Abstract
Regeneration is a complex process that requires an organism to recognize and repair tissue damage, as well as grow and pattern new tissue. Here, we describe a genetic screen to identify novel regulators of regeneration. We ablated the Drosophila melanogaster larval wing primordium by inducing apoptosis in a spatially and temporally controlled manner and allowed the tissue to regenerate and repattern. To identify genes that regulate regeneration, we carried out a dominant-modifier screen by assessing the amount and quality of regeneration in adult wings heterozygous for isogenic deficiencies. We have identified 31 regions on the right arm of the third chromosome that modify the regenerative response. Interestingly, we observed several distinct phenotypes: mutants that regenerated poorly, mutants that regenerated faster or better than wild-type, and mutants that regenerated imperfectly and had patterning defects. We mapped one deficiency region to cap-n-collar (cnc), the Drosophila Nrf2 ortholog, which is required for regeneration. Cnc regulates reactive oxygen species levels in the regenerating epithelium, and affects c-Jun N-terminal protein kinase (JNK) signaling, growth, debris localization, and pupariation timing. Here, we present the results of our screen and propose a model wherein Cnc regulates regeneration by maintaining an optimal level of reactive oxygen species to promote JNK signaling.
Keywords: Cap-n-collar, Nrf2, Drosophila, imaginal disc, regeneration, reactive oxygen species
REGENERATION has long captured the interest of scientists, who seek to understand the complex pathways that enable some animals to replace missing structures after injury. Model organisms across animal phyla have been used to identify genetic regulators of regrowth and repatterning, demonstrating considerable evolutionary conservation in the factors that are used during regeneration, including Wnt, c-Jun N-terminal protein kinase (JNK), Hippo, and reactive oxygen species (ROS) signaling. For example, Wnt signaling plays a vital role in reestablishing axis polarity in the invertebrate flatworm planarians (Petersen and Reddien 2008, 2009), and Wnts are upregulated during head regeneration in the invertebrate cnidarian Hydra (Hobmayer et al. 2000). Furthermore, Wnt signaling is required for regeneration in vertebrate models, including the zebrafish caudal fin (Wehner et al. 2014), the axolotl limb (Kawakami et al. 2006), and the murine liver, where it plays an important role in cellular differentiation (Williams et al. 2010). JNK signaling is also required for regeneration of planarians (Tasaki et al. 2011) and murine liver regeneration (Behrens et al. 2002). Yorkie/YAP, the transcription factor and effector of the Hippo pathway, is required for proper planarian regeneration (Lin and Pearson 2014), for cricket leg regeneration (Bando et al. 2009), and is activated during mammalian liver regeneration (Grijalva et al. 2014).
ROS are also important for tissue repair across phyla. ROS are required in planarians for efficient head and tail regeneration (Pirotte et al. 2015) and in Drosophila for imaginal disc regeneration (Santabárbara-Ruiz et al. 2015), compensatory proliferation (Fogarty et al. 2016), and dorsal closure, a model for wound healing (Muliyil and Narasimha 2014). ROS signals are also required in vertebrates during wound healing (Schäfer and Werner 2008; Niethammer et al. 2010; Yoo et al. 2012) and regeneration (Beyer et al. 2008; Gauron et al. 2013; Love et al. 2013). However, the complex relationship between ROS and other signaling pathways during regeneration remains unclear, as does the mechanism by which ROS are constrained to appropriate levels.
The requirement for the same signaling pathways during regeneration across species strongly suggests that conserved mechanisms regulate many aspects of regeneration and that much can be learned about regeneration by studying model organisms. However, many regeneration models have technical limitations, including lack of a sequenced genome, no ability to make transgenic animals, a significantly long regeneration time, or labor-intensive methods of causing tissue damage. Thus, work in these models would be complemented by an approach in a more genetically tractable model system.
The wing imaginal disc of the fruit fly, Drosophila melanogaster, is capable of substantial regeneration, enabling us to ask questions about the regulation of regeneration using this genetically tractable model system. Experiments in the midtwentieth century by Ernst Hadorn and colleagues discovered that fragmented imaginal discs could regenerate upon transplantation into a young larva or an adult fly abdomen [reviewed in Worley et al. (2012)]. Imaginal discs regenerate by forming a zone of proliferating cells called a blastema at the site of tissue damage, enabling regrowth [Hadorn et al. 1949; Hadorn and Buck 1962; reviewed in Worley et al. (2012)]. These studies were invaluable for understanding consequences of damage to imaginal discs. However, the laborious nature of these experiments made genetic screens challenging.
A few genetic screens have been carried out in imaginal discs to identify regeneration genes. First, a screen of lacZ-containing P-element insertion lines, using a temperature-sensitive cell-lethal mutation to induce damage, identified loci that induced expression of the enhancer-trap lacZ after tissue damage (Brook et al. 1993). Second, the Schubiger lab performed a dominant-modifier genetic screen to identify mutations that modify the frequency of transdetermination, by exploiting the fact that ubiquitous expression of wg in the leg disc recapitulates the leg-to-wing fate changes occasionally seen after tissue damage. They also screened for expression changes of P-element insertion reporter lines in the regeneration blastema (McClure and Schubiger 2008). However, technical limitations did not allow either group to screen for inability to regenerate. Additionally, multiple labs have screened for regulators of compensatory proliferation, a similar but distinct process by which scattered apoptotic cell death can induce proliferation in neighboring cells (Gerhold et al. 2011; Fan et al. 2014; Meserve and Duronio 2015). While many signaling pathways might be involved in both regeneration and compensatory proliferation, these screens were not designed to identify genes that are required to replace a tissue after catastrophic damage.
Instead of manual fragmentation of imaginal discs, overexpression of wg, or induction of scattered cell death, we use genetic tools to induce extensive tissue ablation in the wing primordium (Smith-Bolton et al. 2009). This ablation system enables sophisticated genetic experiments because it provides temporal and spatial control of tissue damage and subsequent regeneration. This system also enables large-scale, unbiased screening for genes that are necessary for the process of regeneration. Indeed, studies using genetically-induced tissue damage have already confirmed roles in imaginal disc regeneration for conserved signaling pathways, including Wnt signaling (Smith-Bolton et al. 2009), JNK signaling (Bergantiños et al. 2010), Hippo/Yki signaling (Grusche et al. 2011; Sun and Irvine 2011), and ROS (Santabárbara-Ruiz et al. 2015; Fogarty et al. 2016).
Two pilot forward genetic screens using an EMS-induced mutant collection and a small portion of a deficiency collection demonstrated that genetically-induced imaginal wing disc ablation can be used to identify genes that are required for regeneration and that restrict regeneration (Smith-Bolton et al. 2009). Genes identified in these pilot screens included the chromatin modifier Trithorax, which regulates regeneration signaling (Skinner et al. 2015), and Taranis, which is a novel regeneration-specific regulator of posterior wing cell fate (Schuster and Smith-Bolton 2015). Thus, genetic screens using this regeneration model successfully identified novel genetic regulators of regeneration that are likely to be conserved in vertebrates.
Here, we present the results of a dominant-modifier deficiency screen covering 20% of the Drosophila genome. We identified 17 regions of chromosome 3R that contain one or more genes that are required for regeneration, and 14 regions that, when hemizygous, cause enhanced regeneration. We have shown that the specific gene within one of the regions that is required for regeneration is cap-n-collar (cnc), which encodes the Drosophila homolog of vertebrate Nrf2. Furthermore, we demonstrate that Cnc coordinates the response to injury-produced ROS, by constraining the ROS signal to appropriate levels and thereby regulating the appropriate signaling response to tissue damage and, thus, the cellular and developmental processes downstream of those early signals.
Materials and Methods
Fly stocks
Flies were reared on standard molasses medium at 25° unless otherwise noted. The line w1118;;rn-GAL4, UAS-reaper, tubGAL80ts/TM6B, tubGAL80 (Brand and Perrimon 1996; McGuire et al. 2003; Smith-Bolton et al. 2009; Schuster and Smith-Bolton 2015) was used to induce ablation in developing wing discs, referred to later as the “ablation chromosome.” Deficiency lines from the DrosDel collection (Ryder 2004), the Exelixis collection (Parks et al. 2004; Thibault et al. 2004), and the Bloomington Drosophila Stock Center collection (Cook et al. 2012) were used to conduct the screen. All deficiency lines were ordered from the Bloomington Drosophila Stock Center, with the exception of Df(3R)ED10951, which came from the Drosophila Genomic Resources Center in Kyoto, Japan. A list of deficiency lines used can be found in Supplemental Material, Table S1. w1118 was used as a control. cnc03921 (Perrimon et al. 1996), cncEY08884 (Bellen et al. 2004), Irk1 MI08404 and Irk1MB08423 (Venken et al. 2011), nubMI05126 (nub-GFP) (Nagarkar-Jaiswal et al. 2015; Khan et al. 2016), UAS-eGFP (Halfon et al. 2002), cncHMS02021 (Ni et al. 2011), and P{CaryP}attP40 (Markstein et al. 2008) were from the Bloomington Drosophila Stock Center. RNA interference (RNAi) lines targeting Cnc (VDRC ID #101235 and #108127) were from the Vienna Drosophila Resource Center (Dietzl et al. 2007). TRE-red (TPA Response Element) (Chatterjee and Bohmann 2012) and UAS-cncC (Sykiotis and Bohmann 2008), were gifts from Dirk Bohmann.
Genetic screen
The screen is outlined in Figure 1. Briefly, deficiency lines were crossed to the ablation chromosome. Embryos were collected on a grape juice plate for 4–5 hr at room temperature in dark conditions. The plates were moved to 18°. Larvae were staged and selected 2 days after egg lay to synchronize development and then moved from the grape juice plate to standard Bloomington cornmeal media that was churned and had yeast paste added. For each genotype, three vials of 40–50 larvae per vial were selected. The vials were then placed at 18° until day 7 after egg lay. On the morning of day 7, when the larvae were in the early third instar as determined by counting mouth hooks, the vials were placed in a 30° water bath for 24 hr, during which ablation of the wing pouch occurred. After 24 hr they were placed in an ice-water bath for one minute to stop ablation and returned to 18°. For screening experiments, the vials remained at 18° until after eclosion, at which point their wing size was scored and they were discarded. Wings from the same genotype and date of egg lay were pooled and scored by relative size compared to a control undamaged wing (Figure 1C). This process was repeated three times to have three independent replicates of the experiment. The average regeneration index was determined by calculating the sum of the products of percentage wing size and the percentage of the population at each size. To determine an absolute wing size, flies of the correct genotype and ablation status were frozen, wings were mounted in Gary’s Magic Mount (Canada Balsam dissolved in methyl salicylate; Sigma [Sigma Chemical], St. Louis, MO), and imaged on an Olympus SZX10 microscope with an Olympus DP21 camera with CellSens Dimensions software. The area was measured in ImageJ (Schneider et al. 2012).
