Significance
Protein arginine methyltransferase 5 (PRMT5) is involved in various developmental processes by globally regulating pre-mRNA splicing of diverse genes, but the underlying mechanism remains elusive. Here we demonstrate for the first time, to our knowledge, that Arabidopsis PRMT5 promotes the recruitment of the NineTeen Complex and splicing factors in the catalytic reactions to the spliceosome, thus promoting global pre-mRNA splicing. Our findings uncover a key molecular mechanism for PRMT5 in the regulation of pre-mRNA splicing, which fills a major gap in understanding of the role for PRMT5 in spliceosome assembly. Due to the conservation of PRMT5 in plants and animals, our finding is likely a fundamental molecular mechanism applicable to all eukaryotes, thereby shedding light on PRMT5 functions and spliceosome activation in animals.
Keywords: arginine methylation, protein arginine methyltransferase, AtPRMT5, pre-mRNA splicing, Prp19C/NTC
Abstract
Protein arginine methylation, catalyzed by protein arginine methyltransferases (PRMTs), is involved in a multitude of biological processes in eukaryotes. Symmetric arginine dimethylation mediated by PRMT5 modulates constitutive and alternative pre-mRNA splicing of diverse genes to regulate normal growth and development in multiple species; however, the underlying molecular mechanism remains largely unknown. A genetic screen for suppressors of an Arabidopsis symmetric arginine dimethyltransferase mutant, atprmt5, identified two gain-of-function alleles of pre-mRNA processing factor 8 gene (prp8-8 and prp8-9), the highly conserved core component of the U5 small nuclear ribonucleoprotein (snRNP) and the spliceosome. These two atprmt5 prp8 double mutants showed suppression of the developmental and splicing alterations of atprmt5 mutants. In atprmt5 mutants, the NineTeen complex failed to be assembled into the U5 snRNP to form an activated spliceosome; this phenotype was restored in the atprmt5 prp8-8 double mutants. We also found that loss of symmetric arginine dimethylation of Sm proteins prevents recruitment of the NineTeen complex and initiation of spliceosome activation. Together, our findings demonstrate that symmetric arginine dimethylation has important functions in spliceosome assembly and activation, and uncover a key molecular mechanism for arginine methylation in pre-mRNA splicing that impacts diverse developmental processes.
Protein arginine methyltransferase 5 (PRMT5), a highly conserved type II protein arginine methyltransferase, transfers methyl groups to arginine residues, generating monomethylarginine and symmetric ω-NG, N′G-dimethylarginine (SDMA) (1–3). In mammals, PRMT5 methylates and interacts with diverse proteins, including histones and other proteins, and has long been implicated in the modulation of a variety of processes, such as transcriptional regulation, RNA metabolism (4), apoptosis (5), signal transduction (6), and germ cell development (7). Both loss-of-function and overexpression of PRMT5 are fatal in mammals (8). AtPRMT5, the Arabidopsis homolog of PRMT5, regulates multiple aspects of plant growth and development, such as flowering time, growth rate, leaf morphology, sensitivity to stress conditions, and circadian rhythm, by modulating transcription, constitutive and alternative precursor mRNA (pre-mRNA) splicing of diverse genes (9–13). AtPRMT5 also mediates the symmetric arginine dimethylation of uridine-rich small nuclear ribonucleoproteins (U snRNPs) AtSmD1, D3, and AtLSm4 proteins, thus linking arginine methylation and splicing (10, 11, 13). However, the exact mechanism remains elusive.
Pre-mRNA splicing occurs in the nucleus, removing introns and ligating exons (14). In eukaryotes, most introns are spliced in a series of reactions catalyzed by the spliceosome, which consists of five subcomplexes of U snRNPs and several non-snRNP factors. Each U snRNP contains distinct splicing factors plus a uridine-rich small nuclear RNA (snRNA), namely U1, U2, U4, U5, or U6 (15). PRMT5 plays important roles in U snRNP assembly, in which U1, 2, 4, 5 snRNP Sm proteins Sm B/B′, D1, D3, and U6 snRNP-specific Sm-like protein LSm4 are symmetrically dimethylated by PRMT5 (16, 17). These methylated Sm proteins can be recognized by the survival motor neuron (SMN) complex or Tudor staphylococcal nuclease (Tudor-SN) and loaded onto U snRNAs, forming U snRNPs (18–20).
The snRNPs assemble dynamically on a pre-mRNA along with the non-snRNP splicing factors and catalyze two sequential transesterification reactions producing a mature mRNA. Briefly, U1 snRNP first recognizes the conserved 5′ splice site sequence in the intron by base pairing. U2 snRNP then binds the conserved branch-point sequence forming the prespliceosome. A preformed U4/U6.U5 tri-snRNP, in which the U4 and U6 snRNAs are base-paired, joins the prespliceosome to form the precatalytic spliceosome. A large structural rearrangement occurs to form an active spliceosome, involving the unwinding of U4/U6 base pairing interaction, the release of U1 and U4 snRNAs, and the addition of a non-snRNP protein complex called the Prp19 complex (Prp19C) or the NineTeen Complex (NTC) (21). Then the branch-point adenosine residue nucleophilically attacks the 5′ splice site for the first transesterification reaction, followed by the second transesterification reaction, resulting in the ligation of two exons. The spliceosome is a highly dynamic structure, assembled by sequential binding of the splicing factors to the pre-mRNA for each round of splicing, and then released for recycling after completion of the reaction (22).
From a genetic screen of second-site suppressors, we identified two neomorphic mutations in the core spliceosome component Prp8 (pre-mRNA processing factor 8) that suppress the pleiotropic developmental and splicing alterations of atprmt5 mutants. Further study demonstrated that the Prp19C/NTC complex failed to be assembled into U5 snRNP to form activated spliceosome in atprmt5 mutants, but was restored in the suppressor. We also showed that loss of symmetric arginine dimethylation on AtSm proteins in atprmt5 failed to efficiently recruit Prp19C/NTC complex, which is essential for spliceosome activation. Our examination of the atprmt5 phenotype and the interaction of atprmt5 and prp8 revealed an essential role for AtPRMT5 in spliceosome assembly and activation in pre-mRNA splicing.
Results
Two Suppressors Rescue the Pleiotropic Developmental Defects of atprmt5 Mutants.
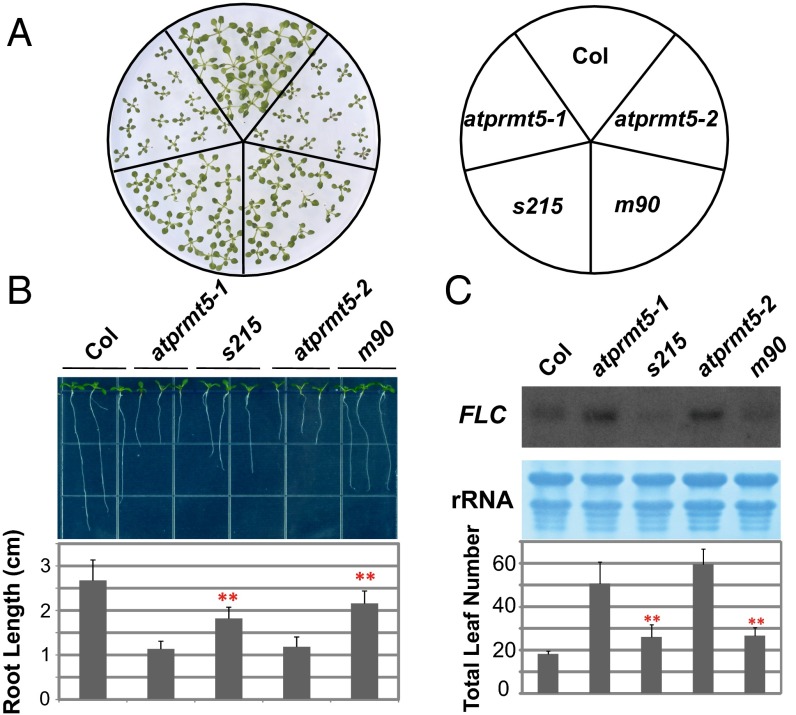
Our previous study showed that atprmt5 mutants exhibit pleiotropic phenotypes, such as dark green and curled leaves, growth retardation (9), and delayed flowering (9). To better understand the links connecting arginine methylation, posttranscriptional regulation, and plant development, and to identify the molecular mechanism by which AtPRMT5 functions in pre-mRNA splicing, we performed a genetic screen for suppressors of atprmt5 mutants. atprmt5-1 and atprmt5-2 seeds were mutagenized with ethylmethane sulfonate (EMS), respectively, and individual M2 families were screened for mutations closed to the wild-type (WT) phenotypes. Among individual M2 families screened from atprmt5 mutants, two allelic mutants, s215 and m90, from atprmt5-1 and atprmt5-2, respectively, partially rescued the pleiotropic phenotypes of atprmt5 mutants. Compared with the atprmt5 mutants, s215 and m90 plants showed less growth retardation, with larger cotyledons and longer primary roots than those of atprmt5 mutants (Fig. 1 A and B). Intriguingly, compared with atprmt5 mutants, the suppressors also showed partial suppression of the atprmt5 delayed flowering phenotype, with decreased total leaf number (including rosette and cauline leaves) and FLOWERING LOCUS C (FLC) expression levels (Fig. 1C). In addition, the suppressors also showed less insensitivity to vernalization (Fig. S1A) and hypersensitivity to salt stress (Fig. S1B) compared with atprmt5 mutants. Therefore, the two suppressors partially rescued the pleiotropic developmental defects of atprmt5 mutants.
Fig. 1.
atprmt5 suppressors partially rescue the pleiotropic developmental defects of atprmt5 mutants. (A) Rescued growth retardation of young seedling leaves at 12 d. (B) Rescued primary roots of s215 and m90 at 9 d. Data are shown as means ± SD (n = 20). Two-sided Student t test between atprmt5 and the suppressors was performed (**P < 0.01). (C) Rescued FLC expression and flowering time of s215 and m90. (Upper) The total RNAs from 12-d-old seedlings of Col, atprmt5, s215, and m90 plants were probed by RNA blot with the full-length coding sequence of FLC. rRNAs were used as a loading control. (Lower) Flowering time was assessed by total leaf number after plants stopped producing new leaves when plants were grown at 23 °C under long day conditions. Data are shown as means ± SD (n = 30). Two-sided Student t test between atprmt5 and the suppressors was performed (**P < 0.01).
Fig. S1.
