Abstract
Group II metabotropic glutamate receptors (mGluR2/3s) have been implicated in stress and trauma related disorders including post-traumatic stress disorder (PTSD). PTSD is characterized by flashbacks, anxiety, and sleep disturbances. While many people are exposed to trauma in their lifetime, only a small percentage go on to develop PTSD, indicating individual differences in stress and emotional processing. Wistar strain rats display directionally different rapid-eye movement sleep (REM) responses to footshock stress, with resilient rats having no change or an increase in REM and vulnerable rats having a significant reduction in REM compared to baseline. The basolateral nucleus of the amygdala (BLA) is key in regulating individual differences in stress-induced alterations in sleep. Group II metabotropic glutamate receptors (mGluR2/3s) negatively modulate glutamate and are implicated in fear, fear memory, and sleep. The current study evaluated the effect of mGluR2/3 agonist LY379268 (LY37) in BLA on stress and fear memory induced changes in sleep, EEG spectra, behavioral fear expression and physiological stress. These data indicate that vulnerable rats treated with LY37 have an attenuation of the REM reductions generally seen in vulnerable rats. Furthermore, LY37 altered EEG spectra in the delta and theta frequency. LY37 did not impact behavioral fear expression or physiological stress. Therefore, mGluR2/3s within BLA are implicated in regulating individual differences in sleep responses to fear- and stress-related memories.
Keywords: Group II metabotropic glutamate receptor, basolateral amygdala, REM sleep, PTSD, stress resilience and vulnerability
1. Introduction
Post-traumatic stress disorder (PTSD) is a stress and trauma related disorder characterized by flashbacks, hypervigilance, nightmares and sleep disturbances, and it is thought to arise from impairments in fear neurocircuitry (American Psychiatric Association, 2013). However, only a small percentage of those who experience or witness a traumatic event go on to develop PTSD, indicating individual differences in how stress is processed. Attempts to increase the validity of PTSD models include separating animals into resilient and vulnerable categories based on directionally different responses to stressors. Several studies have demonstrated individual differences in stress responses in rats (Dulka et al., 2015; Pfau & Russo, 2015; Rana et al., 2016; Sweis et al., 2013); for example, rats bred for a low novelty response were more sensitive to chronic unpredictable mild stress measured by suppressed feeding behavior (Rana et al., 2016). Outbred, Wistar strain rats display variable responses to the elevated plus maze, a behavioral measure of anxiety, with one subset of rats spending more time in the open arms, suggesting resilience, and the other subset spending more time in the closed arms, suggesting vulnerability (Rao & Sadananda, 2016). These findings suggest that understanding individual differences in behavioral responses to fear, fear memories and stress in animal models may provide insight into the factors underlying stress-and trauma-related disorders in humans.
Experimental conditioned fear is one of the primary models used for studying fear memory and disorders related to impaired fear circuitry. In contextual fear conditioning, an association is formed between a situational context and an aversive stimulus, usually footshock (Davis, 1992). Afterwards, the fear context alone can evoke fear and produce behavioral and physiological outcomes similar to those produced by the original aversive stimulus. Changes in sleep can be fear conditioned: fearful memories can produce changes in subsequent sleep, especially rapid-eye movement sleep (REM), that are similar to those following the initial aversive stimulus (Sanford et al., 2003; Tang et al., 2005; Wellman et al. 2008, 2014). These changes are important as REM has been implicated in emotional processing and is known to be disrupted or fragmented in PTSD (Mellman et al., 2002). Outbred, Wistar strain rats display directionally different REM responses to identical stressors, with resilient rats having an increase or no change in REM compared to baseline and vulnerable rats exhibiting significant decreases in REM compared to baseline following footshock training (Sweeten et al., 2019; Wellman et al., 2017, 2018). When re-exposed to the fear context, vulnerable rats continue to exhibit significant reductions in REM compared to baseline sleep, while resilient animals have similar REM levels across experimental days (Wellman et al., 2016, 2017). The individual differences seen in this outbred strain may better model the variability in post-stress sleep and fear processing seen in the human population and provide insights into the factors that regulate stress resiliency and vulnerability.
The amygdala, a key region of the fear circuit, exhibits hyperactivity in patients with PTSD and during fear conditioning (Greco & Liberzon, 2016). Specifically, the basolateral nucleus of the amygdala (BLA) has been implicated in the acquisition, expression and extinction of fear learning (Davis, 1992). BLA regulates stress-induced changes in REM as inhibition of BLA with muscimol, a GABAA receptor agonist, prior to footshock training blocks stress-induced changes in REM and the expression of behavioral fear, measured by freezing behavior (Wellman et al., 2014). BLA mediates individual differences in fear conditioned REM responses as inhibition of BLA after footshock training (Wellman et al., 2016) and prior to context re-exposure (Wellman et al., 2017) attenuates fear memory-induced decreases in REM in vulnerable rats while freezing behavior and increases in core body temperature (stress-induced hyperthermia (SIH), a measure of physiological stress (Olivier et al., 2003)) remain similar across both vulnerable and resilient groups. However, the signaling processes and/or molecular mechanisms in BLA that regulate these differences in REM are not well understood.
Glutamate is the primary excitatory neurotransmitter in the central nervous system (CNS) and glutamate hyper-excitability is implicated in PTSD and other anxiety and stress-and trauma-related disorders (Cortese & Phan, 2005). Metabotropic glutamate receptors (mGluRs) regulate glutamate signaling in the CNS, and specifically group II mGluRs (mGluR2/3s) negatively modulate glutamate neurotransmission pre-and post-synaptically (Conn & Pin, 1997). Group II mGluRs are highly expressed in regions implicated in PTSD including the amygdala, hippocampus and prefrontal cortex (Muly et al., 2007). While group II mGluRs have been implicated in anxiety-based disorders, their possible role in regulating individual differences in processing stress has not been examined. Furthermore, glutamate also plays a central role in regulating sleep and arousal. Systemic administration of the group II mGluR agonist, LY379268 (LY37), results in a dose-dependent suppression of REM, while systemic administration of the group II mGluR antagonist, LY341495 (LY34) increases arousal (Feinberg et al., 2002). Microinjection of mGluR 2/3 agonist LY37 into BLA dose-dependently decreases REM without altering non-rapid eye movement sleep (NREM) or total sleep, while mGluR2/3 antagonist LY34 suppresses NREM and total sleep (Dong et al., 2012). However, microinjection of either mGluR2/3 agonist LY37 or mGluR2/3 antagonist LY34 into the central nucleus of the amygdala (CNA), the primary output nucleus of the amygdala, did not impact sleep (Dong et al., 2012). To date, it is unknown how glutamate signaling is involved in regulating individual differences in fear or fear memory induced changes in sleep.
In this study, we microinjected mGluR 2/3 agonist LY37 into BLA of Wistar rats prior to fear context re-exposure and evaluated sleep, EEG spectra, freezing, and core body temperature to assess SIH. Our goal was to determine whether activation of group II mGluRs in BLA is important for mediating individual differences in fear memory recall as measured by behavior, the stress response and sleep.
2. Materials and Methods
2.1. Animal Procedures
The subjects were 43, ninety-day-old, male Wistar strain rats obtained from Envigo laboratories (Dublin, VA). Upon arrival, the rats were individually housed in polycarbonate cages and given ad lib access to food and water. The rooms were kept on a 12:12-h light/dark cycle with lights on from 0700–1900 h. Light intensity during the light period was 100–110 lux and less than 1 lux during the dark period. Ambient room temperature was maintained at 24.5 ± 0.5°C. All experimental manipulations were conducted during the 4th h of the light period such that sleep recording would begin at the start of the 5th h. This resulted in 8 h of light period recording and 12 h of dark period recording for a total of 20 h on each experimental day. Home cages were changed at least 3 days prior to sleep recording or behavioral testing. The same room was used for animal housing and sleep recording. Behavioral testing was conducted in a separate room from that used for recording.
2.2. Surgery
One week following arrival, the rats were anesthetized with isoflurane (5% induction: 2–3% maintenance). The rats were then implanted with skull screw electrodes for recording their electroencephalogram (EEG) and stainless-steel wire electrodes were sutured to the dorsal neck musculature for recording their electromyogram (EMG). One of the skull screw electrodes was placed over the hippocampus which we have found produces pronounced EEG theta activity during REM. Leads from the recording electrodes were routed to a 9-pin miniature plug that mated to one attached to a recording cable. Bilateral cannulae (26 ga.) for microinjections into BLA were implanted with their tips aimed 1.0 mm above BLA (AP 2.6, ML ± 4.8, DV 8.0) (Paxinos & Watson, 1998). The recording plug and cannulae were affixed to the skull with dental acrylic and stainless-steel anchor screws. During the same surgery, animals were implanted intraperitoneally with SubCue Standard Dataloggers (Canadian Analytical Technologies Inc., Calgary, Alberta, Canada) for recording of body temperature at 15 min intervals during study. Ibuprofen (15 mg/kg) was made available in their water supply 24–48 h prior to surgery and for a minimum of 72 h after surgery for relief of post-operative pain. Animals were assigned to drug or vehicle groups immediately following their 14-day recovery period. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School’s Institutional Animal Care and Use Committee (Protocol # 16–002).
2.3. Microinjections
LY37 was obtained from Tocris. A 1.0 nM solution was prepared in sterile PBS and sonicated for 30 min to ensure that the drug was dissolved completely. The drug dosage was determined based on our previous work (Dong et al., 2012); as this dose did not impact total REM.. A fresh solution was prepared for each experimental day.
