Abstract
Hyperglycemia is the main feature for the diagnosis of diabetes mellitus (DM). Some studies have demonstrated the relationship between DM and dysfunction on neurotransmission systems, such as the purinergic system. In this study, we evaluated the extracellular nucleotide hydrolysis and adenosine deamination activities from encephalic membranes of hyperglycemic zebrafish. A significant decrease in ATP, ADP, and AMP hydrolyses was observed at 111-mM glucose-treated group, which returned to normal levels after 7 days of glucose withdrawal. A significant increase in ecto-adenosine deaminase activity was observed in 111-mM glucose group, which remain elevated after 7 days of glucose withdrawal. The soluble-adenosine deaminase activity was significantly increased just after 7 days of glucose withdrawal. We also evaluated the gene expressions of ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases), ecto-5′-nucleotidase, ADA, and adenosine receptors from encephala of adult zebrafish. The entpd 2a.1, entpd 2a.2, entpd 3, and entpd 8 mRNA levels from encephala of adult zebrafish were decreased in 111-mM glucose-treated and glucose withdrawal groups. The gene expressions of adenosine receptors (adora1, adora2aa, adora2ab, and adora2b) were decreased in 111-mM glucose-treated and glucose withdrawal groups. The gene expression of ADA (ada 2a.1) was decreased in glucose withdrawal group. Maltodextrin, used as a control, did not affect the expression of adenosine receptors, ADA and E-NTPDases 2, 3, and 8, while the expression of ecto-5′-nucleotidase was slightly increased and the E-NTPDases 1 decreased. These findings demonstrated that hyperglycemia might affect the ecto-nucleotidase and adenosine deaminase activities and gene expression in zebrafish, probably through a mechanism involving the osmotic effect, suggesting that the modifications caused on purinergic system may also contribute to the diabetes-induced progressive cognitive impairment.
Keywords: Adenosine deaminase, Hyperglycemia, E-NTPDase, Ecto-5′-nucleotidase, Zebrafish
Introduction
The incidence of diabetes mellitus (DM) is increasing rapidly worldwide, reaching approximately 382 million people [1]. According to International Diabetes Federation (2013) [1], there is an alarming estimate that this number will raise to 582 million people by 2035. DM is a chronic disease, characterized by hyperglycemia, resulting from defects in insulin secretion, insulin action, or both [2, 3]. The effects of this disease are being strongly associated with neurophysiological, neurochemical, and behavioral modifications in brain of patients that are also observed in animal model of DM [4–8].
Several studies have shown that dysfunction of synaptic plasticity in diabetic subjects may be related to the inefficiency of neurotransmitter release [9, 10]. One possible strategy to correct the changes in synaptic efficiencies caused by DM can be through of analysis of the presynaptic neuromodulation systems, as purinergic system [11, 12]. Adenine nucleotides represent an important class of extracellular molecules involved in modulation of signaling pathways that are essential to the normal functioning of the central nervous system (CNS) [13].
Purine nucleotides, ATP, ADP and AMP, and the nucleoside, adenosine, act as extracellular messengers, and their levels are controlled by a cascade of ecto-enzymes, including ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases), ecto-nucleotide pyrophosphohydrolases/phosphodiesterases (ecto-NPPs), ecto-alkaline phosphatases (APs), and ecto-5′nucleotidase [14, 15]. The hydrolysis of ATP to AMP is catalyzed mainly by a family of E-NTPDases. E-NTPDases-targeted therapies have been conducted in an experimental stage based on the increasing of scientific reports about the role of these enzymes on the control of extracellular levels of ATP in pathological conditions, including diabetes [16, 17]. The nucleotide AMP is hydrolyzed to adenosine, an important neuromodulator, by the action of an ecto-5′-nucleotidase (CD73 and EC 3.1.3.5) [18–20]. Extracellular concentrations of adenosine are also regulated by the interplay of equilibrative and concentrative nucleoside transporters with enzymes of adenosine metabolism, as adenosine deaminase [21].
Some authors have suggested that diabetic conditions may alter the purinergic tonus by inducing adaptive changes of the sensibility of adenosine receptors in the brain [22, 23]. Also, Schmatz et al. (2009) [24] demonstrated that ATP, ADP, and AMP hydrolyses were increased in synaptosomes from STZ-induced diabetic rats, and also, Lunkes et al. (2004) [25] demonstrated that diabetic rats show an increase in NTPDase and 5′-nucleotidase activities.
Considering the importance to control nucleotides and nucleosides levels to the properly neurotransmission in CNS functioning and that cognitive impairment is a long-term consequence of diabetes mellitus, the aim of the present study was to evaluate the activity and expression of these enzymes and adenosine receptors in adult zebrafish submitted to a hyperglycemia model.
Material and methods
Animals
Adult zebrafish (Danio rerio) wild type (Tübingen background; 3–5 cm) of both sexes were purchased from commercial suppliers and acclimated for at least 14 days in the experimental room. Animals were housed in groups of 15 fish in 5-L thermostated (28 ± 2 °C) tanks with water of reverse osmosis reconstituted with 0.4-ppt marine salt (Cristalsea™, Marinemix), kept under constant chemical, biological, and mechanical water filtration and aeration (7.20 mg O2/L). Fish were maintained under a 14–10-h day/night photoperiod cycle, fed three times a day with commercial flakes (TetraMin™, NC, USA), and supplemented with live brine shrimp. All fish used in these experiments were randomly chosen from different clusters. The animals were submitted to hypothermia by exposure to ice water flocked followed by decapitation as end point. All protocols were approved by the Institutional Animal Care Committee (12/00310–CEUA PUCRS) and followed Brazilian legislation, the guidelines Federal Council of Veterinary Medicine (CFMV), and the Canadian Council for Animal Care (CCAC) “Guide on the Care and Use of Fish in Research, Teaching, and Testing.”
Drugs
Glucose was purchased from Nuclear™ (São Paulo, Brazil). Trizma base, EDTA, ethylene glycol tetraacetic acid (EGTA), sodium citrate, Coomassie blue, bovine serum albumin, malachite green, ammonium molybdate, polyvinyl alcohol, nucleotides, calcium, and magnesium chloride were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Magnesium chloride, phenol, and sodium nitroprusside were purchased from Merck (Darmstadt, Germany). All other reagents used were from analytical grade.
Hyperglycemia model
Groups of 15 adult animals were placed in 5-L aquariums containing 111 mM glucose solution, which were diluted in water and maintained during 14 days at room temperature as described in previous section according to Capiotti et al. (2014a) [8]. The solutions of glucose were exchanged three times per week to avoid contamination with opportunistic microorganisms. Control group (no glucose added) and dextrin group (4 % maltodextrin dissolved in water) animals were maintained in 5-L aquariums for the same period and conditions as the glucose-treated groups. The glucose-treated and dextrin-treated animals were placed in its respective treatments and monitored for signs of stress as such as difficulty for swimming or excessive gill movement [26].