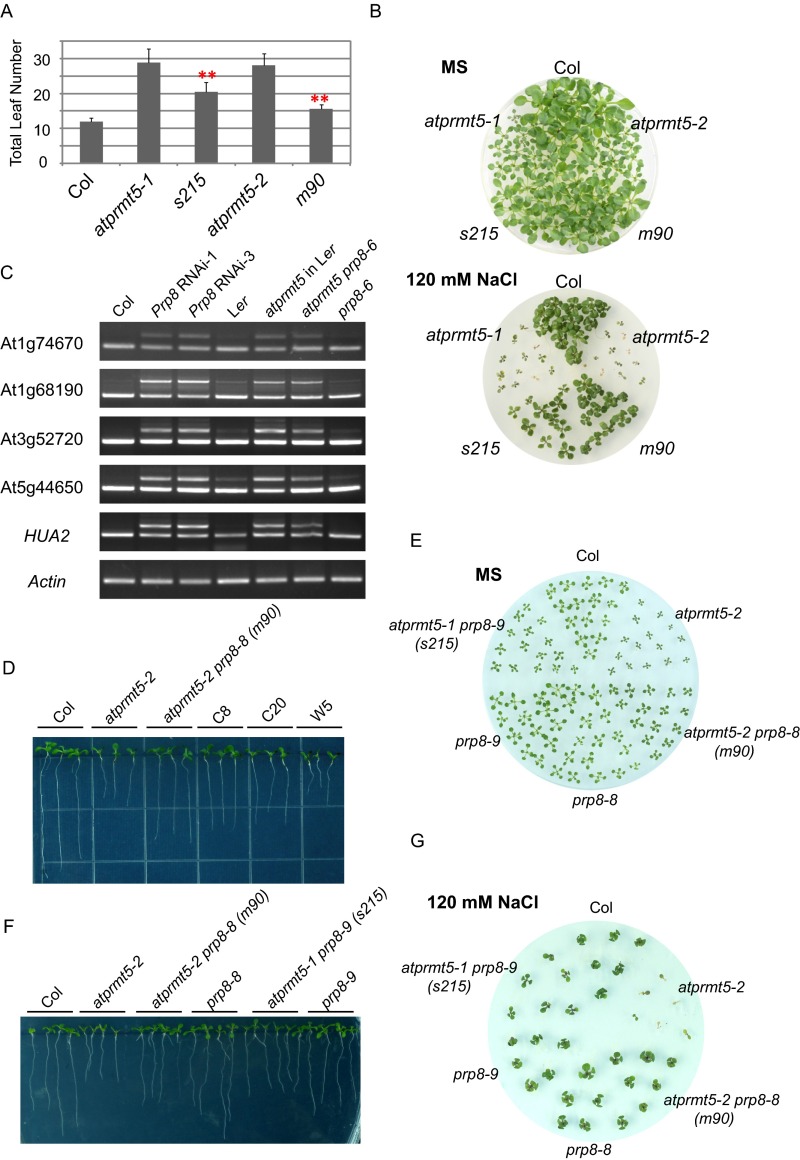
Phenotypic and molecular analysis of atprmt5 suppressors. (A) Flowering time of Col, atprmt5 mutants, s215, and m90, assessed by total leaf number after plants stop producing new leaves. Plants were subjected to vernalization treatment at 4 °C for 6 wk and then transferred to 23 °C and grown under long day conditions. Data are shown as means ± SD (n = 20). Two-sided Student t test between atprmt5 and the suppressors was performed (**P < 0.01). (B) Comparison of the survival rate of the Col, atprmt5 mutants, s215, and m90 plants grown on MS medium containing 120 mM NaCl. Photographs were taken 25 d after seedling transfer to the treatment medium. (C) RT-PCR analysis for Prp8 RNAi lines in atprmt5 background and atprmt5-2 prp8-6 double mutants (in Ler background). (D) Rescued primary roots of the transformation lines of CDS Prp8 clone with P1141S mutation in atprmt5-2 sus2-5 double mutants background at 9 d. (E–G) Phenotypic analysis of the young seedling leaves at 12 d (E), the primary roots at 9 d (F), and the survival rate on MS medium containing 120 mM NaCl (G) for prp8-8 and prp8-9 mutants.
Point Mutations in Prp8 Are Responsible for Suppressors of atprmt5 Mutants.
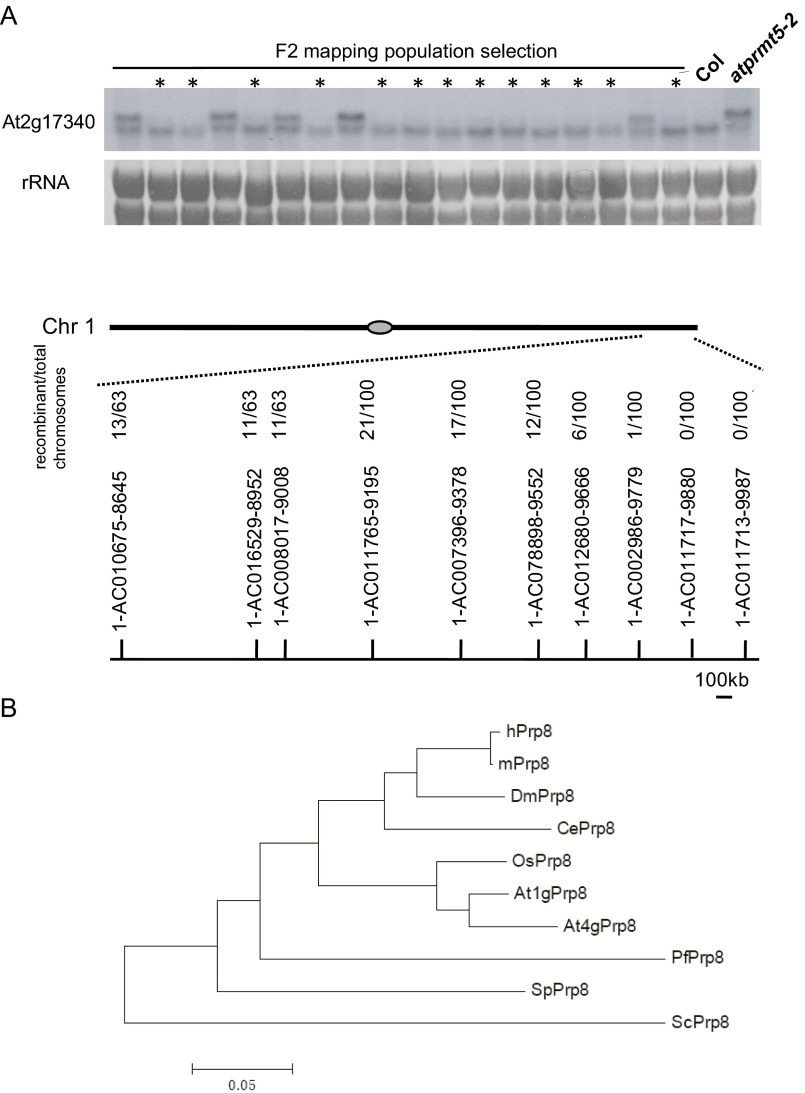
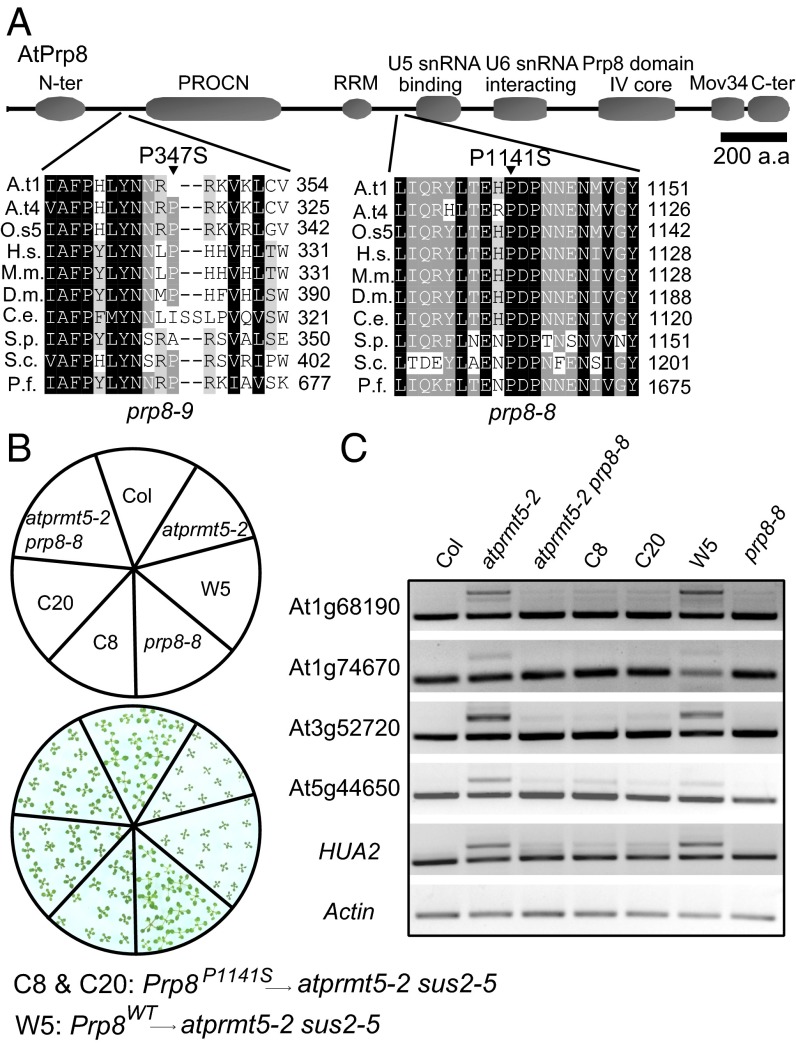
Low-resolution mapping by bulked segregant analysis places m90 and s215 on the long arm of chromosome 1 between simple sequence length polymorphism (SSLP) markers 1-AC002986-9779 and 1-AC011713-9987 (2.5-cM), a region that covered 184 putative genes annotated in TAIR10 (Fig. S2A) (23). To identify the point mutations, we sequenced the genomic DNA of the F3 progeny of m90 by Illumina Genome Analyzer IIx. Eleven homozygous SNPs were clustered in the region of chromosome 1 to where m90 mapped (Table S1). A C3421T (nucleotide) transition mutation that is consistent with EMS mutagenesis was found, which changes a proline (P) to serine (S) at amino acid position 1,141 (P1141S) in the ORF of At1g80070 (referred to from now as prp8-8; Fig. 2A), a locus involved in embryogenesis, as null mutants (prp8-sus2 alleles) result in an abnormal suspensor development and embryonic lethality (24). Meanwhile, conventional DNA sequencing result showed that s215 carried P347S substitution in At1g80070 as well (referred to from now as prp8-9) (Fig. 2A). prp8-8 and prp8-9 are neomorphic alleles of Prp8, because neither prp8-6 (also named sof81, a hypomorphic mutant allele of Prp8) (25) nor Prp8 RNAi lines rescue the splicing alterations of atprmt5 mutants (Fig. S1C). In addition, the rescued phenotypes and splicing alterations in atprmt5 prp8 plants were mimicked by a coding sequence (CDS) of Prp8 clone with P1141S mutation, but not without mutation, in atprmt5-2 sus2-5 double mutants (Fig. 2 B and C and Fig. S1D), indicating that the neomorphic mutation of Prp8 can rescue the pleiotropic phenotypes of atprmt5 mutants. However, there is no obvious physiological and molecular effect in prp8-8 and prp8-9 single mutants compared with wild-type Col (Fig. 2 B and C and Fig. S1 E–G).
Fig. S2.
