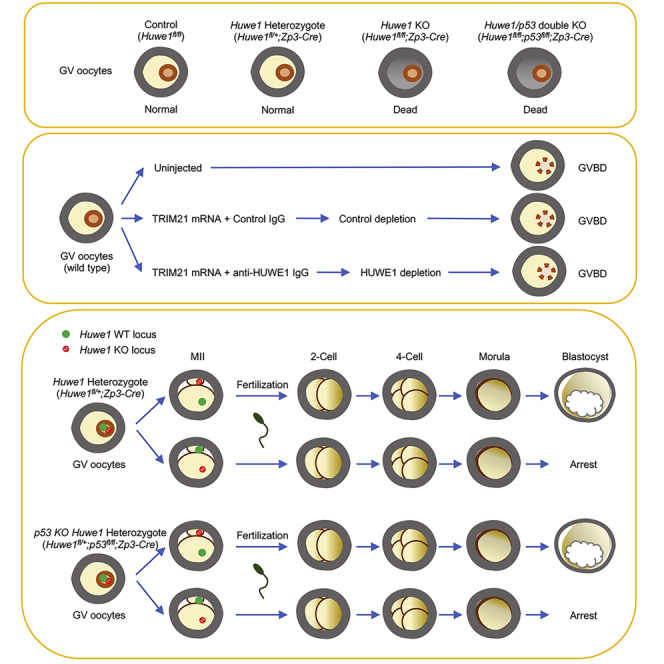
Highlights
-
•
Oocyte-specific Huwe1 KO leads to female infertility in mice
-
•
Homozygous, but not heterozygous, Huwe1 KO kills oocytes before the GV stage
-
•
Maternal Huwe1 is also required for preimplantation embryonic development
-
•
Defects in oocyte and embryonic development of Huwe1 KO mice are independent of p53
Cell Biology; Developmental Biology
Introduction
The development of mammalian oocytes is a tightly controlled cellular process. In adult mice and humans, the majority of oocytes are arrested at the diplotene stage of the first meiotic prophase, commonly referred to as the germinal vesicle (GV) stage. During an estrous cycle, a group of follicles is recruited to grow to their maximum size, while the follicle continues to increase the number of granulosa cells surrounding an oocyte. In response to the luteinizing hormone surge, an oocyte in a preovulatory follicle resumes meiosis. As females are born with a finite number of oocytes, and also because oocytes are single cells, even a single genetic mutation can lead to detrimental outcomes, such as oocyte death, abnormal fertilization, or embryonic lethality, which leads to infertility in females.
HUWE1 (also known as, ARF-BP1, MULE, and HectH9) is a HECT-domain ubiquitin E3 ligase widely expressed in normal tissues, and its gene, Huwe1, is located on the X chromosome in both mice and humans. HUWE1 was initially identified as an E3 ligase that targets the tumor suppressor p53 for degradation (Chen et al., 2005). Consistent with this finding, Huwe1 RNAi knockdown or gene knockout (KO) results in p53 accumulation and the induction of apoptosis in certain tissues/cells (Chen et al., 2005; Hao et al., 2012; Kon et al., 2012; Wang et al., 2014). However, targeted deletion of Huwe1 in other tissues, including keratinocytes, male germ cells, and hematopoietic progenitor cells, does not lead to noticeable p53 activation (Chen et al., 2016; Fok et al., 2017; Inoue et al., 2013; King et al., 2016), suggesting that the role of HUWE1 is complex and may be context dependent.
In this study, we investigated the role of HUWE1 in the development of oocytes and preimplantation embryos by using oocyte-specific Huwe1 and p53 KO mouse models. We also employed Trim-Away technology to promote acute degradation of HUWE1 protein in GV-stage oocytes. Our results uncover critical, p53-independent roles of HUWE1 in the female reproductive system.
