Abstract
BACKGROUND.
PSMA expression in the prostate epithelium is controlled by a cis-element, PSMA enhancer (PSME). PSME contains multiple binding sites for Sox proteins, and in this study, we identified Sox7 protein as a negative regulator of PSMA expression through its interaction with PSME.
METHODS.
The statistical correlation between Sox7 and PSMA mRNA expression was evaluated using five prostate cancer studies from cBioportal. In vitro and in vivo interaction between Sox7 and PSME was evaluated by chromatin immunoprecipitation (ChIP), electrophoretic mobility shift assay (EMSA), and luciferase reporter assay. Synthetic oligonucleotides were generated to define the sites in PSME that interact with Sox7 protein. Sox7 mutants were generated to identify the region of this protein required to regulate PSMA expression. Sox7 was also stably expressed in LNCaP/C4–2 and 22Rv1 cells to validate the regulation of PSMA expression by Sox7 in vivo.
RESULTS.
Sox7 mRNA expression negatively correlated with PSMA/FOLH1 and PSMAL/FOLH1B mRNA expression in Broad/Cornell, TCGA and MSKCC studies, but not in two studies containing only metastatic prostate tumors. PC-3 cells mostly expressed the 48.5 KDa isoform 2 of Sox7, and the depletion of this isoform did not restore PSMA expression. Ectopic expression of canonical, wild-type Sox7 in C4–2 and 22Rv1 cells suppressed PSMA protein expression. ChIP assay revealed that canonical Sox7 protein preferentially interacts with PSME in vivo, and EMSA identified the SOX box sites #2 and #4 in PSME as required for its interaction. Sox7 was capable of directly binding to PSME and suppressed PSME-mediated transcription. The NLS regions of Sox7, but not its β-catenin interacting motif, are essential for this suppressing activity. Furthermore, restoration of wild-type Sox7 expression but not Sox7-NLS mutant in Sox7-null prostate cancer cell lines suppressed PSMA expression.
CONCLUSIONS.
The inactivation of canonical Sox7 is responsible for the upregulated expression of PSMA in non-metastatic prostate cancer.
Keywords: Prostate-specific expression, Transcriptional factor, WNT signaling, Diagnostic and therapeutic marker
1 |. INTRODUCTION
Prostate-specific membrane antigen (PSMA) is a type II integral membrane protein that has been detected primarily in prostatic epithelium.1 While PSMA is moderately expressed in benign and hyperplastic prostate tissues, its expression level has been shown to be associated with increased tumor stage.2,3 An increased level of PSMA expression may directly contribute to prostate cancer development because PSMA induces aneuploidy and activates AKT signaling.4,5 Furthermore, a mouse model study indicated that moderate PSMA expression facilitates prostate carcinogenesis.6 Consequently, PSMA has been evaluated not only as a diagnostic marker for prostate cancer but also as a therapeutic target. PSMA-based targeted therapies include various PSMA antibodies and small molecules that act as PSMA inhibitors.7 Recent studies using PSMA inhibitors with α-emitters have shown dramatic responses in heavily pre-treated patients with castration-resistant prostate cancer.8,9 Therefore, a better understanding of the molecular mechanism underlying the regulation of PMSA expression may facilitate the proper use of these novel agents in the treatment of prostate cancer.
The regulation of PSMA expression has focused on the cis-elements in its promoter and enhancer. The proximal 1-Kb promoter of the FOLH1/PMSA gene can direct reporter gene expression at low levels,10 but the prostate-specific expression of PSMA is controlled by a 331-bp DNA fragment called PSMA enhancer (PSME).11 PSME contains many putative transcription factor binding sites, but NFATc1 is the only transcription factor that has been shown to bind to the AP-3 site in the 5’ end of PSME to facilitate PSMA expression.12 Interestingly, PSME also contains multiple binding sites for Sox proteins, but the role of Sox proteins in the regulation of PSMA has not been demonstrated in any experimental system. PSME-mediated transcription is suppressed by androgen,11 and Ghosh and Heston previously postulated that androgen receptor might sequester tissue-specific transcription factors such as SRY or Sox proteins to inhibit PSME-mediated transcription.13
We previously demonstrated that Sox7 protein contains a β-catenin interaction region, which is capable of suppressing WNT signaling.14 We also discovered that Sox7 is frequently inactivated in colorectal and prostate cancer by promoter hyper-methylation, and we hypothesized that it may act as a tumor suppressor.14 The suppression of WNT by Sox7 has now been observed in several other cancer types, including endometrial cancer, ovarian cancer and renal cell carcinoma.15–17 Because the down-regulation of Sox7 expression also correlated with prostate cancer progression,18 we set to determine whether Sox7 also regulates PSMA expression, and if so, whether this regulation requires the β-catenin interaction region of Sox7 protein.
2 |. MATERIALS AND METHODS
2.1 |. Correlation analysis
We evaluated the RNA expression data for Sox7, FOLH1 and FOLHB in prostate adenocarcinoma from five studies in cBioPortal; Broad/Cornell (n=57), TCGA (n=333), MSKCC (n=216), Michigan/Metastatic (n=61), and SU2C/PCF Dream Team (n=150). We conducted correlation analyses to statistically test the qualitative correlation between the expression of Sox7 and FOLH1 as well as Sox7 and FOLH1B. The normality assumption for Sox7, FOLH1 and FOLH1B was evaluated via PROC UNIVARIATE in SAS, and normal distribution was found in Broad/Cornell, MSKCC, and Michigan/Metastatic studies. For studies with normal distribution, the Pearson Correlation Coefficient (r) and P-value were obtained via PROC CORR in SAS. For studies with non-normal distribution (i.e. TCGA and SU2C/PCF studies), we obtained Spearman Rank Correlation Coefficient. A summary of this correlation analysis is provided in Supplemental Table 1.
