Abstract
Objective:
Nicotine craving is considered an important element in the persistence of cigarette smoking, but little is known about the role of craving in the widely recognized association between variants mapped to the neuronal nicotinic acetylcholine receptor (CHRN) genes on chromosome 15 and nicotine phenotypes.
Method:
The associations between CHRNA5–CHRNA3–CHRNB4 variants and cigarettes per day (CPD), the Fagerström Test for Nicotine Dependence (FTND), and craving were analyzed in data from 662 lifetime smokers from an Israeli adult Jewish household sample. Indirect effects of genotype on nicotine phenotypes through craving were formally tested using regression and bootstrapping procedures.
Results:
At CHRNA3, allele G of rs3743078 was associated with increased craving, CPD, and FTND scores: Participants with one or two copies of the G allele had, on average, higher scores on the craving scale (p = .0025), more cigarettes smoked (p = .0057), and higher scores on the FTND (p = .0024). With craving in the model, variant rs3743078 showed a significant indirect effect through craving on CPD (p = .0026) and on FTND score (p = .0024). A sizeable proportion of the total rs3743078 effect on CPD (56.4%) and FTND (65.2%) was indirect through craving.
Conclusions:
These results suggest that nicotine craving may play a central role in nicotine use disorders and may have utility as a therapeutic target.
Despite concerted public health efforts, cigarette smoking remains a leading preventable cause of global morbidity and mortality (World Health Organization, 2011). Thus, a better understanding of the factors underlying smoking has substantial public health significance. Genetic factors influence the risk for smoking phenotypes (Maes et al., 2004). Because nicotine binds to neuronal nicotinic acetylcholine receptors (Benowitz, 2010; Greenbaum & Lerer, 2009), neuronal nicotinic acetylcholine receptor genes (CHRNs) have been widely studied for their relationship to nicotine phenotypes.
Meta-analyses show a robust association of single nucleotide variants in the chromosome 15 gene cluster encoding receptor subunits α5, α3, and β4 (CHRNA5/A3/B4) with cigarettes smoked per smoking day (CPD; Furberg et al. 2010; Liu et al., 2010; Saccone et al., 2010; Thorgeirsson et al., 2010). These variants also show associations with the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) (Broms et al., 2012; Ware et al., 2012). Both phenotypes are associated with two statistically independent loci in this gene cluster, one tagged by variant rs16969968 (or the strongly correlated rs1051730), and the other tagged by rs578776 (or the correlated rs3743078) (Bierut et al., 2008; Greenbaum et al., 2009; Saccone et al., 2007, 2009a, 2009b, 2010; Stevens et al., 2008). However, few studies have investigated potentially modifiable mechanisms of the widely recognized relationship between CHRNA5/A3/B4 variants and CPD or the FTND. Elucidating such mechanisms may lead to more effective interventions, which is of considerable importance for public health.
Nicotine craving may be one such mechanism. Some consider craving to be the proximal cause of smoking and to play a central role in nicotine dependence (Benowitz, 2010; Tiffany & Wray, 2012). However, little is known about the association of the two CHRNA5/A3/B4 loci with nicotine craving. One study showed an association of rs16969968 with three nicotine craving measures (Chen et al., 2012). Another study showed an association of craving with joint haplotypes of rs578776 and rs16969968, but only among early-onset smokers (Baker et al., 2009). Additional studies are warranted to further elucidate the relationship between nicotine craving and CHRNA5/A3/B4, specifically if craving is associated with the rs578776/rs3743078 locus, which is statistically independent of the rs16969968 locus.
In addition, a sensitization-homeostasis model (DiFranza & Wellman, 2005) posits that among lifetime smokers, binding of nicotine to receptors inhibits craving. According to this theoretical model, genetic variation that affects nicotine binding or receptor activation could alter craving, perhaps leading to more frequent smoking throughout the day (increased CPD) (DiFranza & Wellman, 2005). Similarly, craving is likely to influence dependence, as assessed by the FTND items (e.g., time to first cigarette after morning awakening, difficulty refraining from smoking where prohibited, number of cigarettes usually smoked), so variants influencing craving could affect FTND scores. We therefore hypothesized that the association between CHRNA5/A3/B4 and CPD or FTND is indirect through craving; that is, CHRNA5/A3/B4 is associated with craving, which in turn is associated with CPD or FTND.