Withdrawal of glucose
In order to verify if the effects caused by 111-mM glucose solution treatment would be persistent, a group of 15 fish was kept in glucose-free water by additional 7 days (washout group, no glucose added) [8]. During this period, the animals were maintained at the same conditions of welfare described in previous section. Control group animals were maintained in aquariums with water for the same period and were handled as washout group.
Preparation of soluble and membrane fractions from zebrafish encephalon
The animals were cryoanesthetized and euthanized by decapitation, and encephalon was dissected [27]. Encephalic samples were prepared as previously described, and each independent experiment was performed using biological preparations consisting of a “pool” of five encephala [28–30]. Following the dissection, the whole zebrafish encephalon was homogenized in a glass-Teflon homogenizer according to the protocol for each enzyme assay. For E-NTPDase and ecto-5′-nucleotidase assays, zebrafish encephalon was homogenized in 60 vol (v/w) of chilled Tris–citrate buffer (50 mM Tris–citrate, 2 mM EDTA, 2 mM EGTA, and pH 7.4). For ecto-ADA experiments, encephalon was homogenized in 20 vol (v/w) of chilled phosphate-buffered saline (PBS), with 2 mM EDTA, 2 mM EGTA, and pH 7.4. The encephalic membranes were obtained as previously described [31]. The homogenates were centrifuged at 800×g for 10 min, and the supernatant fraction was subsequently centrifuged for 25 min at 40.000×g. The resultant supernatant and the pellet obtained corresponded to the soluble and membrane fractions, respectively. For soluble ADA activity experiments, the supernatant was collected and kept on ice for enzyme assays. The pellets of membrane preparations were frozen in liquid nitrogen, thawed, resuspended in the respective buffers (to ensure the lysis of the encephalic vesicle membranes), and centrifuged for 20 min at 40.000×g. The final pellets were resuspended and used for assays to ecto-nucleotidases and ecto-ADA. All samples were maintained at 2–4 °C throughout preparations. Protein was measured by the Coomassie blue method [32] using bovine serum albumin as a standard.
Ecto-nucleotidase assays
E-NTPDase and ecto-5′-nucleotidase assays were performed following previous described methods [28, 30]. Zebrafish encephalic membranes (3–5 μg protein) were added to the reaction medium containing 50 mM Tris–HCl (pH 8.0) and 5 mM CaCl2 (for the E-NTPDase activity) or 50 mM Tris–HCl (pH 7.2) and 5 mM MgCl2 (for the ecto-5′-nucleotidase activity) at a total volume of 200 μL. The samples were preincubated for 10 min at 37 °C, and the reaction was started by the addition of the substrate (ATP, ADP, or AMP) to a final concentration of 1 mM. The reaction was terminated after 30 min by the addition of 200 μL trichloroacetic acid at a final concentration of 5 %, and then, the samples were chilled on ice for 10 min. The colorimetric reagent composed by 2.3 % polyvinyl alcohol, 5.7 % ammonium molybdate, and 0.08 % malachite green was added (1 mL) in order to determine the inorganic phosphate released (Pi) [33]. The quantification of Pi released was determined spectrophotometrically at 630 nm, and the specific activity was expressed as nmol of Pi min−1 mg−1 of protein. Controls with the addition of the enzyme preparation after the addition of trichloroacetic acid were used to correct non-enzymatic hydrolysis of the substrates. All enzyme reactions were performed with triplicate samples. At least seven independent experiments were performed.
Adenosine deaminase assays
Ecto- and soluble-ADA activities were determined as previously described [29]. The encephalic fractions (5–10 μg protein) were added to the reaction mixture containing 50 mM sodium phosphate buffer (pH 7.0) and 50 mM sodium acetate buffer (pH 5.0) for soluble and membrane fractions, respectively. The samples were preincubated for 10 min at 37 °C, and the reaction was started by the addition of substrate (adenosine) to a final concentration of 1.5 mM, in a final volume of 200 μL. The reaction was stopped by the addition of 500-μL phenol-nitroprusside reagent (50.4 mg of phenol and 0.4 mg of sodium nitroprusside/ml) after 75 min (soluble fraction) and 120 min (membrane fraction). ADA activity was determined spectrophotometrically by measuring the ammonia produced over a fixed time interval using a Berthelot reaction according to [34]. The reaction mixtures were mixed to 500 μL of alkaline-hypochlorite reagent (sodium hypochlorite to 0.125 % available chlorine, in 0.6 M NaOH) and vortexed. Samples were incubated at 37 °C for 15 min, and the colorimetric assay was carried out at 635 nm. The ADA activity was expressed as nmol of NH3 min−1 mg−1 of protein. Controls with the addition of the enzyme preparation after mixing with phenol-nitroprusside reagent were used to correct the substrates’ non-enzymatic hydrolysis. All enzyme reactions were performed with triplicate samples. At least five independent experiments were performed.
Gene expression analysis by quantitative real-time reverse transcription PCR
The gene expression of ADA subfamilies (ada1, ada2.1, and ada2.2) including an alternative splicing isoform (adaasi) and, an adenosine deaminase-like related gene (adal), adenosine receptor subtypes (adora1, adora2aa, adora2ab, and adora2b), ecto-nucleotidases (entpd1, entpd2a.1, entpd2a.2, entpd2-like, entpd3, and entpd8), and ecto-5′-nucleotidase (nt5e) were determined. Total RNA was isolated with TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. The total RNA was quantified by spectrophotometry, and the complementary DNA (cDNA) was synthesized with ImProm-II™ Reverse Transcription System (Promega, Madison, WI, USA) from 1 μg of total RNA, following the manufacturer’s instructions. Quantitative PCR was performed using SYBR® Green I (Invitrogen) to detect double-strand cDNA synthesis. Reactions were done in a volume of 25 μL using 12.5 μL of diluted cDNA, containing SYBR® Green I 0.2 times diluted (Invitrogen), 100 μM dNTP, 1× PCR buffer, 3 mM MgCl2, 0.25 U Platinum® Taq DNA Polymerase (Invitrogen), and 200 nM of each reverse and forward primers (Table 1) [35–38]. The PCR cycling conditions were an initial polymerase activation step for 5 min at 95 °C, 40 cycles of 15 s at 95 °C for denaturation, 35 s at 60 °C for annealing, and 15 s at 72 °C for elongation. At the end of cycling protocol, a melting-curve analysis was included and fluorescence measured from 60 to 99 °C and showed in all cases one single peak. ef1α and rpl13α were used as reference genes for normalization. Relative expression levels were determined with 7500 and 7500 Fast Real-Time PCR Systems Software v.2.0.6 (Applied Biosystems, CA, USA). The efficiency per sample was calculated using LinRegPCR version 2012.3 software (http://LinRegPCR.nl). Relative messenger RNA (mRNA) expression levels were determined using the 2−∆∆CT method [39, 40].
