Abstract
Background
The aim of this study was to explore the influence of mitofusin-2 (Mfn-2) on phosphatidylinositol transfer protein 3 (PITPNM3) and tumor growth and the potential mechanism behind the regulation of Mfn-2 on PITPNM3 in hepatic carcinoma cell line SMMC-7721.
Material/Methods
We obtained promoter sequence of PITPNM3 gene from University of Santa Cruz (UCSC) genomic database, and we predict transcriptional factor of PITPNM3 genes by JASPAR database. Target transcription factor was determined by comparison of binding sites number for promoter. SMMC-7721 cells were transfected with expression plasmid containing Mfn-2, transcription factor gene and PITPNM3. The cells transfected with empty vector were used as control. Real-time polymerase chain reaction was used to determine the mRNA level of target genes. Co-immunoprecipitation (Co-IP) assay was used to determine the interaction between Mfn-2 and target transcription factor. Chromatin immunoprecipitation assay (ChIP) assay was used to determine the binding of transcription factor with PITPNM3 promoter. Tumorigenicity assay was used to compare the effect of Mfn-2, SP1, and PITPNM3 on tumor development.
Results
SP1 was selected as the target transcriptional factor. In the Co-IP assay, Mfn-2 was shown to interact with SP1. In the ChIP assay Mfn-2 transfection resulted in decreased binding number of SP1 with PITPNM3 promoter. Furthermore, PITPNM3 mRNA levels were significantly increased in SMMC-7721 cells transfected with SP1 but were decreased after transfection with Mfn-2. In nude mice, PITPNM3 and SP1 upregulation lead to larger tumor lump and conversely Mfn-2 upregulation lead to smaller tumor lump.
Conclusions
Mfn-2 could suppress expression of PITPNM3 through interaction with transcription factor SP1; Mfn-2 may have anti-tumor activity; SP1 and PITPNM3 may promote tumor development.
MeSH Keywords: Carcinoma, Acinar Cell; Caspase 1; Drug Screening Assays, Antitumor
Background
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors and ranked the third highest cause of cancer-related mortality globally [1]. Furthermore, the incidence of HCC is rising each year [2]. HCC cells are considered to be highly invasive, which result in advanced metastatic tumors [3]. However, the mechanism behind highly invasive metastatic HCC is not fully understood, and thus, treatment strategies addressing metastatic HCC is still lacking [3].
Mitofusin-2 (Mfn-2) belongs to the large family of mitochondrial transmembrane GTPase and plays important roles in mitochondrial fusion thereby mediating the apoptosis pathway of mitochondrial [4–6]. Some studies had indicated that Mfn-2 can play an important role in growth, proliferation, metastasis, and invasion of various carcinoma cells [7,8]. Specifically, the study performed by Tang et al. showed that silencing the expression of Mfn-2 might increase invasion capability of hepatic cancer cell line SMMC-7721 through regulating PITPNM3 expression [9]. Phosphatidylinositol transfer protein 3 (PITPNM3) gene encodes a member of a family of membrane-associated phosphatidylinositol transfer domain-containing proteins. PITPNM3 had been shown to be closely associated with invasion and metastasis of HCC cells [10,11]. Therefore, in the present study, we tried to validate the influence of Mfn-2 on PITPNM3 and tumor growth in HCC cells and explore the potential action mechanism behind.