Thus, the study goal was to investigate a proposed mechanism for the well-established relationships between CHRNA5/A3/B4 and CPD or FTND: indirect effects through craving. Toward that end, using data on lifetime smokers from a general population sample of adult Israelis (Shmulewitz et al., 2011, 2013), we evaluated the association of nicotine phenotypes (craving, CPD, and FTND scores) with four CHRNA5/A3/B4 variants (rs16969968, rs578776, rs3743078, and rs684513) representing the two associated loci, and the association of nicotine craving with CPD and FTND. Then the total effects (overall association) of the CHRNA5/A3/B4 variants were decomposed into direct effects on CPD or FTND and indirect effects through craving (Hayes, 2013).
Method
Study procedures and sample
Data were collected from 1,349 household residents in 2007–2009 (Hasin et al., 2002; Shmulewitz et al., 2010, 2011, 2013). To maximize genetic homogeneity, Jewish adults (one per household) were selected from the Israeli Population Register. Men were oversampled because alcohol use, the main focus of the overall study, is limited in Israeli women. The study was approved by relevant American and Israeli institutional review boards. Interviewers obtained written informed consent and conducted face-to-face computer-assisted interviews. The response rate was 68.9%. Strict quality-control procedures included structured interviewer training, field observations, reviews of recorded interviews, and telephone verification of responses.
This report focuses on lifetime smokers genotyped for CHRNA5/A3/B4 variant rs3743078 (N = 662). Although sample sizes were slightly different for the other CHRNA5/A3/B4 variants (delineated below), demographic proportions were virtually the same. Lifetime smokers had smoked ≥100 cigarettes, a threshold widely used to indicate substantial smoking (Bondy et al., 2009; Chen et al., 2009, 2012; Saccone et al., 2009a, 2009b). Of these, 82.5% (n = 546) were male; 19.0% (n = 126) were 21–29 years old, 33.1% (n = 219) were 30–44, and 47.9% (n = 317) were 45 years or older; 28.8% (n = 191) were immigrants from the former Soviet Union. Both current (n = 477) and former (n = 185) smokers were included because genetic effects can occur throughout the life span once smoking starts.
Lifetime measures
Number of cigarettes usually smoked per smoking day.
CPD was assessed using the Alcohol Use Disorders and Associated Disabilities Interview Schedule (AUDADIS; Grant et al., 1995, 2003) for three separate periods: past year or the year before quitting, period of daily smoking, and period of heaviest smoking. The AUDADIS CPD measures have excellent test–retest reliability (intraclass correlation coefficients = .74–.83) (Grant et al., 2003). We used the maximum of the three periods, with scores ranging from 1 to 80. Self-report of CPD is commonly used in association studies and generally has good to excellent reliability, even among former smokers (Soulakova et al., 2012).
Fagerström Test for Nicotine Dependence.
The FTND (Heatherton et al., 1991) was assessed using the AUDADIS module used by the National Epidemiologic Survey on Alcohol and Related Conditions (Grant et al., 2003), slightly adapted for use in large-scale epidemiologic studies, with two changes from the standard version: (a) “Did you often smoke just after getting up or shortly after getting up in the morning” replaced “How soon after getting up do you smoke your first cigarette,” and a positive response was worth 2 points, similar to the “6–30 minutes” response to the actual FTND question (“How soon … ”); and (b) “Which cigarette would you hate most to give up” was not assessed, because it showed a lack of utility and poor psychometric properties (Chabrol et al., 2003; Etter et al., 1999; Heatherton et al., 1991). Thus, the FTND score ranged from 0 to 8. The mean FTND (3.4) was within the range of mean scores (2.8–4.6) from 15 general population studies in Europe and the United States (Fagerström & Furberg, 2008) and lower than scores reported in clinical samples (Wessel et al., 2010).
