Abstract
The Liver X Receptors (LXRs) are oxysterol-activated transcription factors that upregulate a suite of genes that together promote coordinated mobilization of excess cholesterol from cells and from the body. The LXRs, like other nuclear receptors, are anti-inflammatory, inhibiting signal-dependent induction of pro-inflammatory genes by nuclear factor-κB, activating protein-1, and other transcription factors. Synthetic LXR agonists have been shown to ameliorate atherosclerosis and a wide range of inflammatory disorders in preclinical animal models. Although this has suggested potential for application to human disease, systemic LXR activation is complicated by hepatic steatosis and hypertriglyceridemia, consequences of lipogenic gene induction in the liver by LXRα. The past several years have seen the development of multiple advanced LXR therapeutics aiming to avoid hepatic lipogenesis, including LXRβ-selective agonists, tissue-selective agonists, and transrepression-selective agonists. Although several synthetic LXR agonists have made it to phase I clinical trials, none have progressed due to unforeseen adverse reactions or undisclosed reasons. Nonetheless, several sophisticated pharmacologic strategies, including structure-guided drug design, cell-specific drug targeting, as well as non-systemic drug routes have been initiated and remain to be comprehensively explored. In addition, recent studies have identified potential utility for targeting the LXRs during therapy with other agents, such as glucocorticoids and rexinoids. Despite the pitfalls encountered to date in translation of LXR agonists to human disease, it appears likely that this accelerating field will ultimately yield effective and safe applications for LXR targeting in humans.
Keywords: Liver X Receptor, Inflammation, Oxysterol, Cholesterol, Atherosclerosis, Reverse Cholesterol Transport
1. Introduction
First identified in the mid-1990s as orphan members of the nuclear receptor (NR) superfamily, the liver X receptors (LXRα and LXRβ) were soon recognized to be activated by endogenous oxysterols (i.e., oxidized cholesterol), and to induce a suite of target genes that correct sterol overload by promoting cholesterol disposal from the cell and from the body (Fessler, 2008; Peet, et al., 1998). The identification of LXRs as master regulators of cholesterol sensing and handling has led to sustained efforts to develop synthetic LXR agonists that might ultimately prove therapeutic against human atherosclerosis. Interest in the LXRs as drug targets has been further elevated by the discovery that they, like other NRs (e.g., the glucocorticoid receptor), have potent anti-inflammatory effects (Steffensen, et al., 2013; Zelcer & Tontonoz, 2006). Indeed, pharmacological activation of LXR has now been shown not only to reduce atherosclerosis in rodent models but also to ameliorate pathology in a wide range of preclinical inflammatory disease models, ranging from atopic dermatitis, to acute lung injury, autoimmune disease, and neurodegenerative disorders (A-Gonzalez, et al., 2009; Cui, et al., 2012; Fowler, et al., 2003; Smoak, et al., 2008). Remarkably, LXR agonists also show early promise in models of cancer and viral infection, two disorders in which disrupted cellular cholesterol homeostasis has been shown to drive pathogenesis (Fessler, 2016; Lin, et al., 2016; Ramezani, et al., 2015).
Development of LXR agonists as therapeutics has proven quite challenging due to their precipitation of hepatic steatosis and hypertriglyceridemia, effects generally thought to arise from activation of LXRα in the liver. This has led the field to a range of innovative strategies, including LXRβ-selective agonists, tissue-selective LXR agonists, and LXR transrepression-selective agents. Further hurdles have arisen in the form of known differences between rodent and human physiology as well as unforeseen differences between non-human primates and humans. To date, the four LXR-targeted drugs that have made it to phase I clinical trials have unfortunately not advanced further. Nonetheless, it appears likely that with further refinement of advanced LXR therapeutics and evolving insights into the role of the LXRs in specialized cell types, opportunities for successful targeting of the LXRs in human disease will ultimately be realized.
2. Background on the LXRs
2.1. Structural features and activation by ligands
LXRα (encoded by NR1H3 on chromosome 11 in humans) and LXRβ (encoded by NR1H2 on chromosome 19 in humans) are ligand-activated transcription factors (i.e., NRs). LXRs are comprised of four principal functional domains: i) an N-terminal AF-1 activation domain; ii) a DNA-binding domain; iii) a ligand-binding domain; and iv) a C-terminal AF-2 domain that regulates transcription through interactions with co-activators and co-repressors (Ma, et al., 2017). The LXRs vary in tissue distribution, with LXRα expressed primarily in macrophages, intestine, liver, adipose, and kidney, whereas LXRβ is more ubiquitous (Repa, et al., 2000). This may create opportunities for targeted therapeutic strategies given that, for example, human lymphocytes express LXRβ but not LXRα (Bensinger, et al., 2008). Although human LXRα (447 amino acids) and LXRβ (460 amino acids) share 77% sequence homology and have just a single amino acid difference in their ligand-binding domains, their crystal structures have revealed a very flexible ligand-binding pocket (Farnegardh, et al., 2003). This perhaps explains the breadth of structurally dissimilar LXR ligands, including LXRα- and -β-selective agents, that have been identified or designed to date, and also suggests potential for rational design of LXR modulators. A further layer of LXR modulation is conferred by a wide range of regulatory post-translational modifications that have been identified for the LXR proteins, including SUMOylation, phosphorylation, acetylation, ubiquitination, and O-GlcNacylation (Jakobsson, et al., 2012).
The major endogenous LXR agonists that have been identified to date are: i) oxysterols [i.e., 20(S)-, 22(R)-, 24(S)-, 25-, and 27-hydroxycholesterol; 24(S),25-epoxycholesterol; cholestenoic acid]; and ii) cholesterol biosynthetic intermediates [i.e, desmosterol, follicular fluid meiosis-activating sterol (FF-MAS), testis meiosis-activating sterol (T-MAS)](Komati, et al., 2017; Mutemberezi, et al., 2016). LXR-active oxysterols are generated from cholesterol substrate by endoplasmic reticulum (ER) enzymes (CYP46, CH25H) and mitochondrial enzymes (CYP27A1), or, in the case of 24(S),25-epoxycholesterol, as a shunt by-product off of the cholesterol synthesis pathway (Mutemberezi, et al., 2016). Confirmation that oxysterols serve as native LXR activators in vivo was provided by the triple knockout mouse for Cyp46, Ch25h, and Cyp27a1, which was found to have deficient LXR-dependent gene expression in the liver in response to high-cholesterol diet (Chen, et al., 2007). Of interest, several exogenous, natural plant-derived compounds have also been shown to have activating (phytosterols, diterpenes, gynosaponin, honokiol, podocarpic acid, cyanidin) or inhibitory (riccardin C and F, naringenin) activity upon the LXRs – in many of these cases, via indirect mechanisms (Komati, et al., 2017). This may suggest that the LXR pathway also serves as a xenobiotic sensor.
As discussed below, the primary function of the LXRs is thought to be cholesterol homeostasis (i.e., correction of cholesterol overload). Target genes induced by LXRs mediate efflux of cholesterol from cells (e.g., the lipid transporters ATP Binding Cassette [ABC] A1 and ABCG1), reduction of cholesterol uptake into cells (e.g., inducible degrader of the low density lipoprotein receptor [IDOL]), and excretion of cholesterol from the body (e.g., ABCG5/8 transporter in the biliary and intestinal epithelium). Interestingly, oxysterols and desmosterol serve as integrators of cellular cholesterol homeostasis, as they not only drive cholesterol efflux via LXR activation, but also coordinately suppress cholesterol accumulation by blocking activation of sterol response element binding protein (SREBP)-2, a master regulatory transcription factor that upregulates the low density lipoprotein receptor (LDLR) and the enzymes of the cholesterol biosynthesis pathway (Fessler, 2016).
In vivo, some oxysterols are thought to be produced predominantly in specific tissues, where they mediate organ-specific functions. For example, 24(S)-hydroxycholesterol derives largely from CYP46-expressing neurons in the central nervous system, where it serves key functions in health and disease (Zhang & Liu, 2015). Cholestenoic acid is thought largely to derive from CYP27A1-expressing alveolar macrophages in humans (Babiker, et al., 1999). These two oxysterols circulate at substantial concentrations in mice and humans. This suggests that oxysterol diffusion from brain and lung may serve as a direct pathway for tissue sterol export, and also that oxysterols may possibly mediate endocrine functions in vivo. While in most cases it remains unclear which LXR agonists are relevant in specific cell types during disease, it was recently shown that desmosterol plays a key role in LXR-dependent gene expression in macrophage foam cells (Spann, et al., 2012).
Several LXR ligands also have activity on other NRs as well as immune receptors, suggesting integration of LXR with other endocrine and immune networks. For example, 27-hydroxycholesterol is also a selective estrogen receptor (ER) modulator (DuSell, et al., 2008), 25-hydroxycholesterol is an ERα activator (Lappano, et al., 2011), and 24(S)-hydroxycholesterol is a retinoid orphan receptor (ROR)-α and ROR-γ inverse agonist (Mutemberezi, et al., 2016). Desmosterol was recently identified as an agonist of RORγt, the master regulator of T helper 17 cell differentiation (Hu, et al., 2015). In some cases, oxysterols have been shown to have remarkably pleiotropic activity that goes well beyond NRs. For example, in different settings, 25-hydroxycholesterol suppresses inflammasomes thereby inhibiting interleukin-1β production (Reboldi, et al., 2014), augments pro-inflammatory gene transcription through supporting binding of AP-1 to promoters (Gold, et al., 2014), and acts as a neutrophil chemoattractant through ligating the chemokine receptor CXCR2 (Raccosta, et al., 2013). LXR-active oxysterols also communicate as metabolic intermediates with other signaling networks. Thus, oxidation of 25-hydroxycholesterol by Cyp7b converts it into 7α,25-hydroxycholesterol, a key chemoattractant that positions dendritic cells and B cells in the spleen via the receptor Gpr183 (Cyster, et al., 2014). Oxidation of 27-hydroxycholesterol by Cyp7b converts it into a RORγt agonist (Soroosh, et al., 2014).
Two non-steroidal, synthetic, dual LXRα/β full agonists that were developed in the early 2000s have now been used widely by the field to probe LXR biochemistry. The first, TO901317, also has activity on several other NRs, including farnesoid X receptor and pregnane X receptor (agonist), and RORα and -γ (inverse agonist), somewhat limiting its utility (Guillemot-Legris, et al., 2016). The second, GW3965, is much more specific for LXR. A key difference between native oxysterol LXR agonists and synthetic LXR agonists such as these is that the latter do not have coordinated activity on SREBPs; thus, LXR gene induction by these compounds is non-physiologically dissociated from inhibition of lipid synthesis. As discussed below, further advances have been made in recent years in the design of more selective LXR-active compounds, including some that do also suppress SREBP activation.
Endogenous LXR antagonists that have been identified include polyunsaturated fatty acids, arachidonic acid, prostaglandin F2α, and 5α,6α-epoxycholesterol (Komati, et al., 2017). The precise mechanism(s) of LXR inhibition remain to be defined in some of these cases. While the physiologic relevance of these antagonists in vivo also remains largely unclear, it has been shown that 25-hydroxycholesterol acquires antagonistic activity for LXR upon sulfation (i.e., into 25-hydroxycholesterol-3-sulfate) by the sulfotransferase Sult2b1b (Ma, et al., 2008). Remarkably, Sult2b1b-mediated inactivation of LXR agonistic oxysterols has been shown to be a key event during T cell proliferation (Bensinger, et al., 2008), and may also promote pro-tumorigenic cholesterol accumulation in cancer cells by uncoupling LXR-dependent sterol sensing from cellular sterol burden (Bovenga, et al., 2015).
2.2. Genomic binding and mechanisms of target gene transactivation
Transactivation of target genes by LXRs requires permissive heterodimerization of LXR with retinoid X receptor (RXR), a retinoic acid-activated NR that dimerizes with several members of the NR superfamily. LXR/RXR dimers bind to so-called ‘DR4’-type LXR response elements (LXREs) in target gene promoters, a binding site composed of a repeating 6-mer core sequence of 5′-AGGTCA-3′ separated by a spacer of any 4 nucleotides (Pehkonen, et al., 2012). In macrophages, binding of LXR to promoters and enhancers requires initial chromatin remodeling by the pioneer factor PU.1 (Heinz, et al., 2010). While recent genome-wide studies support the DR4-type element as the major binding motif for LXR (Pehkonen, et al., 2012), it appears that a substantial percentage of LXR target genes do not have a well-defined DR4 element (Boergesen, et al., 2012; Heinz, et al., 2010). Also, recent studies indicate that some LXREs may downregulate target gene expression upon LXR binding; examples of such negatively regulated genes are CYP51A1 and FDFT1 (encoding lanosterol 14α-demethylase and squalene synthase, respectively, of the cholesterol synthesis pathway)(Wang, et al., 2008). Of interest, negative regulation at LXREs in the promoters for these two genes appears to be LXR ligand-specific, as downregulation is induced by oxysterols but not by TO901317 (Wang, et al., 2008). Further work will be necessary to define the identity and frequency of negative LXREs in the genome given that recent studies indicate that a substantial proportion of target genes are in fact downregulated in macrophages upon LXR activation (Pehkonen, et al., 2012).
