Abstract
The regulation of gene expression controls development, and changes in this regulation often contribute to phenotypic evolution. Drosophila pigmentation is a model system for studying evolutionary changes in gene regulation, with differences in expression of pigmentation genes such as yellow that correlate with divergent pigment patterns among species shown to be caused by changes in cis- and trans-regulation. Currently, much more is known about the cis-regulatory component of divergent yellow expression than the trans-regulatory component, in part because very few trans-acting regulators of yellow expression have been identified. This study aims to improve our understanding of the trans-acting control of yellow expression by combining yeast-one-hybrid and RNAi screens for transcription factors binding to yellow cis-regulatory sequences and affecting abdominal pigmentation in adults, respectively. Of the 670 transcription factors included in the yeast-one-hybrid screen, 45 showed evidence of binding to one or more sequence fragments tested from the 5′ intergenic and intronic yellow sequences from D. melanogaster, D. pseudoobscura, and D. willistoni, suggesting that they might be direct regulators of yellow expression. Of the 670 transcription factors included in the yeast-one-hybrid screen, plus another TF previously shown to be genetically upstream of yellow, 125 were also tested using RNAi, and 32 showed altered abdominal pigmentation. Nine transcription factors were identified in both screens, including four nuclear receptors related to ecdysone signaling (Hr78, Hr38, Hr46, and Eip78C). This finding suggests that yellow expression might be directly controlled by nuclear receptors influenced by ecdysone during early pupal development when adult pigmentation is forming.
Keywords: transcription factor, pigmentation, ecdysone, Hr78, Hr38, Mutant Screen Report
Regulation of gene expression is important for the proper development and physiology of all organisms. Changes in gene expression contribute to phenotypic diversity within a species, as well as divergence between species (Stern and Orgogozo 2008; Martin and Orgogozo 2013). Such heritable changes in gene expression can result from changes in cis-regulatory sequences and/or trans-regulatory factors. Cis-regulatory sequences are typically located in noncoding regions of the genome and work by binding trans-regulatory molecules called transcription factors (TFs). TFs are typically proteins that recognize 6–12 bp long DNA sequences (Spitz and Furlong 2012). Physical interactions between a cis-regulatory sequence and one or more TFs largely determine when, where, and how much a gene is transcribed. Understanding how gene expression is controlled during development, and changes over evolutionary time, thus requires identifying both cis- and trans-regulatory factors, as well as the interactions between them.
To date, much more progress has been made studying cis-regulatory sequences of genes with divergent expression than TFs that bind to these cis-regulatory sequences (Wittkopp and Kalay 2012). This is largely because there has not been a practical way to systematically test TFs for binding to specific pieces of DNA. In recent years, however, yeast-one-hybrid (Y1H) systems have been developed that allow the entire repertoire of an organism’s TFs to be tested for evidence of binding to a particular DNA sequence (Simicevic and Deplancke 2010; Ouwerkerk and Meijer 2011; Reece-Hoyes and Walhout 2012). These Y1H assays use yeast cells as a host for both the putative cis-regulatory DNA sequence as well as each TF. When a TF binds to the region of DNA tested, transcription of a reporter gene occurs, allowing the yeast cells to grow on selective media. TFs that allow growth of the yeast cells are thus identified as possible direct regulators of the DNA-sequence being tested. A list of potential regulators from a Y1H screen can be narrowed further by identifying TFs that affect development of the trait of interest when their activity is reduced, such as with the use of RNA-interference (RNAi) (Hens et al. 2011).
Here, we describe complementary Y1H and RNAi screens designed to identify potential direct regulators of the pigmentation gene yellow. The yellow gene is required for black pigment formation in the genus Drosophila (Wittkopp et al. 2003), and its expression has diverged among species in a manner that correlates with pigmentation divergence (reviewed in Massey and Wittkopp 2016) (Figure 1). Both cis-regulatory (Wittkopp et al. 2002b; Gompel et al. 2005; Prud’homme et al. 2006; Jeong et al. 2006; Kalay and Wittkopp 2010; Ordway et al. 2014) and trans-regulatory (Wittkopp et al. 2002b; Werner et al. 2010; Arnoult et al. 2013) changes have been implicated in this expression divergence; yellow is also required for the wing extension behavior that is part of male courtship in D. melanogaster (Bastock 1956; Burnet et al. 1973), and expression required for this trait is regulated by a dedicated cis-regulatory element (Drapeau et al. 2006). For pigmentation, prior studies have identified the Abdominal-B (Abd-B) protein as a direct regulator of a male-specific body enhancer in D. melanogaster yellow (Jeong et al. 2006), as well as Engrailed (En) (Gompel et al. 2005) and Distal-less (Dll) (Arnoult et al. 2013) proteins as direct regulators of the wing enhancer in D. biarmipes yellow. Much less is known about the regulation of yellow expression for traits other than adult pigmentation, although the overexpression of Fruitless (Fru) protein, which is a major regulator of male courtship behavior (Anand et al. 2001), has been shown to induce Yellow protein expression in the larval brain (Drapeau et al. 2003). Identifying additional direct regulators of yellow will improve our understanding of how this critical pigmentation gene is regulated during development, and facilitate further investigation into how changes in this regulation have evolved.
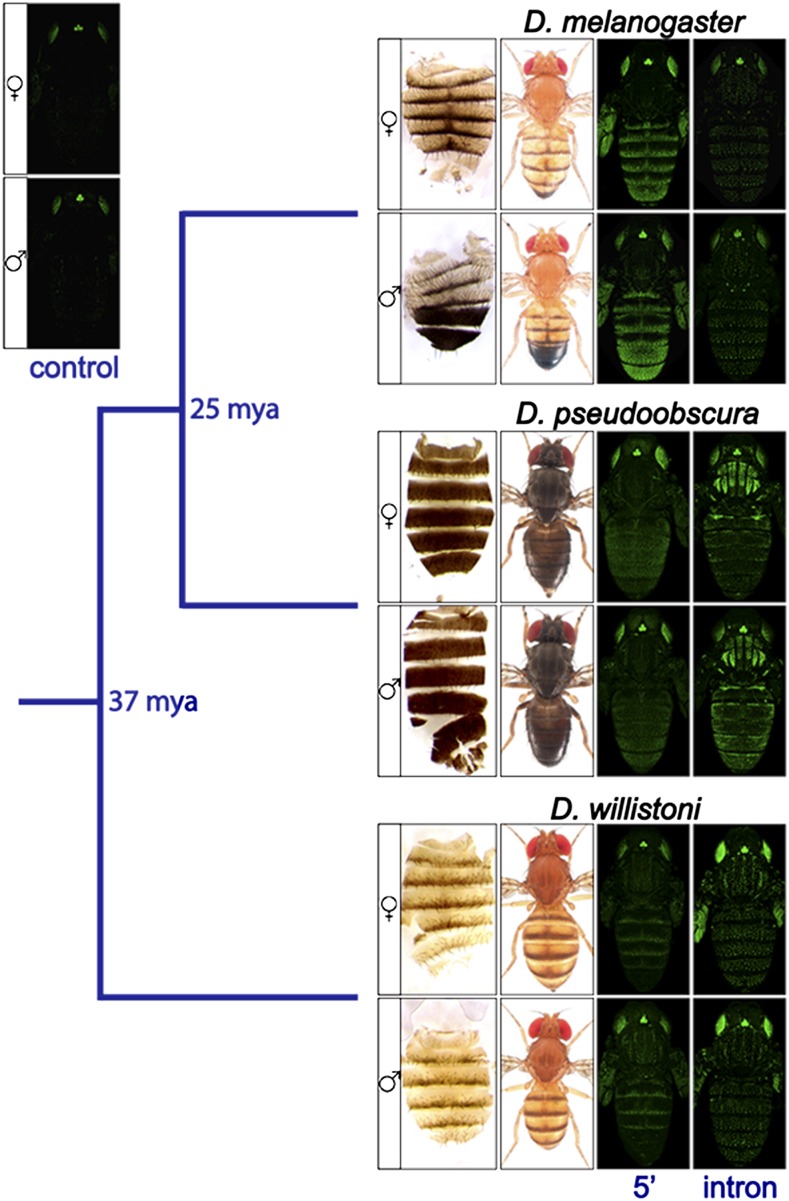
Figure 1.
Differences in adult pigmentation patterns correlate with differences in yellow expression patterns between Drosophila species. Phylogenetic relationship between three species from the Sophophora subgenus, D. melanogaster, D. pseudoobscura, and D. willistoni, are shown. For each species, panels are as follows: from left to right, dissected dorsal abdomen, dorsal view of adult fly, pupal GFP expression in a D. melanogaster host driven by 5′ intergenic sequence upstream of yellow, pupal GFP expression in a D. melanogaster host driven by yellow intronic sequence, with females shown in the top row and males shown in the bottom row. Images of dorsal view of adult flies are courtesy of Nicolas Gompel. The panel labeled “control” shows pupal GFP expression in females (top) and males (bottom) driven by only the basal promoter used to construct all other reporter genes. The GFP used in these constructs is a nuclear enhanced green fluorescent protein (nEGFP). Additional information about these reporter genes can be found in Kalay and Wittkopp (2010), in which these images of adult flies and GFP expression patterns were first published.
Materials and Methods
Overview
To identify potential direct regulators of yellow, we used a collection of 670 TFs from D. melanogaster (∼89% of all known and predicted TFs in this species) (Hens et al. 2011), and tested each one for evidence of binding to each of 26 overlapping fragments from the 5′ intergenic and intronic regions of yellow from D. melanogaster, D. pseudoobscura, and D. willistoni, which last shared a common ancestor 37 million yr ago (Russo et al. 1995) (Figure 1 and Figure 2). In D. melanogaster, the 5′ intergenic and intronic regions drive expression of yellow in the developing body, wings, bristles, larval mouthparts, and denticle belts (Geyer and Corces 1987; Martin et al. 1989; Wittkopp et al. 2002b; Jeong et al. 2006; Kalay and Wittkopp 2010). The 5′ intergenic region also harbors a cis-regulatory element required for proper male courtship behavior (Drapeau et al. 2006). yellow is also expressed in the embryo (Walter et al. 1991) and larval brain (Radovic et al. 2002) of D. melanogaster, although specific cis-regulatory elements driving these expression patterns have not been identified. Prior work has shown that the 5′ intergenic and intronic regions from D. pseudoobscura and D. willistoni also contain cis-regulatory sequences that drive pupal expression when assayed in D. melanogaster transformant flies (Figure 1; Kalay and Wittkopp 2010). Results from the biochemical Y1H screen for TF binding to these enhancers were combined with results from a genetic screen using RNAi to knock down activity of a subset of TFs and test for changes in pigmentation.