Results
Oocyte-Specific Huwe1 KO Mice Are Completely Infertile
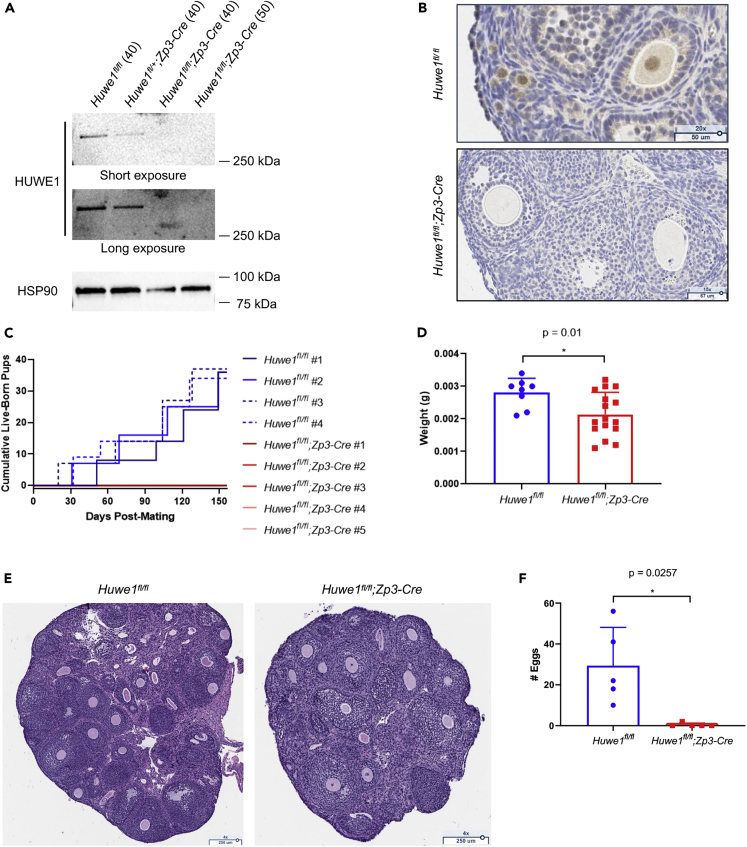
To investigate the role of HUWE1 in the female reproductive system, we employed a Cre-loxP strategy to achieve oocyte-specific disruption of the Huwe1 gene from primary follicle stage. We generated oocyte-specific Huwe1 KO mice by crossing Huwe1 flox (Huwe1fl/fl(y)) mice, in which Huwe1 exon 11 is flanked by loxP sites (Kon et al., 2012), to oocyte-specific Cre-expressing mice (Zp3-Cre). Western blot showed complete depletion of HUWE1 protein in oocytes collected from Huwe1fl/fl;Zp3-Cre mice (Figure 1A). Immunohistochemistry also confirmed the absence of HUWE1 protein in oocytes of KO mice, whereas HUWE1 protein predominantly localized in the nucleus of oocytes in control mice (Figure 1B). When control (Huwe1fl/fl) and Huwe1 KO (Huwe1fl/fl;Zp3-Cre) females were bred with wild-type males, there were no pups obtained from KO females (Figure 1C). Ovaries from Huwe1 KO mice were significantly lighter (Figure 1D) compared with those from control mice. Histological analysis of ovaries showed that ovaries from Huwe1 KO mice contained fewer numbers of follicles at 4 weeks of age (Figure 1E). When superovulation was induced with Equine Chorionic Gonadotropin (eCG) and Human Chorionic Gonadotropin (hCG) injections, KO mice at 8 weeks of age ovulated nearly no eggs, whereas control mice ovulated an average of 29.4 eggs per female (Figures 1F and S1).
Figure 1.
Huwe1 KO Females are Completely Infertile
(A) Western blot performed with the indicated antibodies for whole GV-stage oocytes collected from control (Huwe1fl/fl), Huwe1 heterozygous (Huwe1fl/+;Zp3-Cre), and Huwe1 KO (Huwe1fl/fl;Zp3-Cre) mice. The numbers of oocytes loaded in each lane were indicated in the parentheses.
(B) Representative images of ovaries from 3-week-old control (Huwe1fl/fl) and Huwe1 KO (Huwe1fl/fl;Zp3-Cre) mice stained with the indicated antibodies.
(C) The cumulative number of live-born pups when 4 control (Huwe1fl/fl #1–4) and 5 Huwe1 KO (Huwe1fl/fl;Zp3-Cre #1–5) females were bred with wild-type males, respectively.
(D) The average weight of single ovaries collected from 3-week-old control (Huwe1fl/fl) and Huwe1 KO (Huwe1fl/fl;Zp3-Cre) mice.
(E) Hematoxylin and eosin stain of ovaries collected from control (Huwe1fl/fl) and Huwe1 KO (Huwe1fl/fl;Zp3-Cre) mice.
(F) The average number of eggs collected from control (Huwe1fl/fl) and Huwe1 KO (Huwe1fl/fl;Zp3-Cre) mice after superovulation. In (D) and (F), statistical significance was assessed using Student's t test. The error bars represent the standard deviation.
