Abstract
In animal societies, behavioral idiosyncrasies of the individuals often guide which tasks they should perform. Such personality-specific task participation can increase individual task efficiency, thereby improving group performance. While several recent studies have documented group-level benefits of within-group behavioral (i.e., personality) diversity, how these benefits are realized at the individual level is unclear. Here we probe the individual-level benefits of personality-driven task participation in the social spider Stegodyphus dumicola. In S. dumicola, the presence of at least one highly bold individual catalyzes foraging behavior in shy colony members, and all group constituents heavily compete for prey. We assessed boldness by examining how quickly spiders resumed normal movement after a simulated predator attack. We test here whether (1) participants in collective foraging gain more mass from prey items and (2) whether bold individuals are less resistant to starvation than shy spiders, which would motivate the bold individuals to forage more. Next, we assembled colonies of shy spiders with and without a bold individual, added one prey item, and then tracked the mass gain of each individual spider after this single feeding event. We found that spiders that participated in prey capture (whether bold or shy) gained more mass than nonparticipators, and colonies containing a single bold spider gained more total mass than purely shy colonies. We also found that bold spiders participated in more collective foraging events and were more susceptible to starvation than shy spiders, suggesting that the aggressive foraging of bold individuals may represent a strategy to offset starvation risk. These findings add to the body of evidence that animal personality can shape social organization, individual performance, and group success.
Keywords: animal personality, keystone individuals, social behaviour, task allocation
The fate of social groups often hinges on the mixture of behavioral phenotypes present (Modlmeier and Foitzik 2011; Pinter-Wollman 2012a; Pruitt 2012; Pruitt and Goodnight 2014). The salience of a group’s behavioral composition for group performance stems from a close relationship between behavioral variation and participation in tasks central to group persistence (Modlmeier et al. 2012; Holbrook et al. 2014; Wright et al. 2014, 2015). Here we will refer to consistent individual differences in behavior as animal “personality” (Gosling 2001). In many societies, bold and aggressive individuals specialize on cooperative foraging (Aplin et al. 2014; Wright et al. 2014), or risky anti-predator behaviors (Wright et al. 2014, 2015), whereas shy and docile individuals often specialize on brood care (Kurvers et al. 2009; Wright et al. 2014). These are cases of personality-specific task participation, which refers to any case where individuals of certain personality types spend more time performing a particular task and perform that task more effectively (Wright et al. 2014). However, this relationship is not always straightforward. For instance, in some social insect species, less active and nonaggressive individuals perform seemingly no tasks, whereas aggressive and active individuals perform numerous tasks (Jandt et al. 2012, 2014; Charbonneau et al. 2015). These seemingly maladaptive personality-specific task participation arrangements highlight the importance of probing the fitness consequences of both personality traits and task participation.
In non-eusocial groups, selection can act at both the individual and group/kin levels, and may select for adaptive group compositions (West et al. 2007; Pruitt and Goodnight 2014). Although personality-based task differentiation is known to improve group performance in both ant and social spider colonies (Modlmeier and Foitzik 2011; Wright et al. 2014), the degree to which this personality-driven task participation benefits participants at the individual level remains unclear. For instance, do bold individuals that participate in collective foraging benefit from engaging in this task? If participation in social foraging and brood care (or whatever other task) fails to benefit participants while still benefitting the rest of the group, then these personality specific-specializations may generate conflict between the direct reproductive interests of task participants and their group mates (Giraldeau and Caraco 2000).
Because boldness is defined as the propensity to forage in the presence of danger (Huntingford 1976), bold individuals often exhibit high levels of activity and successful foraging strategies (Sih et al. 2004; Ward et al. 2004; Smith and Blumstein 2008). For instance, boldness gives individuals an advantage in the outcome of contests over food between ninespine Pungitius pungitius and three-spined Gasterosteus aculeatus sticklebacks (Webster et al. 2009). At the same time, existing theoretical (Biro and Stamps 2008; Careau et al. 2008, Stamps 2007) and empirical evidence (Kühbandner et al. 2014; Shearer and Pruitt 2014) indicates that bold individuals have higher metabolic rates than shy individuals. Specifically, bold individuals are less resistant to starvation (Lichtenstein and Pruitt 2015) and exhibit higher heart rates during stressful encounters (Shearer and Pruitt 2014). These findings in tandem with the well-studied link between boldness, activity level, and foraging success (Werner and Anholt 1993; Short and Petren 2008; Smith and Blumstein 2008) hint that bolder individuals might need to consume more food to maintain their active foraging strategies (Stamps 2007; Biro and Stamps 2008). Thus, we argue that bolder animals within societies may initiate foraging in social situations, because they need more food, potentially providing the rest of their group with food as a by-product.