Material and Methods
Strains, plasmids, reagents and experimental animal
Escherichia coli DH5α, vector pCMV-Myc-SPI (SPI is simian virus 40 promoter factor 1), and vector pACT2-Mfn-2 were preserved reagents of our laboratory. Vector pCMV-HA, antibody for Myc-HA were obtained from Clontech Company. Goat anti-rabbit IgG/HRP antibody and chemiluminescence solution were purchased from BOSTER Biological Technology Company. Cell lysis solution were purchased from Santa Cruz Company. Polyvinylidene fluoride (PVDF) membrane were purchased from Millipore Company. Biotinylated protein ladder and anti-biotin HRP-linked antibody were purchased from Aksomics Company. Genetic engineering enzymes were purchased from TaKaRa Company. Plasmid extraction kit and gel extraction kit were purchased from Omega Company. EZ ChIP kit was purchased from Upstate Biotechnology Company. Trytone and yeast extract were purchased from Oxford Company. Agar powder and agarose were purchased from Shanghai Sangon Company. Dulbecco’s Modified Eagle Medium (DMEM) was purchased from Gibco Company. Transfection Reagent Lipofetamine 2000 was purchased from Invitrogen Company. The 5- to 6-week old female nude mice were purchased from the Animal Center of Chinese Academy of Medical Sciences.
PITPNM3 transcription factor screening
We retrieved PITPNM3 promoter using the University of Santa Cruz (UCSC) genomic database (http://genome.ucsc.edu). Then we predicted the transcription factor of PITPNM3 with the promoter sequence using JASPAR database. The scoring threshold was set to 95%. Then we checked UCSC peaks on Cistrome Data Browser (http://cistrome.org/db) and compared the predicted factors with the binding sites to further screen the transcription factor which may regulate PITPNM3. The binding sites numbers of the transcriptional factor candidates were compared and the transcriptional factor with largest number of binding sites was selected as target transcription factor for the present study.
RNA extraction, polymerase chain reaction (PCR) and real-time (RT)-PCR
TRizol (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA in cells. Oligo(dT)12 substrate and reverse transcriptase superscript II (Invitrogen) were used to reverse transcript cDNA. SYBR green dye (Takara Bio Inc., Shiga, Japan) was used to amplify cDNA. Primers for Mfn-2, SP1, PITPNM3, and anti-β-Actin (ACTB) were synthesized from TakaRa. Prism7900 fluorescence quantitative PCR instrument (Applied Biosystems, Foster City, CA, USA) were used to analyze the mRNA expression levels of Mfn-2, SP1, PITPNM3 and reference ACTB. The amplification conditions were as follows: 95°C for 2 minutes, 95°C for 15 seconds, 60°C for 1 minute, for 35 cycles. Delta Ct methods was employed for analysis.
Construction of recombinant vector pCMV-HA-Mfn-2
Plasmid pACT2-Mfn2 and empty vector-HA were digested with enzymes. The digestion product was separated by 0.6% agarose gel electrophoresis. The gel sections containing target segments and vectors were separated and extracted. T4 DNA ligase enzyme was used to ligase the extracted segments (16°C for 1 hour). The vectors were transfected into E. coli DH5α. Single colonies were picked for plasmid amplification. The segment insertion was confirmed by electrophoresis.
Cell transfection
SMMC-7721 cells were single transfected with pCMV-Myc-SP1 and pCMV-HA-Mfn2, or co-transfected with both plasmids. Then 10 ug DNA were mixed with 250 uL antibiotic-free aseptic DMEM and incubated under room temperature for 5 minutes. Then 10 uL Lipofectamine 2000 was mixed with 240 uL antibiotic-free aseptic DMEM and incubated under room temperature for 5 minutes. The 2 solution were mixed together to obtain transfection solution and incubated under room temperature for 15 minutes. The culture medium in culture plates were replaced with 1 mL antibiotic-free aseptic DMEM. Transfection solution was added, mixed gently and placed in CO2 incubator at 37°C, 5% CO2. After 4 hours, cells were cultured continually under 10% DMEM.
Protein extraction and Co-immunoprecipitation (Co-IP)
After 48 hours of transfection, the culture medium was discarded, and subsequent operation was carried out on ice. The cells were scraped off, rinsed twice with cold phosphate-buffered saline (PBS) solution and centrifuged. The supernatant was discarded. 100 uL radio-immunoprecipitation assay (RIPA) lysis buffer was added and ice bathed for 30 minutes. The 1 ug anti-Myc monoclonal antibody was added, rotation mixed at 4°C for 2 hours, and then we added with 20 uL protein A, and continued to mix for 12 hours. After centrifuging, the supernatant was removed. The precipitate was washed with RIPA for 3 times. Then 20 uL 2×Tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer was added and boiled for 5 minutes. Electrophoresis was started after slight centrifuging.
