Highlight
Transcriptional analyses revealed that single AtRLP genes may be involved in multiple physiological processes. Overexpression studies found AtRLP3/11 to be involved in meristem development and AtRLP28 to be involved in salt tolerance.
Key words: Arabidopsis, hormone, overexpression, receptor-like protein, stress, transcriptional regulation.
Abstract
Receptor-like proteins (RLPs) have been implicated in multiple biological processes, including plant development and immunity to microbial infection. Fifty-seven AtRLP genes have been identified in Arabidopsis, whereas only a few have been functionally characterized. This is due to the lack of suitable physiological screening conditions and the high degree of functional redundancy among AtRLP genes. To overcome the functional redundancy and further understand the role of AtRLP genes, we studied the evolution of AtRLP genes and compiled a comprehensive profile of the transcriptional regulation of AtRLP genes upon exposure to a range of environmental stresses and different hormones. These results indicate that the majority of AtRLP genes are differentially expressed under various conditions that were tested, an observation that will help to select certain AtRLP genes involved in a specific biological process for further experimental studies to eventually dissect their function. A large number of AtRLP genes were found to respond to more than one treatment, suggesting that one single AtRLP gene may be involved in multiple physiological processes. In addition, we performed a genome-wide cloning of the AtRLP genes, and generated and characterized transgenic Arabidopsis plants overexpressing the individual AtRLP genes, presenting new insight into the roles of AtRLP genes, as exemplified by AtRLP3, AtRLP11 and AtRLP28. Our study provides an overview of biological processes in which AtRLP genes may be involved, and presents valuable resources for future investigations into the function of these genes.
Introduction
All living organisms exploit cell-surface receptors to perceive extracellular signals that are from self (e.g. endogenous signaling molecules), non-self (e.g. pathogen-derived molecules) or modified-self (e.g. self molecules that are modified by pathogens) (Cook et al., 2015). In plants, most of these receptors contain extracellular leucine-rich repeats (eLRRs) that are thought to mediate protein–protein interactions (Kobe and Kajava, 2001; Matsubayashi, 2003). Receptor-like proteins (RLPs) represent an important class of such cell-surface receptors. Structurally RLPs consist of two eLRR domains interrupted by an island domain, a single-pass transmembrane domain and a short cytoplasmic tail that lacks obvious motifs for intracellular signaling, except for a putative endocytosis motif found in some members (Tör et al., 2009; Wang et al., 2010a).
RLPs have been shown to play important roles in development and disease resistance in several plant species (Kruijt et al., 2005; Tör et al., 2009; Wang et al., 2010a). Two Arabidopsis RLPs, CLAVATA2 (CLV2)/AtRLP10 and TOO MANY MOUTHS (TMM)/AtRLP17, are known to play a role in plant development. While CLV2 is involved in meristem and organ development, TMM regulates stomatal distribution (Jeong et al., 1999; Nadeau and Sack, 2002; Wang et al., 2008; Wang et al., 2010b; Wang et al., 2011). Apart from CLV2 and TMM, most RLPs characterized to date have been found to be involved in disease resistance. These include the Cf proteins, mediating resistance to the fungal pathogen Cladosporium fulvum (Rivas and Thomas, 2005; Thomma et al., 2005; Stergiopoulos and de Wit, 2009); LeEIX, mediating recognition of the ethylene-inducing xylanase (EIX) of the biocontrol fungus Trichoderma viride (Ron and Avni, 2004); HcrVf-2, conferring resistance to the apple scab fungus Venturia inaequalis (Belfanti et al., 2004); LepR3, providing race-specific resistance to the fungal pathogen Leptosphaeria maculans (Larkan et al., 2013); and Ve1, mediating resistance towards Verticillium vascular fungi expressing the avirulence gene Ave1 (Fradin et al., 2009; Fradin et al., 2011).
Over the years, an increasing number of Arabidopsis RLPs (AtRLPs) have been assigned functions in pathogen resistance. We reported previously the assembly of a genome-wide collection of T-DNA insertion lines for the 57 AtRLP genes in the Arabidopsis genome (Wang et al., 2008). After an extensive screening only a few novel phenotypes were discovered, including the reported phenotypes for CLV2 and TMM. While AtRLP41 was found to mediate abscisic acid (ABA) sensitivity, AtRLP30 and AtRLP18 were found to influence non-host resistance towards Pseudomonas syringae pv. phaseolicola (Wang et al., 2008). In addition, AtRLP52 is required for basal defense against the powdery mildew pathogen Erysiphe cichoracearum (Ramonell et al., 2005). SNC2/AtRLP51 and AtRLP55 were suggested to be implicated in basal defense against the bacterial pathogen Pseudomonas syringae pv. tomato DC3000 (Zhang et al., 2010). ReMAX/AtRLP1 was found to provide recognition of eMAX from Xanthomonads (Jehle et al., 2013), while the fungal pattern sensor RBPG1/AtRLP42 confers resistance to fungal endo-polygalacturonases (Zhang et al., 2014). RFO/AtRLP3 has been implicated in resistance to the vascular wilt fungus Fusarium oxysporum forma specialis matthioli (Shen and Diener, 2013). As a final example, AtRLP23 was recently found to perceive a conserved 20-amino-acid fragment present in most necrosis and ethylene-inducing peptide (NEP) 1-like proteins, thereby mediating immune activation that, similar to what was observed for the Cf proteins, is dependent on SOBIR1 and SERK3/BAK1 (Liebrand et al., 2013; Albert et al., 2015; Postma et al., 2016). However, the biological functions of the majority of the AtRLP genes still remain unclear.
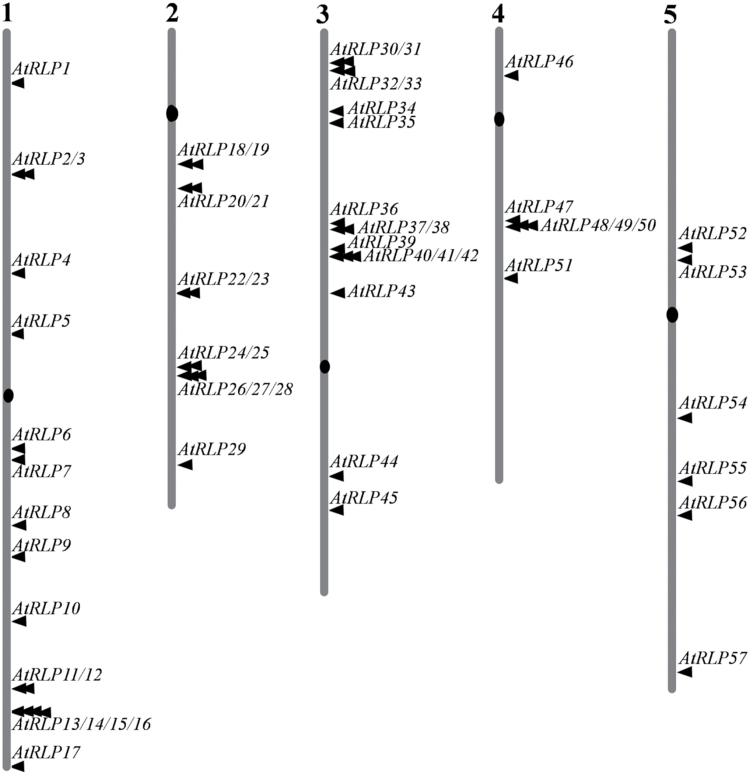
The major challenge currently is to understand the biological function of AtRLP genes that lack an obvious phenotype in a single mutant background (Wang et al., 2008). One reason is the lack of suitable screening conditions in which the phenotype might only be visible in a condition-specific manner. Interestingly, studies on several AtRLP genes have revealed gene expression changes, as well as the emergence of phenotypic alterations, with specific elicitors (Wang et al., 2008; Wang et al., 2010a). Therefore, it may be necessary to test a broad range of physiological conditions, in combination with high-resolution screening for phenotypes. To this end, a comprehensive profile of the transcriptional response of AtRLP genes under various conditions, including exposure to biotic and abiotic stress and hormones, will be very helpful. The lack of assignment of biological functions to AtRLP genes may also be explained by a strong functional redundancy among the various AtRLP genes (Wang et al., 2008). In particular, most of the closely related AtRLP genes are located at one locus on the chromosomes (Fig. 1), making it impossible to generate high-order mutant combinations. RNA interference studies that silence multiple AtRLP genes simultaneously also failed to uncover new biological functions for several sets of closely related AtRLP genes (Ellendorff et al., 2008). As an alternative approach, analysis of the gain-of-function phenotypes has yielded valuable information on the function of AtRLP genes, including TMM, Ve1 and AtRLP23 (Fradin et al., 2009; Fradin et al., 2011; Yan et al., 2014; Albert et al., 2015).