Nicotine craving.
To provide a more nuanced and informative measure of craving, a scale was formed using two questions adapted from Russell’s Smoking Motives Questionnaire (Russell et al., 1974): “When you have run out of cigarettes, do/did you find it almost unbearable until you can get them?” and “Do/did you get a strong desire to smoke when you haven’t smoked for a while?” These items load most strongly on the “addictive” (craving) factor (Russell et al., 1974), assess both craving frequency and intensity, and are similar to items used to assess both moderate and severe craving (Kim et al., 2014; Piper et al., 2004; Shiffman et al, 2004). Responses (4 = always, 3 = often, 2 = sometimes, 1 = infrequently, 0 = never) were summed to create a craving scale, with scores ranging from 0 to 8 (higher values indicating greater craving). Internal consistency (standardized coefficient α = .69) for the scale was acceptable (Kline, 2000).
Genotyping
DNA was extracted from blood or saliva using standard DNA isolation products (Roche Diagnostics, Germany; Qiagen Inc., Valencia, CA; DNA Genotek Inc., Ottawa, Ontario, Canada). Four variants in the CHRNA5/A3/B4 cluster were genotyped (rs16969968, rs684513, rs578776, and rs3743078) via TaqMan, as described previously (Sherva et al., 2010). Because the Jewish population has a unique genetic background (Guha et al., 2012; Ostrer & Skorecki, 2013), these variants were selected to ensure adequate coverage in this Israeli Jewish sample. Samples were run in duplicate; discordant genotypes were discarded. Thirty-two ancestry-informative markers (AIMs) were genotyped to detect population stratification (Listman et al., 2010).
Analysis
Genetic markers.
All variants had minor allele frequencies >0.01 and were included in the preliminary analysis. Using SAS Version 9.3 (SAS Institute Inc., Cary, NC), the chi-square goodness-of-fit test tested for deviations from Hardy–Weinberg equilibrium (HWE) expectations for each variant in all genotyped samples (i.e., not limited to lifetime smokers because these are population-based estimates). Linkage disequilibrium between variants was assessed by r2 and Lewontin’s D'.
Initial genetic analysis.
The study goal was to evaluate indirect effects of CHRNA5/A3/B4 variants via craving on nicotine outcomes (consumption or dependence). Although the predictor (gene variant) does not need to be associated with the outcome to test an indirect effect (Hayes, 2013), association between CHRNA5/A3/B4 variants and all nicotine phenotypes was assessed to inform selection of the genetic models to use in evaluating indirect effects and to place study results in the context of previous studies.