We also conducted simple linear regression (SLR) analysis to measure the direction and strength of linear relationship between the expression of Sox7 and FOLH1 as well as Sox7 and FOLH1B. An estimate of the true slope, p-value, 95% confidence intervals and 95% prediction intervals were obtained via PROC REG in SAS.
The Broad/Cornell (Cell2013) study contains data from 16 paired samples with expression of Sox7, FOLH1 and FOLH1B in both normal and prostate adenocarcinoma from the same patient. We tested for significant differences in expression of Sox7, FOLH1 and FOLH1B between the means of the normal group and prostate adenocarcinoma group using Repeated Measures ANOVA. The F-statistic and p-value were obtained via PROC GLM in SAS.
2.2 |. Quantitative RT-PCR
Sox7 and PSMA mRNA expression were determined by real-time RT-PCR analysis as previously described using primers listed in Supplemental Table 2.19 Reactions were carried out in 20-μl volume using iQ SYBR green supermix (cat# 170–8882, Bio-Rad, Hercules, CA) on a Bio-Rad iCycler. Standard curves for Sox7, PSMA and glyceraldehyde-3-phophate dehydrogenase (GAPDH) were generated, and gene expression levels of PSMA were normalized by a comparative threshold cycle method. Standard deviations were calculated based on values from the duplicates.
2.3 |. Sox7 RNAi analysis
SOX7 siRNA duplexes were purchased from Sigma Chemical Co. (St. Louis, MO) (SASI_Hs01_00119451& SASI_Hs02_00358850). Gene silencing was achieved as described previously.20 Briefly, cells were grown to 50~60% confluence in 6-well plates, Lipofectamine reagent (cat#11668–500, Invitrogen, Grand Island, NY) was incubated with Opti-MEM reduced serum medium (cat#31985, Invitrogen) for 10 mins, and a mixture of siRNA was then added. After incubation for 20 mins at room temperature, the mixture (300 μl) followed by 700 μl Opti-MEM was added to each well. After incubating for 6 hrs, the media was replaced with complete medium. 80 pmol of siRNA was used per well. Cells were treated with the desired chemical for the desired time, and then harvested for immunoblot. Sox7 and PSMA protein expressions were evaluated with Western blot using goat polyclonal anti-Sox7 antibody (cat#AF2766, R&D system, raised against C-terminus of human Sox7, amino acid 249–345) and rabbit polyclonal anti-PSMA antibody (cat#12815, Cell Signaling Technology, Danvers, MA). Mouse monoclonal anti-actin antibody was purchased from Sigma Chemical Co. (St. Louis, MO), and actin level was used as a loading control.
2.4 |. Recombinant Sox7 proteins and electrophoretic mobility shift assay
Sox7-Δ mutant was generated previously14 and Sox7-NLS mutant was generated by site-directed mutagenesis (Stratagene) in CMV-Tag2B-Sox7 plasmid using mutagenesis primers listed in Supplemental Table 2. Mutations were validated by Sanger sequencing, and wild type or mutant Sox7 were then cloned into pENTR-3C vector. His-tagged Sox7 proteins were generated by cloning the wild type or mutant Sox7 into pDEST17 vector using the Invitrogen Gateway system, followed by expression in BL21 E. coli cells and purification using ProBond Purification system (Invitrogen). Sequences of synthetic oligonucleotides are listed in Supplemental Table 2. Oligonucleotides were end-labeled with biotin using Biotin 3’ end labeling kit (Pierce Chemical, Dallas, TX). Electrophoretic mobility shift assay (EMSA) was carried out using Lightshift Chemiluminescent EMSA kit (Pierce Chemical).
2.5 |. Luciferase reporter assay
CMV-Tag2B-Sox7 wild type or mutant plasmids were used in a luciferase reporter assay. Cells were plated at 5×104 per well on 24-well tissue culture plates 24 hrs before transfection. All transfections were carried out with Lipofectamine 2000 (Invitrogen) and pRL-CMV. Luciferase activity was measured in a luminometer (BD Biosciences, San Jose, CA) after 48 hrs, and data were normalized for the background Renilla luciferase activities using the Dual Luciferase Reporter Assay system (Promega, Madison, WI) according to manufacturer’s instructions. For PSME-mediated transcription, LNCaP cells in each well were transfected with 50 ng of pGL3-PSMEPSMA1k and different amounts of pCMV-Tag2B/Sox7, pCMV-Tag2B/Sox7-Δ, or an empty vector control (pCMV-Tag2B). 50 ng of pGL3 or pGL3-PSMA1k was also transfected as negative control.
2.6 |. Generation of C4–2 and 22Rv1 cell line with Sox7 expression
The “Tet-on” LNCaP/C4–2 and 22RV1 cell line was generated using the Tet-On gene expression system from Clonetech (Mountain View, CA). Flag-tagged mouse canonical Sox7, Sox7-Δ or Sox7-NLS were cloned into pTRE2-pur vector and transfected into the “Tet-on” LNCaP or 22Rv1 cell line. Cells were selected in culture media containing puromycin. Stable clones were isolated 14 days after selection began. The Tet-on system was leaky, and the clones isolated all had stable Sox7 expression which was not altered by doxycycline treatment. Sox7 and PSMA protein expressions were evaluated with Western blot using actin level as a loading control.