For microinjections, injection cannulae (33ga.) were secured in place within the guide cannulae and projected 1.0 mm beyond the tip of the guide cannulae for delivery of drug into the target region. The injection cannulae were connected to one end of a section of polyethylene tubing which was connected to 5.0 μL Hamilton syringes. The injection cannulae and tubing were prefilled with LY37 or vehicle alone. Once the cannulae were in place, 0.5 μL of LY37 or vehicle was bilaterally infused over 3 min. The cannulae were left in place one min pre- and post-injection to allow for maximal absorption of the solution. Following microinjections, rats were returned to their home room for 30 minutes.
2.4. Sleep Recording
For recording sleep, each animal, in its home cage, was placed on a rack outfitted for electrophysiological recording and a lightweight, shielded cable was connected to the miniature plug on the rat’s head. The cable was attached to a commutator that permitted free movement of the rat within its cage. EEG and EMG signals were processed by a Grass Model 12 polygraph equipped with model 12A5 amplifiers and routed to an A/D board (Model USB-2533, Measurement Computing) housed in a personal computer. The signals were digitized at 256 Hz and collected in 10 s epochs using the SleepWave™ (Biosoft Studio) data collection program. The rats were allowed a post-surgery recovery period of at least 14 days prior to the beginning of experiments. Once recovered, the rats were habituated to handling procedures necessary for microinjections and the sleep recording cable over 3 days and baseline sleep (Base) was recorded on the 4th day.
2.5. Fear Conditioning
On experimental day 1, individual rats were placed in shock chambers (Coulbourn Habitest cages equipped with grid floors (Model E10–18RF)) that were housed in Coulbourn Isolation Cubicles (Model H10–23)) and were allowed to freely explore for 5 min. Over the next 20 min, they were presented with 20 footshocks (0.8 mA, 0.5 s duration) at 1.0 min intervals. Shock was produced by Coulbourn Precision Regulated Animal Shockers (Model E13–14) and presented via the grid floor of the shock chamber. Five minutes following the last shock, animals were returned to their home cage for sleep recording. The shock training session (ST) lasted 30 minutes.
On experimental day 7, rats were microinjected as described above. They were then allowed to freely explore the shock chamber for 30 min without shock presentation then returned to their home cage for subsequent sleep recording. This context re-exposure (CTX) was used to test for the effects of fear memory recall on immediate behavior (assessed by freezing), the physiological stress response as indicated by SIH and post-exposure alterations in sleep.
The shock chambers were thoroughly cleaned with diluted alcohol following each session. Each session was videotaped using mini video cameras (Weldex, WDH-2500BS, 3.6 mm lens) attached to the center of the ceiling of the shock chamber for subsequent visual scoring of freezing behavior.
2.6. Data Analysis
2.6.1. Sleep
Computerized EEG and EMG records were visually scored by trained observers blind to drug condition in 10 s epochs to determine wakefulness, NREM, and REM. Wakefulness was scored based on the presence of low-voltage, fast EEG and high amplitude, and tonic EMG levels. NREM was characterized by the presence of spindles interspersed with slow waves, lower muscle tone and no gross body movements. REM was scored continuously during the presence of low voltage, fast EEG, theta rhythm, and muscle atonia. Data were collapsed into 1st 4 h of sleep (B1), the total 8 h light period (light), the 12 h dark period (dark), and total 20 h recording. The following sleep parameters were examined in the data analyses: total REM (min), total NREM (min) and total sleep (REM + NREM) (min).
Subsequent to assignment to drug or vehicle groups immediately following recovery from surgery, the rats were further separated into 4 groups based on distinct sleep responses after ST: vehicle-vulnerable (Veh-Vul; n=11), vehicle-resilient (Veh-Res; n=15), LY37-vulnerable (LY37-Vul; n=7), and LY37-resilient (LY37-Res; n=10). The groups were formed based on whether, compared to baseline (Base), the rats showed a 50% or greater decrease in REM during B1 following ST (Wellman et al., 2016). The sleep data were analyzed with two-way mixed factors (Treatment (Veh-Vul; Veh-Res; LY37-Vul; LY37-Res) X Condition (Base; ST; CTX)) ANOVAs with repeated measures on Treatment. The Tukey method was used to determine differences among means as appropriate. Statistical power was evaluated and reached a minimum of 0.8 for all reported comparisons. P-values < 0.05 were considered significant.
2.6.2. EEG Spectral Analysis
EEG spectra were analyzed using a fast Fourier transformation (FFT) algorithm and subsequently sorted by frequency in 0.5 Hz bins from 0 to 20 Hz and normalized to the total power within 0 – 20 Hz for each hour. Five 4 h blocks (B1–B5) were compared across groups to analyze the entire 20 h sleep recording time. The power values in the spectrum of 5–9.5 Hz were summed as an index of relative theta power (θ) during REM, and power values between 0.5–4.5 Hz were summed as an index of relative delta power (δ) during NREM. The power values per h were then averaged within B1–B5. EEG spectra data were analyzed with a two-way mixed factor (Treatment (Veh-Vul; Veh-Res; LY37-Vul; LY37-Res) X Condition (Base; ST; CTX)) ANOVAs with repeated measures on Treatment. The Tukey method was used to determine differences among means as appropriate.
2.6.3. Freezing
Videotapes of the ST and CTX sessions were scored for freezing, defined as the absence of body movement except for respiration (Blanchard & Blanchard, 1969). Freezing was scored by a trained observer blind to condition in 5 s intervals during 1.0 min observation periods over the course of the 5 min pre-ST period (to obtain baseline), 5 min post-ST period and the 30 min of CTX which was separated into three 10 min blocks. The percentage of time spent freezing was calculated (FT%: freezing time/observed time × 100) for each animal during each observation period. Freezing data were analyzed for the observation periods described above and compared to the pre- and post-ST periods across groups. The freezing data were analyzed with a two-way mixed factor ANOVA (Group (Veh-Vul; Veh-Res; LY37-Vul; LY37-Res) by Treatment (Pre-shock; post-ST; CTX) with repeated measures on Treatment. Post-hoc tests were conducted using the Tukey method.
2.6.4. Core Body Temperature
The Subcue Dataloggers were programmed to record an individual animal’s temperature every 15 min over the course of the experiment. To determine the effect of fear and shock on SIH and its relationship to sleep, temperature data for the time in ST, CTX and for the first 4 h of the sleep recording period following testing were compared to the temperature data collected prior to ST (45 min prior to sleep recording) or the injection before CTX (90 min prior to sleep recording) and then across treatment conditions (ST and CTX). The temperature data were analyzed with two-way mixed factors (Group (Veh-Vul; Veh-Res; LY37-Vul; LY37-Res) X Recording Day (Base, ST, CTX) ANOVAs with repeated measures on Recording Day. Post hoc comparisons were conducted with the Tukey method.
2.6.5. Histology
To localize the microinjection sites in BLA, brain slices (40 μm) were made through the amygdala and the sections were mounted on slides and stained with cresyl violet. The sections were then examined in conjunction with a stereotaxic atlas to confirm cannulae placements (Paxinos & Watson, 1998). Though there were rostral-caudal variations in the placements among animals, the histology indicated that LY37 or vehicle would have been infused into BLA and adjacent areas in all the rats, and all animals were used in the data analyses.
3. Results
3.1. Sleep Amounts
3.1.1. mGluR2/3 agonist LY37 in BLA attenuates fear memory-induced REM reductions in vulnerable rats
The rats were grouped based on differences in REM amounts in B1 after ST as described above and the subsequent analysis compared treatment groups (Veh-Res, Veh-Vul, LY37-Res and LY37-Vul) across experimental days. Analysis of B1 REM revealed significant treatment group (F [3, 38] =13.72; p<0.001) and day (F [2, 38] =16.37; p<0.001) main effects and a significant treatment by day (F [6, 38] =1.97; p<0.05) interaction effect. Post-hoc analysis indicated that LY37-Res rats had significantly higher Base REM amounts compared to Veh-Res rats (p=0.012), LY37-Vul rats (p=0.003) and Veh-Vul rats (p=0.018). The other groups did not significantly differ. Due to the higher Base REM in LY37-Res rats, all subsequent comparisons were made solely within treatment groups.
Due to selection criteria, Vul rats had significantly lower REM after ST compared to Base (Veh: p<0.001; LY37: p<0.001). Veh-Vul rats continued to display significant decreases in CTX (p=0.003) compared to Base. By comparison, LY37-Vul rats had similar REM after CTX compared to Base (p=0.782), indicating an attenuation of the REM response to fear memory generally seen in Vul rats (Figure 1A). LY37-Res and Veh-Res rats did not display any significant differences in REM compared to Base across experimental days (Figure 1A; Table 1).