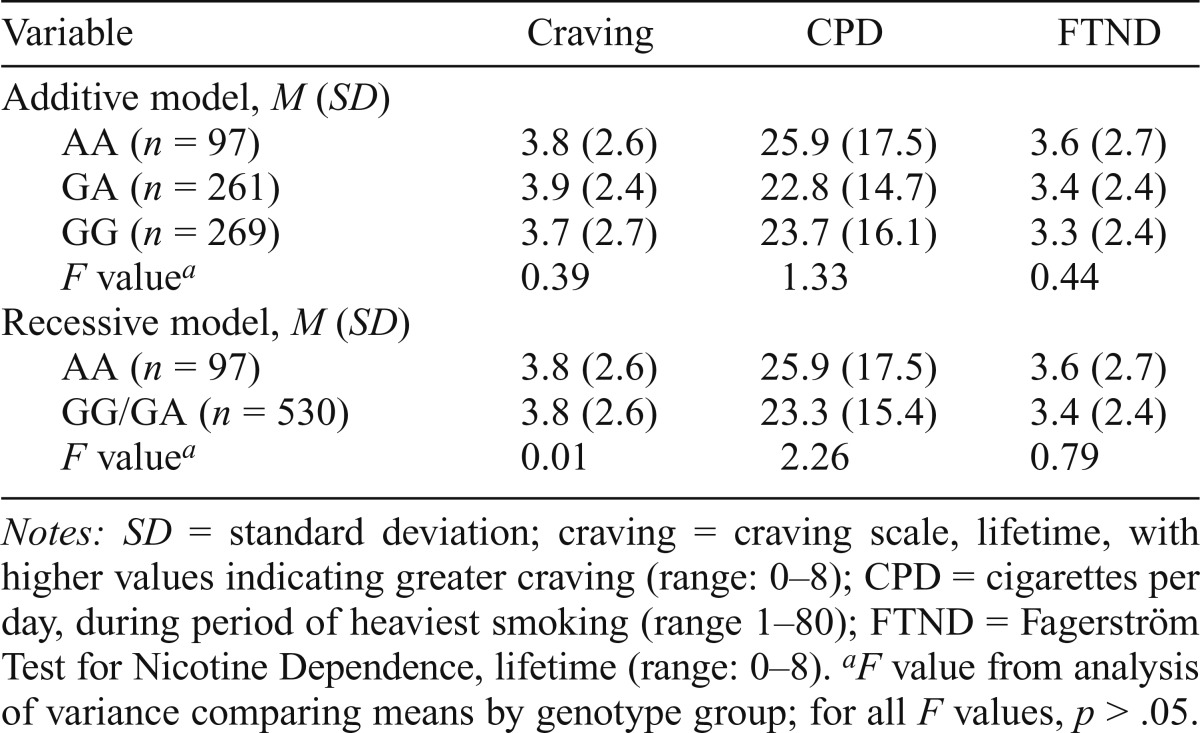
Although previous research with CHRNA5/A3/B4 variants assumed specific genetic models, e.g., the rs578776/rs3743078 locus modeled as additive (Greenbaum et al., 2009; Saccone et al., 2009a, 2009b, 2010; Stevens et al., 2008), genetic effects may differ by population (Carlson et al., 2013). Therefore, for each variant, we determined whether the genetic risk model appeared allele-specific (presence or absence of a particular allele) or additive by inspecting the phenotype values in each genotype group. For rs16969968 (Table 7), inspection of phenotype means by genotype group suggested a recessive model (comparing groups AA and GG/GA) or, less likely, additive (comparing groups AA, AG, and GG), but not a dominant model (AA/GA vs. GG). No significant differences in phenotype means were observed by genotype groups (additive or recessive), indicating no detectable genetic effect in this sample. For the other variants, phenotypic means suggested an allele-specific (recessive) genetic model; e.g., for rs3743078, both genotypes with allele G (GG/CG) had similar phenotype values (Table 3, footnote), higher than CC. Thus, association analyses compared the “high-risk” group (GG/CG) to the “low-risk” group (CC). Similarly, for rs684513, CC/CG was high risk and GG was low risk (Table 5, footnote); for rs578776, GG/GA was high risk and AA was low risk (Table 6, footnote).
Table 7.
Association between nicotine phenotypes and rs16969968 in lifetime smokers (n = 627)
| Variable | Craving | CPD | FTND |
| Additive model, M (SD) | |||
| AA (n = 97) | 3.8 (2.6) | 25.9 (17.5) | 3.6 (2.7) |
| GA (n = 261) | 3.9 (2.4) | 22.8 (14.7) | 3.4 (2.4) |
| GG (n = 269) | 3.7 (2.7) | 23.7 (16.1) | 3.3 (2.4) |
| F valuea | 0.39 | 1.33 | 0.44 |
| Recessive model, M (SD) | |||
| AA (n = 97) | 3.8 (2.6) | 25.9 (17.5) | 3.6 (2.7) |
| GG/GA (n = 530) | 3.8 (2.6) | 23.3 (15.4) | 3.4 (2.4) |
| F valuea | 0.01 | 2.26 | 0.79 |
Notes: SD = standard deviation; craving = craving scale, lifetime, with higher values indicating greater craving (range: 0–8); CPD = cigarettes per day, during period of heaviest smoking (range 1–80); FTND = Fagerström Test for Nicotine Dependence, lifetime (range: 0–8).