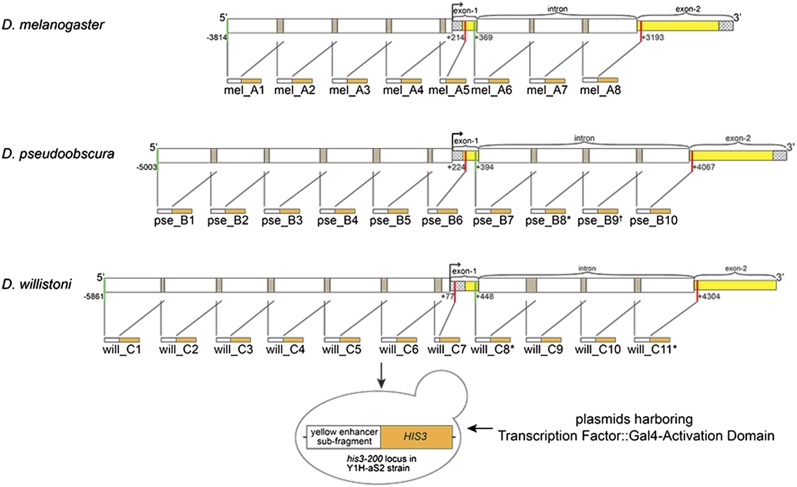
Figure 2.
Fragments from yellow 5′ intergenic and intronic regions tested in yeast-one-hybrid (Y1H) assay. The yellow locus, including the 5′ intergenic and intronic regions from D. melanogaster, D. pseudoobscura, and D. willistoni are shown. Each locus was divided into the fragments indicated by the white boxes, plus the preceding and following gray shaded boxes (if applicable), which show the overlapping regions between neighboring fragments. The broken arrow represents transcription start site (TSS). Checkered boxes represent 5′ untranslated region (UTR) if they come before exon 1, 3′ UTR if they come after exon 2. Yellow boxes indicate yellow coding sequences. Green vertical lines indicate the start, and red vertical lines represent the end, of 5′ intergenic or intronic regions of yellow from each species. The numbers next to green and red vertical lines indicate the start and end position of 5′ intergenic and intronic regions relative to TSS. Each fragment was cloned in front of a HIS3 reporter gene, which is represented by the orange boxes. The name of each fragment is written under each schematic. * indicates fragments that were not tested with Y1H for technical reasons, as described in Materials and Methods. † indicates that the fragment was tested with only half of the available transcription factors in Y1H, again for technical reasons. mel, D. melanogaster; pse, D. pseudoobscura; will, D. willistoni.
Subcloning regions of yellow noncoding sequences
Fragments from the 5′ intergenic and intronic regions of yellow from D. melanogaster, D. pseudoobscura, and D. willistoni were amplified using PCR (see Supplemental Material, Table S1), and each subcloned into pGEMT, pDONR-P4-P1R, and pMW2 vectors. Each enhancer fragment was ∼ 1000 bp long, except for mel_A5, pse_B6, will_C7, which were 423 bp, 641 bp, and 345 bp long, respectively (Figure 2 and Table S1). Each fragment overlapped with the flanking fragments by ∼ 100 bp (Figure 2 and Table S1). A mix of Taq DNA polymerase and Phusion High-Fidelity DNA Polymerase (New England Biolabs) was used for PCR to minimize PCR-introduced mutations. PCR primers (see Table S1) had attB sequences attached to their 5′ ends that were compatible with the attP site in the pDONR-P4-P1R vector (Deplancke et al. 2004). PCR products were first subcloned into a pGEMT vector (Promega) for sequencing. Sequence-confirmed-fragments (see File S1) were cut out of pGEMT vector and subcloned into the pDONR-P4-P1R vector using a BP reaction to create an Entry clone (Gateway, Life Technologies). For each enhancer fragment, an LR reaction (Gateway, Life Technologies) was then used to move the fragment from pDONR-P4-P1R vector into the Y1H compatible pMW2 vector upstream of a basal promoter and coding sequence for the S. cerevisiae HIS3 gene. These final constructs were mini-prepped and transformed into the Y1H-aS2 strain of S. cerevisiae using the lithium acetate (LiAc)–polyethylene glycol (PEG) method (Gietz and Woods 2006), where they were integrated into the mutant his3-200 locus. Three out of the 29 enhancer/bait fragments subcloned into pMW2vector (pse_B8, will_C8 and will_C11) (Figure 2, File S1, and Table S1) could not be integrated into the yeast genome because the restriction enzyme used to linearize the vector harboring the DNAbait::HIS3 fusion construct prior to genome integration also cut within the bait fragment. These three fragments were thus excluded from our analysis. Transformant cells for each of the other 26 baits were selected on synthetic complete medium lacking histidine (SC –His), which allowed growth only of cells that had incorporated the DNAbait::HIS3 fusion construct into their genomes. This protocol follows that described in Hens et al. (2011).
Yeast-one-hybrid (Y1H) assay
Prior to testing for interactions between specific TFs and specific DNA sequences, a self-activation test was conducted for each of the 26 integrated DNA sequence baits to determine whether the endogenous S. cerevisiae transcription factors could activate sufficient expression of the HIS3 reporter gene to allow growth in the absence of any D. melanogaster TF. This test was performed by spotting eight independent transformants for each bait onto SC –His plates containing varying concentrations (0, 10, 20, 40, 60, 80, and 100 mM) of 3-amino-1,2,4-triazole (3AT), which is a competitive inhibitor of the enzyme imidazoleglycerol-phosphate dehydratase, encoded by the HIS3 gene. This enzyme catalyzes the sixth step of histidine production (Fink 1964). The higher the level of 3AT in the medium, the more HIS3 gene product is required for growth, resulting in a more stringent assay for TF binding (Reece-Hoyes and Walhout 2012). For each DNA sequence bait, a single transformant that was unable to grow on plates with 10, 20, and/or 40 mM of 3AT was selected and used for the subsequent Y1H screen.
A previously constructed collection of 670 D. melanogaster TFs fused to the activation domain of the yeast Gal4 protein (GAL4-AD) arrayed across two 384-well plates (Hens et al. 2011) was used in this study. This collection includes ∼89% of all predicted and known TFs in D. melanogaster. Each of these 670 TFs (prey) were combined with each of the 26 yellow fragments (bait) in a 384-well format using the LiAc–PEG method, and plated on plates with synthetic complete medium lacking histidine, and tryptophan (SC –His, –Trp), which ensured growth of successful transformations only. After 3 d of growth, these 384-well format permissive plates were used to generate at least four 1536-well format plates by spotting each transformant genotype four times in a quartet of neighboring wells (Figure 3). One of the four 1536-well SC –His, –Trp plates did not contain any 3AT. The rest of the 1536-well SC –His, –Trp plates contained increasing concentrations of 3AT, starting with the lowest concentration of 3AT that prevented growth in the self-activation test. All plates were incubated at 30°C to allow colony growth, and then imaged using a Bio-Rad Gel Documentation camera. The permissive 1536-well plates lacking 3AT were imaged after 3 d of growth as positive controls, whereas the 1536-well plates containing 3AT were imaged after 7 and 10 d of growth. Two types of negative controls were included on each plate: cells that were not transformed with any TF-GAL4-AD construct (i.e., an empty well in the AD-TF collection), and cells that were transformed with the Gal4-AD construct alone lacking any D. melanogaster TF. The former was used to test if endogenous yeast TFs can activate HIS3, and the latter was used to test whether Gal4-AD can bind to the bait DNA in the absence of a TF and activate HIS3. Sets of quadruplicate cells containing the same TF and bait DNA that showed growth above background levels were inferred to have a direct interaction between the D. melanogaster TF protein and the bait DNA that caused the cells to express the HIS3 gene (Figure 3). One of the two plates of TFs was ultimately excluded for the pse_B9 bait fragment because of contamination, resulting in only ∼50% of the 670 TFs tested for binding to this sequence.
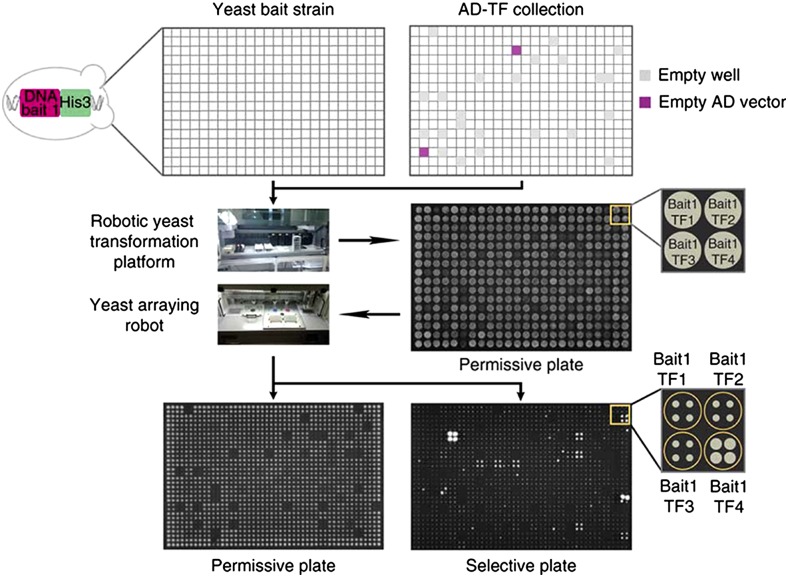
Figure 3.
