Abstract
The follicle stem cell (FSC) lineage in the Drosophila ovary is a highly informative model of in vivo epithelial stem cell biology. Studies over the past 30 years have identified roles for every major signaling pathway in the early FSC lineage. These pathways regulate a wide variety of cell behaviors, including self-renewal, proliferation, survival and differentiation. Studies of cell signaling in the follicle epithelium have provided new insights into how these cell behaviors are coordinated within an epithelial stem cell lineage and how signaling pathways interact with each other in the native, in vivo context of a living tissue. Here, we review these studies, with a particular focus on how these pathways specify differences between the FSCs and their daughter cells. We also describe common themes that have emerged from these studies, and highlight new research directions that have been made possible by the detailed understanding of the follicle epithelium.
Keywords: Follicle stem cells, Drosophila, Wnt signaling, Notch signaling, Hedgehog signaling, EGFR signalling
Introduction
Each Drosophila ovary is composed of long strands of developing follicles, called ovarioles, and oogenesis begins at the anterior tip of the ovariole in a structure called the germarium (Figure 1) [1]. The germarium is divided into four regions, Regions 1, 2a, 2b, and 3, that are defined by the stage of germ cell development. Two to three germline stem cells (GSCs) reside within a niche provided by cap and terminal filament cells in Region 1 and divide during adulthood to continuously produce eggs. GSC daughter cells, called cystoblasts, undergo four rounds of mitosis with incomplete cytokinesis to become a cyst of 16 interconnected cells, with one oocyte and 15 nurse cells. During this time, the cyst moves away from the GSC niche through Regions 1 and 2a, which contain at least three types of inner germarial sheath cells (IGS cells, also called escort cells) [2]. The IGS cells ensheath the developing cysts and provide cues that guide their differentiation [3–6]. Next, the cysts exit the IGS cell region and become encapsulated by prefollicle cells (pFCs), which are produced by a population of follicle stem cells (FSCs) that reside in the middle of the germarium [7]. Normally, the pFCs differentiate into one of three major cell types as the cyst buds off from the germarium to become a follicle: main body follicle cells, which form a single layered epithelium that makes up the majority of the outer surface of the follicle; polar cells, which reside at the anterior and posterior of each follicle; and stalk cells, which connect adjacent follicles to one another. However, several studies, discussed further below, indicate that newly-produced pFCs do not differentiate immediately but instead retain the capacity to either re-enter the niche and acquire the FSC fate or to differentiate into any of the three cell types, depending on the type of signals they receive.
Figure 1: The Drosophila Germarium.
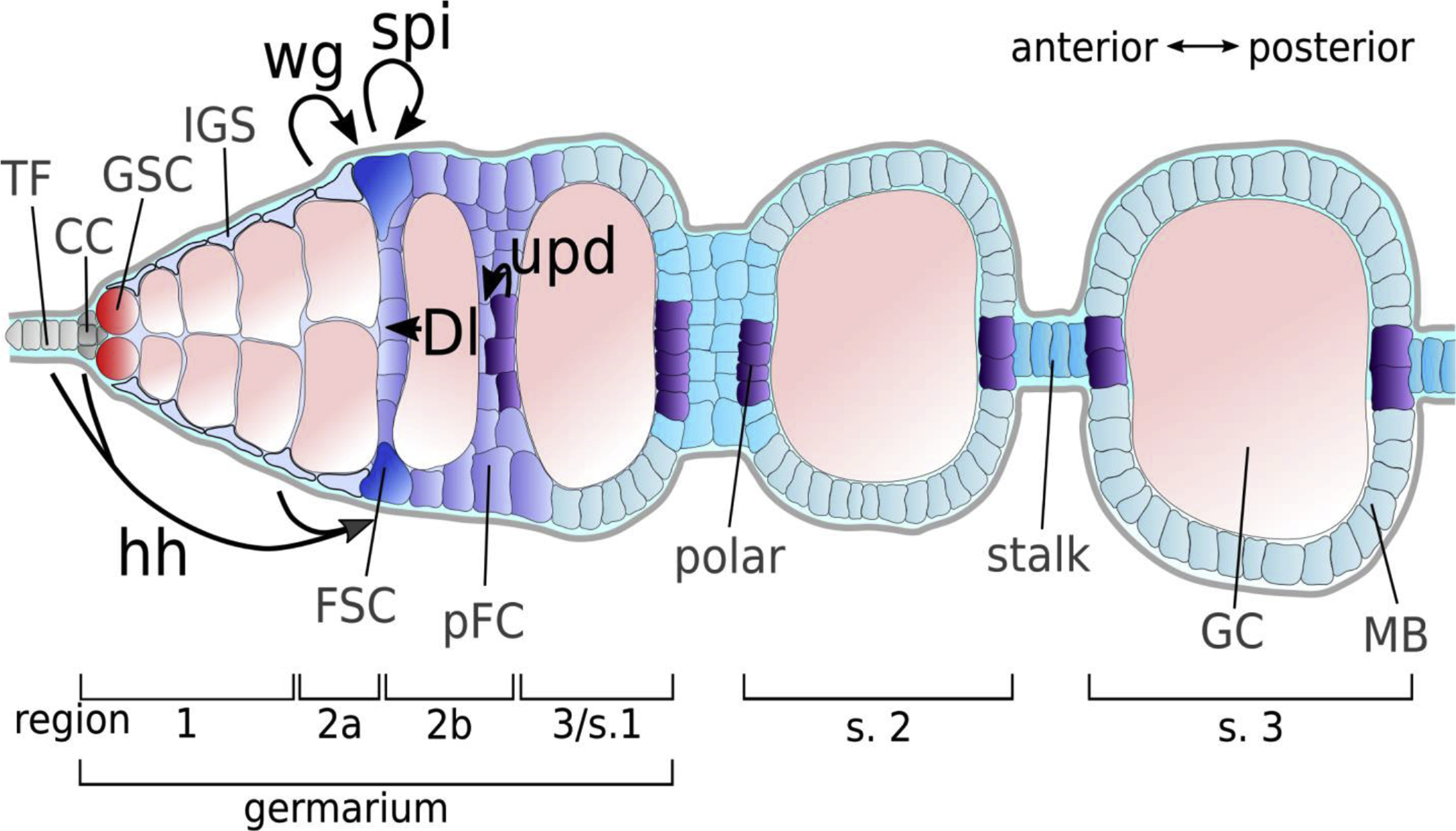
Diagram of the early stages of Drosophila oogenesis and overview of sources of selected signaling ligands implicated in follicle cell development. The Drosophila germarium is divided in four subregions (1, 2a, 2b and 3). The first budded cyst is referred to as stage 2. Anterior-most terminal filament (TF) and cap cells (CC) build the niche for germline stem cells (GSC). Together with the inner germarial sheath (IGS) cells TF and CC provide Hedgehog (Hh) ligand to follicle stem cells (FSC), which are located at the 2a/2b border. IGS cells further provide Wingless (Wg) to FSCs. In response, FSCs and pFCs produce Spitz (Spi). A subset of prefollicle cells (pFC) receives Delta (Dl) from germline cells (GC) and assumes polar cell fate. Polar fated cells produce the JAK-STAT ligand Unpaired (Upd), which specifies stalk cells. To date, no signaling pathways have been identified to induce the earliest steps towards main body (MB) cell fate.