F value from analysis of variance comparing means by genotype group; for all F values, p > .05.
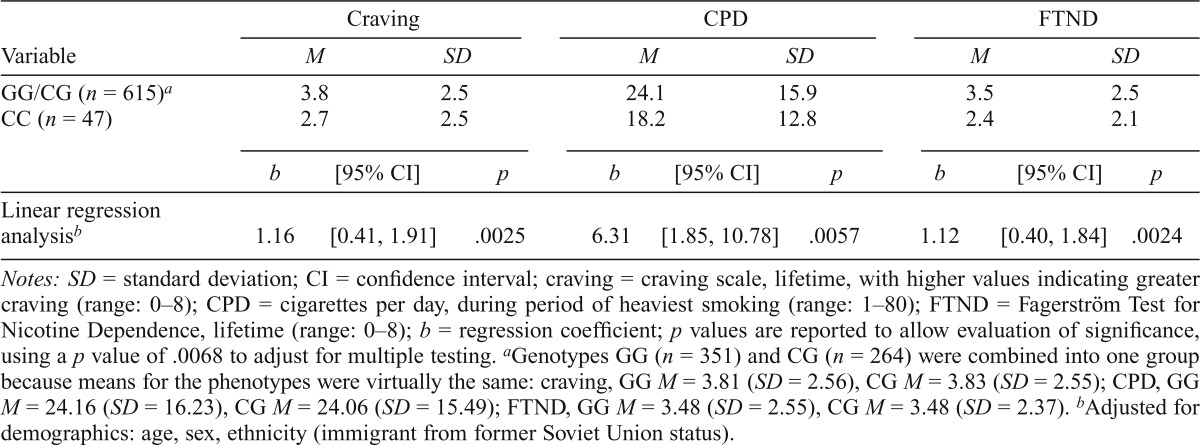
Table 3.
Association between nicotine phenotypes and rs3743078 in lifetime smokers (n = 662)
| Craving |
CPD |
FTND |
|||||||
| Variable | M | SD | M | SD | M | SD | |||
| GG/CG (n = 615)a | 3.8 | 2.5 | 24.1 | 15.9 | 3.5 | 2.5 | |||
| CC (n = 47) | 2.7 | 2.5 | 18.2 | 12.8 | 2.4 | 2.1 | |||
| b | [95% CI] | p | b | [95% CI] | p | b | [95% CI] | p | |
| Linear regression analysisb | 1.16 | [0.41, 1.91] | .0025 | 6.31 | [1.85, 10.78] | .0057 | 1.12 | [0.40, 1.84] | .0024 |
Notes: SD = standard deviation; CI = confidence interval; craving = craving scale, lifetime, with higher values indicating greater craving (range: 0–8); CPD = cigarettes per day, during period of heaviest smoking (range: 1–80); FTND = Fagerström Test for Nicotine Dependence, lifetime (range: 0–8); b = regression coefficient; p values are reported to allow evaluation of significance, using a p value of .0068 to adjust for multiple testing.
Genotypes GG (n = 351) and CG (n = 264) were combined into one group because means for the phenotypes were virtually the same: craving, GG M = 3.81 (SD = 2.56), CG M = 3.83 (SD = 2.55); CPD, GG M = 24.16 (SD = 16.23), CG M = 24.06 (SD = 15.49); FTND, GG M = 3.48 (SD = 2.55), CG M = 3.48 (SD = 2.37).
Adjusted for demographics: age, sex, ethnicity (immigrant from former Soviet Union status).
