Abstract
ZIP (ZRT/IRT-like Protein) and CDF (Cation Diffusion Facilitator) are two large metal transporter families mainly transporting zinc into and out of the cytosol. Several ZIP and CDF transporters have been characterized in mammals and various model organisms, such as yeast, nematode, fruit fly, and zebrafish, and many candidate genes have been identified by genome projects. Unexpected functions of ZIP and CDF transporters have been recently reported in some model organisms, leading to major advances in our understanding of the functions of mammalian counterparts. Here, we review the recent information on the sequence similarity and functional relationship among eukaryotic ZIP and CDF transporters obtained from the representative model organisms.
Key words: zinc transporter, ZIP, CDF, ZnT
Introduction
Zinc is an essential trace element for living organisms, because it is required for the catalytic activity of numerous metalloenzymes (1) and can also serve as a key structural component of a large number of zinc-dependent proteins 2., 3.. Zinc homeostasis in the cells, therefore, is achieved through the coordinate regulation of zinc influx, efflux, and distribution to intracellular organelles (4). Zinc transporters have essential functions in such processes and a number of zinc transporters have been identified in many organisms 4., 5., 6., 7..
Zinc transporters are largely classified into two metal transporter families, the ZIP (ZRT/IRT-like Protein) and CDF (Cation Diffusion Facilitator) families 4., 5., 7.. In bacteria, the ABC transporters and P-type ATPases have been shown to function as zinc transporters (8), but neither of them plays a physiological role in zinc transport in eukaryotes (5). The ZIP and CDF families are also assigned as solute carrier 39 (SLC39A) and SLC30A families 9., 10., and both seem to have a very ancient origin because they are identified in diverse organisms from archeae and eubacteria to eukaryotes (5). ZIP family transporters function in zinc influx into the cytosol, while CDF family transporters mobilize zinc in the opposite direction. All members of both families are thought to transport zinc across the biological membranes, but certain proteins are speculated to transport other metals such as iron, nickel, and manganese as a major substrate. In fact, ZIP and CDF transporters have been shown to transport these metals as physiologically important substrates in certain plants 11., 12., 13., and manganese not zinc is described as a more selective substrate of ZIP8/BIGM103 in the competitive assay of cadmium uptake (14).
Recently, interesting functions of ZIP and CDF transporters have been found in various organisms. A comprehensive deliberation on these functions together with integrative comparison of the sequence similarity within each ZIP and CDF transporter family would provide a clue to speculate functions of the uncharacterized ZIP and CDF proteins and to elicit further functions of the characterized ones. Here we review the physiological and cellular functions of ZIP and CDF transporters with emphasis on these matters. The plant ZIP and CDF proteins are referred to other reviews 15., 16., 17..
ZIP Transporters
Arrangement of ZIP proteins found in the genome sequences of the representative model organisms
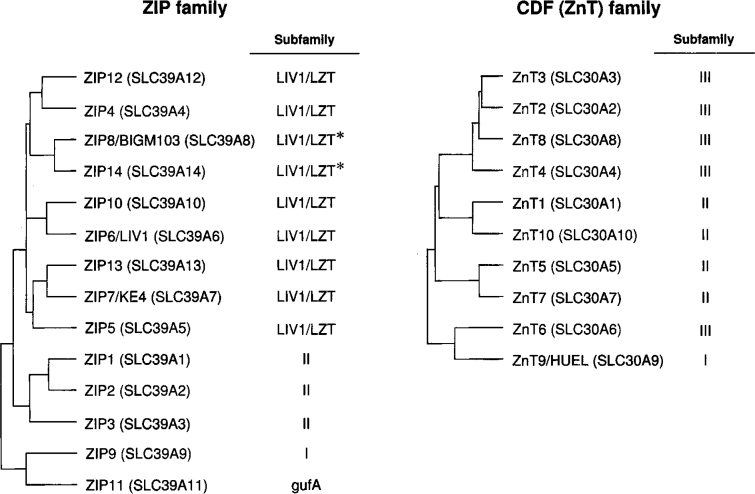
To date, fourteen ZIP proteins have been molecularly characterized or identified in human and mouse 4., 9.. The ZIP family is divided into subfamilies I, II, LIV1/LZT, and gufA, based on their degrees of sequence conservation 5., 18. (Figure 1). The LIV1/LZT subfamily is characterized by having a metalloprotease motif (HEXPHEXGD) around the membrane-spanning domain V. Although the initial histidine in the HEXPHEXGD motif is thought to be requisite for the zinc transport activity of LIV1/LZT transporters, ZIP8/BIGM103 and ZIP14 lacking it have zinc transport activity 19., 20., 21., 22.. ZIP proteins are predicted to have eight membrane-spanning domains with a membrane topology in which the N- and C-terminal ends are located outside the plasma membrane, and have a cytoplasmic His-rich loop between membrane-spanning domains III and IV, which is thought to function as a zinc-binding site. However, ZIP11, ZIP12, and ZIP13 lack the His-rich loop.