Fig. 1.
AtRLP genes scatter over the different chromosomes of the Arabidopsis genome. The numbers at the top indicate the chromosome number.
To overcome the functional redundancy and further understand the role of AtRLP genes, in this work we studied the evolution of AtRLP genes and compiled a comprehensive profile of the transcriptional regulation of AtRLP genes upon exposure to environmental stresses and hormones. This will help to select AtRLP genes that might be involved in a specific biological process for further experimental studies aimed at dissecting AtRLP function. In addition, we performed a genome-wide cloning of AtRLP genes, and generated and characterized transgenic Arabidopsis overexpressing individual AtRLP genes. The data presented in this study provide valuable resources for future investigations into the biological role of AtRLP genes.
Materials and methods
Plant materials and growth conditions
The Arabidopsis ecotypes Columbia (Col-0) and Landsberg erecta (Ler) were used as wild-types (WT) for all phenotypic analyses. The clv2-1 and rlp10-1 mutants were described previously (Kays and Clark, 1998; Wang et al., 2008). Plants were grown in soil in the greenhouse or on 1/2 MS medium supplemented with 1% sucrose under a 16h light–8h dark regime at 22 °C. For the in vitro growth of Arabidopsis plants, seeds were surface-sterilized for 3h by mixing 10mL water, 10mL 99% NaClO and 5mL 99% HCl, and subsequently sown on 1/2 MS solidified with 1% agar. The plates were incubated at 4 °C in the dark for 3 days and transferred to growth chambers.
Chromosomal locations and analysis of duplication of genes in the AtRLP gene family
The chromosomal location of each member of the AtRLP family and its location-related receptor-like kinase (RLK) gene was determined with the Chromosome Map Tool at TAIR (http://www.arabidopsis.org/jsp/ChromosomeMap/tool.jsp). The location of each gene in relation to major chromosomal duplication events in the Arabidopsis genome was determined with tools provided at http://wolfe.gen.tcd.ie/athal/dup and/or defined by Blanc et al. (2003). Tandem duplicated genes were identified based on criteria described by Shiu and Bleecker (2003). Briefly, tandem repeats of AtRLP genes were defined as genes that are located within 30kb or are separated by five or fewer non-homologous spacer genes.
Gene expression data analysis
The gene expression data of each experiment presented in this study were obtained from AtGenExpress (http://www.weigelworld.org/resources/microarray/AtGenExpress) (Kilian et al., 2007; Goda et al., 2008), and were normalized using the GC-RMA method (Wu et al., 2004). Fifty-two of the 57 AtRLP genes were available in the dataset. The fold-change of expression of AtRLP genes under each condition was determined by the expression change relative to the respective controls. Fold-change ratios were subsequently transformed to log2 values to indicate the transcript change. We set a two-fold change threshold as a cut-off to identify differentially expressed genes, in which the false discovery rate was found to be around 0.2% (Zhu and Wang, 2000). Overlapping of AtRLP gene sets is defined as AtRLP genes that display similar responses to the selected conditions.
AtRLP cloning and generation of transgenic plants
The primers to amplify AtRLP genes were designed according to the predicted ORF sequences that were retrieved from the TAIR database (https://www.arabidopsis.org). Total RNA was extracted from Arabidopsis seedlings using the EZNATM Plant RNA Kit (Omega, USA) and reverse-transcribed into cDNA using the RevertAidTM First Strand cDNA Synthesis Kit (MBI, Fermentas, USA). The PCR reaction was conducted using PhusionTM High-Fidelity DNA Polymerase (Finnzymes, Finland). The PCR products were subsequently purified using the Quick DNA Purification Kit (Cwbio, China). The purified PCR products were cloned into pDONR207 and sequenced. After sequence verification, all entry clones were subsequently recombined into the destination vector pGD625 that contained the CaMV 35S promoter through LR recombination reactions. In the case of AtRLP32 and AtRLP46, CaMV 35S promoters containing destination vectors pFAST-R02 and pB2GW7, respectively, were used.
The resulting constructs were introduced into Agrobacterium tumefaciens and transformed into WT plants or the clv2-1 or rlp10-1 mutants using the floral dip method (Clough and Bent, 1998). Seeds from transformed plants were selected using corresponding antibiotics until at least three homozygous transgenic lines were obtained for each AtRLP gene.
Stress induction and gene expression analysis by quantitative real-time RT-PCR (qPCR)
The sterilized seeds were sown in 1/2 MS liquid medium containing 1% (w/v) sucrose. After sowing, the medium was incubated at 4 °C in the dark for 3 d and subsequently grown on a roller shaker for 7 d with 16h light–8h dark at 21 °C. For NaCl and mannitol induction, the seedlings were treated with 150mM NaCl and 400mM mannitol, respectively, and sampled at 0, 3, and 6h. The qPCRs were performed in triplicate with SYBR Green PCR Master Mix (Thermo Scientific) using a Bio-Rad IQ5 (Bio-Rad). The Actin2 gene was used as control to normalize expression levels. For each independent biological replicate, the relative transcript amount was calculated as the mean of three technical replicates. The relative expression levels were normalized to a value of 1 in the respective control samples. All primers used for qPCRs are listed in Supplementary Table S1 at JXB online.
Phenotypic analyses
The carpel number was counted using mature siliques under a dissection microscope. For biological statistics, there was a minimum of 30 plants of which 20 siliques per plant were counted to determine the mean carpel numbers for individual genotypes. For the salt and mannitol tests, seeds were sown on 1/2 MS medium supplemented with different concentrations of salt and mannitol as indicated. The germination rate was analysed in triplicate for each line (around 60 seeds each).
Results and discussion
Duplication of AtRLP genes in the Arabidopsis genome
We found that AtRLP genes are distributed over all five chromosomes with many clusters containing two or more AtRLP genes (Fig. 1), suggesting a major role of tandem duplications in the enlargement of the AtRLP gene family. We therefore investigated the evolutionary relationship and duplication events of AtRLP family members. To this end, we determined the chromosomal locations and the duplicated chromosome segments in which AtRLP genes are found (Fig. 1 and Table 1). A total of 35 AtRLP genes were found to be present in tandem repeats, representing about 60% of all AtRLP genes (Table 1). Out of 57 AtRLP genes, 27 were found in the hypothesized duplicated regions, whereas 30 were located outside these regions (Table 1). Within the duplicated regions, 11 AtRLP genes were found to have duplicated pair(s), whereas the remaining 16 AtRLP genes were found to have no corresponding duplicated pair although their locations were surrounded by duplicated segments (Table 1). Specifically, four pairs of AtRLP genes (AtRLP23 and AtRLP42; AtRLP33 and AtRLP53; AtRLP44 and AtRLP57; and AtRLP51 and AtRLP55) constitute pairs of duplicated genes in segmental duplicated blocks of chromosomes. In addition, three AtRLP genes, AtRLP1, AtRLP4 and AtRLP17/TMM, were found to be duplicated counterparts of the eLRR-containing genes At1g74360 (eLRR-receptor-like kinase (RLK) gene), At2g14440 (eLRR-RLK gene) and At3g126102 (containing an eLRR domain), respectively. No traceable duplication event was found for three genes, namely AtRLP7, AtRLP36, and AtRLP52. Altogether, these results suggest that a major role of the tandem duplications, in conjunction with segmental duplications, has been to contribute to enlargement of the AtRLP gene family in Arabidopsis.
Table 1.