Western blot analysis
Samples were electrophorized by 10% Tricine SDS-PAGE gel. Each well was loaded with 20 ug sample. After transmembrane by semi-dry electrophoresis, 5% skimmed milk was used for blocking for 1 hour. Then 1: 2000 diluted rabbit anti-HA or Myc antibodies were added. After incubation at 4°C overnight, membrane was washed with 0.1% Tris-buffered saline Tween-20 (TBST) 3 times, 5 minutes each time. 1: 10 000 diluted goat anti-rabbit immunoglobulin G (IgG) and 1: 10 000 diluted protein standard products were added to detect antibodies. After incubation at room temperature for 30 minutes, membrane was washed. The chemiluminescence solution was added for developing in the Bio-Rad gel imager.
Chromatin immunoprecipitation assay (ChIP)
EZ ChIP kit was used following the instructions in manual. We used 5×109 SMMC-7721 cells transfected with pCMV-HA-Mfn2, which were cross-liked by 1% formaldehyde and dissolved in 400 uL SDS lysis buffer.When the chromatin was broken, by ultrasound, to about 500 bp in length, SP1 was added for treatment. Then amplification was conducted in a 15 uL system containing DNA template. Fragments of immunoprecipitation product and control were amplified by real-time PCR. The CT values of each group were calculated and compared.
Tumorigenicity assay in nude mice
Nude mice are fed in SPF (specific-pathogen free) barriers. Food, bedding, cages, and drinking water were sterilized by high pressure steam and acid water (pH 3–4) was used. Female nude mice aged 5 to 6 weeks were fed in the SPF environment for 1 week. Cell inoculation experiments were carried out after body weight reached 16–19 g. The SMMC-7721 cells under logarithmic growth state which were single transfected SP1, Mfn-2, PITPNM3, and co-transfected with SP1 plus Mfn-2, and PITPNM3 plus Mfn-2, were digested with pancreatin. After washing with PBS twice, the cells were calculated precisely. Cells were suspended and pipetted with PBS in a concentration of 2×1010 cells/L. The 8 nude mice were divided into 2 group: 4 mice in group 1 was transplanted with SMMC-7721 cells transfected with empty vector, Mfn-2, PITPNM3, and PITPNM3 plus Mfn-2; the 4 mice in the other group were transfected with empty vector, SP1, Mfn-2, and SP1 plus Mfn-2. We used 0.1 mL of suspension to inject subcutaneously into the dorsal right shoulder of each nude mouse, with 2×106 cells for each mouse. After transplantation, the mice were observed cautiously. The volume of the tumor was estimated after the solid mass formed subcutaneously at the inoculated site.
Statistical analysis
SPSS12.0 software was used to analyze the data. All the data were expressed as means±standard deviation (SD). The t-test was used to compare the data between 2 groups. One-way ANOVA was used to compare the data between three or more groups. The difference was statistically significant when P<0.05.
Results
Identification of transcriptional factor for PITPNM3
Totally, 134 binding sites in PITPNM3 promoter with scoring threshold ≥95% were obtained. By comparing with UCSC peaks, 2 transcriptional factor candidates, SP1 and CCAAT/enhancer binding protein beta (CEBPB) were identified. The PITPNM3 promoter relative binding site numbers for SP1 and CEBPB were further compared and SP1 with significantly more binding sites in PITPNM3 was selected as target transcription factor in the present study (Figure 1).
Figure 1.