The conventional model of LXR target gene transactivation holds that unliganded LXRs are tonically bound to LXREs and suppress transcription of target genes by recruiting co-repressors such as nuclear co-repressor 1 (NCoR1) and silencing mediator of retinoic acid and thyroid hormone receptor (SMRT)(Jakobsson, et al., 2012)(Figure 1a). Upon LXR ligation, co-repressors are cleared and exchanged for co-activators such as NR co-activator 1 (NCOA1) and activating signal co-integrator 2 (ASC2), leading to transcription. More recently, it has been recognized that this model, typified by the target gene ABCA1, may characterize just a subset of the LXR transcriptome. Recent genome-wide studies have indicated that in a majority of cases LXR may be recruited de novo to the promoters of target genes upon activation (Boergesen, et al., 2012). In the case of the target gene ABCG1, the co-regulator G-protein pathway suppressor 2 (GPS2) stabilizes an NCoR/histone deacetylase (HDAC)-3 co-repressor complex along with repressive histone 3 lysine 9 dimethylation in the inactive state, whereas, upon LXR ligation, GPS2 facilitates H3K9 de-methylation and recruitment of LXR (Jakobsson, et al., 2009)(Figure 1a).
Figure 1. Molecular mechanisms of LXR action.
Genomic mechanisms, including gene transactivation (A) and transrepression (B), are the best characterized means of LXR activity in cells, whereas recently described non-genomic mechanisms (C) remain somewhat less well understood. In transactivation, two general models, represented by induction of the target genes ATP Binding Cassette (ABC)A1 and ABCG1, have been identified. For ABCA1, it is thought that LXR bound to promoter LXR response elements (LXREs) in the steady state tonically represses gene expression by recruiting co-repressors such as nuclear receptor co-repressor 1 (NCoR). Upon LXR ligand binding, co-repressors are shed in exchange for co-activators, driving gene expression. More recent studies suggest that for a majority of LXR targets including ABCG1, LXRs bind to LXREs only after ligand-induced activation and histone demethylation. In both of these models, LXR binds LXREs directly in the form of a heterodimer with retinoid X receptor (RXR). In the case of transrepression of pro-inflammatory genes (B), two general mechanisms of LXR action have been identified in macrophages/hepatocytes and astrocytes. In both cases, lysines in the ligand-binding domain of LXR are SUMOylated after LXR ligation. In the case of LPS-stimulated macrophages or cytokine-stimulated hepatocytes, SUMOylated LXR binds in monomeric form to a multimolecular co-repressor complex, inhibiting release of co-repressors from gene promoters, thereby blocking gene expression. In astrocytes, SUMOylated LXRs inhibit transcription by blocking the binding of signal transducer and activator of transcription 1 (STAT1) to promoters. Recently, examples of non-genomic (extranuclear) LXRβ action have been identified, as shown in panel C. In colon cancer cells, cytoplasmic LXRβ drives NLRP3- and caspase-1-dependent pyroptotic cell death by inducing pannexin-1-mediated ATP release. In platelets, LXRβ inhibits kinase signaling to degranulation and aggregation downstream of the GPVI receptor. In endothelial cells, lipid raft-localized LXRβ mediates a signaling pathway to cell migration involving estrogen receptor (ER)-α and the kinase AKT. The relative importance of genomic and non-genomic mechanisms remains poorly understood. The distinct binding partners of LXRs in these various contexts, however, creates the exciting potential for targeted, potentially cell type- and gene-specific, pharmacologic interventions. eNOS, endothelial nitric oxide synthase; GPVI, glycoprotein VI; GPS2, G-protein pathway suppressor 2; HDAC, histone deacetylase; iNOS, inducible nitric oxide synthase; P2X7, P2X purinoceptor 7; PIAS1, protein inhibitor of STAT1; SAA, serum amyloid A; SUMO, small ubiquitin-like modifier; TBLR, transducin beta-like 1X-related protein 1; UBC9, SUMO-conjugating enzyme UBC9.
In many cases, LXRα and LXRβ appear to be largely interchangeable in transactivation, with their contribution determined by relative expression level. That said, in murine macrophages, LXRα may be more robust at transactivation and LXRβ more potent at basal target repression (Bischoff, et al., 2010). Also, CD5L/Aim has been identified as an LXRα-specific target gene (Joseph, et al., 2004). In vivo, important systems-level differences have also been identified between the two LXRs. For example, LXRα and not LXRβ is required for development of splenic marginal zone macrophages in mice (A-Gonzalez, et al., 2013).
2.3. Transrepression
LXR activation has also been shown to inhibit the signal-dependent induction of pro-inflammatory genes by transcription factors such as nuclear factor (NF)-κB, signal transducer and activator of transcription (STAT)-1, and activator protein (AP)-1, a mechanism known as ‘transrepression’ (Figure 1b). In macrophages and hepatocytes, LXR agonists induce HDAC4-dependent conjugation of LXR with SUMO-2/3 at specific lysine residues in the LXR ligand-binding domain; SUMOylated LXR then binds as a monomer to the NCoR-SMRT co-repressor complex and prevents its signal-dependent clearance from the promoters of pro-inflammatory genes (Ghisletti, et al., 2009; Ghisletti, et al., 2007; Jakobsson, et al., 2012). LXR thereby suppresses LPS induction of inducible nitric oxide synthase (iNOS), interleukin (IL)-1β, and other genes in macrophages by preventing co-repressor dismissal (Ghisletti, et al., 2009); of interest, transrepression by LXR appears to be selective as it does not inhibit iNOS induction by the Toll-like Receptor (TLR)2 agonist Pam3CSK4 (Ghisletti, et al., 2007). In cultured hepatocytes as well as in vivo in the mouse, induction of acute phase response genes (i.e., serum amyloid A, haptoglobin) by cytokines is similarly suppressed by SUMOylated LXRβ (Venteclef, et al., 2010). Interestingly, in astrocytes, the LXRs transrepress interferon-γ-induced genes by yet a different mechanism, one that does not involve NCoR and that involves differential SUMOylation of the two LXR proteins (Lee, et al., 2009). Specifically, LXRα is SUMO2-conjugated by HDAC4, whereas LXRβ is SUMO1-conjugated by protein inhibitor of activated STAT1 (PIAS1); the two SUMOylated LXRs complex with phosphorylated STAT1 and prevent its binding to gene promoters (Lee, et al., 2009). Taken together, LXRs suppress pro-inflammatory gene induction through mechanisms that are distinct from those that they employ for target gene transactivation. This said, for the most part, the physiological relevance of LXR SUMOylation remains to be clearly demonstrated in vivo. Moreover, it has recently been shown that LXR-dependent repression of inflammatory genes requires both RXR and the LXR DNA-binding domain, and still functions despite mutation of LXR SUMOylation sites and knockdown of the SUMOylation machinery (Ito, et al., 2015). Given this, the SUMOylation model of LXR transrepression remains less well established than the transcriptional model, with less certain physiological significance and relevance.
2.4. Non-genomic mechanisms of LXR action
Recently, as is the case for some other NRs, non-genomic (non-transcriptional) mechanisms of action have also been identified for LXRβ (Figure 1c), further expanding the potential for selective manipulation of the LXRs in health and disease. However, it is important to acknowledge that, given the current relative paucity of publications in this area in comparison to the large number of reports on LXRβ as a nuclear transcription factor, non-genomic roles for LXRβ should be viewed with some caution until these findings are more widely replicated and their physiologic significance more firmly established. In colon cancer cells, LXRβ, upon ligation, binds to and promotes activation of the plasma membrane channel pannexin-1, inducing ATP efflux and subsequent caspase-1-dependent pyroptotic cell death through a pathway involving the P2X7 purinergic receptor and the Nlrp3 inflammasome (Derangere, et al., 2014). Mislocalization of LXRβ to the cytoplasm in colon cancer cells (but not healthy colonic epithelia) may arise from their expression of an abnormal truncated form of RXRα which sequesters LXRβ to this compartment (Courtaut, et al., 2015). In endothelial cells, ligated LXRβ binds to ERα in lipid raft membrane microdomains, thereby initiating a raft-localized signaling cascade via ERα, phosphoinositide-3-kinase, and Akt to activation of endothelial NOS (Ishikawa, et al., 2013). In (anucleate) platelets, LXR agonists inhibit degranulation and aggregation through inducing interactions between LXRβ and signaling proteins immediately downstream of the collagen receptor GPVI (Ishikawa, et al., 2013). Additional studies will be required to define the relative importance of genomic vs. non-genomic roles for LXRβ in inflammatory disease, as well as whether LXRα participates in non-transcriptional signaling events.
3. LXR regulation of reverse cholesterol transport and atherosclerosis
The best-established role for the LXRs in physiology is in transcriptional activation of reverse cholesterol transport (RCT), the coordinated pathway in vivo that protects against atherosclerosis by promoting efflux and bodily excretion of excess cellular cholesterol (Figure 2). Many of the key proteins in RCT, including ABCA1, ABCG1, cholesteryl ester transfer protein (CETP), ABCG5, and ABCG8 are direct LXR target genes and are thus coordinately upregulated by LXR agonists (Jakobsson, et al., 2012). Cholesterol 7α hydroxylase (CYP7A1), the major enzyme of the classical pathway of bile acid synthesis, is an LXR target in mice but not humans.
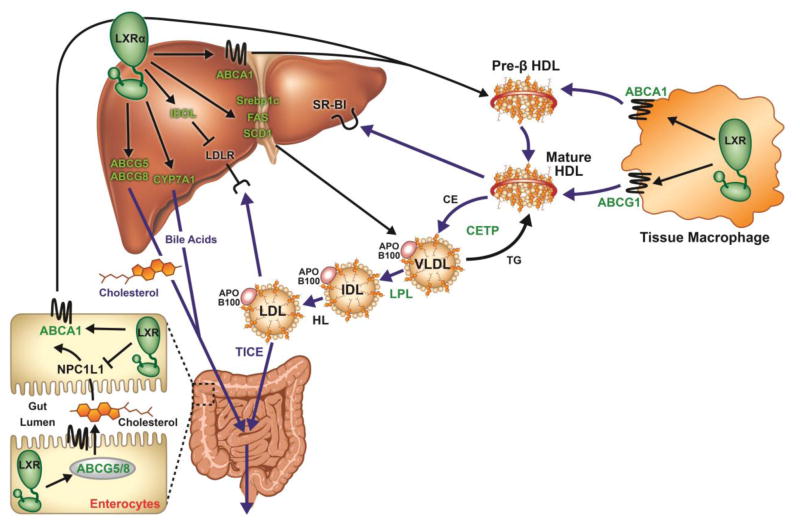
Figure 2. LXRs are master regulators of reverse cholesterol transport.
The in vivo pathways for disposal of excess cellular – in this case, macrophage – cholesterol from the organism, so called ‘reverse cholesterol transport,’ are depicted. Proteins in green font are encoded by LXR target genes, and are thus upregulated by LXR agonists (note, however, that cytochrome P450 family 7 subfamily A member 1 [CYP7A1] is an LXR target in mice and not humans, and cholesteryl ester transfer protein [CETP] is expressed in humans but not mice). ATP Binding Cassette (ABC)A1 in enterocytes and hepatocytes generates plasma high density lipoprotein (HDL) by lipidation of plasma apolipoprotein (apo)A-I. HDL in turn induces cholesterol efflux from macrophages via cooperative interactions with ABCA1 and ABCG1. HDL-associated cholesterol can then either be cleared via hepatocyte scavenger receptor (SR)-BI, or transferred to apoB100-containing lipoproteins via CETP in exchange for triglyceride (TG). Finally, apoB100-lipoprotein cholesterol is cleared from the plasma either by hepatocyte low density lipoprotein receptor (LDLR), or by trans-enterocyte transfer into the gut lumen, the so-called ‘trans-intestinal cholesterol excretion’ (TICE) pathway. Hepatic cholesterol that is not used to assemble very LDL (VLDL) mnplasma particles can be excreted into the biliary system (and from there into the gut lumen), either via ABCG5/8-dependent transport as free cholesterol, or as bile acids, after conversion by CYP7A1. In enterocytes, LXR upregulates ABCA1 (driving HDL production) and ABCG5/8 (promoting cholesterol efflux into gut lumen), and downregulates Niemann Pick C1 like 1 (NPC1L1) protein (reducing uptake of luminal cholesterol). Taken together, enterocyte LXR increases plasma HDL, reduces cholesterol absorption, and promotes cholesterol excretion. Hepatic LXR increases plasma HDL and promotes biliary cholesterol excretion. Hepatic LXR also induces the lipogenic genes sterol response element binding protein (SREBP)-1c, fatty acid synthase (FAS), and stearoyl coA-desaturase (SCD)1, as well as inducible degrader of the LDLR (IDOL). In rodent models, treatment with LXR agonists drives fecal excretion of macrophage-derived cholesterol (ie, RCT). HL, hepatic lipase; IDL, intermediate density lipoprotein; LPL, lipoprotein lipase.