Table 1.
Primer sequences for RT-qPCR experiments included in the study
| Gene | Primer sequences (5′-3′) | Accession number (mRNA) | Amplicon size (bp) |
|---|---|---|---|
| rpl13α a | F-TCTGGAGGACTGTAAGAGGTATGC and R-AGACGCACAATCTTGAGAGCAG | NM_212784 | 147 |
| ef1α a | F-CTGGAGGCCAGCTCAAACAT and R-ATCAAGAAGAGTAGTACCGCTAGCATTAC | NSDART00000023156 | 86 |
| entpd1 b | F-TTATGGCCTACATTTATTTCCGTCG and R-GATTCTTTGAAATGTAAAACCGCTTG | BC078240.1 | 176 |
| entpd2a.1 b | F-TTAAATCCAATGCTATATGCCGGTG and R-TCTGTGATGGATGTGTCGGACAAAGG | BC078419.1 | 103 |
| entpd2a.2 b | F-AAAGTTGAAGACACCTCTGTCGGCTG and R-CCATTCTTTTGGTAGCTTCGCAAC | XM_682630.2 | 188 |
| entpd2-like b | F-AGGCGTCTGTTGGCTGGGCTC and R-GAAACATCAAACCAGTCCATGCTGC | XM_692508.3 | 117 |
| entpd3 b | F-GCTACAATACCTCCATACCTGCAGAGG and R-GATACTCCTGACCAAGGCTTTGCAC | EF446129.1 | 146 |
| entpd8 b | F-GTTGCAGATACAGATATTGGTTGGACG and R-GTAGAGTGAGGAAGAGGGCAAATGC | NM_001002379.2 | 154 |
| nt5e b | F-TGGACGGAGGAGACGGATTCACC and R-GGAGCTGCTGAACTGGAAGCGTC | BC055243.1 | 149 |
| ada1 c | F-GCACAGTGAATGAGCCGGCCAC and R-AATGAGGACTGTATCTGGCTTCAACG | BC076532.1 | 168 |
| ada2.1 c | F-TTCAACACCACACGTATCGGGCAC and R-ATCAGCACTGCAGCCGGATGATC | AF384217.1 | 161 |
| ada2.2 c | F-TTGCAATTGTTCATCATCCCGTAGC and R-TCCCGAATAAACTGGGATCATCG | XM_682627.1 | 186 |
| adaasi c | F-CTTTGTGGTACTTCAAGGACGCTTTG and R-TTGTAGCAGATAAAAGAAGCGAGACG | AF384217.1 | 121 |
| adal c | F-CTCTAATGTGAAAGGTCAAACCGTGC and R-AAGACGCCCTTATCATCCGTGC | NM_001033744.1 | 108 |
| adora1 d | F-GTTCCTCATTTACATTGCCATTCTGC and R-TGGTTGTTATCCAGTCTCTCGCTCG | NM_001128584.1 | 180 |
| adora2aa d | F-GCGAACTGTACGCCGAGCAGAG and R-TTATTCCCAGTGAGCGGCGACTC | NM_001039815.1 | 178 |
| adora2ab2 d | F-GGATTGGGTCATGTACCTGGCCATC and R-GCTGTTTCCAATGGCCAGCCTG | NM_001040036.1 | 160 |
| adora2b d | F-GTTTGTTCGCTCTCTGTTGGCTGC and R-CTAAAAGTGACTCTGAACTCCCGAATG | NM_001039813.2 | 178 |
aAccording to Tang et al. (2007)
bAccording to Capiotti et al. (2013)
cAccording to Leite et al. (2013)
dAccording to design by authors
Statistical analysis
The data are shown as mean ± SEM of at least seven (ecto-nucleotidase assays), five (adenosine deaminase assays), and four (molecular analysis) independent experiments. Data were analyzed by one-way ANOVA followed by Tukey post hoc test or Student’s t test when appropriated. p < 0.05 was considered as significant.
Results
Blood glucose levels and mortality