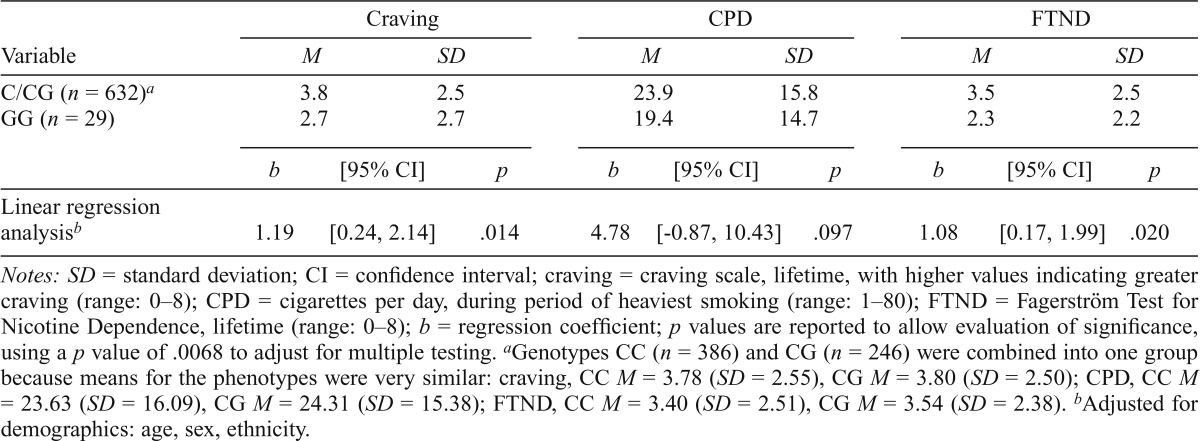
Table 5.
Association between nicotine phenotypes and rs684513 in lifetime smokers (n = 661)
| Variable | Craving |
CPD |
FTND |
||||||
| M | SD | M | SD | M | SD | ||||
| C/CG (n = 632)a | 3.8 | 2.5 | 23.9 | 15.8 | 3.5 | 2.5 | |||
| GG (n = 29) | 2.7 | 2.7 | 19.4 | 14.7 | 2.3 | 2.2 | |||
| b | [95% CI] | p | b | [95% CI] | p | b | [95% CI] | p | |
| Linear regression analysisb | 1.19 | [0.24, 2.14] | .014 | 4.78 | [-0.87, 10.43] | .097 | 1.08 | [0.17, 1.99] | .020 |
Notes: SD = standard deviation; CI = confidence interval; craving = craving scale, lifetime, with higher values indicating greater craving (range: 0–8); CPD = cigarettes per day, during period of heaviest smoking (range: 1–80); FTND = Fagerström Test for Nicotine Dependence, lifetime (range: 0–8); b = regression coefficient; p values are reported to allow evaluation of significance, using a p value of .0068 to adjust for multiple testing.
Genotypes CC (n = 386) and CG (n = 246) were combined into one group because means for the phenotypes were very similar: craving, CC M = 3.78 (SD = 2.55), CG M = 3.80 (SD = 2.50); CPD, CC M = 23.63 (SD = 16.09), CG M = 24.31 (SD = 15.38); FTND, CC M = 3.40 (SD = 2.51), CG M = 3.54 (SD = 2.38).
Adjusted for demographics: age, sex, ethnicity.
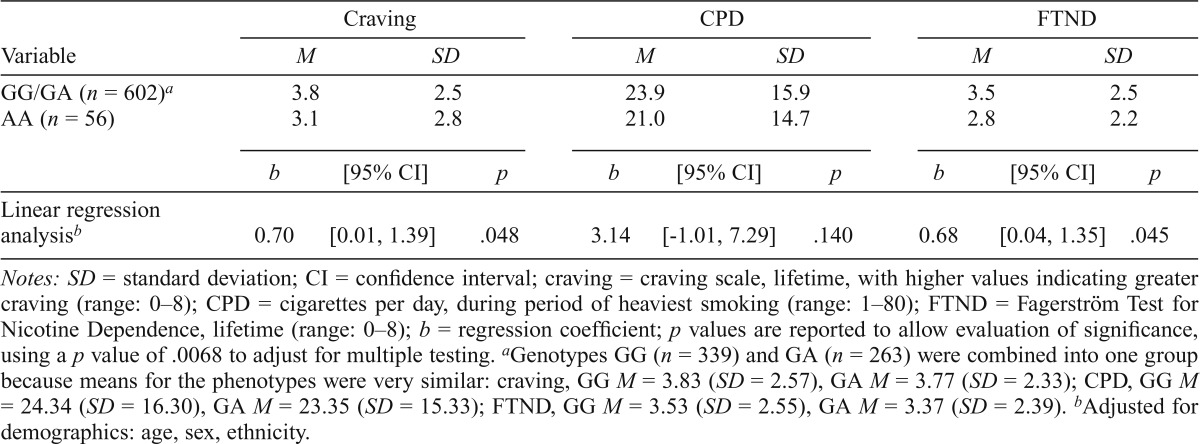
Table 6.