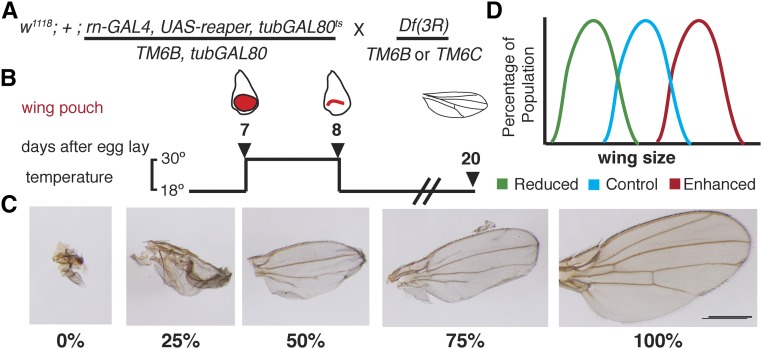
Figure 1.
Outline of the deficiency screen. (A) The genotype of the ablation chromosome and the genetic cross used in the screen. (B) A diagram of the tissue ablation system. Briefly, at 18° GAL80TS inhibits GAL4 and UAS-reaper is not expressed. Shifting to 30° at day 7 after egg lay (AEL) relieves the inhibition and reaper expression induces apoptosis. On day 8 AEL, stocks are shifted back to 18° and regeneration occurs. Starting at approximately day 20 AEL, adult flies eclose, enabling scoring of wing size. (C) A semiquantitative scoring scale for the adult wings that resulted from regenerated wing primordia. (D) Tissue ablation was timed so that the majority of a population of control wings regenerate to 50% of normal size, so that screening could identify populations of wings that were larger or smaller than controls.
Pupariation timing experiments
Flies underwent the same treatment as above with some minor differences. Six sets of 40–50 larvae were selected and moved to standard Bloomington cornmeal media in vials. Three vials underwent the thermal shift as above while three vials remained at 18° to serve as the nonablated control. The ablated and nonablated experiments were performed at the same time to control for environmental variables. Starting at day 9 and continuing to day 15, new pupal cases were counted on the side of each vial every 24 hr. The vials from the same genotype and ablation status were pooled and graphed to show the percentage of the population that had pupariated at each day after egg lay. For Table 1, the time at which 50% of the animals had entered pupariation was interpolated from the graph.
Table 1. Deficiency regions that reduced regenerative capacity.
| Deficiency line | Developmental delay (relative to control) | Expanded recovery period (relative to control) |
|---|---|---|
| Df(3R)BSC678 | None | None |
| Df(3R)ED6361 | 1 day | 1 day |
| Df(3R)ED6265 | None | None |
| Df(3R)ED6280 | None | None |
| Df(3R)ED6103 | None | None |
| Df(3R)BSC677 | None | 1 day |
| Df(3R)ED5331 | None | None |
| Df(3R)ED5156 | None | None |
| Df(3R)Exel6270 | None | None |
| Df(3R)BSC467 | None | None |
| Df(3R)BSC516 | None | None |
| Df(3R)ED5942 | N/A* | N/A* |
| Df(3R)BSC321 | None | None |
| Df(3R)BSC620 | None | 1 day |
| Df(3R)BSC489 | None | None |
| Df(3R)BSC498 | None | None |
| Df(3R)Exel9013 | 1 day | 1 day |
The deficiency regions that, when heterozygous, caused wing discs to regenerate poorly. The developmental timing of pupariation and regeneration-specific timing of pupariation is given in the number of days before or after controls (w1118) that 50% of the animals had entered pupariation (see Materials and Methods). N/A*; deficiency is lethal over the TM6B-Tb balancer, which is necessary for the experiment.
Immunostaining
Immunostaining was performed as previously described (Schuster and Smith-Bolton 2015; Skinner et al. 2015). The Nubbin antibody was a gift from S. Cohen and was used at a 1:200 dilution (Ng et al. 1996). The PH3 antibody came from Millipore (Bedford, MA) and was used at a 1:500 dilution. Anti-dMyc came from Santa Cruz Biotechnology (sc-28207) and was used at a 1:500 dilution. Anti-Wg came from the Developmental Studies Hybridoma Bank (DSHB) and was used at a 1:100 dilution (Brook and Cohen 1996). The DSHB was created by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH) and is maintained at the University of Iowa, Department of Biology, Iowa City, IA 52242. Discs were mounted in Vectashield (Vector Laboratories, Burlingame, CA) and imaged on a Zeiss LSM 700 Confocal microscope (Zeiss [Carl Zeiss], Thornwood, NY].
ROS detection
This protocol was adapted from a previously published protocol (Owusu-Ansah et al. 2008). Briefly, larvae were dissected and collected in Schneider’s Insect Medium (S0146; Sigma). The dihydroethidium (DHE) (D11347; Thermo Fisher Scientific) was reconstituted in DMSO, then 1 μl of DHE was dissolved in 1 ml of Scheider’s Medium. The tissue was incubated in DHE in the dark for 5 min, washed 3 × in Schneider’s Medium, fixed for 7 min in 7% formaldehyde in 1 × PBS, washed once in 1 × PBS, then immediately mounted in Vectashield (Vector Laboratories), and imaged on a Zeiss LSM 700 Confocal.
ROS feeding
This protocol was adapted from previous published protocols (Grover et al. 2009; Santabárbara-Ruiz et al. 2015). To increase ROS levels, food was supplemented with a solution of 1% sucrose and either 0% (control) or 0.5% H2O2. A circle of Whatman paper was placed on top of the food, and 1 ml of solution was added to the Whatman paper at the end of the 24-hr temperature shift, such that regenerating larvae were consuming excess ROS throughout regeneration.
Image analysis
All image analysis was done in ImageJ (NIH). To measure the wing pouch size, the area that immunostained with anti-Nubbin was measured by outlining and measuring in ImageJ. For PH3 measurements, the wing primordium was identified by outlining the Nubbin-positive region, and mitoses were counted using the ImageJ cell-counter tool. Orthogonal images of the imaginal discs were generated using the ImageJ reslice tool on a Z-stack of images. For DHE, eGFP (enhanced GFP), and TRE-Red intensity analysis in orthogonal slices, three equal-sized rectangles were placed in each region (apical, epithelial, and basal), and the average fluorescence intensity was measured. Three measurements were taken per region, per image.
Data availability
All relevant data are included in the paper. Drosophila lines are available upon request. Raw data from primary and secondary screens are available upon request.
Results
Wing disc ablation system and screening methods
To facilitate genetic screening for regeneration genes, we used a method of genetically ablating the wing disc using the GAL4/UAS/GAL80ts system that has been previously described (Figure 1, A and B; see Materials and Methods) (Smith-Bolton et al. 2009). Briefly, apoptosis was induced in the wing-blade primordium by driving expression of the proapoptotic transgene UAS-reaper with rotund-GAL4, which is expressed in the majority of the wing primordium. This ablation was turned on and off with a temperature-sensitive GAL80 (GAL80ts), which represses GAL4 when active. At 18°, GAL4 was inhibited by GAL80ts, preventing apoptosis; shifting to 30° relieved the inhibition and allowed tissue ablation. All three components are on a single chromosome for ease of genetic manipulation, hereafter referred to as the ablation chromosome. The amount of regeneration expected in controls was regulated by adjusting the developmental stage of the larvae at the beginning of ablation and the amount of time for which ablation was allowed to continue. The wing primordium was ablated over the course of 24 hr starting at the early third instar (day 7 after egg laying) (Figure 1B). After the temperature shift back to 18°, the wing discs regenerated and the resulting adult wings were qualitatively scored by assessing the size of each adult wing and counting the numbers of wings that were approximately < 25, 25, 50, 75, or 100% the size of a normal adult wing (Figure 1C). This semiquantitative evaluation enabled the screening of ∼900 wings or 6–10 genotypes per week.
We used this ablation system to conduct a dominant-modifier genetic screen using chromosomal deficiencies. We adjusted the timing of the ablation such that a control population, generated by crossing the ablation chromosome to w1118, had a majority of its wings regenerate to 50% of normal wing size. This moderate amount of regeneration in the control wings enabled us to identify both enhancers and suppressors of regeneration, similar to the previously reported pilot screens (Smith-Bolton et al. 2009) (Figure 1D). Each line was screened three times to ensure reproducible results. We screened three collections of isogenic deficiency lines to maximize coverage of the right arm of the third chromosome: the Bloomington Drosophila Stock Center and Exelixis Deficiency collections (Parks et al. 2004; Cook et al. 2012), which were generated in the same genetic background and were screened together, as well as the DrosDel deficiencies (Ryder 2004), which we screened separately to control for differences in genetic background (Table S1). We found that the proportion of lines that had poor, normal, and enhanced regeneration was similar among the three collections (Figure S1 and Figure S2), indicating that such separate treatment was likely unnecessary. We calculated an average regeneration index number from the semiquantitative scores. Variations in regeneration occurred between experiments due to food batch differences and ambient environmental factors outside of our control (Skinner et al. 2015; Vonesch et al. 2016). Therefore, assessment of regenerative capacity for each deficiency line was determined by its score in comparison to the other lines screened at the same time, not in comparison to the complete set of screened lines, resulting in the intermixing of normal and altered regenerative ability observed in Figure S1 and Figure S2. Seventeen deficiency regions were identified that displayed reduced regenerative capacity (Table 1). Thirty-five deficiency regions were identified that displayed enhanced regenerative capacity (Table 2). Of these 35, two also displayed consistent defects in patterning of the wing.