The social spider Stegodyphus dumicola (Araneae, Eresidae) lives in colonies of highly inbred individuals (Bilde et al. 2007b) that exhibit cooperative behaviors such as prey capture and alloparental care (Henschel et al. 1995; Henschel 1998; Avilés et al. 1999; Bilde et al. 2007a). However, colony members compete heavily for prey (Whitehouse and Lubin 1999; Amir et al. 2000). Furthermore, individual variation in boldness impacts the ability of colonies to capture prey because the presence of just one or few bold individuals expedites collective foraging (Pruitt and Keiser 2014) and increases the participation of shy spiders in collective foraging (Keiser and Pruitt 2014), thus increasing the mass gain of the entire colony (Pruitt and Keiser 2014). This is a prime example of a keystone individual, which is defined as any individual that has a disproportionately large effect on the behavior of a group (Modlmeier et al. 2014a). By cooperating, S. dumicola can consume prey items many times their own body size (e.g., large locusts and beetles), and most prey capture events involve multiple individuals (Lichtenstein JL, Wright CM, personal observation). Despite the cooperative nature of prey capture, colony members can compete intensely for access to prey once it is subdued (Amir et al. 2000), and therefore S. dumicola colonies often exhibit skewed resource allocations among colony members (Whitehouse and Lubin 1999). By capturing prey, individual spiders can gain an advantage in consuming more of a prey item. Here we examine whether colony members who participate in collective foraging have an advantage in competition over prey, which could potentially explain why they participate in this energetically demanding task.
Probing the individual-level fitness benefits of being a keystone individual will further clarify the evolutionary significance of S. dumicola’s personality-driven task allocation. Specifically, we attempt to elucidate whether the benefits of biased task participation are gleaned primarily via direct benefits to task participants or benefits to kin and/or the colony, because S. dumicola societies are composed of highly related individuals (Avilés 1997; Lubin and Bilde 2007). We use mass gain as a proxy for fitness, because body mass and reproductive success are tightly linked among individual spiders (Foelix 2010; Kralj-Fišer and Schneider 2012). To probe the direct fitness consequences of boldness, we tested the following hypotheses: (1) Individuals that participate in collective prey capture will gain more mass from prey than nonparticipating individuals and (2) bold spiders will be less resistant to starvation than shy spiders. To address the indirect fitness consequences of boldness, we retested several previous findings: (3) that bold individuals participate in more collective foraging events than shy individuals and (4) that their presence will increase the mass gained by the group as a whole (Pruitt and Keiser 2014).
Materials and Methods
Study species and collection
Stegodyphus dumicola is a social spider from arid regions of Southern Africa (Kraus and Kraus 1988). Like many other social spider species (Riechert and Roeloffs 1993; Agnarsson et al. 2006), S. dumicola exhibit a high degree of inbreeding (Bilde et al. 2007b). This species builds extensive, 3D, chambered retreats and 2D capture webs extending from the retreat (Henschel 1998). These 3D retreats are composed of matted silk, prey carcasses, and plant debris. Colonies may also have multiple retreats. Spiders spend the majority of their time in the retreat, only leaving to capture prey or repair webbing at dusk. Colonies can contain 2–2000 spiders and exhibit highly female-biased sex ratios (Bilde et al. 2007b). We collected S. dumicola colonies along roadsides near Upington, Northern Cape, South Africa in October 2015, the austral spring. Because juveniles of this species perform obligate matriphagy in the late austral autumn and mature only in December (Schneider 2002), the individuals in our study were all sub-adults post matriphagy. Cooperative prey capture success is essential for the growth and development of immature social spiders (Kim et al. 2005). We collected 6 colonies by trimming the supporting host plant and placing the colony in plastic containers. Colonies were transported to our laboratory in Upington, South Africa and dissected and separated from the plant material by hand. All resident spiders were counted and run through a boldness assay (described below). We then created experimental colonies of known personality composition, taking care not to mix individuals from multiple source colonies to preserve natural within-colony relatedness and familiarity (Ruch et al. 2009) which impact colonies’ collective behavior and success (Laskowski and Pruitt 2014, Modlmeier et al. 2014b). When creating colonies, care was taken to create experimental groups of similar developmental stage and body size (±15% of each other’s prosoma width). We dismantled these source colonies entirely (producing 5–14 colonies from each source colony) into 42 experimental colonies each containing 6 spiders of known boldness. Specifically, 21 colonies were comprised of 6 shy individuals and 21 experimental colonies comprised of 5 shy and 1 bold individual, roughly approximating the proportion of bold spiders in wild colonies (Keiser and Pruitt 2014). We housed experimental colonies in 200-mL containers with 3 twigs Acacia mellifera for structural support. We waited 12 h after colony assembly to begin collective foraging assays (described below). This represents sufficient time for the spiders to build a functional retreat and one or more capture webs. Care was taken during foraging assays not to damage spiders” capture webs.