The details of RCT have been covered in prior comprehensive reviews (Azzam & Fessler, 2012; Temel & Brown, 2010). In brief, RCT is initiated in hepatocytes and enterocytes, where ABCA1-dependent lipidation of apolipoprotein (apo)A-I generates nascent pre-β high density lipoprotein (HDL) particles. Pre-β-HDL (and lipid-free apoA-I) serve as primary plasma acceptors for excess cellular cholesterol, acting, along with mature HDL, to induce cholesterol efflux from macrophages (and other parenchymal cells) in a manner facilitated by the lipid transporters ABCA1 and ABCG1. HDL-associated cholesterol is then cleared from the plasma through three alternate pathways. First, scavenger receptor B type I can mediate selective cholesterol ester uptake from HDL into hepatocytes. Second, CETP (present in humans, but not mice) facilitates transfer of cholesterol ester from HDL to very LDL (VLDL) and LDL, followed by LDL clearance by hepatocytes via LDLR. Lastly, apoB100 lipoprotein-derived cholesterol can also be cleared from the plasma by direct basolateral-to-apical transfer across enterocytes into the intestinal lumen, the so-called ‘transintestinal cholesterol excretion’ (TICE) pathway (Temel & Brown, 2015). Hepatic cholesterol, deriving from HDL uptake from the plasma, diet (chylomicron remnant uptake), and/or in situ synthesis is either used to assemble VLDL particles for release into the plasma, or completes the RCT pathway via excretion into the biliary tract either as free cholesterol (via the biliary epithelial transporter ABCG5/ABCG8), or as bile acids (after processing by CYP7A1). ABCG5/ABCG8 expressed in intestinal epithelial cells also mediates cholesterol efflux directly into the gut lumen. Finally, gut luminal cholesterol is excreted from the body, thus completing the centripetal clearance of cellular cholesterol into the feces, if it escapes short-circuiting reabsorption into enterocytes mediated via Niemann-Pick C1-like 1 protein (NPC1L1). Of interest, LXR agonists reduce intestinal cholesterol absorption by downregulating NPC1L1 (Duval, et al., 2006) and upregulating ABCG5/ABCG8 (Bonamassa & Moschetta, 2013) in the brush border. This enhances successful completion of RCT.
Extensive data now demonstrates that synthetic LXR agonists upregulate RCT and reduce atherosclerosis in preclinical models (Joseph, et al., 2002; Naik, et al., 2006; Tangirala, et al., 2002). Complex roles for both LXRα and LXRβ have now been identified at several sites in the RCT pathway, from peripheral macrophage to intestine, suggesting that multiple therapeutic strategies for activating LXRs to combat atherosclerosis may be available. As further detailed below, a selective strategy will almost certainly be necessary given that activation of hepatic LXRα (the predominant LXR in liver), induces hepatic steatosis and hypertrigycleridemia (through induction of hepatic SREBP-1c, fatty acid synthase, stearoyl-coA desaturase-1, and carbohydrate-responsive element-binding protein) and also increases plasma LDL-cholesterol in non-human primates through IDOL-mediated degradation of hepatic LDLR (Ma, et al., 2017)(Figure 2).
LXR-null mice have severe impairment of RCT (Tangirala, et al., 2002), whereas treatment of wild type mice with synthetic LXR agonists increases RCT (Naik, et al., 2006). A predominant role for LXRα in RCT was suggested by studies showing that LXRα-null mice on the pro-atherogenic Apoe-null background have severe tissue cholesterol overload and augmented atherosclerosis, whereas LXRβ-null counterparts do not (Bradley, et al., 2007; Hong, Bradley, et al., 2012). Similar findings were noted in Ldlr-null background mice on a Western diet, an alternate widely used model of atherosclerosis (Bischoff, et al., 2010). The impact of LXRα deletion on macrophage cholesterol overload appears to arise as a post-macrophage defect perhaps driven by altered hepatic gene expression given that macrophage cholesterol efflux is deficient in vivo but not ex vivo in LXRα-null mice (Hong, Bradley, et al., 2012). In support of this, hepatocyte-specific deletion of LXRα also compromises RCT and increases atherosclerosis on the Ldlr-null background (Zhang, et al., 2012). Both LXR genes are required for maximal atheroprotection in vivo (Feig, et al., 2010). Nonetheless, synthetic LXR agonists reduce atherosclerosis in both global LXRα-null mice (on an Apoe-null background) and hepatocyte-specific LXRα-null mice (on an Ldlr-null background), and do so without inducing hypertriglyceridemia or hepatic steatosis (Bradley, et al., 2007; Zhang, et al., 2012). This suggests that LXRβ-selective agonists may prove efficacious as well as safe as atheroprotective agents. Contrary to this, however, is one report that synthetic LXR agonists reduce aortic atherosclerosis as assessed by en face analysis in Ldlr knockout and Ldlr/Lxrb double knockout mice, but not in Ldlr/Lxra double knockout mice (Bischoff, et al., 2010). Activation of hepatic LXRβ also has the potential to induce steatosis (Korach-Andre & Gustafsson, 2015). Given this, avoidance of all hepatic LXR activation may possibly be required to avoid liver complications.
Investigations to determine the tissue site of LXR activity in atheroprotection have taught us critical lessons. Selective hematopoietic (macrophage) LXR deletion induced by bone marrow transplantation in mice increases atherosclerosis on both Apoe-null and Ldlr-null backgrounds (Levin, et al., 2005; Tangirala, et al., 2002), and impairs atherosclerotic plaque regression (Feig, et al., 2010). Atherosclerosis inhibition by TO901317 in Ldlr-null mice is abolished in the setting of hematopoietic LXR deficiency (Levin, et al., 2005), and GW3965-induced mobilization of cholesterol is attenuated from LXR-null macrophages transplanted into wild type mice (Yasuda, et al., 2010). Collectively, these studies suggest that macrophage LXR is anti-atherogenic and is required for a full in vivo RCT and atheroprotective response to synthetic LXR agonists. On the other hand, cell transplantation studies indicate that macrophage LXR is not sufficient (i.e., in an otherwise LXR-null host) to substantially promote RCT in response to LXR agonists (Yasuda, et al., 2010).
Interestingly, emerging reports suggest that the intestine is a major target tissue in LXR agonist-induced RCT and atheroprotection. LXR agonists dramatically increase TICE (Temel, et al., 2010; van der Veen, et al., 2009), potentially by upregulating the intestinal ABCG5/ABCG8 cholesterol exporter and downregulating intestinal NPC1L1. Selective transgenic overexpression of LXRα in the intestines of mice not only reduces cholesterol absorption and increases plasma HDL, but also enhances RCT from macrophage to feces and reduces atherosclerosis; by contrast, selective overexpression of hepatic LXRα induces hepatic steatosis and hypertriglyceridemia without increasing RCT (Lo Sasso, et al., 2010). LXR agonists increase plasma HDL via activity on intestinal ABCA1 (Brunham, et al., 2006). However, this may not be a critical driver of increased RCT given that LXR agonists reduce atherosclerosis in atherogenic mouse models despite not raising HDL, and HDL-deficient apoAI-null and ABCA1-null mice have intact cholesterol disposal (Temel & Brown, 2015).
Taken together, important roles have been demonstrated for both LXR proteins in several cell types along the RCT pathway. Nonetheless, it does not appear necessary to activate LXR pharmacologically at all steps along the RCT pathway in order to achieve effective RCT and atheroprotection. In principle, avoidance of hepatic LXR activation may avert complicating steatosis without significant compromise to RCT, and, as discussed below, selective activation of intestinal LXR holds promise as an effective strategy. What remains unclear is to what extent selective activation of intestinal LXR will prove efficacious against inflammatory disorders other than atherosclerosis.
4. LXR agonists in preclinical models of inflammatory disease
4.1. Anti-inflammatory mechanisms of LXRs
Although originally identified for their role in cholesterol homeostasis, the LXRs, like other NRs (e.g, the glucocorticoid receptor), are now known to suppress inflammation, and thus represent ripe targets for anti-inflammatory drug development. The LXRs suppress inflammation through multiple mechanisms, direct and indirect, some involving transactivation and others, transrepression. The relative importance of these mechanisms in specific cell types and during specific disease states remains uncertain.
Tontonoz and colleagues were the first to identify that LXR activation suppresses NF-κB-dependent pro-inflammatory gene induction by lipopolysaccharide (LPS)(Joseph, et al., 2003). As discussed above, this effect, along with suppression of AP-1- and STAT1-dependent pro-inflammatory genes, may stem from transrepression (i.e., tethering of co-repressor complexes to gene promoters by SUMOylated LXR). While a recent report has challenged the importance of LXR SUMOylation, suggesting instead that LXR suppresses TLR4 signaling by depleting cholesterol in lipid rafts via ABCA1 transactivation (Ito, et al., 2015), another group has reported that the anti-inflammatory activity of LXR agonists is intact or even increased in Abca1−/−Abcg1−/− macrophages (Kappus, et al., 2014). On other fronts, LXR supports anti-inflammatory programming of macrophages by directly inducing IL-5 (Chen, et al., 2012) and the efferocytosis receptor Mer (A-Gonzalez, et al., 2009), and indirectly promoting arginase induction (Pourcet, et al., 2011). Activation of LXR in macrophages upon internalization of cell corpses induces Mer in a feed-forward fashion, and thereby plays a key role in anti-inflammatory clearance of senescent neutrophils and control of the hematopoietic niche in the bone marrow (Casanova-Acebes, et al., 2013).
In addition to macrophages, the LXRs modulate inflammatory functions in other leukocyte types. Systemic treatment of mice with TO901317 inhibits chemokine-directed neutrophil migration, although it is unclear if this is LXR-dependent (Smoak, et al., 2008). LXR activation in tissue phagocytes during clearance of senescent neutrophils also plays a pivotal role in neutrophil homeostasis by repressing an IL-23/IL-17/G-CSF granulopoietic cytokine cascade (Hong, Kidani, et al., 2012). In lymphocytes, LXR agonists reduce transcription of IL-17 and T helper 1 cytokines (Cui, et al., 2011; Walcher, et al., 2006), suppress cell proliferation (Geyeregger, et al., 2009), and inhibit chemokine-directed migration (Walcher, et al., 2010). LXRα activation also suppresses trafficking of mature dendritic cells from tumors to lymph nodes in mice by reducing expression of CC chemokine receptor-7 (CCR7)(Villablanca, et al., 2010), and inhibits LPS-induced functional maturation of dendritic cells (Geyeregger, et al., 2007). Surprisingly, LXR activation is reported to upregulate Ccr7 in immature human dendritic cells and in murine monocyte-derived cells, and to promote migration of the latter from atherosclerotic plaques (Feig, et al., 2010).
LXR also modulates inflammation through transcriptional reprogramming of lipid metabolism. Plasma HDL, which is increased in vivo in response to LXR agonists, inhibits pro-inflammatory TLR signaling in macrophages and myeloproliferative cytokine signaling in bone marrow progenitors by depleting lipid raft cholesterol (Azzam & Fessler, 2012; Fessler & Parks, 2011). HDL also suppresses pro-inflammatory gene expression through induction of activating transcription factor 3 (De Nardo, et al., 2014). In macrophages, LXRs induce anti-inflammatory omega-3 polyunsaturated fatty acids via upregulation of genes that mediate elongation and unsaturation of fatty acids (Li, et al., 2013). In hepatocytes, induction of lysophosphatidylcholine acyltransferase 3 (Lpcat3) by LXRα reduces ER stress and associated cytokine induction via incorporation of polyunsaturated fatty acids into cellular phospholipids (Rong, et al., 2013).