Table 2. Deficiency regions that enhanced regenerative capacity.
| Deficiency line | Developmental delay (relative to control) | Expanded recovery period (relative to control) |
|---|---|---|
| Df(3R)BSC140 | None | None |
| Df(3R)ED10257 | 1 day | 2 days |
| Df(3R)BSC138 | None | None |
| Df(3R)BSC819 | None | 1 day |
| Df(3R)ED6232 | None | 2 days |
| Df(3R)ED5177 | None | 1 day |
| Df(3R)Exel6269 | 1 day | 1 day |
| Df(3R)Exel7327 | 1 day | 1 day |
| Df(3R)BSC748 | None | 1 day |
| Df(3R)Exel6169 | None | 1 day |
| Df(3R)Exel7320 | 1 day | 1 day |
| Df(3R)ED5642 | 1 day | 2 days |
| Df(3R)Exel6195 | None | 1 day |
| Df(3R)ED5911 | 1 day | 2 days |
| Df(3R)Exel6214 | None | 1 day |
| Df(3R)BSC320 | None | 1 day |
| Df(3R)ED5780 | 1 day | 1 day |
| Df(3R)ED6220 | 1 day | 2 days |
| Df(3R)ED6025 | None | 1 day |
| Df(3R)BSC549 | 1 day | 2 days |
| Df(3R)BSC874 | None | 1 day |
| Df(3R)ED5454 | 3 days | 3 days |
| Df(3R)BSC681 | 1 day | 1 day |
| Df(3R)ED5612 | 1 day | 2 days |
| Df(3R)ED5664 | 1 day | 2 days |
| Df(3R)BSC793 | 1 day | 2 days |
| Df(3R)BSC749 | 2 days | 2 days |
| Df(3R)ED5092 | 2 days | 3 days |
| Df(3R)ED5518 | 3 days | 3 days |
| Df(3R)ED5428 | 3 days | 3 days |
| Df(3R)ED6187 | 2 days | 3 days |
| Df(3R)ED6058 | None | 2 days |
| Df(3R)ED5100 | 2 days | 3 days |
| Df(3R)ED5514 | None | None |
| Df(3R)ED10951 | 4 days | 4 days |
The deficiency regions that, when heterozygous, caused wing discs to regenerate better than controls. The developmental timing of pupariation and regeneration-specific timing of pupariation is given in the number of days before or after controls (w1118) that 50% of the animals had entered pupariation (see Materials and Methods).
Secondary screen for disruption of developmental timing
Regenerative capacity is linked to the developmental age of the tissue and, in turn, damaged tissue can regulate developmental progression. Damaged imaginal discs are capable of extending the third larval instar, delaying metamorphosis to allow sufficient time for regeneration (Halme et al. 2010). These damaged discs secrete an insulin-like peptide, dILP8, that regulates the production in the ring gland of ecdysone, the hormone that regulates the developmental transition between the larval and pupal stages (Colombani et al. 2012; Garelli et al. 2012). Despite this ability to stall development, there is a limited developmental window during which imaginal disc regeneration is possible. If tissue is damaged after a particular point in development, metamorphosis will continue without adequate time for regeneration (Smith-Bolton et al. 2009). This restriction is likely due to a spike in ecdysone levels that occurs before pupariation, as larvae with a reduced ability to synthesize ecdysone have a prolonged window in which to regenerate (Katsuyama and Paro 2013). Epigenetic silencing prevents at least one regeneration-specific enhancer from responding to damage at this stage of development (Harris et al. 2016), suggesting that broader epigenetic silencing of regeneration genes may prevent full regeneration if damage occurs just before metamorphosis.
Given these connections between developmental progression and regenerative capacity, it was important to determine whether deficiency lines with a regeneration phenotype also had defects in developmental or pupariation timing. Note that direct comparisons between regenerating larvae, which spend 24 hr at 30° (Figure 1A), and developing larvae, which remain at 18°, cannot be made due to the effects of temperature on development (Figure 2A). Therefore, we compared the pupariation timing of each deficiency line during regeneration (Figure 2, B–D) or during development (Figure 2. B’–D’) to a w1118 control that experienced the same temperature and ablation treatments.
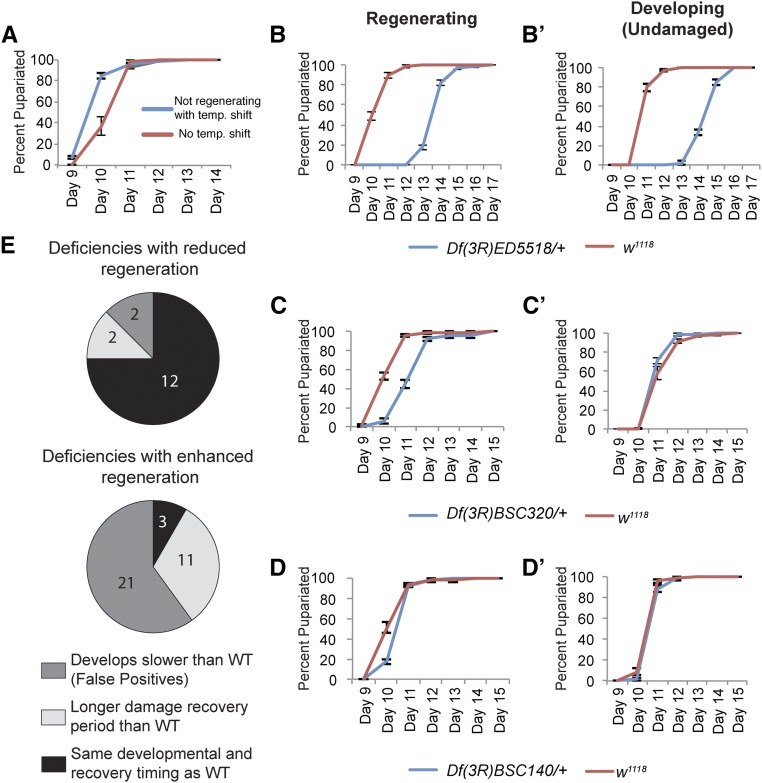
Figure 2.
Secondary screen for defects in developmental and regeneration timing. Categorization of mutant lines according to the rates at which the mutant populations reached pupariation during normal development and after tissue damage. (A) The rate at which larvae at 18° reach pupariation compared to that of larvae experiencing the 24-hr, 30° temperature shift without ablation. (B) Df(3R)ED5518/+ pupariated 4 days later than controls following tissue damage, three independent experiments, w1118 n = 157, Df(3R)ED5518/+ n = 64.; (B’) the same line also delayed pupariation during normal development, three independent experiments, w1118 n = 184 wings, Df(3R)ED5518/+ n = 64 wings. (C) Df(3R)BSC320/+ delayed pupariation 1 day longer than controls following tissue damage, three independent experiments, w1118 n = 165, Df(3R)BSC320/+ n = 96. (C’) the same line developed at the same rate as controls when undamaged, three independent experiments, w1118 n = 173, Df(3R)BSC320/+ n = 96; (D) Df(3R)BSC140/+ pupariated at the same rate as controls following tissue damage, three independent experiments, w1118 n = 128, Df(3R)BSC140/+ n = 69; (D’) the same line also developed at the same rate as controls when undamaged, three independent experiments, w1118 n = 129, Df(3R)BSC140/+ n = 52; (E) The secondary screening results for lines that regenerated poorly compared to controls: 2 lines developed slower than controls, 2 lines developed normally but had an increased recovery period following tissue damage, and 12 lines had no discernible pupariation delay after tissue damage. Data for individual lines are in Table 1. The secondary screening results for lines that regenerated better than controls: 21 lines developed slower than controls, 11 lines developed normally but had an increased recovery time following tissue damage compared to controls, and 3 lines had enhanced regeneration with no discernible change in pupariation timing relative to controls. Data for individual lines are in Table 2. temp., temperature; WT, wild-type.
There are three different ways a mutation can alter apparent regenerative capacity by altering developmental timing. First, poor regeneration can occur if the deficiency contains one or more genes that are responsible for signaling the need for a delay in progression to metamorphosis, resulting in a shortened time for regeneration. Second, enhanced regeneration can occur if the deficiency contains one or more genes that are required for normal development such that the mutant animal develops slowly even without tissue damage, resulting in ablation of a smaller amount of tissue and/or a longer time to regenerate (Figure 2, B and B’). Finally, enhanced regeneration can occur if the deficiency contains one or more genes that restrict the delay in pupariation after tissue damage, resulting in a longer time for regeneration in the mutant (Figure 2, C and C’). By contrast, a mutation that specifically affects regenerative growth would do so without affecting either normal developmental timing or the delay of entry into metamorphosis after tissue damage (Figure 2, D and D’).
We performed a secondary screen of the 51 deficiency regions that scored in our primary screen (Table 1 and Table 2). Based on these results, the animals heterozygous for the deficiencies fell into three categories. In category 1, mutant populations took longer than controls for 50% of the animals to pupariate in the absence of tissue damage, indicating an overall developmental delay. These lines with slow development also had enhanced delays in entering metamorphosis after tissue damage (Figure 2, B and B’). Only two deficiencies that caused poor regeneration (16%) fell into category 1 (Figure 2D). These mutants likely experienced extremely slow growth during normal development and regeneration. Twenty-one deficiencies that caused enhanced regeneration (60%) fell into category 1 (Figure 2E). These mutant animals likely had imaginal discs that were less mature at the time of ablation and had more time to regenerate, accounting for the apparent increase in regenerative capacity. No mutations in category 1 were selected for further analysis.
In category 2, mutant populations developed at the same rate as controls, but had a regeneration-specific delay in metamorphosis (Figure 2, C and C’). Two deficiencies that caused poor regeneration (16%) fell into category 2 (Figure 2E), indicating that loss of a gene or genes within these regions significantly impaired regeneration such that adult wings were smaller than controls even with additional time for regrowth. Eleven deficiencies that caused enhanced regeneration (31%) fell into category 2 (Figure 2E). These regions may contain genes that affect the expression of dILP8, or the systemic response to this signal that regulates entry into metamorphosis. The additional time for regeneration likely accounts for the apparent increase in regenerative capacity.
Surprisingly, none of the poorly regenerating deficiency lines had a reduction in time to pupariation. However, our lab has previously shown that animals heterozygous mutant for the gene trithorax exhibit poor regeneration because of a reduction in dilp8 expression and time before pupariation (Skinner et al. 2015). The additional time for regeneration likely accounts for the apparent increase in regenerative capacity.
In category 3, mutant populations developed normally and delayed metamorphosis at the same rate as controls. These deficiencies were most likely to contain one or more genes that regulate regeneration itself (Figure 2, D and D’). Twelve of the poorly regenerating deficiency lines (75%) had no changes in developmental timing relative to the control line (Figure 2E). Only three of the deficiencies that enhanced regeneration (8.5%) did so without affecting developmental timing relative to the control line (Figure 2E). Below, we describe the identification and characterization of one gene within a deficiency in category 3.