Low-resolution mapping and resequencing of the m90 mutation indicate it has a lesion in At1g80070. (A) (Upper) RNA blot analysis of the splicing patterns of At2g17340 in the F2 mapping population of m90, with rRNA as the loading control. The lanes marked with asterisks represent the population used for low-resolution mapping. The F2 population segregated into WT and mutant phenotypes at a ratio of 3:1, indicating that the mutant phenotype is caused by a single recessive mutation; (Lower) Bulked segregant analysis maps atprmt5-2 prp8-8 to between 1-AC002986-9779 and 1-AC011713-9987 on chromosome 1. (B) Phylogenic analysis of Prp8 from yeast, fly, mouse, worm, human, Arabidopsis, and rice. The species for each protein is designated by the following prefixes: Arabidopsis thaliana (At), Oryza sativa (Os), Homo sapiens (Hs), Mus musculus (Mm), Drosophila melanogaster (Dm), Caenorhabditis elegans (Ce), Schizosaccharomyces pombe (Sp), Saccharomyces cerevisiae (Sc), and Plasmodium falciparum (Pf).
Table S1.
Eleven SNPs in the region of chromosome 1 to which m90 maps
| Coverage | Reference | SNP | Coordinates on Chr. 1 | SNP location in annotation | Annotation of region with SNP | Gene name | Amino acid change |
| 9 | C | T | 29,759,319 | 5′UTR | Pyruvate decarboxylase | At1g79110 | — |
| 8 | C | T | 29,846,184 | Gene | EMB1135 | At1g79350 | No change |
| 3 | A | G | 29,983,760 | Intergenic | — | — | — |
| 3 | T | C | 29,983,779 | Intergenic | — | — | — |
| 11 | C | T | 30,116,432 | Gene | Ubiquitin-like | At1g80060 | R to K |
| 10 | C | T | 30,122,310 | Gene | SUS2 | At1g80070 | P1141S |
| 14 | C | T | 30,148,084 | Gene | Pectin lyase-like | At1g80140 | No change |
| 11 | C | T | 30,159,879 | Intron | PSF1 component | At1g80190 | — |
| 6 | C | T | 30,344,751 | Gene | CMT1 | At1g80740 | No change |
| 4 | C | T | 30,354,337 | Intergenic | — | — | — |
| 12 | C | T | 30,367,988 | Gene | Tudor/PWWP/MBT | At1g80810 | S to L |
—, Indicate that there is no annotated genes or amino acid changes.
Fig. 2.
Point mutations in Prp8 are responsible for suppressors of atprmt5 mutants. (A) Sequence alignment of the conserved Prp8 containing the P347S and P1141S mutations in prp8-9 and prp8-8, respectively. Abbreviations for species are as follows: Arabidopsis thaliana (At), Oryza sativa (Os), Homo sapiens (Hs), Mus musculus (Mm), Drosophila melanogaster (Dm), Caenorhabditis elegans (Ce), Schizosaccharomyces pombe (Sp), Saccharomyces cerevisiae (Sc), and Plasmodium falciparum (Pf). The point mutation sites are labeled by black triangles. (B and C) Rescued growth retardation of young seedling leaves at 12 d (B) and intron retention events (C) by a transformation of CDS Prp8 clone with P1141S mutation in atprmt5-2 sus2-5 double mutants background. C8 and C20 are two individual transgenic lines containing P1141S construct of Prp8. W5 is the control transgenic line containing WT Prp8 construct.
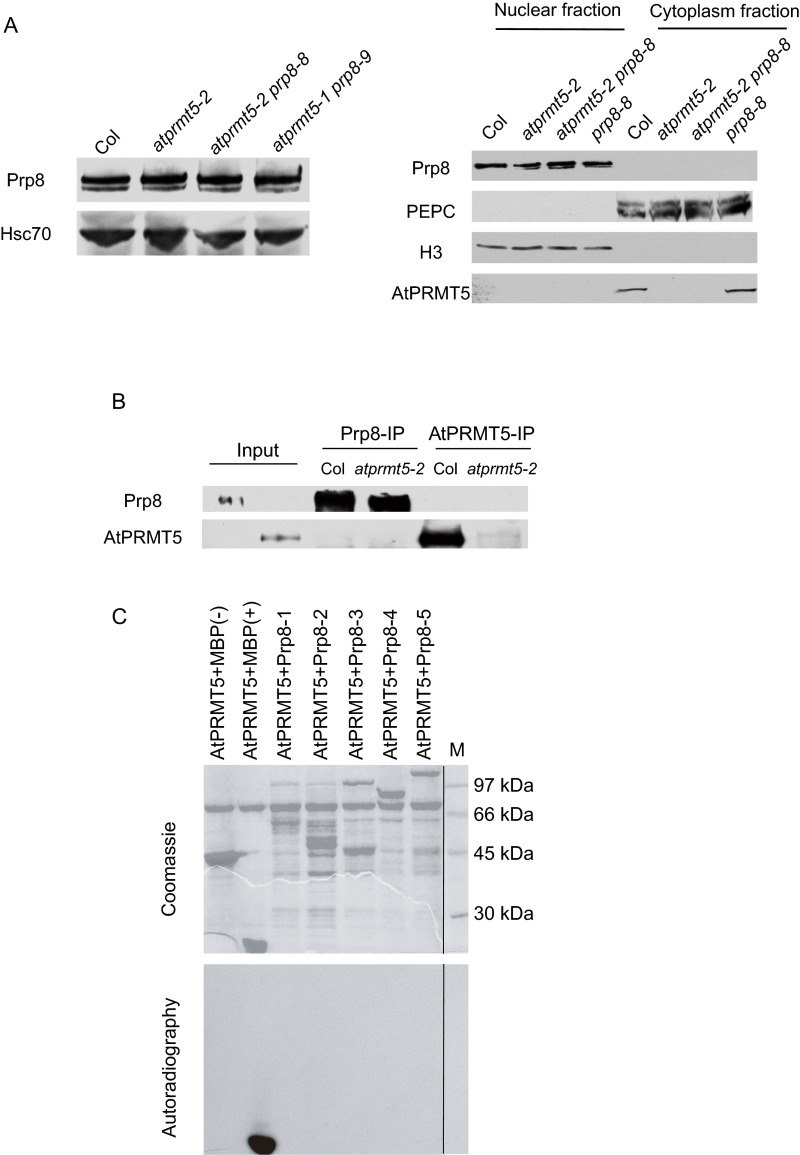
At1g80070 encodes the Arabidopsis homolog of the highly conserved U5 snRNP component (26), Prp8, which plays a central role in the catalytic core of the spliceosome and is highly conserved from yeast to plants and humans (27) (Fig. S2B). A recent study showed that Arabidopsis Prp8 is involved in modulating the splicing of the GFP reporter gene in a genetic screen (28) and long noncoding transcript COOLAIR to regulate FLC transcription (25). Protein sequence alignment of Prp8 homologs showed that P1141 of Prp8 is in the linker region between the RNA recognition motif (RRM) and the U5-snRNA binding domain and is highly conserved from yeast to humans, and P347 is in the linker region between the N-terminal domain and the PROCN domain and is moderately conserved (Fig. 2A). These mutations neither cause a change in Prp8 protein levels nor affect the subcellular localization of Prp8 in Arabidopsis (Fig. S3A). AtPRMT5 could neither methylate nor interact with Prp8 (Fig. S3 B and C).
Fig. S3.
Point mutations in Prp8 do not destabilize protein levels, and AtPRMT5 does not interact with and methylate Prp8. (A) Analysis of Prp8 protein levels and nucleus/cytoplasm distribution by Western blotting using anti-Prp8 polyclonal antibody, with Hsc70, PEPC, and H3 as loading controls for total, cytoplasm, and nucleus, respectively. (B) AtPRMT5 does not interact with Prp8 in vivo. AtPRMT5 and Prp8 immunoprecipitated by anti-AtPRMT5 and anti-Prp8 polyclonal antibodies from Col and atprmt5 mutants were immunoblotted with anti-Prp8 and anti-AtPRMT5 antibodies, respectively. (C) AtPRMT5 does not methylate Prp8 in vitro. Full length of Prp8 is divided into five fragments (1–5), and each of the fragments is subjected to the methyltransferase activity assay with AtPRMT5. The dividing lines indicate noncontiguous images between samples and the protein marker.
The Suppressors Partially Rescue the Splicing Alterations of atprmt5 Mutants.
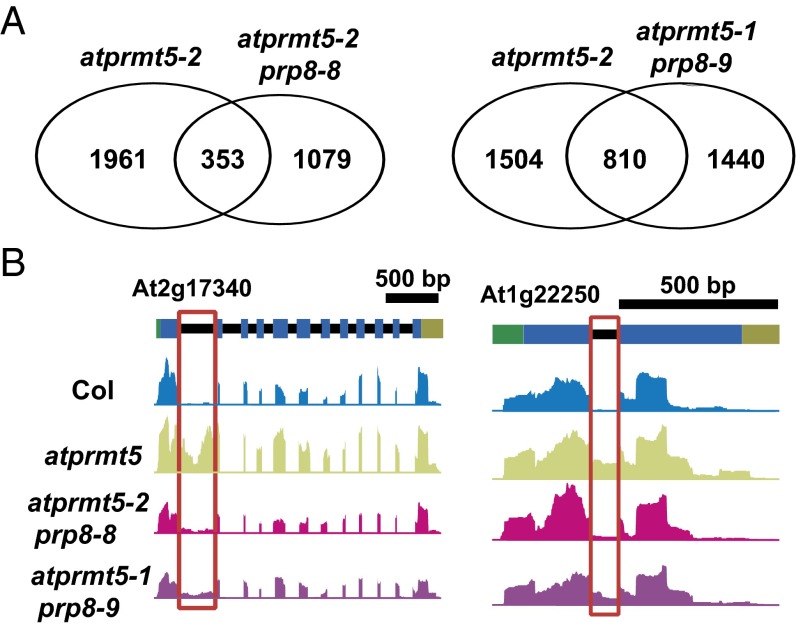
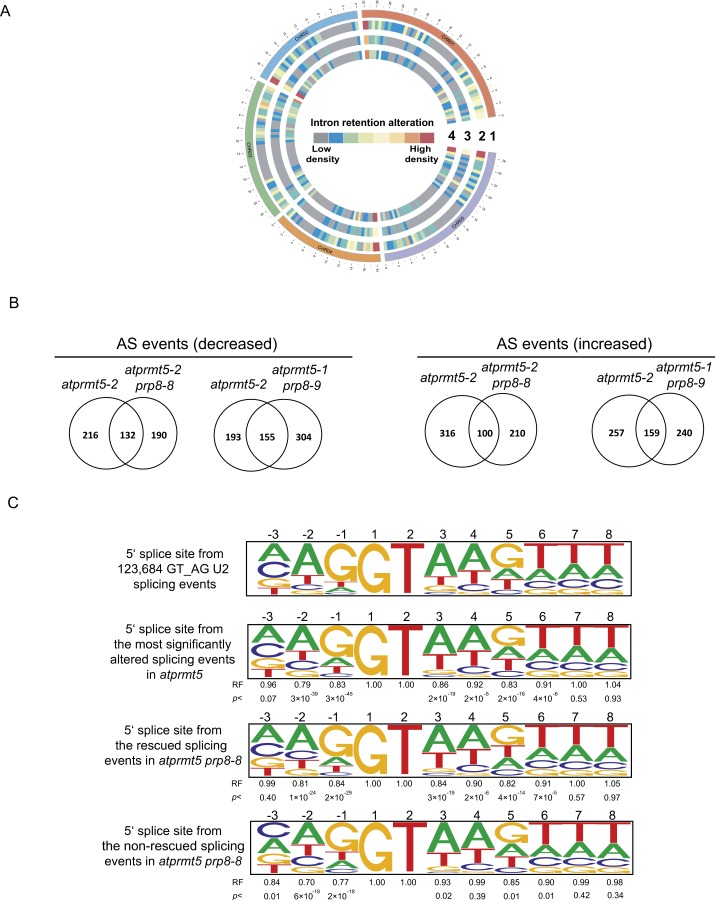
To test whether atprmt5-1 prp8-9 and atprmt5-2 prp8-8 restore the splicing alterations genome-wide, we performed ultrahigh-throughput RNA sequencing (RNA-seq) to examine the global pre-mRNA splicing (Table S2). Compared with Col, we identified 2,314 intron retention events in atprmt5 mutants. By contrast, we found that 84.7% (1,961 of 2,314) intron retention events in atprmt5-2 prp8-8 and 65.0% (1,504 of 2,314) in atprmt5-1 prp8-9 (P < 0.01) were rescued, respectively (Fig. 3A and Fig. S4A). Fig. 3B shows two examples of mRNAs with rescued intron retention events detected by RNA-seq. In addition, we analyzed alternative splicing (AS) events and identified 348 decreased and 416 increased AS events in atprmt5 mutants, among which 62% (216 of 348) and 55% (193 of 348) of decreased AS events, and 76% (316 of 416) and 62% (257 of 416) of increased AS events were rescued in atprmt5-2 prp8-8 and atprmt5-1 prp8-9, respectively (Fig. S4B).