Figure 1:
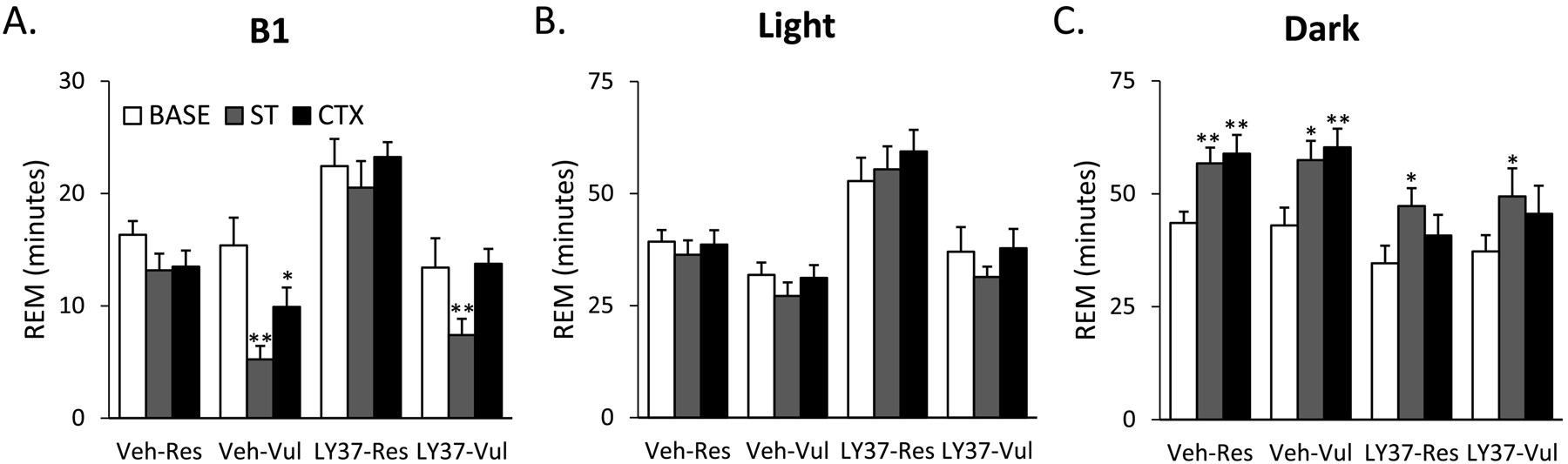
Total duration of rapid eye movement sleep (REM) for baseline (BASE), shock training (ST), and context re-exposure (CTX) for the different treatment groups. Bars represent REM (min) during (A) the first 4 hours (B1), (B) 8-hour light period and (C) 12-hour dark period. Error bars represent SEM. *p<0.05 compared to BASE, **p<0.001 compared to BASE. Veh-Res: vehicle treated resilient, Veh-Vul: vehicle treated vulnerable, LY37-Res: LY37 treated resilient, LY37-Vul: LY37 treated vulnerable.
Analysis of the 8h light period, the primary sleeping period for rodents, revealed no significant group or treatment effects for REM (Figure 1B; Supplementary Table 1). To determine the effects of LY37 on REM during the primary active period for rodents, REM was analyzed during the 12 h dark period. ANOVA revealed significant treatment (F [3, 38] =3.80; p=0.018) and day (F [2, 38] =25.35; p<0.001) main effects. Post-hoc Tukey test revealed that Veh rats had a significant increase in REM from Base to ST (Res: p<0.001; Vul: p=0.002)) and from Base to CTX (Res: p<0.001; Vul: p<0.001) (Figure 1C; Supplementary Table 1). LY37 rats displayed a significant increase in REM from Base to ST (Res: p=0.006; Vul: p=0.042), but no differences in REM from Base to CTX (Figure 1C; Supplementary Table 1). LY37 treated rats did not display the REM recovery in the dark period that was seen in the Veh treated rats after CTX.
3.1.2. LY37 in BLA minimally impacts Non-Rapid-Eye Movement Sleep
ANOVA of B1 revealed a significant day (F [2, 38] =12.66; p<0.001) effect for NREM. LY37-Vul rats had higher NREM after CTX compared to Base (p=0.05) (Figure 2A; Supplementary Table 1). ANOVA of the 8h light period revealed a significant day (F [2, 38] =24.84; p<0.001) effect for NREM. Both LY37-Res and LY37-Vul rats had increased NREM from Base to CTX (p=0.03 and p=0.007 respectively) (Figure 2B; Supplementary Table 1). No significant differences were found within LY37-Res or LY37-Vul rats during the dark period (Figure 2C; Supplementary Table 1).
Figure 2:
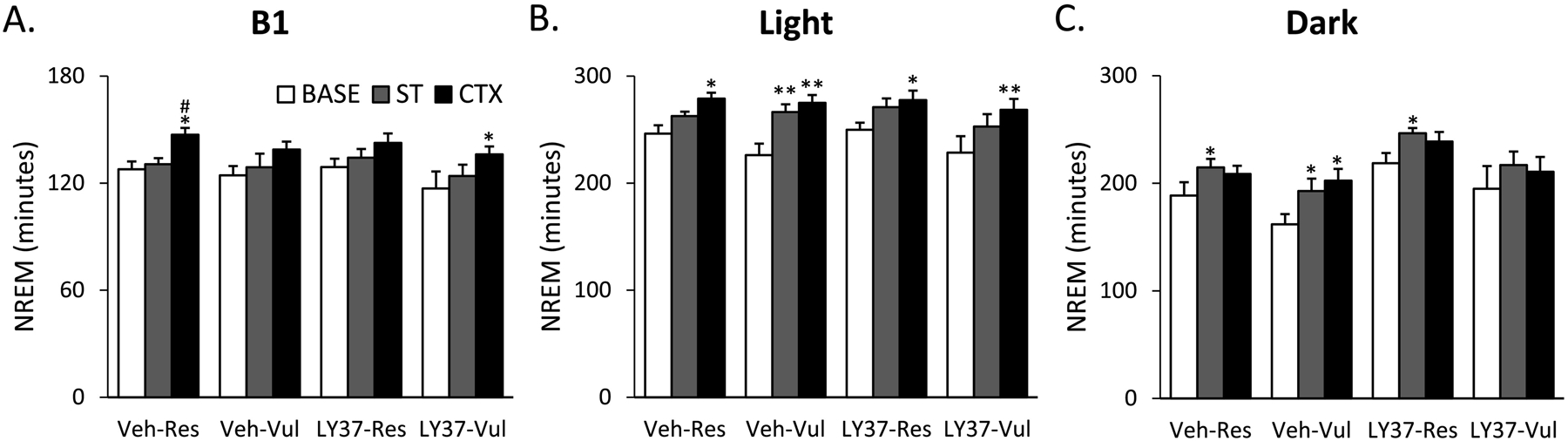
Total duration of non-rapid eye movement sleep (NREM) for baseline (BASE), shock training (ST), and context re-exposure (CTX) for the different treatment groups. Bars represent NREM (min) during (A) the first 4 hours (B1), (B) 8-hour light period and (C) 12-hour dark period. Error bars represent SEM.*p<0.05 compared to BASE, **p<0.001 compared to BASE, # p<0.05 compared to ST. Veh-Res: vehicle treated resilient, Veh-Vul: vehicle treated vulnerable, LY37-Res: LY37 treated resilient, LY37-Vul: LY37 treated vulnerable.
3.1.3. LY37 in BLA increased total sleep
ANOVA of B1 revealed significant treatment (F [3, 38] =5.81; p=0.002) and day (F [2, 38] =11.04; p<0.001) main effects. There were differences in total sleep in B1 Base, therefore like the REM analysis, comparisons were made within groups. LY37-Vul rats had significantly higher total sleep after CTX higher compared to Base (p<0.001). ANOVA of the 8h light period revealed significant treatment (F [3, 38] =8.34; p<0.001) and day (F [2, 38] =22.54; p<0.001) main effects. Post-hoc Tukey test indicated that LY37 rats displayed increases in total sleep after CTX compared to Base (Res: p<0.001; Vul: p<0.001). ANOVA for the 12h dark period revealed significant treatment (F [3, 38] =3.54; p=0.02) and day (F [2, 38=37.37; p<0.001) main effects. LY37 treated rats all displayed increases in total sleep after CTX compared to Base during the light (Res: p<0.001; Vul: p<0.001) and dark periods (Res: p<0.001; Vul: p<0.001). These data suggest that LY37 may increase total sleep after fear recall (Figure 3; Supplementary Table 1).
Figure 3:
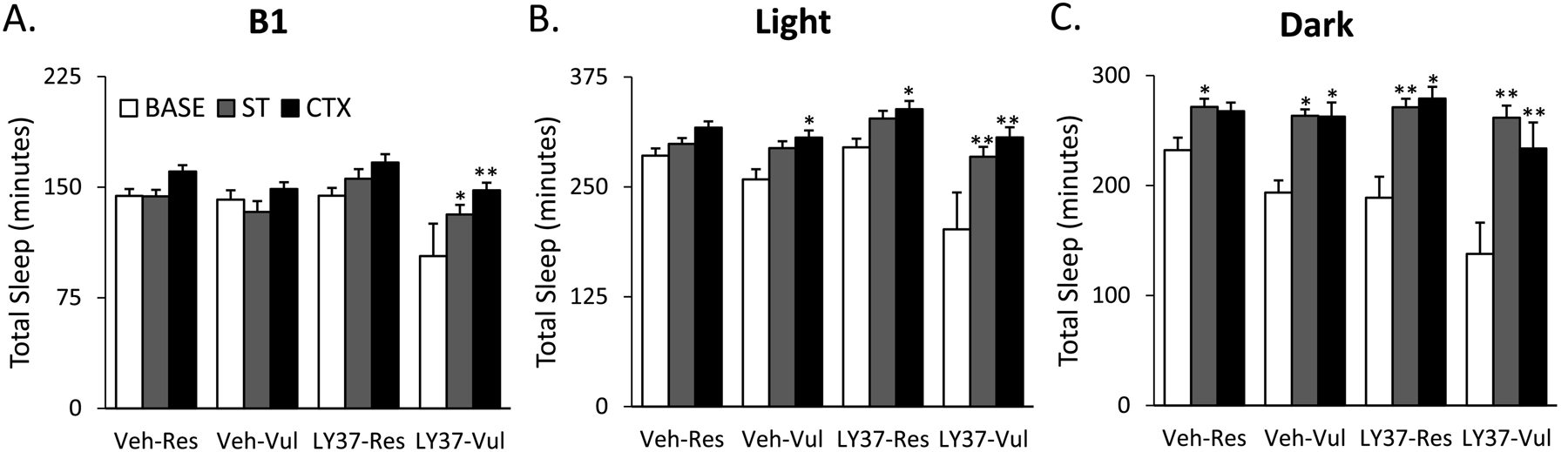
Total duration of sleep (REM + NREM) for baseline (BASE), shock training (ST), and context re-exposure (CTX) for the different treatment groups. Bars represent total sleep (min) during (A) the first 4 hours (B1), (B) 8-hour light period and (C) 12-hour dark period. Error bars represent SEM. *p<0.05 compared to BASE, **p<0.001 compared to BASE, ## p<0.001 compared to ST. Veh-Res: vehicle treated resilient, Veh-Vul: vehicle treated vulnerable, LY37-Res: LY37 treated resilient, LY37-Vul: LY37 treated vulnerable.