Identification of cnc as a Drosophila regeneration gene
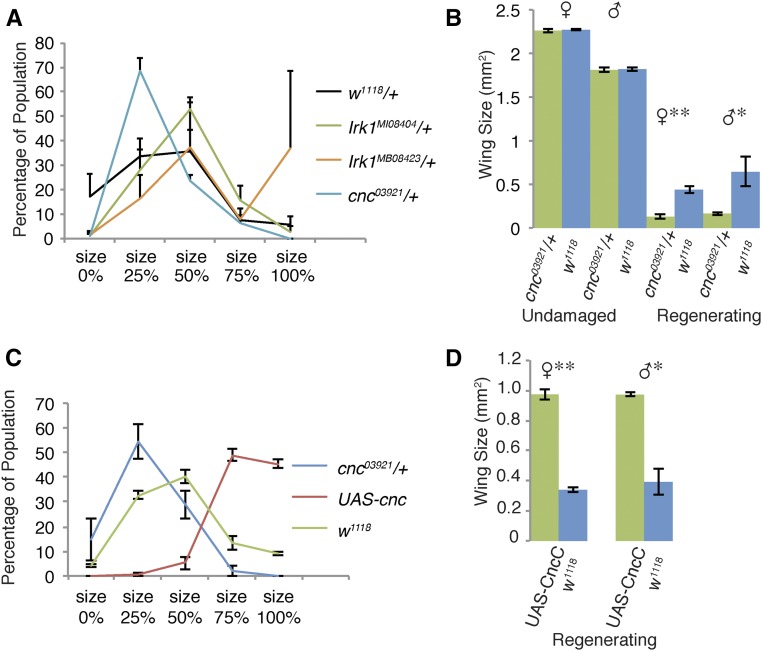
To identify the genes that are responsible for regulating regeneration, we mapped the regeneration phenotypes to smaller regions and then to individual genes. We first mapped the phenotype of Df(3R)ED6103 because it displayed a strong phenotype. We tested three overlapping deficiency lines for a regeneration phenotype (Figure S3, A and B), and identified a smaller region, which contained two genes, that was likely responsible for the phenotype. We tested mutants of the two genes, the transcription factor cnc (Mohler et al. 1991), a homolog of vertebrate Nrf2, and Inwardly Rectifying Potassium Channel-1 (Irk1) (MacLean et al. 2002) for altered regenerative capacity. The majority of the population of flies with cnc03921/+ wings only regenerated to 25% of normal size (Figure 3A), whereas the irk1/+ mutants regenerated comparably to controls, indicating that cnc is most likely the gene responsible for the phenotype. Similar results were obtained for a second, independently derived allele, cncEY08884 (Figure S3C). Measurement of absolute wing size showed that heterozygous mutants did not regenerate to the extent controls did, despite not having a size difference in the absence of tissue damage (Figure 3B), indicating that cnc is required for regeneration.
Figure 3.
Cnc (cap-n-collar) is required for regeneration. (A) Comparison of populations of adult wings after regeneration for control (w1118), cnc03921/+, Irk1MI08404/+, and Irk1MB08423/+ animals. Error bars display SEM. Three independent experiments, w1118 n = 228 wings, cnc03921/+ n = 122 wings, Irk1MI08404/+ n = 160 wings, and Irk1MB08423/+ n = 116 wings. (B) Measurement of adult wing sizes resulting from undamaged or ablated imaginal discs, in square millimeters. cnc03921/+ (undamaged male n = 56 wings, undamaged female n = 84 wings, regenerated male n = 53 wings, and regenerated female n = 82 wings) and w1118 (undamaged male n = 103 wings, undamaged female n = 165 wings, regenerated male n = 105 wings, and regenerated female n = 154 wings). Male and female wings were separated because of sexually dimorphic adult wing size. There was no significant difference between undamaged cnc and w1118 wing sizes (P > 0.5); however, there was a significant difference in wing size following tissue damage in both males and females using the Student’s t-test. * P < 0.05 and ** P < 0.005. Error bars represent SEM. (C) Comparison of populations of adult wings after imaginal disc ablation with the genotypes w1118, cnc03921/+, and UAS-cncC. Error bars represent SEM. Three independent experiments, w1118 n = 233 wings, cnc03921/+ n = 206 wings, and UAS-cncC n = 176 wings. (D) Measurement of w1118 and UAS-cncC adult wing sizes resulting from undamaged or regenerated imaginal discs in square millimeters. w1118 male n = 70 wings, female n = 131 wings, and UAS-cncC male n = 155 wings, female n = 204 wings. Error bars represent SEM. * P < 0.05, ** P < 0.005, using the Student’s t-test.
Approximately 5% of rn-GAL4-expressing cells survive ablation and contribute to the regenerating tissue (Smith-Bolton et al. 2009; Skinner et al. 2015), enabling expression of transgenic constructs in our regeneration system. Larvae with cnc levels reduced in the wing pouch via RNAi also regenerated poorly (Figure S3D). Interestingly, the severity of the phenotype was comparable between the heterozygous mutant and the RNAi knockdown. By contrast, overexpression of Cnc via UAS-cncC, the isoform known to respond to stress (Sykiotis and Bohmann 2008), led to better regeneration than in controls as assessed by our semiquantitative assay (Figure 3C), further supporting a role for Cnc in regulating the regeneration of the wing primordium. To confirm that ablation was not reduced in flies expressing UAS-Cnc, we measured the size of the wing pouch at the end of the temperature shift. At R0, the average wing pouch size of UAS-Cnc animals was the same as controls, indicating they experienced equivalent amounts of cell death (Figure S4). The enhanced regeneration induced by overexpression of Cnc was confirmed by measurement of absolute wing size (Figure 3D).
The basic leucine zipper transcription factor Cnc is a well-characterized stress response gene that is activated in the presence of ROS and xenotoxic compounds. It subsequently activates transcription of antioxidant enzymes and detoxifying proteins (reviewed in Sykiotis and Bohmann 2010). There are three protein isoforms, although CncA has no known function. The CncB isoform is required for head development (Gellon et al. 1997; McGinnis et al. 1998). CncC regulates intestinal stem cell proliferation (Hochmuth et al. 2011), the 26S proteasome (Grimberg et al. 2011), aging (Rahman et al. 2013; Tsakiri et al. 2013), and occupies the early-ecdysone puffs during metamorphosis (Deng and Kerppola 2013). Nrf2, the vertebrate ortholog of Cnc, is also required for epidermal wound healing (Beyer et al. 2007) and murine liver regeneration (Beyer et al. 2008). Interestingly, hyperactivation of Nrf2 also negatively impacts murine liver regeneration (Köhler et al. 2014), indicating that further insight into how Cnc/Nrf2 regulates regeneration is necessary. We characterized the cnc03921/+ regenerating tissue to clarify how this transcription factor regulates regeneration in Drosophila.
Cnc is required for the initial growth response to tissue damage
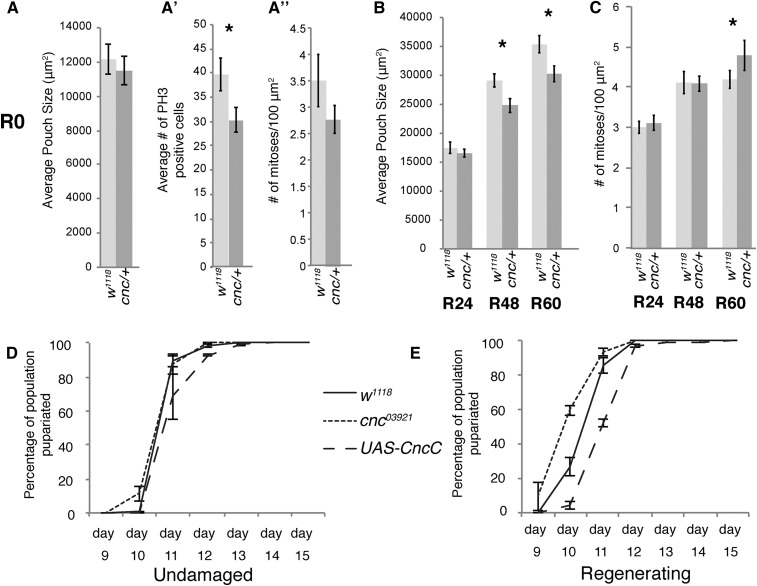
One of the early steps of regeneration is organization of a zone of proliferation at the damage site called a blastema. To test if cnc03921/+ animals fail to regenerate due to a defect in proliferation, we measured the differences in blastema growth between cnc03921/+ and control regenerating wing discs. We measured the size of the developing wing primordium during regeneration as marked by expression of the wing primordium gene nubbin. We did not observe a significant difference in the size of the primordium between cnc03921/+ and control animals at the beginning of or after 24 hr of regeneration (R0 and R24) (Figure 4,A and B). We next measured the number of mitotic nuclei in the blastema by immunostaining with anti-phospho-histone H3 (PH3). At R0, there were significantly fewer PH3+ nuclei in the cnc03921/+ wing primordia than in controls (Figure 4A’). Calculating the mitotic rate showed that cnc03921/+ discs had 20% fewer mitotic nuclei per 100 μm2 of blastema (Figure 4A’’), suggesting that the cnc03921/+ discs were slower to begin proliferation in response to the massive tissue loss.
Figure 4.
Cnc (cap-n-collar) is required for early blastema proliferation and regulates entry to metamorphosis. (A) Average wing pouch size in control and cnc03921/+ regenerating discs at R0, as marked by anti-Nubbin immunostaining. n = between 11 and 15 discs for each sample, from at least two independent experiments. * P < 0.05. (A’) Average number of mitoses, as marked by anti-phospho histone H3 (PH3), per wing pouch, as marked by anti-Nubbin, in the same discs as (A). (A’’) The average number of mitoses per 100 μm2, calculated from the same discs. Error bars represent SEM. (B) Average wing pouch size in control and cnc03921/+ regenerating discs at R24, R48, and R60, as marked by anti-Nubbin immunostaining. n = between 12 and 22 discs for each sample, from at least two independent experiments. * P < 0.05, Student’s t-test. (C) Average number of mitoses per 100 μm2, calculated from the same discs. Error bars represent SEM. (D) The percentage of normally developing animals that had formed pupae on the side of the vial each day after egg lay. These animals contained the ablation chromosome but were reared at 18° and so did not induce ablation. Three independent experiments, total n for w1118 = 205, UAS-cncC = 207, and cnc03921/+ = 103. (E) The percentage of animals with damaged imaginal discs that had formed pupae on the side of the vial each day after egg lay. The thermal shift to 30° accelerated development such that the timing in (D) cannot be compared to the timing in (E). Three independent experiments, total n for w1118 = 177, UAS-cncC = 154, and cnc03921/+ = 95. Error bars are SEM.
Such a subtle difference in proliferation rate may require several rounds of cell division before it affects the overall size of the blastema. Indeed, while the average regenerating cnc03921/+ wing pouch size was the same as controls at R24, it began lagging behind controls at R48 and continued to be smaller than controls at R60 (Figure 4B). Interestingly, the mitotic rate in the cnc03921/+ wing pouch was equal to that of controls at R24 and R48, indicating that the cnc03921/+ blastema was able to proliferate appropriately after the early stages of regeneration (Figure 4C). The mitotic rate was actually higher in the cnc03921/+ regenerating wing pouch at R60, which is shortly before the animals enter metamorphosis. These late rounds of proliferation were insufficient to make up for the slow initial regenerative growth. Thus, the cnc03921/+ discs grew slowly during the early stages of regeneration, likely because of a defect in activating proliferation due to insufficient expression of Cnc targets.