2.7 |. Chromatin immunoprecipitation (ChIP) assay
Cells were cross-linked for 10 mins in 1% formaldehyde at room temperature. ChIP assay was carried out using EZ-ChIP™ Chromatin Immunoprecipitation Kit (Cat# 17–371, Millipore, Burlington, MA). Immunoprecipitated DNA was analyzed by quantitative real-time PCR using iTaq™ Universal SYBR Green Supermix (cat#172–5121, BioRad,). Reactions were carried out in triplicates, and each reaction (15 μl) contained 1 μl of DNA, 0.2 μM primers, and 7.5 μl of SYBR Green Supermix. The reaction was subjected to a hot start for 3 mins at 95 °C and 43 cycles of 95 °C, 10 s; 57 °C, 30 s. Melt curve analysis was performed to verify a single product species. Starting quantities were determined relative to a common standard curve generated using LNCaP genomic DNA. Fold enrichment is calculated as the percent of input DNA immunoprecipitated by the goat anti-Sox7 antibody (cat# AF2766, R&D system) relative to that of a normal goat IgG control (cat# AB-108-C, R&D system) at each primer pair. Primers are listed in Supplemental Table 2 and Supplemental Figure 2.
3 |. RESULTS
The expression of Sox7 negatively correlates with PSMA (FOLH1) and PSMAL (FOLH1B) expression in tumor samples from prostate cancer patients.
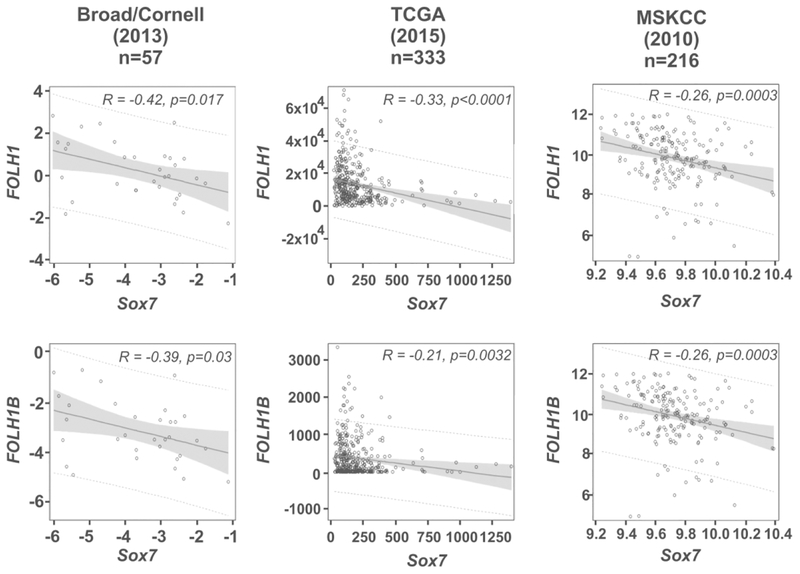
PSMA protein is encoded by the FOLH1 gene in the human genome, and we first evaluated the correlation between Sox7 and FOLH1 mRNA expression in five prostate cancer studies from cBioPortal. In the Broad/Cornell study, TCGA study, and MSKCC study, there was significant negative linear association between the expression of Sox7 and FOLH1. The correlation coefficients (r) were −0.41899 (P=0.017), −0.33047 (P<0.0001), and −0.26230 (P=0.0003), respectively. For each unit increase in expression of Sox7, the expression of FOLH1 was expected to decrease 0.40005, 17.70385, and 1.72427, respectively (Figure 1). This correlation, however, was not observed at a statistically significant level in studies that only focused on metastatic prostate cancer, such as the Michigan/Metastatic study and SU2C/PCF Dream Team study (Supplemental Figure 1).
FIGURE 1.
Sox7 expression negatively correlates with FOLH1 or FOLH1B expression in prostate cancer studies. RNA expression data was retrieved from cBioPortal. The Pearson Correlation Coefficient (r) and P-value were obtained via PROC CORR in SAS for Broad/Cornell and MSKCC studies. Spearman Rank Correlation Coefficient was obtained for TCGA study because of non-normal data distribution. We also conducted simple linear regression (SLR) analysis to measure the direction and strength of the linear relationship between the expression of Sox7 and FOLH1 as well as Sox7 and FOLH1B. An estimate of the true slope (solid line), P-value, 95% confidence intervals (shaded area) and 95% prediction intervals (dotted line) were obtained via PROC REG in SAS.
PSME is also present in the promoter of FOLH1B which encodes prostate-specific membrane antigen-like protein (PSMAL). The DNA sequence of PSME in the FOLH1B promoter differs from that in FOLH1 at five positions, two of which altered ATF/CREB and C/EBP binding sites (Supplemental Figure 2). Because none of these alterations occurred inside the Sox binding sites, we also evaluated the correlation of Sox7 and FOLH1B mRNA expression in these studies. In the Broad/Cornell study, TCGA study and MSKCC study, there was significant negative linear association between the expression of Sox7 and FOLH1B. The r values were −0.39201(P=0.0265), −0.21146 (P<0.0001) and −0.26249(P=0.0003), respectively. For each unit increase in expression of Sox7, the expression of FOLH1B was expected to decrease 0.34496, 0.43116 and 1.67752, respectively (Figure 1). Therefore, Sox7 expression also negatively correlates with FOLH1B expression, suggesting that the PSME may be the potential link for this observed correlation. This correlation was not observed in the Michigan/Metastatic study and SU2C/PCF Dream Team study (Supplemental Figure 1), indicating that it cannot be observed with studies containing only metastatic prostate cancer.