Association between nicotine phenotypes and rs578776 in lifetime smokers (n = 658)
| Variable | Craving |
CPD |
FTND |
||||||
| M | SD | M | SD | M | SD | ||||
| GG/GA (n = 602)a | 3.8 | 2.5 | 23.9 | 15.9 | 3.5 | 2.5 | |||
| AA (n = 56) | 3.1 | 2.8 | 21.0 | 14.7 | 2.8 | 2.2 | |||
| b | [95% CI] | p | b | [95% CI] | p | b | [95% CI] | p | |
| Linear regression analysisb | 0.70 | [0.01, 1.39] | .048 | 3.14 | [-1.01, 7.29] | .140 | 0.68 | [0.04, 1.35] | .045 |
Notes: SD = standard deviation; CI = confidence interval; craving = craving scale, lifetime, with higher values indicating greater craving (range: 0-8); CPD = cigarettes per day, during period of heaviest smoking (range: 1–80); FTND = Fagerström Test for Nicotine Dependence, lifetime (range: 0–8); b = regression coefficient; p values are reported to allow evaluation of significance, using a p value of .0068 to adjust for multiple testing.
Genotypes GG (n = 339) and GA (n = 263) were combined into one group because means for the phenotypes were very similar: craving, GG M = 3.83 (SD = 2.57), GA M = 3.77 (SD = 2.33); CPD, GG M = 24.34 (SD = 16.30), GA M = 23.35 (SD = 15.33); FTND, GG M = 3.53 (SD = 2.55), GA M = 3.37 (SD = 2.39).
Adjustedfor demographics: age, sex, ethnicity.
To account for multiple testing in evaluating the association of CHRNA5/A3/B4 variants and nicotine phenotypes, the p value for declaring significance was adjusted for the number of independent tests, following a procedure to quantify the number of tests (Nyholt, 2004a), as used in He et al. (2009) and Vaillancourt et al. (2012). For rs578776, rs3743078, and rs684513, one genetic model (allelic) was chosen based on phenotypic means. Matrix spectral decomposition estimated that these three correlated variants effectively reflected 1.66 independent “variants” (Nyholt, 2004b). Two models (additive and allelic) were tested for rs16969968 since phenotypic means were not significantly different by genotype (Table 4). Thus, there were 3.66 “variant-models” (one model for 1.66 “variants” plus two models for one variant). Matrix spectral decomposition (Nyholt, 2004b) estimated that the three correlated phenotypes (craving, CPD, FTND) reflected 2.00 independent “phenotypes”; 3.66 “variant-models” for 2.00 “phenotypes” yields 7.32 tests; the conservative Bonferroni-corrected p value was 0.05/7.32 = .0068. We also report p values to allow readers to evaluate the significance.
Table 4.