4.2. Efficacy of LXR agonists in disease models
LXR agonists have now been shown to ameliorate pathology in multiple preclinical rodent models of inflammatory disease (Table 1). Most studies to date have used the first-generation dual LXRα/β agonists TO901317 and GW3965. Given this, the generalizability of these findings to advanced LXR therapeutics (discussed below) is uncertain, and in many cases even the specificity of the observed effects for LXRs remains unproven.
Table 1.
Synthetic dual LXRα/β agonists in preclinical models of inflammatory disease*
| Disease model | LXR agonist | Effect | Reference |
|---|---|---|---|
| Autoimmune disease | |||
| Collagen-induced arthritis | GW3965 | ↓clinical and pathological severity | (Park, et al., 2010) |
| GW3965, T0901317 | ↑inflammation; ↑cartilage destruction | (Asquith, et al., 2009; Asquith, et al., 2011) | |
| Experimental autoimmune uveitis | T0901317 | ↓retinal cytokines; ↓clinical severity | (Yang, et al., 2014) |
| EAE (multiple sclerosis model) | T0901317 | ↓CNS inflammation, demyelination, clinical severity | (Cui, et al., 2011) |
| Lupus (lpr/lpr mouse) | GW3965 | ↓lymphadenopathy; ↓glomerular antibody deposition | (A-Gonzalez, et al., 2009) |
|
| |||
| Neurologic disease | |||
| Alzheimer’s disease (APP/PS1 tg) | T0901317 | ↓glial inflammation; ↓cognitive impairment | (Cui, et al., 2012) |
| (APP23) | T0901317 | ↓Aβ levels | (Terwel, et al., 2011) |
| Acute brain ischemia | GW3965 | ↓inflammation; ↓injury | (Sironi, et al., 2008) |
| GW3965, T0901317 | ↓infarct volume; ↓inflammation; ↑neurologic function | (Morales, et al., 2008) | |
| Cerebral hemorrhage | T0901317 | ↓inflammation; ↓brain damage; ↓functional deficits | (Wu, et al., 2016) |
| Spinal cord trauma | T0901317 | ↓inflammation; ↓tissue injury; ↑limb function | (Paterniti, et al., 2010) |
| MPTP-induced Parkinson’s disease | T0901317 | ↓ inflammation | (Paterniti, et al., 2017) |
|
| |||
| Lung disease | |||
| Acute lung injury (LPS) | T0901317 | ↓neutrophil recruitment, ↓airspace TNFα | (Smoak, et al., 2008) |
| Acute lung injury (bleomycin) | T0901317 | ↓inflammation; ↓fibrosis | (Shi, et al., 2016) |
| Asthma (OVA) | GW3965 | ↔ inflammation; ↔ mucus; ↑AHR; ↑ASM thickness | (Birrell, et al., 2008) |
| (OVA) | T0901317 | ↔ inflammation; ↓mucus; ↓IgE; ↓AHR; ↓ASM thickness | (Shi, et al., 2014) |
| (OVA, HDM) | GW3965 | ↑eosinophils; ↑T helper 2 cytokines | (Smet, et al., 2016) |
|
| |||
| Ischemia/reperfusion injury | |||
| Myocardial | GW3965 | ↓infarct size; ↓oxidative stress; ↓ventricular dysfunction | (He, et al., 2014) |
| Splanchnic | T0901317 | ↓neutrophil infiltration; ↓tissue injury; ↑survival | (Crisafulli, et al., 2010) |
|
| |||
| Miscellaneous | |||
| DSS- and TNBS-induced colitis | GW3965 | ↓inflammation; ↑body weight recovery; ↑survival | (Jakobsson, et al., 2014) |
| Diabetic nephropathy (STZ model) | T0901317 | ↓macrophage infiltration; ↓cytokines; ↓urinary albumin | (Tachibana, et al., 2012) |
| Renal transplantation | GW3965 | ↓graft inflammation and dysfunction | (Kiss, et al., 2011) |
| Irritant and allergic dermatitis | GW3965 | ↓skin thickness and inflammation | (Fowler, et al., 2003) |
This table lists select reports of GW3965 and TO901317 efficacy across preclinical inflammatory disease models.
AHR, airway hyperresponsiveness; APP, amyloid precursor protein; ASM, airway smooth muscle; DSS, dextran sulfate sodium; EAE, experimental autoimmune encephalomyelitis; HDM, house dust mite; Ig, immunoglobulin; LPS, lipopolysaccharide; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MS, multiple sclerosis; OVA, ovalbumin sensitization and challenge; PS-1, presenilin-1; STZ, streptozotocin; tg, transgenic mouse; TNBS, 2,4,6-Trinitrobenzenesulfonic acid.
Of note, while anti-inflammatory efficacy has been demonstrated for LXR agonists in a wide range of disorders, in at least two disease models – collagen-induced arthritis (CIA; a rodent model for rheumatoid arthritis) and ovalbumin sensitization/challenge (a rodent model for allergic asthma) – divergent results have been reported (Table 1). Two groups have shown that GW3965 reduces the incidence and severity of CIA in DBA/1 mice (Huang, et al., 2015; Park, et al., 2010). By contrast, another group has shown that TO90137 and GW3965 exacerbate CIA in DBA/1 mice (Asquith, et al., 2009), and that GW3965 worsens clinical and histopathological severity in C57BL/6 wild type but not LXRα−/−, LXRβ−/−, or LXRα/β−/− mice (Asquith, et al., 2011). Technical discrepancies presumably account for these differences. In an ovalbumin model of asthma in BALB/C mice, GW3965 was reported to worsen airway hyperresponsiveness without affecting inflammation (Birrell, et al., 2008). By contrast, another group has shown that LXR-null mice from a different genetic background have reduced eosinophilic inflammation, mucus, and airway hyperresponsiveness in both the ovalbumin and house dust mite models of asthma, whereas GW3965 aggravates eosinophilia (Smet, et al., 2016).
Caution is warranted in extrapolating results derived from rodent models to humans. Importantly, TLR4 is a direct target gene in human, but not rodent cells; thus, LXR agonists upregulate TLR4 in primary human macrophages and augment LPS induction of pro-inflammatory genes in human macrophages and dendritic cells (Fontaine, et al., 2007; Torocsik, et al., 2010). Augmentation of inflammation in macrophages is dependent on the duration of pretreatment with LXR agonist, with <12h pretreatment inhibiting, but 24–48h pretreatment augmenting LPS-induced cytokines (Fontaine, et al., 2007). LXR regulation of eicosanoids also differs between murine and human cells. Whereas LXR agonists reduce LPS induction of prostaglandin E2 in mouse macrophages potentially through Lpcat3-dependent effects on arachidonic acid availability (Rong, et al., 2013), LXR-induced Lpcat3 enhances LPS-induced eicosanoid release from human macrophages (Ishibashi, et al., 2013).
Finally, another important in vivo difference between rodent and primate LXRs lies in induction in the liver of the LXR target IDOL, an E3 ubiquitin ligase that induces degradation of LDLR and family members VLDLR and apoER2 (Hong, et al., 2010). Hepatic LDLR is a major clearance site for plasma LDL and thus a key determinant of plasma LDL levels. In mice, LXR induces Idol in peripheral tissues (e.g., macrophages) but not in liver, and thus does not alter plasma LDL; by contrast, in cynomolgus monkeys, LXR agonists induce hepatic IDOL, and thereby reduce LDLR protein levels, elevating plasma LDL (Hong, et al., 2014). The increase in LDL-cholesterol in non-human primates (and its potential to occur in humans) may also be driven by induction of CETP, an LXR target gene expressed in primates but not mice. CETP supports transfer of cholesterol ester from HDL to LDL particles (Figure 2). Clearly, more work will be required to define species- and context-specific effects of LXR agonists. In principle, dual LXR therapies such as co-treatment with a hepatic IDOL antagonist and/or a statin could be tested. Of interest, it was recently shown that co-treatment of mice with the peroxisome proliferator-activated receptor (PPAR)α agonist fenofibrate neutralized the hepatic steatosis and hypertriglyceridemia induced by an LXR agonist (Boergesen, et al., 2012).
5. Advanced LXR therapeutics
In recent years, several pharmacological attempts have been made to harness the pro-RCT and anti-inflammatory actions of LXR agonists without activating hepatic LXRα (and thereby causing hepatic steatosis, hypertriglyceridemia, and elevated LDL-cholesterol in primates). These include: i) LXRβ-selective agonists; ii) tissue-selective LXR agonists; and iii) transrepression-selective LXR agonists. To date, four synthetic LXR agonists have entered phase I clinical trials, most of these with the ultimate aim of treating atherosclerosis (Hong & Tontonoz, 2014; Ma, et al., 2017). These agents are: LXR-623 (Wyeth Pharmaceuticals – now Pfizer); CS-8080 (Daichii Sankyo); BMS-779788 (XL-652; Exelixis and Bristol-Myers Squibb); and BMS-852927 (XL-041; Exelixis and Bristol-Myers Squibb). Unfortunately, none of these drugs have progressed, either due to adverse effects (LXR-623, BMS-852927) as discussed below, or for other reasons (not disclosed by sponsor). A fifth agent, VTP-38543 (Vitae Pharmaceuticals – now Allergan), was tested recently in a phase IIa trial for topical treatment of mild-to-moderate atopic dermatitis (ClinicalTrials.gov identifier: NCT02655679). The trial has been completed but the results are yet to be disclosed.
5.1. LXRβ-selective agonists
Recent efforts in industry have yielded several agonists with relative selectivity for LXRβ overLXRα. Selective carboxylic acid-based quinoline agonists were developed, but proved to have PPAR agonism (Hu, et al., 2008). Quinolone-3-carboxamide-containing sulfones with good LXRβ-selectivity were developed but found to have an insufficient dose window between ABCA1 upregulation in macrophages versus triglyceride accumulation in hepatocytes (Hu, Bernotas, et al., 2010). Additional agents identified to have LXRβ-selectivity include cinnolines/quinolines and N-aryl-3,3,3-trifluoro-2-hydroxy-2-methylproprionamides (Hu, et al., 2009; Swahn, et al., 2009), among other molecules.
LXRβ-selective agents have recently been tested in human subjects. The first was LXR-623, an agonist with modest (~7-fold) selectivity for LXRβ which had been shown to reduce atherosclerosis in Ldlr-null mice without hepatic lipogenesis and to reduce plasma LDL-cholesterol in non-human primates with only transient effects on plasma triglycerides (Quinet, et al., 2009). In a single ascending dose study in humans, although LXR-623 did increase LXR target gene expression in peripheral blood cells, it was associated with central nervous system and psychiatric adverse events at the two highest doses, precluding further development (Katz, et al., 2009). It remains unclear whether this was an off-target effect, or LXR-mediated. On other fronts, introduction of a substituted phenylsulfone into a series of biphenyl imidazole LXR agonists was recently reported to yield two novel LXRβ-selective partial agonists, BMS-779788 and BMS-852927, with a therapeutic window (i.e., blood cell ABCG1 upregulation but modest elevation of plasma triglycerides) in cynomolgus monkeys (Kick, et al., 2016; Kirchgessner, et al., 2015). Of these, testing of BMS-779788 in a phase I clinical trial was completed (ClinicalTrials.gov identifier: NCT00836602), but the results have not been disclosed. For BMS-852927, a wide therapeutic index in cynomolgus monkeys and mice and successful induction of RCT genes in peripheral blood of healthy subjects and hypercholesterolemic patients was observed. Despite this, increased plasma and hepatic triglyceride, increased plasma LDL-cholesterol, and decreased circulating neutrophils – effects not initially predicted by the preclinical monkey model – were observed in human volunteers in a phase I clinical study (Kirchgessner, et al., 2016).
Recently, the application of structure-based drug design technology to a phenylsulfone fragment led to development of a novel, orally bioavailable LXRβ-selective partial agonist (Zheng, et al., 2016). This prototype has reportedly been further optimized for human trials (Zheng, et al., 2016).
5.2. Tissue-selective agonists
One of the most promising areas in LXR drug development is tissue-selective LXR activation. In some cases, tissue selectivity has been conferred through pharmacologic strategies (e.g., drug inactivation in plasma, low blood-brain barrier penetration, cell-specific uptake), whereas in others, specificity arises from tissue- or even gene-specific interaction of the LXR/agonist complex with differential co-activators/co-repressors. On the latter note, a recent study has shown that the co-activator thyroid hormone receptor-associated protein 80 (TRAP80) selectively promotes transcription of SREBP-1c but not ABCA1 in hepatic cell lines and that liver-selective knockdown of TRAP80 in mice ameliorates the hepatic steatosis and hypertriglyceridemia induced by an LXR agonist without impairing RCT stimulation (Kim, et al., 2015).