Fig. 1.
A dendrogram showing the sequence similarity and the class of subfamily of human ZIP and CDF (ZnT) family members. The dendrogram was generated by using the GENETYX-MAC software, and the class of subfamily follows the assignment by Gaither and Eide (5). The class of members of the LIV1/LZT subfamily lacking the initial histidine in the HEXPHEXGD motif is indicated as LIV1/LZT* subfamily. [This figure has been modified from Kambe et al. (4) with permission from Birkhäuser Publishing, Basel, Switzerland.]
Table 1 shows the ZIP proteins found in the genome sequences of human, mouse, chicken, zebrafish, fruit fly, nematode, and yeast according to the similarity to the human ZIP proteins. As shown in Table 1, most LIV1/LZT proteins in the indicated organisms are arranged to each human LIV1/LZT member except in yeast, where the LIV1/LZT protein is only found as the homologous protein to ZIP7/KE4. In LIV1/LZT subfamily, ZIP12 and ZIP4, ZIP8/BIGM103 and ZIP14, ZIP10 and ZIP6/LIV1, or ZIP13 and ZIP7/KE4 are similar (Figure 1), but not completely homologous. For example, ZIP12 lacks the His-rich loop between membrane-spanning domains III and IV while ZIP4 has; ZIP13 lacks histidine residues in N-terminal portion and between membrane-spanning domains II and III, or III and IV, but ZIP7/KE4 has many histidine residues in these portions. ZIP8/BIGM103 and ZIP14, or ZIP10 and ZIP6/LIV1 have a high identity (48% or 38% identity, respectively) and are homologous in the length of amino acid sequence, the property of the His-rich loop, and the distribution of histidine residues in their sequences 19., 23.. The expression of only one or the other of ZIP8/BIGM103 and ZIP14 in nematode, or ZIP10 and ZIP6/LIV1 in fruit fly and nematode, may be sufficient for the biological function, judging from the genome sequences (Table 1). Compared with LIV1/LZT subfamily, ZIPII subfamily has similar amino acid length, topology, and subcellular localization (at the plasma membrane) 4., 5., 24.. Interestingly, the numbers of homologous proteins of ZIP1, ZIP2, or ZIP3 found are: one in chicken and zebrafish, two in yeast, and six in nematode (Table 1), which may be linked to the fact that nematode has extra CDF proteins (see Table 2 and below). All eukaryotes have proteins with similarity to ZIP9 of ZIPI subfamily or ZIP11 of gufA subfamily, which suggests that they may have retained important functions during evolution.
Table 1.