Duplication of AtRLP genes in the Arabidopsis genome
| Outside the duplication region | Within the duplication region | ||
|---|---|---|---|
| With duplicated genes | Without duplicated genes | ||
| Singular | 3 | 8 | 11 |
| Tandem repeats | 27 | 3 | 5 |
| Total | 30 | 11 | 16 |
In a previous study, Shiu and Bleecker (2003) found that some of the AtRLP genes locate close to an RLK. We identified nine AtRLP genes that were located in relatively close proximity (10 predicted genes) to an RLK gene (see Supplementary Table S2 at JXB online), thus resulting in RLP–RLK combinations. To determine a possible functional significance of such associations, the expression patterns of these genes were compared using ATTED-II (Obayashi et al., 2014). Unfortunately, co-expression could not be confirmed for any of the AtRLP–RLK combinations, suggesting that these RLP–RLK combinations do not have a biological relevance. Indeed, for instance, sequence comparison revealed that AtRLP52 and At5g25930, encoding an RLK, show a high degree of similarity in their extracellular domains (see Supplementary Fig. S1 at JXB online), while they do not exhibit an overlap in expression patterns. It is also possible that several of these AtRLP genes that are located in RLK gene clusters may have arisen through unequal crossovers (Shiu and Bleecker, 2003), as might be the case for AtRLP52 and At5g25930.
AtRLP genes display comprehensive and distinct transcriptional regulation upon exposure to external stimuli and hormones
It was suggested that the majority of AtRLP genes are involved in plant defense, as has been shown by phylogenomic analysis (Fritz-Laylin et al., 2005). The data suggest potential transcriptional regulation of AtRLP genes upon exposure to environmental stimuli. Therefore, we started to investigate the transcriptional regulation of the entire AtRLP gene family by external stimuli and hormones. The availability of microarray datasets allowed us to identify specifically regulated AtRLP genes. To deepen our understanding of the transcriptional regulation of the AtRLP genes, we explored and visualized the expression of the genes under various growth conditions by using AtGenExpress (Kilian et al., 2007). AtRLP27, AtRLP38, AtRLP50, AtRLP51 and AtRLP53 are not present in the AtGenExpress, which resulted in a total of 52 AtRLP genes that were analysed in our study. AtRLP8 was found not to exhibit a response to any treatment tested (Supplementary Table S3). An overview of the differentially expressed AtRLP genes for all conditions tested can be found in Supplementary Tables S4–S7 at JXB online. As the environmental stimuli tested were by no means comprehensive, it is possible that AtRLP8 expression is responsive to other environmental factors. Only AtRLP29 was differentially expressed upon various light treatments (Supplementary Table S7), suggesting that the expression of most AtRLP genes is not perturbed by light. To confirm the validity of the microarray data, we selected AtRLP23, AtRLP28, AtRLP30, AtRLP33 and AtRLP37, based on our study interests, to examine their expression in response to NaCl and mannitol at different time points. By qPCR, we confirmed that out of 20 samples tested, 14 showed similar expression patterns to the microarray data (Supplementary Fig. S2 at JXB online), which represents most of the genes and the two treatments.
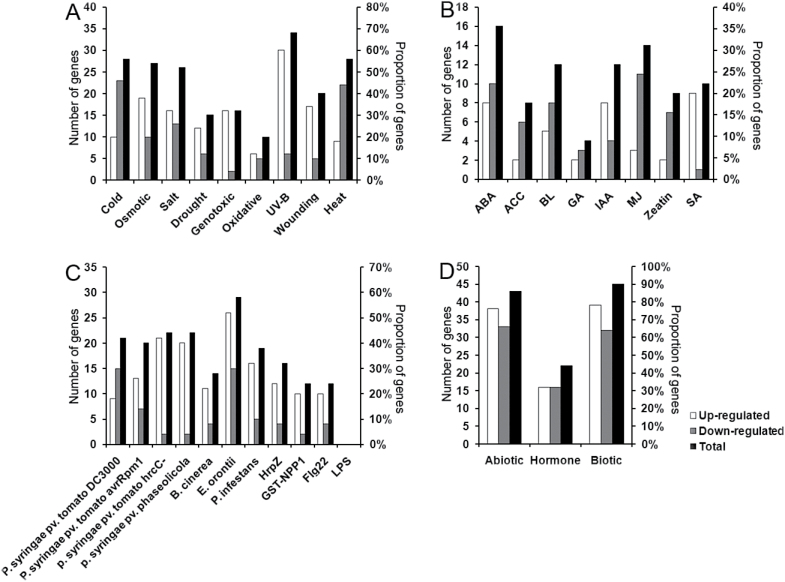
A total of 51 AtRLP genes exhibited differential expression, with a two-fold change or more, under at least one of the tested conditions (Fig. 2, Table 2 and Supplementary Tables S4–S7). A list of AtRLP genes showing the highest differential expression per treatment is presented in Table 2. We found that a number of up-regulated AtRLP genes were significantly over-represented for multiple stress conditions and hormones tested (Fig. 2, Table 2 and Supplementary Tables S4–S7). In particular, the up-regulated AtRLP genes were significantly enriched among seven of the nine abiotic stress conditions and nine of the 11 biotic stress conditions analysed (Fig. 2, Table 2 and Supplementary Tables S4–S7). Amongst all conditions tested, biotic stresses perturbed the expression of the largest proportion of AtRLP genes (87%), compared with the abiotic stresses (80%) and hormone treatments (40%) (Fig. 2). In more detail, the largest proportion of differentially expressed AtRLP genes was found for the treatments of UV-B, cold, heat, osmotic stress, salt stress, ABA, bacterial pathogens and bacterial pattern HrpZ (Fig. 2 and Supplementary Tables S4–S7).
Fig. 2.
Overview of the amounts of differentially expressed AtRLP genes per treatment. The number and proportion of AtRLP genes that are differentially expressed per treatment are indicated. An AtRLP with a two-fold change or greater is considered as a differentially expressed AtRLP gene. (A) Number and proportion of differentially expressed AtRLP genes which are regulated by abiotic treatments. (B) Number and proportion of differentially expressed AtRLP genes that are regulated by hormonal treatments. (C) Number and proportion of differentially expressed AtRLP genes that are regulated by biotic treatments. (D) Number and proportion of differentially expressed AtRLP genes in three major classes of treatments, abiotic stress, hormones, and biotic stress. The total number represents the sum of number of up-regulated and down-regulated genes. As a result of dynamic responses, the total number in some cases was smaller than the sum of up-regulated and down-regulated genes.
Table 2.