Boldness assays
To assess the boldness of individual spiders, we puffed them with 2 jets of air from an infant ear cleaning bulb (Target home brand) after we placed each spider in a 10-cm diameter circular arena for 30 s to acclimate. Jets of air nearly always produce a huddle response in these spiders, likely because they are intended to simulate the approach of an aerial predator (Hedrick and Riechert 1989; Riechert and Hedrick 1990, 1993). We then measured the latency of each spider to move 1 body length after this aversive stimulus, with bolder spiders exhibiting lower latencies. If spiders did not move after 600 s, they were assigned a latency of 600 s. We subtracted the latency of each spider to move from 600s to obtain a boldness score in which higher values represent greater boldness. We categorized all individuals with boldness scores of 1–200 as “shy,” 201–400 as “moderate,” and 401–600 as “bold” after Pruitt et al. (2013) and Keiser and Pruitt (2014). Distributions of bold, shy, and moderate individuals in S. dumicola colonies are reported in Pinter-Wollman et al. (2016). Responses to this test are highly repeatable in this species (R = 0.63) (Keiser et al. 2014a) relative to repeatabilities seen for other kinds of traits and taxa (Bell et al. 2009). Because of our need to process very large numbers of spiders (>8,000 over the course of this field season), it was not feasible to measure each of these spiders multiple times before assigning them to a behavioral type (shy, moderate, bold). Admittedly, this procedure does increase statistical noise in our datasets; however, this merely means that the results herein should tend to underestimate the observed patterns and their corresponding effects sizes. We used paint pens (Sharpie®) to individually mark each spider with a unique color ID. After the paint dried, we weighed each spider such that the mass of the paint could not confound with any other observed weight changes.
Collective foraging assays and feeding events
Our collective foraging assay is designed to simulate a winged prey item caught in the web (Grinsted et al. 2013; Pruitt et al. 2013). To quantify the collective foraging participation of spiders within the colonies, we placed a dummy prey item (a 1-cm2 piece of paper) on the capture web of each colony to produce a consistent prey cue across trials, which cannot be controlled for when using live prey. After waiting 30 s to allow the spiders to adjust to this minor disturbance to their web, we began vibrating the paper with a handheld vibratory device (GoVibe) using a quickly pulsating setting until a spider investigated the paper by coming into contact with it—typically these individuals seize the paper with their chelicerae. We recorded the color ID of each attacking spider along with 2 aspects of collective aggressiveness: the latency for the 1st spider to reach the paper and the number of attackers that were moving toward the paper at the moment of contact. We performed this test 3 times for each colony over 2 days by spacing assays 12 h apart. Thus, we quantified the collective foraging participation for each spider and summed the instances in which they attacked the paper, yielding a measurement of participation ranging from 0–3 attacks per individual. We deemed colonies that reached the paper more quickly and with more attackers to be more aggressive. If no spider reached the paper after 300 s, then we assigned the colony a latency of 300 s.
To test whether participation in collective prey capture is linked with feeding success, we fed each colony 3 days after colony assembly, and 1 day after the last prey trial, by adding 1 live termite to each colony in the evening. We then reweighed each spider the next morning.
Starvation resistance
Spiders used for our starvation experiment were different from those in the collective foraging experiments described above. To test the resistance of spiders to starvation, we fed 26 bold spiders and 26 shy an ad libitum meal of termite workers while they were in 6-spider colonies containing 3 bold and 3 shy individuals, and then ceased feeding them. This ad libitum feeding approach inundated colonies with prey ensuring that all colony members could feed for 24 h until each spider had abandoned their partially consumed prey carcass without interference from other colony members. We then isolated spiders into 30-mL containers. Each morning, we recorded which all spiders had died until the last spider died. During the course of these starvation trials, we were blind to the personalities of the spiders.
Statistical methods
First, we assessed the relationship between collective foraging participation and individual mass gain by comparing the number of times each individual assisted in prey capture with their change in mass using a normally distributed GLMM with “colony ID” and “source colony ID” as random effects. We used individuals’ change in body mass as our response variable and the number of times they participated in staged prey capture events as our predictor variable. We then used post hoc Tukey tests to assess pairwise mass change differences between spiders that participated 0-3 times. Next, we tested whether bold spiders (putative keystones) participated in more collective foraging trials than shy individuals. We used a GLMM with “colony ID” and “source colony ID” as random effects, individual personality as a predictor variable (shy/bold), and participation in prey capture (0–3) as our response variable. For this analysis, we modeled a zero-inflated negative binomial error distribution. Finally, we used a GLMM to assess whether bold or shy spiders gained more mass within our mixed-colony treatment (5 shy, 1 bold). We modeled this GLMM with a Gaussian error distribution and “colony ID” and “source colony ID” as random effects in our model.
Finally to ascertain whether the presence of bold spiders increased mass gain of all colony members, we compared total colony mass gain between colonies with and without bold individuals, using a normally distributed GLMM with “Source Colony ID” as a random effect. Colony ID was not included as a random effect in this model because we analyzed only 1 combined statistic per experimental colony. We also compared colonies’ average latency to attack and the number of attackers that responded to the simulated prey in colonies with and without bold spiders and the latency of colonies to attack with total colony mass change. Here again we modeled a Gaussian error distribution with “Source colony ID” as a random effect; colony ID was not modeled as a random effect in this model because we used only one average metric per colony for our analysis. The summary of the results for our random effects is detailed in our Supplementary Document 1. We performed all statistics in JMP version 12.0 (SAS).