The GSC niche was among the first to be characterized at a single cell level and contributed significantly to the early understanding in the field of how adult stem cell niches function in vivo [8,9]. GSC divisions are oriented perpendicular to the niche and are inherently asymmetric, producing two daughter cells that contain unequal cytoplasmic contents and positions relative to the niche. Specifically, one daughter cell remains anchored to the cap cells through adherens junctions and retains the majority of a cytoplasmic structure called the fusome [10] while the other daughter is formed on the side of the GSC opposite the niche and thus does not have any connections to cap cells. This type of rigid niche architecture provides a straightforward mechanism for robustly segregating the stem cell and daughter cell fates at every stem cell division. In addition, these inherent asymmetries make it possible to unambiguously determine the number and location of stem cells in the tissue.
However, as more adult stem cell niches have been characterized, it is becoming clear that other types of stem cell niches do not operate in such a rigid manner. The stem cell niches in epithelial tissues have been particularly elusive, and the much more subtle differences between stem cells and daughter cells in these tissues has led to many debates over the number and position of stem cells in epithelial tissues. Nonetheless, intensive studies of stem cell based epithelial tissues have provided significant insights into the mechanisms that govern cell fate in epithelial stem cell lineages. In this review, we summarize the advances made in understanding how cell signaling pathways promote FSC behaviour and coordinate the differentiation of pFCs.
FSC number and location
The FSCs were discovered by Margolis and Spradling, and their study was among the first to use site-specific DNA recombination to identify tissue-resident stem cells [7]. By inducing sparse clones during adulthood and analyzing the clone patterns at multiple time points after clone induction, they determined that there are two mitotically active FSCs per germarium and that there are a maximum of 8 divisions downstream of the FSC division. This study provided a foundation for understanding the FSC lineage and indeed, many subsequent studies are consistent with this model. However, a recent study challenged this model and instead proposed that there are 14–16 mitotically active FSCs per germarium that each contribute to a much smaller fraction of the follicle epithelium [11]. We investigated the claims of this study and identified several flaws, such as the use of an unreliable clonal marking system and the assumption that heat shock induced flippase expression causes FRT recombination to occur in every mitotic cell with 100% efficiency [12]. We also developed a method to quantify clone size throughout the entire ovariole, and our measurements of clone size following sparse clonal labeling re-confirmed the original Margolis and Spradling model, though our data would be consistent with a range of 2–4 FSCs per germarium. In accordance, we recently showed that 2–3 cells at the Region 2a/2b boundary express low levels of Wnt4-Gal4, and these cells behave like FSCs and are capable of populating the entire follicle epithelium [2].
The precise location of the FSCs has also been debated. Margolis and Spradling noticed that clones originated from the border between Regions 2a and 2b of the germarium and extended toward the posterior, suggesting that the anterior-most cells in an FSC clone are the FSCs. Subsequent analysis confirmed that the anterior-most cells in FSC clones are located at the Region 2a/2b border, at the boundary of Fas3 expression, and that these cells are typically Fas3+ [13–15]. In contrast, Reilein et al. proposed that FSCs are organized within three rings, one at the boundary of Fas3 expression, and two additional rings located anterior to the boundary of Fas3 expression [11]. However, this is inconsistent with several studies that have identified the Fas3− somatic cells in Region 2a as IGS cells based on their distinct shape and function in promoting germ cell differentiation [3–6,15,16]. We recently re-examined the Fas3 status of cells in an FSC clone and found that all cells in the clone are Fas3+ in the large majority of cases [12]. In addition, we found that the FSCs expressing low levels of Wnt4-Gal4 are Fas3+, and that the expression profile of the Fas3− somatic cells in Region 2a is distinct from that of the Fas3+ cells in Region 2b [2]. Taken together, these findings indicate that each germarium contains 2–4 FSCs that are located within a single ring at the edge of the Fas3 expression boundary.
The regulation of cell fate decisions in the FSC lineage
The signaling pathways that regulate self-renewal, differentiation or proliferation of the FSCs or their daughter cells intersect in multiple ways, forming at least three distinct signaling networks. First, Wnt signaling acts upstream of EGFR signaling in FSCs and Notch signaling in pFCs (Figure 2) [17,18]. There is a steep gradient of Wnt signaling in the follicle epithelium, with high levels in FSCs and pFCs at the Region 2a/2b border and low or undetectable levels of pathway activity in pFCs that have moved into Region 2b [17,19,20]. This gradient is achieved by localized delivery of the Wnt ligand, Wingless (Wg), to the FSCs from neighboring IGS cells [17], combined with the action of the glypican, Dally-like (Dlp), that concentrates Wnt ligands in the niche region and the matrix metalloproteinase, Mmp2 [21], which degrades Dlp [22]. In the FSCs, Wnt signaling promotes self-renewal and proliferation, at least in part, by activating expression of the EGF ligand, Spitz, which induces the MAPK pathway, presumably through activation of EGFR [17]. EGFR signaling, in turn, functions upstream of the Lkb1-AMPK pathway to regulate cell polarity [23] and represses the transcriptional corepressor, groucho (gro) through phosphorylation by dpERK [18]. In pFCs, the active (unphosphorylated) form of Gro promotes Notch signaling in differentiating pFCs. Interestingly, the phosphorylated form of gro persists for a short period of time after pFCs exit the niche and downregulate dpERK. This may provide these cells with a molecular “memory” of niche signaling that delays differentiation and allows these cells to participate in stem cell replacement or to increase in number before committing to a cell fate choice [18].
Figure 2: Wingless and EGFR signaling pathways.