The most transcriptionally responsive AtRLP genes per treatment
| Treatment | Gene | Accession no. | Log2 | Treatment | Gene | Accession no. | Log2 |
|---|---|---|---|---|---|---|---|
| Cold | AtRLP33 | At3g05660 | 7.3 | Heat | AtRLP37 | At3g23110 | –3.0 |
| AtRLP37 | At3g23110 | –3.8 | AtRLP9 | At1g58190 | –2.2 | ||
| AtRLP32 | At3g05650 | 2.8 | AtRLP6 | At1g45616 | 2.1 | ||
| AtRLP49 | At4g13900 | 2.6 | AtRLP45 | At3g53240 | –2.1 | ||
| AtRLP18 | At2g15040 | –2.4 | AtRLP54 | At5g40170 | –2.0 | ||
| Osmotic | AtRLP33 | At3g05660 | 5.4 | P. syringae | AtRLP22 | At2g32660 | 5.1 |
| AtRLP23 | At2g32680 | 5.1 | AtRLP21 | At2g25470 | 4.4 | ||
| AtRLP49 | At4g13900 | 3.7 | AtRLP20 | At2g25440 | 4.3 | ||
| AtRLP46 | At4g04220 | 3.5 | AtRLP40 | At3g24954 | 4.3 | ||
| AtRLP28 | At2g33080 | 3.5 | AtRLP26 | At2g33050 | –4.1 | ||
| Salt | AtRLP33 | At3g05660 | 4.3 | P. infestans | AtRLP19 | At2g15080 | 2.8 |
| AtRLP7 | At1g47890 | 3.2 | AtRLP30 | At3g05360 | 2.6 | ||
| AtRLP49 | At4g13900 | 3.2 | AtRLP49 | At4g13900 | 2.4 | ||
| AtRLP37 | At3g23110 | 3.0 | AtRLP18 | At2g15040 | 2.3 | ||
| AtRLP36 | At3g23010 | 2.6 | AtRLP46 | At4g04220 | 2.2 | ||
| AtRLP39 | At3g24900 | 3.9 | AtRLP18 | At2g15040 | –4.6 | ||
| Drought | AtRLP37 | At3g23110 | 3.9 | B. cinerea | AtRLP30 | At3g05360 | 3.1 |
| AtRLP18 | At2g15040 | –2.2 | AtRLP49 | At4g13900 | 2.4 | ||
| AtRLP32 | At3g05650 | 2.1 | AtRLP25 | At2g33030 | 2.1 | ||
| AtRLP33 | At3g05660 | 1.9 | AtRLP20 | At2g25440 | 1.8 | ||
| Genotoxic | AtRLP49 | At4g13900 | 3.8 | E. orontii | AtRLP41 | At3g25010 | 3.7 |
| AtRLP37 | At3g23110 | 3.1 | AtRLP18 | At2g15040 | 3.2 | ||
| AtRLP23 | At2g32680 | 2.3 | AtRLP23 | At2g32680 | 3.1 | ||
| AtRLP7 | At1g47890 | 1.9 | AtRLP35 | At3g11080 | 2.9 | ||
| AtRLP34 | At3g11010 | 1.7 | AtRLP30 | At3g05360 | 2.6 | ||
| Oxidative | AtRLP37 | At3g23110 | 2.9 | HrpZ | AtRLP30 | At3g05360 | 3.1 |
| AtRLP49 | At4g13900 | 1.9 | AtRLP22 | At2g32660 | 3.1 | ||
| AtRLP30 | At3g05360 | 1.8 | AtRLP21 | At2g25470 | 3.0 | ||
| AtRLP23 | At2g32680 | –1.4 | AtRLP12 | At1g71400 | 2.6 | ||
| AtRLP41 | At3g25010 | –1.4 | AtRLP40 | At3g24954 | 2.1 | ||
| UV-B | AtRLP23 | At2g32680 | 6.0 | GST-NPP1 | AtRLP22 | At2g32660 | 2.8 |
| AtRLP30 | At3g05360 | 5.5 | AtRLP12 | At1g71400 | 2.4 | ||
| AtRLP46 | At4g04220 | 4.8 | AtRLP30 | At3g05360 | 2.3 | ||
| AtRLP37 | At3g23110 | 4.8 | AtRLP7 | At1g47890 | 1.9 | ||
| AtRLP49 | At4g13900 | 4.7 | AtRLP23 | At2g32680 | 1.8 | ||
| Wounding | AtRLP33 | At3g05660 | 4.6 | Flg22 | AtRLP24 | At2g33020 | 3.8 |
| AtRLP40 | At3g24954 | 4.1 | AtRLP22 | At2g32660 | 3.5 | ||
| AtRLP6 | At1g45616 | 3.5 | AtRLP21 | At2g25470 | 3.2 | ||
| AtRLP32 | At3g05650 | 2.3 | AtRLP40 | At3g24954 | 2.6 | ||
| AtRLP37 | At3g23110 | 2.1 | AtRLP30 | At3g05360 | 2.4 | ||
| ABA | AtRLP32 | At3g05650 | 4.0 | IAA | AtRLP23 | At2g32680 | 2.3 |
| AtRLP10 | At1g65380 | –3.0 | AtRLP17 | At1g80080 | 2.0 | ||
| AtRLP12 | At1g71400 | –3.0 | AtRLP46 | At4g04220 | 1.8 | ||
| AtRLP33 | At3g05660 | 2.7 | AtRLP1 | At1g07390 | 1.7 | ||
| AtRLP46 | At4g04220 | –2.2 | AtRLP21 | At2g25470 | 1.7 | ||
| ACC | AtRLP33 | At3g05660 | –2.2 | MJ | AtRLP33 | At3g05660 | –2.9 |
| AtRLP25 | At2g33030 | 1.7 | AtRLP1 | At1g07390 | –2.5 | ||
| AtRLP1 | At1g07390 | 1.4 | AtRLP46 | At4g04220 | –2.3 | ||
| AtRLP17 | At1g80080 | –1.4 | AtRLP22 | At2g32660 | 2.1 | ||
| AtRLP7 | At1g47890 | –1.3 | AtRLP12 | At1g71400 | –1.7 | ||
| BL | AtRLP23 | At2g32680 | 2.6 | Zeatin | AtRLP33 | At3g05660 | 1.9 |
| AtRLP33 | At3g05660 | –2.6 | AtRLP23 | At2g32680 | 1.7 | ||
| AtRLP17 | At1g80080 | 2.4 | AtRLP17 | At1g80080 | 1.2 | ||
| AtRLP37 | At3g23110 | 2.1 | AtRLP54 | At5g40170 | –1.2 | ||
| AtRLP41 | At3g25010 | 2.0 | AtRLP46 | At4g04220 | –1.1 | ||
| GA | AtRLP33 | At3g05660 | –2.7 | SA | AtRLP17 | At1g80080 | –4.8 |
| AtRLP10 | At1g65380 | –1.7 | AtRLP34 | At3g11010 | 3.9 | ||
| AtRLP7 | At1g47890 | –1.3 | AtRLP37 | At3g23110 | 3.6 | ||
| AtRLP37 | At3g23110 | 1.3 | AtRLP33 | At3g05660 | 2.7 | ||
| AtRLP30 | At3g05360 | 1.9 |
Interestingly, the down-regulated AtRLP genes were also enriched in four abiotic conditions and two biotic conditions (Fig. 2, Table 2 and Supplementary Tables S4–S7). Surprisingly, no AtRLP gene was differentially expressed upon treatment with the pathogen elicitor lipopolysaccharide (Fig. 2 and Supplementary Table S5). Nevertheless, a number of AtRLP genes were differentially expressed under different hormone treatments (Supplementary Table S6). More than half (53%) of the AtRLP genes were not perturbed by hormones, whereas only seven and nine AtRLP genes were not responsive to biotic and abiotic stresses, respectively (Fig. 2 and Supplementary Table S3). This is consistent with the hypothesis that the majority of AtRLP genes are involved in plant stress signaling, as was suggested by phylogenomic analyses (Fritz-Laylin et al., 2005). Indeed, most of the functionally characterized AtRLP genes are involved in pathogen defense, whereas only a few of them, e.g. AtRLP41, are involved in the response to hormone treatments (Wang et al., 2008).
It was found that with respect to abiotic stresses the most notable perturbation was observed for many AtRLP genes in the aerial parts of Arabidopsis seedlings. However, this strong induction was not seen in roots (see Supplementary Table S4 at JXB online). These data indicate that the response of AtRLP genes to abiotic stresses possibly is tissue or stage specific. In addition, we found that AtRLP genes, as a whole, tend to be up-regulated by avirulent P. syringae, especially in the case of treatments with the avirulent strains P. syringae pv. tomato DC3000 HrcC, P. syringae pv. tomato DC3000 avrRpm1 and P. syringae pv. phaseolicola (Fig. 2 and Supplementary Table S5). However, many AtRLP genes were significantly down-regulated by P. syringae pv. tomato DC3000, a virulent bacterial strain capable of infecting Arabidopsis (Fig. 2 and Supplementary Table S5). These observations suggest that AtRLP genes are involved in basal defense networks that are suppressed by DC3000. Notably, several AtRLP genes, including AtRLP30, were significantly induced by flg22, a peptide corresponding to the most conserved domain of bacterial flagellin (Supplementary Table S5). It would be of great interest to examine whether those AtRLPs are also involved in mediating flagellin signaling.
Generally, our results indicate that a large number of AtRLP genes are responsive to abiotic and biotic stresses, as well as to hormones. In addition, our study provides an overview of the biological processes in which AtRLP genes may be involved. Given the fact that phenotypic changes often are observed under suitable physiological conditions, the specific treatments and corresponding AtRLP gene expression profiles identified here serve as a resource for targeted screening of individual AtRLP genetic mutants.
Individual AtRLP genes are transcriptionally regulated by multiple external stimuli and hormones
It has been suggested previously that some AtRLP genes might be responsive to several factors and thereby participate in different signaling pathways (Wang et al., 2008; Fradin et al., 2011). To elucidate this phenomenon, we firstly counted the number of treatments in which individual AtRLP genes were differentially expressed across all the treatments tested (see Supplementary Fig. S3 at JXB online). Of the 52 AtRLP genes, only AtRLP8 displayed no response to any of the treatments tested (Supplementary Table S3), and six AtRLP genes displayed an increase or decrease in expression under only one condition (see Supplementary Fig. S3 and Supplementary Tables S4–S7). The remaining AtRLP genes exhibited an increased or decreased expression upon more than one treatment (Supplementary Fig. S3 and Supplementary Tables S4–S7), suggesting that a single AtRLP gene may be involved in several physiological processes or in a common process initiated by more than one condition. Our observation that a large number (45 out of 52) of AtRLP genes respond to more than one treatment also supports the existence of extensive cross-talk and signal integration among different signaling pathways.