Huwe1 KO Oocytes Fail to Mature
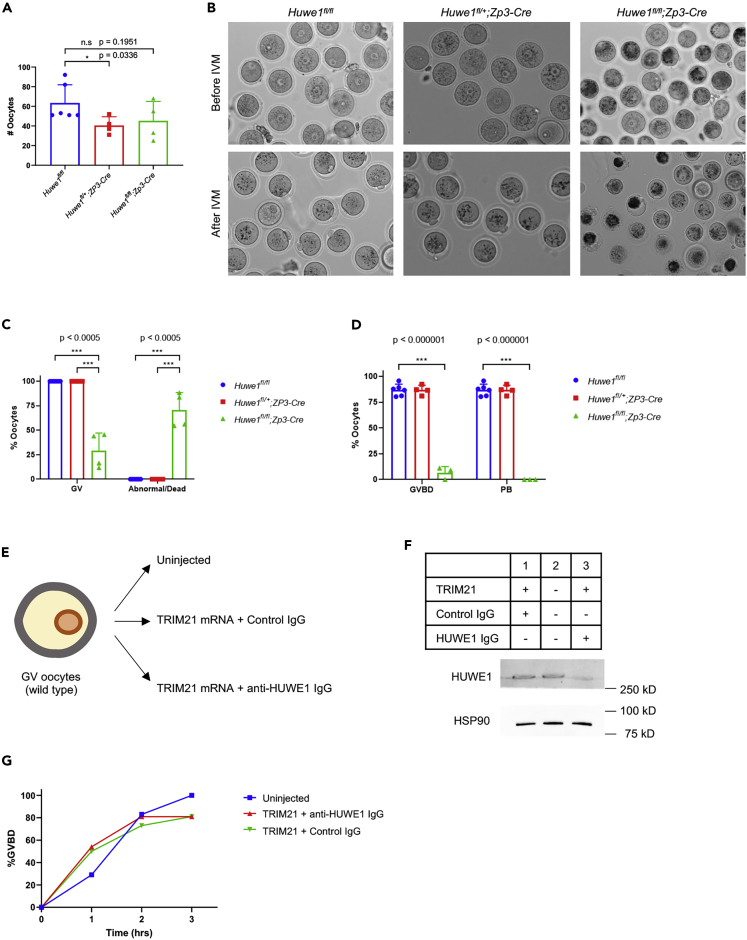
The failure in superovulation prompted us to analyze oocytes in Huwe1 KO mice. GV-stage oocytes were retrieved from ovaries from control (Huwe1fl/fl) and Huwe1 KO (Huwe1fl/fl;Zp3-Cre) mice following eCG administration. The total number of oocytes retrieved from ovaries was not statistically different between Huwe1 KO mice and littermate control mice (Figure 2A). However, many of the oocytes collected from Huwe1 KO mice showed morphological abnormalities, such as the lack of a clear GV and the presence of dark spots in the cytoplasm (Figures 2B and 2C). Accordingly, upon in vitro maturation (IVM), the vast majority of Huwe1 KO oocytes did not complete GV breakdown (GVBD) and none of the KO oocytes extruded the first polar body (Figure 2D).
Figure 2.
Huwe1 KO Oocytes Fail IVM
(A) The number of oocytes collected from antral follicles of control (Huwe1fl/fl), Huwe1 heterozygous (Huwe1fl/+;Zp3-Cre), and Huwe1 KO (Huwe1fl/fl;Zp3-Cre) mice.
(B) Representative images of oocytes collected from control (Huwe1fl/fl), Huwe1 heterozygous (Huwe1fl/+;Zp3-Cre), and Huwe1 KO (Huwe1fl/fl;Zp3-Cre) mice before and after in vitro maturation (IVM).
(C) The percentage of morphologically normal GV-stage oocytes and abnormal oocytes collected from antral follicles of control (Huwe1fl/fl), Huwe1 heterozygous (Huwe1fl/+;Zp3-Cre), and Huwe1 KO (Huwe1fl/fl;Zp3-Cre) mice.
(D) The percentage of GV-stage oocytes that underwent GVBD or extruded the first polar body (PB) after in vitro maturation. In (A), (C), and (D), statistical significance was assessed using Student's t test. The error bars represent the standard deviation.
(E) Scheme of the Trim-Away method.
(F) Western blot performed with the indicated antibodies for whole GV-stage oocytes after Trim-Away.
(G) The percentage of oocytes undergoing GVBD over time after Trim-Away.
Interestingly, compared with control mice, Huwe1 heterozygous mice (Huwe1fl/+;Zp3-Cre) also produced significantly fewer GV oocytes (Figure 2A). However, unlike Huwe1 KO oocytes, oocytes collected from Huwe1 heterozygous mice were morphologically indistinguishable from control oocytes (Figure 2B) and successfully matured in vitro (Figures 2C and 2D), indicating that one copy of the Huwe1 gene is sufficient to support oocyte maturation. It should be noted that compared with control oocytes, approximately 50% less Huwe1 protein was detected in Huwe1 heterozygous oocytes (Figure 1A).