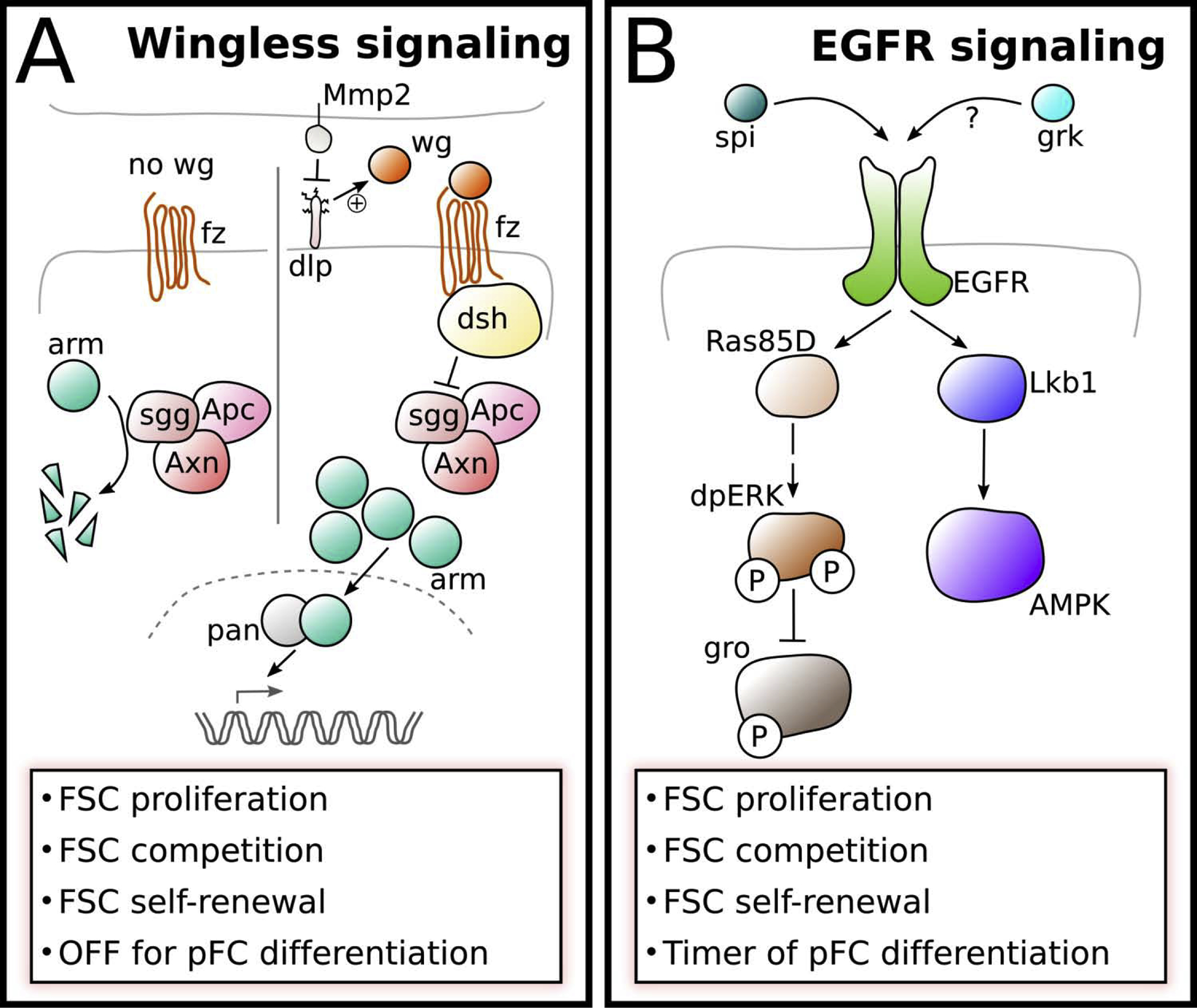
A) Wg is provided to FSCs by IGS cells leading to high levels of Wnt pathway activity in FSCs, which changes dynamically [17,19]. The glypican Dlp aids to concentrate Wg in the niche region and is counteracted by Matrix metalloproteinase 2 (Mmp2). In the absence of Wg ligand, Arm is subject to proteasomal degradation induced by a destruction complex consisting of Apc, Shaggy (Sgg) and Axin (Axn). When Wg binds to its receptor Frizzled (Fz), active Dishevelled (Dsh) represses destruction complex activity allowing arm to interacts with Pangolin (Pan) to activate target gene expression.
B) The expression of the EGFR ligand Spitz (Spi) is induced by Wnt signaling in the FSC. It is unclear whether Gurken (Grk) may also function as an EGFR ligand in the FSCs. EGFR signaling is active in FSCs, uniformly low in newly produced pFCs and active again in older pFCs and main body follicle cells. In FSCs activated EGFR signaling induces the MAP-Kinase pathway resulting in the dual-phosphorylation and activation of ERK. One target of dpERK is Groucho (Gro), which is repressed by ERK-mediated phosphorylation and functions as a molecular timer regulating pFC differentiation. Active EGFR signaling is also required for the establishment of cell polarity via the Lkb1-AMPK pathway.
In the early pFCs, low levels of Wnt signaling promote receptivity to Notch signaling [20]. pFCs at this stage that are correctly positioned to receive a Delta signal from the germline activate the Notch pathway and progress toward the polar cell fate, whereas pFCs that do not receive a Delta signal before they downregulate Wnt signaling become refractory to Notch signals [20]. Thus, Wnt signaling must be high in FSCs to promote self-renewal and downregulated in pFCs to allow for differentiation, suggesting that a steep gradient of Wnt signaling is important for controlling FSC number and position.
Second, Hedgehog (Hh) signaling controls proliferation and differentiation in the FSC lineage by regulating Yorkie (Yki), the downstream effector in the Hippo signaling pathway, leading to Yki nuclear accumulation and activation of target genes, like Cyclin E (Figure 3) [24]. The Hh ligand is produced by cap cells, terminal filament cells, and IGS cells in a gradient that decreases in an anterior-to-posterior direction [19,25]. RNAi knockdown of Hh in either cap cells and terminal filament cells or IGS cells causes follicle formation phenotypes [19,26], indicating that Hh is required from multiple sources for optimal performance of the tissue. In cap and terminal filament cells, the release of Hh from the apical surface of the cells is inhibited by the transmembrane protein, Brother of ihog (Boi) [26]. This is counteracted by cholesterol in the diet, which binds to the nuclear hormone, Hr96 in cap and terminal filament cells and stimulates the release of Hh from Boi, thus helping to coordinate egg production with nutrient availability [27]. The mir-310 family of microRNAs also helps to coordinate the level of Hh signaling in response to dietary cues by repressing Hr96 and vesicle trafficking of the Hh ligand [28]. The gradient of Hh ligand availability produces a steep gradient of Hh pathway activity in the FSC lineage, with high levels in the FSCs and rapidly decreasing levels in newly-produced pFCs [20,29,30]. Hh signaling regulates early pFC differentiation through a network of mutually repressive interactions with two transcription factors, castor and eyes absent [31,32]. However, the gradient of Hh activity in pFCs appears not to be important for spatial patterning of pFC fate [33]. These observations indicate that Hh signaling regulates follicle cell production, FSC self-renewal, and early pFC differentiation but is unlikely to play a direct role in the specification of FSC number or position. Interestingly, several studies suggest that Hh signaling in the FSC does not function via the canonical pathway: mastermind, which is usually a Notch signaling component, is a positive regulator of Hh signaling in FSCs [34], and Hh signaling in the FSC (but not pFCs) is independent of fused [35]. In addition, some types of Hh signaling in the FSC lineage appear to be independent of Smo [36]. Further, unlike in embryogenesis, Hh signaling does not interact with Wingless signaling in the FSC [30].
Figure 3: Hedgehog and Hippo signaling pathways.