Arrangement of ZIP Proteins Found in the Genome Sequences of the Representative Model Organisms*
The representative model organisms include: human (Homo sapiens), mouse (Mus musculus), chicken (Gallus gallus), zebrafish (Danio rerio), fruit fly (Drosophila melanogaster), nematode (Caenorhabditis elegans), and yeast (Saccharomyces cerevisiae). ZIP proteins in the indicated organisms are arranged according to the similarity to each human member from the homology search using NCBI BLAST server (http://www.ncbi.nlm.nih.gov/BLAST/) and available information. The upper number indicates the identity (%) between the sequence and the corresponding human counterpart. The lower number indicates the accession number with the name in the parentheses if known.
Partial sequence but shows significant similarity to ZIP4
Has a short N-terminal portion and does not conserve the HEXPHEXGD sequence
Two highly homologous proteins are found
The sequences include NP_493626, NP_491660, NP_496876, NP_495126, NP_500517, and NP_001026796. They are not arranged because of almost equal similarity to ZIP1, ZIP2, and ZIP3
Affinity of ZIP1 and ZIP2 to zinc is unknown although Zrt1 (high affinity) and Zrt2 (low affinity) are arranged.
Table 2.
Arrangement of CDF Proteins Found in the Genome Sequences of the Representative Model Organisms*
CDF proteins in the indicated organisms are arranged according to the similarity to each human member as described in Table 1.
Indicates the absence of the corresponding proteins in human ZnTs
Partial sequence but shows significant similarity to ZnT10
Has long N- and C-terminal portions and shows higher similarity to ZnT2 (44%)
Sequence name indicated in parentheses is taken from Yoder et al. (51)
Shows almost equal similarity to ZnT2 and ZnT8
May functionally be arranged to ZnT4 orthologue
Shows the highest similarity to ZnT1
Not found by homology search using BLASTP but functionally homologous between ZnT6 and Zrg17
Shows weak similarity to yeast Mft1 and Mft2.
Interesting characteristics of ZIP transporters obtained from the model organisms
An interesting function of ZIP6/LIV1 was found in zebrafish (25). Zebrafish ZIP6/LIV1 is essential for epithelial-mesenchymal transition (EMT), which is one of the central events of embryonic development, organ and tissue remodeling, and cancer metastasis, by regulating the nuclear localization of the zinc-finger transcription factor Snail, a master regulator of EMT, because it represses the transcription of E-cadherin (25). The expression of zebrafish ZIP6/LIV1 is dependent on STAT3, which is required for the cell migration, and this characteristic is conserved in human and mouse ZIP6/LIV1 (25). As ZIP6/LIV1 was identified as an estrogen-regulated gene in breast cancer cells (26) and was shown to be significantly associated with the spread of breast cancer to the lymph nodes (27), the presented function of ZIP6/LIV1 is very interesting in that it may be a novel therapeutic target for improving tumor therapy (28).
FOI, a homologous protein of ZIP10 in fruit fly, can act as a zinc transporter (29), and is required for both germ cell ensheathment and gonad morphogenesis in order to control germ cell migration without affecting gonad cell identity (30). It controls the level of E-cadherin in the gonad that is essential for the cell-cell adhesion (31). FOI was reported as the closely related protein to ZIP6/LIV1 (30), but its sequence is the most homologous to ZIP10 (Table 1). ZIP10 and ZIP6/LIV1 are homologous in amino acid sequence (38% identity) and the property of many histidine residues in the His-rich loop or N-terminal portion, therefore they are likely to have very similar functions. Either of them may function as a backup system if expressed simultaneously.
Another LIV1/LZT protein, Catsup, a fruit fly ZIP7/KE4 orthologue, down-regulates tyrosine hydroxylase activity (32). Interestingly, IAR1, an Arabidopsis ZIP7/KE4 homologue, is supposed to regulate auxin conjugate hydroxylase activity by exporting inhibitory metal (zinc) out of the secretory pathway (33). Actually, ZIP7/KE4 is localized to the endoplasmic reticulum (ER) and the Golgi apparatus 34., 35. and transport zinc out of the Golgi apparatus (35). Since the expression of mouse ZIP7/KE4 cDNA complements the defects of iar1 mutant (33), ZIP7/KE4 and all of its orthologues may export zinc out of the secretory compartments to fine-tune the activity of zinc-requiring enzymes and other metal-requiring enzymes like hydroxylases.