Cnc is required for proper timing of pupariation after tissue damage
To determine the extent to which Cnc regulates the systemic signaling that delays metamorphosis, another process required for regeneration after tissue damage, we quantified the rate of pupariation in control, cnc03921/+, and UAS-cncC animals with regenerating wing discs. cnc03921/+ and UAS-CncC animals developed at the same rate as controls when undamaged (Figure 4D). However, cnc03921/+ did not delay pupariation as long as controls after the tissue had been damaged, while UAS-CncC delayed pupariation longer than controls (Figure 4E). The difference in time of entry to metamorphosis was approximately 12 hr early for cnc03921/+. Interestingly, the original Df that scored in our genetic screen did not cause a reduction in time to pupariation. However, this large Df removes many genes, and may remove additional loci that impact pupariation timing. By contrast, the UAS-CncC regenerating wing pouches had an extra 12 hr before pupariation. These animals were not different in size from controls at R60 (Figure S4), suggesting that this difference in pupariation timing may account for most or all of the differences in the final adult wing size and, thus, regenerative capacity induced by overexpression of Cnc. Because pupariation delay after tissue damage is regulated by dilp8, which is regulated by JNK signaling (Colombani et al. 2012; Katsuyama et al. 2015), the differences in pupariation timing in cnc03921/+ and UAS-cncC could be due to misregulation of JNK signaling in Cnc mutants. Thus, the cnc03921/+ mutants regenerate poorly due to a combination of reduced growth early in regeneration, and reduced time for regeneration caused by premature pupariation, both of which are consistent with defects in JNK signaling.
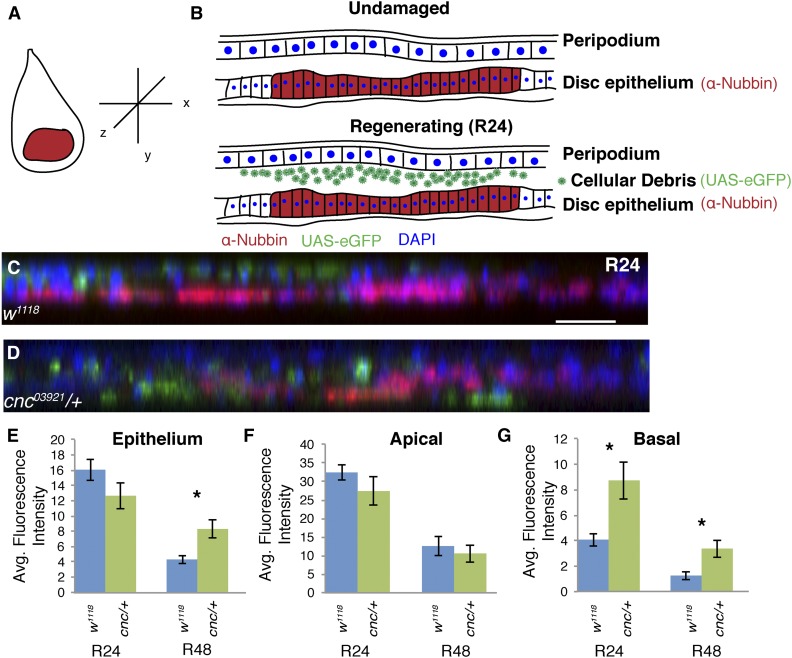
Cnc regulates debris localization after tissue ablation
Another early step in regeneration is reestablishing tissue continuity and clearing cellular debris. We noticed that there was a difference in the distribution of cellular debris among the different genotypes. Because the cellular debris may indicate differences in wound closure and debris clearance, and the debris itself may signal to the regenerating epithelium and impact regeneration signaling, we quantified the distribution of cellular debris following ablation. To mark the debris, we expressed UAS-eGFP with the ablation chromosome, such that eGFP was transiently expressed in the same cells that expressed reaper. Thus, GFP was found in dead and dying cells, as well as any rn-GAL4-expressing cells that survived the ablation (Figure 5, A and B).
Figure 5.
Cnc prevents persistence of basally localized cellular debris. Distribution of cellular debris marked by UAS-eGFP expression in regenerating discs. (A) Typical view of a wing imaginal disc in the XY-plane. (B) Schematic of wing discs in the YZ-plane, which is the orientation of all confocal images shown (orthogonal slices). UAS-eGFP is expressed during the 24-hr ablation period; 24 hr after ablation has ended, the visible GFP is in the debris in the lumen between the peripodium and disc epithelium. (C and D) Orthogonal slices showing the debris fields (eGFP, green) and regenerating epithelia (anti-Nubbin, red; DAPI, blue) at R24. In w1118, the debris is apical to the disc epithelium (C). In cnc03921/+ mutants, the debris is located both apical and basal to the disc epithelium (D). The bar in (C) is equal to 20 μm. (E–G) Quantification of fluorescence intensity in the epithelium (E), and regions apical (F) and basal (G) to the epithelium of the two genotypes at R24 and R48. Error bars are SEM. * P < 0.05, Student’s t-test. Avg., average; Cnc, cap-n-collar; eGFP, enhanced GFP; UAS, upstream activating sequence.
To obtain a more complete view of the debris location, we examined orthogonal slices of the wing primordium during regeneration, noting the location of the GFP-containing debris. We measured fluorescence intensity within the region apical to the epithelial sheet and below the peripodium, within the epithelium as marked by the Nubbin antibody, and within the region basal to the epithelial sheet (Figure 5, C and D). The amount of GFP in the epithelium was not significantly different between the genotypes at R24 (Figure 5E), indicating that the number of cells surviving ablation was similar. However, the GFP levels in the cnc03921/+ mutant epithelium at R48 were significantly higher than in the w1118 epithelium. Given that Cnc/Nrf2 can regulate proteasomal and lysosomal degradation in other contexts (Grimberg et al. 2011; Nagy et al. 2013; Pickering et al. 2013), the persistent GFP in the epithelium suggests that the cnc03921/+ mutants may have been unable to degrade the GFP or, if the epithelial cells ingest debris, they were less able to degrade the debris they were clearing.
In the ablation system used here, the large field of debris was extruded apically (Figure 5, C and F), which is contrary to most single-cell extrusion from epithelial sheets and may occur because such a large number of cells die simultaneously. Interestingly, in cnc03921/+-ablated discs, there was significantly more debris in the region basal to the epithelium (Figure 5, D and G), suggesting that these regenerating discs may have an impaired ability to move dying cells and debris to the apical surface, or that there is a difference in wound closure, resulting in an atypical distribution of debris. This failure to remove debris directionally and efficiently may affect regeneration by imposing physical constraints or by impairing or changing the location of any pro-regeneration signals emitted by dying cells.
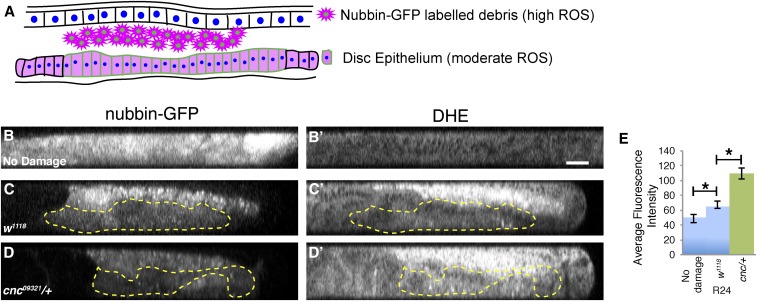
ROS levels are higher in regenerating tissue with reduced Cnc
Cnc/Nrf2 is closely associated with changes in expression of antioxidant enzymes in response to ROS (Itoh et al. 2004; Sykiotis and Bohmann 2008). Damage to imaginal discs leads to ROS production by the resulting cellular debris and by the healing epithelium [Santabárbara-Ruiz et al. 2015; Fogarty et al. 2016; reviewed in Serras (2016)]. To determine the extent to which Cnc affects levels of ROS in the regeneration blastema, we used DHE staining (Owusu-Ansah et al. 2008) to observe ROS levels in control and cnc03921/+ regenerating discs after 24 hr of regeneration. ROS levels were high in the cellular debris, but also present in the epithelium, which is the area of interest (Figure 6A). Undamaged samples had very low levels of ROS (Figure 6B’). Damaged samples had higher levels of ROS in the regenerating epithelium compared to an undamaged disc (Figure 6C’), as previously reported (Santabárbara-Ruiz et al. 2015). However, cnc03921/+ mutants had significantly higher levels of ROS in the regenerating epithelium compared to controls (Figure 6, C–G), indicating that Cnc is required to control ROS levels in the blastema during regeneration. The elevated ROS in the cnc03921/+ regenerating epithelium could have several deleterious effects, including increasing damage and cell death in the blastema and interfering with regeneration signaling, contributing to the poor regeneration observed in the cnc03921/+ tissue.
Figure 6.
Reactive oxygen species (ROS) levels are higher in the blastema when Cnc (cap-n-collar) levels are reduced. (A) Schematic of a disc in the XY plane. A nubbin-GFP enhancer trap labels the debris and the regenerating epithelium. Dihydroethidum (DHE) fluorescence marking ROS is present in high levels in the cellular debris and lower levels in the disc epithelium. (B–D) Wing discs presented in the YZ-plane expressing a nubbin-GFP reporter to mark the wing primordium and debris (B–D) with DHE staining to indicate ROS levels (B’–D’). Discs are w1118 undamaged (B and B’), w1118 R24 (C and C’), or cnc03921/+ R24 (D and D’). Yellow dotted lines outline the diffuse nub-GFP expression in the regenerating wing pouch, and exclude the bright GFP with puncta in the debris. Bar = 20 μm. (E) DHE levels were quantified by measuring fluorescence intensity in three equal-sized boxes in the epithelial layer of each disc. Undamaged, n = 13; w1118, n = 15; and cnc03921, n = 15. Error bars are SEM. * P < 0.05, Student’s t-test.