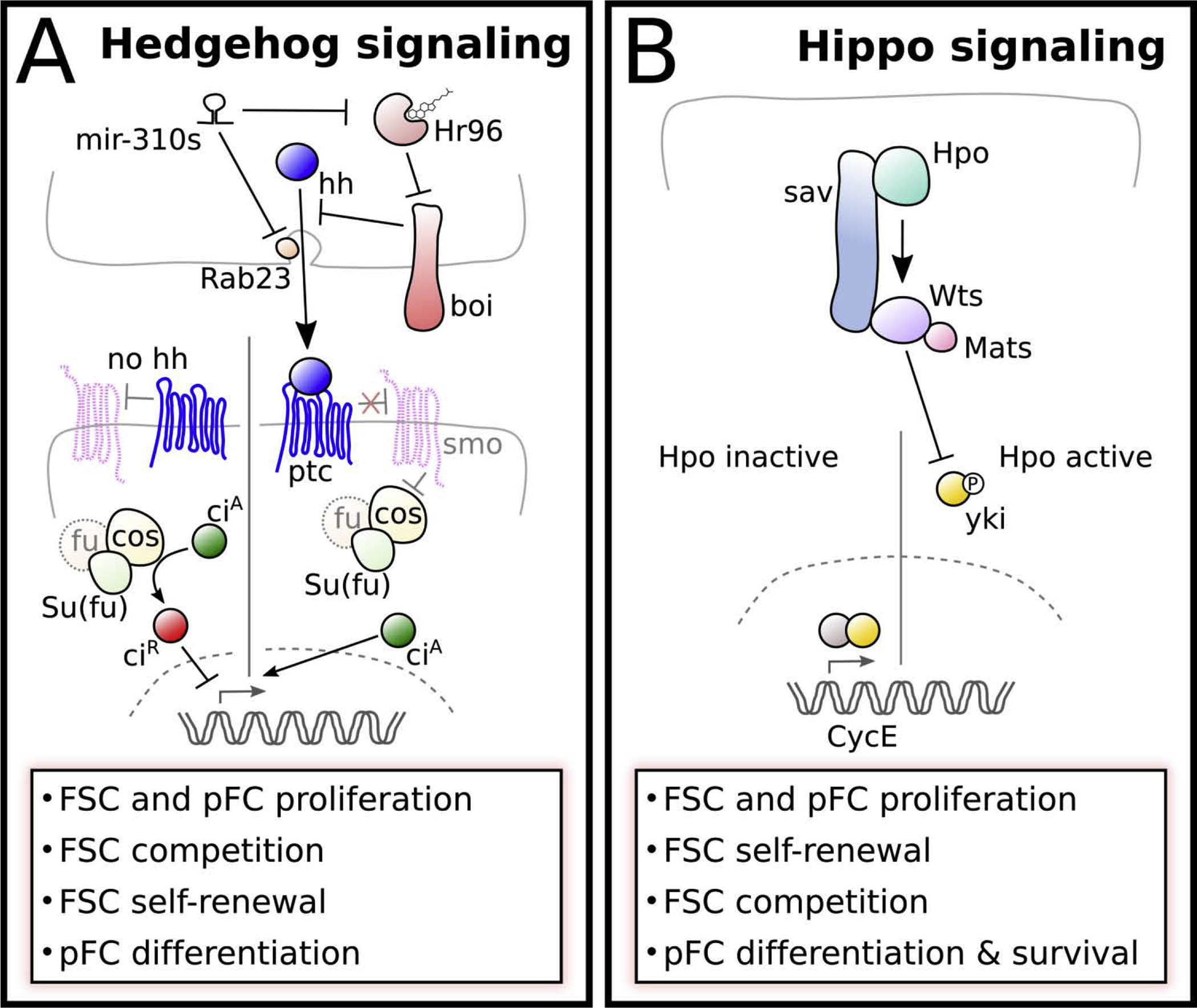
A) Hh signaling in the early follicle cell lineage. Hh is provided for FSCs from terminal filament cells, cap cells, and IGS cells. Brother of ihog (Boi) regulates hh release by sequestration. Under rich diet, cholesterol binds and activates the negative regulator of boi, Hormone receptor 96 (Hr96), allowing hh release. The micro-RNA family mir-310 represses Hh release by targeting Hr96, and Rab23, which functions in Hh transport. In canonical Hh signaling extracellular hh binds to its receptor Patched (Ptc) and prevents Ptc-mediated repression of Smoothened (Smo). This allows Costal (Cos), which interacts with Fused (Fu) and Supressor of fused (Su(fu)), to induce proteolytic cleavage of the transcription factor Cubitus interruptus (Ci), producing the repressive form (CiR). CiR acts as a repressor of Hh target gene expression. In the presence of Hh, Smo represses Cos allowing the active form of Ci (CiA) to activate Hh target gene expression.
B) Core components of the Hippo signaling pathway. When Hippo signaling is active the Hippo (Hpo) kinase phosphorylates itself and the components of the kinase cassette: Salvador (Sav), which acts as a scaffold between Hippo and Warts (Wts), as well as the Warts cofactor Mob as tumor suppressor (Mats). The activated Warts kinase phosphorylates Yorkie (Yki) and prevents its nuclear translocation. When the kinase cassette is inactive, Yki enters the nucleus and interacts acts as a transcriptional cofactor to induce target gene expression. In the FSC, Yki is regulated by the Hh signaling pathway both transcriptionally as well as post-transcriptionally and induces the expression of the cell cycle gene Cyclin E [24,76].
Third, Notch signaling cooperates with JAK-STAT signaling to specify polar and stalk cell fates (Figure 4). Notch signaling is not required for FSC self-renewal [14,34] but provides the earliest-known differentiation signal in pFCs [20,37,38], and constitutive activation of Notch causes an increased rate of FSC loss from the niche [34], presumably due to premature differentiation. A subset of newly-produced pFCs with low levels of Wnt signaling and high levels of fringe expression receive a Delta signal from the anterior surface of a germ cell cyst that is moving into Region 2b [14,20,38–41]. This initiates a program of differentiation toward the polar cell fate in which 2 polar cells are selected from a cluster of 4–6 “polar-fated” cells. As the cyst begins to bud from the germarium, the polar-fated cells secrete a JAK-STAT signal to induce nearby pFCs to differentiate into stalk cells, which then contribute to the establishment of the polar cells on the posterior surface of the next younger cyst [42–44]. Within the cluster of polar-fated cells, one cell becomes refractory to Notch signaling, resistant to apoptosis, and upregulates Delta. This activates high levels of Notch in a neighboring polar-fated cell, while the remaining cells are eliminated by apoptosis, leaving a single pair of polar cells on the anterior surface of the cyst by Stage 5 [45,46]. In this way, Notch signaling initiates a stepwise chain reaction of events that ensures the polar and stalk cells are formed in the correct time and place on two adjacent follicles. Cells that do not adopt either fate differentiate into main body follicle cells.
Figure 4: Notch and JAK-STAT signaling.