In yeast, a high-affinity zinc uptake transporter, Zrt1, is rapidly endocytosed from plasma membrane through a ubiquitin-mediated mechanism and degraded in vacuoles in response to high levels of extracellular zinc 36., 37.. This type of posttranslational distribution operates in mammalian ZIP proteins; not only in the Zrt1 homologous proteins ZIP1 and ZIP3 38., 39. but also in the LIV1/LZT protein ZIP4 more clearly 40., 41.. These characteristics of ZIP transporters indicate that the traffic of ZIP proteins in response to extracellular zinc would be essential for physiological and cellular zinc homeostasis.
CDF Transporters
Arrangement of CDF proteins found in the genome sequences of the representative model organisms
To date, ten CDF proteins designated as ZnT (Zn Transporter) proteins of human or murine origin have been molecularly characterized or identified 7., 10., 42.. CDF transporters are divided into three subgroups, CDF subfamilies I, II, and III, based on their sequence similarities (5). Most eukaryotic members are assigned to subfamilies II and III (5) but ZnT9/HUEL and its homologous proteins are classified into CDF subfamily I (Figure 1), which contains mostly prokaryotic members from both eubacterial and archeael sources (5). There are sequence similarities among ZnT2, ZnT3, ZnT4, and ZnT8, between ZnT1 and ZnT10, and between ZnT5 and ZnT7 (Figure 1), which suggests that these closely related proteins have similar functions in the cells. CDF transporters have the same predicted membrane topology of six membrane-spanning domains with both N- and C-terminal ends thought to reside intracellularly and a cytoplasmic His-rich loop between membrane-spanning domains IV and V, although ZnT5 and its homologous proteins have a long N-terminal portion with extra membrane-spanning domains (43). In ZnT6 and its orthologues, the His-rich loop is not rich in histidine residues but retains serine residues instead (44). In ZnT10 and its orthologues, the loop lacks histidine residues but bears a long loop rich in serine and basic amino acid residues (42). The His-rich loop is thought to function as a metal-binding site and is shown to have essential functions (45). ZnT9/HUEL has a cation efflux domain (pfam01545); therefore, it has been assigned to the CDF family 7., 10.. However, ZnT9/HUEL and its homologous proteins have significant homology to the DNA-binding domain and the nuclear receptor interaction motif (46). Furthermore, ZnT9/HUEL is predominantly localized to the cytoplasm and translocates to the nucleus in a cell cycle-dependent manner (46). Thus, whether ZnT9/HUEL belongs to the CDF family remains open to question.
The CDF proteins found in the human, mouse, chicken, zebrafish, fruit fly, nematode, and yeast genome sequences are arranged in Table 2 according to the similarity to human ZnT proteins as in Table 1. Compared with ZIP proteins, CDF proteins in the indicated organisms are arranged to each human ZnT member except for ZnT3. ZnT3 is specifically expressed in the brain, which suggests that ZnT3 has important neural functions in mammals (47). Since the zinc transported by ZnT3 into the synaptic vesicles is implicated in β-amyloid plaque formation (48), the expression level of ZnT3 may be an important factor in the incidence of Alzheimer’s disease.
The sequences of ZnT5 and ZnT6 are found simultaneously in all organisms except for fruit fly (Table 2), which is consistent with their characteristic to form hetero-oligomeric complexes (49). In fruit fly, the ZnT7 homologous protein is found (Table 2). As ZnT5 and ZnT7 have similar functions in the secretory pathway (see below and ref. 45), the expression of either ZnT5 (with ZnT6) or ZnT7 would be sufficient in fruit fly, nematode, and yeast.