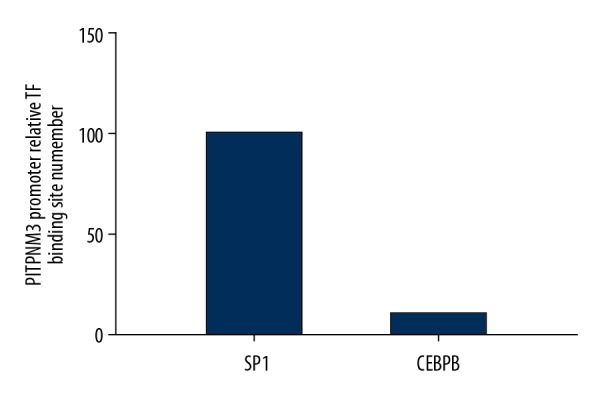
The relative binding site number of SP1 and CEBPB with PITPNM3 promoter. SP1 – simian virus 40 promoter factor 1; PITPNM3 – phosphatidylinositol transfer protein 3; CEBPB – CCAAT/enhancer binding protein beta.
Interaction between Mfn-2 and SP1 measured by Co-IP test
Total proteins of SMMC-7721 cells transfected with Mfn-2 and SP1 were extracted and co-precipitated by HA or Myc antibodies. Immune mixture was collected and separated by SDS-PAGE electrophoresis. After film transfer, the interacting proteins were detected by western blot analysis. The results were shown in Figure 2. In the cells transfected with Mfn-2, HA-conjugated-Mfn2 could be co-precipitated by HA antibodies, and SP1 protein was also detected in the precipitation. In the cells transfected with SP1, Myc-conjugated SP1 could be co-precipitated by Myc antibodies, and Mfn-2 was also detected in the precipitation. The results suggested that Mfn-2 and SP1 can interact with each other.
Figure 2.
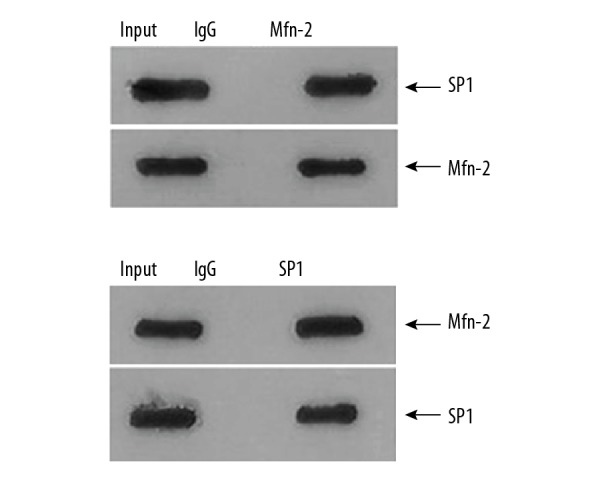
SP1 and Mfn-2 were both detected in the precipitation for SMMC-7721 cells transfected with Mfn-2 and SP1 respectively in Co-IP assay. Input lane represents sample of total extracted protein; IgG lane represents sample of negative control of IgG protein; Mfn-2 lane represents sample of immune mixture precipitated by HA antibody; SP1 lane represents sample of immune mixture precipitated by Myc antibody; SP1 beside arrow represents the protein for the detected band; Mfn-2 beside arrow represents the protein for the detected band. SP1 – simian virus 40 promoter factor 1; PITPNM3 – phosphatidylinositol transfer protein 3; Co-IP – co-immunoprecipitation.