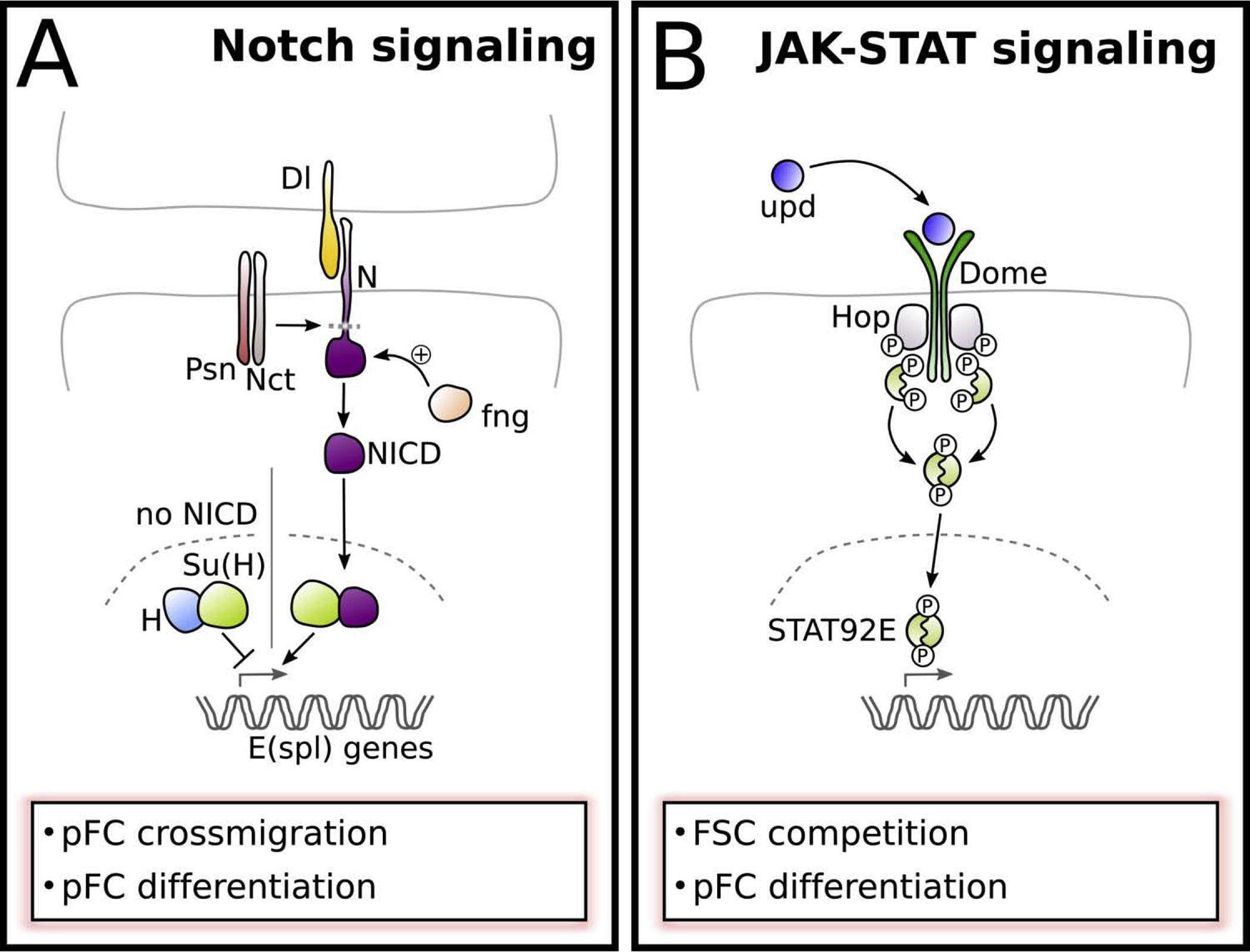
A) Notch signaling provides the earliest-known differentiation signal in pFCs but is inactive in FSCs. A subset of pFCs receives Delta (Dl) signal from neighboring germline cells. Dl interaction with Notch (N) is enhanced by high levels of the glycosyltransferase Fringe (Fng). Dl binding to N leads to proteolytic cleavage release of the Notch intracellular domain (NICD) by the gamma secretase complex subunits, including Presilin (Psn) and Nicastrin (Nct). NICD enters the nucleus and triggers the release of Suppressor of Hairless (Su(H)) from its corepressor Hairless (H). Together with Su(H) NICD induces the expression of target genes, including Enhancer of split (E(spl)) genes.
B) JAK-STAT signaling functions in FSCs as well as in pFC differentiation. The ligand Unpaired (Upd) is produced by polar fated cells and binds to the receptor Dome. Upon unpaired binding, bound Hopscotch (Hop, Drosophila JAK) is autophosphorylated and phosphorylates Dome, allowing binding of STAT92E. STAT92E is then phosphorylated, dimerizes and transfers to the nucleus to induce gene expression.
No inductive cues of main body follicle cell fate have been identified thus far, suggesting that it may be a default fate for pFCs that are not directed toward the polar or stalk cell fates. Notch signaling antagonizes JAK-STAT signaling in the follicle epithelium and Notch mutant main body cells exhibit nuclear accumulation of Stat, so Notch signaling may help to maintain this default fate by inhibiting JAK-STAT signaling [43]. However, the levels of Notch signaling in main body cells are not high enough to activate Notch reporters such as Su(H)-LacZ or m7-LacZ [18,20,43]. Indeed, even overexpression of the constitutively active Notch intracellular domain is not sufficient to activate Su(H)-LacZ in all main body and stalk cells [18,20]. Thus, it may be that these Notch pathway reporters are not effective in main body follicle cells or that additional mechanisms, such as regulation at the level of endocytic trafficking of the Notch ligand [46,47] or through interactions with Gro [18] attenuate Notch signaling in these cells.
Proliferation in the FSC lineage
FSC proliferation must be tightly controlled so that the production of new follicle cells matches the rate of new cysts entering the follicle epithelium. This coordination between the germ cell and follicle cell lineages was revealed by studies of insulin signaling, which found that loss of the insulin receptor from GSCs causes a decrease in GSC proliferation, and that this induces a non-autonomous decrease in FSC proliferation [48]. Within the FSC lineage, there are distinct modes of regulation in the FSC, pFC, and main body follicle cell populations. Specifically, proliferation in FSCs is regulated by the Wnt-EGFR and Hh-Hippo signaling networks described above as well as by BMP and TOR signaling [49,50], whereas the pFCs do not appear to require Wnt, EGFR, or TOR signaling for proliferation. Indeed Wnt and EGFR signaling are not active in early pFCs, though EGFR signaling becomes detectable again starting in Region 3. However, the Endosomal Sorting Complex Required for Transport (ESCRT) machinery has been shown to regulate the proliferation of pFCs but not main body follicle cells outside of the germarium [51]. Understanding why follicle cells rely on distinct modes of regulation as they progress through differentiation will be an interesting area for future study.
Regulation of FSC self-renewal and competition for the niche
Adult stem cells are defined by their ability to undergo self-renewing divisions in which one daughter retains the stem cell fate and the other daughter differentiates. In some stem cell lineages, such as the GSCs, this segregation of cell fate occurs during mitosis, producing two unequal daughter cells. In contrast, in most epithelial tissues, stem cell divisions are not inherently asymmetric but instead produce two daughter cells that appear to have an equal potential to acquire either the stem cell or daughter cell fate [52–54]. The ultimate fate of each cell is determined by stochastic events, such as the position of each cell relative to the niche and the behavior of cells produced by neighboring stem cell lineages. The size of the niche limits how many cells can have the stem cell identity at any moment in time, but which cells have this identity is relatively fluid. This mode of self-renewal is referred to as population asymmetry because the mechanisms that govern the segregation of cell fate do not act at the level of each stem cell division but instead on the population of cells produced by multiple stem cells dividing in close proximity to one another [55–57]. The patterns of this type of stem cell loss and replacement can be described with statistical models of neutral competition that have been adapted from those used in population genetics [58–60].