Mft1 and Mft2, which were identified as mitochondrial iron transporters in yeast (50), and SUR7, which is the nematode CDF protein involved in Ras signaling (51), are not classified into human members because of low homology and different subcellular localization and functions (Table 2). Nematode has five more CDF proteins that fail to show similarity to human members (Table 2). Further investigation is needed to identify their functions and the relationship between these unclassified CDF proteins and mammalian members.
Interesting characteristics of CDF transporters obtained from the model organisms
Like the ZIP proteins, interesting CDF functions have been found in the model organisms. In yeast, Msc2 and Zrg17 form hetero-oligomeric complexes and have essential functions to maintain homeostasis in the ER by transporting zinc into the ER (52). They are counterpart proteins of ZnT5 and ZnT6, although Msc2 is homologous to ZnT5 only in the C-terminal portion including six membrane-spanning domains 43., 53. and Zrg17 is the distant homologue of ZnT6 (52). The hetero-oligomeric formation of ZnT5 and ZnT6 has been evidenced by using chicken DT40 cells deficient in ZnT5, ZnT6, and ZnT7 (49). ZnT7 is homologous to ZnT5 in cation efflux domains (pfam01545), but it fails to form hetero-oligomeric complexes with ZnT6 (unpublished data), instead, it forms homo-oligomeric complexes (49). In vertebrates, these two different zinc transport complexes, ZnT5/ZnT6 hetero-oligomeric complexes and ZnT7 homo-oligomeric complexes, operate to activate zinc-requiring enzymes like alkaline phosphatases that are synthesized and activated by binding with zinc in the secretory pathway (49). Moreover, since Msc2 and Zrg17 are involved in the unfolded protein response (UPR) because the mutant yeast strains lacking neither or either of the genes are defective in the ER-associated degradation (ERAD) and show the increased UPR under low-zinc conditions 52., 54., ZnT5, ZnT6, ZnT7, and their orthologues may have such functions. In fact, zinc deficiency can up-regulate the UPR in mammalian cells (54).
CDF1, a nematode ZnT1 orthologue, positively regulates the Ras-Raf-MEK-ERK signal transduction by promoting zinc efflux and reducing the concentration of cytosolic zinc 55., 56.. CDF1 binds to Raf-1 and promotes the biological and enzymatic activity of Raf-1 (57). This interaction occurs between the intracellular C-terminal tail of CDF1 and the N-terminal regulatory portion of Raf-1. ZnT1 complements all of these characteristics of CDF1 55., 57.. As the binding of ZnT1 to Raf-1 is inhibited by zinc (57), it is plausible that ZnT1 lowers the cytosolic zinc, which promotes its binding to Raf-1 and facilitates Raf-1 activation. However, it has not been elucidated whether the mammalian Ras-mediated signaling pathway is fine-tuned by ZnT1 in physiological condition.
The divergent CDF protein in nematode, SUR7, which is probably localized to the ER, also positively regulates Ras signaling through modulating the activity of kinase suppressor of Ras (KSR; ref. 51), suggesting that other CDF proteins may regulate Ras signaling in nematode. In fact, the toc-1 protein (ZC395.3) that shows homology to ZnT6 is reported to be involved in Ras signaling (51). The toc-1 protein seems to have essential functions of supplying zinc to proteins in the secretory pathway by forming hetero-oligomeric complexes with the ZnT5 orthologue protein (Y105E8A.3), because the ZnT7 gene is not found in the nematode genome sequence (Table 2). The putative hetero-oligomeric complexes may have important functions in Ras signaling in nematode.
Conclusion
Various roles of ZIP or CDF transporters have been clarified, but further studies are needed to fully elucidate their physiological functions. A comprehensive comparison of similarities and differences in the functions and regulations in transcription, translation, trafficking, and turnover of homologous proteins of ZIP and CDF among mammals and other organisms should help elucidate the true role of each transporter in zinc homeostasis. By elucidating which of the redundant transporters is the principal or the backup, and identifying which transporter forms homo-oligomeric complexes or hetero-oligomeric complexes to express zinc transport activity, we should be able to ultimately solve the intriguing question why living organisms including humans need so many zinc transporters to survive.