The Broad/Cornell (Cell2013) study contains sixteen paired samples with both normal and prostate adenocarcinoma from the same patient. We evaluated significant differences in expression of these genes between normal prostate and prostate tumors using Repeated Measures ANOVA. The true mean expression of Sox7 was significantly lower among the prostate adenocarcinoma group than the normal group (P=0.0004). The true mean expression of FOLH1 and FOLH1B were both significantly higher among the prostate adenocarcinoma group than the normal group (P =0.0012 and 0.0007 respectively (Supplemental Figure 3). These results were consistent with our previous finding that the expression of Sox7 is suppressed during the oncogenic transformation of prostate epithelium.14
Induction of canonical Sox7 expression in LNCaP/C4–2 cells suppresses PSMA expression.
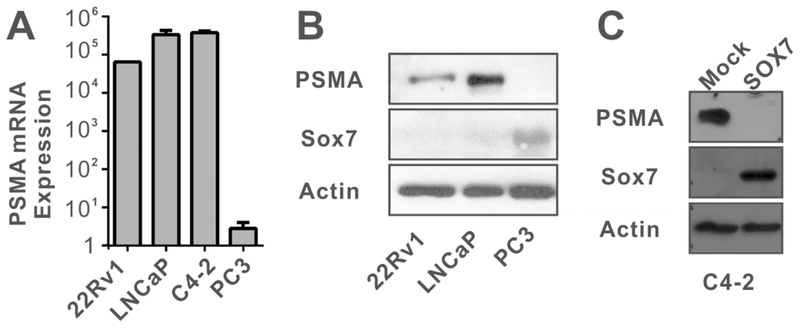
The negative correlation between Sox7 and PSMA expression was also observed in several prostate cancer cell lines. We have previously demonstrated that Sox7 expression was down-regulated by promoter hypermethylation in LNCaP and 22Rv1 cells but not in PC-3 cells.14 We used quantitative real-time PCR to determine the mRNA expression of PSMA in several prostate cancer cell lines, and our analysis indicated that PSMA mRNA expression levels were high in 22Rv1, LNCaP and LNCaP-derived C4–2 cells but not in PC-3 cells (Figure 2A). Our data is consistent with several published studies21–23 and expression data from the Broad Cancer Cell Line Encyclopedia (Supplemental Table 3). A similar trend was observed in immunoblot analysis of PSMA and Sox7 protein levels (Figure 2B). Interestingly, anti-Sox7 antibody detected two bands in PC-3 cells (Supplemental Figure 4). The weakly expressed band at 42 KDa corresponded to the canonical Sox7 (isoform 1, Q9BT81–1), and the strongly expressed band at 48.5 KDa may be a splicing isoform predicted by UniProt (isoform 2, Q9BT81–2). These two proteins differ in their N-terminal end, and isoform 2 lacks the bipartite NLS (Supplemental Figure 4A). One of our qRT-PCR primer resided in the bipartite NLS region, so mRNA for isoform 2 cannot be detected by real-time PCR method. We carried out RNAi analysis with Sox7 siRNA in PC-3 cells. Control scrambled siRNA resulted in slight suppression of Sox7 isoform 2 expression, and two different siRNA against Sox7 resulted in complete elimination of Sox7 isoform 2 (Supplemental Figure 4). However, both siRNA failed to suppress isoform 1 expression, and the elimination of Sox7 isoform 2 did not restore PSMA expression in PC-3 cells. Therefore, the presence of Sox7 isoform 2 is not responsible for the suppression of PSMA expression in PC-3 cells.
FIGURE 2.
Sox7 negatively correlates with PSMA expression in prostate cancer cell lines. A. Expression of PSMA mRNA was determined by quantitative real time PCR normalized by GAPDH expression. B. Sox7 and PSMA protein levels were determined by immunoblot using the indicated antibodies. C. Immunoblot analysis of C4–2 or C4–2 cells that constitutively express a mouse wild-type Sox7 protein (lane 2).
We next sought to determine whether the expression of the canonical Sox7 (isoform 1) suppressed the expression of PSMA protein. C4–2 cells are derived from LNCaP24, and we generated a C4–2 cell line that constitutively expresses a mouse Sox7 protein. The stable expression of Sox7 in C4–2 cells significantly suppressed PSMA expression, suggesting that the canonical Sox7 protein negatively regulates PSMA expression (Figure 2C), and we will refer the canonical Sox7 as Sox7 in the remaining text.
Sox7 interacts with PSME in vivo and negatively regulates PSME-mediated transcription.
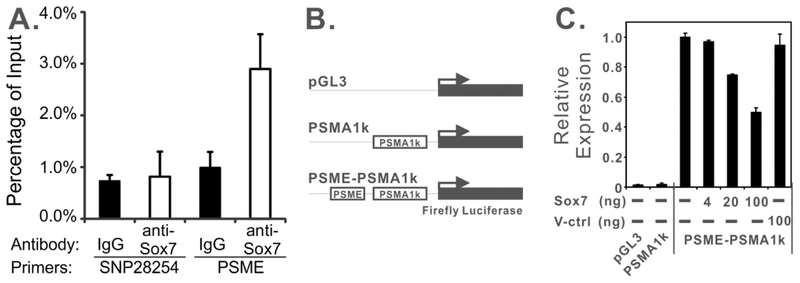
PSMA expression is stimulated by an enhancer element, PSME, in prostate cells, which contains several potential binding sites for Sox proteins.11 To determine whether Sox7 physically interacts with PSME in vivo, we carried out ChIP analysis in C4–2 cells with ectopic Sox7 expression using a ChIP-validated anti-Sox7 antibody.25 Our data indicated that anti-Sox7 antibody preferentially precipitated the genomic region containing PSME compared to IgG control antibody (Figure 3A). Furthermore, this difference was not observed when we used the same antibody pairs against a genomic region on chromosome 8 (SNP28254).26 These data indicate that Sox7 protein preferentially interacts with PSME in vivo.