Selective activation of LXR in the intestine is particularly promising as it carries the potential to decrease dietary cholesterol absorption (via reduced intestinal NPC1L1 and increased intestinal ABCG5/ABCG8), increase plasma HDL (via increased intestinal ABCA1), and increase RCT, all in the absence of hepatic steatosis (Lo Sasso, et al., 2010). GW6340, an intestine-specific ester version of the LXR agonist GW3965, was shown to promote fecal excretion of macrophage-derived cholesterol (i.e., RCT) in mice without changing hepatic LXR target genes or hepatic triglyceride content, although the magnitude of RCT was less than that induced by GW3965 (Yasuda, et al., 2010). YT-32, a phytosterol derivative [(22E)-ergost-22-ene-1α,3β-diol] LXRα/β agonist, was also shown to selectively activate intestinal LXR target genes in mice upon oral administration, thereby reducing cholesterol absorption without increasing plasma triglycerides (Kaneko, et al., 2003).
WYE-672, a phenylsulfone-substituted quinoxaline, is an LXRβ-selective agonist with tissue-selectivity, activating LXRβ in kidney (HEK293) cells and macrophages (THP-1), but not liver cells (huh-7); treatment of Ldlr-null mice reduced aortic arch atherosclerosis with no plasma or hepatic triglyceride increase (Hu, Unwalla, et al., 2010). Generally similar findings were noted for ATI-829, a potent synthetic steroidal LXR agonist which induced ABCA1 robustly in THP-1 macrophages but SREBP-1c to only a minor extent in HepG2 hepatoma cells; treatment of Ldlr-null mice activated LXR target genes in intestine and macrophages but not liver, reducing atherosclerosis without hepatic or plasma triglyceride increase (Peng, et al., 2008).
Finally, alternative LXR agonist formulations have recently succeeded in tissue-selective drug delivery. In a recent immunologically targeted strategy, an aminooxy-modified LXR agonist conjugated to anti-CD11a IgG achieved potent, macrophage-selective LXR activation without a significant effect in hepatocytes (Lim, et al., 2015). Nanoparticle formulations containing GW3965 have recently also been shown to have increased anti-inflammatory potency against macrophage-mediated inflammation in vitro and in vivo, with systemic injection achieving effective uptake by atherosclerotic plaque macrophages with negligible induction of lipogenic genes in the liver (Gadde, et al., 2014; Zhang, et al., 2015). Although intestinal-specific LXR agonists may in principle suppress inflammation in peripheral macrophages by increasing plasma HDL, direct activation of macrophage LXR may have greater anti-inflammatory effect.
5.3. Transrepression-dissociated agonists
LXR agonists have recently been developed that dissociate transrepression from target gene transactivation in an effort to selectively harness the anti-inflammatory activity of LXRs (Figure 1). As an example, structure-guided design of N-phenyl tertiary amines using the crystal structure of TO901317 bound to LXRβ led to the identification of a compound, GSK9772, with >10-fold selectivity for transrepression over transactivation (Chao, et al., 2008). GSK9772 suppressed LPS-induced iNOS and IL-6 through a mechanism involving LXRβ SUMOylation and inhibition of NCoR clearance, and did so with only very weak transactivation of ABCA1 and SREBP-1c (Chao, et al., 2008). N,N-dimethyl-3β-hydroxycholenamide (DMHCA), a steroidal LXR agonist, and its more potent analog, methylpiperidinyl-3β-hydroxycholenamide (MePipHCA), both reduce inflammation in colitis and traumatic brain injury models without inducing hepatic steatosis (Yu, et al., 2016). The minimal induction of lipogenic genes by DMHCA stems not from its inefficacy at transactivation but rather from its inhibition of the lipogenic transcription factor SREBP-1c (Yu, et al., 2016). This likely arises from its structural similarity to desmosterol, a native cholesterol pathway intermediate with coordinate LXR agonism and SREBP inhibitory activity. Interestingly, DMHCA also reportedly increases desmosterol levels and reduces cholesterol through inhibiting the cholesterol biosynthetic enzyme 3β-hydroxysterol-Δ24-reductase (Pfeifer, et al., 2011). In an analogous vein, ATI-111, a steroidal LXR agonist modified from ATI-829 (discussed above), was reported to reduce atherosclerosis in Ldlr-null mice while lowering plasma triglycerides, potentially also through inhibition of SREBP-1c activation (Peng, et al., 2011). Taken together, DMHCA and ATI-111 dissociate transactivation of RCT-active LXR target genes from lipogenic LXR target genes by blocking processing of the LXR target SREBP-1c. In this regard, they are similar to native LXR agonists (desmosterol and oxysterols), which coordinately inhibit SREBP activation through actions in the ER (Fessler, 2016).
5.4. LXR inverse agonists and antagonists
Recently, potential applications for inhibition of LXR have been proposed. Non-alcoholic fatty liver disease is a common condition that in its more severe form can progress to steatohepatitis and cirrhosis (Vizuete, et al., 2017). There is currently no specific, proven effective pharmacological therapy. Recently, SR9238, a liver-selective (due to inclusion of an ester group labile to plasma enzymes) dual LXRα/β inverse agonist was shown to suppress hepatic lipid accumulation and inflammation in a mouse model of non-alcoholic hepatosteatosis, suggesting potential application to human fatty liver disease (Griffett, et al., 2013). On a different front, it was recently shown that the LXR antagonist GSK2033 suppresses dexamethasone-induced upregulation of gluconeogenic genes (i.e., phosphoenolpyruvate carboxykinase [Pepck]) in liver cells without attenuating dexamethasone anti-inflammatory effects; this effect arises from inhibition of LXRβ-dependent recruitment of the glucocorticoid receptor to the Pepck promoter (Patel, et al., 2017). Moreover, LXR antagonism reduced dexamethasone-induced hepatic steatosis (Patel, et al., 2017; Patel, et al., 2011). This suggests potential for adjunctive use of LXR antagonists during glucocorticoid therapy in order to attenuate the metabolic side effects of glucocorticoids while preserving anti-inflammatory efficacy. On an analogous front, bexarotene, an RXR agonist, has been used as a therapy for refractory cutaneous T-cell lymphoma, but induces complicating hypertriglyceridemia in the majority of patients (Huen & Kim, 2015). It was recently reported that bexarotene-induced hypertriglyceridemia and hepatic steatosis are abrogated in LXR-null mice; this is due to bexarotene-induced recruitment of RXRs to LXREs (via permissive LXR heterodimerization) in the promoters of lipogeneic genes in the liver, including Srebp-1c and fatty acid synthase (Lalloyer, et al., 2009). This suggests the possibility that LXR antagonists may be useful adjuncts during bexarotene therapy.
Inhibition of hepatic LXR has the potential to impair RCT (through downregulation of hepatic ABCA1, ABCG5, and ABCG8) and thereby theoretically aggravate atherosclerosis. Given this, it remains unclear whether LXR antagonism will prove a safe pharmacological strategy in humans. Indeed, increased plasma levels of 25-hydroxycholesterol-3-sulfate, a native metabolite with antagonistic activity on LXR, are found in patients with obesity and metabolic syndrome (Mutemberezi, et al., 2016).
6. Conclusions and future perspectives
Studied now for ~20 years, the LXRs have posed exciting potential opportunities for the therapy of atherosclerosis and a growing number of inflammatory diseases, but have so far eluded successful translation to humans. Recently, several advanced pharmacologic strategies have been pursued, including LXRβ-selective agonists, tissue-selective LXR agonists, and transrepression-selective LXR agonists, among others. Several of these more selective strategies appear promising, although early studies in humans with some of these agents have unfortunately been complicated by adverse effects not predicted even by non-human primate models. Non-systemic routes of drug delivery with reduced risk for toxicity (e.g., topical administration) are also under study, and have not yet been comprehensively explored. In addition, dual therapy of LXR agonists with other agents that mitigate lipogenesis (e.g., IDOL antagonists) remain as possibilities. As testing of LXR agonists in preclinical disease models and structure-guided drug development both continue, it appears likely that effective and safe applications for LXR targeting in human disease will ultimately be realized.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES102005).
Abbreviations
- ABC
ATP-Binding Cassette
- DR4
direct repeat 4
- ER
endoplasmic reticulum
- HDL
high density lipoprotein
- LDLR
low density lipoprotein receptor
- LXR
Liver X Receptor
- LXRE
LXR response element
- NCoR
nuclear co-repressor
- NPC1L1
Niemann-Pick C1-like 1
- NR
nuclear receptor
- RCT
reverse cholesterol transport
- ROR
retinoid orphan receptor
- RXR
retinoid X receptor
- SREBP
sterol response element binding protein
- STAT1
signal transducer and activator of transcription 1
- TICE
transintestinal cholesterol excretion
- TLR
Toll-like Receptor
Footnotes
Conflict of Interest statement: The author declares that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A-Gonzalez N, Guillen JA, Gallardo G, Diaz M, de la Rosa JV, Hernandez IH, Casanova-Acebes M, Lopez F, Tabraue C, Beceiro S, Hong C, Lara PC, Andujar M, Arai S, Miyazaki T, Li S, Corbi AL, Tontonoz P, Hidalgo A, Castrillo A. The nuclear receptor LXRalpha controls the functional specialization of splenic macrophages. Nat Immunol. 2013;14:831–839. doi: 10.1038/ni.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asquith DL, Miller AM, Hueber AJ, McKinnon HJ, Sattar N, Graham GJ, McInnes IB. Liver X receptor agonism promotes articular inflammation in murine collagen-induced arthritis. Arthritis Rheum. 2009;60:2655–2665. doi: 10.1002/art.24717. [DOI] [PubMed] [Google Scholar]
- Asquith DL, Miller AM, Reilly J, Kerr S, Welsh P, Sattar N, McInnes IB. Simultaneous activation of the liver X receptors (LXRalpha and LXRbeta) drives murine collagen-induced arthritis disease pathology. Ann Rheum Dis. 2011;70:2225–2228. doi: 10.1136/ard.2011.152652. [DOI] [PubMed] [Google Scholar]
- Azzam KM, Fessler MB. Crosstalk between reverse cholesterol transport and innate immunity. Trends Endocrinol Metab. 2012;23:169–178. doi: 10.1016/j.tem.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiker A, Andersson O, Lindblom D, van der Linden J, Wiklund B, Lutjohann D, Diczfalusy U, Bjorkhem I. Elimination of cholesterol as cholestenoic acid in human lung by sterol 27-hydroxylase: evidence that most of this steroid in the circulation is of pulmonary origin. J Lipid Res. 1999;40:1417–1425. [PubMed] [Google Scholar]
- Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell MA, De Alba J, Catley MC, Hardaker E, Wong S, Collins M, Clarke DL, Farrow SN, Willson TM, Collins JL, Belvisi MG. Liver X receptor agonists increase airway reactivity in a model of asthma via increasing airway smooth muscle growth. J Immunol. 2008;181:4265–4271. doi: 10.4049/jimmunol.181.6.4265. [DOI] [PubMed] [Google Scholar]