As our results showed that Huwe1 KO oocytes were already dead at or before the GV stage, we next investigated the impact of acute deletion of HUWE1 protein in GV-stage oocytes. Toward this end, we used the “Trim-Away” technology to force degradation of HUWE1 protein (Clift et al., 2018, 2017). In brief, wild-type mouse oocytes were co-injected with mRNA encoding TRIM21 and control IgG or an anti-HUWE1 antibody (Figure 2E). Western blot analysis confirmed that a substantial reduction of HUWE1 protein was induced by the anti-HUWE1 antibody (Figure 2F). Interestingly, the depletion of HUWE1 protein did not affect the morphology of the oocytes (Figure S2). Moreover, oocytes underwent GVBD normally despite HUWE1 depletion (Figures 2G and S2). Taken together, these results suggest that HUWE1 is required before GV formation.
Maternal HUWE1 Is Also Required for Early Embryogenesis
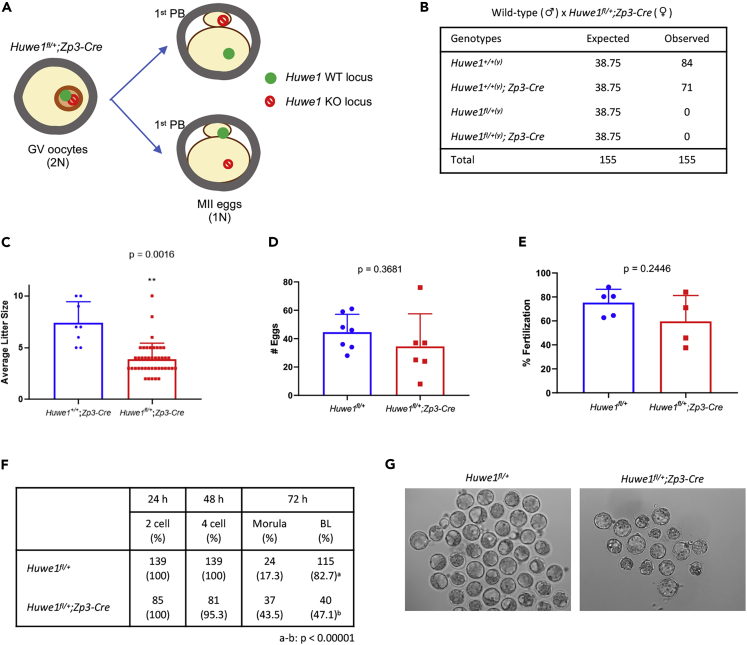
The aforementioned studies showed that one copy of the Huwe1 gene is sufficient to support full oocyte development (Figure 3A). Therefore, Huwe1 heterozygous mice (Huwe1fl/+;Zp3-Cre) were fertile when bred with a wild-type male. However, all the offspring derived from the heterozygous mice carried the Huwe1 wild-type allele, but not the Huwe1 flox allele (Figure 3B). Moreover, the number of offspring from Huwe1 heterozygous mice (3.9 per female) was approximately half of that from control (Huwe1+/+;Zp3-Cre) female mice (7.4 per female) (Figure 3C). These results suggest that in addition to oocyte death, the lack of maternal Huwe1 would result in embryonic lethality. Thus, we next performed in vitro fertilization (IVF) to investigate how the lack of Huwe1 impacts fertilization and early embryonic development. Huwe1 heterozygous mice (Huwe1fl/+;Zp3-Cre) responded normally to the superovulation treatment, although the average number of ovulated eggs was slightly smaller than that of control mice (Huwe1fl/+) (Figure 3D). Likewise, eggs from Huwe1 heterozygous mice fertilized and developed to the 4-cell stage at a similar rate to eggs from control mice (Figures 3E and 3F). Interestingly, whereas 82.7% fertilized control eggs developed to blastocysts by 72 h post-IVF, only 41.7% eggs from Huwe1 heterozygous mice reached the blastocyst stage (Figures 3F and 3G). At 72 h post-IVF, 43.5% eggs from Huwe1 heterozygous mice were still at the morula stage, whereas only 17.3% control eggs remained at this stage (Figures 3F and 3G). It is expected that 50% eggs derived from Huwe1 heterozygous mice carry the Huwe1 KO locus (Figure 3A). Importantly, it was confirmed by PCR that the majority of the morulae (23/30) carried the Huwe1 KO locus. In contrast, 6 of 20 blastocysts were positive for Huwe1 deletion. These results indicated that the lack of maternal Huwe1 impairs the development of preimplantation embryos.
Figure 3.
The Lack of Maternal Huwe1 Leads to Impaired Blastocyst Formation In Vitro
(A) Schematic of the transition from the GV stage to the second stage of meiosis (MII) in oocytes carrying a Huwe1 KO locus.
(B) Analysis of offspring from the cross between Huwe1 heterozygous (Huwe1fl/+;Zp3-Cre) females and wild-type males.