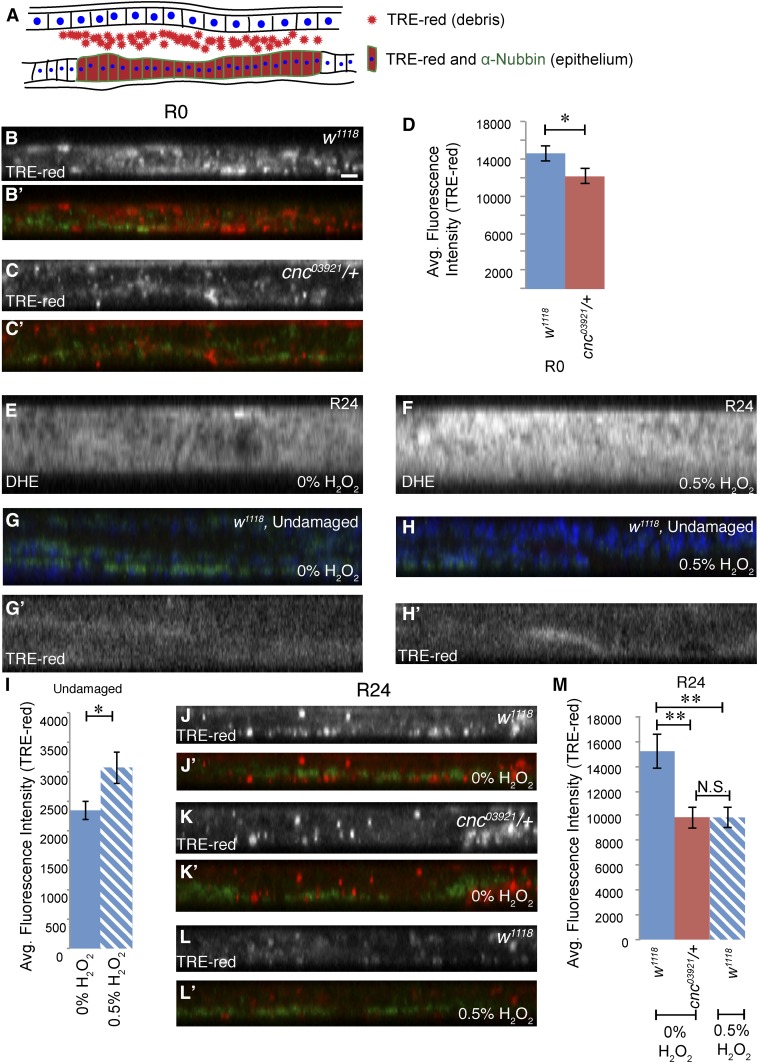
Cnc regulates regeneration signaling
ROS presence in damaged tissue induces would healing and activation of JNK signaling (Santabárbara-Ruiz et al. 2015; Fogarty et al. 2016). Additionally, the cnc03921 mutants showed defects in wound healing, blastema formation, growth, and pupariation timing, processes that are regulated by JNK signaling (Bosch et al. 2005; Mattila et al. 2005; Bergantiños et al. 2010; Colombani et al. 2012; Garelli et al. 2012). Thus, reduction of Cnc levels might affect JNK signaling, either directly through misregulation of its transcriptional targets or indirectly through misregulation of ROS levels. To examine JNK signaling, we quantified expression of a TRE-red transcriptional reporter (Chatterjee and Bohmann 2012). While expression levels were high in the cellular debris, our region of interest was the regenerating epithelium (Figure 7A). This reporter was higher in the epithelium of control discs immediately after tissue ablation (R0) than in cnc03921/+ regenerating discs at R0 (Figure 7B–D). Thus, cnc03921/+ damaged tissue did not have the same initial levels of JNK signaling as control damaged tissue. Interestingly, after 48 hr of regeneration, levels of JNK signaling were comparable in cnc03921/+ regenerating discs and controls, further suggesting that Cnc activity is more important early in regeneration (Figure S5, A–C).
Figure 7.
JNK signaling is reduced during regeneration by exposure to excessive ROS. (A) Schematic of a wing disc in the YZ plane. A reporter for JNK signaling, TRE-red, is present at high levels in the debris and at lower levels in the disc epithelium, which is identified by Nubbin antibody (green). (B and C) Wing discs at the end of the ablation period (R0) with TRE-red (red) and anti-Nubbin (green) of the genotypes w1118 (B) and cnc03921/+ (C). Bar in (B) = 20 μm. (D) Quantification of TRE-red fluorescence levels in the disc epithelium (w1118 n = 20 and cnc03921/+ n = 16). (E and F) DHE staining of wing imaginal discs from larvae fed 0 and 0.5% H2O2. Note that feeding 0.5% H2O2 to the larvae results in higher ROS levels in the wing discs. (G and H) Undamaged w1118 wing discs from larvae fed 0% H2O2 (G and G’), or 0.5% H2O2 (H and H’) with anti-Nubbin (green), TRE-Red (red), and DAPI (blue). (G’ and H’) The TRE-Red alone, adjusted for brightness and contrast to enhance visibility. (I) Quantification of TRE-Red performed on unadjusted images (see Materials and Methods): 0% H2O2, w1118 n = 14 and 0.5% H2O2, w1118 n = 20. (J–M) TRE-Red (red) and anti-Nubbin (green) in wing discs after 24 hr of regeneration. (J and J’) w1118, fed 0% H2O2; (K and K’) cnc03921/+, fed 0% H2O2; and (L and L’) w1118, fed 0.5% H2O2. (M) Quantification of (J–L). (0% H2O2, w1118 n = 31, cnc03921/+ n = 25; R24 0.5% H2O2, w1118 n = 30). Note that TRE-Red levels in cnc03921/+ discs are equal to those in w1118 discs with ectopic 0.5% H2O2. Error bars are SEM. * P < 0.05, ** P < 0.005, Student’s t-test. Avg., average; DHE, dihydroethidum; JNK, c-Jun N-terminal protein kinase; N.S., not significant; ROS, reactive oxygen species.
These data suggest that Cnc regulates JNK signaling during the early stages of regeneration. There are two possible mechanisms through which Cnc could be required for JNK signaling, which are not mutually exclusive. First, direct genetic targets of Cnc may promote JNK signaling. Second, there may be an optimal level of ROS to activate JNK signaling, and excessive amounts of ROS might decrease JNK activation. To determine whether elevated ROS can inhibit JNK signaling, we exposed regenerating tissue to ectopic ROS and assessed the effects on JNK activation. We supplemented the food with 0.5% H2O2 and stained with DHE to confirm that consumption of H2O2 by the larvae increased the amount of ROS in regenerating discs (Figure 7, E and F). We also observed that increased ROS in undamaged discs did activate JNK expression, as expected (Santabárbara-Ruiz et al. 2015) (Figure 7, G–I). Interestingly, ectopically increasing ROS for the first 24 hr of regeneration lowered JNK activation (Figure 7, J–M), supporting the idea that excess ROS can dampen the JNK signal. Thus, Cnc ensures proper levels of JNK signaling at least in part through maintaining optimal ROS levels, although additional Cnc-dependent mechanisms of regulating JNK have not been ruled out.
To assess the influence of Cnc on regeneration signaling and gene expression downstream of JNK, we assessed Wg and Myc expression levels in cnc03921/+ regenerating discs via immunostaining. Quantification of staining intensity showed statistically significant reduction in levels of both Wg and Myc in cnc03921/+ regenerating discs at R24 compared to controls (Figure S5, D–I). These small yet significant reductions in Wg and Myc may be a result of the reduction in JNK signaling. Expression of Myc was comparable to controls by R48 (Figure S5F). The cnc03921/+ regenerating discs resolved Wg expression to normal third instar patterning by R48.
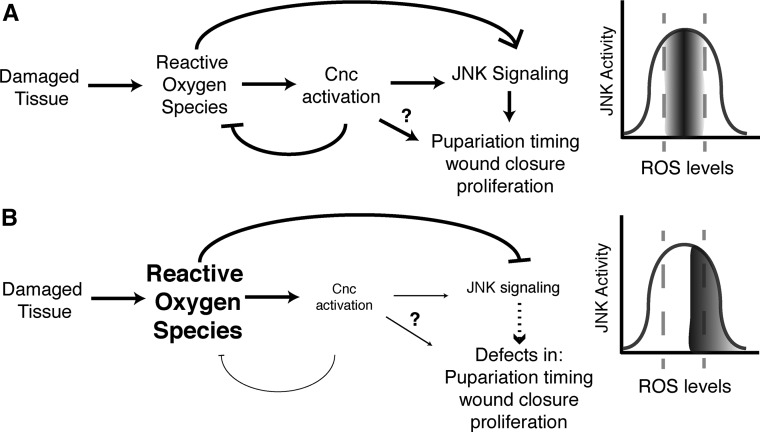
All together, these data led us to a model wherein ROS is necessary to activate JNK signaling after tissue damage, but too much ROS reduces JNK signaling, and Cnc is required to maintain ROS at the appropriate level (Figure 8A). When Cnc levels are lowered, ROS levels increase such that JNK signaling is lowered, leading to regeneration defects (Figure 8B). Our data do not rule out a role for Cnc in activating the JNK pathway independently of ROS levels; indeed, it is possible that Cnc regulates JNK through both mechanisms. Furthermore, we have not ruled out the possibility that Cnc affects regeneration independently of JNK.
Figure 8.
Model for Cnc activity during regeneration. (A) Proposed relationship between ROS levels, Cnc, JNK signaling, and regenerative capacity. Injury induces ROS, which in turn activate JNK and Cnc activity. Cnc transcriptional targets constrain ROS levels and may also regulate JNK activity. Thus, there is an ideal range of ROS levels at which regeneration is most efficient. (B) Model where Cnc levels are reduced. Injury induces ROS, which in turn activate JNK and Cnc. Less Cnc activity results in high ROS levels and low JNK activation, due to an inhibitory effect of high ROS and possibly due to reduction of other important Cnc targets. Cnc, cap-n-collar; c-Jun N-terminal protein kinase; ROS, reactive oxygen species.
Discussion
The first regeneration gene mapped and identified from our screen of chromosome arm 3R was cnc. In contrast to other regulators identified through our pilot genetic screen (Smith-Bolton et al. 2009; Schuster and Smith-Bolton 2015; Skinner et al. 2015), reduction of Cnc levels affected multiple factors during regeneration. Our data demonstrate that Cnc has an important role in maintaining proper ROS levels during regeneration, presumably through regulating expression of antioxidant genes [reviewed in Pitoniak and Bohmann (2015)]. ROS are one of the earliest signals that tissue damage has occurred (Niethammer et al. 2010; Yoo et al. 2012). However, ROS themselves are also agents of tissue damage (Fridovich 1978). Thus, there is likely an ideal level of ROS for stimulating regeneration, and too much or too little ROS could impair regenerative capacity (Figure 8A). Recent work has shown that ROS activates JNK signaling during regeneration (Santabárbara-Ruiz et al. 2015). However, we show here that the reduction of Cnc results in increased ROS (Figure 6) but decreased JNK signaling (Figure 7). Thus, there is not a direct linear relationship between ROS levels and the amount of JNK activation, and there is a point at which excess ROS dampens JNK activation (Figure 8B). Interestingly, our data also show that Cnc affects many regenerative processes that are regulated by JNK signaling, including wound closure and debris localization, early blastema proliferation, and stalling entry to pupariation. We propose a model where cnc has important regulatory roles during regeneration. First, it regulates the response to the presence of ROS and keeps ROS at the appropriate levels for efficient regeneration. Second, it regulates JNK signaling, via ROS levels and possibly via additional transcriptional targets (Figure 8B). Third, it may regulate regenerative processes independently of JNK (Figure 8).