Overview of high-throughput Y1H assay. Each yeast strain with a yellow enhancer fragment (DNA bait)-HIS3 reporter construct integrated into its genome (Yeast bait strain) was transformed with a library of transcription factors (TFs) fused to a Gal4 activation domain (AD-TF collection) using 384-well plates. As negative controls, each TF plate contained empty wells (gray squares in AD-TF collection plate) as well as constructs with activation domains (AD) without a TF attached (magenta squares in AD-TF collection plate) to test for activation in the absence of a TF. Then, each “bait-TF” combination was quadruplicated using a yeast arraying robot, first onto a permissive plate (no 3AT), then onto selective plates with increasing amounts of 3AT. After 3 d of growth, the permissive plate (no 3AT) was imaged as a reference. After 7 and then 10 d of growth, the selective plates were imaged and colony growth was scored as a readout of DNA bait–TF interaction. This figure is modified from Hens et al. (2011).
Analysis of Y1H data
To determine which TFs showed evidence of binding to yellow enhancer fragments, the images from each 1536-well plate were analyzed using an R package called Gitter (Wagih and Parts 2014)—an image analysis tool for processing of colony-based screens. Raw plate images used for this analysis are included as File S2. This program detects the grid of colonies on each plate, and then uses the contrast between the colony and the medium to determine the area of the colony, the degree to which the colony is circular, and whether the colony appears to overlap with other colonies. This method was used as an alternative to the TIDY analysis pipeline for Y1H data described in Hens et al. (2011), which identified significant interactions much more liberally.
To ensure optimal processing, plate images were first manually cropped to remove the plate edges, which sometimes created glare that interfered with Gitter’s grid detection. Contaminant colonies, visible on some plates after 7 or 10 d of growth (see File S3), were also manually removed from the images at this stage by replacing them with gray regions of equal darkness to neighboring noncolony pixels. Gitter was then run in batch mode across all of these preprocessed images using default parameters with the exception of setting the ‘fast’ image scaling parameter to 3000, and the ‘autorotate’ parameter to ‘T.’
Each potential TF-enhancer interaction was represented on each plate by a quartet of colonies. Colonies flagged by Gitter as noncircular were screened from further analysis. Areas of colonies were calculated as the natural log of the number of pixels detected to be in the colony. Quartets were excluded from further analysis if they: (a) had only one detectable circular colony, (b) had mean area of < 2, or (c) had an index of dispersion among the quartet colony areas of > 0.2. The value of each quartet was recorded as the mean of the quartet’s colonies’ areas.
Z-scores were assigned to each quartet by comparing the quartet’s value to an outlier-screened and edge-corrected distribution of values of quartets across the plate. Outlier screening was performed by using Grubb’s test for outliers on the quartet values. To detect if the plate showed an edge effect, a t-test was used to compare the values of quartets in the outermost two rows and columns against the values of the inner quartets. If there was a significant difference between edge and interior quartets (alpha = 0.05), the values of the exterior quartets were adjusted downward by the difference between the mean values of edge and interior quartets. Z-scores were then calculated for each quartet, including outlier quartets, on the basis of these edge-corrected values. Y1H output files created by Gitter (see File S3) were analyzed using custom R scripts that are included in File S4. A complete summary of results is provided in Table S2, and with graphical summaries shown in the main text.
RNAi screening
To identify TFs that affect pigmentation in adult D. melanogaster flies, we used RNAi to decrease TF activity, and examined body color in the dorsal abdominal cuticle. For this purpose, 153 RNAi lines were obtained from the Bloomington Drosophila Stock Center (BDSC), and one line (RNAi for bric-a-brac1) was obtained from Vienna Drosophila Stock Center (VDRC) (see Table S3). These lines were used to test the function of 124 different TFs that were also tested in the Y1H screen, as well as one transcription factor (fru) that was previously shown to be genetically upstream of yellow (Drapeau et al. 2003). The lines ordered from BDSC belong to the TRiP collection (Transgenic RNAi Project, Harvard Medical School), in which UAS-RNAi transgenes are integrated into an attP2 site on the third chromosome using the phiC31 site-directed integration (Ni et al. 2008, 2009). The integration of each of these UAS-RNAi transgenes into the same genomic location reduces variability in expression among these lines by controlling for position effects. RNAi lines targeting pigmentation genes ebony, yellow, and tan were also included as controls. The ebony RNAi line (part of TRiP collection) was ordered from the BDSC, and the yellow and tan RNAi lines were ordered from the VDRC (see Table S3).
Flies homozygous for a UAS-RNAi transgene were crossed to flies heterozygous for the pannier-Gal4 driver (pnr-Gal4) and a TM3 balancer chromosome (Figure 4). Flies inheriting the pnr-Gal4 driver, which expresses Gal4 in the dorsal midline during pupal stages when adult pigmentation is developing (Calleja et al. 2000; Wittkopp et al. 2002a) (Figure 6A), were tested for effects on pigmentation by comparing their pigmentation to that of siblings that inherited the TM3 balancer chromosome. More specifically, virgin females from RNAi lines with the genotype y1, sc1, v1; P{y+t7.7 = CaryP}attP2, P{UAS-RNAi y+ v+} were crossed to males with the genotype y1, w1118; P{w+mW.hs = GawB}pnrMD237/TM3, P{w+mC = UAS-y.C}MC2, Ser1. From their progeny, females with the genotype y1, sc1, v1 /y1, w1118 ; P{y+t7.7 = CaryP}attP2, P{UAS-RNAi y+ v+}/ P{w+mW.hs = GawB}pnrMD237, and males with the genotype y1, sc1, v1; P{y+t7.7 = CaryP}attP2, P{UAS-RNAi y+ v+}/ P{w+mW.hs = GawB}pnrMD237, were identified based on the lack of a humeral bristle phenotype caused by a mutation on the TM3 balancer chromosome. These flies, which inherited both the UAS-RNAi and pnr-GAL4 transgenes, were the “test” flies. Their siblings, which had the genotype y1, sc1, v1/y1, w1118 ; P{y+t7.7 = CaryP}attP2, P{UAS-RNAi y+ v+}/TM3, P{w+mC = UAS-y.C}MC2, Ser1 in females, and y1, sc1, v1; P{y+t7.7 = CaryP}attP2, P{UAS-RNAi y+ v+}/TM3, P{w+mC = UAS-y.C}MC2, Ser1 in males, and therefore inherited a UAS-RNAi transgene without the pnr-Gal4 driver required for its expression, carried the humeral bristle phenotype and were used as controls. All flies were raised at room temperature (∼22°) on cornmeal and yeast-based medium; 42.5 liters of this medium contains 39 liters of water, 675 g of yeast (SafPro Relax+YF deactivated dry yeast from Lesaffre Yeast Corp.), 390 g of soy flour (ADM, Protein Specialties Division), 2850 g of yellow cornmeal, 225 g of agar (MoorAgar, Inc.), 3 liters of light corn syrup (Karo light syrup), and 188 ml of propionic acid. Adult progeny from each cross were collected the day that they emerged, sorted based on sex and humeral bristle phenotype, aged 3–5 d, and scored for pigmentation by eye. If a difference in pigmentation was detected by eye between the control and test genotypes under the microscope, the flies were placed in a 1:10 glycerol:ethanol solution. After being stored in the glycerol:ethanol mix at least for 3 d, abdominal cuticle of test and control flies from both sexes were dissected for each line, mounted in polyvinyl alcohol mounting medium (BioQuip), baked at 65° overnight, and imaged using a Schott Leica MZ6 stereoscope with camera and “Scion Visicapture” version 1.2 software under identical lighting conditions. To improve the consistency between digital images and visual observations, we decreased background color and increased contrast by applying color adjustment to all cuticle images in each panel using Adobe Photoshop CS6.
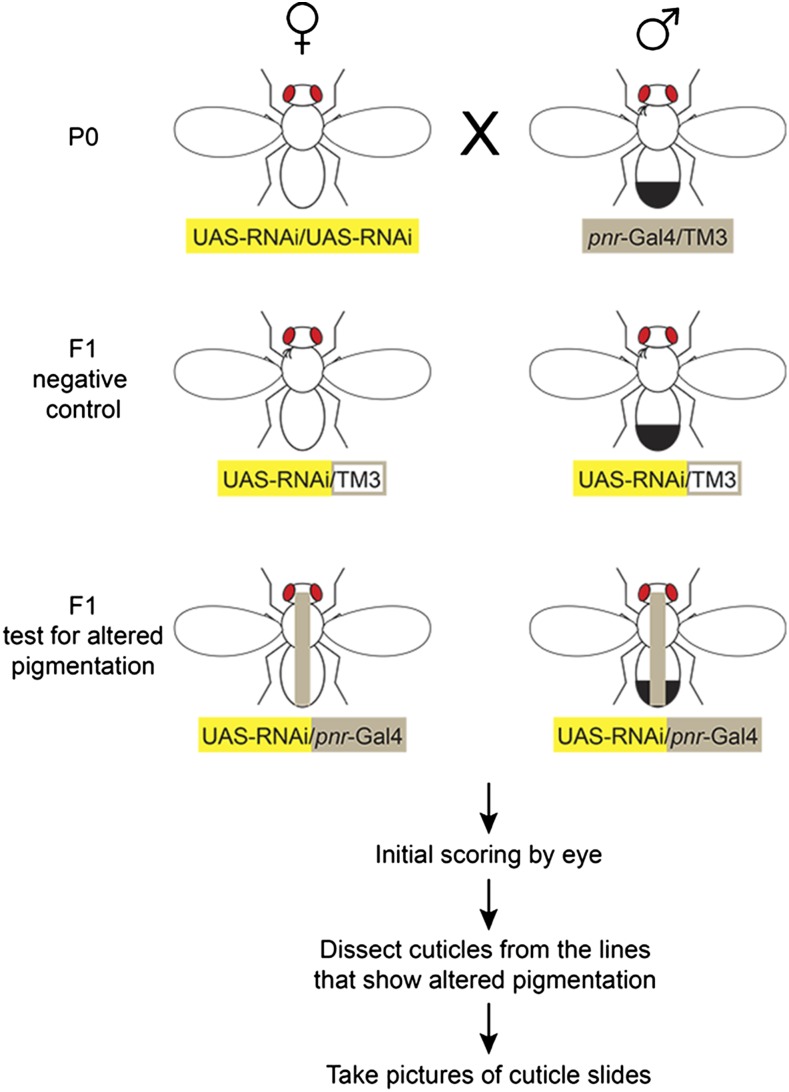
Figure 4.