To probe the starvation resistance of bold and shy spiders, we used a Kaplan–Meier survival analysis (Kaplan and Meier 1958). We used log-rank tests because we anticipated that differences in mortality would emerge later in the experiment and log-rank tests are sensitive to later term mortality differences.
Results
Foraging participation and weight gain
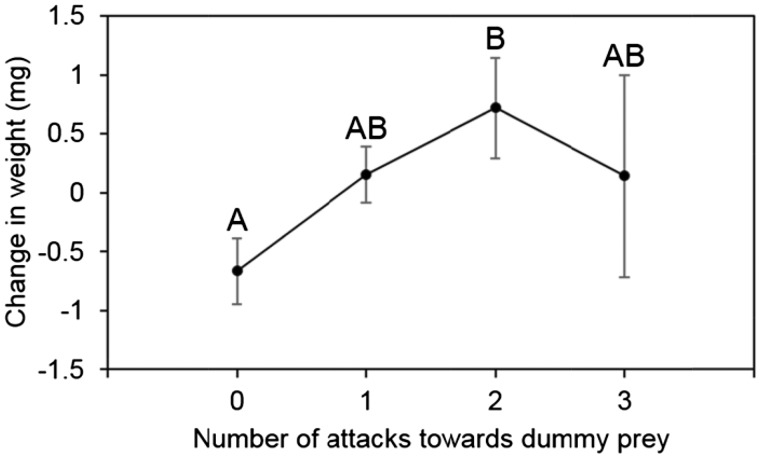
Spiders participated in collective foraging 0–3 times across 3 foraging trials, and although 55% of all spiders participated at least once, fewer individuals engaged in each successive number of prey capture events (n0 = 115, n1 = 93, n2 = 40, n3 = 8). Individuals that participated in more attacks of a dummy prey gained more mass during later interactions with live prey (termites) (GLMM: F1, 216.9 = 5.13, P = 0.0107). Spiders that participated in 2 prey capture events gained the most mass and those that never participated in prey capture gained the least amount of mass (Figure 1).
Figure 1.
The extent to which Stegodyphus dumicola participate in collective foraging predicts how much weight they gain from prey. Categories with different letters differ significantly according to a post hoc Tuckey test. Error bars represent standard errors.
Bold spiders were more likely to participate in collective foraging than shy spiders (GLMM: F1, 246.9 = 6.72, P = 0.0131). However, bold and shy spiders did not differ significantly in mass gain (GLMM: F1, 232 = 0.78, P = 0.72). For random effect estimates, please refer to Supplementary Document 1.
Colony-level weight gain
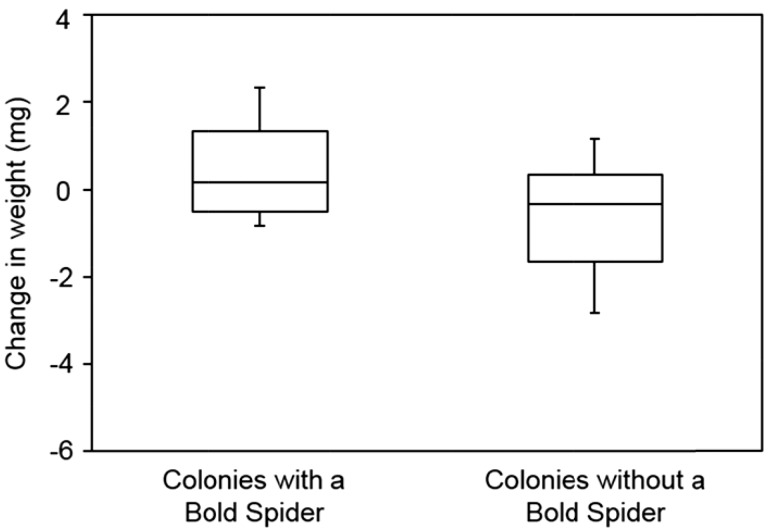
We found that colonies containing 1 bold spider gained on average 1.37 ± 0.09 SE (standard error) mg more than shy colonies (Figure 2; GLMM: F1, 37.65 = 5.65, P = 0.01). For colonies with a bold individual the latency to attack was 39.70 ± 9.86 s and number of attackers 1.84 ± 0.12 and for all-shy colonies the latency to attack was 44.16 ± 7.36 s and number of attackers 1.59 ± 0.08. However, colonies with and without a bold individual did not differ significantly in either their latency to attack (GLMM: F1, 39.39 = 0.0227, P = 0.71) or number of attackers (GLMM: F1, 0 = 2.92, P = 0.81). Furthermore, neither a colony’s average latency to attack (GLMM: F1, 39.75 = 1.88, P = 0.71) nor its average number of attackers (GLMM: F1, 37.78 = 0.0261, P = 0.81) were significantly correlated with total colony weight gain.
Figure 2.