While work to date in Drosophila has focused on the role of Cnc in the cellular response to stress and to xenobiotic factors (Deng and Kerppola 2013, 2014), as well as oxidative stress related to aging and aging-related neurological disorders (Sykiotis and Bohmann 2008, 2010; Barone et al. 2011; Rahman et al. 2013), work in vertebrates has identified Nrf2 as an important regulator of epidermal wound healing (Beyer et al. 2007; Schäfer and Werner 2008) and liver regeneration (Beyer et al. 2008; Beyer and Werner 2008; Wakabayashi et al. 2010; Dayoub et al. 2013; Zou et al. 2014). Thus, by establishing a complementary model to explore the role of Cnc in imaginal disc repair and regeneration, we will be able to gain deeper understanding of the role of this transcription factor in both invertebrate and vertebrate tissue repair. For example, overactivated Nrf2 negatively impacts liver regeneration (Köhler et al. 2014). Given the similarities in the imaginal disc, where both too much and too little ROS can result in insufficient JNK signaling for regeneration, further elucidation of the mechanism relating ROS, Cnc/Nrf2, and JNK signaling in response to damage or other stressors may offer insight into the protective mechanisms at work in vertebrate organisms. Systematic identification of the transcriptional targets of Cnc during wound healing and regeneration, which is beyond the scope of this work, will be tremendously informative concerning the regulation of regeneration in this and other systems.
In addition to the chromosomal deficiency that removed cnc, the genetic screen described here has isolated chromosomal deficiencies that remove a variety of important regeneration genes, likely including wound response genes, growth regulators, genes required for patterning, systemic signaling factors, and novel genes that have heretofore unknown roles in regeneration. The secondary screen we carried out provides some clues to the different processes regulated by each mutant. For example, the genes identified by mapping the phenotypes within the deficiencies that affect the timing of metamorphosis will further characterize the mechanism of systemic signaling that coordinates growth regulation and metamorphosis after tissue damage (Colombani et al. 2012; Garelli et al. 2012; Jaszczak et al. 2015). The secondary screen also narrowed down the number of deficiencies that likely contain negative regulators of regeneration to three. These negative regulators may act as a brake on cellular growth, but their reduction did not result in obvious overgrowth. They regenerated faster but still respected endogenous organ size control mechanisms. Such factors are of particular interest because inhibition of their vertebrate orthologs might have the potential to enhance regeneration without inducing tumor formation.
We have only begun to characterize the genes identified in this screen. As the number of mapped deficiency regions increases and we identify and characterize novel regeneration genes, this screen will prove to be a powerful tool in our quest to characterize regeneration. Furthermore, we expect that by expanding this screen to other chromosome arms, we will generate a more complete understanding of the regulation of regeneration in the Drosophila imaginal wing disc.
Supplementary Material
Supplemental material is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.196832/-/DC1.
Acknowledgments
The authors thank R. Roberts-Galbraith and S. J. Khan for critical reading of the manuscript and helpful comments; D. Bohmann and S. Cohen for reagents; the Bloomington Drosophila Stock Center [National Institutes of Health (NIH) P40-OD018537], the Vienna Drosophila Resource Center, and the Kyoto Stock Center for Drosophila lines; and the Developmental Studies Hybridoma Bank (Eunice Kennedy Shriver National Institute of Child Health and Human Development, University of Iowa) for antibodies. This work was funded by a Young Investigator Award from the Roy J. Carver Charitable Trust (#12-4041) and a grant from the NIH (National Institute of General Medical Sciences R01-GM107140).
Footnotes
Communicating editor: R. J. Duronio
Literature Cited
- Bando T., Mito T., Maeda Y., Nakamura T., Ito F., et al. , 2009. Regulation of leg size and shape by the Dachsous/Fat signalling pathway during regeneration. Development 136: 2235–2245. [DOI] [PubMed] [Google Scholar]
- Barone M. C., Sykiotis G. P., Bohmann D., 2011. Genetic activation of Nrf2 signaling is sufficient to ameliorate neurodegenerative phenotypes in a Drosophila model of Parkinson’s disease. Dis. Model. Mech. 4: 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A., Sibilia M., David J. P., Möhle-Steinlein U., Tronche F., et al. , 2002. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. EMBO J. 21: 1782–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen H. J., Levis R. W., Liao G., He Y., Carlson J. W., et al. , 2004. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167: 761–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergantiños C., Corominas M., Serras F., 2010. Cell death-induced regeneration in wing imaginal discs requires JNK signalling. Development 137: 1169–1179. [DOI] [PubMed] [Google Scholar]
- Beyer T. A., Werner S., 2008. The cytoprotective Nrf2 transcription factor controls insulin receptor signalling in the regenerating liver. Cell Cycle 7: 874–879. [DOI] [PubMed] [Google Scholar]
- Beyer T. A., Auf dem Keller U., Braun S., Schäfer M., Werner S., 2007. Roles and mechanisms of action of the Nrf2 transcription factor in skin morphogenesis, wound repair and skin cancer. Cell Death Differ. 14: 1250–1254. [DOI] [PubMed] [Google Scholar]
- Beyer T. A., Xu W., Teupser D., Auf dem Keller U., Bugnon P., et al. , 2008. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 27: 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M., Serras F., Martin-Blanco E., Baguñà J., 2005. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev. Biol. 280: 73–86. [DOI] [PubMed] [Google Scholar]
- Brand A., Perrimon N., 1996. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 138: 401–415. [DOI] [PubMed] [Google Scholar]
- Brook W. J., Cohen S. M., 1996. Antagonistic interactions between wingless and decapentaplegic responsible for dorsal-ventral pattern in the Drosophila leg. Science 273: 1373–1377. [DOI] [PubMed] [Google Scholar]
- Brook W. J., Ostafichuk L. M., Piorecky J., Wilkinson M. D., Hodgetts D. J., et al. , 1993. Gene expression during imaginal disc regeneration detected using enhancer-sensitive P-elements. Development 117: 1287–1297. [DOI] [PubMed] [Google Scholar]
- Chatterjee N., Bohmann D., 2012. A versatile ΦC31 based reporter system for measuring AP-1 and Nrf2 signaling in Drosophila and in tissue culture. PLoS One 7: e34063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J., Andersen D. S., Leopold P., 2012. Secreted peptide Dilp8 coordinates. Science 582: 582–585. [DOI] [PubMed] [Google Scholar]
- Cook R. K., Christensen S. J., Deal J. A., Coburn R. A., Deal M. E., et al. , 2012. The generation of chromosomal deletions to provide extensive coverage and subdivision of the Drosophila melanogaster genome. Genome Biol. 13: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayoub R., Vogel A., Schuett J., Lupke M., Spieker S. M., et al. , 2013. Nrf2 activates augmenter of liver regeneration (ALR) via antioxidant response element and links oxidative stress to liver regeneration. Mol. Med. 19: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Kerppola T. K., 2013. Regulation of Drosophila metamorphosis by xenobiotic response regulators. PLoS Genet. 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Kerppola T. K., 2014. Visualization of the Drosophila dKeap1-CncC interaction on chromatin illumines cooperative, xenobiotic-specific gene activation. Development 141: 3277–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K.-C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156. [DOI] [PubMed] [Google Scholar]
- Fan Y., Wang S., Hernandez J., Yenigun V. B., Hertlein G., et al. , 2014. Genetic models of apoptosis-induced proliferation decipher activation of JNK and identify a requirement of EGFR signaling for tissue regenerative responses in Drosophila. PLoS Genet. 10: e1004131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty C. E., Diwanji N., Lindblad J. L., Bru K., Fan Y., et al. , 2016. Apoptosis-induced proliferation via Drosophila article extracellular reactive oxygen species drive apoptosis-induced proliferation via Drosophila macrophages. Curr. Biol. 26: 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I., 1978. The biology of oxygen radicals. Science 201: 875–880. [DOI] [PubMed] [Google Scholar]
- Garelli A., Gontijo A. M., Miguela V., Caparros E., Dominguez M., 2012. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336: 579–582. [DOI] [PubMed] [Google Scholar]
- Gauron C., Rampon C., Bouzaffour M., Ipendey E., Teillon J., et al. , 2013. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci. Rep. 3: 2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellon G., Harding K. W., McGinnis N., Martin M. M., McGinnis W., 1997. A genetic screen for modifiers of Deformed homeotic function identifies novel genes required for head development. Development 124: 3321–3331. [DOI] [PubMed] [Google Scholar]
- Gerhold A. R., Richter D. J., Yu A. S., Hariharan I. K., 2011. Identification and characterization of genes required for compensatory growth in Drosophila. Genetics 189: 1309–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grijalva J. L., Huizenga M., Mueller K., Rodriguez S., Brazzo J., et al. , 2014. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 307: G196–G204. [DOI] [PubMed] [Google Scholar]
- Grimberg K. B., Beskow A., Lundin D., Davis M. M., Young P., 2011. Basic leucine zipper protein Cnc-C is a substrate and transcriptional regulator of the Drosophila 26S proteasome. Mol. Cell. Biol. 31: 897–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover D., Ford D., Brown C., Hoe N., Erdem A., et al. , 2009. Hydrogen peroxide stimulates activity and alters behavior in Drosophila melanogaster. PLoS One 4: e7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusche F. A., Degoutin J. L., Richardson H. E., Harvey K. F., 2011. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev. Biol. 350: 255–266. [DOI] [PubMed] [Google Scholar]
- Hadorn E., Buck D., 1962. On the differentiation of transplanted wing imaginal disc fragments of Drosophila melanogaster. Rev. Suisse Zool. 69: 302–310. [Google Scholar]
- Hadorn E., Bertani G., Gallera J., 1949. Regulative capacity and field organization of male genital discs in Drosophila melanogaster. Roux’s Arch. Entwickl. 144: 31–70. [DOI] [PubMed] [Google Scholar]
- Halfon M. S., Gisselbrecht S., Lu J., Estrada B., Keshishian H., et al. , 2002. New fluorescent protein reporters for use with the Drosophila Gal4 expression system and for vital detection of balancer chromosomes. Genesis 34: 135–138. [DOI] [PubMed] [Google Scholar]
- Halme A., Cheng M., Hariharan I. K., 2010. Retinoids regulate a developmental checkpoint for tissue regeneration in Drosophila. Curr. Biol. 20: 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. E., Setiawan L., Saul J., Hariharan I. K., 2016. Localized epigenetic silencing of a damage-activated WNT enhancer limits regeneration in mature Drosophila imaginal discs. eLife 5: e11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobmayer B., Rentzsch F., Kuhn K., Happel C. M., Cramer von Laue C., et al. , 2000. WNT signalling molecules act in axis formation in the diploblastic metazoan Hydra. Nature 407: 186–189. [DOI] [PubMed] [Google Scholar]