The blood glucose levels of animals were already published by Capiotti et al. (2014b) [41]. The profile of blood glucose levels from the experimental groups showed an increase of 150 % in the 111-mM glucose-treated animals in relation to control group (control group 3.524 ± 0.2370 mM and glucose-treated group 8.83 ± 0.663 mM; p < 0.0001), while after 7 days of glucose withdrawal, the animals kept 50 % of increase of blood glucose levels in relation to the specific control (p = 0.02). The dextrin group exhibited a slight decrease on blood glucose level in relation to control group (2.25 ± 0.1237 mM; p < 0.01). Only the 111-mM glucose-treated groups exhibited mortality during the period of treatment (20 %).
Nucleotide hydrolysis from encephalic membrane of zebrafish after hyperglycemia
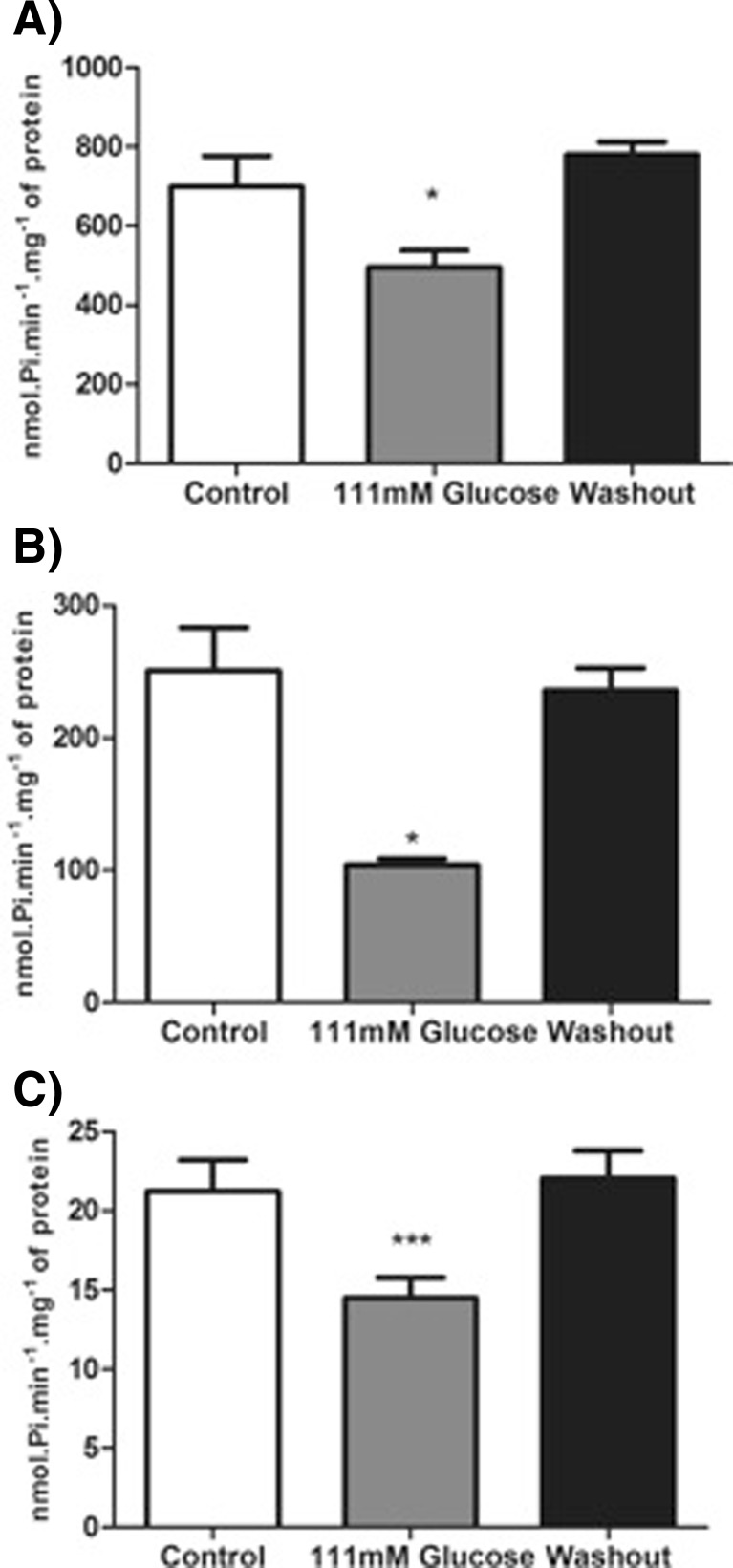
We evaluated the influence of hyperglycemia on ATP, ADP, and AMP hydrolysis activities from encephalic membrane of adult zebrafish. Figure 1a–c shows that adult zebrafish exposed to 111 mM glucose significantly decreased the ATP (29 %) [F(2; 28) = 7.212; p = 0.003], ADP (58 %) [F(2; 24) = 11.59; p = 0.0003] and AMP (32 %) [F(2; 27) = 4.991; p = 0.0143] hydrolysis when compared to control groups. After 7 days of glucose withdrawal, the nucleotide hydrolysis returned to levels similar to control group (Fig. 1a–c).
Fig. 1.
Effect of hyperglycemia model on ATP (a), ADP (b), and AMP (c) hydrolyses in zebrafish brain membranes. Bars represent the mean ± SEM of at least seven independent experiments performed in triplicate. The asterisk represents a significant difference from control group (one-way ANOVA, followed by Tukey test as post hoc, P ≤ 0.05). The specific enzyme activity is reported as nmol of Pi min−1 mg−1 of protein
Nucleoside hydrolysis from encephalic membrane of zebrafish after hyperglycemia
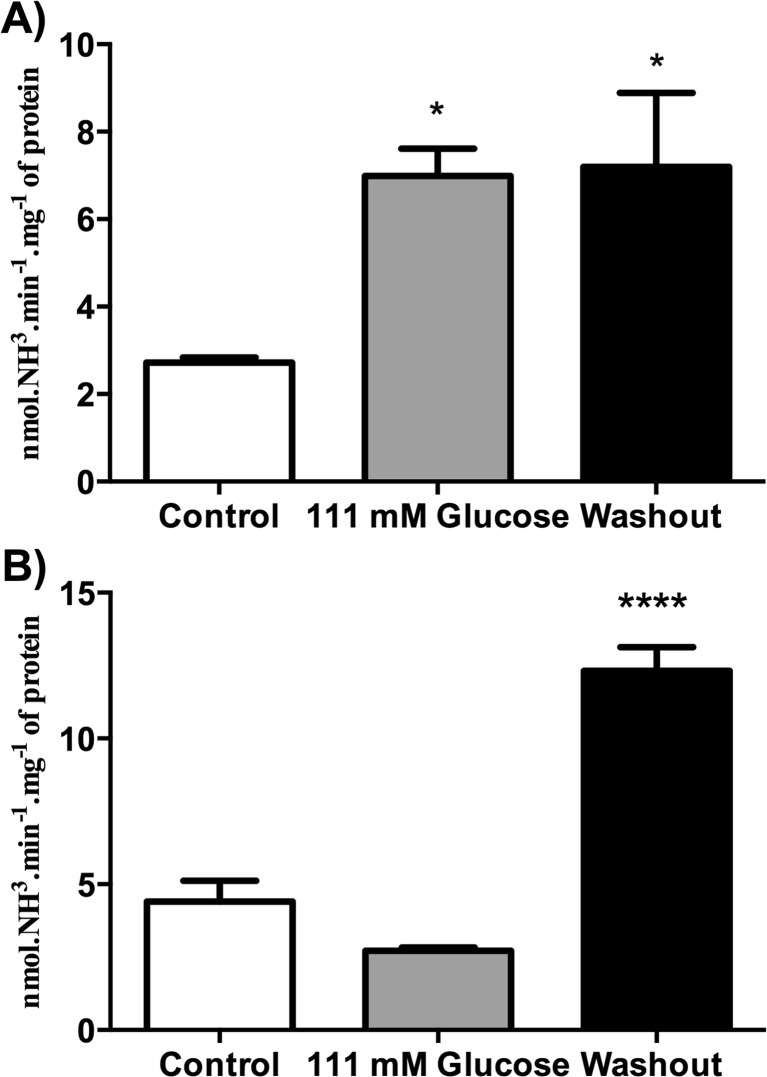
After verifying that hyperglycemia modifies the ATP, ADP, and AMP hydrolyses from encephala of zebrafish, we observed whether the hyperglycemia was able to promote changes on ecto-ADA and soluble-ADA activities from encephalic membrane of adult zebrafish. Our results demonstrated that ecto-ADA activity was increased (61 %) in 111-mM glucose-treated animals (Fig. 2a) [F(2; 17) = 5.594; p = 0.0136], when compared to control group. After 7 days of glucose withdrawal, the ecto-ADA activity remained increased (62 %) [F(2; 17) = 5.594; p = 0.0136] (Fig. 2a). We also evaluated soluble-ADA activity. Our results demonstrated that glucose-treated animals had their soluble-ADA activity unaltered, while after 7 days of glucose withdrawal, the soluble-ADA activity was significantly increased (38 %) [F(2; 19) = 53.87; p < 0.0001], when compared to control group (Fig. 2b).
Fig. 2.