3.2. EEG Spectral Analysis
3.2.1. LY37 in BLA decreased relative theta power during REM in vulnerable rats
For analysis of REM θ for Base and ST, rats were separated into Res and Vul groups independent of LY37 or Veh treatment, as rats were not microinjected until immediately prior to context re-exposure. There were no significant differences in θ between groups for Base or ST. However, during B1 there was a trend towards Vul rats having reduced θ compared to Res rats (p=0.07). For analysis of REM θ following CTX, rats were separated into LY37-Res, LY37-Vul, Veh-Res, and Veh-Vul groups. ANOVA revealed no significant differences in θ between groups during B1 for CTX. ANOVA revealed a significant treatment effect for B2 (F [3, 38] = 7.64; p=0.009), B3 (F [3, 38] =4.84; p=0.035), and B4 (F [3, 38] =5.54; p=0.025). Post-hoc Tukey test revealed LY37-Vul rats had lower REM θ compared to Veh-Vul rats during B2 (p=0.01), B3 (p=0.04), and B4 (p=0.03). There were no significant differences in REM θ during B5 (Figure 4). There were no significant differences in REM θ between LY37-Res and Veh-Res rats.
Figure 4:
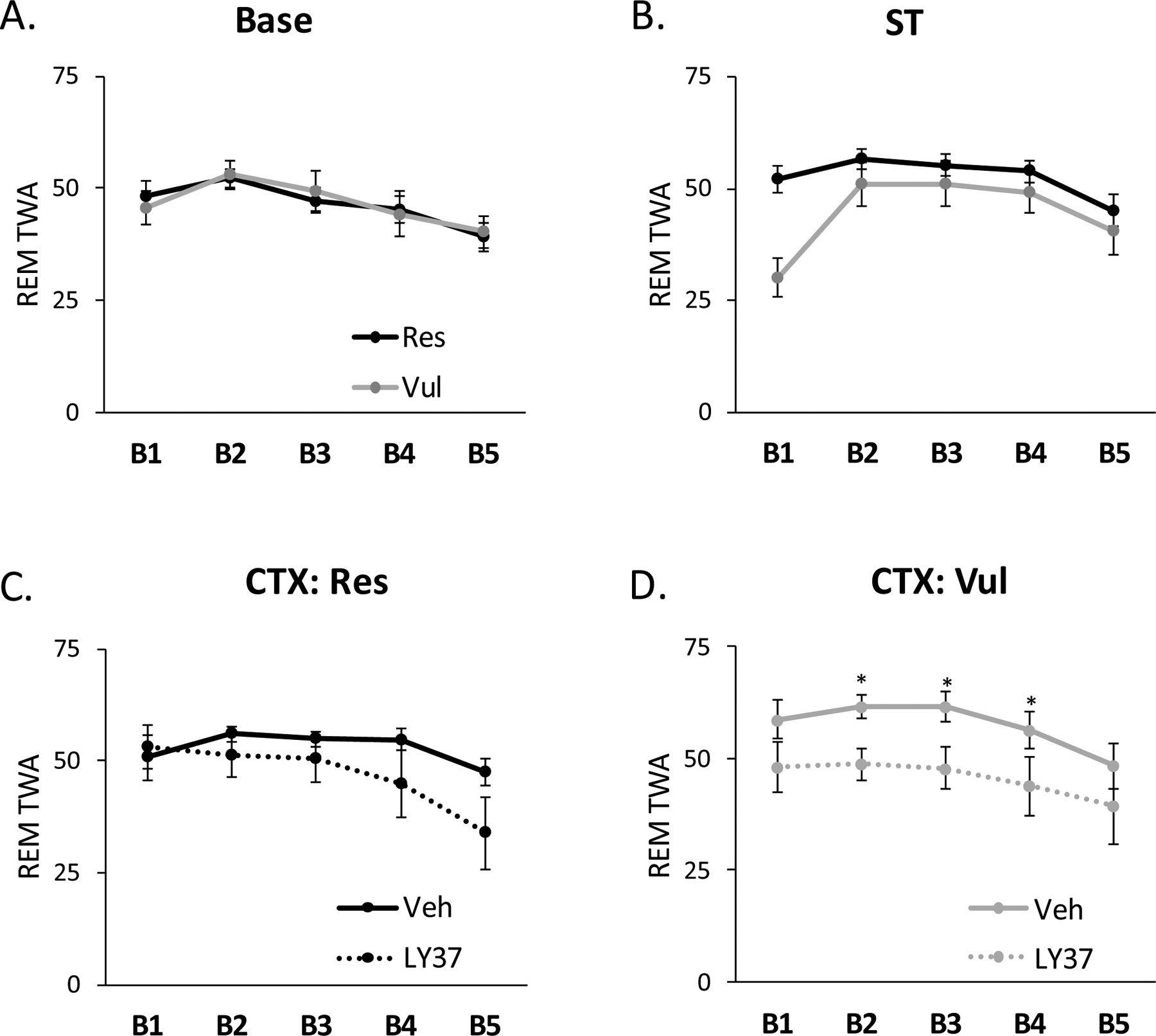
EEG spectral analysis for relative theta wave amplitude (θ) during rapid eye movement sleep (REM) for the entire 20h sleep recording separated into 4h blocks. B1: H1–H4, B2: H5–H8, B3: H9–H12, B4: H13–16, and B5: H17–20. Lines represent REM θ in resilient and vulnerable rats during (A) baseline (Base) and (B) shock training (ST). REM θ was compared in (C) resilient (Res) or (D) vulnerable (Vul) rats treated with LY37 or vehicle prior to context re-exposure (CTX). Error bars represent SEM.*p<0.05 LY37-Vulnerable vs Vehicle vulnerable. Veh-Res: vehicle treated resilient, Veh-Vul: vehicle treated vulnerable, LY37-Res: LY37 treated resilient, LY37-Vul: LY37 treated vulnerable.
3.2.2. mGluR2/3 agonist LY37 decreased relative delta power during NREM in resilient rats after fear context re-exposure
Similar to the analysis above, for NREM δ, animals were separated into Res and Vul groups independent of treatment for Base and ST, and were separated into LY37-Res, LY37-Vul, Veh-Res, and Veh-Vul groups for analysis of CTX. ANOVA revealed no significant differences between Res and Vul rats in NREM δ for Base or ST. ANOVA revealed a significant treatment effect (F [3, 38] =5.24; p=0.029) for B1 (F [3, 38] =5.24; p=0.029), B2(F [3, 38] =5.74; p=0.023), B3(F [3, 38] =4.17; p=0.049) and B5 (F [3, 38] =7.23; p=0.001) after CTX. Post-hoc Tukey tests indicated that LY37-Res rats had significantly lower NREM δ compared to Veh-Res rats during B1 (p=0.021), B2 (p=0.009), B3 (p=0.028), and B5 (p=0.024). No differences were found in δ for B4 after CTX. Furthermore, no differences were found in δ between Veh-Vul and LY37-Vul rats after CTX (Figure 5).