Stegodyphus dumicola colonies that contained a single bold spider, which gained more weight than colonies composed entirely of shy spiders. The center line of each box represents the median; the upper and lower margins of the of the box represents the 3rd and 1st quartiles respectively, and the whiskers represent the 10th and 90th percentile.
Starvation resistance
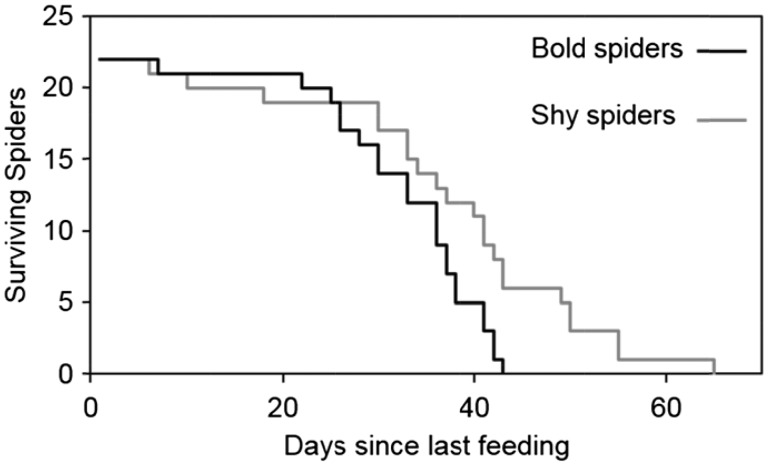
Shy spiders were more resistant to starvation than bold spiders (Figure 3; Log-rank test: χ2 = 6.2214, P = 0.0124). Spiders did not begin to die in large numbers until 20 days after their ad libitum feeding ended, and some of the hardiest shy spiders survived for over 2 months without feeding. Conversely, no bold spider survived past day 43 (Figure 3).
Figure 3.
Shy Stegodyphus dumicola (gray line) are more resistant to starvation than bold individuals (black line) (Log-rank test: χ2 = 6.2214, P = 0.0124).
Discussion
The personality type of colony members often predicts the tasks they perform (Grinsted et al. 2013; Wright et al. 2014, 2015; Walton and Toth 2016) and how they interact with fellow group members (Pinter-Wollman 2015; Knotts and Griffen 2016). This personality-related task differentiation can have a variety of benefits for group performance (Modlmeier et al. 2012; Holbrook et al. 2014). Yet, to what extent individuals’ participation in various tasks influences their own success (mass gain, survival, etc.) remains unclear. Here we found that individuals’ tendency to participate in cooperative prey capture was associated with greater mass gain during collective feeding events. Specifically, individuals that attacked a dummy prey item twice gained more weight than individuals that never attacked. In S. dumicola, bold individuals tend to perform the majority of the prey capture behavior for their colonies (Wright et al. 2014, 2015). We reason that bold spiders may actually participate more in prey capture because they are more susceptible to starvation (Figure 3), consistent with the pace-of-life theory (Careau et al. 2008; Réale et al. 2010). This theory argues that bolder and more aggressive individuals will tend to have higher metabolic rates than their shy counterparts, a prediction that received empirical support in spiders and other taxa (Careau et al. 2010; Shearer and Pruitt 2014). We therefore propose that bold individuals’ tendency to participate in prey capture (and thus gain an advantage in competition over prey) may actually be driven by their need to fuel a higher metabolic rate. However, the relationship between metabolic rate and boldness remains untested in this species. The finding that bold spiders do not weigh more than shy spiders here (P = 0.72), suggests that any advantage gleaned by bold spiders by participating in prey capture is ultimately offset by some other factor (e.g., higher activity levels, high basal metabolic rates, etc.).
Bold S. dumicola often act as keystone individuals, which are defined as individuals that exhibit a disproportionately large effect on group behavior (Modlmeier et al. 2014a). Specifically, the addition of a single bold spider to a colony increases the personality variation of a colony (Pruitt and Keiser 2014). We found here that in S. dumicola colonies just 1 bold individual increases colony-wide mass gain from a single feeding event. Colonies without any bold individuals tended to lose weight and colonies containing just 1 bold individual gained significant amounts of mass (Figure 2). These findings support considerable existing literature suggesting that personality diversity increases group performance (Pinter-Wollman 2012b) in ants (Modlmeier and Foitzik 2011; Modlmeier et al. 2012) and other social spider species (Lichtenstein and Pruitt 2015). At the same time, they contradict evidence demonstrating that behavioral diversity does not increase group performance (Jandt and Dornhaus 2014). Pruitt and Keiser (2014) found that the positive effects of a keystone individual stem from the fact that bold individuals increase the collective aggressiveness of their colony mates. However, we failed to detect an effect of bold individuals on any metric of collective aggressiveness considered here (e.g., colony latency to attack), and colony foraging aggressiveness was not associated with greater mass gain in this study. It is possible that the experimental colonies in this study were not established for long time to allow the long-lasting behavioral changes that keystone individuals have been observed to impose on other group members (Pruitt and Keiser 2014; Pruitt and Pinter-Wollman proc B 2015). Still, we show here that the benefits of bold individuals on colony success in the form of mass gain are almost immediate and are established before a shift in colony aggressiveness. The mechanisms driving this outcome are uncertain.