- Bischoff ED, Daige CL, Petrowski M, Dedman H, Pattison J, Juliano J, Li AC, Schulman IG. Non-redundant roles for LXRalpha and LXRbeta in atherosclerosis susceptibility in low density lipoprotein receptor knockout mice. J Lipid Res. 2010;51:900–906. doi: 10.1194/jlr.M900096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boergesen M, Pedersen TA, Gross B, van Heeringen SJ, Hagenbeek D, Bindesboll C, Caron S, Lalloyer F, Steffensen KR, Nebb HI, Gustafsson JA, Stunnenberg HG, Staels B, Mandrup S. Genome-wide profiling of liver X receptor, retinoid X receptor, and peroxisome proliferator-activated receptor alpha in mouse liver reveals extensive sharing of binding sites. Mol Cell Biol. 2012;32:852–867. doi: 10.1128/MCB.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonamassa B, Moschetta A. Atherosclerosis: lessons from LXR and the intestine. Trends Endocrinol Metab. 2013;24:120–128. doi: 10.1016/j.tem.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Bovenga F, Sabba C, Moschetta A. Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer. Cell Metab. 2015;21:517–526. doi: 10.1016/j.cmet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Bradley MN, Hong C, Chen M, Joseph SB, Wilpitz DC, Wang X, Lusis AJ, Collins A, Hseuh WA, Collins JL, Tangirala RK, Tontonoz P. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J Clin Invest. 2007;117:2337–2346. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham LR, Kruit JK, Pape TD, Parks JS, Kuipers F, Hayden MR. Tissue-specific induction of intestinal ABCA1 expression with a liver X receptor agonist raises plasma HDL cholesterol levels. Circ Res. 2006;99:672–674. doi: 10.1161/01.RES.0000244014.19589.8e. [DOI] [PubMed] [Google Scholar]
- Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chevre R, NAG, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, Weber C, Nagasawa T, Frenette PS, Castrillo A, Hidalgo A. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao EY, Caravella JA, Watson MA, Campobasso N, Ghisletti S, Billin AN, Galardi C, Wang P, Laffitte BA, Iannone MA, Goodwin BJ, Nichols JA, Parks DJ, Stewart E, Wiethe RW, Williams SP, Smallwood A, Pearce KH, Glass CK, Willson TM, Zuercher WJ, Collins JL. Structure-guided design of N-phenyl tertiary amines as transrepression-selective liver X receptor modulators with anti-inflammatory activity. J Med Chem. 2008;51:5758–5765. doi: 10.1021/jm800612u. [DOI] [PubMed] [Google Scholar]
- Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 2007;5:73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Duan Y, Kang Y, Yang X, Jiang M, Zhang L, Li G, Yin Z, Hu W, Dong P, Li X, Hajjar DP, Han J. Activation of liver X receptor induces macrophage interleukin-5 expression. J Biol Chem. 2012;287:43340–43350. doi: 10.1074/jbc.M112.403394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtaut F, Derangere V, Chevriaux A, Ladoire S, Cotte AK, Arnould L, Boidot R, Rialland M, Ghiringhelli F, Rebe C. Liver X receptor ligand cytotoxicity in colon cancer cells and not in normal colon epithelial cells depends on LXRbeta subcellular localization. Oncotarget. 2015;6:26651–26662. doi: 10.18632/oncotarget.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisafulli C, Di Paola R, Mazzon E, Paterniti I, Galuppo M, Genovese T, Bramanti P, Cappellani A, Cuzzocrea S. Liver X receptor agonist treatment reduced splanchnic ischemia and reperfusion injury. J Leukoc Biol. 2010;87:309–321. doi: 10.1189/jlb.0609438. [DOI] [PubMed] [Google Scholar]
- Cui G, Qin X, Wu L, Zhang Y, Sheng X, Yu Q, Sheng H, Xi B, Zhang JZ, Zang YQ. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Invest. 2011;121:658–670. doi: 10.1172/JCI42974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Sun Y, Wang Z, Xu C, Peng Y, Li R. Liver X receptor activation attenuates inflammatory response and protects cholinergic neurons in APP/PS1 transgenic mice. Neuroscience. 2012;210:200–210. doi: 10.1016/j.neuroscience.2012.02.047. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Dang EV, Reboldi A, Yi T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol. 2014;14:731–743. doi: 10.1038/nri3755. [DOI] [PubMed] [Google Scholar]
- De Nardo D, Labzin LI, Kono H, Seki R, Schmidt SV, Beyer M, Xu D, Zimmer S, Lahrmann C, Schildberg FA, Vogelhuber J, Kraut M, Ulas T, Kerksiek A, Krebs W, Bode N, Grebe A, Fitzgerald ML, Hernandez NJ, Williams BR, Knolle P, Kneilling M, Rocken M, Lutjohann D, Wright SD, Schultze JL, Latz E. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol. 2014;15:152–160. doi: 10.1038/ni.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derangere V, Chevriaux A, Courtaut F, Bruchard M, Berger H, Chalmin F, Causse SZ, Limagne E, Vegran F, Ladoire S, Simon B, Boireau W, Hichami A, Apetoh L, Mignot G, Ghiringhelli F, Rebe C. Liver X receptor beta activation induces pyroptosis of human and murine colon cancer cells. Cell Death Differ. 2014;21:1914–1924. doi: 10.1038/cdd.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval C, Touche V, Tailleux A, Fruchart JC, Fievet C, Clavey V, Staels B, Lestavel S. Niemann-Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem Biophys Res Commun. 2006;340:1259–1263. doi: 10.1016/j.bbrc.2005.12.137. [DOI] [PubMed] [Google Scholar]
- Farnegardh M, Bonn T, Sun S, Ljunggren J, Ahola H, Wilhelmsson A, Gustafsson JA, Carlquist M. The three-dimensional structure of the liver X receptor beta reveals a flexible ligand-binding pocket that can accommodate fundamentally different ligands. J Biol Chem. 2003;278:38821–38828. doi: 10.1074/jbc.M304842200. [DOI] [PubMed] [Google Scholar]
- Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, Gautier EL, Rubinstein D, Hong C, Liu J, Wu C, van Rooijen N, Bhardwaj N, Garabedian M, Tontonoz P, Fisher EA. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler MB. Liver X Receptor: Crosstalk Node for the Signaling of Lipid Metabolism, Carbohydrate Metabolism, and Innate Immunity. Curr Signal Transduct Ther. 2008;3:75–81. doi: 10.2174/157436208784223170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler MB. The Intracellular Cholesterol Landscape: Dynamic Integrator of the Immune Response. Trends Immunol. 2016;37:819–830. doi: 10.1016/j.it.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J Immunol. 2011;187:1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine C, Rigamonti E, Nohara A, Gervois P, Teissier E, Fruchart JC, Staels B, Chinetti-Gbaguidi G. Liver X receptor activation potentiates the lipopolysaccharide response in human macrophages. Circ Res. 2007;101:40–49. doi: 10.1161/CIRCRESAHA.106.135814. [DOI] [PubMed] [Google Scholar]
- Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, Collins JL, Willson TM, Mangelsdorf DJ, Elias PM, Feingold KR. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- Gadde S, Even-Or O, Kamaly N, Hasija A, Gagnon PG, Adusumilli KH, Erakovic A, Pal AK, Zhang XQ, Kolishetti N, Shi J, Fisher EA, Farokhzad OC. Development of therapeutic polymeric nanoparticles for the resolution of inflammation. Adv Healthc Mater. 2014;3:1448–1456. doi: 10.1002/adhm.201300688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyeregger R, Shehata M, Zeyda M, Kiefer FW, Stuhlmeier KM, Porpaczy E, Zlabinger GJ, Jager U, Stulnig TM. Liver X receptors interfere with cytokine-induced proliferation and cell survival in normal and leukemic lymphocytes. J Leukoc Biol. 2009;86:1039–1048. doi: 10.1189/jlb.1008663. [DOI] [PubMed] [Google Scholar]
- Geyeregger R, Zeyda M, Bauer W, Kriehuber E, Saemann MD, Zlabinger GJ, Maurer D, Stulnig TM. Liver X receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood. 2007;109:4288–4295. doi: 10.1182/blood-2006-08-043422. [DOI] [PubMed] [Google Scholar]
- Ghisletti S, Huang W, Jepsen K, Benner C, Hardiman G, Rosenfeld MG, Glass CK. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23:681–693. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold ES, Diercks AH, Podolsky I, Podyminogin RL, Askovich PS, Treuting PM, Aderem A. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc Natl Acad Sci U S A. 2014;111:10666–10671. doi: 10.1073/pnas.1404271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffett K, Solt LA, El-Gendy Bel D, Kamenecka TM, Burris TP. A liver-selective LXR inverse agonist that suppresses hepatic steatosis. ACS Chem Biol. 2013;8:559–567. doi: 10.1021/cb300541g. [DOI] [PubMed] [Google Scholar]
- Guillemot-Legris O, Mutemberezi V, Muccioli GG. Oxysterols in Metabolic Syndrome: From Bystander Molecules to Bioactive Lipids. Trends Mol Med. 2016;22:594–614. doi: 10.1016/j.molmed.2016.05.006. [DOI] [PubMed] [Google Scholar]
- He Q, Pu J, Yuan A, Lau WB, Gao E, Koch WJ, Ma XL, He B. Activation of liver-X-receptor alpha but not liver-X-receptor beta protects against myocardial ischemia/reperfusion injury. Circ Heart Fail. 2014;7:1032–1041. doi: 10.1161/CIRCHEARTFAILURE.114.001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Bradley MN, Rong X, Wang X, Wagner A, Grijalva V, Castellani LW, Salazar J, Realegeno S, Boyadjian R, Fogelman AM, Van Lenten BJ, Reddy ST, Lusis AJ, Tangirala RK, Tontonoz P. LXRalpha is uniquely required for maximal reverse cholesterol transport and atheroprotection in ApoE-deficient mice. J Lipid Res. 2012;53:1126–1133. doi: 10.1194/jlr.M022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Duit S, Jalonen P, Out R, Scheer L, Sorrentino V, Boyadjian R, Rodenburg KW, Foley E, Korhonen L, Lindholm D, Nimpf J, van Berkel TJ, Tontonoz P, Zelcer N. The E3 ubiquitin ligase IDOL induces the degradation of the low density lipoprotein receptor family members VLDLR and ApoER2. J Biol Chem. 2010;285:19720–19726. doi: 10.1074/jbc.M110.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Kidani Y, NAG, Phung T, Ito A, Rong X, Ericson K, Mikkola H, Beaven SW, Miller LS, Shao WH, Cohen PL, Castrillo A, Tontonoz P, Bensinger SJ. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J Clin Invest. 2012;122:337–347. doi: 10.1172/JCI58393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Marshall SM, McDaniel AL, Graham M, Layne JD, Cai L, Scotti E, Boyadjian R, Kim J, Chamberlain BT, Tangirala RK, Jung ME, Fong L, Lee R, Young SG, Temel RE, Tontonoz P. The LXR-Idol axis differentially regulates plasma LDL levels in primates and mice. Cell Metab. 2014;20:910–918. doi: 10.1016/j.cmet.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014;13:433–444. doi: 10.1038/nrd4280. [DOI] [PubMed] [Google Scholar]
- Hu B, Bernotas R, Unwalla R, Collini M, Quinet E, Feingold I, Goos-Nilsson A, Wilhelmsson A, Nambi P, Evans M, Wrobel J. Quinoline-3-carboxamide containing sulfones as liver X receptor (LXR) agonists with binding selectivity for LXRbeta and low blood-brain penetration. Bioorg Med Chem Lett. 2010;20:689–693. doi: 10.1016/j.bmcl.2009.11.062. [DOI] [PubMed] [Google Scholar]
- Hu B, Quinet E, Unwalla R, Collini M, Jetter J, Dooley R, Andraka D, Nogle L, Savio D, Halpern A, Goos-Nilsson A, Wilhelmsson A, Nambi P, Wrobel J. Carboxylic acid based quinolines as liver X receptor modulators that have LXRbeta receptor binding selectivity. Bioorg Med Chem Lett. 2008;18:54–59. doi: 10.1016/j.bmcl.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Hu B, Unwalla R, Collini M, Quinet E, Feingold I, Goos-Nilsson A, Wihelmsson A, Nambi P, Wrobel J. Discovery and SAR of cinnolines/quinolines as liver X receptor (LXR) agonists with binding selectivity for LXRbeta. Bioorg Med Chem. 2009;17:3519–3527. doi: 10.1016/j.bmc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Hu B, Unwalla RJ, Goljer I, Jetter JW, Quinet EM, Berrodin TJ, Basso MD, Feingold IB, Nilsson AG, Wilhelmsson A, Evans MJ, Wrobel JE. Identification of phenylsulfone-substituted quinoxaline (WYE-672) as a tissue selective liver X-receptor (LXR) agonist. J Med Chem. 2010;53:3296–3304. doi: 10.1021/jm100034x. [DOI] [PubMed] [Google Scholar]
- Hu X, Wang Y, Hao LY, Liu X, Lesch CA, Sanchez BM, Wendling JM, Morgan RW, Aicher TD, Carter LL, Toogood PL, Glick GD. Sterol metabolism controls T(H)17 differentiation by generating endogenous RORgamma agonists. Nat Chem Biol. 2015;11:141–147. doi: 10.1038/nchembio.1714. [DOI] [PubMed] [Google Scholar]