(C) The average litter size of control (Huwe1+/+;Zp3-Cre) and Huwe1 heterozygous (Huwe1fl/+;Zp3-Cre) mice when crossed with wild-type males.
(D) The number of MII eggs collected from control (Huwe1fl/+) and Huwe1 heterozygous (Huwe1fl/+;Zp3-Cre) mice after superovulation.
(E) The in vitro fertilization rates of eggs from control (Huwe1fl/+) and Huwe1 heterozygous (Huwe1fl/+;Zp3-Cre) mice with wild-type mouse sperm. In (C) and (D), statistical significance was assessed using Student's t test. The error bars represent the standard deviation.
(F) Analysis of preimplantation embryo development after IVF. Statistical significance in the blastocyst (BL) formation was assessed using chi-square test (a-b: p < 0.00001).
(G) Representative images of preimplantation embryo 72 h after IVF.
Roles of Huwe1 in Oogenesis and Embryonic Development Are p53 Independent
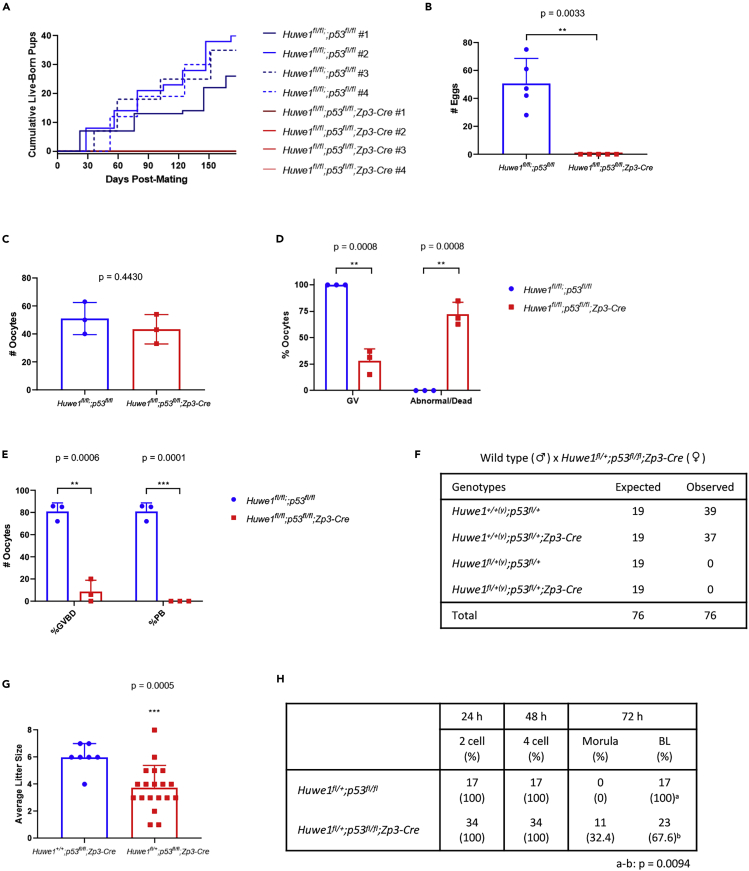
Among the known key substrates of HUWE1 is p53, which regulates cell death. Oocyte-specific deletion of MDM2, another major E3 ligase for p53, results in female infertility due to oocyte death caused by abundant p53 (Livera et al., 2016). Hence, we examined whether co-deletion of p53 could rescue the infertility in Huwe1-deficient female mice. Interestingly, oocyte-specific Huwe1/p53 double KO female mice (Huwe1fl/fl;p53fl/fl;Zp3-Cre) were still completely infertile (Figure 4A) and did not release any eggs upon superovulation (Figure 4B). When the number of total GV-stage oocytes in ovaries was analyzed after eCG administration, there were no significant differences between control and double KO mice (Figure 4C). However, similar to what was observed in Huwe1 single KO mice (Figure 2B), more than 70% oocytes from double KO mice were found to be abnormal or dead (Figures 4D and S3A). Upon IVM, very few double KO oocytes underwent GVBD and none of them extruded the first polar body (Figures 4E and S3A).
Figure 4.
Concomitant Deletion of p53 Does Not Recover the Fertility of Huwe1 KO Females
(A) The cumulative number of live-born pups when 4 control (Huwe1fl/fl;p53fl/fl, #1–4) and 4 Huwe1/p53 double KO (Huwe1fl/fl;p53fl/fl;Zp3-Cre, #1–4) females were bred with wild-type males, respectively.
(B) The average number of oocytes released from control (Huwe1fl/fl;p53fl/fl) and Huwe1 KO (Huwe1fl/fl;p53fl/fl;Zp3-Cre) mice following superovulation.