Several studies on S. dumicola have shown that bold individuals are more likely to participate in prey capture, but is this behavior self-serving or is it costly? We show here that engaging in prey capture is associated with greater individual mass gain. We also obtained data to suggest that bold S. dumicola may require more food to survive, because bold individuals are more susceptible to starvation than shy individuals. One possible explanation for this result is that bold individuals are merely in poorer body condition than their shy counterparts (Dall et al. 2004; Smith and Blumstein; 2008). However, data from multiple studies on S. dumicola have failed to recover any association between boldness and body condition (Keiser et al. 2014b; Keiser and Pruitt 2014). Thus, we instead propose that variation in metabolic rate may underlie both the tendency for bold individuals to engage in prey capture and their increased susceptibility to starvation. Bold individuals may actually need to engage in prey capture to help ensure that they acquire more resources and thus reduce starvation risk. Shy individuals, in contrast, are predicted to require less food and thus can forgo participating in prey capture. Shy individuals also tend to join feeding groups later and scrounge the foraging efforts of their bold colony mates (Wright et al. 2015). Although we infer these relationships from our starvation data, they have never been explicitly tested. We therefore tentatively conclude that bold individuals’ tendency to participate in prey capture is not overtly altruistic, and is instead self-motivated in the interest to help circumvent starvation. Shy individuals merely appear to piggyback off of this tendency.
Supplementary Material
Acknowledgments
We are indebted to the Northern Cape of South Africa for issuing research and collection permits (FAUNA 1060/2012, FAUNA 1072, 2013). We also thank four anonymous reviewers for their comments that improved the quality of this manuscript.
Funding
Funding for this research was generously provided by NSF IOS [grants 1352705, 1455895, and 1456010] and NIH [GM115509 to JNP and NPW].
Supplementary Material
Supplementary material can be found at http://www.cz.oxfordjournals.org/.
References
- Agnarsson I, Avilés L, Coddington JA, Maddison WP, 2006. Sociality in theridiid spiders: repeated origins of an evolutionary dead end. Evolution 60:2342–2351. [PubMed] [Google Scholar]
- Amir N, Whitehouse ME, Lubin Y, 2000. Food consumption rates and competition in a communally feeding social spider Stegodyphus dumicola (Eresidae). J Arachnol 28:195–200. [Google Scholar]
- Aplin LM, Farine DR, Mann RP, Sheldon BC, 2014. Individual-level personality influences social foraging and collective behaviour in wild birds. Proc R Soc Lond B Biol Sci 281:20141016.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilés L, 1997. Causes and consequences of cooperation and permanent-sociality in spiders. The evolution of social behavior in insects and arachnids In: Choe J, Crespi B, editors. Evolution of Social Behaviour in Insects and Arachnids. Cambridge: Cambridge University Press, 476–498. [Google Scholar]
- Avilés L, Varas C, Dyreson E, 1999. Does the African social spider Stegodyphus dumicola control the sex of individual offspring? Behav Ecol Sociobiol 46:237–243. [Google Scholar]
- Bell AM, Hankison SJ, Laskowski KL, 2009. The repeatability of behaviour: a meta-analysis. Anim Behav77(4):771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilde T, Coates K, Birkhofer K, Bird T, Maklakov A. et al. , 2007a. Survival benefits select for group living in a social spider despite reproductive costs. J Evol Biol 20:2412–2426. [DOI] [PubMed] [Google Scholar]
- Bilde T, Coates K, Birkhofer K, Bird T, Maklakov AA. et al. , 2007b. Survival benefits select for group living in a social spider despite reproductive costs. J Evol Biol 20:2412–2426. [DOI] [PubMed] [Google Scholar]
- Biro PA, Stamps JA, 2008. Are animal personality traits linked to life-history productivity? Trends Ecol Evol 23:361–368. [DOI] [PubMed] [Google Scholar]
- Careau, Thomas D, Humphries M, Réale D, 2008. Energy metabolism and animal personality. Oikos 117:641–653. [Google Scholar]
- Careau V, Réale D, Humphries MM, Thomas DW, 2010. The pace of life under artificial selection: personality, energy expenditure, and longevity are correlated in domestic dogs. Am Nat 175:753–758. [DOI] [PubMed] [Google Scholar]
- Charbonneau D, Hillis N, Dornhaus A, 2015. “ Lazy”in nature: ant colony time budgets show high “inactivity”in the field as well as in the lab. Insectes Soc 62:31–35. [Google Scholar]
- Dall SR, Houston AI, McNamara JM, 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol Lett 7:734–739. [Google Scholar]
- Foelix R, 2010. Biology of Spiders. Oxford University Press. [Google Scholar]
- Giraldeau L-A, Caraco T, 2000. Social Foraging Theory. Princeton University Press. [Google Scholar]