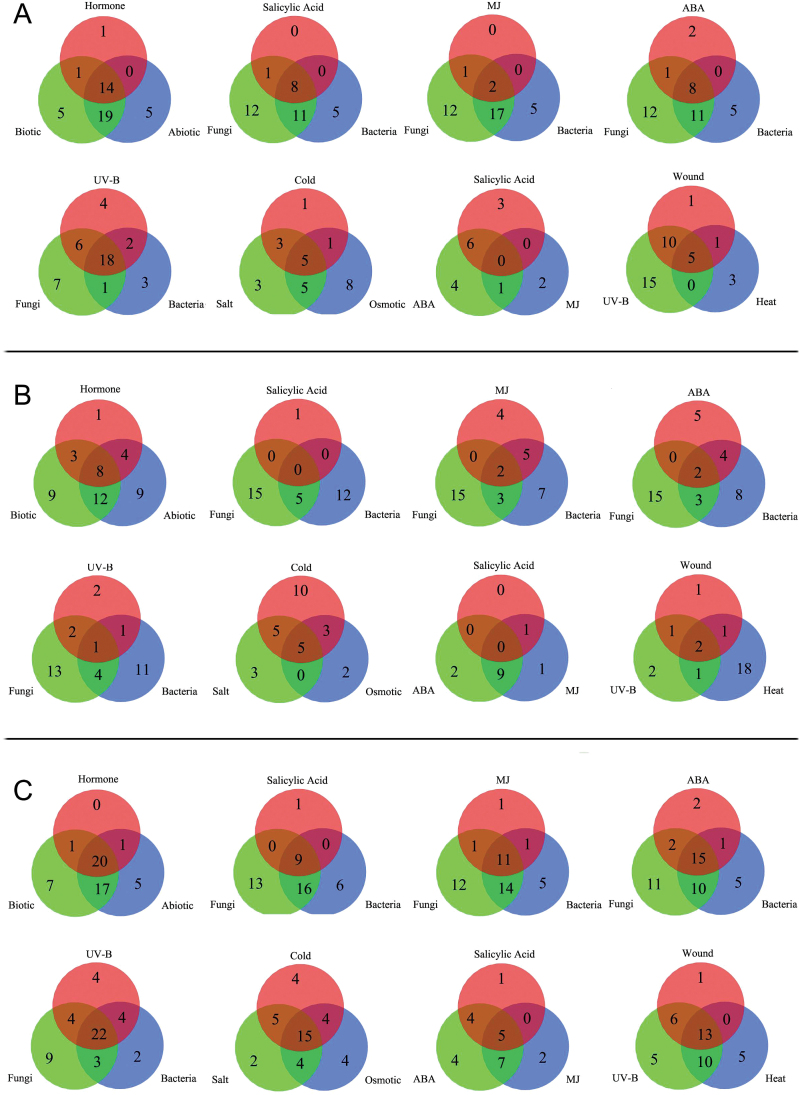
Next, we compared sets of three treatments that are known to induce physiological responses to identify overlapping AtRLP genes that display multiple responses (Fig. 3). In general, we found a large number of the same AtRLP genes had increased expression, while only a few overlapping AtRLP genes showed decreased expression upon any of the three treatments we examined (Fig. 3). Thus, no clear stimulus-specific AtRLP gene expression patterns could be deduced. Treatment with UV-B, fungi and bacteria showed the largest number of overlapping AtRLP genes with increased expression, namely 18, which was followed by 14 shared AtRLP genes upon exposure to hormones, biotic and abiotic stresses (Fig. 3). Osmotic stress, salt stress, and cold are intricately linked in various physiological processes (Xiong and Zhu, 2002; Zhu, 2002). With these three treatments there were five AtRLP genes with increased response and five AtRLP genes with decreased expression (Fig. 3). Eight AtRLP genes showed increased expression, while no AtRLP showed decreased expression upon exposure to SA, fungi and bacteria (Fig. 3). Among the abiotic stress-related treatments, wounding, UV-B and heat treatment shared five respondents with increased expression and two with decreased expression (Fig. 3). ABA, SA, and methyl jasmonate (MJ) are well known to have cooperative effects in plant development and plant defense (Cutler et al., 2010). Surprisingly, we found that exposure to ABA, SA, and MJ revealed no overlapping genes with either increased or decreased expression (Fig. 3). Similarly, there was very little overlap with either increased or decreased expression found for MJ, fungi, and bacteria (Fig. 3). This may indicate that some of the AtRLP genes are conditionally responsive or play a role outside of the cross-talk networks. Nevertheless, the various conditions that we analysed are not exhaustive and more overlap might be found upon additional treatments.
Fig. 3.
The number of overlapping AtRLP genes showing differential regulation in response to different treatments. (A) The number of up-regulated overlapping AtRLP genes in three selective treatment sets. (B) The number of down-regulated overlapping AtRLP genes in three selective treatment sets as shown in (A). (C) The number of differentially expressed AtRLP genes in three selective treatment sets as shown in (A) and (B). Differentially expressed AtRLP genes represent the sum of up-regulated and down-regulated genes. As a result of dynamic responses, the number of differentially expressed AtRLP genes in some cases was smaller than the sum of up-regulated and down-regulated genes.
Around ten of the 57 AtRLP genes have already been implicated in various physiological programs, as has been discussed (Tör et al., 2009; Wang et al., 2010a). Among them, we found that the expression of ReMAX/AtRLP1, RFO/AtRLP3, AtRLP18, AtRLP23, AtRLP30 and RBPG1/AtRLP42, but not AtRLP55, is perturbed by a broad set of external stimuli and hormones (Fig. 2 and Supplementary Tables S4–S7). Surprisingly, two developmental AtRLP genes, CLV2 and TMM, are differentially expressed upon several external stimuli (Supplementary Tables S4–S7), suggesting dual functions of the encoded proteins in plant development and in the response to stress. For example, CLV2 is repressed by virulent P. syringae pv. tomato DC3000 and Phytophthora infestans, while TMM is repressed by osmotic stress (Supplementary Tables S4–S5). However, AtPDO2/AtRLP4, another putative developmentally related AtRLP gene identified through phylogenomic analysis (Fritz-Laylin et al., 2005), exhibited no alteration in expression under most external conditions tested (Supplementary Tables S4–S7).
In conclusion, our observations reveal that a large number of AtRLP genes display differential expression upon more than one treatment, indicating that a single AtRLP gene may be involved in several physiological processes. We found that 14 AtRLP genes with increased expression and eight AtRLP genes with decreased expression, a sum of 22 AtRLP genes showing differential expression, are shared among the three major classes of treatments, abiotic stress, biotic stress, and hormones (Fig. 3). The results thus reveal a large number of overlapping AtRLP genes responding to the examined treatments. Notably, our analysis also highlights that several known AtRLP genes exhibit distinct responses to specific treatments, which has not been described previously.
Cloning of AtRLP genes and sequence analysis of the isolated AtRLP genes
The cDNA fragments containing the respective coding sequences of individual AtRLP genes were obtained by RT-PCR (Supplementary Tables S8 and S9 at JXB online). AtRLP18 and AtRLP49 were not amplified, as they were annotated as pseudogenes in the TAIR10 release (see Supplementary Table S9). PCR products were obtained for 51 of the remaining 54 predicted AtRLP genes, while AtRLP1, AtRLP8, AtRLP15, and AtRLP21 failed to amplify by RT-PCR and were excluded from our study (Supplementary Table S9). Purified PCR products were introduced into pDONR207 to produce the entry clones. Plasmid DNA from entry clones was subsequently recombined into the CaMV 35S promoter containing binary vector pGD625, pFAST-R02 or pB2GW7 to generate over-expression constructs.