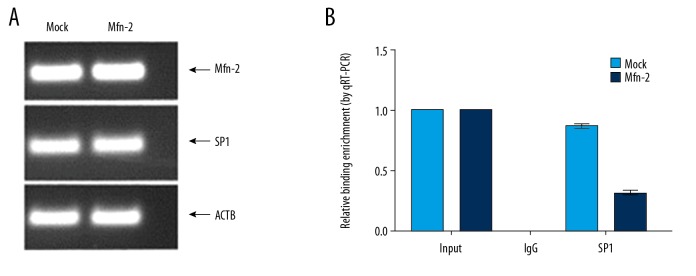
Effect of upregulation of Mfn-2 on SP1-binding with PITPNM3 promoter
Transfection result was determined by PCR as shown in Figure 3A. The Mfn-2 mRNA level increased and SP1 mRNA decreased in SMMC-7721 cells after transfection Mfn-2. Chromatin immunoprecipitation assay was used to validate the binding of SP1 and PITPNM3 and analyze the effect of Mfn-2 protein upregulation on the binding of SP1 to PITPNM3 promoter. The results of ChIP assay are shown in Figure 3B. The results show that the precipitation induced by SP1 antibody contained PITPNM3 promoter sequence; the relative binding enrichment of SP1 with PITPNM3 promoter decreased significantly after the upregulation of Mfn-2.
Figure 3.
(A) mRNA levels of Mfn-2 and SP1 in SMMC-7721 cells transfected with Mfn-2. (B) SP1 binding with PITPNM3 promoter was significantly reduced in SMMC-7721 cells after transfection with Mfn-2 as determined by ChIP analysis. Mfn-2 – mitofusin-2; SP1 – simian virus 40 promoter factor 1; PITPNM3 – phosphatidylinositol transfer protein 3; ChIP – chromatin immunoprecipitation.
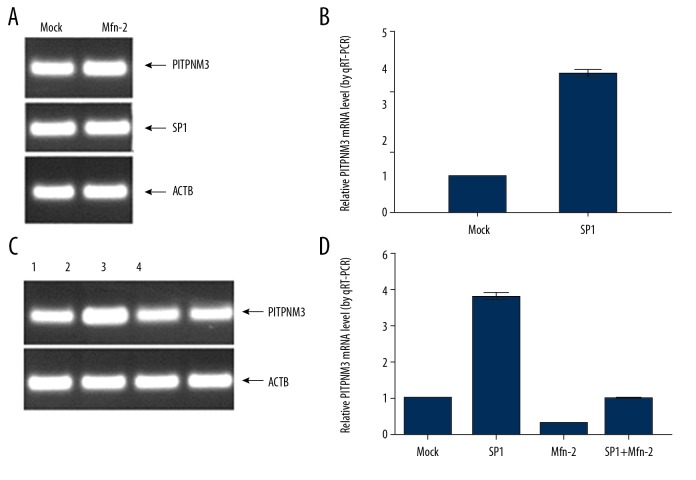
Influence of upregulation of Mfn-2 or SP1 on mRNA expression level of PITPNM3
RT-PCR was employed to determine mRNA levels of Mfn-2, SP1, and PITPNM3 after transfection with target genes. The results are shown in Figure 4. PITPNM3 mRNA levels was significantly increased in SMMC-7721 cells transfected with SP1, which indicated that PITPNM3 mRNA expression level can be enhanced by SP1. PITPNM3 mRNA levels were further compared in SMMC-7721 cells transfected with SP1, Mfn-2 alone, and both combined. As a result, PITPNM3 mRNA levels was increased in the SP1 transfection group and decreased in the Mfn-2 transfection group. The level of PITPNM3 mRNA in SMMC-7721 cells transfected with both SP1 and Mfn-2 were comparable with that in the control group.
Figure 4.
(A) mRNA levels of PITPNM3 and SP1 in SMMC-7721 cells transfected with SP1. (B) PITPNM3 mRNA levels were significantly increased in SMMC-7721 cells transfected with SP1. (C) mRNA levels of PITPNM3 in SMMC-7721 cells transfected with SP1, Mfn-2, and both. (D) PITPNM3 mRNA levels were significantly increased in SMMC-7721 cells transfected with SP1 and decreased in SMMC-7721 cells transfected with Mfn-2. 1=mock; 2=SP1; 3=Mfn-2; 4=SP1+Mfn-2. PITPNM3 – phosphatidylinositol transfer protein 3; SP1 – simian virus 40 promoter factor 1; Mfn-2 – mitofusin-2.
