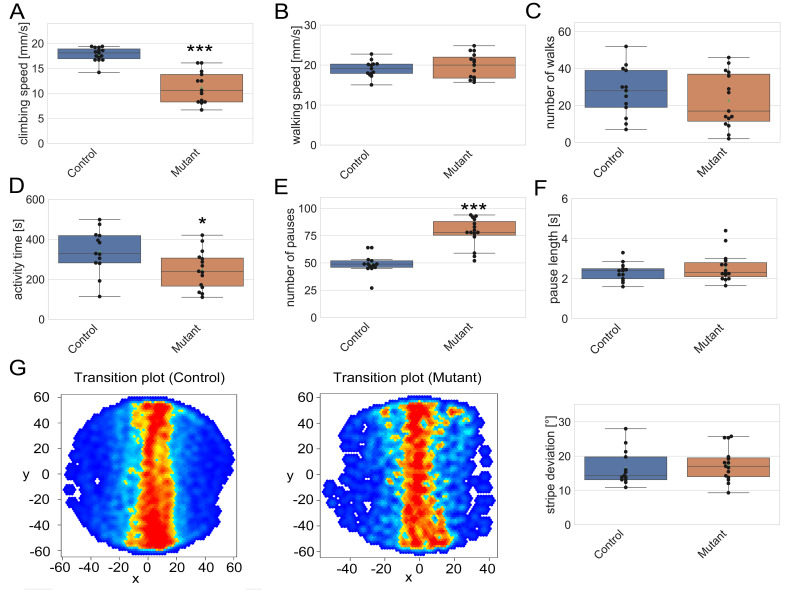
Figure 1. Loss of fuss results in reduced locomotor activity.
(A) Climbing speed of homozygous fuss mutant flies is reduced in contrast to control flies (mean speed (Mutant): 11.2 mm/s; mean speed (Control): 17.8 mm/s; Student’s t-test: p < 0.001). (B) In a Buridan’s assay, walking speed during active times is similar between genotypes (mean speed (Mutant): 19.7 mm/s, mean speed (Control): 19.1 mm/s). (C) Number of walks between stripes is slightly but insignificantly reduced in fuss mutant flies compared to controls (mean number of walks (Mutant): 22.7; mean number of walks (Control): 27.4). (D) Activity time is significantly reduced in fuss mutant flies compared to controls (mean activity time (Mutant): 248.1 s; mean activity time (Control): 340.3 s; Student’s t-test: p < 0.05). (E) Number of pauses is strongly increased in fuss mutant flies (mean number of pauses (Mutant): 78.0; mean number of pauses (Control): 49.1; Student’s t-test: p < 0.001). (F) Pause length is similar between fuss mutant flies and controls (mean pause length (Mutant): 2.6s; mean pause length (Control): 2.3s). (G) Stripe perception of fuss mutant flies is similar to control flies as shown by transition plots and stripe deviation (mean stripe deviation (Mutant): 17.6°; mean stripe deviation (Control): 16.6°). n = 13 – 15 for each genotype.
Description
In Drosophila melanogaster, two members of the Ski/Sno protein family exist, the Ski novel oncogene (Snoo) (Barrio et al. 2007) and the functional Smad suppressing element (Fuss) (Fischer et al. 2012). We have previously shown that Fuss is specifically expressed in gustatory receptor neurons (GRNs) and interneurons in the central nervous system (CNS). We created a loss of function allele fussdelDS and showed that loss of fuss had no impact on survival rate during development or on adult life span. In fact, fussdelDS flies display an impaired bitter GRN development and bitter taste reception (Rass et al. 2019).
To address if fussdelDS shows other behavioural phenotypes we tested these flies in different behavioural assays (circadian rhythm, optomotor response, climbing and Buridan’s paradigm), but we only found phenotypes in a climbing (Botella et al. 2004) and Buridan’s paradigm assay (Bülthoff et al. 1982; Colomb et al. 2012). In the climbing assay, flies were allowed to climb as high as possible in a pipette within 12 seconds. Interestingly, although fuss is not expressed in motoneurons, a strong reduction of the mean climbing speed of homozygous mutant fussdelDS flies was detected if compared to heterozygous controls (fussdelDS /+) (Fig 1, A). Upon closer examination of this phenotype, we noticed that the flies did not show any impairment in climbing capability but rather exhibited an increased number of pauses during testing. To record several different traits, we chose to test the flies using the Buridan’s paradigm assay. One day old flies’ wings were clipped, flies were allowed to recover for one night and underwent testing the next day. Flies were placed on a platform surrounded by water. The arena is homogenously illuminated except for two black stripes on opposite sides. If flies perceive the stripes, they will try to reach them mainly in an alternating fashion. The movement of every fly was recorded for 10 minutes with BuriTrack and analysed with the CeTrAn analysis software (Colomb et al. 2012). Interestingly, no difference between mutant and control flies in speed could be observed anymore, because the speed in this assay is only measured during activity (Fig 1, B). Furthermore, the number of walks between stripes was not significantly different between genotypes and showed only a slight tendency to a lesser number of walks between stripes in mutant flies (Fig 1, C). However, the overall activity time of mutant flies was significantly reduced in contrast to control flies (Fig 1, D). Similar to our previous observation, the number of pauses was strongly increased in mutant flies (Fig 1, E), whereas the pause length was unchanged (Fig 1, F). Overall, we can say that, due to the increased number of parameters recorded via BuriTrack and analysed with CeTrAn, the reduced climbing speed observed in the climbing assay is not the consequence of slower walking, but rather due to an increased number of pauses during walking, which ultimately leads to a reduced locomotor activity. Additionally, we have found that mutant fussdelDS flies have no problems in perceiving the black stripes as shown by the average trajectory plot and the stripe deviation (Fig 1, G).