FIGURE 3.
Sox7 interacts with PSME in vivo and negatively regulates PSME-mediated transcription. A. ChIP analysis of C4–2/Sox7 cell line with anti-Sox7 antibody or IgG control antibody at PSME or SNP28254. Quantitative real-time PCR was carried out in triplicate, and error bars represent one standard deviation. B. Schematic drawing of luciferase reporters for PSMA promoter and enhancer (PSME) activity. PSMA1k only contains a 1-Kb promoter region. PSME-PSMA1k contains both the promoter and enhancer. C. Sox7 suppresses PSME-mediated transcription. Indicated amount of CMV-Tag2B/Sox7 (Sox7) or CMV-Tag2B (V-ctrl) plasmids were transfected into LNCaP cells with 100 ng of PSME-PSMA1k luciferase reporter construct. Control reactions were carried out by transfecting 100 ng of pGL3 or PSMA1K plasmids. Reactions were carried out in duplicates, and error bars represent one standard deviation.
We also evaluated the role of Sox7 protein in PSME-mediated transcription. The proximal 1-Kb promoter region of FOLH1/PSMA was cloned into a pGL3-basic luciferase report plasmid (Figure 3B), and the inclusion of this promoter region did not significantly alter luciferase activity in LNCaP cells (Figure 3C), consistent with previous studies.11 The inclusion of PSME in this reporter construct enhanced luciferase activity by 250-fold (Figure 3C). Co-transfection of a plasmid expressing a wild-type Sox7, but not a vector control, suppressed PSME-mediated transcription in a dose-dependent manner (Figure 3C). These data suggest that over-expression of Sox7 suppresses PSME-mediated transcription in LNCaP cells.
Sox7 directly binds to PSME in vitro.
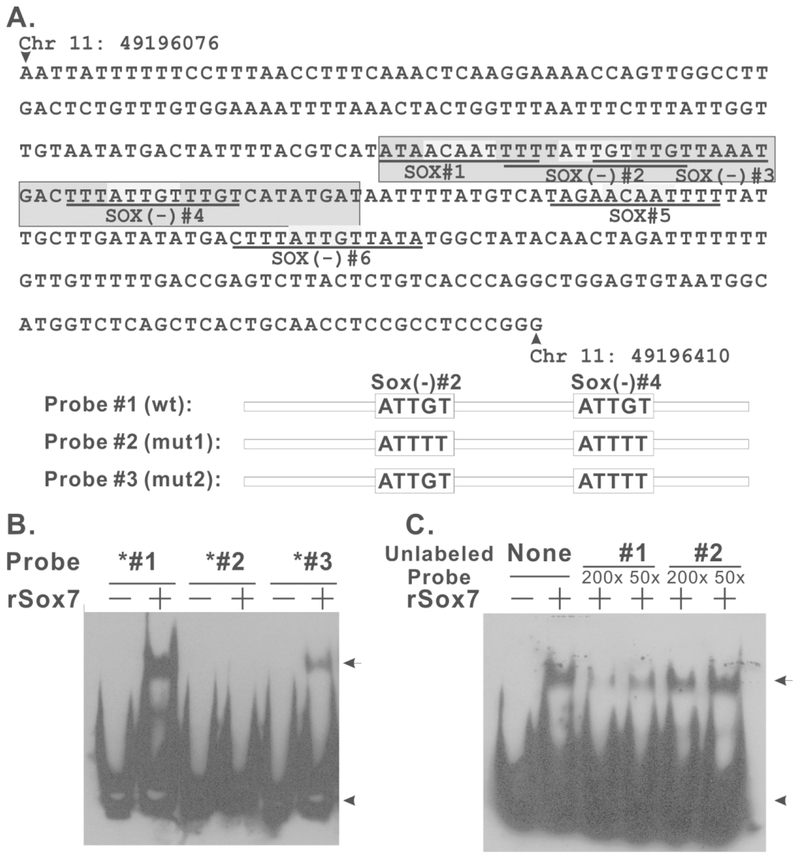
We next generated and purified recombinant His-tagged Sox7 protein (rSox7) and tested whether Sox7 directly interacts with PSME using EMSA. The potential binding sites for Sox7 in PSME are depicted in Figure 4A. A synthetic oligonucleotide duplex was generated to contain Sox binding sites 1 through 4 (Figure 4A, probe #1). Recombinant Sox7 formed a complex with this duplex DNA and caused a mobility shift (Figure 4B, lane 2). Mutations in both sites 2 and 4 from ATTGT to ATTTT (probe #2) eliminated Sox7 binding, indicating that these two sites are required for Sox7 binding (Figure 4B, lane 4). Probe #3 only contained a mutation in site 4 but not in site 2. Even though this probe was still capable of interacting with rSox7, we observed significant reduction in the amount of shifted complex, supporting the notion that both sites 2 and 4 are involved in Sox7 binding. The binding between Sox7 and this DNA duplex was also specific because it was disrupted by excess unlabeled probe #1 but not probe #2 (Figure 4C). Therefore, recombinant Sox7 directly interacts with PSME through Sox binding sites 2 and 4.
FIGURE 4.