Overview of RNAi screen for transcription factors affecting pigmentation in D. melanogaster. To determine whether a given TF affects abdominal pigmentation in D. melanogaster, one or more homozygous UAS-RNAi lines reducing activity of that TF were each crossed to a line heterozygous for the pannier-Gal4 (pnr-Gal4), which drives expression in the dorsal midline (gray stripe), and a TM3 balancer on the 3rd chromosome (P0 cross). Half of the F1 progeny inherited the UAS-RNAi construct and the TM3 balancer (control), whereas the other half inherited the UAS-RNAi construct and the pnr-Gal4 driver (knockdown). The presence or absence, respectively, of a bristle phenotype on the humeral (shoulder) region of adult flies caused by a mutation on the TM3 balancer chromosome was used to distinguish control and knockdown flies. After scoring initially for possible abdominal pigmentation differences by eye, cuticles were dissected from lines that showed potentially altered abdominal pigmentation. Dissected cuticles were mounted on microscope slides and imaged as described in Materials and Methods.
Figure 6.
Altering expression of yellow, tan, and ebony in D. melanogaster alters pigmentation. For comparison to the effects of knocking down TFs with unknown effects, we examined the pigmentation phenotypes caused by altering genes required for pigment synthesis with our dissection and imaging protocols. Specifically, we examined the effects of altering the yellow, ebony, and tan genes, which are required for pigment synthesis (Massey and Wittkopp 2016). Changes in abdominal pigmentation were caused by loss-of-function mutations as well as Gal4-driven increases and decreases in expression for all three genes. The Gal4 driver used for this work was pnr-Gal4, which activates expression along the dorsal midline, as shown by a UAS-GFP construct in (A). Note that pnr-Gal4 activates expression in a subset of the dorsal abdominal cuticle [outlined in red in (A)], allowing pigmentation outside of the pnr-Gal4 expression domain to be used as an internal control within each cuticle. Wild-type D. melanogaster abdominal cuticle from females (B) and males (C) are also shown for comparison. (D–F) Loss-of-function mutations in yellow (D), ebony (E), and tan (F) altered pigmentation throughout the abdominal cuticle, with yellow mutants showing decreased black pigments, ebony mutants showing decreased yellow pigments, and tan mutants showing decreased brown pigments (G–I) Reducing activity of yellow (G), ebony (H), and tan (I) using RNAi caused similar changes in pigmentation, but only in the pnr-GAL4 expression domain. (J–L) Increasing activity of yellow (J), ebony (K), and tan (L) in the pnr-Gal4 expression domain had opposite effects on pigmentation. Images shown in this panel were taken on multiple days, causing imaging conditions to vary among genotypes.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results and Discussion
Y1H screen identifies potential direct regulators of the yellow gene
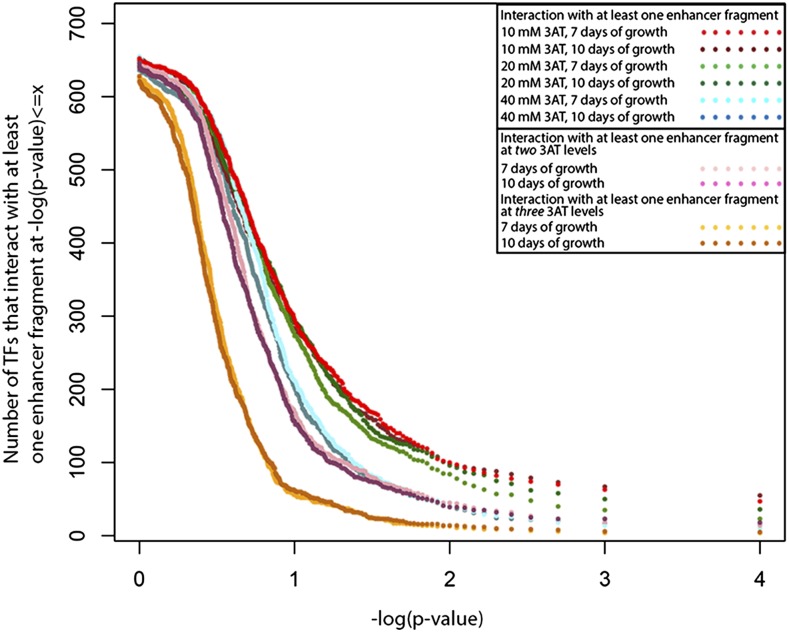
Each of the 670 TFs was tested for evidence of binding to each of the 26 yellow enhancer fragments from D. melanogaster, D. pseudoobscura, and D. willistoni for a total of 17,420 unique tests. As expected, the number of TFs showing evidence of a significant interaction with a DNA fragment in the Y1H screen depended upon the statistical threshold used to call significance and the stringency of selection used (controlled by altering the amount of 3AT in the media, see Materials and Methods). The number of TFs showing a significant interaction with at least one of the DNA fragments generally decreased as 3AT concentrations (and thus the strength of selection) increased, regardless of the significance threshold used (Figure 5). Increasing the number of days of growth allowed before scoring from 7 to 10 had little effect on the number of interactions detected (Figure 5). Requiring a significant interaction at two or three levels of 3AT tested for a particular enhancer/TF pair reduced the number of significant interactions at all p-value thresholds (Figure 5). For all conditions, the rate of decrease in the number of TFs that showed evidence of an interaction with increasing statistical stringency slowed around –log10(p-value) = 2, which corresponds to a p-value of 0.01 (Figure 5).
Figure 5.
Effects of selection strength, incubation time, and significance threshold on interactions observed between TFs and yellow fragments. The number of TFs with a significant interaction with at least one yellow enhancer fragment (y-axis) at a given significance threshold or smaller [x-axis, –log(p-value)] is shown. The different colored dots show results using plates with different levels of inhibitor (10 mM, 20 mM, 40 mM of 3AT), two different incubation times (7 or 10 d of growth allowed), and when we required evidence of an interaction with at least one, two, or three levels of 3AT inhibitor, as indicated in the figure inset. Note that the rate of decrease in the number of TFs that showed a significant interaction with at least one yellow enhancer fragment slowed down around –log(p-value) = 2, which corresponds to p-value ≤ 0.01. Results discussed in the manuscript were based on a statistical threshold of p-value ≤ 0.005, or –log(p-value) = 2.3 and the requirement that a given TF-enhancer fragment interaction was observed with at least two 3AT levels.
To generate a list of possible regulators, we used a significance threshold of p-value < 0.005, corresponding to 2.3 in Figure 5. We also required that a specific TF show evidence of an interaction with the same DNA fragment for at least two of the 3AT concentrations tested for that fragment after 7 d of growth. Using these criteria, we found that 45 (6.7%) of the 670 TFs tested showed evidence of binding at least one enhancer fragment (Table 1 and Table S2), with ∼9% of these TFs (four of 45) showing a significant interaction with more than one of the 26 yellow enhancer fragments (Table 1 and Table S2). Nineteen TFs showed interactions with at least one of the eight yellow enhancer fragments from D. melanogaster, seven TFs with at least one of the nine fragments from D. pseudoobscura, and 21 with at least one of the nine fragments from D. willistoni. The TF interacting with the highest number of fragments (6/26) was Hormone-receptor-like in 78 (Hr78), which has been shown to repress ecdysone signaling (Zelhof et al. 1995). This TF interacted with at least one enhancer fragment from each of the three species, suggesting that it might be a conserved regulator. Hr78 (also known as DHR78) encodes a nuclear receptor involved in larval molting, particularly cuticle formation in the larval tracheal system, and its absence leads to larval death at the third instar larval stage (Astle et al. 2003; King-Jones and Thummel 2005). CG8765, Hormone-receptor-like in 38 (Hr38) (also known as DHR38), and intermediate neuroblasts defective (ind) were tied for the next most commonly binding TFs. They each showed evidence of interaction with two of the 26 fragments for at least two different 3AT levels. To the best of our knowledge, none of these three genes have previously been implicated in regulating yellow expression, and thus represent new candidates for direct regulators of yellow. The full list of TFs with the number of 3AT levels that showed a significant (p-value < 0.005) interaction with each DNA fragment is shown in Table S2. These genes include Abd-B, which was previously shown to directly bind to D. melanogaster yellow cis-regulatory sequences (Jeong et al. 2006), as well as Doublesex (dsx), which has been shown to directly regulate, along with Abd-B, another gene that impacts pigmentation, bric-a-brac 1 (bab1) (Williams et al. 2008).