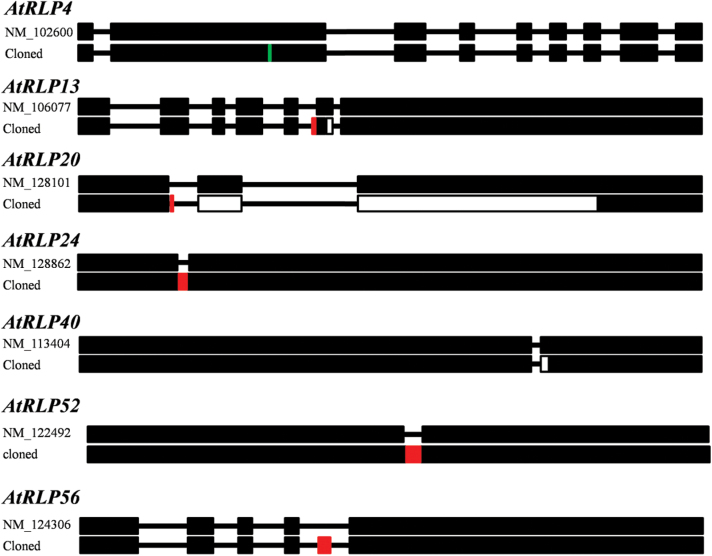
A total of 51 cDNA sequences were successfully cloned into pDONR207 and sequenced (Supplementary Table S9). Among them, 44 out of the 51 isolated clones carrying cDNA sequences were identical to those predicted in TAIR, whereas the other seven isolated sequences differed from their corresponding predictions in TAIR (Fig. 4; Supplementary Table S9). The isolated sequence of AtRLP4 showed single base substitution, which caused a non-synonymous mutation (Fig. 4 and Supplementary Fig. S4 at JXB online). The isolated sequences of AtRLP13, AtRLP20, and AtRLP40 showed different intron–exon boundaries as compared with the predicted sequences, resulting in different gene products (Fig. 4 and Supplementary Fig. S4). The predicted introns of AtRLP24 and AtRLP52 were eliminated in their isolated sequences and have integrated as a part of the exon, which results in the presence of one single exon instead of two exons (Fig. 4 and Supplementary Fig. S4). An unpredicted exon was found in the isolated sequence of AtRLP56 (Fig. 4 and Supplementary Fig. S4). These observations indicate that the annotations are probably not correct. It is also possible that the isolated and the predicted sequences are both present in planta, which may suggest that some AtRLP genes have undergone alternative splicing, probably in different tissues and organs, or upon applying different stimuli.
Fig. 4.
Schematic comparisons of cloned AtRLP sequences and predicted mRNA sequences derived from TAIR. Black boxes indicate exons, and lines between exons represent introns. Red boxes represent new exon sequences in the cloned AtRLP gene, and open boxes show the missed exon sequences in the cloned AtRLP gene. The vertical blue line indicates a single base substitution.
In this study, a total of 51 AtRLP cDNAs containing complete coding sequences were generated, and these will provide useful tools for further functional analyses of this important gene family. For instance, the entry clones can be recombined into any Gateway-compatible destination vector and introduced into Arabidopsis to dissect the resulting phenotypes, which will indicate the possible functions of target genes. It was revealed previously that some conserved residues and motifs of RLPs are of functional significance (Fritz-Laylin et al., 2005; Wang et al., 2008; Wang et al., 2010b). Therefore, the cloned genes could also be mutated in these conserved residues and/or motifs by site-directed mutagenesis to create mutant variants. In line with this hypothesis, it has been shown that transgenic plants expressing a mutation in the conserved GxxxG motif, which is known to aid in protein–protein interactions, that is located on the transmembrane domain of SNC2/AtRLP51 exhibit constitutively activated defense responses (Zhang et al., 2010). In summary, the resources generated in this study will provide useful tools for future functional examination of AtRLP genes.
Generation of transgenic Arabidopsis plants overexpressing AtRLP genes
To facilitate functional analysis, we generated transgenic plants overexpressing the individual AtRLP genes. To this end, the resulting CaMV 35S-driven expression constructs were transformed into wild-type (Col-0 and/or Ler) plants and/or the clv2 mutant (see Supplementary Table S10 at JXB online). For each construct, at least 20 independent transgenic lines were initially analysed in the T2 generation, and then at least three independent homozygous lines (T3 or T4 plants) were obtained for each AtRLP gene (Supplementary Table S10). Altogether, we generated a collection of 167 homozygous overexpression (AtRLP-OX) lines for 51 AtRLP genes. This collection of transgenic plants could be used for the analysis of developmental aspects and studies on RLP function in pathogen defense and stress conditions, thus providing a valuable resource for future investigations into the biological role of AtRLPs.
Overexpression of AtRLP3 and AtRLP11 rescues the clv2-1 mutant
A phenotypical analysis of 4- to 6-week-old homozygous AtRLP-OX lines with respect to their growth and development under normal growth conditions did not reveal any abnormalities. Therefore, additional tests need to be performed on these AtRLP-OX lines to study the phenotype of various organs at multiple growth and developmental stages.
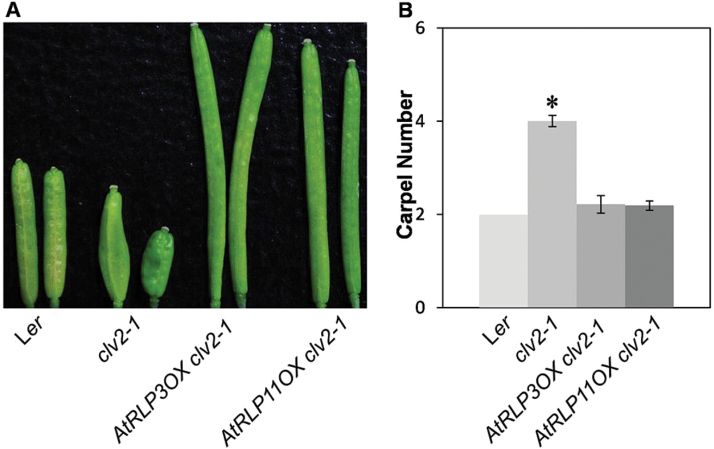
We reported previously that two AtRLPs, AtRLP2 and AtRLP12, which share high sequence similarity to CLV2, are able to rescue the clv2 mutant phenotype when expressed under the control of the CLV2 promoter, suggesting that the specialization among CLV2, AtRLP2 and AtRLP12 is largely ascribed to differences in their expression patterns (Wang et al., 2010b). Intriguingly, AtRLP3 and AtRLP11 are duplicated genes of AtRLP2 and AtRLP12, respectively, which may suggest a similar function for these paralogues. To test this hypothesis and to investigate the biological role of AtRLP3 and AtRLP11, we analysed the transgenic plants overexpressing AtRLP3 and AtRLP11 in either the wild-type plants or the clv2 mutant (Supplementary Table S10). Wild-type plants developed an invariant two carpels per flower, while clv2-1 mutants produced multiple carpels per flower (Kayes and Clark, 1998; Wang et al., 2008). Interestingly, AtRLP3-OX and AtRLP11-OX transformed into the clv2-1 mutant completely complemented its phenotype, showing a mean carpel number that is comparable to the wild-type plants (Fig. 5), which is similar to what has been shown for AtRLP2 and AtRLP12 (Wang et al., 2010b). The overall growth and appearance of AtRLP3-OX and AtRLP11-OX in the wild-type were indistinguishable from wild-type plants grown under normal growth conditions. Additionally, the atrlp3-1 and atrlp11-1 mutants displayed no meristem defects (Wang et al., 2008), despite our observation that AtRLP3-OX and AtRLP11-OX are able to rescue the phenotype of the clv2-1 mutant. These results suggest that the functional diversity among these closely related genes is primarily due to divergence of gene expression, rather than of their protein-coding regions. CLV2 exhibited overlapping expression with AtRLP2, AtRLP3, AtRLP11 and AtRLP12 in some organs, suggesting that CLV2 may have overlapping functions with these members in those organs.
Fig. 5.
AtRLP3-OX and AtRLP11-OX rescue the clv2-1 mutant. (A) Representative siliques of Ler, clv2-1, AtRLP3-OX in clv2-1 and AtRLP11-OX in clv2-1 plants. (B) The mean number of carpels for multiple independent transgenic lines of AtRLP3-OX and AtRLP11-OX that were transformed into clv2-1 relative to the wild-type Ler and the clv2-1 mutant. For each genotype, a minimum of 30 transgenic plants with 20 siliques per plant were analysed. An asterisk indicates a significant difference (P<0.01) compared with the wild-types.
It has been shown that RFO2/AtRLP3 confers resistance to the vascular wilt fungus Fusarium oxysporum, whereas AtRLP2 does not (Shen and Diener, 2013). The eLRRs of RFO2/AtRLP3 and AtRLP2 are interchangeable for resistance, while the less conserved membrane-proximal domains of RFO2/AtRLP3 specify resistance (Shen and Diener, 2013). It was thus suggested that AtRLP2 was a non-functional pseudogene, similar to the case where a loss-of-function polymorphism accounts for the susceptible allele of Ve1 (Fradin et al., 2009). Conversely, ectopic expression of AtRLP2 could suppress the clv2 mutant, suggesting that AtRLP2 is functional. Combined with our results, this suggests that, unlike AtRLP2, AtRLP3 has a dual function in plant development and immunity (Fig. 5; Shen and Diener, 2013).