Sox7 directly interacts with PSME. A. Sequence of the 331-enhancer core of PSME. This panel was modified from Watt. et al, Genomics 73, 243–254, 2001, and potential Sox7 binding sites (ATTGT) are highlighted by light gray color. Dark gray indicates the DNA sequence used in EMSA analysis. Schematic drawing of probe #1–3 is provided to indicate changes in Sox binding sites. B. Sox7 EMSA analysis with wild-type and mutant PSME probes. 0.6 μg of recombinant Sox7 protein was incubated with 0.1 pmol of biotinylated probes for 30 mins at 4 °C. DNA/protein complex was resolved by 6% native PAGE. Arrow heads indicated unbounded probe, and arrows indicated shifted complex. C. Sox7 EMSA analysis with 200- or 50-fold excess of unlabeled probe #1 or #2.
The HMG domain of Sox7 is required for PSME binding and the regulation of PSME-mediated transcription.
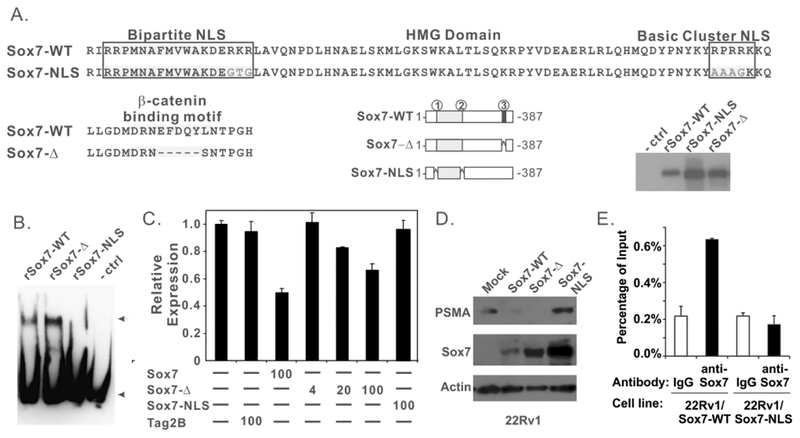
Sox7 has several evolutionarily conserved protein sequence motifs, and we have previously identified a DxxEFDQYL motif in its C-terminus to be essential for the suppression of β-catenin mediated transcription and WNT signaling.14 The deletion of this sequence motif in Sox7, however, did not alter the binding of recombinant Sox7 protein to PSME (Figure 5B, lane 2). Furthermore, ectopic expression of Sox7-Δ was still capable of inhibiting PSME-mediated transcription in a dose-dependent manner (Figure 5C), indicating that the β-catenin interaction motif is not required for this suppressing activity. Sox7 also contains an HMG motif with a bipartite nuclear localization sequence (NLS) and a basic cluster NLS (Figure 5A). We used site-directed mutagenesis to convert RKR in the bipartite NSL to GTG and RPRRK in the basic cluster NLS to AAAGK (Figure 5A). Mutation of these two NLS motifs in other Sox proteins results in complete exclusion of the protein from the nucleus27. Interestingly, our data indicated that mutation of these sites in Sox7-NLS mutant also abolished the direct binding of purified recombinant Sox7 to PSME in vitro (Figure 5B). Therefore, these sites may also directly participate in PSME binding. In addition, ectopic expression of Sox7-NLS failed to suppress PSME-mediated transcription (Figure 5C). Hence, mutations in the NLS regions disrupt the interaction between Sox7 and PSME and the ability of Sox7 to suppress PSME-mediated transcription.
FIGURE 5.
The NLS region but not the β-catenin interacting motif of Sox7 is essential for the regulation of PSMA expression. A. Schematic drawing of wild type and mutant recombinant Sox7 proteins. Areas 1 and 2 represent the bipartite nuclear localization sequence and basic cluster NLS in the HMG domain, respectively. Area 3 denotes the β-catenin interacting motif. Immunoblot analysis of these recombinant proteins is included in the bottom/right of this panel. B. EMSA analysis of wild-type and mutant recombinant Sox7 proteins with probe #1. Reaction conditions were similar to those described in Figure 4B. C. Sox7-Δ but not Sox7-NLS suppresses PSME-mediated transcription. Reaction conditions were similar to those described in Figure 4B. D. Immunoblot analysis of 22Rv1, 22Rv1/Sox7-wt, 22Rv1/Sox7-Δ and 22Rv1/Sox7-NLS cell lines. Cell lysates were harvested and analyzed by Immunoblot for PSMA and Sox7 proteins; actin was used as loading control. E. ChIP analysis of indicated 22Rv1 cell lines with anti-Sox7 antibody or IgG control antibody at PSME locus. Quantitative real-time PCR was carried out in triplicate, and error bars represent one standard deviation.
To confirm this finding in vivo, we expressed these Sox7 mutants in a 22Rv1/Teton cell line. The Tet-on promoter, however, was leaky in this cell line, and we observed a constitutive level of Sox7 expression under untreated conditions (Figure 5D). While PSMA protein was readily detected in the 22Rv1-parental and 22Rv1/Sox7-NLS cell line, it was not observed in 22Rv1/Sox7-WT and 22Rv1/Sox7-Δ cells (Figure 5D). We also carried out ChIP analysis in 22Rv1/Sox7-WT and 22Rv1/Sox7-NLS cells to evaluate the interaction between wild-type/mutant Sox7 and PSME. While enhanced interaction between Sox7-WT and PSME was observed in 22Rv1/Sox7-WT cells, it was not observed between Sox7-NLS mutant and PSME (Figure 5E). These data are consistent with our in vitro observations that the NLS regions are essential for Sox7 to suppress PSMA expression.