Effect of hyperglycemia model on membrane-bound (a) and soluble (b) ADA activity from zebrafish brain. Bars represent the mean ± SEM of at least five independent experiments performed in triplicate. The asterisk represents a significant difference from control group (one-way ANOVA, followed by Tukey test as post hoc, P ≤ 0.05). The specific enzyme activity is reported as nmol of NH3 min−1 mg−1 of protein
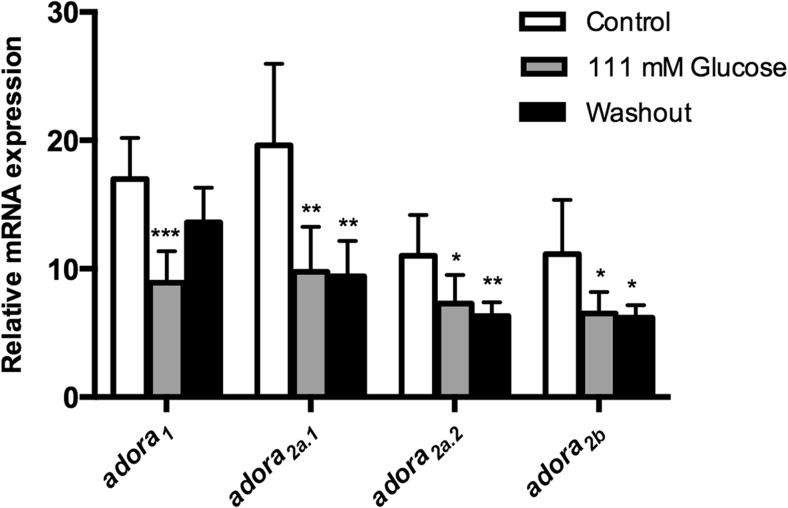
Effect of hyperglycemia on E-NTPDases, nt5e, ADA, and Adora gene expressions from encephala of adult zebrafish
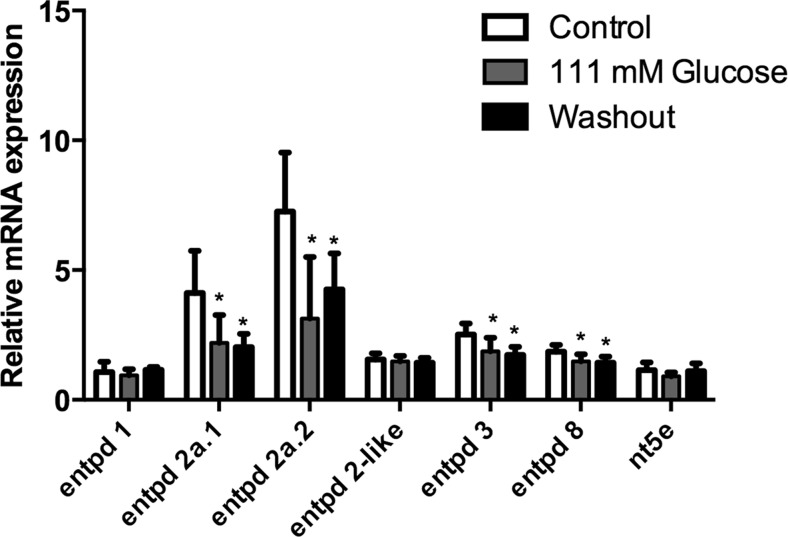
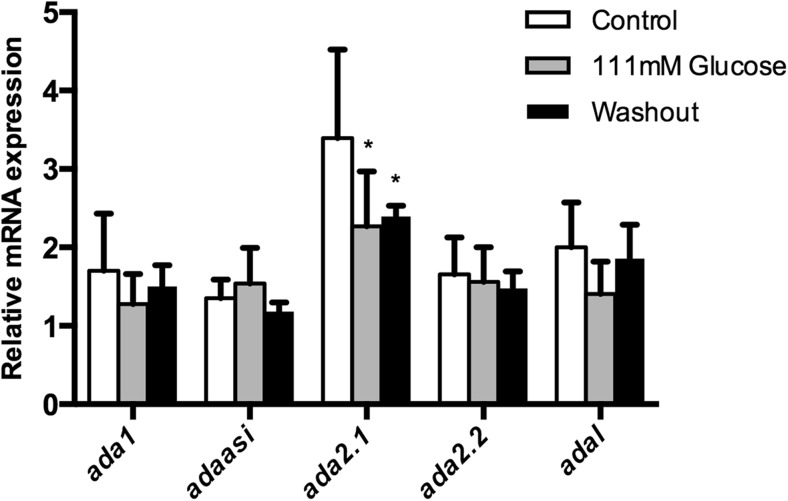
Quantitative reverse transcription (RT)-PCR experiments were performed to verify whether the hyperglycemia could alter the expression of ecto-nucleotidases, ADA-related genes (ada1, ada2.1, ada2.2, adaasi, and adal) and adenosine receptors (adora1, adora2aa, adora2ab, and adora2b). The osmotic control, dextrin-treated animals, had also these selected gene expressions evaluated. Our results showed a decrease of the entpd 2a.1, entpd 2a.2, entpd 3, and entpd 8 gene expressions in both groups analyzed, 111-mM glucose and washout groups. Nevertheless, the entpd 1, entpd 2-like, and nt5e gene expressions did not change (Fig. 3). Regarding ADA-related genes expression, we observed a decrease of the ada2.1 gene expression, while no changes were observed in the other isoforms of ada (Fig. 4). Furthermore, the evaluation of adenosine receptors in encephala of zebrafish demonstrated that adora1, adora2a.1, adora2a.2, and adora2b were decreased in 111-mM glucose group. After 7 days of glucose withdrawal, only adora1 adenosine receptor returned to levels similar to control group (Fig. 5). Dextrin-treated animals had no effects on those genes affected by glucose in the glucose-treated animals (ada isoforms; adora isoforms; and entpd 2a.1, entpd 2a.2, entpd 3, and entpd 8), while nt5e expression was slightly increased (12 % in relation to control group; p = 0.048) and entpd1 decreased (30 % in relation to control group; p = 0.016).
Fig. 3.
Effect of hyperglycemia on entpd1, entpd2a.1, entpd 2a.2, entpd2-like, entpd3, entpd8, and nt5e gene expressions in 111 mM glucose and glucose washout. Data are expressed as mean ± SEM of four independent experiments performed in quadruplicate
Fig. 4.
Effect of hyperglycemia on ada gene expression pattern in 111 mM glucose and glucose washout group. Data are expressed as mean ± SEM of four independent experiments performed in quadruplicate
Fig. 5.
Effect of hyperglycemia on adora gene expression pattern in 111 mM glucose and glucose washout group. Data are expressed as mean ± SEM of four independent experiments performed in quadruplicate
Discussion
The purine-based therapy has long been encouraged by purine receptors agonism and antagonism, although the enzyme activity control has recently been receiving attention, especially the NTPDases [41]. In the present study, we evaluated the E-NTPDases, ecto-5′-nucleotidase, and ADA activities from encephala in a model of hyperglycemia using zebrafish. We also evaluated the same biochemical parameters after 7 days of glucose withdrawal. After, we performed the evaluation of the E-NTPDases, ada-related, and adora gene expressions from encephala of adult zebrafish. The blood glucose levels from glucose-treated and washout animals demonstrated the same profile already developed in previous works [8, 42].