Table S2.
Summary of high-throughput RNA sequencing analysis
| Library | Total reads | Total matches (% of total)* | Reads mapped to splice junctions (% of total) | Unique matches (% of total matches)† | Multiple matches (% of total matches)‡ | Coverage of intron-containing genes (RPKM > 3)§ | Coverage of genes (RPKM > 3)§ |
| Col | 29,505,966 | 27,908,233 (94.6%) | 8,769,813 (29.5%) | 27,440,647 (98.3%) | 467,586 (1.7%) | 13,308 | 15,300 |
| atprmt5-2 | 31,472,542 | 29,483,401 (93.7%) | 9,334,131 (29.5%) | 28,919,312 (98.1%) | 564,089 (1.9%) | 13,345 | 15,225 |
| atprmt5-2 prp8-8 | 30,418,678 | 28,703,813 (94.3%) | 8,948,627 (29.3%) | 28,170,586 (98.1%) | 533,227 (1.9%) | 13,178 | 15,077 |
| atprmt5-1 prp8-9 | 30,146,556 | 28,116,182 (93.3%) | 8,427,608 (27.9%) | 27,669,011 (98.4%) | 447,171 (1.6%) | 12,254 | 13,915 |
RPKM, reads per kilobase per million mapped reads.
Number of reads that aligned to the A. thaliana genome and annotated splicing junction sequences.
Number of reads that aligned to a unique location of the TAIR9 reference genome.
Number of mapped reads that aligned to more than one genomic location.
Number of intron-containing genes covered by the unique reads (>3 RPKM).
Fig. 3.
atprmt5 suppressors partially rescue the splicing alterations of atprmt5 mutants. (A) Overlapping analysis of significantly altered constitutive splicing events between atprmt5-2 and the suppressors. (B) Two examples of intron retention events rescued in atprmt5-1 prp8-9 and atprmt5-2 prp8-8. Annotated gene structures are shown (Upper), with thick lines representing exons and thin lines representing introns. Wiggle plots representing the normalized reads coverage in a logarithmic scale (log2) are shown in blue for Col, in green for atprmt5 mutants, in pink for atprmt5-2 prp8-8, and in purple for atprmt5-1 prp8-9. The red frames indicate the retained introns rescued in atprmt5-1 prp8-9 and atprmt5-2 prp8-8.
Fig. S4.
Impact of atprmt5-2, atprmt5 prp8-8, and atprmt5 prp8-9 on genome-wide constitutive splicing and alternative splicing. (A) The global splicing defects of atprmt5 mutants are partially suppressed in atprmt5-1 prp8-9 and atprmt5-2 prp8-8 plants. The heatmap view of the density of pre-mRNA splicing defects in a 600-kb window in atprmt5-2 (circle 2), atprmt5-2 prp8-8 (circle 3), and atprmt5-1 prp8-9 (circle 4). Circle 1 represents the chromosome. (B) Overlap between significantly altered alternative splicing events. (C) Bioinformatic analysis of donor splice-site sequences. Pictograms showing the frequency distribution of nucleotides at the 5′ splice site of 123,684 GT_AG_U2 Arabidopsis introns, the most significantly intron retention events whose splicing were altered simultaneously in atprmt5 and the significantly rescued and nonrescued intron retention events in atprmt5 prp8-8. The representation factor (RF) is the frequency of interest divided by the total frequency. For each RF, a P value was calculated using the hypergeometric test.
Moreover, we also compared the 5′ splice-site sequence in atprmt5 and atprmt5-2 prp8-8 (including rescued events and nonrescued events) compared with the 5′ splice-site consensus sequence of all introns present in the Arabidopsis genome. Compared with the Arabidopsis 5′ splice-site consensus sequence (Fig. S4C, first panel), atprmt5 displayed a significant decrease in the frequency of the dominant consensus G and A at the −1 and −2 positions, respectively, and a tendency toward randomization of the nucleotides at the +5 positions (Fig. S4C, second panel), consistent with the previous results from Yanovsky’s laboratory (10, 12). Then we compared the 5′ splice-site sequence in the rescued and nonrescued splicing events in atprmt5-2 prp8-8 and found that the nonrescued splicing events in atprmt5-2 prp8-8 showed a more tendency toward randomization of the nucleotides at the −3, −2, and −1 positions (Fig. S4C, third and fourth panels), suggesting that the P1141S point mutation is more inclined to rescue the intron retention events with less random 5′ splice-site in atprmt5. These results showed that the suppressors partially rescued the constitutive and alternative splicing alterations of atprmt5 mutants, consistent with their recovered developmental phenotypes. Because of the higher conservation of the affected amino acid, and the higher rescued splicing alterations efficiency in the mutant plants, we used atprmt5-2 prp8-8 (m90) for the following studies.
Prp19C/NTC and Step-Specific Splicing Factors Fail to Incorporate into the Spliceosomal Core in atprmt5 Mutants.
The highly dynamic spliceosome changes in composition and conformation during complex assembly, catalytic activation, active site remodeling, and complex disassembly, providing accuracy and flexibility in pre-mRNA splicing (15, 29). Prp8, a highly conserved and essential component of the U5 snRNP, occupies a central position in the catalytic core of the spliceosome, interacts with the pre-mRNA, U snRNA, and numerous protein factors, and has been implicated in several structural and compositional rearrangements during splicing (27). The recently reported crystal structure of yeast Prp8 reveals that the mutated amino acid in prp8-8 occurs on the outer surface of Prp8, which may affect interactions with surrounding components within the spliceosome (30). Therefore, we hypothesized that AtPRMT5 may be required for proper complex composition during spliceosome formation and activation, and the neomorphic mutation of atprmt5-2 prp8-8 might overcome the requirement for AtPRMT5.
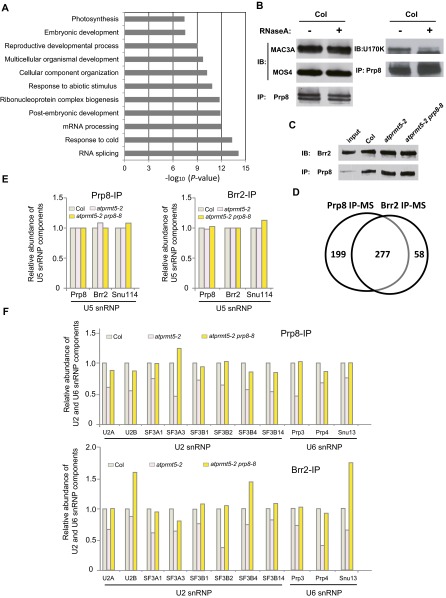
To explore the alterations of spliceosome composition in atprmt5 mutants, we characterized Prp8-associated proteins by MS. We extracted nuclear proteins from Col, atprmt5-2, atprmt5-2 prp8-8, and prp8-8 plants and immunoprecipitated with anti-Prp8 antibodies, and then used MS to identify the components that interact with Prp8. We used spectral counts to quantify protein abundance in various spliceosomal complexes and normalized the counts for each protein to the spectral counts for Prp8 to estimate the relative abundance of proteins associating with Prp8. We identified 477 proteins that each had more than five spectral counts. Gene Ontology (GO) analysis revealed that these interacting proteins function in multiple biological processes (Fig. S5A).
Fig. S5.
Prp8 interacts with Brr2 and share similar interaction proteins. (A) Gene ontology terms enriched in genes associated with Prp8. (B) The interactions between Prp8 and Prp19C/NTC are independent of RNA (Left), whereas the interaction between Prp8 and U1 snRNP (U1-70K) is partially dependent on RNA (Right). (C) Prp8 immunoprecipitated by anti-Prp8 antibody was immunoblotted with anti-Brr2 antibody. (D) Venn diagram of numbers of Prp8-associated and Brr2-associated proteins, as identified by MS. (E and F) The association of Prp8 (and Brr2) within U5 snRNP (E) and U2 and U6 snRNPs (F) in Col, atprmt5-2, and atprmt5-2 prp8-8.