Tumorigenicity analysis of SMMC-7721 in nude mice
After 7 days, a tumor lump could be observed for all mice (Figure 5A). In group 1 mice, PITPNM3 upregulation lead to a larger size tumor lump and conversely Mfn-2 upregulation lead to smaller size tumor lump (Figure 5B). In group 2 mice, transfection with SP1 lead to a larger size of tumor lump, which is opposite to the effect of Mfn-2 (Figure 5C). These results suggested that Mfn-2 had an anti-tumor effect while PITPNM3 and SP1 had a promoting tumor effect; Mfn-2 might suppress tumorigenicity of PITPNM3 and SP1 in nude mice.
Figure 5.
The results of tumorigenicity assay of SMMC-7721 in nude mice. (A) At 14 days after subcutaneous cell injection, tumor lumps were formed in nude mice; (B) dissected tumor lump in mice of group 1; (C) dissected tumor lump in mice of group 2.
Discussion
A previous study indicated Mfn-2 might be involved in invasion of hepatic carcinoma cells by regulating expression of PITPNM3. To further validate the influence of Mfn-2 on PITPNM3 and explore the associated mechanism, we performed the present study using the hepatic carcinoma cell line SMMC-7721.
Using UCSC genomic database, SP1 was identified as a transcriptional factor for PITPNM3 promoter with a relatively larger binding site number. RT-PCR result indicated that SP1 upregulation resulted in elevated PITPNM3 expression, which confirmed that SP1 can regulate PITPNM3 expression significantly. According to the results of Co-IP assay, there was obvious interaction between Mfn-2 protein and SP1. According to the results of ChIP assay, upregulation of Mfn-2 might suppress the binding of SP1 to PITPNM3 promoter. Moreover, Mfn-2 regulation resulted in significantly reduced PITPNM3 mRNA levels as shown in RT-PCR results. In the tumorigenicity assay in nude mice, SP1 and PITPNM3 transfection resulted in tumors with a larger volume and conversely Mfn-2 transfection lead to smaller size of tumors. Based on these findings, we might conclude that Mfn-2 could suppress expression of PITPNM3 through interaction with transcription factor SP1; Mfn-2 might have anti-tumor activity.
According to recent studies, PITPNM3 might play an important role in tumor invasion and metastasis. PITPNM3 expression was significantly elevated in HCC tissues [10]. Silencing the expression of PITPNM3 markedly attenuated the invasive and metastatic abilities of HCC cells, whereas upregulation of PITPNM3 significantly increased HCC cell mobility [10]. Similarly, in breast cancer cells, upregulation of PITPNM3 also promoted invasion and metastasis of breast cancer cells [11]. According to founding by Zhu et al., CC-chemokine ligand 18 (CCL18) in SMMC-7721 could combine with receptor PITPNM3 and further activated downstream signal pathway related with cell adhesion, thereby improve the invasion and metastasis capacity of hepatic carcinoma cells [12,13]. In respect to Mfn-2, studies in lung cancer and gastric cancer cells have indicated that Mfn-2 played important roles in growth, proliferation, metastasis, and invasion of carcinoma cells [7,8]. In vitro studies demonstrated that Mfn-2 was a downstream gene of P53 and overexpression of Mfn-2 could effectively suppress proliferation of hepatic cancer cells [14,15]. The study performed by Tang et al. elucidated that silencing expression of Mfn-2 can promote invasion capacity of hepatic carcinoma cells through upregulation of PITPNM3 [9]. The results of our study were consistent with the previous founding and further revealed that the regulation of Mfn-2 on PITPNM3 was medicated by interaction between Mfn-2 and transcriptional factor SP1.