- Hochmuth C. E., Biteau B., Bohmann D., Jasper H., 2011. Redox regulation by keap1 and Nrf2 controls intestinal stem cell proliferation in drosophila. Cell Stem Cell 8: 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Tong K. I., Yamamoto M., 2004. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 36: 1208–1213. [DOI] [PubMed] [Google Scholar]
- Jaszczak J. S., Wolpe J. B., Dao A. Q., Halme A., 2015. Nitric oxide synthase regulates growth coordination during. Genetics 4: 1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuyama T., Paro R., 2013. Innate immune cells are dispensable for regenerative growth of imaginal discs. Mech. Dev. 130: 112–121. [DOI] [PubMed] [Google Scholar]
- Katsuyama T., Comoglio F., Seimiya M., Cabuy E., Paro R., 2015. During Drosophila disc regeneration, JAK/STAT coordinates cell proliferation with Dilp8-mediated developmental delay. Proc. Natl. Acad. Sci. USA 112: E2327–E2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Rodriguez C., Raya M., Kawakami H., Dubova I., et al. , 2006. Wnt/β-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 20: 3232–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. J., Abidi S. N. F., Tian Y., Skinner A., Smith-Bolton R. K., 2016. A rapid, gentle and scalable method for dissociation and fluorescent sorting of imaginal disc cells for mRNA sequencing. Fly (Austin) 10: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler U. A., Kurinna S., Schwitter D., Marti A., Schäfer M., et al. , 2014. Activated Nrf2 impairs liver regeneration in mice by activation of genes involved in cell-cycle control and apoptosis. Hepatology 60: 670–678. [DOI] [PubMed] [Google Scholar]
- Lin A. Y. T., Pearson B. J., 2014. Planarian yorkie/YAP functions to integrate adult stem cell proliferation, organ homeostasis and maintenance of axial patterning. Development 141: 1197–1208. [DOI] [PubMed] [Google Scholar]
- Love N. R., Chen Y., Ishibashi S., Kritsiligkou P., Lea R., et al. , 2013. Europe PMC Funders Group Amputation-induced reactive oxygen species (ROS) are required for successful Xenopus tadpole tail regeneration. Nat. Cell Biol. 15: 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean S. J., Andrews B. C., Verheyen E. M., 2002. Characterization of Dir: a putative potassium inward rectifying channel in Drosophila. Mech. Dev. 116: 193–197. [DOI] [PubMed] [Google Scholar]
- Markstein M., Pitsouli C., Villalta C., Celniker S. E., Perrimon N., 2008. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat. Genet. 40: 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blanco E., Gampel A., Ring J., Virdee K., Kirov N., et al. , 1998. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 12: 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila J., Omelyanchuk L., Kyttälä S., Turunen H., Nokkala S., 2005. Role of Jun N-terminal Kinase (JNK) signaling in the wound healing and regeneration of a Drosophila melanogaster wing imaginal disc. Int. J. Dev. Biol. 49: 391–399. [DOI] [PubMed] [Google Scholar]
- McClure K. D., Schubiger G., 2008. A screen for genes that function in leg disc regeneration in Drosophila melanogaster. Mech. Dev. 125: 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis N., Ragnhildstveit E., Veraksa A., McGinnis W., 1998. A cap ‘n’ collar protein isoform contains a selective Hox repressor function. Development 125: 4553–4564. [DOI] [PubMed] [Google Scholar]
- McGuire S. E., Le P. T., Osborn A. J., Matsumoto K., Davis R. L., 2003. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302: 1765–1768. [DOI] [PubMed] [Google Scholar]
- Meserve J. H., Duronio R. J., 2015. Scalloped and Yorkie are required for cell cycle re-entry of quiescent cells after tissue damage. Development 142: 2740–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler J., Vani K., Leung S., Epstein A., 1991. Segmentally restricted, cephalic expression of a leucine zipper gene during Drosophila embryogenesis. Mech. Dev. 34: 3–10. [DOI] [PubMed] [Google Scholar]
- Muliyil S., Narasimha M., 2014. Mitochondrial ROS regulates cytoskeletal and mitochondrial remodeling to tune cell and tissue dynamics in a model for wound healing. Dev. Cell 28: 239–252. [DOI] [PubMed] [Google Scholar]
- Nagarkar-Jaiswal S., Lee P., Campbell M. E., Chen K., Anguiano-Zarate S., et al. , 2015. A library of MiMICs allows tagging of genes and reversible, spatial and temporal knockdown of proteins in Drosophila. eLife 4: e05338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy P., Varga Á., Pircs K., Hegedus K., Juhász G., 2013. Myc-driven overgrowth requires unfolded protein response-mediated induction of autophagy and antioxidant responses in Drosophila melanogaster. PLoS Genet. 9: e1003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M., Diaz-Benjumea F. J., Vincent J. P., Wu J., Cohen S. M., 1996. Specification of the wing by localized expression of wingless protein. Nature 381: 316–318. [DOI] [PubMed] [Google Scholar]
- Ni J. Q., Zhou R., Czech B., Liu L., Holderbaum L., et al. , 2011. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 8: 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer P., Grabher C., Look A. T., Timothy J., Hospital C., 2010. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459: 996–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E., Yavari A., Banerjee U., 2008. A protocol for in vivo detection of reactive oxygen species. Protoc. Exch. Available at: https://www.nature.com/protocolexchange/protocols/414. [Google Scholar]
- Parks A. L., Cook K. R., Belvin M., Dompe N. A., Fawcett R., et al. , 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36: 288–292. [DOI] [PubMed] [Google Scholar]
- Perrimon N., Lanjuin A., Arnold C., Elizabeth N., 1996. Zygotic lethal mutations with maternal effect phenotypes. Genetics 144: 1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. P., Reddien P. W., 2008. Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 319: 327–330. [DOI] [PubMed] [Google Scholar]
- Petersen C. P., Reddien P. W., 2009. A wound-induced Wnt expression program controls planarian regeneration polarity. Proc. Natl. Acad. Sci. USA 106: 17061–17066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering A. M., Staab T. A., Tower J., Sieburth D., Davies K. J. A., 2013. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative-stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. J. Exp. Biol. 216: 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotte N., Stevens A., Fraguas S., Plusquin M., Van Roten A., et al. , 2015. Reactive oxygen species in planarian regeneration: an upstream necessity for correct patterning and brain formation. Oxid. Med. Cell. Longev. 2015: 392476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitoniak A., Bohmann D., 2015. Mechanisms and functions of Nrf2 signaling in Drosophila. Free Radic. Biol. Med. 88B: 302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. M., Sykiotis G. P., Nishimura M., Bodmer R., Bohmann D., 2013. Declining signal dependence of Nrf2-MafS-regulated gene expression correlates with aging phenotypes. Aging Cell 12: 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder E., 2004. The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167: 797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santabárbara-Ruiz P., López-Santillán M., Martínez-Rodríguez I., Binagui-Casas A., Perez L., et al. , 2015. ROS-induced JNK and p38 signaling is required for unpaired cytokine activation during Drosophila regeneration. PLoS Genet. 11: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer M., Werner S., 2008. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 58: 165–171. [DOI] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S., Eliceiri K. W., 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster K. J., Smith-Bolton R. K., 2015. Taranis protects regenerating tissue from fate changes induced by the wound response in Drosophila. Dev. Cell 34: 119–128. [DOI] [PubMed] [Google Scholar]
- Serras F., 2016. The benefits of oxidative stress for tissue repair and regeneration. Fly (Austin) 0: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner A., Khan S. J., Smith-Bolton R. K., 2015. Trithorax regulates systemic signaling during Drosophila imaginal disc regeneration. Development 142: 3500–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Bolton R. K., Worley M. I., Kanda H., Hariharan I. K., 2009. Regenerative growth in Drosophila imaginal discs is regulated by wingless and Myc. Dev. Cell 16: 797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G., Irvine K. D., 2011. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev. Biol. 350: 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis G., Bohmann D., 2008. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 14: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis G. P., Bohmann D., 2010. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci. Signal. 3: re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki J., Shibata N., Sakurai T., Agata K., Umesono Y., 2011. Role of c-Jun N-terminal kinase activation in blastema formation during planarian regeneration. Dev. Growth Differ. 53: 389–400. [DOI] [PubMed] [Google Scholar]
- Thibault S. T., Singer M. A., Miyazaki W. Y., Milash B., Dompe N. A., et al. , 2004. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat. Genet. 36: 283–287. [DOI] [PubMed] [Google Scholar]
- Tsakiri E. N., Sykiotis G. P., Papassideri I. S., Gorgoulis V. G., Bohmann D., et al. , 2013. Differential regulation of proteasome functionality in reproductive vs. somatic tissues of Drosophila during aging or oxidative stress. FASEB J. 27: 2407–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken K. J. T., Schulze K. L., Haelterman N. A., Pan H., He Y., et al. , 2011. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods 8: 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonesch S. C., Lamparter D., Mackay T. F. C., Bergmann S., Hafen E., 2016. Genome-wide analysis reveals novel regulators of growth in Drosophila melanogaster. PLoS Genet. 12: e1005616 10.1371/journal.pgen.1005616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N., Shin S., Slocum S. L., Agoston E. S., Wakabayashi J., et al. , 2010. Regulation of notch1 signaling by nrf2: implications for tissue regeneration. Sci. Signal. 3: ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner D., Cizelsky W., Vasudevaro M., Özhan G., Haase C., et al. , 2014. Wnt/β-catenin signaling defines organizing centers that orchestrate growth and differentiation of the regenerating zebrafish caudal fin. Cell Rep. 6: 467–481. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Oh S.-H., Jorgensen M., Steiger N., Darwiche H., et al. , 2010. The role of the Wnt family of secreted proteins in rat oval “stem” cell-based liver regeneration: Wnt1 drives differentiation. Am. J. Pathol. 176: 2732–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley M. I., Setiawan L., Hariharan I. K., 2012. Regeneration and transdetermination in Drosophila imaginal discs. Annu. Rev. Genet. 46: 289–310. [DOI] [PubMed] [Google Scholar]
- Yoo S. K., Freisinger C. M., Lebert D. C., Huttenlocher A., 2012. Early redox, Src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J. Cell Biol. 199: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Lee J., Nambiar S. M., Hu M., Rui W., et al. , 2014. Nrf2 is involved in maintaining hepatocyte identity during liver regeneration. PLoS One 9: e107423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are included in the paper. Drosophila lines are available upon request. Raw data from primary and secondary screens are available upon request.