Figure 5:
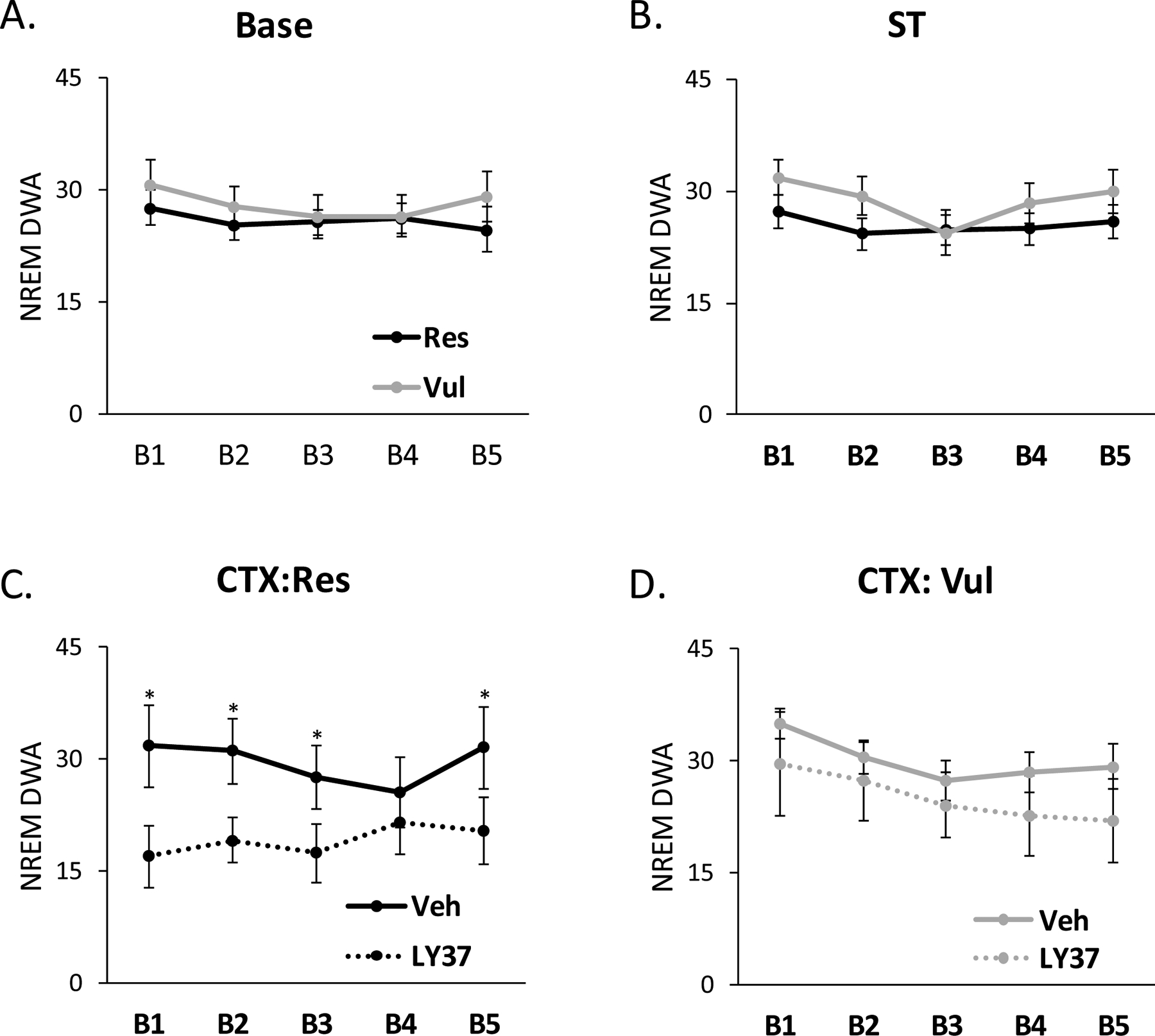
EEG spectral analysis for relative delta wave amplitude (δ) during non-rapid eye movement sleep (NREM) for the entire 20h sleep recording separated into 4h blocks. B1: H1–H4, B2: H5–H8, B3: H9–H12, B4: H13–16, and B5: H17–20. Lines represent NREM δ in resilient and vulnerable rats during (A) baseline (Base) and (B) shock training (ST). NREM δ was compared in (C) resilient (Res) or (D) vulnerable (Res) rats treated with LY37 or vehicle prior to context re-exposure (CTX). Error bars represent SEM. *p<0.05 LY37-Res vs Veh-Res. Veh-Res: vehicle treated resilient, Veh-Vul: vehicle treated vulnerable, LY37-Res: LY37 treated resilient, LY37-Vul: LY37 treated vulnerable.
3.3. Behavioral Fear and Physiological Stress Response
3.3.1. mGluR2/3 agonist LY37 did not impact behavioral fear expression or the physiological stress response
Minimal freezing occurred during the 5 min pre-ST period. All rats displayed more freezing in the 5 min post-ST and CTX compared to the 5 min pre-ST period. ANOVA revealed a significant treatment (F [3, 38] =9.57; p<0.001) effect. Post-hoc Tukey test revealed that during the 5 min post-ST period, rats subsequently treated with LY37, independent of Res or Vul grouping, showed significantly higher freezing compared to the Veh-treated rats (LY37-Res vs Veh-Res: p<0.001; LY37-Vul vs Veh-Vul: p<0.001). This was unexpected as these rats had not yet been treated with drug. LY37 treated rats, independent of Res or Vul grouping displayed significantly higher freezing compared to vehicle treated rats during CTX (LY37-Res vs Veh-Res: p<0.01; LY37-Vul vs Veh-Vul: p<0.01)(Figure 6).
Figure 6:
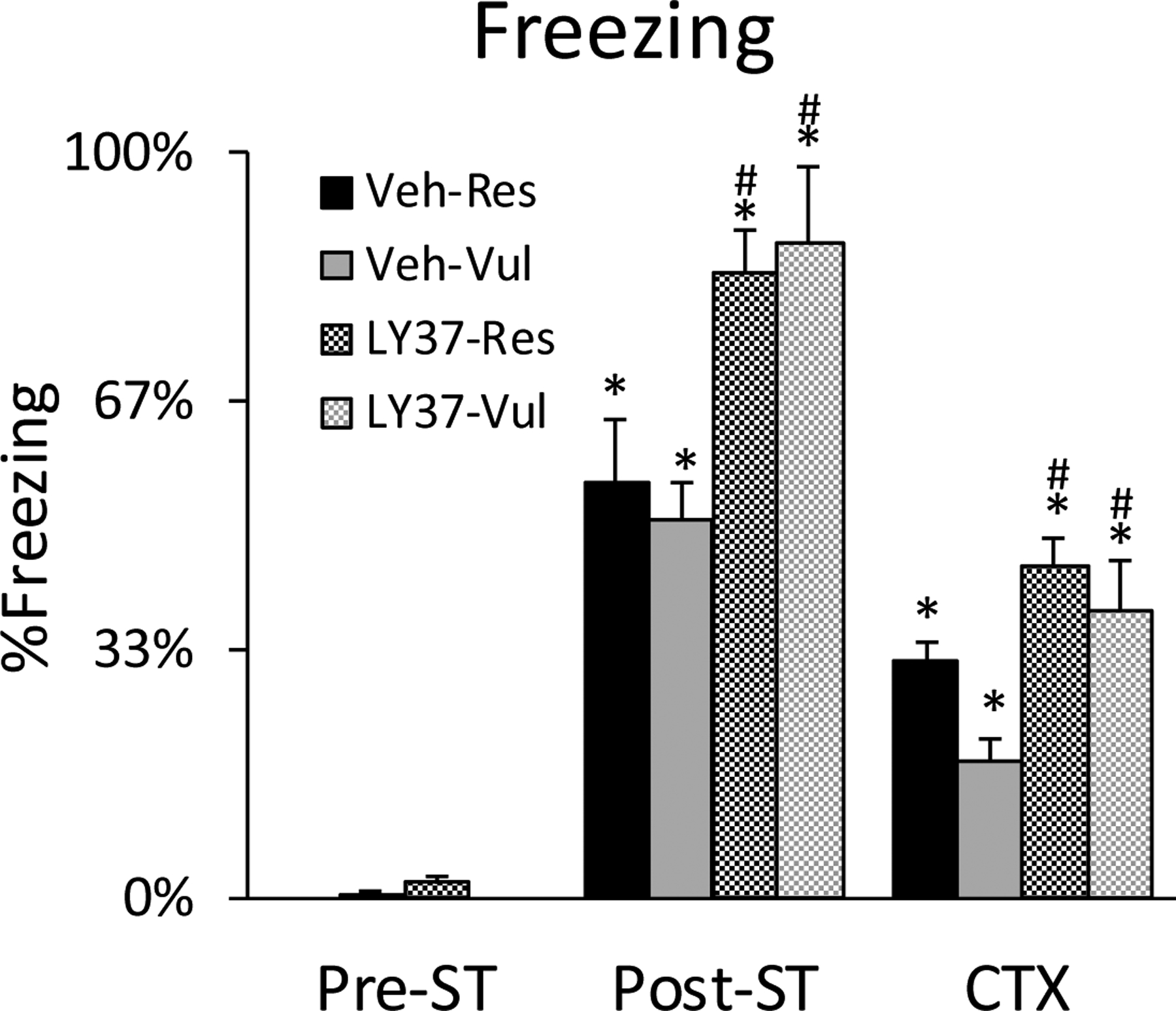
Freezing as a measure of behavioral fear expression. Bars represent percent time freezing in the 5 min pre-shock training period (pre-ST), the 5-minute post-shock training period (post-ST), and the 30 minutes of context re-exposure (CTX). Error bars represent SEM. *p<0.05 compared to pre-ST, #p<0.05 LY37 compared to vehicle. Veh-Res: vehicle treated resilient, Veh-Vul: vehicle treated vulnerable, LY37-Res: LY37 treated resilient, LY37-Vul: LY37 treated vulnerable
Core body temperature did not differ between treatment groups in the 15 min prior to ST (LY37-Res: 36.8°C; LY37-Vul: 36.8°C; Veh-Res: 36.8°C; Veh-Vul: 36.9°C). ANOVA revealed a significant treatment effect (F [3, 38] =169.80, p<0.001) and post-hoc Tukey tests indicated that all treatment groups displayed significant increases in core body temperature during ST compared to the 15 min prior to ST (LY37-Res: p<0.001; LY37-Vul: p<0.001; Veh-Res: p<0.001, Veh-Vul: p<0.001) which normalized within approximately 4 h (Figure 7A). Core body temperature did not differ between treatment groups in the 15 min prior to the microinjection before CTX (LY37-Res: 37.0°C; LY37-Vul: 36.8C; Veh-Res: 36.8°C; Veh-Vul: 36.9°C). All treatment groups displayed significant increases in core body temperature during CTX compared to the 15 min prior to the microinjection (LY37-Res: p<0.001; LY37-Vul: p<0.001; Veh-Res: p<0.001; Veh-Vul: p<0.001) (Figure 7B). There were no differences between treatment groups for core body temperature during or following ST or CTX (Figure 7).