- Gosling SD, 2001. From mice to men: what can we learn about personality from animal research? Psychol Bull 127:45.. [DOI] [PubMed] [Google Scholar]
- Grinsted L, Pruitt JN, Settepani V, Bilde T, 2013. Individual personalities shape task differentiation in a social spider. Proc R Soc Lond B Biol Sci 280:20131407.. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hedrick AV, Riechert SE, 1989. Genetically-based variation between two spider populations in foraging behavior. Oecologia 80:533–539. [DOI] [PubMed] [Google Scholar]
- Henschel J, Lubin Y, Schneider J, 1995. Sexual competition in an inbreeding social spider Stegodyphus dumicola (Araneae: Eresidae). Insectes Soc 42:419–426. [Google Scholar]
- Henschel JR, 1998. Predation on social and solitary individuals of the spider Stegodyphus dumicola (Araneae, Eresidae). J Arachnol 61–69. [Google Scholar]
- Holbrook CT, Wright CM, Pruitt JN, 2014. Individual differences in personality and behavioural plasticity facilitate division of labour in social spider colonies. Anim Behav 97:177–183. [Google Scholar]
- Huntingford FA, 1976. The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback Gasterosteus aculeatus. Anim Behav 24:245–260. [Google Scholar]
- Jandt J, Robins N, Moore R, Dornhaus A, 2012. Individual bumblebees vary in response to disturbance: a test of the defensive reserve hypothesis. Insectes Soc 59:313–321. [Google Scholar]
- Jandt JM, Bengston S, Pinter-Wollman N, Pruitt JN, Raine NE. et al. , 2014. Behavioural syndromes and social insects: personality at multiple levels. Biol Rev 89:48–67. [DOI] [PubMed] [Google Scholar]
- Jandt JM, Dornhaus A, 2014. Bumblebee response thresholds and body size: does worker diversity increase colony performance? Anim Behav 87:97–106. [Google Scholar]
- Kaplan EL, Meier P, 1958. Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481. [Google Scholar]
- Keiser CN, Jones DK, Modlmeier AP, Pruitt JN, 2014a. Exploring the effects of individual traits and within-colony variation on task differentiation and collective behavior in a desert social spider. Behav Ecol Sociobiol 68:839–850. [Google Scholar]
- Keiser CN, Modlmeier AP, Singh N, Jones DK, Pruitt JN, 2014b. Exploring how a shift in the physical environment shapes individual and group behavior across two social contexts. Ethology 120:825–833. [Google Scholar]
- Keiser CN, Pruitt JN, 2014. Personality composition is more important than group size in determining collective foraging behaviour in the wild. Proc R Soc Lond B Biol Sci 281:20141424.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Krafft B, Choe JC, 2005. Cooperative prey capture by young subsocial spiders. Behav Ecol Sociobiol 59:92–100. [Google Scholar]
- Knotts ER, Griffen BD, 2016. Individual movement rates are sufficient to determine and maintain dynamic spatial positioning within Uca pugilator herds. Behav Ecol Sociobiol 1–8. [Google Scholar]
- Kralj-Fišer S, Schneider JM, 2012. Individual behavioural consistency and plasticity in an urban spider. Anim Behav 84:197–204. [Google Scholar]
- Kraus O, Kraus M, 1988. The genus Stegodyphus (Arachnida, Araneae): sibling species, species groups, and parallel origin of social living. Verhandlungen Des Naturwissenschaftlichen Vereins in Hamburg (NF) 30:151–254. [Google Scholar]
- Kühbandner S, Modlmeier AP, Foitzik S, 2014. Age and ovarian development are related to worker personality and task allocation in the ant Leptothorax acervorum. Curr Zool 60:392–400. [Google Scholar]
- Kurvers RH, Prins HH, van Wieren SE, van Oers K, Nolet BA. et al. , 2009. The effect of personality on social foraging: shy barnacle geese scrounge more. Proc R Soc Lond B Biol Sci 1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski KL, Pruitt JN, 2014. Evidence of social niche construction: persistent and repeated social interactions generate stronger personalities in a social spider. Proc R Soc Lond B Biol Sci 281:20133166. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lichtenstein JL, Pruitt JN, 2015. Similar patterns of frequency-dependent selection on animal personalities emerge in three species of social spiders. J Evol Biol [DOI] [PubMed] [Google Scholar]
- Lubin Y, Bilde T, 2007. The evolution of sociality in spiders. Adv Stud Behav 37:83–145. [Google Scholar]
- Modlmeier AP, Foitzik S, 2011. Productivity increases with variation in aggression among group members in Temnothorax ants. Behav Ecol 22:1026–1032. [Google Scholar]
- Modlmeier AP, Keiser CN, Watters JV, Sih A, Pruitt JN, 2014a. The keystone individual concept: an ecological and evolutionary overview. Anim Behav 89:53–62. [Google Scholar]