Table 1. TFs interacted with at least one yellow enhancer fragment in Y1H.
| Gene Name | D. melanogaster | D. pseudoobscura | D. willistoni |
|---|---|---|---|
| Hr78a,b | xxxx | x | x |
| CG8765 | xx | ||
| Hr38a,b | xx | ||
| ind | xx | ||
| ab | x | ||
| Abd-Bb | x | ||
| bigmax | x | ||
| C15b | x | ||
| caup | x | ||
| CG11085 | x | ||
| CG1233 | x | ||
| CG1529 | x | ||
| CG1621 | x | ||
| CG1647 | x | ||
| CG17806 | x | ||
| CG31666 | x | ||
| CG33695 | x | ||
| CG5591 | x | ||
| CG7101 | x | ||
| CG7928 | x | ||
| CG8216 | x | ||
| CG9437 | x | ||
| CG9650 | x | ||
| crm | x | ||
| d4 | x | ||
| dsxb | x | ||
| Eip78Ca,b | x | ||
| Gsc | x | ||
| Hey | x | ||
| HLH4C | x | ||
| Hr46a,b | x | ||
| Lim3b | x | ||
| NfI | x | ||
| Oaz | x | ||
| Oli | x | ||
| otp | x | ||
| p53 | x | ||
| pdm2 | x | ||
| pfk | x | ||
| pntb | x | ||
| ro | x | ||
| slp2 | x | ||
| stwl | x | ||
| Su(var)3-7 | x | ||
| toe | x |
All transcription factors that showed a significant interaction with at least one yellow enhancer fragment from the 5′ intergenic and/or intronic regions from D. melanogaster, D. pseudoobscura, and/or D. willistoni for more than one 3AT levels are shown. Each “x” indicates evidence of an interaction between the TF and a yellow cis-regulatory sequence from the species indicated. The number of “x”s indicated shows the number of yellow enhancer fragments a given TF interacted with for that species.
TFs that are nuclear receptors.
TFs showing altered abdominal pigmentation when knocked down with RNAi.
Y1H screens are perhaps the best method currently available to systematically search for TFs binding to a cis-regulatory sequence of interest, but they are known to have an important portion of false positives and false negatives (Deplancke et al. 2004; Hens et al. 2011). False positives can be technical, where the reporter gene becomes active independent of an interaction between a TF and a DNA fragment, or they can be biological, where a TF and DNA fragment that interacted in the yeast cell do not interact in their native biological setting for one of many reasons, including lack of competition among TFs (Walhout 2011). We have tried to minimize technical false positives by testing for self-activation of each DNA fragment and indiscriminate activation by the vector used to express TFs, assaying each potential interaction in quadruplicate, using stringent statistical thresholds, and requiring evidence of binding at multiple levels of inhibitor (3AT). Testing a TF-DNA fragment interaction at multiple 3AT levels is a “semi-independent” way of confirming a given interaction, because even though independent transformations are not conducted, the same transformant colony has to grow in a new, perhaps more stringent, environment. Presence of growth in multiple 3AT levels makes it more likely that a given TF-DNA fragment interaction is not a technical false positive. These stringent criteria may cause us to miss some true interactions, however, especially in cases where an interaction activates a low level reporter gene expression, such as when a repressor binds to the DNA fragment (Deplancke et al. 2004). These cases can be detected only at low levels of 3AT, where growth environment is less stringent. The raw Y1H data (plate images) are provided in File S2 so that other researchers can analyze them with different analysis criteria.
The false positive and false negative rates are unknown for our data, but a previous Y1H experiment using the same reagents as our study found that 72–77% of the TFs detected as binders of specific DNA fragments in a Y1H screen were also detected with a secondary direct binding assay (Hens et al. 2011). A false negative rate was also reported in this prior study, with 14 of the 19 previously reported direct regulators not detected using the automated Y1H system (Hens et al. 2011). Consequently, we anticipate that most of the TFs identified in our Y1H screen are likely to truly be able to bind to yellow cis-regulatory sequences in yeast at least, but many other factors might also bind that we failed to identify. We also anticipate that only a subset of TFs that are capable of binding to the sequences tested will actually regulate yellow expression in D. melanogaster. For these reasons, we think it is premature to interpret similarities and differences in TF binding among species, even thought this was one of the original goals of our experiment.
RNAi screen identifies new regulators of adult pigmentation
As described in the introduction, yellow is a pleiotropic gene required for pigmentation in larvae and adults as well as mating behavior and potentially other traits (reviewed in Wittkopp and Beldade 2009). The cis-regulatory sequences controlling yellow expression during the pupal stage when adult pigmentation is forming have been studied most extensively (Geyer and Corces 1987; Martin et al. 1989; Wittkopp et al. 2002b; Jeong et al. 2006; Kalay and Wittkopp 2010), but potential direct regulators identified by the Y1H screen could affect any aspect of yellow expression. To identify TFs that might specifically affect yellow expression during the pupal stages and contribute to pigmentation development, we used UAS-RNAi transgenes (Ni et al. 2008, 2009) to reduce the activity of 124 of the 670 TFs tested in the Y1H screen (see Table S3) plus fru, which is genetically upstream of yellow, along the dorsal midline of the developing fly with a pnr-Gal4 driver (Calleja et al. 1996). We then looked for effects on pigmentation in dissected cuticle from adult abdomens by comparing flies with both the UAS and Gal4 transgenes to their siblings that lacked one or both of these components (Figure 4). During pupal development, pnr-Gal4 is expressed in a stripe along the dorsal midline throughout the abdomen (Figure 6A), in cells that produce black, brown, and yellow pigments in both females (Figure 6B) and males (Figure 6C). As positive controls and reference points for this analysis, we also used pnr-Gal4 to modify activity of three genes required for pigment synthesis: yellow, ebony, and tan. yellow mutants lack black pigment (Figure 6D), ebony mutants lack yellow pigment (Figure 6E), and tan mutants lack brown pigment (Figure 6F). Reducing activity of these three genes in the pnr-Gal4 expression domain using UAS-RNAi resulted in a loss of the expected pigment for each gene (Figure 6, G–I), whereas overexpressing each of these genes using pnr-Gal4 and UAS-yellow, UAS-ebony, or UAS-tan resulted in a gain of black, yellow, and brown pigments, respectively (Figure 6, J–L). Note that overexpression of yellow primarily darkens black pigmentation where it already exists (described in more detail in Wittkopp et al. 2002b) whereas overexpression of ebony or tan is sufficient to produce yellow or brown pigment, respectively, in all cells within the pnr-Gal4 expression domain.
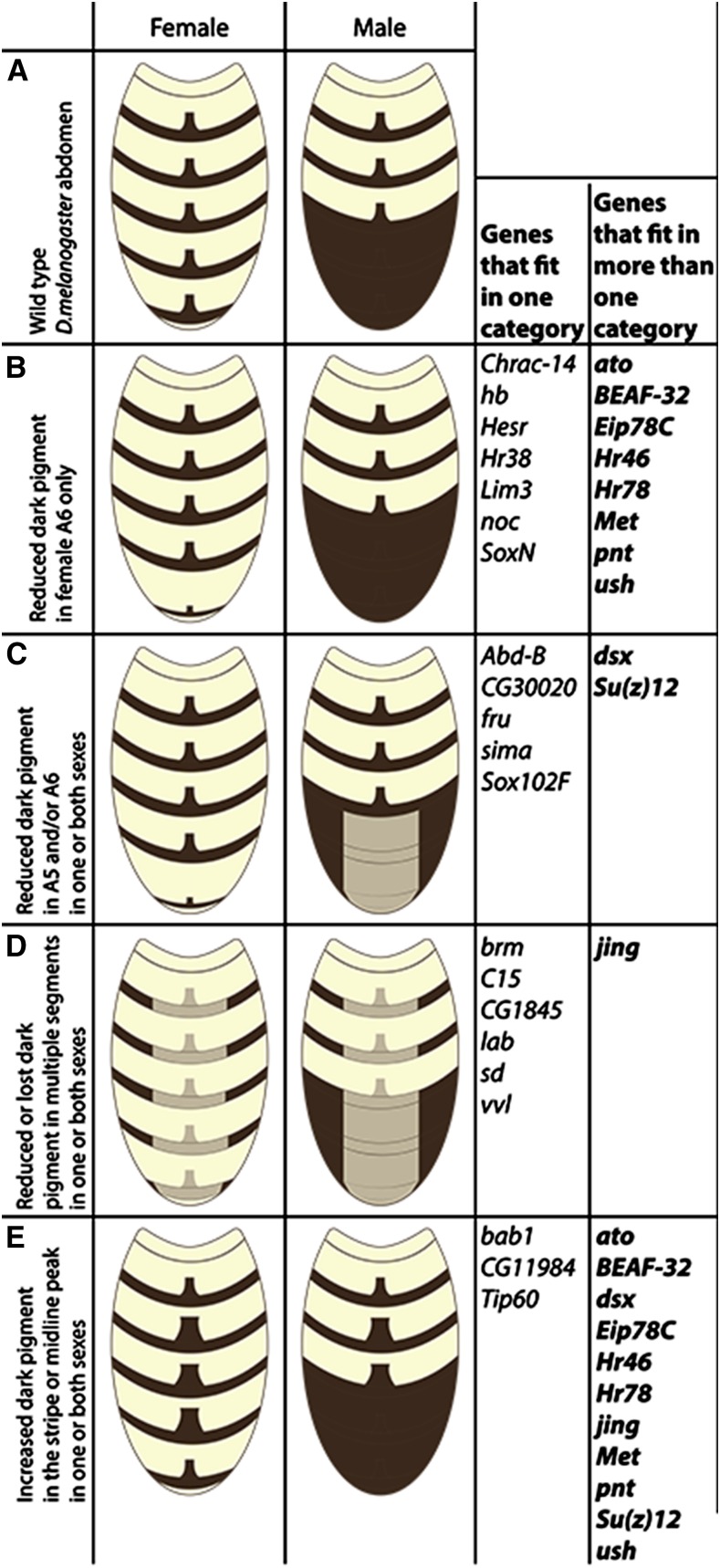
Among the 125 RNAi lines targeting TFs we tested, we found that 32 (∼25%) affected abdominal pigmentation when activated by pnr-Gal4 (Figure 7, Table S4, and File S5). Reducing activity of seven of these TFs, Chromatin accessibility complex 14kD protein (Chrac-14), hunchback (hb), Hes-related (Hesr), Hr38, Lim3 (Lim3), no ocelli (noc), and SoxNeuro (SoxN), reduced dark pigment only in the A6 abdominal segment of females (Figure 7B), whereas reducing activity of five other TFs Abd-B, CG30020, fru, similar (sima), Sox102F (Sox102F), resulted in reduced or partially lost dark pigment in the A5 and/or A6 abdominal segments of one or both sexes (Figure 7C). As previously shown (Celniker et al. 1990; Hopmann et al. 1995), males with Abd-B knocked down in the dorsal midline, lost male specific pigmentation in abdominal segments A5 and A6. The knock-down of the above 12 TFs had no noticeable effect on pigmentation in other segments. Six TFs reduced abdominal pigmentation in multiple segments when knocked down, with each having a unique effect (Figure 7D, Table S4, and File S5). Knock down of brahma (brm) resulted in loss of pigmentation in abdominal segments A2–A6 in males, including the midline peak, but had no visible effect on pigmentation in females. C15, when knocked down, resulted in loss of the midline peak in female abdominal segments A2–A6 and in male abdominal segments A2, A3, and A4, and also removed part of the dark pigmentation in the anterior part of male abdominal segment A5. This knockdown also led to overall thinner dark stripes in all segments that have it. Knockdown of CG1845 resulted in close to complete loss of dark pigment in the dorsal midline of the dark stripe in female abdominal segments A2–A6 and abdominal segments A2, A3, and A4 in males. This knockdown also led to close to complete loss of the midline peak of all segments that normally have it, and it resulted in reduced dark pigment in the dorsal midline of male abdominal segments A5 and A6. Knock down of labial (lab) resulted in partial or complete loss of dark pigment in the dorsal midline of female abdominal segments A2–A6, and male abdominal segments A2, A3, and A4. This knockdown did not affect the midline peak of dark stripe in any of the segments except the female abdominal segment A6, where it resulted in a close to complete loss. Knock down of scalloped (sd) reduced dark pigment in male abdominal segments A2–A6, and led to close to complete loss of dark pigment in female abdominal segment A6. Finally, knock down of ventral veins lacking (vvl) resulted in reduced dark pigment in the pnr-Gal4 expression domain in abdominal segments A2–A6 in both males and females. Three TFs increased pigmentation in both males and females when knocked down (Figure 7E): Knockdown of Tat interactive protein 60kda (Tip60) and CG11984 increased pigmentation by broadening the midline peak in abdominal segments A3–A6 in females and in abdominal segments A3 and A4 in males, whereas bab1 broadened the midline peak in abdominal segments A2 and A3 of males and females, as well as led to gain of male specific pigmentation in female abdominal segments A4, A5, and A6 and male abdominal segment A4.