(C) The number of oocytes collected from antral follicles of control (Huwe1fl/fl;p53fl/fl) and Huwe1 KO (Huwe1fl/fl;p53fl/fl;Zp3-Cre) mice.
(D) The percentage of morphologically normal GV-stage oocytes and abnormal oocytes collected from antral follicles of control (Huwe1fl/fl;p53fl/fl) and Huwe1 KO (Huwe1fl/fl;p53fl/fl;Zp3-Cre) mice.
(E) The percentage of GV-stage oocytes that underwent GVBD or extruded the first polar body (PB) after in vitro maturation.
(F) Analysis of offspring from the cross between p53 KO Huwe1 heterozygous (Huwe1fl/+;p53fl/fl;Zp3-Cre) females and wild-type males.
(G) The average litter size of p53 KO (Huwe1+/+;p53fl/fl;Zp3-Cre) females and p53 KO Huwe1 heterozygous (Huwe1fl/+;p53fl/fl;Zp3-Cre) females when crossed with wild-type males. In (B), (C), (D), (E), and (G), statistical significance was assessed using Student's t test. The error bars represent the standard deviation.
(H) Analysis of preimplantation embryo development after IVF. Statistical significance in the blastocyst (BL) formation was assessed using Fisher's exact test (a-b: p = 0.0094).
We next examined how the concomitant deletion of p53 affected impaired blastocyst formation of eggs from Huwe1 heterozygous mice. Interestingly, deletion of p53 did not impact the survival of embryos that lacked maternal Huwe1, as Huwe1 heterozygous p53 KO females (Huwe1fl/+;p53fl/fl;Zp3-Cre) only produced pups with wild-type Huwe1 allele(s) (Figure 4F). As seen in Huwe1 heterozygous females (Figure 3B), even after concomitant deletion of p53, the average litter size of Huwe1 heterozygous females (3.7 per female) was still significantly smaller than that of control mice (6.0 per female) (Figure 4G). IVF experiments showed that eggs from Huwe1 heterozygous p53 KO females (Huwe1fl/+;p53fl/fl;Zp3-Cre) developed to blastocysts at a significantly lower rate than eggs from control females (Huwe1fl/+;p53fl/fl) (Figures 4H and S3B). These results indicate that the functions of HUWE1 in oogenesis and embryonic development are independent of p53.
Discussion
In the present study, we demonstrated that Huwe1 KO leads to complete infertility due to death of GV-stage oocytes. We also showed that although one copy of Huwe1 is sufficient for oocytes to undergo maturation, ovulation, and fertilization, the lack of maternal Huwe1 still hampers the subsequent development and results in early embryonic lethality. Together, our results indicate that HUWE1 is a critical maternal factor that plays important roles in both oocyte maturation and preimplantation embryo development.
We showed that the deletion of the Huwe1 gene at the primary follicle stage, when Cre recombinase is expressed under the Zp3 promoter (Lan et al., 2004), killed the vast majority of oocytes before they reached the antral follicle stage (Figures 2B–2D). Interestingly, one copy of Huwe1 is sufficient to protect oocytes during this process (Figures 2B–2D). Moreover, the acute depletion of HUWE1 protein in the GV-stage oocytes isolated from antral follicles did not affect GVBD (Figures 2G and S2). These results suggest that either HUWE1 is indispensable only for the early oocyte maturation process or the loss of Huwe1 in the primary follicle stage leads to the accumulation of HUWE1 substrates over time, which causes oocyte death at a later stage of folliculogenesis. Interestingly, it is reported that HUWE1 is required for the transition from gonocytes to spermatogonia, as well as for the maintenance of spermatogonia (Bose et al., 2017; Fok et al., 2017). Thus, these results indicate that the requirement of HUWE1 is shared in both spermatogenesis and oogenesis.
Previously, it was shown that small interfering RNA (siRNA)-mediated Huwe1 knockdown in fertilized mouse eggs caused apoptosis and resulted in poor embryonic development in vitro (Chen et al., 2016). It is interesting that siRNA-mediated HUWE1 depletion only affected the late stage (i.e., blastocyst formation) of preimplantation embryos in vitro (Chen et al., 2016). Our results support this previous observation as the loss of one copy of maternal Huwe1 mainly impacted the transition from morulae to blastocysts (Figure 3F). Although it is tempting to speculate that the majority of embryos that lack the maternal Huwe1 die before implantation, this needs to be tested by directly analyzing early post-implantation embryos in the uterus. It is interesting to note that another earlier study demonstrated that when Huwe1fl/+ female mice were crossed with Rosa26-Cre homozygote males, Huwe1 KO embryos (Huwe1fl/y;Rosa26-Cre) survived until E14.5 when they died due to hemorrhage (Kon et al., 2012). This may be ascribed to a different Cre system used in the study. The Zp3-Cre system deletes Huwe1 specifically in oocytes of primary follicles, whereas sperm-delivered Cre recombinase may not be fully expressed until the 2-cell stage or later, which would result in genetic mosaics of Huwe1 in the embryos. Regardless, our finding is consistent with the results from these earlier studies, highlighting the critical role of HUWE1 during embryogenesis.