Figure 7:
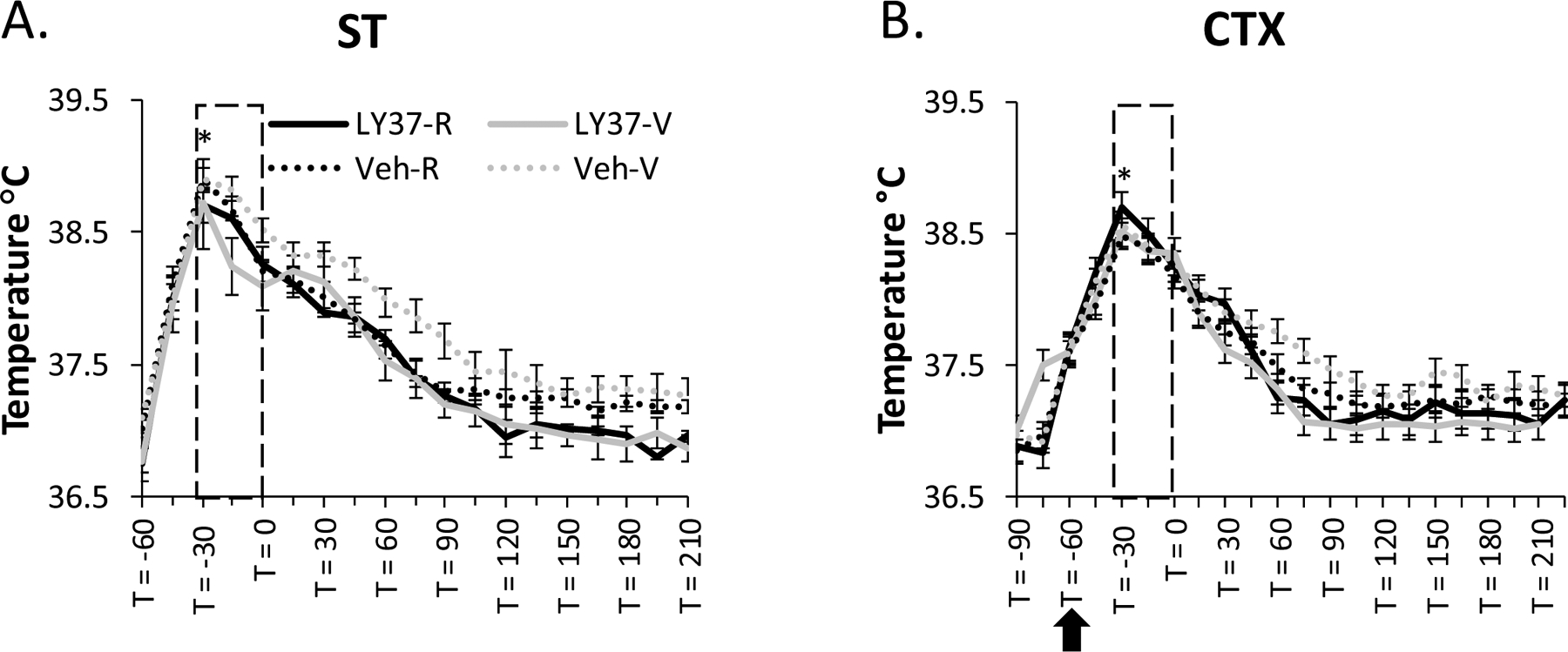
Stress-induced hyperthermia (SIH) as a measure of physiological stress. Lines represent averaged core body temperature recordings for each treatment group for (A) shock training (ST) and (B) context re-exposure (CTX). The measurements were collected in 15-minute intervals beginning 30 minutes prior to ST (−60) and 30 minutes prior to the microinjection (−90). Boxes represent time in chamber; arrow represents when microinjections were delivered. Error bars represent SEM. *p<0.05 represents all treatment groups having a significant increase in SIH compared to pre-ST or pre-CTX. Veh-Res: vehicle treated resilient, Veh-Vul: vehicle treated vulnerable, LY37-Res: LY37 treated resilient, LY37-Vul: LY37 treated vulnerable
4. Discussion
The factors that regulate individual differences in responses to outwardly identical stressors are not well understood. Therefore, evaluating signaling pathways that may regulate individual variations in stress resilience and vulnerability is critical for fully understanding and developing more effective therapies for stress-related disorders. In the current study, we investigated the effects of activating mGluR2/3s in BLA prior to fear memory recall on behavioral fear expression, physiological stress, sleep amounts and EEG spectra. The results indicate that outbred, Wistar strain rats display phenotypically distinct REM responses to stress and fear, separating into resilient and vulnerable groups with respect to REM amounts. Vulnerable rats microinjected with mGluR2/3 agonist LY37 in BLA prior to context re-exposure, display an attenuation of fear memory recall-induced reductions in REM generally seen in vulnerable rats, yet REM was not altered in resilient rats. By comparison, the stress response (measured by SIH) and freezing behavior were virtually identical in resilient and vulnerable rats, within drug and control groups, and were not predictive of subsequent sleep.
This study expands on our previous work examining individual differences in an animal model of fear conditioning. This variability is likely important as only a small percentage of individuals develop persistent PTSD-like symptoms following trauma exposure while others only have transient effects (Mellman et al., 2002). The relationship between sleep and stress is complex, as virtually any stressor can produce acute disturbances in sleep; however, traumatic stress may cause long-term problems such as nightmares, insomnia, and fragmented sleep in some individuals (American Psychiatric Association, 2013). REM is associated with emotional processing, and can be reduced and fragmented in the early onset of PTSD (Cowdin et al., 2014; Mellman et al., 2002) which may exacerbate symptoms. Glutamate signaling in the amygdala has also been implicated in emotional learning and processing, as disruption of glutamatergic transmission in the amygdala across all classes of glutamate receptor impairs fear conditioning (Cortese & Phan, 2005; Gillespie & Ressler, 2005; Walker & Davis, 2002). Group II mGluRs are highly expressed within BLA and appear to be involved in mediating adaptive or maladaptive sleep responses to stress as well as sleep and arousal states (Jones, 2005). Systemic treatment with mGluR 2/3 agonist LY37 dose-dependently suppressed REM (Feinberg et al., 2002), and treatment with a different group II mGluR agonist, LY35470 (LY35; structurally similar to glutamate with specificity for mGluR2/3) also dose-dependently suppressed REM and prolonged onset latency (Ahnaou et al., 2009). REM amounts were not affected by mGluR 2/3 agonist LY35 in mGluR2−/− mice suggesting mGluR2 may be more important in sleep and arousal regulation compared to mGluR3 (Ahnaou et al., 2009). While there are no current agents that directly target mGluR2 or mGluR3 independently, positive allosteric modulators (PAM) of mGluR2 have been developed, and oral delivery of the mGluR2 PAM JNJ-42153605 dose dependently reduced REM sleep and did not show tolerance compared to mGluR2/3 agonist LY35 (Ahnaou et al. 2015). Further studies are necessary to delineate the role of mGluR2 PAMs in stress and fear induced alterations in REM.
Changes in sleep can be fear conditioned; these changes are regulated by BLA, as inhibition of BLA prior to CTX attenuated fear memory recall-induced reductions in REM in Vul rats, independent of freezing or SIH (Wellman et al., 2017). These changes in REM most notably occur in the 1st 4 hours of sleep post-ST and after CTX (Wellman et al., 2016, 2017). Our previous work determined that Group II mGluRs are important for amygdalar regulation of sleep, as microinjections of mGluR 2/3 agonist LY37 into BLA selectively decreased REM at mM and nM concentrations (Dong et al., 2012). Conversely, microinjections of mGluR 2/3 antagonist LY34 increased wakefulness without altering REM solely at the highest concentration tested of 60nM (Dong et al., 2012). Interestingly, microinjection of either mGluR2/3 agonist LY37 or mGluR 2/3 antagonist LY34 into CNA did not significantly alter sleep or arousal (Dong et al., 2012). For the current study we used a low dose of the mGluR 2/3 agonist, LY37, which did not cause changes in REM when administered alone (Dong et al., 2012), in order to evaluate the role of activating mGluR2/3s on fear memory recall-induced changes in sleep. Therefore, we attributed the changes in sleep seen here to the interaction of fear memory recall and mGluR2/3 activation. Differences in REM, NREM and total sleep following mGluR 2/3 agonist LY37 microinjection in BLA prior to fear memory recall were observed. During B1, LY37-Vul rats had similar REM to Base, indicating an attenuation of the REM response normally seen in Vul rats. Total sleep time was increased in both LY37-Res and LY37-Vul rats during the 8h light period, 12 h dark period, and total 20h sleep recording period. LY37-Vul rats also had an increase in total sleep from ST to CTX. This is consistent with previous work indicating that rats treated with mGluR2/3 antagonists have increased wakefulness, suggesting that mGluR2/3 agonists may increase total sleep and decrease wakefulness (Hanley et al., 2019). Furthermore, deletion of mGluR2/3s in mice disrupts sleep and circadian rhythms (Pritchett et al., 2015). These results support a role for mGluR2/3s in BLA in regulating stress-and fear-induced changes in sleep and arousal.