Nucleotides are important extracellular messengers in both physiological and pathological conditions [11]. After its release in the synaptic cleft, ATP can be catabolized to ADP, AMP, and adenosine. E-NTPDases and ecto-5′-nucleotidase action regulate the concentrations of ATP, ADP, and AMP by increasing/decreasing their hydrolysis with a consequent increase/decrease in adenosine levels [15]. Extracellular concentrations of adenosine might be regulated by adenosine uptake via bi-directional transporters [43]. Adenosine uptake followed by its phosphorylation to AMP by adenosine kinase or deamination to inosine by ecto-adenosine deaminase are two possible mechanisms able to promote the inactivation of adenosine signaling [44–46].
Previous studies demonstrated that purinergic signaling was altered in rats submitted to diabetes mellitus. Schmatz et al. (2009a) [16] showed that extracellular nucleotide hydrolysis was increased in synaptosomes from the cerebral cortex of rats submitted to streptozotocin-induced diabetes. Lunkes et al. (2004) and Miron et al. (2007) [25, 47] also demonstrated an increase on the activities of the enzymes E-NTPDase and ecto-5′-nucleotidase in platelets and synaptosomes from the cerebral cortex of rats with alloxan-induced diabetes. In contrast to these results, our findings showed that hyperglycemia reduces significantly ATP, ADP, and AMP hydrolyses promoted by E-NTPDases and ecto-5′-nucleotidase in zebrafish encephalic membranes. Likewise, Duarte et al. (2007) [48] demonstrated that ATP catabolism was decreased in diabetic rats, possibly due to decreased activity of ecto-nucleotidases.
The effect observed in ATP hydrolysis in encephalic membranes of zebrafish suggests that specific extracellular nucleotide-hydrolyzing enzymes may be involved in the effects observed. While we do not have specific information about the catalytic properties of ecto-nucleotidase isoforms expressed in zebrafish, assuming data from mammals and amphibian, the decrease in ATP hydrolysis seems here could involve NTPDase 2, due to hydrolysis ratio 30:1 (ATP:ADP) that contributes more effectively to ATP hydrolysis when compared with other NTPDases [15, 49, 50]. In fact, the expression of E-NTPDase 2 isoforms (entpd2a.1 and entpd2a) was significantly decreased in glucose-treated zebrafish. In this same way, the decrease in ADP hydrolysis could indicate a response to enptd3- and entpd8-reduced expressions in glucose-treated animals. Interestingly, the gene expression levels of nt5e have not changed, suggesting that the decrease of AMP hydrolysis observed is probably not directly related to nt5e gene expression control but possible by stoichiometric mechanism, since ADP and ATP are strong inhibitors of ecto-5′-nuleotidase [51]. Nucleotide hydrolyses were fully recovered after 7 days of glucose withdrawal, probably in function of the partial recovery of glycemic control.
ADA is located both in the cytosol and in the cell membrane, and the regulation of brain adenosine levels might be promoted by distinct ada members (ada1, ada2, and adal) [29, 52, 53]. Evidences suggest that ecto-ADA (ADA1) can be anchored to the cell membrane by the A1R adenosine receptor as well, in order to downregulate the signal produced by adenosine [54]. Our results demonstrated that ecto-ADA activity was significantly increased in 111-mM glucose group, suggesting a decrease in adenosine levels in synaptic cleft. There is some consensus that a decrease of adenosine levels could be related with cognitive impairment observed in diabetic rats [8, 55, 56], which also could be suggested occurring in the zebrafish hyperglycemic model.
At least in human plasma, the soluble ADA has a high KM value for the reaction of deamination, suggesting that this enzyme requires a high adenosine concentration for its activity [57]. Previous studies have reported that the regulation of adenosine levels in intracellular and extracellular fractions in the zebrafish encephala might be promoted by distinct ada members (ada1, ada2, and adal), which have diverse gene expression patterns and activity properties [25, 53] and might contribute for the regulation of adenosine levels in different manners.
The results demonstrated that the relative gene expression levels of ada (ada2.1) were significantly decreased in 111 mM glucose, suggesting that the increase of adenosine hydrolysis promoted by soluble ADA is probably not directly related to a reduced ada gene expression. The transcription machinery is continuously controlled by a complex signaling system, creating a set of signals able to adjust gene expression profile of the cell. The signal transduction can be exerted by proteins, products of enzyme reactions, or even toxins able to regulate transcription factors [58]. The phenomenon known as negative feedback loop [58, 59], which is situated at the interface of genetic and metabolic networks, could explain, at least in part, the simultaneous increase of adenosine hydrolysis and the decrease of ada2.1 transcripts in zebrafish encephala.
Regarding the relative gene expression of adenosine receptors, we verified a decrease in mRNA transcripts of adora1, adora2aa, adora2ab, and adora2b receptors in encephala of hyperglycemic zebrafish. Previous studies reported a change of adenosine sensitivity in the hippocampus of diabetic rats [22, 23, 60], suggesting that diabetic conditions may also induce an adaptive change of the density of adenosine receptors in the brain. Faulhaber-Walter et al. (2011) [61] demonstrated that the decrease of adora1 adenosine receptor signaling is strongly linked to impaired glucose tolerance and insulin resistance.
Osmotic insults and oxidative stress are important issues in the pathophysiology of diabetes [62] and probably are working together with other glucose effects, like glycation, to promote effects on protein or gene expression control. In fact, dextrin-treated zebrafish had no effect on adora, ada, and E-NTPD isoforms affected by glucose treatment, indicating that the osmotic imbalance promoted by glucose can operate in the effects seen here.
Previous studies from our group demonstrated that hyperglycemic zebrafish has impaired memory, probably by the incapacity to keep proper cholinergic signaling as a result of increased acetylcholinesterase activity [41]. As cholinergic system is closely related to purinergic system, based on the co-release of acetylcholine and ATP, and in the adenosine control of acetylcholine release [63], the alteration on nucleotide and nucleoside metabolism detected after the hyperglycemic status could contribute to the neurophysiological disturbance that could implicate in the development of cognitive impairment.
Conclusions
The present study provides evidences that the purinergic signaling system is compromised in the central nervous system of zebrafish treated with 111 mM glucose. Molecular changes are observed even after 7 days glucose free. The modifications could lead to alterations in the modulation of neurotransmission, which may also contribute to the diabetes-induced progressive cognitive impairment.
Acknowledgments
This work was supported by DECIT/SCTIE-MS through Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) (proclamation 10/0036-5, conv. no. 700545/2008–PRONEX). K.M.C. is recipient of FAPERGS/Capes fellowship.
Compliance with ethical standards
Conflict of interest
The authors declare that not potential conflict of interests is relevant to this article.
Disclosure statement
The authors declare that no competing financial interests exist.
Contributor Information
Katiucia Marques Capiotti, Email: katucapiotti@gmail.com.
Anna Maria Siebel, Email: annasiebel.siebel@gmail.com.