Several mutations have been identified that cause non-neutral competition, in which mutant lineages displace wildtype lineages at disproportionately low or high rates (referred to as hypocompetition or hypercompetition, respectively) [18,22–24,57,58,61–63]. The identification of hypercompetition mutations is particularly interesting as it indicates that stem cells can exert non-cell autonomous effects on their neighbors, in which the presence of a fitter (mutant) lineage increases the chance that a neighboring less-fit (wildtype) lineage will be lost prematurely. This form of stem cell replacement has been observed in many different types of Drosophila and mammalian tissues [58–60,64–68], and attention in the field is now turning to the question of whether niche competition provides a useful function for the tissue and what the bases are for the selection of one lineage over another. The most likely function for niche competition would be to eliminate unhealthy stem cells from the niche, just as cell competition eliminates unhealthy cells from tissues [69,70]. Consistent with this possibility, several forward genetic screens have revealed that a wide range of mutations are selected against by the niche competition process [58,61,62]. These include mutations in components of the major signaling pathways, as might be expected, but also in cell adhesion components, mitochondrial genes, vesicle trafficking components, and many other types of genes. Studies of mutations that cause hypercompetition suggest that proliferation rate is positively selected for by the niche competition process [57,71]. Consistent with this, overexpression of Cyclin E or string is sufficient to cause hypercompetition and can suppress some hypocompetition phenotypes [36,57].
However, Hh pathway mutants that cause a sustained increase in the rate of egg production in young flies also cause an increase in the rate of FSC loss in older flies, suggesting that hyperactivity of FSCs also leads to early stem cell exhaustion-like behavior [36]. Taken together these observations suggest that, proliferation may need to be tightly regulated and that both increased and decreased proliferation rates can impair FSC self-renewal. Other hypercompetition mutations do not increase proliferation but instead impair pFC differentiation [18,58], suggesting that there are multiple independent causes of hypercompetition. However, additional studies are needed to confirm that impaired differentiation is sufficient to cause hypercompetition and to determine how the biased selection occurs in these cases.
Concluding remarks
Collectively, research over the past 30 years has found that every major developmental signaling pathway is utilized at some point in the early FSC lineage lineage. These studies have demonstrated that the spatial and temporal patterns of cell fate specification in the early FSC lineage are established by signaling pathways that intersect in complex ways to produce precise yet flexible outcomes. A particularly elegant example of this is the coordinated action of Hh signaling, low levels of Wnt signaling, and the perdurance of phosphorylated Gro to specify a narrow window of time and space for the specification of polar cell differentiation. In addition to providing insights into the signaling pathways themselves, the careful descriptions of where and when each pathway operates in the FSC lineage has provided a foundation for investigations into new and understudied aspects of epithelial cell behavior, such as a unique form of parasitic bacterial tropism specifically to the stem cell niche region [72,73], plasticity in the sexual identity of somatic cells in the gonad [74], and the role of intracellular pH dynamics in the specification of cell fate [29,75]. Continued study of the FSC lineage is sure to provide many more insights into epithelial stem cell biology.
Highlights.
Signaling pathways have different effects in FSC and their daughter cells
Individual pathways induce distinct outcomes through signaling gradients
Additional specificity and control is provided by pathway interactions
Many FSCs behaviors are regulated non-cell autonomously by external cues
Acknowledgements
We are grateful to Sumitra Tatapudy and the reviewers for helpful comments on this manuscript. K.R. is supported by the Deutsche Forschungsgesellschaft (DFG, project number 419293565), and T.N. is supported by a grant from the National Institutes of Health, GM097158.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References
- 1.Koch EA, King RC: The origin and early differentiation of the egg chamber of Drosophila melanogaster. J Morphol 1966, 119:283–303. [DOI] [PubMed] [Google Scholar]
- 2. **.Rust K, Byrnes L, Yu KS, Park JS, Sneddon JB, Tward AD, Nystul TG: A Single-Cell Atlas and Lineage Analysis of the Adult Drosophila Ovary. bioRxiv 2019, doi: 10.1101/798223. This study uses a combination of single-cell RNA-sequencing, in vivo validation, and lineage tracing to provide new insights into cell identity and behavior in the adult Drosophila ovary. The authors identify new subtypes of IGS cells, a marker that can be used to identify FSCs, and several Gal4 lines with specific expression patterns in the ovary. The authors also discover an unexpected form of plasticity in which IGS cells, convert to follicle stem cells as part of a natural physiological response to starvation.
- 3.Kirilly D, Wang S, Xie T: Self-maintained escort cells form a germline stem cell differentiation niche. Development 2011, 138:5087–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu T, Wang S, Gao Y, Mao Y, Yang Z, Liu L, Song X, Ni J, Xie T: COP9-Hedgehog axis regulates the function of the germline stem cell progeny differentiation niche in the Drosophila ovary. Development 2015, 142:4242–4252. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Gao Y, Song X, Ma X, Zhu X, Mao Y, Yang Z, Ni J, Li H, Malanowski KE, et al. : Wnt signaling-mediated redox regulation maintains the germ line stem cell differentiation niche. Elife 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Upadhyay M, Kuna M, Tudor S, Martino Cortez Y, Rangan P: A switch in the mode of Wnt signaling orchestrates the formation of germline stem cell differentiation niche in Drosophila. PLoS Genet 2018, 14:e1007154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margolis J, Spradling A: Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 1995, 121:3797–3807. [DOI] [PubMed] [Google Scholar]
- 8.Xie T, Spradling AC: decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 1998, 94:251–260. [DOI] [PubMed] [Google Scholar]
- 9.Xie T, Spradling AC: A niche maintaining germ line stem cells in the Drosophila ovary. Science 2000, 290:328–330. [DOI] [PubMed] [Google Scholar]
- 10.de Cuevas M, Spradling AC: Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development 1998, 125:2781–2789. [DOI] [PubMed] [Google Scholar]
- 11.Reilein A, Melamed D, Park KS, Berg A, Cimetta E, Tandon N, Vunjak-Novakovic G, Finkelstein S, Kalderon D: Alternative direct stem cell derivatives defined by stem cell location and graded Wnt signalling. Nat Cell Biol 2017, 19:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fadiga J, Nystul T: The Drosophila follicle epithelium is maintained by a small number of stem cells. Elife in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nystul TG, Spradling A: An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell 2007, 1:277–285. [DOI] [PubMed] [Google Scholar]
- 14.Nystul TG, Spradling A: Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics 2010, 184:503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spradling AC, de Cuevas M, Drummond-Barbosa D, Keyes L, Lilly M, Pepling M, Xie T: The Drosophila germarium: stem cells, germ line cysts, and oocytes. Cold Spring Harb Symp Quant Biol 1997, 62:25–34. [PubMed] [Google Scholar]
- 16.Morris LX, Spradling AC: Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development 2011, 138:2207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. *.Kim-Yip RP, Nystul TG: Wingless promotes EGFR signaling in follicle stem cells to maintain self-renewal. Development 2018, 145. Wingless is an essential FSC niche factor and, though wingless is produced by multiple cell types in the germarium, the authors demonstrate that a short-range wingless signal produced by neighboring IGS cells is sufficient. In addition, they demonstrate that Wnt signaling operates genetically upstream of EGFR signaling in FSCs by inducing the expression of the EGFR ligand, spitz.