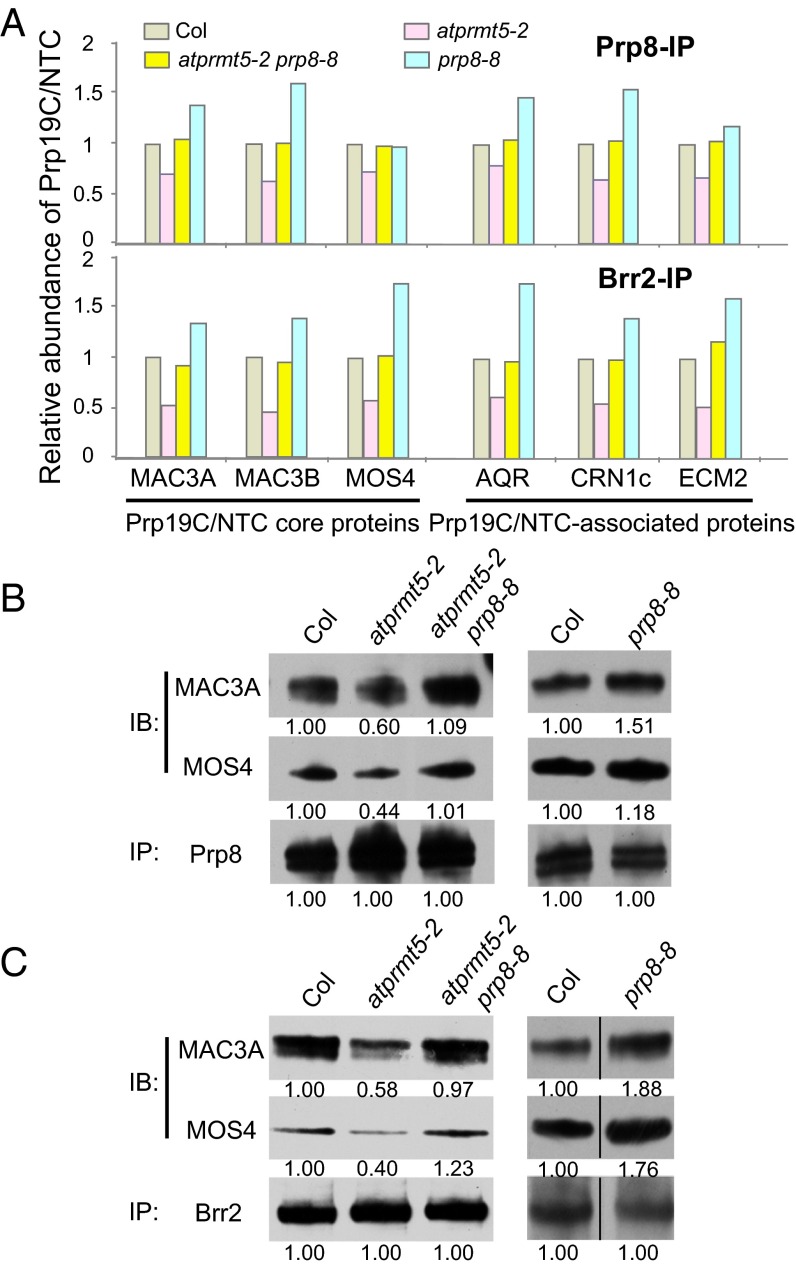
Intriguingly, among the proteins that interact with Prp8, our MS results identified a set of splicing factors, which form the Prp19 complex (Prp19C), also known as NTC (21, 31, 32). Compared with Col, the atprmt5 mutants showed significantly reduced normalized spectral counts from the Prp19C/NTC core proteins, including MAC3A and MAC3B (human Prp19 homolog in Arabidopsis), MOS4 (human SPF27 homolog in Arabidopsis), CDC5, and PRL1, and the Prp19C/NTC-associated proteins (SYF1, AQR, CRN1c, SKIP, ECM2, ISY1, and PPI) (Fig. 4A, Upper, and Table S3), suggesting that Prp19C/NTC cannot efficiently interact with Prp8 in atprmt5 mutants. atprmt5-2 prp8-8 showed restored counts for these Prp19C/NTC core proteins, whereas prp8-8 displayed a slightly increased interaction with Prp19C/NTC (Fig. 4A, Upper, and Table S3), indicating that the Prp8P1141S version can restore the pre-mRNA splicing defects of atprmt5 mutants by increased recruiting Prp19C/NTC to the spliceosome core. To further substantiate the MS results, immunoprecipitation of nuclear extracts with anti-Prp8 antibodies in Col, atprmt5-2, atprmt5-2 prp8-8, and prp8-8, followed by immunoblotting with anti-MAC3A and anti-MOS4 antibodies revealed the decreased interaction of Prp8 with Prp19C/NTC in atprmt5-2 mutants, and the restored interaction in atprmt5-2 prp8-8 mutants, which are independent of RNA (Fig. S5B) and consistent with the MS results (Fig. 4B).
Fig. 4.
The recruitment of Prp19C/NTC to the spliceosome is impaired in atprmt5 mutants and restored in atprmt5-2 prp8-8. (A) The association of Prp19C/NTC and Prp8 (and Brr2) decreased in atprmt5 mutants and was rescued in atprmt5-2 prp8-8 mutants. Nuclear extracts from Col, atprmt5-2, atprmt5-2 prp8-8, and prp8-8 were immunoprecipitated by anti-Prp8 (Upper) and anti-Brr2 (Lower) polyclonal antibodies, and the interacting proteins were identified by MS. The spectral counts of Prp19C/NTC from atprmt5-2, atprmt5-2 prp8-8, and prp8-8 were divided by that from Col, and the ratios are shown in the histogram. (B and C) Prp8- or Brr2-associated proteins were identified in Col, atprmt5, atprmt5-2 prp8-8, and prp8-8 by MS. Prp8 (B) and Brr2 (C) were immunoprecipitated by anti-Prp8 and anti-Brr2 antibodies, respectively, and immunoblotting was performed with anti-MAC3A and anti-MOS4 antibodies for Prp19C/NTC. The dividing lines in C, Right indicate noncontiguous images between Col and prp8-8.
Table S3.
Prp8- and Brr2-associated proteins identified in Col, atprmt5, and atprmt5 prp8-8 (m90) and prp8-8 by MS
| AGI | Protein | Prp8 IP-MS | Brr2 IP-MS | ||||
| atprmt5/Col | m90/Col | prp8-8/Col | atprmt5/Col | m90/Col | prp8-8/Col | ||
| Prp19C/NTC core proteins | |||||||
| At1g04510 | MAC3A | 0.70 | 1.05 | 1.39 | 0.51 | 0.91 | 1.34 |
| At2g33340 | MAC3B | 0.62 | 1.01 | 1.61 | 0.45 | 0.95 | 1.39 |
| At3g18165 | MOS4 | 0.72 | 0.98 | 0.97 | 0.57 | 1.02 | 1.75 |
| At1g09770 | AtCDC5 | 0.66 | 0.98 | 1.33 | 0.48 | 1.06 | 1.88 |
| At4g15900 | AtPRL1 | 0.56 | 0.98 | 1.20 | 0.71 | 1.24 | 1.49 |
| Prp19C/NTC-associated proteins | |||||||
| At5g28740 | AtSyf1 | 0.68 | 0.99 | 1.32 | 0.42 | 0.90 | 1.86 |
| At2g38770 | AtAQR | 0.79 | 1.05 | 1.47 | 0.61 | 0.97 | 1.76 |
| At5g41770 | Syf3/AtCRN1c | 0.64 | 1.03 | 1.55 | 0.55 | 0.99 | 1.41 |
| At1g77180 | AtSKIP | 0.74 | 1.07 | 1.20 | 0.67 | 1.02 | 1.41 |
| At1g07360 | AtECM2 | 0.66 | 1.03 | 1.18 | 0.51 | 1.17 | 1.61 |
| At3g18790 | AtISY1 | 0.62 | 0.84 | 1.15 | 0.54 | 0.90 | 0.97 |
| At2g36130 | AtPPI | 0.65 | 1.01 | 1.01 | 0.84 | 1.47 | 1.58 |
| Splicing factors in the first and second catalytic steps | |||||||
| At1g32490 | AtPrp2 | 0.50 | 0.84 | 1.82 | 0.23 | 0.79 | 2.81 |
| At3g49601 | AtCwf21 | 0.62 | 1.00 | 0.58 | 0.47 | 1.41 | 1.22 |
| At1g80930 | AtCWC22 | 0.63 | 0.94 | 1.26 | 0.79 | 1.34 | 2.06 |
| At1g10580 | AtPrp17 | 0.63 | 0.97 | 1.03 | 0.47 | 1.01 | 2.00 |
As an integral component of active spliceosome, Prp19C/NTC associates with the spliceosome during pre-mRNA splicing and participates in modulating spliceosome composition and conformation (22). In addition to the Prp19C/NTC components, we also found that atprmt5-2 mutants showed decreased association between Prp8 and the specific splicing factors in the first (Prp2, Cwf21, and CWC22) and the second (Prp17 and Prp22) catalytic steps; these associations were recovered in atprmt5-2 prp8-8 mutants (Table S3). These results suggest that AtPRMT5 is required for the recruitment of Prp19C/NTC and specific splicing factors to the catalytic core of the spliceosome, which is essential for spliceosome activation.
To verify that Prp19C/NTC cannot be efficiently recruited to the spliceosome core in atprmt5 mutants, we immunoprecipitated Brr2, a DEAH-box ATPase that interacts with Prp8 (Fig. S5C), and used MS to identify the components that interacted with Brr2. We identified 336 proteins, 277 of which overlapped with Prp8-associated components (P = 2.36 × 10−13; Fig. S5D). Consistent with our Prp8 results, the interactions between Brr2 and Prp19C/NTC, the specific splicing factors in the first and second catalytic steps, decreased in atprmt5 mutants, but were restored in atprmt5-2 prp8-8 (Fig. 4 A, Lower, and C and Table S3), supporting the idea that AtPRMT5 deficiency impairs the interaction between Prp19C/NTC and the spliceosome core and suggesting the essential role for AtPRMT5 in activating the spliceosome.
Prp19C/NTC Recruitment to the Spliceosome Requires Symmetric Dimethylation of AtSm Proteins.
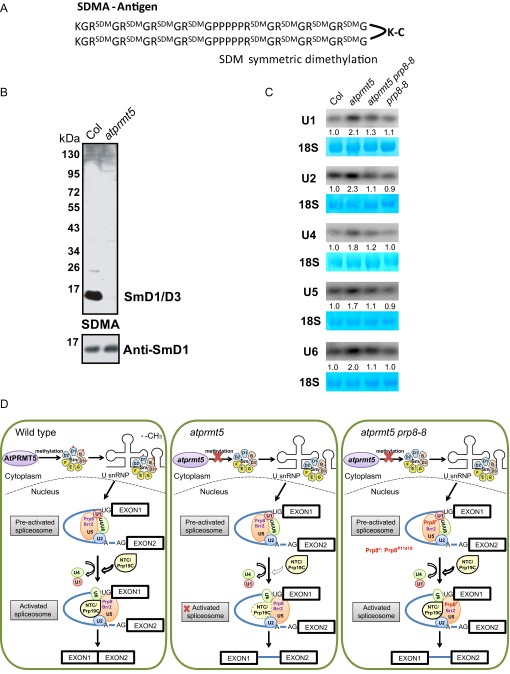
Our previous study showed that AtPRMT5 symmetrically di-methylates AtSmD1, D3, and AtLSm4 proteins, which form the basic core components of U snRNP and have essential functions in splicing (11). To distinguish the methylation status of these AtSm proteins, we used low complex GAR motifs harboring SDMA as the antigen to generate antibodies that specifically detect these methylation marks (Fig. S6A). To characterize the antibody, we tested it on total proteins from Col and atprmt5 mutants. We observed that the signal recognized by the SDMA-specific antibody (mainly AtSmD1 and AtSmD3) dramatically decreased in atprmt5 mutants (Fig. S6B), suggesting that AtPRMT5 is responsible for symmetric dimethylation of arginine residues of AtSm proteins in vivo.
Fig. S6.
Preparation of anti-SDMA antibody, U snRNAs analysis, and working model. (A) Low complex GAR motifs harboring symmetric dimethylarginines (SDMA). (B) Total proteins extracted from Col and atprmt5 mutants were immunoblotted with anti-SDMA and anti-SmD1 antibodies. (C) Levels of U1, U2, U4, U5, and U6 snRNAs are increased in atprmt5 mutants and restored in atprmt5 prp8-8. 18S rRNAs were used as a loading control. (D) Model for the role of AtPRMT5 in Prp19C/NTC complex recruitment to the spliceosome during pre-mRNA splicing.