Figure 7.
Summary of RNAi knockdown phenotypes observed for 32 transcription factors. Schematic representations of abdominal pigmentation in wild type D. melanogaster (A), and after knocking down activity of 32 transcription factors (B–E) are shown. Four classifications were used to describe the pigmentation phenotypes of RNAi knockdown flies: reduced dark pigment in female segment A6 only (B), reduced dark pigment in A5 and/or A6 in one or both sexes (C), reduced or lost dark pigment in multiple segments in one or both sexes (D), and increased dark pigment in stripes and/or midline peak in one or both sexes (E). Knockdown phenotypes of 21 transcription factors each fell into only one of these four categories, whereas knockdown phenotypes of 11 transcription factors were more complex and fell into more than one category.
Interestingly, 11 of the TFs tested [atonal (ato), Boundary element-associated factor of 32kD (BEAF-32), dsx, Ecdysone-induced protein 78C (Eip78C), Hormone receptor 3 (Hr46), Hr78, jing (jing), Methoprene-tolerant (Met), pointed (pnt), Su(z)12 (Su(z)12), and u-shaped (ush)] had opposite effects on pigmentation in different segments of the abdomen or in different sexes when knocked down with RNAi (Figure 7, Table S4, and File S5). Consequently, they are listed in more than one category in Figure 7. For example, knockdowns of ato, Eip78C, Hr46, Hr78, Met, and ush increased dark pigmentation in female abdominal segments A3, A4, and A5, and male abdominal segments A3 and A4 (also A2 in the case of ush) (Figure 7E), but reduced or eliminated dark pigmentation in female abdominal segment A6 (Figure 7B). Reducing activity of Su(z)12 resulted in broader midline peaks in female abdominal segments A3, A4, and A5, and male abdominal segments A3 and A4 (Figure 7E), but less dark pigmentation in female abdominal segment A6, and male abdominal segments A5 and A6 (Figure 7C). Knockdown of pnt or BEAF-32 increased dark pigmentation in male abdominal segments A3 and A4 (Figure 7E), but also decreased dark pigmentation in female abdominal segment A6 (Figure 7B). Knockdown of jing resulted in reduced dark pigment in abdominal segments A2 through A6 in both sexes (Figure 7D), but this knockdown also increased the thickness of the pigmented stripe and broadened the midline peak in all female abdominal segments A2–A6 and male abdominal segments A3 and A4 (Figure 7E). Similar to Abd-B, when knocked-down, jing also resulted in loss of male-specific pigmentation in males. Finally, knocking down the activity of dsx resulted in gain of male specific pigmentation in female abdominal segment A6 (Figure 7E), but this knockdown also resulted in partial loss of dark pigment in male abdominal segments A5 and A6 (Figure 7C).
Two of the TFs that affected abdominal pigmentation in our RNAi screen are necessary for proper male courtship behavior in D. melanogaster. The first TF, fru, was not included in our Y1H screen, but has previously been shown to be genetically upstream of yellow in the 3rd instar larval brain (Radovic et al. 2002; Drapeau et al. 2003). fru has also been shown to be a master regulator of male courtship behavior in D. melanogaster (Anand et al. 2001)— a trait for which we know yellow is also necessary (Bastock 1956; Burnet et al. 1973). Mutants lacking the Fru zinc finger domain lack yellow expression in the 3rd instar larval brain (Drapeau et al. 2003). According to the summary of mutant phenotypes on FlyBase (Attrill et al. 2016), effects of fru on adult body pigmentation have not been described. We found that when fru was knocked down in D. melanogaster using the pnr-Gal4 driver, dark pigment was almost completely lost in the dorsal midline of abdominal segment A6 in females, and was reduced in abdominal segment A5 of both sexes (Figure 7C, Table S4, and File S5). The second TF, ato, was tested with Y1H, but did not show a statistically significant interaction with any of the yellow enhancer fragments with the inclusion criteria and significance thresholds used in our analysis. Knocking down ato in the RNAi screen, however, reduced pigmentation in female abdominal segment A6, and increased pigmentation in the midline peak of female A3, A4, A5, and male A3 and A4 abdominal segments (Figure 7, B and E, Table S4, and File S5). Interestingly, and similar to fru and yellow, this gene is also necessary for proper male mating behavior, specifically the production of courtship song (Tauber and Eberl 2001). These observations suggest that the pathways regulating pigmentation and male courtship might have more shared components than currently appreciated. For a more detailed description of all pigmentation phenotypes observed in this study, please refer to Table S4.
Three of the 32 TFs that affected adult abdominal pigmentation in our RNAi screen have previously had their effects on pigmentation described in detail: Abd-B (Jeong et al. 2006), dsx (Williams et al. 2008), and bab-1 (Kopp et al. 2000; Williams et al. 2008). Three more of these TFs (Sox102F, jing, and vvl) were also described as having effects on pigmentation in another recent screen that used a pnr-Gal4 driver and UAS-RNAi lines from the TRiP collection (Rogers et al. 2013). Additionally, overexpression of ush in the thorax has been shown to cause pigmentation to darken (Calleja et al. 2002), and localized knockdowns of Hr78 and Hr46 have previously been described as affecting abdominal pigmentation (Lam et al. 1999). The remaining 23 TFs [ato, BEAF-32, brm, C15, CG1845, CG11984, CG30020, Chrac-14, Eip78C, fru, hb, Hesr, Hr38, lab, Lim3, Met, noc, pnt, sd, sima, SoxN, Su(z)12, and Tip60] are, to the best of our knowledge, implicated in development of adult body pigmentation for the first time here, although Hr38 has previously been shown to affect immune-related melanization (Sekine et al. 2011). With the exception of Tip60, CG11984, and Chrac-14, these 23 TFs plus ush, Hr78, and Hr46 were also tested for effects on pigmentation in Rogers et al. (2013), but reported not to affect pigmentation. This difference in interpretation likely resulted from the fact that we scored pigmentation phenotypes after dissecting and mounting abdominal cuticles, whereas Rogers et al. (2013) scored pigmentation by looking at whole flies; the phenotypes we observed in pnr-Gal4;UAS-RNAi lines for these TFs were subtle, and likely not visible without dissection. In all, 115 of the 125 TFs we tested in our RNAi screen were also tested in Rogers et al. (2013), with six (Abd-B, dsx, bab1, jing, Sox102F, and vvl) found to affect adult abdominal pigmentation in both screens, 23 found to affect pigmentation only in our study, and 86 found to have no effect on adult abdominal pigmentation in either study (see Table S2, Table S3, and Table S4).
Nine genes (Abd-B, dsx, C15, Eip78C, Hr38, Hr46, Hr78, Lim3, and pnt) with effects on pigmentation in our RNAi screen also showed a significant biochemical interaction with at least one yellow enhancer fragment in our Y1H screen (Table 1, Table S2, and Table S4), suggesting that they might be direct regulators of yellow. The other TFs with effects on pigmentation described above might alter body color by affecting expression and/or activity of pigmentation genes other than yellow or might directly regulate yellow but not affect pigmentation because of shadow enhancers, but the high false negative rate of Y1H screens and the stringent criteria we used when analyzing the Y1H data prevent us from excluding these 23 genes as regulators of yellow. Additional genetic and biochemical experiments are needed to test these hypotheses. However, a recent study comparing evidence of TF binding and phenotypes resulting from reducing activity of these TFs on development of the Caenorhabditis elegans intestine also found very limited overlap between these sets of genes, suggesting that the phenotypic effects of many TFs are indirect (MacNeil et al. 2015).
Nuclear receptors and ecdysone signaling: direct regulators of yellow expression and pigmentation?