p53 is a transcription factor that can induce apoptosis and cell-cycle arrest in response to cytotoxic or genotoxic stimuli. The ubiquitin E3 ligase MDM2 is the major inhibitor of p53 (Wade et al., 2010). Mdm2 KO induces embryonic lethality in mice due to fatal p53 activation (Jones et al., 1995; Montes de Oca Luna et al., 1995). Moreover, oocyte-specific Mdm2 KO mice are infertile because of oocyte death in the early phase of folliculogenesis (Livera et al., 2016). Importantly, the infertility can be rescued by co-KO of p53, indicating that death in Mdm2 KO oocytes is ascribed to lethal p53 activation (Livera et al., 2016). Similar to MDM2, HUWE1 is also a p53-targeting E3 ligase (Chen et al., 2005). However, in the present study, we determined that co-depletion of p53 does not recover the fertility of Huwe1 KO female mice, demonstrating that defects in oocyte maturation and embryonic development of the KO female mice are independent of p53. The role of HUWE1 in p53 regulation appears to be complex and may change during development or depending on stress signals or cell types. It is likely that the infertile phenotype of Huwe1 KO female mice is secondary to the upregulation of a HUWE1 substrate other than p53. Indeed, HUWE1 ubiquitinates a wide range of cellular substrates, including MYC and MCL1 (Cassidy et al., 2020; Chen et al., 2005; Herold et al., 2008; Zhao et al., 2008; Zhong et al., 2005). It is suggested that the “net effect” of Huwe1 depletion is determined by the availability of its substrates as well as the function of each substrate. In this regard, the major substrate of HUWE1 that causes defects in oocyte maturation and embryogenesis remains to be determined.
Limitations of the Study
This study demonstrated that the ubiquitin E3 ligase HUWE1 is essential for oocyte maturation and preimplantation embryo development. The specific substrate(s) of HUWE1 that regulates these cellular processes remains to be identified.
Resource Availability
Lead Contact
Further information and requests should be directed to and will be fulfilled by the Lead Contact, Manabu Kurokawa (mkurokaw@kent.edu).
Materials Availability
New unique reagents were not generated in this study.
Data and Code Availability
The data in this study are available from the corresponding author upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We would like to thank Mark Moser, Sandeep Kaur, Rachel Tinkey, Cora Day, Grace Jones, Gabrielle Murray, and Rebecca Brady for their assistance with the animal work. We would also like to thank Becky O'Meara for preparation of histology samples. This study was supported by an NIH Career Development Award R00 CA140948 and an NIH R03 CA230828 (to M.K.), and by R03 HD099378 and R01 HD096037 (to L.M.M.). E.M.M. and K.J.C. are the SURE program scholars at Kent State University. E.M.M. and K.J.C. are also recipients of the Gary Larkin Scholarship and the Harold R. Papiska Scholarship at Kent State University, respectively.
Author Contributions
Conception and Design, M.K.; Development of Methodology, A.A.E., S.B., N.K., W.G., and L.M.M.; Acquisition of Data, A.A.E., S.B., K.J.C., E.M.M., W.W.F., W.W., and L.M.M.; Analysis and Interpretation of Data, A.A.E., S.B., K.J.C., E.M.M., W.W.F., W.W., L.M.M., and M.K.; Writing of the Manuscript; A.A.E., S.B., K.J.C., and M.K.; Administrative, Technical, or Material Support, S.D., W.W., N.K., W.G., L.M.M., and S.V.; Study Supervision: M.K.
Declaration of Interests
The authors declare no competing interests.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101523.