- Huang Y, Fu X, Lyu X, Xu Z, He Z, Zhang Y, Zeng Y, He F, Huang G. Activation of LXR attenuates collagen-induced arthritis via suppressing BLyS production. Clin Immunol. 2015;161:339–347. doi: 10.1016/j.clim.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Huen AO, Kim EJ. The Role of Systemic Retinoids in the Treatment of Cutaneous T-Cell Lymphoma. Dermatol Clin. 2015;33:715–729. doi: 10.1016/j.det.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Varin A, Filomenko R, Lopez T, Athias A, Gambert P, Blache D, Thomas C, Gautier T, Lagrost L, Masson D. Liver x receptor regulates arachidonic acid distribution and eicosanoid release in human macrophages: a key role for lysophosphatidylcholine acyltransferase 3. Arterioscler Thromb Vasc Biol. 2013;33:1171–1179. doi: 10.1161/ATVBAHA.112.300812. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Yuhanna IS, Umetani J, Lee WR, Korach KS, Shaul PW, Umetani M. LXRbeta/estrogen receptor-alpha signaling in lipid rafts preserves endothelial integrity. J Clin Invest. 2013;123:3488–3497. doi: 10.1172/JCI66533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Hong C, Rong X, Zhu X, Tarling EJ, Hedde PN, Gratton E, Parks J, Tontonoz P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife. 2015;4:e08009. doi: 10.7554/eLife.08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson T, Treuter E, Gustafsson JA, Steffensen KR. Liver X receptor biology and pharmacology: new pathways, challenges and opportunities. Trends Pharmacol Sci. 2012;33:394–404. doi: 10.1016/j.tips.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Jakobsson T, Vedin LL, Hassan T, Venteclef N, Greco D, D’Amato M, Treuter E, Gustafsson JA, Steffensen KR. The oxysterol receptor LXRbeta protects against DSS- and TNBS-induced colitis in mice. Mucosal Immunol. 2014;7:1416–1428. doi: 10.1038/mi.2014.31. [DOI] [PubMed] [Google Scholar]
- Jakobsson T, Venteclef N, Toresson G, Damdimopoulos AE, Ehrlund A, Lou X, Sanyal S, Steffensen KR, Gustafsson JA, Treuter E. GPS2 is required for cholesterol efflux by triggering histone demethylation, LXR recruitment, and coregulator assembly at the ABCG1 locus. Mol Cell. 2009;34:510–518. doi: 10.1016/j.molcel.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O’Connell RM, Cheng G, Saez E, Miller JF, Tontonoz P. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, Tran J, Tippin TK, Wang X, Lusis AJ, Hsueh WA, Law RE, Collins JL, Willson TM, Tontonoz P. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko E, Matsuda M, Yamada Y, Tachibana Y, Shimomura I, Makishima M. Induction of intestinal ATP-binding cassette transporters by a phytosterol-derived liver X receptor agonist. J Biol Chem. 2003;278:36091–36098. doi: 10.1074/jbc.M304153200. [DOI] [PubMed] [Google Scholar]
- Kappus MS, Murphy AJ, Abramowicz S, Ntonga V, Welch CL, Tall AR, Westerterp M. Activation of liver X receptor decreases atherosclerosis in Ldlr(-)/(-) mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells. Arterioscler Thromb Vasc Biol. 2014;34:279–284. doi: 10.1161/ATVBAHA.113.302781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A, Udata C, Ott E, Hickey L, Burczynski ME, Burghart P, Vesterqvist O, Meng X. Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X-receptor agonist, in healthy participants. J Clin Pharmacol. 2009;49:643–649. doi: 10.1177/0091270009335768. [DOI] [PubMed] [Google Scholar]
- Kick EK, Busch BB, Martin R, Stevens WC, Bollu V, Xie Y, Boren BC, Nyman MC, Nanao MH, Nguyen L, Plonowski A, Schulman IG, Yan G, Zhang H, Hou X, Valente MN, Narayanan R, Behnia K, Rodrigues AD, Brock B, Smalley J, Cantor GH, Lupisella J, Sleph P, Grimm D, Ostrowski J, Wexler RR, Kirchgessner T, Mohan R. Discovery of Highly Potent Liver X Receptor beta Agonists. ACS Med Chem Lett. 2016;7:1207–1212. doi: 10.1021/acsmedchemlett.6b00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GH, Oh GS, Yoon J, Lee GG, Lee KU, Kim SW. Hepatic TRAP80 selectively regulates lipogenic activity of liver X receptor. J Clin Invest. 2015;125:183–193. doi: 10.1172/JCI73615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner TG, Martin R, Sleph P, Grimm D, Liu X, Lupisella J, Smalley J, Narayanan R, Xie Y, Ostrowski J, Cantor GH, Mohan R, Kick E. Pharmacological characterization of a novel liver X receptor agonist with partial LXRalpha activity and a favorable window in nonhuman primates. J Pharmacol Exp Ther. 2015;352:305–314. doi: 10.1124/jpet.114.219923. [DOI] [PubMed] [Google Scholar]
- Kirchgessner TG, Sleph P, Ostrowski J, Lupisella J, Ryan CS, Liu X, Fernando G, Grimm D, Shipkova P, Zhang R, Garcia R, Zhu J, He A, Malone H, Martin R, Behnia K, Wang Z, Barrett YC, Garmise RJ, Yuan L, Zhang J, Gandhi MD, Wastall P, Li T, Du S, Salvador L, Mohan R, Cantor GH, Kick E, Lee J, Frost RJ. Beneficial and Adverse Effects of an LXR Agonist on Human Lipid and Lipoprotein Metabolism and Circulating Neutrophils. Cell Metab. 2016;24:223–233. doi: 10.1016/j.cmet.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Kiss E, Popovic Z, Bedke J, Wang S, Bonrouhi M, Gretz N, Stettner P, Teupser D, Thiery J, Porubsky S, Adams J, Grone HJ. Suppression of chronic damage in renal allografts by Liver X receptor (LXR) activation relevant contribution of macrophage LXRalpha. Am J Pathol. 2011;179:92–103. doi: 10.1016/j.ajpath.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komati R, Spadoni D, Zheng S, Sridhar J, Riley KE, Wang G. Ligands of Therapeutic Utility for the Liver X Receptors. Molecules. 2017;22 doi: 10.3390/molecules22010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korach-Andre M, Gustafsson JA. Liver X receptors as regulators of metabolism. Biomol Concepts. 2015;6:177–190. doi: 10.1515/bmc-2015-0007. [DOI] [PubMed] [Google Scholar]
- Lalloyer F, Pedersen TA, Gross B, Lestavel S, Yous S, Vallez E, Gustafsson JA, Mandrup S, Fievet C, Staels B, Tailleux A. Rexinoid bexarotene modulates triglyceride but not cholesterol metabolism via gene-specific permissivity of the RXR/LXR heterodimer in the liver. Arterioscler Thromb Vasc Biol. 2009;29:1488–1495. doi: 10.1161/ATVBAHA.109.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappano R, Recchia AG, De Francesco EM, Angelone T, Cerra MC, Picard D, Maggiolini M. The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor alpha-mediated signaling in cancer cells and in cardiomyocytes. PLoS One. 2011;6:e16631. doi: 10.1371/journal.pone.0016631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Park SM, Kim OS, Lee CS, Woo JH, Park SJ, Joe EH, Jou I. Differential SUMOylation of LXRalpha and LXRbeta mediates transrepression of STAT1 inflammatory signaling in IFN-gamma-stimulated brain astrocytes. Mol Cell. 2009;35:806–817. doi: 10.1016/j.molcel.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Levin N, Bischoff ED, Daige CL, Thomas D, Vu CT, Heyman RA, Tangirala RK, Schulman IG. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol. 2005;25:135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- Li P, Spann NJ, Kaikkonen MU, Lu M, Oh DY, Fox JN, Bandyopadhyay G, Talukdar S, Xu J, Lagakos WS, Patsouris D, Armando A, Quehenberger O, Dennis EA, Watkins SM, Auwerx J, Glass CK, Olefsky JM. NCoR repression of LXRs restricts macrophage biosynthesis of insulin-sensitizing omega 3 fatty acids. Cell. 2013;155:200–214. doi: 10.1016/j.cell.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RK, Yu S, Cheng B, Li S, Kim NJ, Cao Y, Chi V, Kim JY, Chatterjee AK, Schultz PG, Tremblay MS, Kazane SA. Targeted Delivery of LXR Agonist Using a Site-Specific Antibody-Drug Conjugate. Bioconjug Chem. 2015;26:2216–2222. doi: 10.1021/acs.bioconjchem.5b00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Vedin LL, Steffensen KR. The emerging roles of liver X receptors and their ligands in cancer. Expert Opin Ther Targets. 2016;20:61–71. doi: 10.1517/14728222.2015.1081169. [DOI] [PubMed] [Google Scholar]
- Lo Sasso G, Murzilli S, Salvatore L, D’Errico I, Petruzzelli M, Conca P, Jiang ZY, Calabresi L, Parini P, Moschetta A. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010;12:187–193. doi: 10.1016/j.cmet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Ma Y, Xu L, Rodriguez-Agudo D, Li X, Heuman DM, Hylemon PB, Pandak WM, Ren S. 25-Hydroxycholesterol-3-sulfate regulates macrophage lipid metabolism via the LXR/SREBP-1 signaling pathway. Am J Physiol Endocrinol Metab. 2008;295:E1369–1379. doi: 10.1152/ajpendo.90555.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Deng C, Hu W, Zhou J, Fan C, Di S, Liu D, Yang Y, Wang D. Liver X Receptors and their Agonists: Targeting for Cholesterol Homeostasis and Cardiovascular Diseases. Curr Issues Mol Biol. 2017;22:41–64. doi: 10.21775/cimb.022.041. [DOI] [PubMed] [Google Scholar]
- Morales JR, Ballesteros I, Deniz JM, Hurtado O, Vivancos J, Nombela F, Lizasoain I, Castrillo A, Moro MA. Activation of liver X receptors promotes neuroprotection and reduces brain inflammation in experimental stroke. Circulation. 2008;118:1450–1459. doi: 10.1161/CIRCULATIONAHA.108.782300. [DOI] [PubMed] [Google Scholar]
- Mutemberezi V, Guillemot-Legris O, Muccioli GG. Oxysterols: From cholesterol metabolites to key mediators. Prog Lipid Res. 2016;64:152–169. doi: 10.1016/j.plipres.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MP, Billheimer JT, Rothblat GH, Rader DJ. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113:90–97. doi: 10.1161/CIRCULATIONAHA.105.560177. [DOI] [PubMed] [Google Scholar]
- Park MC, Kwon YJ, Chung SJ, Park YB, Lee SK. Liver X receptor agonist prevents the evolution of collagen-induced arthritis in mice. Rheumatology (Oxford) 2010;49:882–890. doi: 10.1093/rheumatology/keq007. [DOI] [PubMed] [Google Scholar]
- Patel R, Magomedova L, Tsai R, Angers S, Orellana A, Cummins CL. Separating the Anti-Inflammatory and Diabetogenic Effects of Glucocorticoids Through LXRbeta Antagonism. Endocrinology. 2017;158:1034–1047. doi: 10.1210/en.2017-00094. [DOI] [PubMed] [Google Scholar]
- Patel R, Patel M, Tsai R, Lin V, Bookout AL, Zhang Y, Magomedova L, Li T, Chan JF, Budd C, Mangelsdorf DJ, Cummins CL. LXRbeta is required for glucocorticoid-induced hyperglycemia and hepatosteatosis in mice. J Clin Invest. 2011;121:431–441. doi: 10.1172/JCI41681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterniti I, Campolo M, Siracusa R, Cordaro M, Di Paola R, Calabrese V, Navarra M, Cuzzocrea S, Esposito E. Liver X receptors activation, through TO901317 binding, reduces neuroinflammation in Parkinson’s disease. PLoS One. 2017;12:e0174470. doi: 10.1371/journal.pone.0174470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterniti I, Genovese T, Mazzon E, Crisafulli C, Di Paola R, Galuppo M, Bramanti P, Cuzzocrea S. Liver X receptor agonist treatment regulates inflammatory response after spinal cord trauma. J Neurochem. 2010;112:611–624. doi: 10.1111/j.1471-4159.2009.06471.x. [DOI] [PubMed] [Google Scholar]
- Peet DJ, Janowski BA, Mangelsdorf DJ. The LXRs: a new class of oxysterol receptors. Curr Opin Genet Dev. 1998;8:571–575. doi: 10.1016/s0959-437x(98)80013-0. [DOI] [PubMed] [Google Scholar]