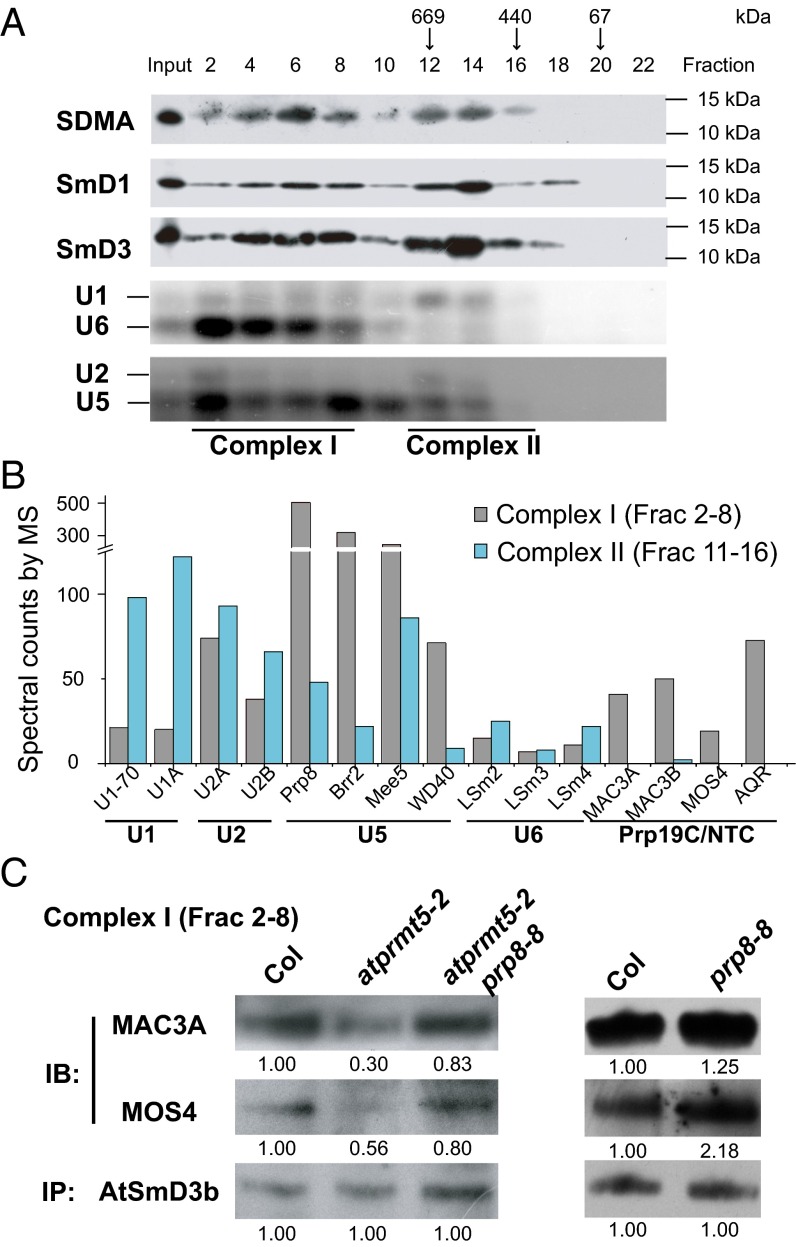
To better understand the role of these symmetrically dimethylated AtSm proteins in spliceosome assembly, we first used size exclusion chromatography to analyze their elution profiles in extracts from Col. We observed that the symmetrically dimethylated AtSm proteins (mainly AtSmD1 and AtSmD3) roughly distributed into two peaks, which we termed complex I (larger than 669 kDa, fractions 2–8) and complex II (around 669 kDa, fractions 11–16), supported by U snRNA profiles (Fig. 5A). To examine the protein composition of complex I and complex II, we combined the corresponding eluted fractions, immunoprecipitated with SDMA antibody, and used MS to identify the protein components. Among the protein components identified, complex I contained U2, U5, and U6 snRNPs, and Prp19C/NTC; complex II contained U1 and U2 snRNPs (Fig. 5B). The partial composition analysis suggests that complex I and complex II are more consistent with activated and preactivated forms of the spliceosome, respectively.
Fig. 5.
Decreased recruitment of Prp19C/NTC to the spliceosome in atprmt5 mutants. (A) The elution profiles of symmetric dimethylated AtSmD1 and AtSmD3 proteins. Cell lysates from Col were fractionated by gel filtration chromatography as described in Materials and Methods and were immunoblotted to visualize symmetric dimethylated AtSmD1 and AtSmD3. The molecular weights of the calibration standards and their elution positions are indicated. (B) The distribution of the spliceosomal proteins in gel filtration chromatography. Fractions 2–8 and 11–16 were collected and immunoprecipitated with SDMA antibody, and then the associated proteins were identified by MS. The spectral counts of U snRNPs and Prp19C/NTC are shown in the histogram. (C) The interaction status between AtSmD3b and Prp19C/NTC in complex I. Fractions 2–8 were collected from Col, atprmt5-2, atprmt5-2 prp8-8, and prp8-8, and AtSmD3b was immunoprecipitated by anti-AtSmD3b polyclonal antibody, and then immunoblotting was performed with anti-MAC3A and anti-MOS4 polyclonal antibodies for Prp19C/NTC and anti-AtSmD3b antibody for loading controls.
AtSmD3 stably exists in U1, U2, U4, and U5 snRNPs, and is involved in almost every state of the spliceosome during pre-mRNA splicing. To explore whether the symmetrical di-methylation of Sm core proteins affects spliceosome assembly and activation, we collected complex I fractions from Col, atprmt5-2, atprmt5-2 prp8-8, and prp8-8, and then performed immunoprecipitation with anti-AtSmD3b antibodies, followed by immunoblotting with anti-MAC3A and anti-MOS4 antibodies. The results further confirmed the decreased interaction between AtSmD3b and Prp19C/NTC in atprmt5-2 mutants, which was restored in atprmt5-2 prp8-8 (Fig. 5C), consistent with the Prp8 IP-MS results, again verifying that Prp19C/NTC cannot be efficiently recruited to the activated spliceosome, which leads to the constitutive and alternative splicing alterations in atprmt5 mutants.
Discussion
Extensive studies have shown that PRMT5 deficiency produces diverse phenotypes in different organisms. We and others have demonstrated that AtPRMT5 is essential for constitutive and alternative pre-mRNA splicing of diverse genes, but how AtPRMT5 functions in pre-mRNA splicing has remained unclear (10–12, 33). Here we showed that AtPRMT5 promotes the recruitment of Prp19C/NTC and key first step and second step splicing factors to the spliceosome, thus enabling global pre-mRNA splicing under normal growth conditions (Fig. S6D).
PRMT5 can symmetrically dimethylate the C-terminal tails of the U snRNP Sm proteins B/B′, D1, D3, and LSm4 in multiple organisms, which may facilitate the recognition of a subset of 5′ splice sites (10, 11, 33–36). Here atprmt5 mutants show a dramatic impairment of the interaction between Prp19C/NTC and AtSmD3b, revealing a previously unidentified function of symmetric arginine dimethylation in the recruitment of Prp19C/NTC to the spliceosome. In the Prp8 and Brr2 IP-MS data, it is interesting to find that the interactions between Prp8/Brr2 and the components in U2 and U6 snRNP, but not in U5 snRNP (Fig. S5 E and F), decreased in atprmt5 mutants, and were partially restored in atprmt5-2 prp8-8, suggesting the defects of spliceosome assembly to the introns in atprmt5 mutants. Although the U snRNAs (including U1, U2, U4, U5, and U6 snRNAs) levels were significantly increased in atprmt5 mutants (Fig. S6C), it cannot rescue the splicing alterations, reflecting a feedback of impaired spliceosome assembly in some of introns in atprmt5. The crystal structure of yeast Prp8 shows that the conserved proline mutated in prp8-8 is located on the outer surface of the RT/En domain, a crucial protein-binding surface, which may affect interactions with surrounding components within the spliceosome (30), and the increased interaction between U5 snRNP with Prp8P1141S and Prp19C/NTC in prp8-8 mutants proved this structural hypothesis. Because AtPRMT5 deficiency results in inefficient recruitment of Prp19C/NTC to the spliceosome, it makes sense that the Prp8P1141S version can restore the pre-mRNA splicing defects in atprmt5 mutants by increased recruiting Prp19C/NTC to the spliceosome core. We therefore hypothesized that, in atprmt5 mutants, the loss of symmetric arginine dimethylation of Sm core proteins alters their binding interactions with other spliceosomal components by changing protein surface structure and hydrophobicity. Mutations in Prp8 may slightly reposition its interacting proteins within the spliceosome to facilitate the optimization of conformation, thereby reversing the splicing alterations in atprmt5 (Fig. S6D).
RNA splicing is a critical posttranscriptional regulatory mechanism, and ∼60% of noninfectious human diseases arise from splicing disorders; thus, the study of pre-mRNA splicing mechanisms has a high priority for human health (37). Splicing involves highly dynamic, organized, and exceptional compositional and structural rearrangements within the spliceosome during complex assembly, catalytic activation, and disassembly (38). Therefore, any mutations in the genes involved in RNA splicing machinery may give rise to serious diseases (37). For example, perturbation of the SMN gene results in widespread splicing defects and thus causes spinal muscular atrophy, a motor neuron disease (39). Despite increasing evidence showing that PRMT5 deficiency leads to genome-wide splicing alterations in different species, our study is the first attempt, to our knowledge, to dissect the underlying mechanism, showing that AtPRMT5 is indispensable for the recruitment of Prp19C/NTC and splicing factors in the catalytic reactions to the spliceosome and a potential conserved mechanism for PRMT5 in pre-mRNA splicing. Additionally, PRMT5 deficiency is (at least in part) involved in Huntington’s disease pathogenesis (40), whereas PRMT5 overexpression occurs in a large number of cancers (41). Thus, PRMT5 plays multifarious roles in human diseases. Due to the evolutionary conservation of PRMT5 in different species, our findings provide a fundamental molecular mechanism applicable to all eukaryotes, thereby shedding light on PRMT5 functions and spliceosome activation in animals.
Materials and Methods
Plant Materials.
The atprmt5-1 and atprmt5-2 mutants were characterized previously (9), and atprmt5-2 prp8-8 and atprmt5-1 prp8-9 were isolated in this study. Further details can be found in SI Materials and Methods.
RNA Sequencing and Data Analysis.
Total RNA was subjected to polyA selection (Invitrogen), and strand-specific RNA-seq libraries were constructed using SMART method (42). The data analysis method was according to the previously reported method (11, 12). RT-PCR is performed using intron-flanking primers (Table S4) for validation. Further details can be found in SI Materials and Methods.
Table S4.