Supplemental Information
References
- Bose R., Sheng K., Moawad A.R., Manku G., O’Flaherty C., Taketo T., Culty M., Fok K.L., Wing S.S. Ubiquitin ligase Huwe1 modulates spermatogenesis by regulating spermatogonial differentiation and entry into meiosis. Sci. Rep. 2017;7:17759. doi: 10.1038/s41598-017-17902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy K.B., Bang S., Kurokawa M., Gerber S.A. Direct regulation of Chk1 protein stability by E3 ubiquitin ligase HUWE1. FEBS J. 2020;287:1985–1999. doi: 10.1111/febs.15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Kon N., Li M., Zhang W., Qin J., Gu W. ARF-BP1/Mule is a critical mediator of the ARF tumor suppressor. Cell. 2005;121:1071–1083. doi: 10.1016/j.cell.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Chen L., Xu W., Yang M., Wang K., Chen Y., Huang X., Ma Q. HUWE1 plays important role in mouse preimplantation embryo development and the dysregulation is associated with poor embryo development in humans. Sci. Rep. 2016;6:37928. doi: 10.1038/srep37928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift D., McEwan W.A., Labzin L.I., Konieczny V., Mogessie B., James L.C., Schuh M. A method for the acute and rapid degradation of endogenous proteins. Cell. 2017;171:1692–1706.e18. doi: 10.1016/j.cell.2017.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift D., So C., McEwan W.A., James L.C., Schuh M. Acute and rapid degradation of endogenous proteins by Trim-Away. Nat. Protoc. 2018;13:2149–2175. doi: 10.1038/s41596-018-0028-3. [DOI] [PubMed] [Google Scholar]
- Fok K., Bose R., Sheng K., Chang C.-W., Katz-Egorov M., Culty M., Su S., Yang M., Ruan Y., Chan H.-C. Huwe1 regulates the establishment and maintenance of spermatogonia by suppressing DNA damage response. Endocrinology. 2017;158:4000–4016. doi: 10.1210/en.2017-00396. [DOI] [PubMed] [Google Scholar]
- Hao Z., Duncan G.S., Su Y.-W.W., Li W.Y., Silvester J., Hong C., You H., Brenner D., Gorrini C., Haight J. The E3 ubiquitin ligase Mule acts through the ATM-p53 axis to maintain B lymphocyte homeostasis. J. Exp. Med. 2012;209:173–186. doi: 10.1084/jem.20111363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold S., Hock A., Herkert B., Berns K., Mullenders J., Beijersbergen R., Bernards R., Eilers M. Miz1 and HectH9 regulate the stability of the checkpoint protein, TopBP1. EMBO J. 2008;27:2851–2861. doi: 10.1038/emboj.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Hao Z., Elia A.J., Cescon D., Zhou L., Silvester J., Snow B., Harris I.S., Sasaki M., Li W.Y. Mule/Huwe1/Arf-BP1 suppresses Ras-driven tumorigenesis by preventing c-Myc/Miz1-mediated down-regulation of p21 and p15. Genes. Dev. 2013;27:1101–1114. doi: 10.1101/gad.214577.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.N., Roe A.E., Donehower L.A., Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- King B., Boccalatte F., Moran-Crusio K., Wolf E., Wang J., Kayembe C., Lazaris C., Yu X., Aranda-Orgilles B., Lasorella A., Aifantis I. The ubiquitin ligase Huwe1 regulates the maintenance and lymphoid commitment of hematopoietic stem cells. Nat. Immunol. 2016;17:1312–1321. doi: 10.1038/ni.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon N., Zhong J., Qiang L., Accili D., Gu W. Inactivation of arf-bp1 induces p53 activation and diabetic phenotypes in mice. J. Biol. Chem. 2012;287:5102–5111. doi: 10.1074/jbc.M111.322867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Z.-J., Xu X., Cooney A.J. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic Mice1. Biol. Reprod. 2004;71:1469–1474. doi: 10.1095/biolreprod.104.031757. [DOI] [PubMed] [Google Scholar]
- Livera G., Uzbekov R., Jarrier P., Fouchécourt S., Duquenne C., Parent A., Marine J., Monget P. Loss of oocytes due to conditional ablation of Murine double minute 2 (Mdm2) gene is p53-dependent and results in female sterility. FEBS Lett. 2016;590:2566–2574. doi: 10.1002/1873-3468.12275. [DOI] [PubMed] [Google Scholar]
- Montes de Oca Luna M., Wagner D.S., Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- Wade M., Wang Y.V., Wahl G.M. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20:299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Luk C.T., Schroer S.A., Smith A.M., Li X., Cai E.P., Gaisano H., MacDonald P.E., Hao Z., Mak T.W., Woo M. Dichotomous role of pancreatic HUWE1/MULE/ARF-BP1 in modulating beta cell apoptosis in mice under physiological and genotoxic conditions. Diabetologia. 2014;57:1889–1898. doi: 10.1007/s00125-014-3295-8. [DOI] [PubMed] [Google Scholar]
- Zhao X., Heng J.I., Guardavaccaro D., Jiang R., Pagano M., Guillemot F., Iavarone A., Lasorella A. The HECT-domain ubiquitin ligase Huwe1 controls neural differentiation and proliferation by destabilizing the N-Myc oncoprotein. Nat. Cell Biol. 2008;10:643–653. doi: 10.1038/ncb1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q., Gao W., Du F., Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this study are available from the corresponding author upon request.