Acknowledgements
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Y.Y.-I. and T.K.) and by the Mishima Kaiun Memorial Foundation (to T.K.). We thank Wataru Matsuura for support in preparing tables.
References
- 1.Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Berg J.M., Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271:1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 3.Krishna S.S. Structural classification of zinc fingers: survey and summary. Nucleic Acids Res. 2003;31:532–550. doi: 10.1093/nar/gkg161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kambe T. Overview of mammalian zinc transporters. Cell. Mol. Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaither L.A., Eide D.J. Eukaryotic zinc transporters and their regulation. Biometals. 2001;14:251–270. doi: 10.1023/a:1012988914300. [DOI] [PubMed] [Google Scholar]
- 6.Chimienti F. Zinc homeostasis-regulating proteins: new drug targets for triggering cell fate. Curr. Drug Targets. 2003;4:323–338. doi: 10.2174/1389450033491082. [DOI] [PubMed] [Google Scholar]
- 7.Liuzzi J.P., Cousins R.J. Mammalian zinc transporters. Annu. Rev. Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 8.Hantke K. Bacterial zinc transporters and regulators. Biometals. 2001;14:239–249. doi: 10.1023/a:1012984713391. [DOI] [PubMed] [Google Scholar]
- 9.Eide D.J. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 10.Palmiter R.D., Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004;447:744–751. doi: 10.1007/s00424-003-1070-7. [DOI] [PubMed] [Google Scholar]
- 11.Eide D. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persans M.W. Functional activity and role of cation-efflux family members in Ni hyperaccumulation in Thlaspi goesingense. Proc. Natl. Acad. Sci. USA. 2001;98:9995–10000. doi: 10.1073/pnas.171039798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delhaize E. Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell. 2003;15:1131–1142. doi: 10.1105/tpc.009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalton T.P. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc. Natl. Acad. Sci. USA. 2005;102:3401–3406. doi: 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maser P. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanikenne M. A comparative inventory of metal transporters in the green alga Chlamydomonas reinhardtii and the red alga Cyanidioschizon merolae. Plant Physiol. 2005;137:428–446. doi: 10.1104/pp.104.054189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer U. MTP1 mops up excess zinc in Arabidopsis cells. Trends Plant Sci. 2005;10:313–315. doi: 10.1016/j.tplants.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Taylor K.M., Nicholson R.I. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim. Biophys. Acta. 2003;1611:16–30. doi: 10.1016/s0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 19.Begum N.A. Mycobacterium bovis BCG cell wall and lipopolysaccharide induce a novel gene, BIGM103, encoding a 7-TM protein: identification of a new protein family having Zn-transporter and Zn-metalloprotease signatures. Genomics. 2002;80:630–645. doi: 10.1006/geno.2002.7000. [DOI] [PubMed] [Google Scholar]
- 20.Liuzzi J.P. Interleukin-6 regulates the zinc transporter Zipl4 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA. 2005;102:6843–6848. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tominaga K. SLC39A14, a LZT protein, is induced in adipogenesis and transports zinc. FEBS J. 2005;272:1590–1599. doi: 10.1111/j.1742-4658.2005.04580.x. [DOI] [PubMed] [Google Scholar]
- 22.Taylor K.M. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 2005;579:427–432. doi: 10.1016/j.febslet.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Taylor K.M. LIV-1 breast cancer protein belongs to new family of histidine-rich membrane proteins with potential to control intracellular Zn2+ homeostasis. IUBMB Life. 2000;49:249–253. doi: 10.1080/15216540050033087. [DOI] [PubMed] [Google Scholar]