Oligonucleotides used in this study
| Gene | Primer sequences (5′ to 3′) |
| At1g68190-F | TCGTTAGAGCAATCTTTTC |
| At1g68190-R | TTGATGCCACAACTGATT |
| At5g05010-F | CCTCGAGTACGAAGCCTCGT |
| At5g05010-R | AACTCCATTGAGCCA CTGCG |
| At1g74670-F | CAAGAGCTAGTCATGGCCAA |
| At1g74670-R | TTGTCCTCCACATT GGTATG |
| At2g17340-F | ATGGAGAGCGATTCAGAGA |
| At2g17340-R | GAATCCCAGCATAT CTTTC |
| At3g48360-F | CTCCGGCGTTCTG GCTTC |
| At3g48360-R | ACATCGGGTGCGTCGCAG |
| At3g52720-F | AAACCCTCCACAG AGATT |
| At3g52720-R | CGAAGAACATGGCAACAT |
| At1g78630-F | TGGATGTTCGACGAAGAAGA |
| At1g78630-R | TTCTCAGCATTCAC CACAA |
| At5g55125-F | CCTTTAGTCAGATCGACA |
| At5g55125-R | GCAAGGTTCATAGTAAATG |
| At5g44650-F | GCCAAATTTAGAG GTTGA |
| At5g44650-R | AAAACATCAATGATGGGTC |
| ACTIN-F | CTCAGCACCTTCCAACAG ATGTGGA |
| ACTIN-R | CCAAAAAAATGAACCAAGGACCAAA |
| PRP8 (prp8-9)-F | TTTTCCCCATCTCTACAATAATGGG |
| PRP8 (prp8-9)-R | AGTTGGGTATCTCTTAGCAAAGGTT |
| PRP8 (prp8-8)-F | ATTCAGCGATATCTTACGGAGGAT |
| PRP8 (prp8-8)-R | GAGACACAACATGAACATCTAATG |
| HX2030 | CGGGGTACCATGTGGAACAACAACGAT |
| HX2031 | CGGGGTACCAGTAAATGTGTCCTCGCG |
| Hx3697 (U1 probe) | CCCCCTCTGCCACAAATAATGACG |
| Hx3698 (U2 probe) | CTGATAAGAACAGATACTACACTTG |
| Hx3699 (U4 probe) | CCTCATTAGCTGCGTCATTGC |
| Hx3700 (U5 probe) | CACGGTATTCTTTAGTAAAAGGC |
| Cx3256 (U6 probe) | TGTATCGTTCCAATTTTATCGGATGT |
| Hx6345 (Prp8 5′ artificial miRNA) | GGGGTACCTGACGATGGAAGACTCAGAAGGAAAACATGCCACATGAGTTGAGCAGGGTA |
| Hx6346 (Prp8 5′ artificial miRNA) | CATGCCATGGATGACTCAGAAGGAGGACATGCCAGAAGAGTAAAAGCCA |
Proteomic Analysis.
Affinity purified protein complexes were separated on a 4–12% Bis-Tris gel (Life Technologies), and the proteins bands were subjected to in-gel digestion as described previously (43). Further details can be found in SI Materials and Methods.
Additional methods are available in SI Materials and Methods.
SI Materials and Methods
Plant Strains and EMS Mutagenesis.
atprmt5-1 and atprmt5-2 mutants were characterized previously (9), and atprmt5-2 prp8-8 and atprmt5-1 prp8-9 were isolated in this study. The sus2-5 heterozygous mutant (CS16073) was from the SALK T-DNA mutant collection. prp8-6 (in Ler background) was from Caroline Dean’s laboratory.
An EMS mutagenesis screen was carried out in the atprmt5-1 and atprmt5-2 backgrounds. Ten thousand atprmt5-1 and atprmt5-2 seeds were treated with 0.3% EMS and 0.01% Tween 20 for 15 h and then planted on soil. M2 seeds were collected from single M1 plants. In the M2 families from the atprmt5-1 and atprmt5-2 background, screened for the rescued pleiotropic developmental defects of atprmt5 mutants, two lines were isolated that were later found to contain mutant alleles at the Prp8 locus. atprmt5-2 prp8-8 was backcrossed to Col for five times before further genetic analysis, and atprmt5-1 prp8-9 was backcrossed for three times.
Transgenes.
Primer sequences used in this study can be found in Table S4. The Prp8 CDS was amplified from Col and atprmt5-2 prp8-8 with primers HX2030 and HX2031, digested by KpnI, and ligated to UBQ10:HA. The constructs were transformed into Agrobacterium tumefaciens cells (strain EHA105). The UBQ10:Prp8WT-HA and UBQ10:Prp8P1141S-HA transgenes were introduced into atprmt5 −/− sus2-5+/− mutants by the floral dip method. T1 Arabidopsis seeds were planted in MS with Hygromycin.
RNA Sequencing and Data Analysis.
Total RNA was extracted with TRIzol Reagent (15596-026; Invitrogen). RNA was subjected to polyA selection (T-61006; Invitrogen), and strand-specific RNA-seq libraries were constructed using the SMART method following the standard protocol (42) and sequenced on the Illumina HiSeq2000 platform to generate high-quality paired-end reads of 100 nt. A. thaliana genome sequences and annotated gene models were downloaded from TAIR10 (The Arabidopsis Information Resource, version 10) (www.arabidopsis.org/). The raw reads were aligned to the genome sequences using TopHat-2.0.11 (44) with anchor length more than 8 nt for spliced alignments, trimming off a nucleotide each from the 5′ and 3′ ends and allowing up to two mismatches. Reads mapped to multiple locations were discarded, and only uniquely mapped reads were used for subsequent analysis. Gene read densities and bin (exon-bin, intron-bin, and AS-bin) read densities were calculated following previous methods (12). We discarded genes with read densities less than 0.05 and genes with splicing index (bin read density/gene read density) less than 0.05 in both WT and mutants. The cutoff for the splicing index ratio (splicing index in the mutant/splicing index in WT) was 2. At last fold change of the bin read density (bin read density in mutant/bin read density in WT) values >2 and false discovery rate (FDR) <0.01 were considered to be the threshold for identification of the differentially splicing events.
Map-Based Cloning of Prp8.
The atprmt5-1 and atprmt5-2 mutant plants were crossed to WT plants of Ler ecotype. The s215 and m90 plants were crossed to atprmt5-1 and atprmt5-2 in Ler, respectively. F2 seeds were harvested from single F1 plants. The F2 families that segregated s215 and m90 were identified by rescue of the pleiotropic developmental defects and pre-mRNA splicing defects of atprmt5. Genomic DNA was isolated from each of these plants. Initial mapping with 40 such plants using SSLP markers located the suppressor gene to chromosome 1. Additional SSLP markers between Ler and Col according to the Arabidopsis Mapping Platform (AMP) (23), allowed the mapping of the suppressor gene to a single bacterial artificial chromosome (BAC). Information on these markers can be obtained at the web site (amp.genomics.org.cn/).
Genomic DNA Sequencing and Data Analysis.
To identify the point mutations, we sequenced the genomic DNA of the F3 progeny from a backcross of m90 to Col-0 (homozygous for the m90) by high-throughput sequencing. The genomic DNA from m90 (homozygous strains) was extracted, and the fragment library was prepared according to the manufacturer’s protocol (Illumina) and sequenced on the Illumina GAIIx platform to generate high-quality single-end reads of 76 nucleotides in length. The sequencing reads were mapped to the Arabidopsis genome (www.arabidopsis.org/) using bowtie-0.12.7 (45) to produce alignment files. In total, 18.7 million single-end reads were produced, in which 17.2 million (92.0%) contiguous reads were aligned to the Arabidopsis genome (TAIR10); thus, the average base coverage was 10-fold. Raw SNPs were extracted using samtools-0.1.7 pileup based on the sorted alignment files. The output pileup formatted, with consensus calls were further filtered by the thresholds of SNP quality ≥20 (less than 1% error rate) to exclude error-prone variant calls and derive a high-confidence set of SNPs. To identify the authentic SNP in m90, we removed all SNPs that were identified in the intergenic region in TAIR10 reference and only used the homozygous SNPs mapped to the annotated genes to determine if they changed amino acids. Eleven homozygous SNPs were clustered in the region of chromosome 1 where m90 mapped; three of these SNPs were from intergenic region, and eight mapped to annotated genes, in which one was in an intron, one was in the 5′ UTR, three were synonymous mutations, and three were missense mutations.
Genotyping.
prp8-8, prp8-9, and prp8-6 were genotyped by derived cleaved amplified polymorphic sequences (dCAPS) markers with the oligonucleotides shown in Table S4 and according to the previous paper (25), followed by digestion of the PCR products with ApaI and BamHI, respectively. Both ApaI and BamHI cleave the WT sequence.
Protein sequence alignment and phylogenic analysis.
Database searching was performed at National Center for Biotechnology Information (NCBI) (www.ncbi.nlm.nih.gov/) and TAIR10 (www.arabidopsis.org/). Multiple alignments of protein sequences were performed with ClustalX and were manually edited with the GeneDoc program. Evolutionary analyses were conducted with Maximum Likelihood method in MEGA 6.06.
Methyltransferase Activity.
Methyltransferase activity assays were performed as described previously (9). Briefly, recombinant GST-AtPRMT5 and MBP-Prp8 fragments were expressed in Escherichia coli strain BL21 and purified using glutathione Sepharose beads (Amersham Biosciences, for GST tag). Purified GST-AtPRMT5 was incubated with the indicated substrates in the presence of the methyl donor S-adenosyl-l-[methyl-3H] Met (Amersham Biosciences), in HMT buffer (20 mM Tris⋅HCl, pH 8.0, 4 mM EDTA, 1 mM PMSF, 0.5 mM DTT) for 3 h at 30 °C. Proteins were separated by 12% SDS/PAGE and visualized by Coomassie staining. Gels were then treated with Amplifier (Amersham Biosciences) for 15–30 min, dried, and exposed to Kodak Biomax MS film at −80 °C for the appropriate time.
Nuclear Extraction.
Approximately 2 g seedlings was ground thoroughly using a pestle and mortar in Honda lysis buffer (25 mM Tris⋅HCl, pH 7.4, 2.5% Ficoll 400, 5% dextran T40, 0.4 M sucrose, 10 mM MgCl2, 5 mM DTT, protease inhibitors) at 4 °C. The homogenate was filtered through 60 μm mesh nylon netting, and Triton X-100 was added to a final concentration of 0.5%. The nuclei were then pelleted by centrifugation at 1,500 × g for 5 min. The pellet was gently resuspended twice with nuclei resuspension buffer (10 mM Tris⋅HCl, pH 8.0, 0.25 M sucrose, 10 mM MgCl2, 5 mM β-mercaptoethanol, 1% Triton X-100, 0.1 mM PMSF, protease inhibitors) and centrifuged at 1,500 × g for 5 min. Then the nuclei were resuspended in ice-cold nuclei lysis buffer [200 mM (NH4)2SO4, 20% glycogen, 25 mM Hepes, pH 7.9, 5 mM DTT, 1 mM PMSF, protease inhibitors] and gently mixed for 2 h at 4 °C, releasing the nuclear proteins into the supernatant. The nuclear fraction was centrifuged at 17,950 × g for 10 min to remove debris. For the RNase treatment, RNase A (5 μg) was added to the nuclear fraction, and treated at 37 °C for 10 min before immunoprecipitation.
Proteomic Analysis.