- 18. *.Johnston MJ, Bar-Cohen S, Paroush Z ‘ev, Nystul TG: Phosphorylated Groucho delays differentiation in the follicle stem cell lineage by providing a molecular memory of EGFR signaling in the niche. Development 2016, 143:4631–4642. The authors identify two proteins, groucho and Six4, that are required for Notch signaling and polar cell differentiation. Groucho is inhibited by EGFR signaling, and they provide evidence that perdurance of the inhibited form of Groucho provides a “memory” of the EGFR niche signal in newly-produced pFCs.
- 19.Sahai-Hernandez P, Nystul TG: A dynamic population of stromal cells contributes to the follicle stem cell niche in the Drosophila ovary. Development 2013, 140:4490–4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. **.Dai W, Peterson A, Kenney T, Burrous H, Montell DJ: Quantitative microscopy of the Drosophila ovary shows multiple niche signals specify progenitor cell fate. Nat Commun 2017, 8:1244. The authors used careful quantitative imaging of pathway reporters in the germarium to investigate the interactions between Wnt, Hh, and Notch signaling. They show that Wnt and Hh signaling operate independently and that low levels of Wnt signaling bias pFCs to adopt a polar cell fate by transiently inhibiting eyes absent.
- 21.Wang X, Page-McCaw A: A matrix metalloproteinase mediates long-distance attenuation of stem cell proliferation. J Cell Biol 2014, 206:923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. *.Su T-Y, Nakato E, Choi PY, Nakato H: Drosophila Glypicans Regulate Follicle Stem Cell Maintenance and Niche Competition. Genetics 2018, 209:537–549. The authors demonstrate that two glypicans, Dally and Dlp have opposite roles in FSC self-renewal, with Dally mutations causing an increase in the rate of FSC loss from the niche and Dlp mutations causing a niche hypercompetition phenotype. In addition, they show that Dally and Dlp contribute to the regulation of JAK-STAT, Wnt, and Hedgehog signaling in the FSC lineage.
- 23.Castanieto A, Johnston MJ, Nystul TG: EGFR signaling promotes self-renewal through the establishment of cell polarity in Drosophila follicle stem cells. Elife 2014, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Kalderon D: Coupling of Hedgehog and Hippo pathways promotes stem cell maintenance by stimulating proliferation. J Cell Biol 2014, 205:325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forbes AJ, Lin H, Ingham PW, Spradling AC: hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development 1996, 122:1125–1135. [DOI] [PubMed] [Google Scholar]
- 26.Hartman TR, Zinshteyn D, Schofield HK, Nicolas E, Okada A, O’Reilly AM: Drosophila Boi limits Hedgehog levels to suppress follicle stem cell proliferation. J Cell Biol 2010, 191:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartman TR, Strochlic TI, Ji Y, Zinshteyn D, O’Reilly AM: Diet controls Drosophila follicle stem cell proliferation via Hedgehog sequestration and release. J Cell Biol 2013, 201:741–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. *.Çiçek IÖ, Karaca S, Brankatschk M, Eaton S, Urlaub H, Shcherbata HR: Hedgehog Signaling Strength Is Orchestrated by the mir-310 Cluster of MicroRNAs in Response to Diet. Genetics 2016, 202:1167–1183. The authors demonstrate that the mir-310 family of miRNAs regulate the release of Hh from the cap and terminal filament cells in response to dietary cues. They show that mir-310 miRNAs act by repressing the expression of multiple targets that promote Hh release, including Rab23, Hr96, and tramtrack.
- 29.Ulmschneider B, Grillo-Hill BK, Benitez M, Azimova DR, Barber DL, Nystul TG: Increased intracellular pH is necessary for adult epithelial and embryonic stem cell differentiation. J Cell Biol 2016, 215:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forbes AJ, Spradling AC, Ingham PW, Lin H: The role of segment polarity genes during early oogenesis in Drosophila. Development 1996, 122:3283–3294. [DOI] [PubMed] [Google Scholar]
- 31.Chang Y-C, Jang AC-C, Lin C-H, Montell DJ: Castor is required for Hedgehog-dependent cell-fate specification and follicle stem cell maintenance in Drosophila oogenesis. Proc Natl Acad Sci U S A 2013, 110:E1734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai J, Montell D: Eyes absent, a key repressor of polar cell fate during Drosophila oogenesis. Development 2002, 129:5377–5388. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Kalderon D: Regulation of cell proliferation and patterning in Drosophila oogenesis by Hedgehog signaling. Development 2000, 127:2165–2176. [DOI] [PubMed] [Google Scholar]
- 34.Vied C, Kalderon D: Hedgehog-stimulated stem cells depend on non-canonical activity of the Notch co-activator Mastermind. Development 2009, 136:2177–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Besse F, Busson D, Pret A-M: Fused-dependent Hedgehog signal transduction is required for somatic cell differentiation during Drosophila egg chamber formation. Development 2002, 129:4111–4124. [DOI] [PubMed] [Google Scholar]
- 36. **.Singh T, Lee EH, Hartman TR, Ruiz-Whalen DM, O’Reilly AM: Opposing Action of Hedgehog and Insulin Signaling Balances Proliferation and Autophagy to Determine Follicle Stem Cell Lifespan. Dev Cell 2018, 46:720–734.e6. The authors demonstrate that, although increased proliferation induced by overexpression of string enhances FSC self-renewal and fecundity, increased Hh signaling by knockdown of boi or overexpression of Hh decreases FSC lifespan and fecundity in older flies. This effect is counteracted by insulin signaling, which may work antagonistically with Hh signaling to promote optimal levels of FSC output. This study also provides evidence in support of a new idea that some types of signaling downstream of Ptc are Smo-independent.