- 24.Dufner-Beattie J. Structure, function, and regulation of a subfamily of mouse zinc transporter genes. J. Biol. Chem. 2003;278:50142–50150. doi: 10.1074/jbc.M304163200. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita S. Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature. 2004;429:298–302. doi: 10.1038/nature02545. [DOI] [PubMed] [Google Scholar]
- 26.Manning D.L. Effects of oestrogen on the expression of a 4.4 kb mRNA in the ZR-75-1 human breast cancer cell line. Mol. Cell. Endocrinol. 1988;59:205–212. doi: 10.1016/0303-7207(88)90105-0. [DOI] [PubMed] [Google Scholar]
- 27.Manning D.L. Oestrogen-regulated genes in breast cancer: association of pLIV1 with lymph node involvement. Eur. J. Cancer. 1994;30:675–678. doi: 10.1016/0959-8049(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 28.Taylor K.M. Zinc transporter LIV-1: a link between cellular development and cancer progression. Trends Endocrinol. Metab. 2004;15:461–463. doi: 10.1016/j.tem.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Mathews W.R. Drosophila fear of intimacy encodes a Zrt/IRT-like protein (ZIP) family zinc transporter functionally related to mammalian ZIP proteins. J. Biol. Chem. 2005;280:787–795. doi: 10.1074/jbc.M411308200. [DOI] [PubMed] [Google Scholar]
- 30.van Doren M. Fear of intimacy encodes a novel transmembrane protein required for gonad morphogenesis in Drosophila. Development. 2003;130:2355–2364. doi: 10.1242/dev.00454. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins A.B. Drosophila E-cadherin is essential for proper germ cell-soma interaction during gonad morphogenesis. Development. 2003;130:4417–4426. doi: 10.1242/dev.00639. [DOI] [PubMed] [Google Scholar]
- 32.Stathakis D.G. The catecholamines up (Catsup) protein of Drosophila melanogaster functions as a negative regulator of tyrosine hydroxylase activity. Genetics. 1999;153:361–382. doi: 10.1093/genetics/153.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lasswell J. Cloning and characterization of IAR1, a gene required for auxin conjugate sensitivity in Arabidopsis. Plant Cell. 2000;12:2395–2408. doi: 10.1105/tpc.12.12.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor K.M. Structure-function analysis of HKE4, a member of the new LIV-1 subfamily of zinc transporters. Biochem. J. 2004;377:131–139. doi: 10.1042/BJ20031183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang L. The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J. Biol. Chem. 2005;280:15456–15463. doi: 10.1074/jbc.M412188200. [DOI] [PubMed] [Google Scholar]
- 36.Gitan R.S. Zinc-induced inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation. J. Biol. Chem. 1998;273:28617–28624. doi: 10.1074/jbc.273.44.28617. [DOI] [PubMed] [Google Scholar]
- 37.Gitan R.S., Eide D.J. Zinc-regulated ubiquitin conjugation signals endocytosis of the yeast ZRT1 zinc transporter. Biochem. J. 2000;346:329–336. [PMC free article] [PubMed] [Google Scholar]
- 38.Kelleher S.L., Lonnerdal B. Zn transporter levels and localization change throughout lactation in rat mammary gland and are regulated by Zn in mammary cells. J. Nutr. 2003;133:3378–3385. doi: 10.1093/jn/133.11.3378. [DOI] [PubMed] [Google Scholar]
- 39.Wang F. Zinc-stimulated endocytosis controls activity of the mouse ZIP1 and ZIP3 zinc uptake transporters. J. Biol. Chem. 2004;279:24631–24639. doi: 10.1074/jbc.M400680200. [DOI] [PubMed] [Google Scholar]
- 40.Kim B.E. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J. Biol. Chem. 2004;279:4523–4530. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 41.Wang F. Acrodermatitis enteropathica mutations affect transport activity, localization and zinc-responsive trafficking of the mouse ZIP4 zinc transporter. Hum. Mol. Genet. 2004;13:563–571. doi: 10.1093/hmg/ddh049. [DOI] [PubMed] [Google Scholar]