AtRLP28-OX lines show enhanced salt stress tolerance in Arabidopsis
In addition to developmental phenotyping, we initiated an assay of the AtRLP-OX lines to test the involvement of individual AtRLP in the response to salt stress. Our transcriptional analyses have shown that several AtRLP genes are responsive to salt (see Supplementary Table S4). However, no evidence is available on their physiological roles in coping with salt stress.
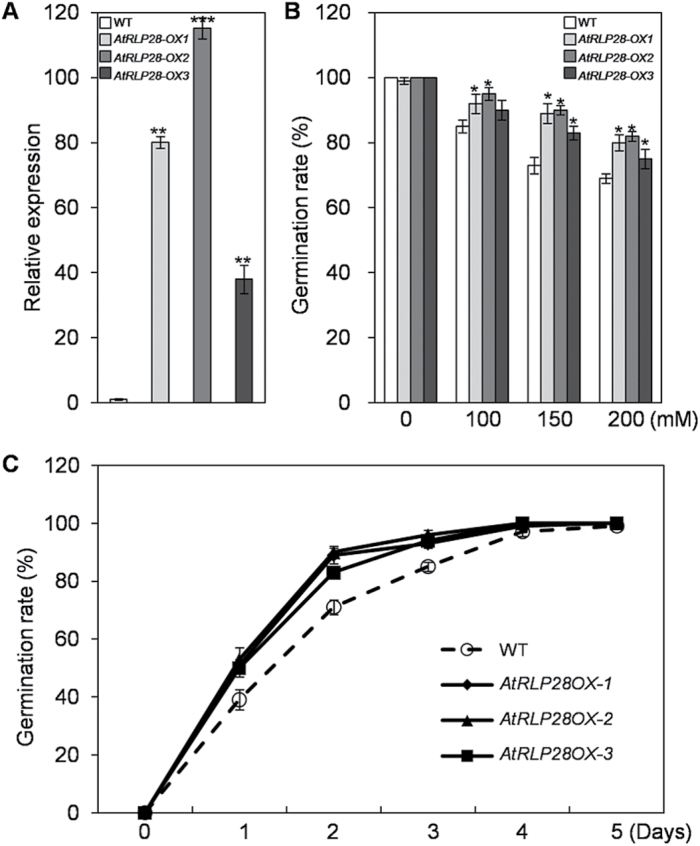
To determine whether any AtRLP gene plays a role in tolerance to salt stress, we have tested the AtRLP-OX lines for their ability to germinate, compared with wild-type seeds, on medium in the presence NaCl. Three independent AtRLP28-OX lines exhibited significantly higher germination rates as compared with wild-type seeds (Fig. 6), implying that AtRLP28 is involved in the tolerance to salt stress. However, the germination rate of AtRLP28-OX lines is comparable to that of WT in the presence of mannitol (see Supplementary Fig. S5 at JXB online). The elevated expression of AtRLP28 was confirmed by quantitative RT-PCR for these independent lines (Fig. 6).
Fig. 6.
Germination phenotype of the wild-type (WT) and AtRLP28-OX lines in response to NaCl treatment. (A) Expression levels of AtRLP28 in WT and three independent transgenic lines overexpressing AtRLP28 were determined by qPCR. (B) Germination percentages of WT and three independent AtRLP28-OX seeds grown for 2 d on the 1/2 MS medium supplemented with different concentrations of NaCl. Asterisks indicate statistically significant differences compared with WT (* indicates P<0.05). (C) Germination percentages of WT and three independent AtRLP28-OX seeds grown on 1/2 MS medium containing 150mM NaCl at the indicated times.
To further determine a possible link between AtRLP28 expression and salt tolerance, the expression of AtRLP28 was evaluated on exposure to NaCl and mannitol by qPCR. AtRLP28 transcripts were up-regulated significantly in response to NaCl and mannitol (see Supplementary Fig. S2), which is partially inconsistent with the microarray data. The discrepancy may be due to the difference in the samples collected. Indeed, in a previous study, AtRLP28 expression was shown to be significantly up-regulated under NaCl treatment (Jung et al., 2008). The data thus confirm our qPCR analyses. In conclusion, these results indicate that high levels of AtRLP28 expression enhance plant salt tolerance. However, how AtRLP28 mediates salt stress tolerance requires further biological investigation.
Conclusions
The Arabidopsis genome contains 57 AtRLP genes, the majority of which have yet to be assigned biological roles. In this study, we compiled a detailed expression profile of the transcriptional regulation of AtRLP genes upon exposure to a broad range of environmental stresses and hormones. Our results indicate that a large number of AtRLP genes are differentially regulated upon various conditions tested, thus providing an overview of the processes in which AtRLP genes may be involved. Furthermore, our data revealed that a large number of AtRLP genes display differential expression upon more than one treatment, indicating that a single AtRLP gene may be involved in multiple physiological processes. The specific processes and the alteration of the expression of the corresponding AtRLP genes identified here serve as a tool for targeted screenings of individual AtRLP mutants and AtRLP-OX lines. In addition, we performed a genome-wide cloning of AtRLP genes, and generated and characterized transgenic plants overexpressing individual AtRLP genes. As an initial attempt to elucidate the biological role of AtRLP genes using these AtRLP-OX lines, we found that AtRLP3-OX and AtRLP11-OX are able to rescue the phenotype of the clv2-1 mutant, which indicates that, similar to their duplicated genes AtRLP2 and AtRLP12, the functional specificity of these genes is determined at the level of their transcriptional regulation. Furthermore, AtRLP28 was found to mediate salt stress tolerance. Taken as a whole, the comprehensive profile and the generated AtRLP-OX lines provide valuable resources for future investigations into the biological role of AtRLP genes.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. The sequence comparison of the extracellular domains of AtRLP52 and At5g25930.
Figure S2. The expression of AtRLP23, AtRLP28, AtRLP30, AtRLP33 and AtRLP37 in response to NaCl and mannitol at indicated times.
Figure S3. Number of treatments in which a given AtRLP gene is up-regulated, down-regulated and differentially expressed.
Figure S4. Sequence comparisons of cloned AtRLP sequences, genomic DNA sequences and predicted mRNA sequences derived from TAIR.
Figure S5. Osmotic effects on the seeds germination of WT and AtRLP28-OX lines using mannitol.
Table S1. A list of quantitative real-time PCR primers used in this study.
Table S2. AtRLP genes which locate close to an RLK gene.
Table S3. AtRLP genes displaying no transcriptional responses to the experimental conditions.
Table S4. Gene expression of AtRLPs under abiotic stress.
Table S5. Gene expression of AtRLPs under biotic stress.
Table S6. Gene expression of AtRLPs upon treatment with hormones.
Table S7. Gene expression of AtRLPs under different light conditions.
Table S8. A list of primers used in the cloning of AtRLP genes.
Table S9. Overview of the cloning results of AtRLP genes.
Table S10. Summary of the AtRLP-OX transgenic plants.
Acknowledgements
We are grateful to Dr Pierre de Wit (Wageningen University) for critically reading the manuscript and helpful discussions, and Dr Bart Thomma (Wageningen University) for giving helpful comments when the project was initiated. We thank Dr Chun-Ming Liu (Institute of Botany, Chinese Academy of Sciences) for his generosity in sharing his lab facilities. We also thank Yin Song (Wageningen University) and Huibin Han (The Institute of Science and Technology Austria) for their technical assistance and useful discussions. Research in our group was supported by the National Natural Science Foundation of China (31271575; 31200902), by the Fundamental Research Funds for the Central Universities (GK201103005), by the Specialized Research Fund for the Doctoral Program of Higher Education from the Ministry of Education of China (20120202120009), and by the Natural Science Basic Research Plan in Shaanxi Province of China (2014JM3064).