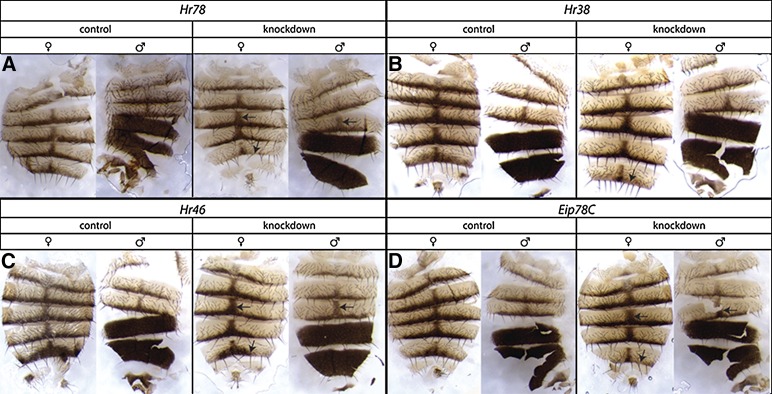
The most striking pattern to emerge from our Y1H and RNAi screens is the high proportion of nuclear receptors related to ecdysone signaling identified as potential direct regulators of yellow. We tested 11 of the 18 nuclear receptors in the D. melanogaster genome (King-Jones and Thummel 2005) for evidence of binding to yellow cis-regulatory sequences in our Y1H screen, and found that four of them (Hr78, Hr38, Hr46, and Eip78C) (∼36%) were among the 45 TFs that showed a statistically significant interaction with at least one yellow enhancer subfragment tested (Table 1 and Table S2). Two of these receptors, Hr78 and Hr38, were among the four TFs with evidence of binding to more than one yellow fragment tested (Table 1 and Table S2). All four of these nuclear receptors were also found to alter pigmentation when their activity was reduced using RNAi (Figure 8 and File S5). Another nuclear receptor, Hr4, which was not included in our study, was also reported to have effects on pigmentation when activity was reduced by RNAi in Rogers et al. (2013). Taken together, these data suggest that one or more of these nuclear receptors might directly regulate yellow expression. Hr78 showed evidence of binding to fragments from all three of the species tested, whereas each of the other three nuclear receptors (Eip78C, Hr46, and Hr38) showed evidence of binding to one or more yellow fragments in only one of the three species tested. These differences in binding among species might result from false positives and/or negatives, but could also be caused by developmental systems drift or divergent activities.
Figure 8.
RNAi knockdown phenotypes for nuclear receptor genes showing evidence of binding to yellow fragments in yeast-one-hybrid screen. Abdominal pigmentation phenotypes in both control and knockdown flies of both sexes are shown for four nuclear receptors, Hr78, Hr38, Hr46, and Eip78C. Changes in pigmentation observed consistently in knockdown relative to control flies are marked with arrows and described further in Table S4. Briefly, knockdown of Hr78, Hr46, and Eip78C resulted in increased dark pigment in female segments A3, A4, A5, and male segments A3 and A4 as well as reduced dark pigment in female segment A6. Knockdown of Hr38 only led to reduced dark pigment in female segment A6.
Nuclear receptors respond to a ligand by translocating to the nucleus and directly regulating transcription of target genes. The ligands for most nuclear receptors in D. melanogaster have not been identified, but many appear to be affected by ecdysone signaling, which controls development and metamorphosis (King-Jones and Thummel 2005). Hr38 can even substitute for the ecdysone receptor (EcR) (Riddiford et al. 2000). Seven of the 18 nuclear receptors are transcriptionally regulated by the active form of ecdysone, hydroxyecdysone (20E), including the other three nuclear receptors showing evidence of binding to yellow sequences in our Y1H screen (Hr78, Hr46, and Eip78C) (King-Jones and Thummel 2005). During metamorphosis, ecdysone signaling controls processes such as tissue-specific cell proliferation, differentiation and programmed cell death, reproductive and behavioral changes, and cuticle deposition (Thummel 1995; King-Jones and Thummel 2005). This last function is particularly intriguing given that (i) yellow is expressed during pupal development at the same time that deposition of the adult cuticle begins (Walter et al. 1991); (ii) Yellow protein is exported from the epidermal cells and incorporated into the developing cuticle (Walter et al. 1991; Wittkopp et al. 2002a); and (iii) yellow mutants show significant changes, both increase and decrease, in the amount of molecules involved in chitin biosynthesis, which is necessary for proper cuticle formation and pigmentation (Bratty et al. 2012). Indeed, in the swallowtail butterfly, Pailio xuthus, a link between ecdysone signaling and yellow expression was established by showing that exposure to a high titer of 20E increased yellow expression (Futahashi and Fujiwara 2007).
Additional support for the hypothesis that one or more of these nuclear receptors affect pigmentation, and might do so by regulating yellow expression, comes from prior studies of these genes. For example, Eip78C, Hr46 (also known as DHR3), and Hr4 are all expressed early in pupal development at the end of the ecdysone peak (King-Jones and Thummel 2005), shortly before yellow expression begins (Walter et al. 1991). Localized knockdowns of Hr78 and Hr46 have previously been described as affecting pigmentation (Zelhof et al. 1995; Lam et al. 1999). HR38 directly regulates expression of Ddc (Davis et al. 2007), another gene required for pigment synthesis (Wright 1987), and affects yellow-dependent melanization caused by the immune response (Sekine et al. 2011). Finally, one of the other TFs showing an effect on pigmentation when knocked down by RNAi, Ventral veins lacking (Vvl), regulates expression of ecdysone biosynthetic enzymes (Danielsen et al. 2014), providing another potential link between ecdysone signaling and pigmentation.
Conclusions
The goal of this study was to identify potential direct regulators of the pigmentation gene yellow by using complementary biochemical and genetic approaches. We tested yellow enhancer fragments from three Drosophila species, D. melanogaster, D. pseudoobscura, and D. willistoni, against 670 TFs using a biochemical assay (Y1H), which identified 45 potential regulators of yellow. We also tested 124 of these 670 TFs, plus another TF known to be genetically upstream of yellow in the nervous system with RNAi to determine the effects of reducing their activity on adult abdominal pigmentation. We identified 32 TFs that altered adult abdominal pigmentation in D. melanogaster when knocked down by RNAi, 23 of which have not previously been implemented in pigmentation development, and nine of which were also identified in the Y1H screen as potential direct regulators of yellow. Taken together, these data provide the largest list of putative regulators of yellow identified to date, while also revealing unexpected links among pigmentation, male-courtship behavior, and ecdysone signaling. This information sets the stage for studies testing possible functional relationships between these TFs and yellow, as well as provides additional candidates for the trans-regulatory factors contributing to divergent yellow expression among species.
Supplementary Material
Acknowledgments
We would like to thank Jonathan David Gruber for extensive help with R scripting and discussion of data analysis, Antonina Iagovitina for development of the TIDY analysis software, Jean Daniel Feuz for help with conducting high throughput yeast transformations, Alisha John for help with imaging cuticle slides, Jennifer Lachowiec for comments on the manuscript, and members of the Wittkopp Lab for technical and intellectual support. This work was supported by the National Institutes of Health (GM089736).
Footnotes
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.032607/-/DC1
Communicating editor: J. M. Comeron
Literature Cited
- Anand A., Villella A., Ryner L. C., Carlo T., Goodwin S. F., et al. , 2001. Mole-cular genetic dissection of the sex-specific and vital functions of the Drosophila melanogaster sex determination gene fruitless. Genetics 158: 1569–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult L., Su K. F. Y., Manoel D., Minervino C., Magriña J., et al. , 2013. Emergence and diversification of fly pigmentation through evolution of a gene regulatory module. Science 339: 1423–1426. [DOI] [PubMed] [Google Scholar]
- Astle J., Kozlova T., Thummel C. S., 2003. Essential roles for the Dhr78 orphan nuclear receptor during molting of the Drosophila tracheal system. Insect Biochem. Mol. Biol. 33: 1201–1209. [DOI] [PubMed] [Google Scholar]
- Attrill H., Falls K., Goodman J. L., Millburn G. H., Antonazzo G., et al. , 2016. FlyBase: establishing a gene group resource for Drosophila melanogaster. Nucleic Acids Res. 44: D786–D792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock M., 1956. A gene mutation which changes a behavior pattern. Evolution 10: 421–439. [Google Scholar]
- Bratty M. A., Chintapalli V. R., Dow J. A. T., Zhang T., Watson D. G., 2012. Metabolomic profiling reveals that Drosophila melanogaster larvae with the y mutation have altered lysine metabolism. FEBS Open Bio 2: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet B., Connolly K., Harrison B., 1973. Phenocopies of pigmentary and behavioral effects of the yellow mutant in Drosophila induced by α-dimethyltyrosine. Science 181(4104): 10591060. [DOI] [PubMed] [Google Scholar]
- Calleja M., Moreno E., Pelaz S., Morata G., 1996. Visualization of gene expression in living adult Drosophila. Science 274: 252–255. [DOI] [PubMed] [Google Scholar]
- Calleja M., Herranz H., Estella C., Casal J., Lawrence P., et al. , 2000. Generation of medial and lateral dorsal body domains by the pannier gene of Drosophila. Development 127: 3971–3980. [DOI] [PubMed] [Google Scholar]
- Calleja M., Renaud O., Usui K., Pistillo D., Morata G., et al. , 2002. How to pattern an epithelium: lessons from achaete-scute regulation on the notum of Drosophila. Gene 292: 1–12. [DOI] [PubMed] [Google Scholar]
- Celniker S. E., Sharma S., Keelan D. J., Lewis E. B., 1990. The molecular genetics of the bithorax complex of Drosophila: cis-regulation in the abdominal-B domain. EMBO J. 9: 4277–4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. T., Moeller M. E., Dorry E., Komura-Kawa T., Fujimoto Y., et al. , 2014. Transcriptional control of steroid biosynthesis genes in the Drosophila prothoracic gland by ventral veins lacking and knirps. PLoS Genet. 10: e1004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Yang P., Chen L., O’Keefe S. L., Hodgetts R. B., 2007. The orphan nuclear receptor DHR38 influences transcription of the DOPA decarboxylase gene in epidermal and neural tissues of Drosophila melanogaster. Genome 50: 1049–1060. [DOI] [PubMed] [Google Scholar]