- 42.Seve M. In silico identification and expression of SLC30 family genes: an expressed sequence tag data mining strategy for the characterization of zinc transporters’ tissue expression. BMC Genomics. 2004;5:32. doi: 10.1186/1471-2164-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kambe T. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J. Biol. Chem. 2002;277:19049–19055. doi: 10.1074/jbc.M200910200. [DOI] [PubMed] [Google Scholar]
- 44.Huang L. Functional characterization of a novel mammalian zinc transporter, ZnT6. J. Biol. Chem. 2002;277:26389–26395. doi: 10.1074/jbc.M200462200. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki T. Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J. Biol. Chem. 2005;280:637–643. doi: 10.1074/jbc.M411247200. [DOI] [PubMed] [Google Scholar]
- 46.Sim del L.C. The novel human HUEL (C4orfl) protein shares homology with the DNA-binding domain of the XPA DNA repair protein and displays nuclear translocation in a cell cycle-dependent manner. Int. J. Biochem. Cell. Biol. 2002;34:487–504. doi: 10.1016/s1357-2725(01)00156-x. [DOI] [PubMed] [Google Scholar]
- 47.Palmiter R.D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. USA. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J.Y. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc. Natl. Acad. Sci. USA. 2002;99:7705–7710. doi: 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki T. Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatase in vertebrate cells. J. Biol. Chem. 2005;280:30956–30962. doi: 10.1074/jbc.M506902200. [DOI] [PubMed] [Google Scholar]
- 50.Li L., Kaplan J. Characterization of two homologous yeast genes that encode mitochondrial iron transporters. J. Biol. Chem. 1997;272:28485–28493. doi: 10.1074/jbc.272.45.28485. [DOI] [PubMed] [Google Scholar]
- 51.Yoder J.H. Modulation of KSR activity in Caenorhabditis elegans by Zn ions, PAR-1 kinase and PP2A phosphatase. EMBO J. 2004;23:111–119. doi: 10.1038/sj.emboj.7600025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ellis C.D. Heteromeric protein complexes mediate zinc transport into the secretory pathway of eukaryotic cells. J. Biol. Chem. 2005;280:28811–28818. doi: 10.1074/jbc.M505500200. [DOI] [PubMed] [Google Scholar]
- 53.Li L., Kaplan J. The yeast gene MSC2, a member of the cation diffusion facilitator family, affects the cellular distribution of zinc. J. Biol. Chem. 2001;276:5036–5043. doi: 10.1074/jbc.M008969200. [DOI] [PubMed] [Google Scholar]
- 54.Ellis C.D. Zinc and the Msc2 zinc transporter protein are required for endoplasmic reticulum function. J. Cell. Biol. 2004;166:325–335. doi: 10.1083/jcb.200401157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruinsma J.J. Zinc ions and cation diffusion facilitator proteins regulate Ras-mediated signaling. Dev. Cell. 2002;2:567–578. doi: 10.1016/s1534-5807(02)00151-x. [DOI] [PubMed] [Google Scholar]
- 56.Hajnal A. Fine-tuning the RAS signaling pathway: Zn2+ makes the difference. Mol. Cell. 2002;9:927–928. doi: 10.1016/s1097-2765(02)00535-x. [DOI] [PubMed] [Google Scholar]
- 57.Jirakulaporn T., Muslin A.J. Cation diffusion facilitator proteins modulate Raf-1 activity. J. Biol. Chem. 2004;279:27807–27815. doi: 10.1074/jbc.M401210200. [DOI] [PubMed] [Google Scholar]
- 58.Qiu A. Molecular cloning and functional characterization of a high-affinity zinc importer (DrZIP1) from zebrafish (Danio rerio) Biochem. J. 2005;388:745–754. doi: 10.1042/BJ20041807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu A., Hogstrand C. Functional expression of a low-affinity zinc uptake transporter (FrZIP2) from pufferfish (Takifugu rubripes) in MDCK cells. Biochem. J. 2005;390:777–786. doi: 10.1042/BJ20050568. [DOI] [PMC free article] [PubMed] [Google Scholar]