References
- Albert I, Böhm H, Albert M, et al. 2015. An RLP23–SOBIR1–BAK1 complex mediates NLP-triggered immunity. Nature Plants 1, 15140. [DOI] [PubMed] [Google Scholar]
- Blanc G, Hokamp K, Wolfe KH. 2003. A recent polyploidy superimposed on older large-scale duplications in the Arabidopsis genome. Genome Research 13, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfanti E, Silfverberg-Dilworth E, Tartarini S, Patocchi A, Barbieri M, Zhu J, Vinatzer BA, Gianfranceschi L, Gessler G, Sansavini S. 2004. The HcrVf2 gene from a wild apple confers scab resistance to a transgenic cultivated variety. Plant Biology 101, 886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Cook DE, Mesarich CH, Thomma BPHJ. 2015. Understanding plant immunity as a surveillance system to detect invasion. Annual Review of Phytopathology 53, 541–563. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. 2010. Abscisic acid: Emergence of a core signaling network. Annual Review of Plant Biology 61, 651–679. [DOI] [PubMed] [Google Scholar]
- Ellendorff U, Zhang Z, Thomma BPHJ. 2008. Gene silencing to investigate the roles of receptor-like proteins in Arabidopsis . Plant Signaling and Behavior 3, 893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin EF, Abd-El-Haliem A, Masini L, van den Berg GC, Joosten MH, Thomma BPHJ. 2011. Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis. Plant Physiology 156, 2255–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin EF, Zhang Z, Ayala JC, Castroverde CC, Nazar RN, Robb J, Liu CM, Thomma BPHJ. 2009. Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1 . Plant Physiology 150, 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz-Laylin LK, Krishnamurthy N, Tör MT, Sjölander KV, Jones JDG. 2005. Phylogenomic analysis of the receptor-like proteins of rice and Arabidopsis . Plant Physiology 138, 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda H, Sasaki E, Akiyama K, et al. 2008. The AtGenExpress hormone and chemical treatment data set: experimental design, data evaluation, model data analysis and data access. The Plant Journal 55, 526–542. [DOI] [PubMed] [Google Scholar]
- Jehle AK, Lipschis M, Albert M, Fallahzadeh-Mamaghani V, Fürst U, Mueller K, Felix G. 2013. The receptor-like protein ReMAX of Arabidopsis detects the microbe-associated molecular pattern eMax from Xanthomonas. The Plant Cell 25, 2330–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Trotochaud AE, Clark SE. 1999. The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. The Plant Cell 11, 1925–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD, Cheong JJ. 2008. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiology 146, 623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayes JM, Clark SE. 1998. CLAVATA2, a regulator of meristem and organ development in Arabidopsis . Development 125, 3843–3851. [DOI] [PubMed] [Google Scholar]
- Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D’Angelo C, Bornberg-Bauer E, Kudla J, Harter K. 2007. The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. The Plant Journal 50, 347–363. [DOI] [PubMed] [Google Scholar]
- Kobe B, Kajava AV. 2001. The leucine-rich repeat as a protein recognition motif. Current Opinion in Structural Biology 11, 725–732. [DOI] [PubMed] [Google Scholar]
- Kruijt M, de Kock MJD, de Wit PJGM. 2005. Receptor-like proteins involved in plant disease resistance. Molecular Plant Pathology 6, 85–97. [DOI] [PubMed] [Google Scholar]
- Larkan NJ, Lydiate DJ, Parkin IA, Nelson MN, Epp DJ, Cowling WA, Rimmer SR, Borhan MH. 2013. The Brassica napus blackleg resistance gene LepR3 encodes a receptor-like protein triggered by the Leptosphaeria maculans effector AVRLM1. New Phytologist 197, 595–605. [DOI] [PubMed] [Google Scholar]
- Liebrand TW, van den Berg GC, Zhang Z, et al. 2013. Receptor-like kinase SOBIR1/EVR interacts with receptor-like proteins in plant immunity against fungal infection. Proceedings of the National Academy of Sciences of the United States of America 110, 10010–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi Y. 2003. Ligand-receptor pairs in plant peptide signaling. Journal of Cell Science 116, 3863–3870. [DOI] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD. 2002. Control of stomatal distribution on the Arabidopsis leaf surface. Science 296, 1697–1700. [DOI] [PubMed] [Google Scholar]
- Obayashi T, Okamura Y, Ito S, Tadaka S, Aoki Y, Shirota M, Kinoshita K. 2014. ATTED-II in 2014: evaluation of gene coexpression in agriculturally important plants. Plant and Cell Physiology 55, e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma J, Liebrand TW, Bi G, Evrard A, Bye RR, Mbengue M, Kuhn H, Joosten MH, Robatzek S. 2016. Avr4 promotes Cf-4 receptor-like protein association with the BAK1/SERK3 receptor-like kinase to initiate receptor endocytosis and plant immunity. New Phytologist 210, 627–642. [DOI] [PubMed] [Google Scholar]
- Ramonell K, Berrocal-Lobo M, Koh S, Wan J, Edwards H, Stacey G, Somerville S. 2005. Loss-of-function mutations in chitin responsive genes show increased susceptibility to the powdery mildew pathogen Erysiphe cichoracearum . Plant Physiology 138, 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas S, Thomas CM. 2005. Molecular interactions between tomato and the leaf mold pathogen Cladosporium fulvum . Annual Review of Phytopathology 43, 395–436. [DOI] [PubMed] [Google Scholar]
- Ron M, Avni A. 2004. The receptor for the fungal elicitor ethylene inducing xylanase is a member of a resistance-like gene family in tomato. The Plant Cell 16, 1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Diener AC. 2013. Arabidopsis thaliana RESISTANCE TO FUSARIUM OXYSPORUM 2 implicates tyrosine-sulfated peptide signaling in susceptibility and resistance to root infection. PLoS Genetics 9, e1003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. 2003. Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis . Plant Physiology 132, 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopoulos I, de Wit PJGM. 2009. Fungal effector proteins. Annual Review of Phytopathology 47, 233–263. [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, van Esse HP, Crous PW, de Wit PJGM. 2005. Cladosporium fulvum (syn. Passalora fulva), a highly specialized plant pathogen as a model for functional studies on plant pathogenic Mycosphaerellaceae. Molecular Plant Pathology 6, 379–393. [DOI] [PubMed] [Google Scholar]
- Tör M, Lotze MT, Holton N. 2009. Receptor-mediated signaling in plants: molecular patterns and programmes. Journal of Experimental Botany 60, 3645–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Ellendorff U, Kemp B, et al. 2008. A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis . Plant Physiology 147, 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Fiers M, Ellendorff U, Wang Z, de Wit PJGM, Angenent G, Thomma BPHJ. 2010. a. The diverse roles of extracellular leucine-rich repeat-containing receptor-like proteins in plants. Critical Reviews in Plant Sciences 29, 285–299. [Google Scholar]
- Wang G, Long Y, Thomma BPHJ, de Wit PJGM, Angenent G, Fiers M. 2010. b. Functional analyses of the CLAVATA2-like proteins and their domains that contribute to CLAVATA2 specificity. Plant Physiology 152, 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Zhang Z, Angenent G, Fiers M. 2011. New aspects of CLV2, a versatile gene in the regulation of Arabidopsis development. Journal of Plant Physiology 168, 403–407. [DOI] [PubMed] [Google Scholar]
- Wu Z, Irizarry RA, Gentleman R, Martinez-Murillo F, Spencer F. 2004. A model-based background adjustment for oligonucleotide expression arrays. Journal of the American Statistical Association 99, 909–917. [Google Scholar]
- Xiong L, Zhu JK. 2002. Salt tolerance. Arabidopsis Book 1, e0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Cheng X, Jia R, Qin Q, Guan L, Du H, Hou S. 2014. New phenotypic characteristics of three tmm alleles in Arabidopsis thaliana . Plant Cell Reports 33, 719–731. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kars I, Essenstam B, Liebrand TWH, Wagemakers L, Elberse J, Tagkalaki P, Tjoitang D, van den Ackerveken G, van Kan JA. 2014. Fungal endopolygalacturonases are recognized as microbe-associated molecular patterns by the Arabidopsis receptor-like protein RESPONSIVENESS TO BOTRYTIS POLYGALACTURONASES1. Plant Physiology 164, 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang Y, Fang B, Gannon P, Ding P, Li X, Zhang Y. 2010. Arabidopsis snc2-1D activates receptor-like protein-mediated immunity transduced through WRKY70. The Plant Cell 22, 3153–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. 2002. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology 53, 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Wang X. 2000. Large-scale profiling of the Arabidopsis transcriptome. Plant Physiology 124, 1472–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.