In future experiments, it will be of particular interest how the loss of fuss impairs the interneurons on a molecular and cellular level and if these interneurons are somehow synaptically connected to motoneurons, which would explain the increased number of walking pauses. In humans a reduced expression of the fuss homolog Skor1 has been shown to be linked to Restless Legs Syndrome (RLS) (Catoire et al. 2018). However, it is rather unlikely that fly fuss has a similar function as human Skor1, or that fuss mutant flies could serve as a model for RLS, because a loss of function dBTBD9 RLS fly model is associated with a higher activity and a reduced number of pauses in Buridan’s assay (Freeman et al. 2012).
Methods
Generation and characterisation of the fussdelDS (FBal0349586) allele has been described before (Rass et al. 2019). The fussdelDS allele has been introduced into wild-type Berlin (WTB) background by backcrossing it five times to WTB flies. In the behavioural assays, only male flies underwent testing and homozygous fussdelDS flies were crossed to WTB and heterozygous progeny served as controls. fussdelDS/+ control flies did not show differences compared to WTB males. In climbing and Buridan´s assays, thirteen control flies and fifteen mutant flies have been analysed. For Buridan´s assay, we used the devices and software described previously in Colomb et al. (2012). For data visualisation the Python libraries pandas, matplotlib and seaborn have been used. For statistical testing the Python library SciPy was used.
Acknowledgments
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (FI2133/1-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Barrio R, López-Varea A, Casado M, de Celis JF. Characterization of dSnoN and its relationship to Decapentaplegic signaling in Drosophila. Dev Biol. 2007 Mar 01;306(1):66–81. doi: 10.1016/j.ydbio.2007.02.039. [DOI] [PubMed] [Google Scholar]
- Botella JA, Ulschmid JK, Gruenewald C, Moehle C, Kretzschmar D, Becker K, Schneuwly S. The Drosophila carbonyl reductase sniffer prevents oxidative stress-induced neurodegeneration. Curr Biol. 2004 May 01;14(9):782–786. doi: 10.1016/j.cub.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Bülthoff, H., Götz, K. G., and Herre, M. Recurrent inversion of visual orientation in the walking fly,Drosophila melanogaster. (1982). J. Comp. Physiol. 148 (4): 471–81.
- Catoire H, Sarayloo F, Mourabit Amari K, Apuzzo S, Grant A, Rochefort D, Xiong L, Montplaisir J, Earley CJ, Turecki G, Dion PA, Rouleau GA. A direct interaction between two Restless Legs Syndrome predisposing genes: MEIS1 and SKOR1. Sci Rep. 2018 Aug 15;8(1):12173–12173. doi: 10.1038/s41598-018-30665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomb J, Reiter L, Blaszkiewicz J, Wessnitzer J, Brembs B. Open source tracking and analysis of adult Drosophila locomotion in Buridan's paradigm with and without visual targets. PLoS One. 2012 Aug 01;7(8):e42247–e42247. doi: 10.1371/journal.pone.0042247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Bayersdorfer F, Harant E, Reng R, Arndt S, Bosserhoff AK, Schneuwly S. fussel (fuss)--A negative regulator of BMP signaling in Drosophila melanogaster. PLoS One. 2012 Aug 01;7(8):e42349–e42349. doi: 10.1371/journal.pone.0042349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A, Pranski E, Miller RD, Radmard S, Bernhard D, Jinnah HA, Betarbet R, Rye DB, Sanyal S. Sleep fragmentation and motor restlessness in a Drosophila model of Restless Legs Syndrome. Curr Biol. 2012 May 31;22(12):1142–1148. doi: 10.1016/j.cub.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass M, Oestreich S, Guetter S, Fischer S, Schneuwly S. The Drosophila fussel gene is required for bitter gustatory neuron differentiation acting within an Rpd3 dependent chromatin modifying complex. PLoS Genet. 2019 Feb 01;15(2):e1007940–e1007940. doi: 10.1371/journal.pgen.1007940. [DOI] [PMC free article] [PubMed] [Google Scholar]