4 |. DISCUSSION
The expression of PSMA is controlled by a cis-element PSME, which is located in the third intron of PSMA/FOLH1. PSMAL/FOHL is related to PSMA/FOLH1 through gene duplication,28 and a PSME element is also present in the promoter of this gene. Even though these two PSME elements differ by 5 nucleotides, they share the same binding sites for Sox proteins, and Sox7 expression negatively correlates with both PSMA/FOLH1 and PSMAL/FOLH1B expression in several large scale prostate cancer studies. This correlation led us to evaluate whether Sox7 negatively regulates PSMA expression through PSME. Our data indicated that Sox7 directly interacts with PSME, and is capable of suppressing PSME-mediated transcription. In addition, the direct interaction between Sox7 and PSME is mediated through Sox binding sites 2 and 4. Because these two sites are separated by 20 base pairs, i.e. two B-DNA double helix turns, they should face the same direction, and it will be interesting to determine in the future whether Sox7 binds to PSME as a dimer.
We also demonstrated that the restoration of canonical, wild-type Sox7 expression in LNCaP/C4–2 and 22Rv1 cells suppresses PSMA protein production. Thus, somatic inactivation of Sox7 by either promoter methylation or biallelic deletion in prostate tumors may lead to the up-regulation of PSMA expression. Sox7 negatively regulates WNT signaling,29,30 and we and others have shown that tumor-specific inactivation of Sox7 is involved in the aberrant activation of WNT signaling in colorectal and prostate cancers.14,31 Even though Sox7 negatively regulates both β-catenin and PSME-mediated transcription, the mechanisms underlying these regulations are different. Protein-to-protein interactions between Sox7 and β-catenin are essential for Sox7 to inhibit β-catenin-mediated transcription, as the disruption of this interaction abolishes the inhibitory effect14. In contrast, the inhibition of PSMA expression does not require β-catenin interaction because a Sox7 mutant that lacks the β-catenin interaction motif is still capable of suppressing PSME-mediated transcription, and the expression of this mutant in 22Rv1 cells suppressed PSMA expression.
The Sox7 antibody used in this study also detected a larger protein in PC-3 cells. The molecular weight of this protein is consisted with the putative, splicing isoform 2 of Sox7 which contains an alternative N-terminus and lacks the bipartite NLS. The presence of this isoform is not responsible for the suppressed PSMA expression in PC-3 cell because RNAi depletion of this isoform did restore PSMA expression.
Accumulating evidence indicates that the up-regulation of PSMA expression promotes prostate carcinogenesis. PSMA is an enzyme with folate hydrolase and N-acetylated-α-linked-acidic dipeptidase (NAALAdase) activities, and an in vitro study indicated that the expression of PSMA promotes tumor invasiveness at physiologically relevant folate conditions.23 This finding was further supported by a mouse model study which indicated that moderate PSMA expression facilitates prostate carcinogenesis in vivo.6 Increased PSMA expression has been shown to induce aneuploidy and activate AKT signaling4,5, and the over-expression of PSMA may promote prostate carcinogenesis through several different mechanisms. Our data suggest that inactivation of Sox7 may provide multiple advantages for prostate carcinogenesis because it leads to the aberrant activation of WNT signaling and the over-expression of PSMA. The notion that Sox7 is a tumor suppressor in prostate cancer is now further supported by TCGA prostate cancer study, which indicated that approximately 11.4% of prostate tumors contain deep deletion of Sox7.
In conclusion, we have discovered that the canonical Sox7 negatively regulates PSMA expression in prostate cancer through its interaction with PSME, and the inactivation of Sox7 in prostate tumors not only leads to aberrant WNT signaling but also increases PSMA expression to promote prostate cancer progression.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. Anthea Hammond for editing this manuscript and Mrs. Doris R. Powell for her help with ChIP analysis. WZ is Georgia Cancer Coalition distinguished Cancer Scholar, American Cancer Research Scholar and Anise McDaniel Brock Scholar.
Funding information
National Cancer Institute, Grant number: R01-CA203928, R01-CA140571, R01-CA194027; American Cancer Society, Grant number: RSG-05-038-01-CCE; the US Department of Defense Prostate Cancer Research Program (PCRP), Grant number: W81XWH-05-1-0042; Key laboratory of Precision Oncology in Suzhou, Grant number: SZS201618; Chinese Natural Science Foundation Project, Grant number:81672970 Georgia Cancer Coalition; Second Affiliated Hospital Of Soochow University Preponderant Clinic Discipline Group Project funding.
Footnotes
Disclosure Statement
Competing interests: The author(s) declare that they have no competing interests.