- Pehkonen P, Welter-Stahl L, Diwo J, Ryynanen J, Wienecke-Baldacchino A, Heikkinen S, Treuter E, Steffensen KR, Carlberg C. Genome-wide landscape of liver X receptor chromatin binding and gene regulation in human macrophages. BMC Genomics. 2012;13:50. doi: 10.1186/1471-2164-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D, Hiipakka RA, Dai Q, Guo J, Reardon CA, Getz GS, Liao S. Antiatherosclerotic effects of a novel synthetic tissue-selective steroidal liver X receptor agonist in low-density lipoprotein receptor-deficient mice. J Pharmacol Exp Ther. 2008;327:332–342. doi: 10.1124/jpet.108.142687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D, Hiipakka RA, Xie JT, Dai Q, Kokontis JM, Reardon CA, Getz GS, Liao S. A novel potent synthetic steroidal liver X receptor agonist lowers plasma cholesterol and triglycerides and reduces atherosclerosis in LDLR(−/−) mice. Br J Pharmacol. 2011;162:1792–1804. doi: 10.1111/j.1476-5381.2011.01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer T, Buchebner M, Chandak PG, Patankar J, Kratzer A, Obrowsky S, Rechberger GN, Kadam RS, Kompella UB, Kostner GM, Kratky D, Levak-Frank S. Synthetic LXR agonist suppresses endogenous cholesterol biosynthesis and efficiently lowers plasma cholesterol. Curr Pharm Biotechnol. 2011;12:285–292. doi: 10.2174/138920111794295774. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Pourcet B, Feig JE, Vengrenyuk Y, Hobbs AJ, Kepka-Lenhart D, Garabedian MJ, Morris SM, Jr, Fisher EA, Pineda-Torra I. LXRalpha regulates macrophage arginase 1 through PU.1 and interferon regulatory factor 8. Circ Res. 2011;109:492–501. doi: 10.1161/CIRCRESAHA.111.241810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinet EM, Basso MD, Halpern AR, Yates DW, Steffan RJ, Clerin V, Resmini C, Keith JC, Berrodin TJ, Feingold I, Zhong W, Hartman HB, Evans MJ, Gardell SJ, DiBlasio-Smith E, Mounts WM, LaVallie ER, Wrobel J, Nambi P, Vlasuk GP. LXR ligand lowers LDL cholesterol in primates, is lipid neutral in hamster, and reduces atherosclerosis in mouse. J Lipid Res. 2009;50:2358–2370. doi: 10.1194/jlr.M900037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raccosta L, Fontana R, Maggioni D, Lanterna C, Villablanca EJ, Paniccia A, Musumeci A, Chiricozzi E, Trincavelli ML, Daniele S, Martini C, Gustafsson JA, Doglioni C, Feo SG, Leiva A, Ciampa MG, Mauri L, Sensi C, Prinetti A, Eberini I, Mora JR, Bordignon C, Steffensen KR, Sonnino S, Sozzani S, Traversari C, Russo V. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013;210:1711–1728. doi: 10.1084/jem.20130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani A, Dubrovsky L, Pushkarsky T, Sviridov D, Karandish S, Raj DS, Fitzgerald ML, Bukrinsky M. Stimulation of Liver X Receptor Has Potent Anti-HIV Effects in a Humanized Mouse Model of HIV Infection. J Pharmacol Exp Ther. 2015;354:376–383. doi: 10.1124/jpet.115.224485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Dang EV, McDonald JG, Liang G, Russell DW, Cyster JG. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 2014;345:679–684. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong X, Albert CJ, Hong C, Duerr MA, Chamberlain BT, Tarling EJ, Ito A, Gao J, Wang B, Edwards PA, Jung ME, Ford DA, Tontonoz P. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 2013;18:685–697. doi: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chen Q, Yan H, Gu W. The effect of a liver-X-receptor ligand on bleomycin induced pulmonary fibrosis in mice. Int Immunopharmacol. 2016;41:116–121. doi: 10.1016/j.intimp.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Shi Y, Xu X, Tan Y, Mao S, Fang S, Gu W. A liver-X-receptor ligand, T0901317, attenuates IgE production and airway remodeling in chronic asthma model of mice. PLoS One. 2014;9:e92668. doi: 10.1371/journal.pone.0092668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi L, Mitro N, Cimino M, Gelosa P, Guerrini U, Tremoli E, Saez E. Treatment with LXR agonists after focal cerebral ischemia prevents brain damage. FEBS Lett. 2008;582:3396–3400. doi: 10.1016/j.febslet.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smet M, Van Hoecke L, De Beuckelaer A, Vander Beken S, Naessens T, Vergote K, Willart M, Lambrecht BN, Gustafsson JA, Steffensen KR, Grooten J. Cholesterol-sensing liver X receptors stimulate Th2-driven allergic eosinophilic asthma in mice. Immun Inflamm Dis. 2016;4:350–361. doi: 10.1002/iid3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoak K, Madenspacher J, Jeyaseelan S, Williams B, Dixon D, Poch KR, Nick JA, Worthen GS, Fessler MB. Effects of liver X receptor agonist treatment on pulmonary inflammation and host defense. J Immunol. 2008;180:3305–3312. doi: 10.4049/jimmunol.180.5.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroosh P, Wu J, Xue X, Song J, Sutton SW, Sablad M, Yu J, Nelen MI, Liu X, Castro G, Luna R, Crawford S, Banie H, Dandridge RA, Deng X, Bittner A, Kuei C, Tootoonchi M, Rozenkrants N, Herman K, Gao J, Yang XV, Sachen K, Ngo K, Fung-Leung WP, Nguyen S, de Leon-Tabaldo A, Blevitt J, Zhang Y, Cummings MD, Rao T, Mani NS, Liu C, McKinnon M, Milla ME, Fourie AM, Sun S. Oxysterols are agonist ligands of RORgammat and drive Th17 cell differentiation. Proc Natl Acad Sci U S A. 2014;111:12163–12168. doi: 10.1073/pnas.1322807111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, Raetz CR, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AH, Jr, Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, Glass CK. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen KR, Jakobsson T, Gustafsson JA. Targeting liver X receptors in inflammation. Expert Opin Ther Targets. 2013;17:977–990. doi: 10.1517/14728222.2013.806490. [DOI] [PubMed] [Google Scholar]
- Swahn BM, Macsari I, Viklund J, Ohberg L, Sjodin J, Neelissen J, Lindquist J. Liver X receptor agonists with selectivity for LXRbeta; N-aryl-3,3,3-trifluoro-2-hydroxy-2-methylpropionamides. Bioorg Med Chem Lett. 2009;19:2009–2012. doi: 10.1016/j.bmcl.2009.02.039. [DOI] [PubMed] [Google Scholar]
- Tachibana H, Ogawa D, Matsushita Y, Bruemmer D, Wada J, Teshigawara S, Eguchi J, Sato-Horiguchi C, Uchida HA, Shikata K, Makino H. Activation of liver X receptor inhibits osteopontin and ameliorates diabetic nephropathy. J Am Soc Nephrol. 2012;23:1835–1846. doi: 10.1681/ASN.2012010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, Wang X, Lusis AJ, Tontonoz P, Schulman IG. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel RE, Brown JM. A new framework for reverse cholesterol transport: non-biliary contributions to reverse cholesterol transport. World J Gastroenterol. 2010;16:5946–5952. doi: 10.3748/wjg.v16.i47.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel RE, Brown JM. A new model of reverse cholesterol transport: enTICEing strategies to stimulate intestinal cholesterol excretion. Trends Pharmacol Sci. 2015;36:440–451. doi: 10.1016/j.tips.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel RE, Sawyer JK, Yu L, Lord C, Degirolamo C, McDaniel A, Marshall S, Wang N, Shah R, Rudel LL, Brown JM. Biliary sterol secretion is not required for macrophage reverse cholesterol transport. Cell Metab. 2010;12:96–102. doi: 10.1016/j.cmet.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwel D, Steffensen KR, Verghese PB, Kummer MP, Gustafsson JA, Holtzman DM, Heneka MT. Critical role of astroglial apolipoprotein E and liver X receptor-alpha expression for microglial Abeta phagocytosis. J Neurosci. 2011;31:7049–7059. doi: 10.1523/JNEUROSCI.6546-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torocsik D, Barath M, Benko S, Szeles L, Dezso B, Poliska S, Hegyi Z, Homolya L, Szatmari I, Lanyi A, Nagy L. Activation of liver X receptor sensitizes human dendritic cells to inflammatory stimuli. J Immunol. 2010;184:5456–5465. doi: 10.4049/jimmunol.0902399. [DOI] [PubMed] [Google Scholar]
- van der Veen JN, van Dijk TH, Vrins CL, van Meer H, Havinga R, Bijsterveld K, Tietge UJ, Groen AK, Kuipers F. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J Biol Chem. 2009;284:19211–19219. doi: 10.1074/jbc.M109.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venteclef N, Jakobsson T, Ehrlund A, Damdimopoulos A, Mikkonen L, Ellis E, Nilsson LM, Parini P, Janne OA, Gustafsson JA, Steffensen KR, Treuter E. GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response. Genes Dev. 2010;24:381–395. doi: 10.1101/gad.545110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, Sanvito F, Ponzoni M, Valentinis B, Bregni M, Prinetti A, Steffensen KR, Sonnino S, Gustafsson JA, Doglioni C, Bordignon C, Traversari C, Russo V. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16:98–105. doi: 10.1038/nm.2074. [DOI] [PubMed] [Google Scholar]
- Vizuete J, Camero A, Malakouti M, Garapati K, Gutierrez J. Perspectives on Nonalcoholic Fatty Liver Disease: An Overview of Present and Future Therapies. J Clin Transl Hepatol. 2017;5:67–75. doi: 10.14218/JCTH.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcher D, Kummel A, Kehrle B, Bach H, Grub M, Durst R, Hombach V, Marx N. LXR activation reduces proinflammatory cytokine expression in human CD4-positive lymphocytes. Arterioscler Thromb Vasc Biol. 2006;26:1022–1028. doi: 10.1161/01.ATV.0000210278.67076.8f. [DOI] [PubMed] [Google Scholar]
- Walcher D, Vasic D, Heinz P, Bach H, Durst R, Hausauer A, Hombach V, Marx N. LXR activation inhibits chemokine-induced CD4-positive lymphocyte migration. Basic Res Cardiol. 2010;105:487–494. doi: 10.1007/s00395-010-0092-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rogers PM, Su C, Varga G, Stayrook KR, Burris TP. Regulation of cholesterologenesis by the oxysterol receptor, LXRalpha. J Biol Chem. 2008;283:26332–26339. doi: 10.1074/jbc.M804808200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Chen CC, Lai CY, Hung TH, Lin CC, Chao M, Chen SF. Treatment with TO901317, a synthetic liver X receptor agonist, reduces brain damage and attenuates neuroinflammation in experimental intracerebral hemorrhage. J Neuroinflammation. 2016;13:62. doi: 10.1186/s12974-016-0524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zheng S, Qiu Y, Yang Y, Wang C, Yang P, Li Q, Lei B. Activation of liver X receptor alleviates ocular inflammation in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2014;55:2795–2804. doi: 10.1167/iovs.13-13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Grillot D, Billheimer JT, Briand F, Delerive P, Huet S, Rader DJ. Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler Thromb Vasc Biol. 2010;30:781–786. doi: 10.1161/ATVBAHA.109.195693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Li S, Henke A, Muse ED, Cheng B, Welzel G, Chatterjee AK, Wang D, Roland J, Glass CK, Tremblay M. Dissociated sterol-based liver X receptor agonists as therapeutics for chronic inflammatory diseases. FASEB J. 2016;30:2570–2579. doi: 10.1096/fj.201600244R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 2015;6:254–264. doi: 10.1007/s13238-014-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Even-Or O, Xu X, van Rosmalen M, Lim L, Gadde S, Farokhzad OC, Fisher EA. Nanoparticles containing a liver X receptor agonist inhibit inflammation and atherosclerosis. Adv Healthc Mater. 2015;4:228–236. doi: 10.1002/adhm.201400337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Breevoort SR, Angdisen J, Fu M, Schmidt DR, Holmstrom SR, Kliewer SA, Mangelsdorf DJ, Schulman IG. Liver LXRalpha expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J Clin Invest. 2012;122:1688–1699. doi: 10.1172/JCI59817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Zhuang L, Fan KY, Tice CM, Zhao W, Dong C, Lotesta SD, Leftheris K, Lindblom PR, Liu Z, Shimada J, Noto PB, Meng S, Hardy A, Howard L, Krosky P, Guo J, Lipinski K, Kandpal G, Bukhtiyarov Y, Zhao Y, Lala D, Van Orden R, Zhou J, Chen G, Wu Z, McKeever BM, McGeehan GM, Gregg RE, Claremon DA, Singh SB. Discovery of a Novel, Orally Efficacious Liver X Receptor (LXR) beta Agonist. J Med Chem. 2016;59:3264–3271. doi: 10.1021/acs.jmedchem.5b02029. [DOI] [PubMed] [Google Scholar]