Microinjections of mGluR 2/3 agonist LY37 into BLA prior to CTX produced changes in EEG spectra following fear memory recall. These changes included reductions in REM θ in Vul rats and reductions in NREM δ in Res rats. Glutamatergic signaling has been implicated in regulating oscillatory rhythms (Draguhn & Buzsáki, 2004; Tamas et al., 2000). Pharmacological studies have shown that mGluR2/3 agonism can suppress theta and gamma oscillations, and antagonism of mGluR2/3s can increase theta and gamma oscillations (Feinberg et al., 2002; Ahnaou et al., 2009). Also, mGluR 2/3 agonist LY37 has been shown to attenuate ketamine (a NMDA antagonist) induced dysregulation of gamma oscillations, further supporting a role for mGluR2/3s in the regulation of oscillatory rhythms (Hikichi et al., 2015; Hiyoshi et al., 2014). The hippocampus, which has reciprocal connections with BLA, is a regulator of θ oscillations during REM (Bland, 1986), and higher REM θ has been associated with stress resilience, as patients with PTSD display lower REM θ compared to trauma exposed patients without PTSD (Cowdin et al., 2014). REM θ may serve as a potential index of emotional processing. Previously, our lab (Sweeten et al., 2019) and others (Nedelcovych et al., 2015) have shown that Res animals display higher REM θ compared to Vul animals at certain time points immediately following footshock training. Interestingly, the effects seen in this study were observed in the second 4 h block of the light period and the first block of the dark period suggesting these changes occur when the sleep drive is less intense. Previous studies support our results as systemic mGluR 2/3 agonist LY37 treatment reduced REM θ in Wistar rats at 3 mg/kg and 10 mg/kg doses (Wood et al., 2018). These effects were not seen in Han Wistar rats, which do not express mGluR2, suggesting that mGluR2s may be more important than mGluR3s in modulating oscillatory rhythms (Wood, et al., 2018). Dysregulation of glutamate signaling within the fear circuit by the mGluR2/3 agonist LY37 may impair the fine balance of excitatory/inhibitory signaling which may alter stress and fear processing. NREM δ rhythms are thought to reflect the brain’s homeostatic sleep drive, and can be altered by sleep deprivation and changes in glutamatergic signaling (Ahnaou et al., 2009). While little work has been done investigating Group II mGluR’s in NREM δ, the role of the other mGluRs have been assessed. mGluR5 knockout mice, which have increased glutamate in the synapse, display decreases in NREM δ (Ahnaou et al., 2009). The current study found decreases in NREM δ in Res rats treated with the mGluR2/3 agonist LY37 in BLA. Therefore, there may be individual differences in the role of glutamatergic signaling for both NREM δ, acting to regulate sleep homeostasis, and REM θ, a potential index of emotional processing. These effects are likely region dependent as different results are found in systemic versus region specific treatment.
Group II mGluRs are implicated in stress and fear related disorders (Muly et al., 2007; Gillespie, C., Ressler, 2005), as these disorders may involve dysregulation of excitatory signaling within the fear circuitry. Patients with PTSD often have amygdalar hyperactivity and hippocampal hypertrophy that could be related to excessive glutamate release resulting in excitotoxicity-induced cell atrophy and death (Bremner, 2006). BLA consists of primarily glutamatergic neurons that send projections to the hippocampus, CNA, the primary output nucleus of the amygdala, and medial prefrontal cortex (mPFC) (McDonald & Mott, 2017). Group II mGluRs act to decrease glutamate signaling and are at moderate to high levels of expression within BLA (Muly et al., 2007) and may be important in individual resilience and vulnerability. Mice prophylactically treated with mGluR2/3 antagonist LY34 via microinjections into mPFC displayed stress resilience to inescapable footshock-induced escape deficits and chronic social defeat stress-induced anhedonia, whereas mice microinjected into mPFC with mGluR 2/3 agonist LY37 were more susceptible to these stress paradigms (Highland et al., 2019). In contrast, rats microinjected with a different group II mGluR agonist, DCG-IV, into BLA, displayed a reduction in fear-potentiated startle behavior suggesting that group II mGluR activation may have anxiolytic properties (Lin et al., 2005). The anxiolytic effects of group II mGluR agonists have also been shown in human subjects, as treatment with mGluR 2/3 agonist LY35 reduced fear-potentiated startle without producing the sedation effects often seen with certain anti-anxiety medications including benzodiazepines (Grillon et al., 2003). The variation seen across these studies may be due to differences in dose, delivery method, region of activation, or stress paradigm. For example, the group II mGluR agonist L-CCG-I significantly increased open arm entries in the elevated plus maze (EPM) when microinjected into the dentate gyrus but not CA1 region of the hippocampus in rats (Smialowska et al., 2007). Conversely, a group III mGluR agonist, L-SOP, increased open arm entries in the EPM when microinjected into the CA1 region but not dentate gyrus of rats (Smialowska et al., 2007). While there are no current pharmacological agents that directly target mGluR2 or mGluR3 independently, allosteric modulators of mGluR2 have been developed. The mGluR2 PAM, Biphenyl-indanone A (BINA), has been shown to decrease anxiety-like behavior in mice. Mice prophylactically treated with BINA had more open arm entries in the elevated plus maze and it prevented SIH (Galici et al. 2006). Furthermore, the mGluR2 PAM (+)-TFMPIP, but not the mGluR2/3 agonist LY35, reduced stress-evoked glutamate release in the PFC of rats, suggesting that PAMs may have potential use as anxiolytics (Hascup et al. 2012). Overall, glutamate signaling is tightly regulated throughout the fear circuit and can induce differential effects based on receptor type and region specificity.
Greater freezing behavior in rodents has long been considered indicative of greater associative learning as well as stronger fear responses. Sleep, particularly REM, has been thought to be important in fear memory consolidation (Helm & Walker, 2010; Walker & Davis, 2002). This is generally found in studies with brief or mild fearful experiences, yet there is no evidence that sleep is necessary for the formation of contextual fear memories associated with intensely stressful experiences as modeledby our experiments. For example, extensive escapable and inescapable footshock training produces differences in REM yet virtually identical freezing (Sanford et al., 2010; Liu et al., 2010; Yang et al., 2011). This study, as well as our previous work, show directionally different REM responses yet similar freezing. We also found that freezing levels differed in rats prior to drug treatment, indicating that freezing levels immediately post-ST can vary extensively, further supporting that freezing is not a clear indicator of stronger fear responses when used in isolation. Therefore, it is unlikely that mGluR2/3 agonist LY37 treatment altered freezing behavior when considering the post-ST freezing differences. Furthermore, freezing was not predictive of subsequent changes in sleep. Thus, while freezing is an attractive, simple behavioral measure, it does not fully reflect the complex relationships between fear learning and the sleep and stress systems, or how those relationships are modulated by individual differences in physiological systems. Both the initial stressor and memories of the stressful environment can produce increases in core body temperature (SIH), increased corticosterone, and increases in heart rate and respiration (Olivier et al., 2003; Vianna & Carrive, 2005; Yang et al., 2011). Here, we examined SIH as an index of the stress response and found that all rats, irrespective of Res or Vul grouping or drug treatment, had virtually identical stress responses. Yet, similar to freezing, it was not predictive of subsequent sleep. Therefore, these measures are not fully reflective of stress and fear responses when used in isolation. Future studies should consider the wide range of stress and fear processes and include multiple measures of stress and fear to fully understand the stress and fear processing in animal models relevant for PTSD and other stress- and trauma-related disorders.
5. Conclusion
Our data demonstrate that group II mGluRs in BLA are involved in the regulation of individual differences in post-stress sleep. Outbred rats display significant individual differences in post-stress and fear sleep that are independent of behavioral markers of fear and activation of the peripheral nervous system. The data further support the use of REM as a potential biomarker of stress resilience and vulnerability as freezing and SIH do not differ between resilient or vulnerable groups. Future research is necessary to fully understand the role of glutamatergic signaling in regulating individual differences in stress and fear induced alterations in sleep as well as how glutamate regulates regional communication in the fear circuit. Furthermore, work is needed to clearly delineate the association of increased or decreased REM in adaptive or maladaptive stress outcomes and the role of glutamatergic signaling in the amygdala in regulating these outcomes.
Supplementary Material
Acknowledgements:
This work was funded by National Institutes of Health Grant MH64827.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical Statement
The authors have read and agree with the journal’s ethical standards. We declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. All animal procedures were approved by the Eastern Virginia Medical School Institutional Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author.
Conflict of Interest
The authors do not have any conflicts of interest to disclose.
Disclosures:
BLW Sweeten, AM Adkins, LL Wellman and LD Sanford have nothing to disclose.