Nuclear proteins were immunoprecipitated with indicated antibodies. Affinity purified protein complexes of each sample were separated on a 4–12% Bis-Tris gel (NuPAGE, NP0321BOX; Life Technologies), and the proteins bands (except the heavy and light chains of the antibody) were subjected to in-gel digestion as described previously (43). The digested peptides were analyzed on the LTQ Orbitrap Velos tandem mass spectrometer coupled to a Dionex Ultimate 3000 RLSCnano System (Thermo Scientific). Label-free quantification was performed with the MaxQuant software version 1.3.0 using the TAIR10 database. Peptide and protein FDRs were set to 1%. MS/MS identifications were transferred between LC-MS/MS runs with the “Match between runs” option in which the maximal retention time window was set to 2 min. The quantification is based on the extracted ion current. Immunoblotting analysis was performed using antibodies against SmD3b (Abclonal), Prp8, Brr2, MAC3A, MOS4, AtPRMT5, PEPC (AS09458), and H3 (ab1791).
Gel Filtration.
Twelve-day-old seedlings of Col plants were ground thoroughly using a pestle and mortar in liquid nitrogen, lysed with PBS buffer plus 0.1% Nonidet P-40, and centrifuged at 17,950 × g for 30 min twice at 4 °C. The supernatant was further clarified by filtration (0.22 μm). We loaded 500 μL cell extract onto a Superose 6 10/300 GL column (GE Healthcare) and equilibrated it with PBS buffer. We collected 0.5-mL fractions and precipitated the proteins with ethyl alcohol before SDS/PAGE and protein blot analysis.
Acknowledgments
We thank Dr. Haiyan Zheng (Rutgers University) for conducting the MS analysis, Dr. Caroline Dean for providing prp8-6 seeds, Dr. Marcelo J. Yanovsky for sharing the data analysis pipelines, and the Arabidopsis Biological Resource Center for providing SALK T-DNA insertion lines. This work was supported by National Natural Science Foundation of China Grants 31330020 and 31210103901 (to X.C.), 31200900 (to X.D.), and 31370770 (to C.L.), National Basic Research Program of China Grant 2011CB915401 (to X.C.), and the State Key Laboratory of Plant Genomics.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. GSE62611).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522458113/-/DCSupplemental.
References
- 1.Sun L, et al. Structural insights into protein arginine symmetric dimethylation by PRMT5. Proc Natl Acad Sci USA. 2011;108(51):20538–20543. doi: 10.1073/pnas.1106946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad A, Cao X. Plant PRMTs broaden the scope of arginine methylation. J Genet Genomics. 2012;39(5):195–208. doi: 10.1016/j.jgg.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Lu F, Cui X, Cao X. Histone methylation in higher plants. Annu Rev Plant Biol. 2010;61:395–420. doi: 10.1146/annurev.arplant.043008.091939. [DOI] [PubMed] [Google Scholar]
- 4.Bedford MT, Clarke SG. Protein arginine methylation in mammals: Who, what, and why. Mol Cell. 2009;33(1):1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang M, et al. Caenorhabditis elegans protein arginine methyltransferase PRMT-5 negatively regulates DNA damage-induced apoptosis. PLoS Genet. 2009;5(6):e1000514. doi: 10.1371/journal.pgen.1000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wrighton KH. Cell signalling: PRMT5 restricts ERK activity. Nat Rev Mol Cell Biol. 2011;12(11):689. doi: 10.1038/nrm3213. [DOI] [PubMed] [Google Scholar]
- 7.Ancelin K, et al. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol. 2006;8(6):623–630. doi: 10.1038/ncb1413. [DOI] [PubMed] [Google Scholar]
- 8.Tee WW, et al. Prmt5 is essential for early mouse development and acts in the cytoplasm to maintain ES cell pluripotency. Genes Dev. 2010;24(24):2772–2777. doi: 10.1101/gad.606110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pei Y, et al. Mutations in the Type II protein arginine methyltransferase AtPRMT5 result in pleiotropic developmental defects in Arabidopsis. Plant Physiol. 2007;144(4):1913–1923. doi: 10.1104/pp.107.099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez SE, et al. A methyl transferase links the circadian clock to the regulation of alternative splicing. Nature. 2010;468(7320):112–116. doi: 10.1038/nature09470. [DOI] [PubMed] [Google Scholar]
- 11.Deng X, et al. Arginine methylation mediated by the Arabidopsis homolog of PRMT5 is essential for proper pre-mRNA splicing. Proc Natl Acad Sci USA. 2010;107(44):19114–19119. doi: 10.1073/pnas.1009669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernando CE, Sanchez SE, Mancini E, Yanovsky MJ. Genome wide comparative analysis of the effects of PRMT5 and PRMT4/CARM1 arginine methyltransferases on the Arabidopsis thaliana transcriptome. BMC Genomics. 2015;16:192. doi: 10.1186/s12864-015-1399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, et al. Arabidopsis floral initiator SKB1 confers high salt tolerance by regulating transcription and pre-mRNA splicing through altering histone H4R3 and small nuclear ribonucleoprotein LSM4 methylation. Plant Cell. 2011;23(1):396–411. doi: 10.1105/tpc.110.081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green MR. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- 15.Wahl MC, Will CL, Lührmann R. The spliceosome: Design principles of a dynamic RNP machine. Cell. 2009;136(4):701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Brahms H, Meheus L, de Brabandere V, Fischer U, Lührmann R. Symmetrical dimethylation of arginine residues in spliceosomal Sm protein B/B′ and the Sm-like protein LSm4, and their interaction with the SMN protein. RNA. 2001;7(11):1531–1542. doi: 10.1017/s135583820101442x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friesen WJ, et al. The methylosome, a 20S complex containing JBP1 and pICln, produces dimethylarginine-modified Sm proteins. Mol Cell Biol. 2001;21(24):8289–8300. doi: 10.1128/MCB.21.24.8289-8300.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meister G, Fischer U. Assisted RNP assembly: SMN and PRMT5 complexes cooperate in the formation of spliceosomal UsnRNPs. EMBO J. 2002;21(21):5853–5863. doi: 10.1093/emboj/cdf585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neuenkirchen N, Chari A, Fischer U. Deciphering the assembly pathway of Sm-class U snRNPs. FEBS Lett. 2008;582(14):1997–2003. doi: 10.1016/j.febslet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Gao X, et al. Tudor staphylococcal nuclease (Tudor-SN) participates in small ribonucleoprotein (snRNP) assembly via interacting with symmetrically dimethylated Sm proteins. J Biol Chem. 2012;287(22):18130–18141. doi: 10.1074/jbc.M111.311852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chanarat S, Sträßer K. Splicing and beyond: The many faces of the Prp19 complex. Biochim Biophys Acta. 2013;1833(10):2126–2134. doi: 10.1016/j.bbamcr.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Matera AG, Wang Z. A day in the life of the spliceosome. Nat Rev Mol Cell Biol. 2014;15(2):108–121. doi: 10.1038/nrm3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou X, et al. A platform of high-density INDEL/CAPS markers for map-based cloning in Arabidopsis. Plant J. 2010;63(5):880–888. doi: 10.1111/j.1365-313X.2010.04277.x. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz BW, Yeung EC, Meinke DW. Disruption of morphogenesis and transformation of the suspensor in abnormal suspensor mutants of Arabidopsis. Development. 1994;120(11):3235–3245. doi: 10.1242/dev.120.11.3235. [DOI] [PubMed] [Google Scholar]
- 25.Marquardt S, et al. Functional consequences of splicing of the antisense transcript COOLAIR on FLC transcription. Mol Cell. 2014;54(1):156–165. doi: 10.1016/j.molcel.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lossky M, Anderson GJ, Jackson SP, Beggs J. Identification of a yeast snRNP protein and detection of snRNP-snRNP interactions. Cell. 1987;51(6):1019–1026. doi: 10.1016/0092-8674(87)90588-5. [DOI] [PubMed] [Google Scholar]
- 27.Grainger RJ, Beggs JD. Prp8 protein: At the heart of the spliceosome. RNA. 2005;11(5):533–557. doi: 10.1261/rna.2220705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki T, et al. An Rtf2 domain-containing protein influences pre-mRNA splicing and is essential for embryonic development in Arabidopsis thaliana. Genetics. 2015;200(2):523–535. doi: 10.1534/genetics.115.176438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan C, et al. Structure of a yeast spliceosome at 3.6-angstrom resolution. Science. 2015;349(6253):1182–1191. doi: 10.1126/science.aac7629. [DOI] [PubMed] [Google Scholar]
- 30.Galej WP, Oubridge C, Newman AJ, Nagai K. Crystal structure of Prp8 reveals active site cavity of the spliceosome. Nature. 2013;493(7434):638–643. doi: 10.1038/nature11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koncz C, Dejong F, Villacorta N, Szakonyi D, Koncz Z. The spliceosome-activating complex: Molecular mechanisms underlying the function of a pleiotropic regulator. Front Plant Sci. 2012;3:9. doi: 10.3389/fpls.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang BB, Brendel V. The ASRG database: Identification and survey of Arabidopsis thaliana genes involved in pre-mRNA splicing. Genome Biol. 2004;5(12):R102. doi: 10.1186/gb-2004-5-12-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bezzi M, et al. Regulation of constitutive and alternative splicing by PRMT5 reveals a role for Mdm4 pre-mRNA in sensing defects in the spliceosomal machinery. Genes Dev. 2013;27(17):1903–1916. doi: 10.1101/gad.219899.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chari A, et al. An assembly chaperone collaborates with the SMN complex to generate spliceosomal SnRNPs. Cell. 2008;135(3):497–509. doi: 10.1016/j.cell.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Abovich N, Rosbash M. A biochemical function for the Sm complex. Mol Cell. 2001;7(2):319–329. doi: 10.1016/s1097-2765(01)00180-0. [DOI] [PubMed] [Google Scholar]
- 36.Weber G, Trowitzsch S, Kastner B, Lührmann R, Wahl MC. Functional organization of the Sm core in the crystal structure of human U1 snRNP. EMBO J. 2010;29(24):4172–4184. doi: 10.1038/emboj.2010.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Zhang J, Li K, Zhao W, Cui Q. SpliceDisease database: Linking RNA splicing and disease. Nucleic Acids Res. 2012;40(Database issue):D1055–D1059. doi: 10.1093/nar/gkr1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valadkhan S. A snRNP’s ordered path to maturity. Genes Dev. 2011;25(15):1563–1567. doi: 10.1101/gad.17311211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, et al. SMN deficiency causes tissue-specific perturbations in the repertoire of snRNAs and widespread defects in splicing. Cell. 2008;133(4):585–600. doi: 10.1016/j.cell.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratovitski T, Arbez N, Stewart JC, Chighladze E, Ross CA. PRMT5- mediated symmetric arginine dimethylation is attenuated by mutant huntingtin and is impaired in Huntington’s disease (HD) Cell Cycle. 2015;14(11):1716–1729. doi: 10.1080/15384101.2015.1033595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stopa N, Krebs JE, Shechter D. The PRMT5 arginine methyltransferase: Many roles in development, cancer and beyond. Cell Mol Life Sci. 2015;72(11):2041–2059. doi: 10.1007/s00018-015-1847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levin JZ, et al. Comprehensive comparative analysis of strand-specific RNA sequencing methods. Nat Methods. 2010;7(9):709–715. doi: 10.1038/nmeth.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rappsilber J, Mann M. 2007. Analysis of the topology of protein complexes using cross-linking and mass spectrometry. CSH Protoc 2007(2):4594.
- 44.Trapnell C, Pachter L, Salzberg SL. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]