- Deplancke B., Dupuy D., Vidal M., Walhout A. J. M., 2004. A gateway-compatible yeast one-hybrid system. Genome Res. 14: 2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau M. D., Radovic A., Wittkopp P. J., Long A. D., 2003. A gene necessary for normal male courtship, yellow, acts downstream of fruitless in the Drosophila melanogaster larval brain. J. Neurobiol. 55: 53–72. [DOI] [PubMed] [Google Scholar]
- Drapeau M. D., Cyran S. A., Viering M. M., Geyer P. K., Long A. D., 2006. A cis-regulatory sequence within the yellow locus of Drosophila melanogaster required for normal male mating success. Genetics 172: 1009–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R., 1964. Gene-enzyme relations in histidine biosynthesis in yeast. Science 146: 525–527. [DOI] [PubMed] [Google Scholar]
- Futahashi R., Fujiwara H., 2007. Regulation of 20-hydroxyecdysone on the larval pigmentation and the expression of melanin synthesis enzymes and yellow gene of the swallowtail butterfly, Papilio xuthus. Insect Biochem. Mol. Biol. 37: 855–864. [DOI] [PubMed] [Google Scholar]
- Geyer P. K., Corces V. G., 1987. Separate regulatory elements are responsible for the complex pattern of tissue-specific and developmental transcription of the yellow locus in Drosophila melanogaster. Genes Dev. 1: 996–1004. [DOI] [PubMed] [Google Scholar]
- Gietz, R. D., and R. A. Woods, 2006 Yeast transformation by the LiAc/SS Carrier DNA/PEG method. Methods Mol. Biol. 313: 107–120. [DOI] [PubMed] [Google Scholar]
- Gompel N., Prud’homme B., Wittkopp P. J., Kassner V. A., Carroll S. B., 2005. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature 433: 481–487. [DOI] [PubMed] [Google Scholar]
- Hens K., Feuz J.-D., Isakova A., Iagovitina A., Massouras A., et al. , 2011. Automated protein-DNA interaction screening of Drosophila regulatory elements. Nat. Methods 8: 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopmann R., Duncan D., Duncan I., 1995. Transvection in the iab-5,6,7 region of the bithorax complex of Drosophila: homology independent interactions in trans. Genetics 139: 815–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S., Rokas A., Carroll S. B., 2006. Regulation of body pigmentation by the Abdominal-B Hox protein and its gain and loss in Drosophila evolution. Cell 125: 1387–1399. [DOI] [PubMed] [Google Scholar]
- Kalay G., Wittkopp P. J., 2010. Nomadic enhancers: tissue-specific cis-regulatory elements of yellow have divergent genomic positions among Drosophila species. PLoS Genet. 6: e1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Jones K., Thummel C. S., 2005. Nuclear receptors—a perspective from Drosophila. Nat. Rev. Genet. 6: 311–323. [DOI] [PubMed] [Google Scholar]
- Kopp A., Duncan I., Godt D., Carroll S. B., 2000. Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature 408: 553–559. [DOI] [PubMed] [Google Scholar]
- Lam G., Hall B. L., Bender M., Thummel C. S., 1999. DHR3 is required for the prepupal–pupal transition and differentiation of adult structures during Drosophila metamorphosis. Dev. Biol. 212: 204–216. [DOI] [PubMed] [Google Scholar]
- MacNeil L. T., Pons C., Arda H. E., Giese G. E., Myers C. L., et al. , 2015. Transcription factor activity mapping of a tissue-specific in vivo gene regulatory network. Cell Syst. 1: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Orgogozo V., 2013. The Loci of repeated evolution: a catalog of genetic hotspots of phenotypic variation. Evolution 67: 1235–1250. [DOI] [PubMed] [Google Scholar]
- Martin M., Meng Y. B., Chia W., 1989. Regulatory elements involved in the tissue-specific expression of the yellow gene of Drosophila. Mol. Gen. Genet. 218: 118–126. [DOI] [PubMed] [Google Scholar]
- Massey J. H., Wittkopp P. J., 2016. The genetic basis of pigmentation differences within and between Drosophila species. Curr. Top. Dev. Biol. 119: 27–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J.-Q., Markstein M., Binari R., Pfeiffer B., Liu L.-P., et al. , 2008. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat. Methods 5: 49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J.-Q., Liu L.-P., Binari R., Hardy R., Shim H.-S., et al. , 2009. A Drosophila resource of transgenic RNAi lines for neurogenetics. Genetics 182: 1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway A. J., Hancuch K. N., Johnson W., Wiliams T. M., Rebeiz M., 2014. The expansion of body coloration involves coordinated evolution in cis and trans within the pigmentation regulatory network of Drosophila prostipennis. Dev. Biol. 392: 431–440. [DOI] [PubMed] [Google Scholar]
- Ouwerkerk P. B. F., Meijer A. H., 2011. Yeast one-hybrid screens for detection of transcription factor DNA interactions. Methods Mol. Biol. 678: 211–227. [DOI] [PubMed] [Google Scholar]
- Prud’homme B., Gompel N., Rokas A., Kassner V. A., Williams T. M., et al. , 2006. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature 440: 1050–1053. [DOI] [PubMed] [Google Scholar]
- Radovic A., Wittkopp P. J., Long A. D., Drapeau M. D., 2002. Immunoh-istochemical colocalization of Yellow and male-specific Fruitless in Drosophila melanogaster neuroblasts. Biochem. Biophys. Res. Commun. 293: 1262–1264. [DOI] [PubMed] [Google Scholar]
- Reece-Hoyes J. S., Walhout A. J. M., 2012. Gene-centered yeast one-hybrid assays. Methods Mol. Biol. 812: 189–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford L. M., Cherbas P., Truman J. W., 2000. Ecdysone receptors and their biological actions. Vitam. Horm. 60: 1–73. [DOI] [PubMed] [Google Scholar]
- Rogers W. A., Grover S., Stringer S. J., Parks J., Rebeiz M., et al. , 2013. A survey of the trans-regulatory landscape for Drosophila melanogaster abdominal pigmentation. Dev. Biol. 385(2): 417–432. [DOI] [PubMed] [Google Scholar]
- Russo C. A., Takezaki N., Nei M., 1995. Molecular phylogeny and divergence times of drosophilid species. Mol. Biol. Evol. 12: 391–404. [DOI] [PubMed] [Google Scholar]
- Sekine Y., Takagahara S., Hatanaka R., Watanabe T., Oguchi H., et al. , 2011. p38 MAPKs regulate the expression of genes in the dopamine synthesis pathway through phosphorylation of NR4A nuclear receptors. J. Cell Sci. 124: 3006–3016. [DOI] [PubMed] [Google Scholar]
- Simicevic J., Deplancke B., 2010. DNA-centered approaches to characterize regulatory protein–DNA interaction complexes. Mol. Biosyst. 6: 462–468. [DOI] [PubMed] [Google Scholar]
- Spitz F., Furlong E. E. M., 2012. Transcription factors: from enhancer binding to developmental control. Nat. Rev. Genet. 13: 613–626. [DOI] [PubMed] [Google Scholar]
- Stern D. L., Orgogozo V., 2008. The loci of evolution: how predictable is genetic evolution? Evolution 62: 2155–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber E., Eberl D. F., 2001. Song production in auditory mutants of Drosophila: the role of sensory feedback. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 187: 341–348. [DOI] [PubMed] [Google Scholar]
- Thummel C. S., 1995. From embryogenesis to metamorphosis: the regulation and function of Drosophila nuclear receptor superfamily members. Cell 83: 871–877. [DOI] [PubMed] [Google Scholar]
- Wagih O., Parts L., 2014. gitter: a robust and accurate method for quantification of colony sizes from plate images. G3 (Bethesda) 4: 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhout A. J. M., 2011. What does biologically meaningful mean? A perspective on gene regulatory network validation. Genome Biol. 12: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. F., Black B. C., Afshar G., Kermabon A. Y., Wright T. R., et al. , 1991. Temporal and spatial expression of the yellow gene in correlation with cuticle formation and dopa decarboxylase activity in Drosophila development. Dev. Biol. 147: 32–45. [DOI] [PubMed] [Google Scholar]
- Werner T., Koshikawa S., Williams T. M., Carroll S. B., 2010. Gener-ation of a novel wing colour pattern by the Wingless morphogen. Nature 464: 1143–1148. [DOI] [PubMed] [Google Scholar]
- Williams T. M., Selegue J. E., Werner T., Gompel N., Kopp A., et al. , 2008. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 134: 610–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopp P. J., Beldade P., 2009. Development and evolution of insect pigmentation: genetic mechanisms and the potential consequences of pleiotropy. Semin. Cell Dev. Biol. 20: 65–71. [DOI] [PubMed] [Google Scholar]
- Wittkopp P. J., Kalay G., 2012. Cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence. Nat. Rev. Genet. 13: 59–69. [DOI] [PubMed] [Google Scholar]
- Wittkopp P. J., True J. R., Carroll S. B., 2002a Reciprocal functions of the Drosophila yellow and ebony proteins in the development and evolution of pigment patterns. Development 129: 1849–1858. [DOI] [PubMed] [Google Scholar]
- Wittkopp P. J., Vaccaro K., Carroll S. B., 2002b Evolution of yellow gene regulation and pigmentation in Drosophila. Curr. Biol. 12: 1547–1556. [DOI] [PubMed] [Google Scholar]
- Wittkopp P. J., Carroll S. B., Kopp A., 2003. Evolution in black and white: genetic control of pigment patterns in Drosophila. Trends Genet. 19: 495–504. [DOI] [PubMed] [Google Scholar]
- Wright T. R., 1987. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv. Genet. 24: 127–222. [PubMed] [Google Scholar]
- Zelhof A. C., Yao T. P., Evans R. M., McKeown M., 1995. Identification and characterization of a Drosophila nuclear receptor with the ability to inhibit the ecdysone response. Proc. Natl. Acad. Sci. USA 92: 10477–10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.