- Modlmeier AP, Laskowski KL, DeMarco AE, Coleman A, Zhao K. et al. , 2014b. Persistent social interactions beget more pronounced personalities in a desert–dwelling social spider. Biol Lett 10:20140419. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Modlmeier AP, Liebmann JE, Foitzik S, 2012. Diverse societies are more productive: a lesson from ants. Proc R Soc Lond B Biol Sci:rspb20112376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter-Wollman N, 2012a. Personality in social insects: how does worker personality determine colony personality. Curr Zool 58:579–587. [Google Scholar]
- Pinter-Wollman N, 2012b. Personality in social insects: how does worker personality determine colony personality? Curr Zool 58:580–588. [Google Scholar]
- Pinter-Wollman N, 2015. An introduction to the special column on animal social networks. Curr Zool 61:42–44. [Google Scholar]
- Pinter-Wollman N, Keiser CN, Wollman R, Pruitt JN, Wcislo WT. et al. , 2016. The effect of keystone individuals on collective outcomes can be mediated through interactions or behavioral persistence. Am Nat 188:000–000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt JN, 2012. Behavioural traits of colony founders affect the life history of their colonies. Ecol Lett 15:1026–1032. [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Goodnight CJ, 2014. Site-specific group selection drives locally adapted group compositions. Nature 514:359–362. [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Grinsted L, Settepani V, 2013. Linking levels of personality: personalities of the “average” and “most extreme” group members predict colony-level personality. Anim Behav 86:391–399. [Google Scholar]
- Pruitt JN, Keiser CN, 2014. The personality types of key catalytic individuals shape colonies” collective behaviour and success. Anim Behav 93:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réale D, Garant D, Humphries MM, Bergeron P, Careau V. et al. , 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil Trans R Soc B 365:4051–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechert SE, Hedrick AV, 1990. Levels of predation and genetically based anti-predator behaviour in the spider, Agelenopsis aperta. Anim Behav 40:679–687. [Google Scholar]
- Riechert SE, Hedrick AV, 1993. A test for correlations among fitness-linked behavioural traits in the spider Agelenopsis aperta (Araneae, Agelenidae). Anim Behav 46:669–675. [Google Scholar]
- Riechert SE, Roeloffs RM, 1993. Evidence for and consequences of inbreeding in the cooperative spiders. In: The Natural History of Inbreeding and Outbreeding 283–303. [Google Scholar]
- Ruch J, Heinrich L, Bilde T, Schneider JM, 2009. Relatedness facilitates cooperation in the subsocial spider Stegodyphus tentoriicola. BMC Evol Biol 9:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JM, 2002. Reproductive state and care giving in Stegodyphus (Araneae: Eresidae) and the implications for the evolution of sociality. Anim Behav 63:649–658. [Google Scholar]
- Shearer TA, Pruitt JN, 2014. Individual differences in boldness positively correlate with heart rate in orb-weaving spiders of genus Larinioides. Curr Zool 60:387–391. [Google Scholar]
- Short KH, Petren K, 2008. Boldness underlies foraging success of invasive Lepidodactylus lugubris geckos in the human landscape. Anim Behav 76:429–437. [Google Scholar]
- Sih A, Bell A, Johnson JC, 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. [DOI] [PubMed] [Google Scholar]
- Smith BR, Blumstein DT, 2008. Fitness consequences of personality: a meta-analysis. Behav Ecol 19:448–455. [Google Scholar]
- Stamps JA, 2007. Growth-mortality tradeoffs and “personality traits” in animals. Ecol 10:355–363. [DOI] [PubMed] [Google Scholar]
- Walton A, Toth AL, 2016. Variation in individual worker honey bee behavior shows hallmarks of personality. Behav Ecol Sociobiol 1–12. [Google Scholar]
- Ward AJ, Thomas P, Hart PJ, Krause J, 2004. Correlates of boldness in three-spined sticklebacks Gasterosteus aculeatus. Behav Ecol Sociobiol 55:561–568. [Google Scholar]
- Webster M, Ward A, Hart P, 2009. Individual boldness affects interspecific interactions in sticklebacks. Behav Ecol Sociobiol 63:511–520. [Google Scholar]
- Werner EE, Anholt BR, 1993. Ecological consequences of the trade-off between growth and mortality rates mediated by foraging activity. Am Nat 242–272. [DOI] [PubMed] [Google Scholar]
- West SA, Griffin AS, Gardner A, 2007. Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. J Evol Biol 20:415–432. [DOI] [PubMed] [Google Scholar]
- Whitehouse M, Lubin Y, 1999. Competitive foraging in the social spider Stegodyphus dumicola. Anim Behav 58:677–688. [DOI] [PubMed] [Google Scholar]
- Wright CM, Holbrook CT, Pruitt JN, 2014. Animal personality aligns task specialization and task proficiency in a spider society. Proc Natl Acad Sci 111:9533–9537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wright CM, Keiser CN, Pruitt JN, 2015. Personality and morphology shape task participation, collective foraging and escape behaviour in the social spider Stegodyphus dumicola. Anim Behav 105:47–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.