Invasion and metastasis of hepatic carcinoma is a very important procedure during disease progression, which is complicated and not fully understood. Mfn-2, SP1, and PITPNM3 might be involved in this process through their interactions. In our study we validated the effect of Mfn-2 on PITPNM3 in HCC cells, and further elucidated the regulation effect that might be exerted through promoter SP1. The results of our study might help further the understanding on HCC metastasis and progression and provide potential intervention targets for HCC prevention and treatment.
Conclusions
Mfn-2 could suppress expression of PITPNM3 through interaction with transcription factor SP1; Mfn-2 might have anti-tumor activity; SP1 and PITPNM3 might promote tumor development. The result of our study might provide a basis for further understanding the mechanism of HCC and the development of intervention targets for HCC treatment.
Footnotes
Source of support: This study was by funded by Shenzhen Science and Technology Planning Project, No. JCYJ20170306140945736
Conflict of interest
The authors declare no conflict of interest
References
- 1.Costentin C. Hepatocellular carcinoma surveillance. Presse Med. 2017;46(4):381–85. doi: 10.1016/j.lpm.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Mullath A, Krishna M. Hepatocellular carcinoma – time to take the ticket. World J Gastrointest Surg. 2019;11(6):287–95. doi: 10.4240/wjgs.v11.i6.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neureiter D, Stintzing S, Kiesslich T, Ocker M. Hepatocellular carcinoma: Therapeutic advances in signaling, epigenetic and immune targets. World J Gastroenterol. 2019;25(25):3136–50. doi: 10.3748/wjg.v25.i25.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Yang L, Gao YF, et al. MicroRNA-106b induces mitochondrial dysfunction and insulin resistance in C2C12 myotubes by targeting mitofusin-2. Mol Cell Endocrinol. 2013;381:230–40. doi: 10.1016/j.mce.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Bach D, Pich S, Soriano FX, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–97. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 6.Filadi R, Pendin D, Pizzo P. Mitofusin-2: from functions to disease. Cell Death Dis. 2018;9(3):330. doi: 10.1038/s41419-017-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lou Y, Zhang Y, Li R, et al. Transcriptional profiling revealed the anti-proliferative effect of Mfn-2 deficiency and identified risk factors in lung adenocarcinoma. Tumour Biol. 2016;37(7):8643–55. doi: 10.1007/s13277-015-4702-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G-E, Jin H-L, Lin X-K, et al. Anti-tumor effects of Mfn-2 in gastric cancer. Int J Mol Sci. 2013;14(7):13005–21. doi: 10.3390/ijms140713005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang T, Tao X, Bao X, et al. Influence of Mfn-2 silence on hepatoma cell invasion and PITPNM3 expression. J Clin Exp Med. 2018;7:703–6. [Google Scholar]
- 10.He C, Su S, Chen F, et al. Overexpression of PITPNM3 promotes hepatocellular carcinoma cell metastasis. Chin Sci Bull. 2014;59:1326–33. [Google Scholar]
- 11.Chen J, Yao Y, Gong C, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cel. 2011;19(006):541–55. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu B. CCL18 promotes invasion and metastasis of human hepatic carcinoma cell line SMMC-7721 through PITPNM3. Guangzhou: Guangzhou Medical School; 2013. [Google Scholar]
- 13.Lin Z, Li W, Zhang H, Wu W, et al. CCL18/PITPNM3 enhances migration, invasion, and EMT through the NF-κB signaling pathway in hepatocellular carcinoma. Tumour Biol. 2016;37(3):3461–68. doi: 10.1007/s13277-015-4172-x. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Zhu F, Wang S, et al. HSG provides antitumor efficacy on hepatocellular carcinoma both in vitro and in vivo. Oncol Rep. 2010;4:183–88. [PubMed] [Google Scholar]
- 15.Wang W, Cheng X, Lu J, et al. Mitofusin-2 is a novel direct target of p53. Biochem Biophys Res Commun. 2010;400:587–92. doi: 10.1016/j.bbrc.2010.08.108. [DOI] [PubMed] [Google Scholar]