- 37.Ruohola H, Bremer KA, Baker D, Swedlow JR, Jan LY, Jan YN: Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell 1991, 66:433–449. [DOI] [PubMed] [Google Scholar]
- 38.Grammont M, Irvine KD: fringe and Notch specify polar cell fate during Drosophila oogenesis. Development 2001, 128:2243–2253. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Schier H, St Johnston D: Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev 2001, 15:1393–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grammont M, Irvine K: Organizer activity of the polar cells during Drosophila oogenesis. Development 2002, 129:5131–5140. [DOI] [PubMed] [Google Scholar]
- 41.Althauser C, Jordan KC, Deng W-M, Ruohola-Baker H: Fringe-dependent notch activation and tramtrack function are required for specification of the polar cells inDrosophila oogenesis. Dev Dyn 2005, 232:1013–1020. [DOI] [PubMed] [Google Scholar]
- 42.Torres IL, López-Schier H, St Johnston D: A Notch/Delta-dependent relay mechanism establishes anterior-posterior polarity in Drosophila. Dev Cell 2003, 5:547–558. [DOI] [PubMed] [Google Scholar]
- 43.Assa-Kunik E, Torres I, Schejter E, Johnston D, Shilo B: Drosophila follicle cells are patterned by multiple levels of Notch signaling and antagonism between the Notch and JAK/STAT pathways. Development 2007, 134:1161–1169. [DOI] [PubMed] [Google Scholar]
- 44.McGregor JR, Xi R, Harrison DA: JAK signaling is somatically required for follicle cell differentiation in Drosophila. Development 2002, 129:705–717. [DOI] [PubMed] [Google Scholar]
- 45.Besse F, Pret A-M: Apoptosis-mediated cell death within the ovarian polar cell lineage of Drosophila melanogaster. Development 2003, 130:1017–1027. [DOI] [PubMed] [Google Scholar]
- 46.Vachias C, Couderc J-L, Grammont M: A two-step Notch-dependant mechanism controls the selection of the polar cell pair in Drosophila oogenesis. Development 2010, 137:2703–2711. [DOI] [PubMed] [Google Scholar]
- 47.Nagaraj R, Banerjee U: Regulation of Notch and Wingless signalling by phyllopod, a transcriptional target of the EGFR pathway. EMBO J 2009, 28:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LaFever L, Drummond-Barbosa D: Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science 2005, 309:1071–1073. [DOI] [PubMed] [Google Scholar]
- 49.Kirilly D, Spana EP, Perrimon N, Padgett RW, Xie T: BMP signaling is required for controlling somatic stem cell self-renewal in the Drosophila ovary. Dev Cell 2005, 9:651–662. [DOI] [PubMed] [Google Scholar]
- 50.LaFever L, Feoktistov A, Hsu H-J, Drummond-Barbosa D: Specific roles of Target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development 2010, 137:2117–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. **.Berns N, Woichansky I, Friedrichsen S, Kraft N, Riechmann V: A genome-scale in vivo RNAi analysis of epithelial development in Drosophila identifies new proliferation domains outside of the stem cell niche. J Cell Sci 2014, 127:2736–2748. The authors demonstrate that the ESCRT machinery is required for proliferation of pFC outside of the stem cell niche but not for more differentiated follicle cells. They conclude that there are at least three domains in the early follicle cell lineage with distinct regulation of proliferation and suggest that the regulation of proliferation changes as the stem cell potential decreases.
- 52.Clayton E, Doupé DP, Klein AM, Winton DJ, Simons BD, Jones PH: A single type of progenitor cell maintains normal epidermis. Nature 2007, 446:185–189. [DOI] [PubMed] [Google Scholar]
- 53.Philpott A, Winton DJ: Lineage selection and plasticity in the intestinal crypt. Curr Opin Cell Biol 2014, 31:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein AM, Simons BD: Universal patterns of stem cell fate in cycling adult tissues. Development 2011, 138:3103–3111. [DOI] [PubMed] [Google Scholar]
- 55.Watt FM, Hogan BL: Out of Eden: stem cells and their niches. Science 2000, 287:1427–1430. [DOI] [PubMed] [Google Scholar]
- 56.Simons BD, Clevers H: Strategies for homeostatic stem cell self-renewal in adult tissues. Cell 2011, 145:851–862. [DOI] [PubMed] [Google Scholar]
- 57.Reilein A, Melamed D, Tavaré S, Kalderon D: Division-independent differentiation mandates proliferative competition among stem cells. Proc Natl Acad Sci U S A 2018, 115:E3182–E3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kronen MR, Schoenfelder KP, Klein AM, Nystul TG: Basolateral junction proteins regulate competition for the follicle stem cell niche in the Drosophila ovary. PLoS One 2014, 9:e101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vermeulen L, Morrissey E, van der Heijden M, Nicholson AM, Sottoriva A, Buczacki S, Kemp R, Tavaré S, Winton DJ: Defining stem cell dynamics in models of intestinal tumor initiation. Science 2013, 342:995–998. [DOI] [PubMed] [Google Scholar]
- 60.Snippert HJ, Schepers AG, van Es JH, Simons BD, Clevers H: Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep 2014, 15:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang ZA, Huang J, Kalderon D: Drosophila follicle stem cells are regulated by proliferation and niche adhesion as well as mitochondria and ROS. Nat Commun 2012, 3:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cook MS, Cazin C, Amoyel M, Yamamoto S, Bach E, Nystul T: Neutral Competition for Drosophila Follicle and Cyst Stem Cell Niches Requires Vesicle Trafficking Genes. Genetics 2017, 206:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vied C, Reilein A, Field NS, Kalderon D: Regulation of Stem Cells by Intersecting Gradients of Long-Range Niche Signals. Dev Cell 2012, 23:836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Navascués J, Perdigoto CN, Bian Y, Schneider MH, Bardin AJ, -Arias AM, Simons BD: Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J 2012, doi: 10.1038/emboj.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amoyel M, Simons BD, Bach EA: Neutral competition of stem cells is skewed by proliferative changes downstream of Hh and Hpo. EMBO J 2014, 33:2295–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin Z, Kirilly D, Weng C, Kawase E, Song X, Smith S, Schwartz J, Xie T: Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell 2008, 2:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, Matunis E: JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science 2009, 326:153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicholson AM, Olpe C, Hoyle A, Thorsen A-S, Rus T, Colombé M, Brunton-Sim R, Kemp R, Marks K, Quirke P, et al. : Fixation and Spread of Somatic Mutations in Adult Human Colonic Epithelium. Cell Stem Cell 2018, 22:909–918.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Beco S, Ziosi M, Johnston LA: New frontiers in cell competition. Dev Dyn 2012, 241:831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amoyel M, Bach EA: Cell competition: how to eliminate your neighbours. Development 2014, 141:988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang ZA, Kalderon D: Cyclin E-dependent protein kinase activity regulates niche retention of Drosophila ovarian follicle stem cells. Proc Natl Acad Sci U S A 2009, 106:21701–21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frydman HM, Li JM, Robson DN, Wieschaus E: Somatic stem cell niche tropism in Wolbachia. Nature 2006, 441:509–512. [DOI] [PubMed] [Google Scholar]
- 73.Toomey ME, Panaram K, Fast EM, Beatty C, Frydman HM: Evolutionarily conserved Wolbachia-encoded factors control pattern of stem-cell niche tropism in Drosophila ovaries and favor infection. Proc Natl Acad Sci U S A 2013, 110:10788–10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma Q, de Cuevas M, Matunis EL: Chinmo is sufficient to induce male fate in somatic cells of the adult Drosophila ovary. Development 2016, 143:754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benitez M, Tatapudy S, Liu Y, Barber DL, Nystul T: Drosophila anion exchanger 2 is required for proper ovary development and oogenesis. Dev Biol 2019, doi: 10.1016/j.ydbio.2019.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsu T-H, Yang C-Y, Yeh T-H, Huang Y-C, Wang T-W, Yu J-Y: The Hippo pathway acts downstream of the Hedgehog signaling to regulate follicle stem cell maintenance in the Drosophila ovary. Sci Rep 2017, 7:4480. [DOI] [PMC free article] [PubMed] [Google Scholar]