Luiza Wilges Kist, Email: lwkist@gmail.com.
Maurício Reis Bogo, Email: mbogo@pucrs.br.
Carla Denise Bonan, Email: cbonan@pucrs.br.
Rosane Souza Da Silva, Phone: + 55 51 3353 4158, Email: rosane.silva@pucrs.br.
References
- 1.Aguiree F, Brown A, Cho N, Dahlquist G (2013) IDF diabetes atlas. International Diabetes Federation
- 2.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Harris MI. Classification and diagnostic criteria for diabetes mellitus and other categories of glucose intolerance. Prim Care. 1988;15:205–225. [PubMed] [Google Scholar]
- 4.Motta M, Sorace S, Restuccia S, et al. Cognitive impairment in the elderly diabetics. Arch Gerontol Geriatr. 1996;22(Suppl 1):43–46. doi: 10.1016/0167-4943(96)86911-1. [DOI] [PubMed] [Google Scholar]
- 5.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Launer LJ, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10:969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Zhong C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol. 2013;108:21–43. doi: 10.1016/j.pneurobio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Capiotti KM, Antonioli R, Kist LW, et al. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp Biochem Physiol B Biochem Mol Biol. 2014;171:58–65. doi: 10.1016/j.cbpb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Guyot LL, Diaz FG, O’Regan MH, et al. The effect of streptozotocin-induced diabetes on the release of excitotoxic and other amino acids from the ischemic rat cerebral cortex. Neurosurgery. 2001;48:385–391. doi: 10.1097/00006123-200102000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Yamato T, Misumi Y, Yamasaki S, et al. Diabetes mellitus decreases hippocampal release of neurotransmitters: an in vivo microdialysis study of awake, freely moving rats. Diabetes Nutr Metab. 2004;17:128–136. [PubMed] [Google Scholar]
- 11.Burnstock G, Novak I. Purinergic signalling and diabetes. Purinergic Signal. 2013;9:307–324. doi: 10.1007/s11302-013-9359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefanello N, Schmatz R, Pereira LB, et al. Effects of chlorogenic acid, caffeine, and coffee on behavioral and biochemical parameters of diabetic rats. Mol Cell Biochem. 2014;388:277–286. doi: 10.1007/s11010-013-1919-9. [DOI] [PubMed] [Google Scholar]
- 13.Gibb AJ, Halliday FC. Fast purinergic transmission in the central nervous system. Semin Neurosci. 1996;8:225–232. doi: 10.1006/smns.1996.0029. [DOI] [Google Scholar]
- 14.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783(5):673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Schmatz R, Mazzanti CM, Spanevello R, et al. Ectonucleotidase and acetylcholinesterase activities in synaptosomes from the cerebral cortex of streptozotocin-induced diabetic rats and treated with resveratrol. Brain Res Bull. 2009;80:371–376. doi: 10.1016/j.brainresbull.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Roszek K, Czarnecka J. Is ecto-nucleoside triphosphate diphosphohydrolase (NTPDase)-based therapy of central nervous system disorders possible? Mini-Rev Med Chem. 2014;14(14):1–16. doi: 10.2174/1389557515666150219114416. [DOI] [PubMed] [Google Scholar]
- 18.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernst PB, Garrison JC, Thompson LF. Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J Immunol. 2010;185:1993–1998. doi: 10.4049/jimmunol.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Augusto E, Matos M, Sévigny J, et al. Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J Neurosci. 2013;33:11390–11399. doi: 10.1523/JNEUROSCI.5817-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sala-Newby GB, Skladanowski a C, Newby a C. The mechanism of adenosine formation in cells: cloning of cytosolic 5′-nucleotidase-I. J Biol Chem. 1999;274:17789–17793. doi: 10.1074/jbc.274.25.17789. [DOI] [PubMed] [Google Scholar]
- 22.Morrison PD, Mackinnon MW, Bartrup JT, et al. Changes in adenosine sensitivity in the hippocampus of rats with streptozotocin-induced diabetes. Br J Pharmacol. 1992;105:1004–1008. doi: 10.1111/j.1476-5381.1992.tb09092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duarte JM, Oliveira CR, Ambrósio AF, Cunha RA. Modification of adenosine A1 and A2A receptor density in the hippocampus of streptozotocin-induced diabetic rats. Neurochem Int. 2006;48:144–150. doi: 10.1016/j.neuint.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Schmatz R, Schetinger MRC, Spanevello RM, et al. Effects of resveratrol on nucleotide degrading enzymes in streptozotocin-induced diabetic rats. Life Sci. 2009;84:345–350. doi: 10.1016/j.lfs.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Lunkes GIL, Lunkes DS, Morsch VM, et al. NTPDase and 5′-nucleotidase activities in rats with alloxan-induced diabetes. Diabetes Res Clin Pract. 2004;65:1–6. doi: 10.1016/j.diabres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Pedroso GL, Hammes TO, Escobar TD, Fracasso LB, Forgiarini LF, da Silveira TR. Blood collection for biochemical analysis in adult zebrafish. J Vis Exp. 2012;26 doi: 10.3791/3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson JM, Bunte RM, Carty AJ. Evaluation of rapid cooling and tricaine methanesulfonate (MS222) as methods of euthanasia in zebrafish (Danio rerio) J Am Assoc Lab Anim Sci. 2009;48:785–789. [PMC free article] [PubMed] [Google Scholar]
- 28.Rico EP, Senger MR, da G Fauth M, et al. ATP and ADP hydrolysis in brain membranes of zebrafish (Danio rerio) Life Sci. 2003;73:2071–2082. doi: 10.1016/S0024-3205(03)00596-4. [DOI] [PubMed] [Google Scholar]
- 29.Rosemberg DB, Rico EP, Senger MR, et al. Kinetic characterization of adenosine deaminase activity in zebrafish (Danio rerio) brain. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:96–101. doi: 10.1016/j.cbpb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Senger MR, Rico EP, Dias RD, et al. Ecto-5′-nucleotidase activity in brain membranes of zebrafish (Danio rerio) Comp Biochem Physiol B Biochem Mol Biol. 2004;139:203–207. doi: 10.1016/j.cbpc.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Barnes JM, Murphy PA, Kirkham D, Henley JM. Interaction of guanine nucleotides with [3H] kainate and 6-[3H] cyano-7-nitroquinoxaline-2,3-dione binding in goldfish brain. J Neurochem. 1993;61:1685–1691. doi: 10.1111/j.1471-4159.1993.tb09804.x. [DOI] [PubMed] [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Chan KM, Delfert D, Junger KD. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem. 1986;157:375–380. doi: 10.1016/0003-2697(86)90640-8. [DOI] [PubMed] [Google Scholar]