REFERENCES
- 1.O’Keefe DS, Bacich DJ, Huang SS, Heston WDW. A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J Nucl Med. 2018;59(7):1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy GP, Barren RJ, Erickson SJ, et al. Evaluation and comparison of two new prostate carcinoma markers. Free-prostate specific antigen and prostate specific membrane antigen. Cancer. 1996;78(4):809–818. [DOI] [PubMed] [Google Scholar]
- 3.Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59(13):3192–3198. [PubMed] [Google Scholar]
- 4.Rajasekaran SA, Christiansen JJ, Schmid I, et al. Prostate-specific membrane antigen associates with anaphase-promoting complex and induces chromosomal instability. Mol Cancer Ther. 2008;7(7):2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caromile LA, Dortche K, Rahman MM, et al. PSMA redirects cell survival signaling from the MAPK to the PI3K-AKT pathways to promote the progression of prostate cancer. Sci Signal. 2017;10(470). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao V, Parwani A, Maier C, Heston WD, Bacich DJ. Moderate expression of prostate-specific membrane antigen, a tissue differentiation antigen and folate hydrolase, facilitates prostate carcinogenesis. Cancer Res. 2008;68(21):9070–9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wustemann T, Haberkorn U, Babich J, Mier W. Targeting prostate cancer: Prostate-specific membrane antigen based diagnosis and therapy. Med Res Rev. 2018. [DOI] [PubMed] [Google Scholar]
- 8.Kratochwil C, Schmidt K, Afshar-Oromieh A, et al. Targeted alpha therapy of mCRPC: Dosimetry estimate of (213)Bismuth-PSMA-617. Eur J Nucl Med Mol Imaging. 2018;45(1):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sathekge M, Knoesen O, Meckel M, Modiselle M, Vorster M, Marx S. (213)Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44(6):1099–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Keefe DS, Su SL, Bacich DJ, et al. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim Biophys Acta. 1998;1443(1–2):113–127. [DOI] [PubMed] [Google Scholar]
- 11.Watt F, Martorana A, Brookes DE, et al. A tissue-specific enhancer of the prostate-specific membrane antigen gene, FOLH1. Genomics. 2001;73(3):243–254. [DOI] [PubMed] [Google Scholar]
- 12.Lee SJ, Lee K, Yang X, et al. NFATc1 with AP-3 site binding specificity mediates gene expression of prostate-specific-membrane-antigen. J Mol Biol. 2003;330(4):749–760. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh A, Heston WD. Understanding Prostate-Specific Membrane Antigen and Its Implication in Prostate Cancer In: LaRochelle WJ, Shimkets RA, eds. The Oncogenomics Handbook. Totowa, NJ: Springer; 2007:597–615. [Google Scholar]
- 14.Guo L, Zhong D, Lau S, et al. Sox7 is an independent checkpoint for beta-catenin function in prostate and colon epithelial cells. Mol Cancer Res. 2008;6(9):1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan DW, Mak CS, Leung TH, Chan KK, Ngan HY. Down-regulation of Sox7 is associated with aberrant activation of Wnt/b-catenin signaling in endometrial cancer. Oncotarget. 2012;3(12):1546–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Yan ZQ, Li B, et al. Reduced expression of SOX7 in ovarian cancer: a novel tumor suppressor through the Wnt/beta-catenin signaling pathway. J Ovarian Res. 2014;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Fan Y, Zhang L, et al. Classic SRY-box protein SOX7 functions as a tumor suppressor regulating WNT signaling and is methylated in renal cell carcinoma. FASEB J. 2018: [DOI] [PubMed] [Google Scholar]
- 18.Zhong WD, Qin GQ, Dai QS, et al. SOXs in human prostate cancer: implication as progression and prognosis factors. BMC Cancer. 2012;12:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong D, Morikawa A, Guo L, et al. Homozygous deletion of SMAD4 in breast cancer cell lines and invasive ductal carcinomas. Cancer Biol Ther. 2006;5(6):601–607. [DOI] [PubMed] [Google Scholar]
- 20.Zhong D, Xiong L, Liu T, et al. The glycolytic inhibitor 2-deoxyglucose activates multiple prosurvival pathways through IGF1R. J Biol Chem. 2009;284(35):23225–23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laidler P, Dulinska J, Lekka M, Lekki J. Expression of prostate specific membrane antigen in androgen-independent prostate cancer cell line PC-3. Arch Biochem Biophys. 2005;435(1):1–14. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh A, Wang X, Klein E, Heston WD. Novel role of prostate-specific membrane antigen in suppressing prostate cancer invasiveness. Cancer Res. 2005;65(3):727–731. [PubMed] [Google Scholar]
- 23.Yao V, Bacich DJ. Prostate specific membrane antigen (PSMA) expression gives prostate cancer cells a growth advantage in a physiologically relevant folate environment in vitro. Prostate. 2006;66(8):867–875. [DOI] [PubMed] [Google Scholar]
- 24.Thalmann GN, Anezinis PE, Chang SM, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54(10):2577–2581. [PubMed] [Google Scholar]
- 25.Zhang Y, Stovall DB, Wan M, et al. SOX7 Target Genes and Their Contribution to Its Tumor Suppressive Function. Int J Mol Sci. 2018;19(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou W, Goodman SN, Galizia G, et al. Counting alleles to predict recurrence of early-stage colorectal cancers. Lancet. 2002;359(9302):219–225. [DOI] [PubMed] [Google Scholar]
- 27.Sudbeck P, Scherer G. Two independent nuclear localization signals are present in the DNA-binding high-mobility group domains of SRY and SOX9. J Biol Chem. 1997;272(44):27848–27852. [DOI] [PubMed] [Google Scholar]
- 28.Rinker-Schaeffer CW, Hawkins AL, Su SL, et al. Localization and physical mapping of the prostate-specific membrane antigen (PSM) gene to human chromosome 11. Genomics. 1995;30(1):105–108. [DOI] [PubMed] [Google Scholar]
- 29.Takash W, Canizares J, Bonneaud N, et al. SOX7 transcription factor: sequence, chromosomal localisation, expression, transactivation and interference with Wnt signalling. Nucleic Acids Res. 2001;29(21):4274–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowsky MW, Varmus HE. Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell. 1999;4(4):487–498. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Huang S, Dong W, et al. SOX7, down-regulated in colorectal cancer, induces apoptosis and inhibits proliferation of colorectal cancer cells. Cancer Lett. 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.