References
- Ahnaou A, Dautzenberg FM., Geys H, Imogai H, Gibelin A, Moechars D, Steckler T, Drinkenburg W (2009). Modulation of group II metabotropic glutamate receptor(mGlu2) elicits common changes in rat and mice sleep-wake architecture. Eur J. Pharmacol, 603, 62–72. [DOI] [PubMed] [Google Scholar]
- Association AP (2013). Diagnostic and statistical manual of mental disorders (5th ed.). [Google Scholar]
- Blanchard RJ, & Blanchard DC (1969). Crouching as an index of fear. Journal ofComparative and Physiological Psychology, 67(3), 370–375. doi: 10.1037/h0026779 [DOI] [PubMed] [Google Scholar]
- Bland BH (1986). The physiology and pharmacology of hippocampal formation theta rhythms. Progress in Neurobiology, 26(1), 1–54. doi: 10.1016/0301-0082(86)90019-5 [DOI] [PubMed] [Google Scholar]
- Bremner JD (2006). Traumatic stress: Effects on the brain. Dialogues in Clinical Neuroscience, 8(4), 445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, & Pin J-P (1997). Pharmacology and Functions of Metabotropic Glutamate Receptors. Annual Review of Pharmacology and Toxicology, 37(1), 205–237. doi: 10.1146/annurev.pharmtox.37.1.205 [DOI] [PubMed] [Google Scholar]
- Cortese BM, & Phan KL (2005). The role of glutamate in anxiety and related disorders. CNS Spectrums, 10(10), 820–830. doi: 10.1017/S1092852900010427 [DOI] [PubMed] [Google Scholar]
- Cowdin N, Kobayashi I, & Mellman TA (2014). Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Experimental Brain Research, 232(5), 1479–1485. doi: 10.1007/s00221-014-3857-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M (1992). The Role of the Amygdala in Fear and Anxiety. Annual Review of Neuroscience, 15(1), 353–375. [DOI] [PubMed] [Google Scholar]
- Dong E, Wellman L, Yang L, and Sanford L (2012). Effects of Microinjections of Group II Metabatropic Glutamate Agents into the Amygdala on Sleep. Brain Research2, 1452, 85–95. doi: 10.1016/j.brainres.2012.03.003.Effects [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, & Buzsáki G (2004). Neuronal Oscillations in Cortical Networks. Science, 304(June), 1926–1930. [DOI] [PubMed] [Google Scholar]
- Dulka BN, Lynch JF, Latsko MS, Mulvany JL, & Jasnow AM (2015). Phenotypic responses to social defeat are associated with differences in cued and contextual fear discrimination. Behavioural Processes. doi: 10.1016/j.beproc.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Muly C, Mania I & Guo J (2007). Group II Metabotropic Glutamate Receptors in Anxiety Circuitry: Correspondence of Physiological Response and Subcellular Distribution. The Journal of Comparative Neurology, 505, 682–700. doi: 10.1002/cne [DOI] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG., Schoepp DD, Anderson K (2002). The selective group mGlu2/3 receptor agonist LY379268 suppresses REM sleep and fast EEG in the rat. Pharmacol. Biochem. Behav, 73, 467–474. [DOI] [PubMed] [Google Scholar]
- Gillespie C, Ressler K (2005). Emotional Learning and Glutamate: Translational Perspectives. CNS Spectrums, 10(10), 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco JA, & Liberzon I (2016). Neuroimaging of Fear-Associated Learning. Neuropsychopharmacology, 41(1), 320–334. doi: 10.1038/npp.2015.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Codova J, Levine LR., Morgan C (2003). Anxiolytic effects of a novel group II metabotropic glutamate receptor agonist (LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology, 168, 446–454. [DOI] [PubMed] [Google Scholar]
- Hanley N, Paulissen J, Eastwood BJ., Gilmour G, Loomis S, Wafford KA, McCarthy A (2019). Pharmacological Modulation of Sleep Homeostasis in Rat: Novel Effects of an mGluR2/3 Antagonist. Sleep, 42(9), 1–13. [DOI] [PubMed] [Google Scholar]
- Helm E. Van Der, & Walker MP (2010). Overnight therapy? The role of sleep in emotional processing. Psychological Bulletin, 135(5), 731–748. doi: 10.1037/a0016570.Overnight [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highland JN. Zanos P, Georgiou P, Gould T (2019). Group II metabotropic glutatmate receptor blockade promotes stress resilience in mice. Neuropsychopharmacology, 44, 1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikichi H, Hiyoshi T, Marumo T, & Tomishima Y (2015). Antipsychotic profiles of TASP0443294, a novel and orally active positive allosteric modulator of metabotropic glutamate 2 receptor. Journal of Pharmacological Science, 127(3), 352–361. doi: 10.1016/j.jphs.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Hiyoshi T, Kikichi H, Karasawa J, Chaki S (2014). Metabotropic glutamate receptors regulate cortical gamma hyperactivities elicited by ketamine in rats. Neuroscience Letters, 264, 30–34. [DOI] [PubMed] [Google Scholar]
- Jones B (2005). Principles and Practice of Sleep Medicine. [Google Scholar]
- Kruger L Saporta S, Swanson L (1995). Photographic atlas of the rat brain. New York: Cambrige University Press. [Google Scholar]
- Lin C, Lee C, Huang Y, Wang S, Gean P (2005). Activation of group II metabotropic glutatmate receptors induces depotentiation in amygdala slices and reduces fear-potentiated statle in rats. Learning and Memory, 12, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A, Mott D (2017). Functional Neuroanatomy of Amygdalohippocampal Interconnections and Their Role in Learning and Memory. J Neurosci Res, 95(3), 797–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman TA, Bustamante V, Fins AI, Pigeon WR, & Nolan B (2002). REM sleep and the early development of posttraumatic stress disorder. American Journal of Psychiatry, 159(10), 1696–1701. doi: 10.1176/appi.ajp.159.10.1696 [DOI] [PubMed] [Google Scholar]
- Miao Y, Feixas F, Eun C, & Mccammon JA (2016). HHS Public Access. 36(20), 1536–1549. doi: 10.1002/jcc.23964.Accelerated [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelcovych MT, Gould RW, Zhan X, Bubser M, Gong X, Grannan M, … Jones CK (2015). A rodent model of traumatic stress induces lasting sleep and quantitative electroencephalographic disturbances. ACS Chemical Neuroscience, 6(3). doi: 10.1021/cn500342u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier B, Zethof T, Pattij T, Van Boogaert M, Van Oorschot R, Leahy C, … Groenink L (2003). Stress-induced hyperthermia and anxiety: Pharmacological validation. European Journal of Pharmacology, 463(1–3), 117–132. doi: 10.1016/S0014-2999(03)01326-8 [DOI] [PubMed] [Google Scholar]
- Paxinos G & Watson C (1998). The Rat Brain in Stereotaxic Coordinates: Fourth Edition. San Diego, CA: Academic Press [Google Scholar]
- Pfau ML, & Russo SJ (2015). Peripheral and central mechanisms of stress resilience. Neurobiology of Stress, 1(1), 66–79. doi: 10.1016/j.ynstr.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett D, Jagannath A, Brown L, Tam SK., Hasan S, Gatti S, Harrison P, Bannerman D, Foster R, Peirson S (2015). Deletion of Metabotropic Glutamate Receptors 2 and 3 (mGluR2 & mGluR3) in Mice Disrupts Sleep and Wheel-Running Activity, and Increase the Sensitivity of the Circadian System to Light. PLOS ONE2, 10(5), e012552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S, Ham H, Glover M, Adkil H, Watson S, Clinton S, and Kerman I (2016). Protective Effects of Chronic Mild Stress during Adolescence in the Low Novelty Responder Rat. Stress, 19(1), 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RM, & Sadananda M (2016). Influence of state and/or trait anxieties of wistar rats in an anxiety paradigm. Annals of Neurosciences, 23(1), 44–50. doi: 10.1159/000443555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LD, Yang L, & Tang X (2003). Influence of contextual fear on sleep in mice: A strain comparison. Sleep, 26(5), 527–540. doi: 10.1093/sleep/26.5.527 [DOI] [PubMed] [Google Scholar]
- Sanford LD, Yang L, Wellman LL, Liu X, & Tang X (2010). Differential effects of controllable and uncontrollable footshock stress on sleep in mice. Sleep, 33(5), 621–630. doi: 10.1093/sleep/33.5.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smialowska M, Wieronska J, Domin H, Zieba B (2007). The Effect of Intrahippocampal Injection of Group II and III Metabotropic Glutamate Recetpro Agonists on Anxiety; the Role of Neuropeptide Y. Neuropsychopharmacology2, 32, 1242–1250. [DOI] [PubMed] [Google Scholar]
- Sweeten BLW, Sutton AM, Wellman LL, & Sanford LD (2019). Predicting Stress Resilience and Vulnerability: Brain-Derived Neurotrophic Factor and Rapid Eye Movement Sleep as Potential Biomarkers of Individual Stress Responses. Sleep, (September), 1–12. doi: 10.1093/sleep/zsz199 [DOI] [PubMed] [Google Scholar]
- Sweis BM, Veverka KK, Dhillon ES, Urban JH, & Lucas LR (2013). Individual differences in the effects of chronic stress on memory: Behavioral and neurochemical correlates of resiliency. Neuroscience, 246(2013), 142–159. doi: 10.1016/j.neuroscience.2013.04.052 [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl E, Lorincz A, Somogyi P (2000). Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nature Neuroscience, 3, 366–371. [DOI] [PubMed] [Google Scholar]
- Tang X, Liu X, Yang L, S. L (2005). Rat strain differences in sleep after acute mild stressors and short-term sleep loss. Behav Brain Res2, 160(1), 60–71. [DOI] [PubMed] [Google Scholar]
- Vianna DML, & Carrive P (2005). Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. European Journal of Neuroscience, 21(9), 2505–2512. doi: 10.1111/j.1460-9568.2005.04073.x [DOI] [PubMed] [Google Scholar]
- Walker D, Davis M (2002). The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacology, Biochemistry, and Behavior, 71, 379–392. [DOI] [PubMed] [Google Scholar]
- Wellman Laurie L., Fitzpatrick Mairen E., Machida Mayumi & Sanford LD (2014). The Basolateral Amygdala Determined the Effects of Fear Memory on Sleep in an Animal Model of PTSD. Experimental Brain Research, 232(5), 1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Fitzpatrick ME, Hallum OY, Sutton AM, Williams BL, & Sanford LD (2016). Individual differences in animal stress models: Considering resilience, vulnerability, and the amygdala in mediating the effects of stress and conditioned fear on sleep. Sleep, 39(6). doi: 10.5665/sleep.5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Fitzpatrick ME, Hallum OY, Sutton AM, Williams BL, & Sanford LD (2017). The basolateral amygdala can mediate the effects of fear memory on sleep independently of fear behavior and the peripheral stress response. Neurobiology of Learning and Memory, 137. doi: 10.1016/j.nlm.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Fitzpatrick ME, Sutton AM, Williams BL, Machida M, & Sanford LD (2018). Antagonism of corticotropin releasing factor in the basolateral amygdala of resilient and vulnerable rats: Effects on fear-conditioned sleep, temperature and freezing. Hormones and Behavior, 100. doi: 10.1016/j.yhbeh.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Yang L, Tang X, S. L (2008). Contextual Fear Extinction Ameliorates Sleep Disturbances Found Following Fear Conditioning in Rats. Sleep, 31(7), 1035–1042. [PMC free article] [PubMed] [Google Scholar]
- Wood C, Wafford K, McCarthy A, Hewes N, Shanks E, Lodge D, Robinson E (2018). Investigating the role of mGluR2 versus mGluR3 in antipsychotic-like effects, sleep-wake architecture and network oscillatory activity using novel Han Wistar rats lacking mGluR2 expression. Neuropharmacology, 140, 246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wellman LL, Ambrozewicz M. a, & Sanford LD (2011). Effects of stressor predictability and controllability on sleep, temperature, and fear behavior in mice. Sleep, 34(6), 759–771. doi: 10.5665/SLEEP.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