- 34.Weisman MI, Caiolfa VR, Parola AH. Adenosine deaminase-complexing protein from bovine kidney, isolation of two distinct subunits. J Biol Chem. 1988;263:5266–5270. [PubMed] [Google Scholar]
- 35.Pereira VM, Bortolotto JW, Kist LW, et al. Endosulfan exposure inhibits brain AChE activity and impairs swimming performance in adult zebrafish (Danio rerio) Neurotoxicology. 2012;33:469–475. doi: 10.1016/j.neuro.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Tang R, Dodd A, Lai D, et al. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin. 2007;39:384–390. doi: 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capiotti KM, Menezes FP, Nazario LR, et al. Early exposure to caffeine affects gene expression of adenosine receptors, DARPP-32 and BDNF without affecting sensibility and morphology of developing zebrafish (Danio rerio) Neurotoxicol Teratol. 2011;33:680–685. doi: 10.1016/j.ntt.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Tseng Y-C, Chen R-D, Lee J-R, et al. Specific expression and regulation of glucose transporters in zebrafish ionocytes. Am J Physiol Regul Integr Comp Physiol. 2009;297:R275–R290. doi: 10.1152/ajpregu.00180.2009. [DOI] [PubMed] [Google Scholar]
- 39.Oggier DM, Weisbrod CJ, Stoller AM, et al. Effects of diazepam on gene expression and link to physiological effects in different life stages in zebrafish Danio rerio. Environ Sci Technol. 2010;44:7685–7691. doi: 10.1021/es100980r. [DOI] [PubMed] [Google Scholar]
- 40.Nery LR, Rodrigues MM, Rosemberg DB, et al. Regulation of E-cadherin expression by growth factor receptors in cancer cells. J Surg Oncol. 2011;104:220–221. doi: 10.1002/jso.21898. [DOI] [PubMed] [Google Scholar]
- 41.Roszek K, Czarnecka J. Is ecto-nucleoside triphosphate diphosphohydrolase (NTPDase)-based therapy of central nervous system disorders possible? Mini-Rev Med Chem. 2015;15(1):5–20. doi: 10.2174/1389557515666150219114416. [DOI] [PubMed] [Google Scholar]
- 42.Capiotti KM, De Moraes DA, Menezes FP, et al. Hyperglycemia induces memory impairment linked to increased acetylcholinesterase activity in zebrafish (Danio rerio) Behav Brain Res. 2014;274:319–325. doi: 10.1016/j.bbr.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 43.Pinto-Duarte A, Coelho JE, Cunha RA, et al. Adenosine A2A receptors control the extracellular levels of adenosine through modulation of nucleoside transporters activity in the rat hippocampus. J Neurochem. 2005;93:595–604. doi: 10.1111/j.1471-4159.2005.03071.x. [DOI] [PubMed] [Google Scholar]
- 44.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 45.Fredholm BB, Chen J-F, Masino SA, Vaugeois J-M. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- 46.Boison D, Shen H-Y. Adenosine kinase is a new therapeutic target to prevent ischemic neuronal death. Open Drug Discov J. 2010;2:108–118. [PMC free article] [PubMed] [Google Scholar]
- 47.Miron VR, Bauermann L, Morsch ALB, et al. Enhanced NTPDase and 5′-nucleotidase activities in diabetes mellitus and iron-overload model. Mol Cell Biochem. 2007;298:101–107. doi: 10.1007/s11010-006-9357-6. [DOI] [PubMed] [Google Scholar]
- 48.Duarte JMN, Oses JP, Rodrigues RJ, Cunha RA. Modification of purinergic signaling in the hippocampus of streptozotocin-induced diabetic rats. Neuroscience. 2007;149:382–391. doi: 10.1016/j.neuroscience.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Kukulski F, Lévesque SA, Lavoie ÉG, et al. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 2005;1:193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massé K, Bhamra S, Eason R, Dale N, Jones EA. Purine-mediated signalling triggers eye development. Nature. 2007;449(7165):1058–1062. doi: 10.1038/nature06189. [DOI] [PubMed] [Google Scholar]
- 51.Sträter N. Ecto-5′-nucleotidase: structure function relationships. Purinergic Signal. 2006;2:343–350. doi: 10.1007/s11302-006-9000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maier SA, Galellis JR, McDermid HE. Phylogenetic analysis reveals a novel protein family closely related to adenosine deaminase. J Mol Evol. 2005;61:776–794. doi: 10.1007/s00239-005-0046-y. [DOI] [PubMed] [Google Scholar]
- 53.Rosemberg DB, Rico EP, Guidoti MR, et al. Adenosine deaminase-related genes: molecular identification, tissue expression pattern and truncated alternative splice isoform in adult zebrafish (Danio rerio) Life Sci. 2007;81:1526–1534. doi: 10.1016/j.lfs.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 54.Franco R, Valenzuela A, Lluis C, Blanco J. Enzymatic and extraenzymatic role of ecto-adenosine deaminase in lymphocytes. Immunol Rev. 1998;161:27–42. doi: 10.1111/j.1600-065X.1998.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 55.Cox DJ, Kovatchev BP, Gonder-Frederick LA, et al. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care. 2005;28:71–77. doi: 10.2337/diacare.28.1.71. [DOI] [PubMed] [Google Scholar]
- 56.Duarte JMN, Agostinho PM, Carvalho RA, Cunha RA. Caffeine consumption prevents diabetes-induced memory impairment and synaptotoxicity in the hippocampus of nonczno10/ltj mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zavialov AV, Engström A. Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity. Biochem J. 2005;391:51–57. doi: 10.1042/BJ20050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salgado H, Santos-Zavaleta A, Gama-Castro S, et al. RegulonDB (version 3.2): transcriptional regulation and operon organization in Escherichia coli K-12. Nucleic Acids Res. 2001;29:72–74. doi: 10.1093/nar/29.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keseler IM, Collado-Vides J, Gama-Castro S et al (2005) EcoCyc: a comprehensive database resource for Escherichia coli. Nucleic Acids Res 33 [DOI] [PMC free article] [PubMed]
- 60.Artola A, Kamal A, Ramakers GM, et al. Synaptic plasticity in the diabetic brain: advanced aging? Prog Brain Res. 2002;138:305–314. doi: 10.1016/S0079-6123(02)38084-1. [DOI] [PubMed] [Google Scholar]
- 61.Faulhaber-Walter R, Jou W, Mizel D, et al. Impaired glucose tolerance in the absence of adenosine A1 receptor signaling. Diabetes. 2011;60:2578–2587. doi: 10.2337/db11-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamagishi S, Ueda S, Okuda S. Food-derived advanced glycation end products (AGEs): a novel therapeutic target for various disorders. Curr Pharm Des. 2007;13(27):2832–2836. doi: 10.2174/138161207781757051. [DOI] [PubMed] [Google Scholar]
- 63.Zimmermann H. ATP and acetylcholine, equal brethren. Neurochem Int. 2008;52:634–648. doi: 10.1016/j.neuint.2007.09.004. [DOI] [PubMed] [Google Scholar]