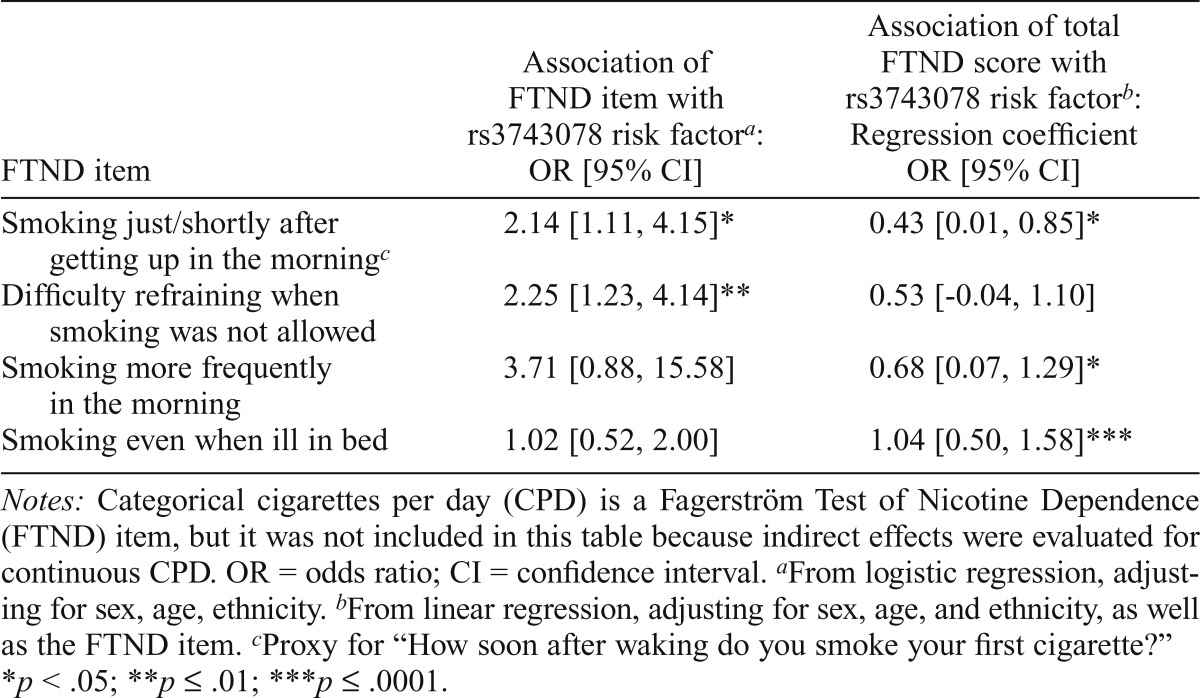
Association between FTND items, FTND score, and rs3743078, in lifetime smokers (n = 662)
| FTND item | Association of FTND item with rs3743078 risk factora: OR [95% CI] | Association of total FTND score with rs3743078 risk factorb: Regression coefficient OR [95% CI] |
| Smoking just/shortly after getting up in the morningc | 2.14 [1.11, 4.15]* | 0.43 [0.01, 0.85]* |
| Difficulty refraining when smoking was not allowed | 2.25 [1.23, 4.14]** | 0.53 [-0.04, 1.10] |
| Smoking more frequently in the morning | 3.71 [0.88, 15.58] | 0.68 [0.07, 1.29]* |
| Smoking even when ill in bed | 1.02 [0.52, 2.00] | 1.04 [0.50, 1.58]*** |
Notes: Categorical cigarettes per day (CPD) is a Fagerström Test of Nicotine Dependence (FTND) item, but it was not included in this table because indirect effects were evaluated for continuous CPD. OR = odds ratio; CI = confidence interval.
From logistic regression, adjusting for sex, age, ethnicity.
From linear regression, adjusting for sex, age, and ethnicity, as well as the FTND item.
Proxy for “How soon after waking do you smoke your first cigarette?”
p < .05;
p < .01;
p < .0001.
Linear regression procedures (SAS 9.3) investigated the association of each variant (rs3743078 [GG/CG vs. CC]; rs684513 [CC/CG vs. GG]; rs578776 [GG/GA vs. AA]) with continuous scores for nicotine craving, CPD, and FTND, and of craving with CPD and FTND. All analyses adjusted for demographic covariates (sex, age, and ethnicity [former Soviet Union immigrants vs. others]), as smoking behavior differs by these subgroups in Israel (Baron-Epel et al., 2004). For rs684513 and rs578776, analysis was repeated adjusting for rs3743078 to determine if the signals for rs684513 and rs578776 were most likely due to correlation with rs3743078.
Indirect effects estimation (mediation)
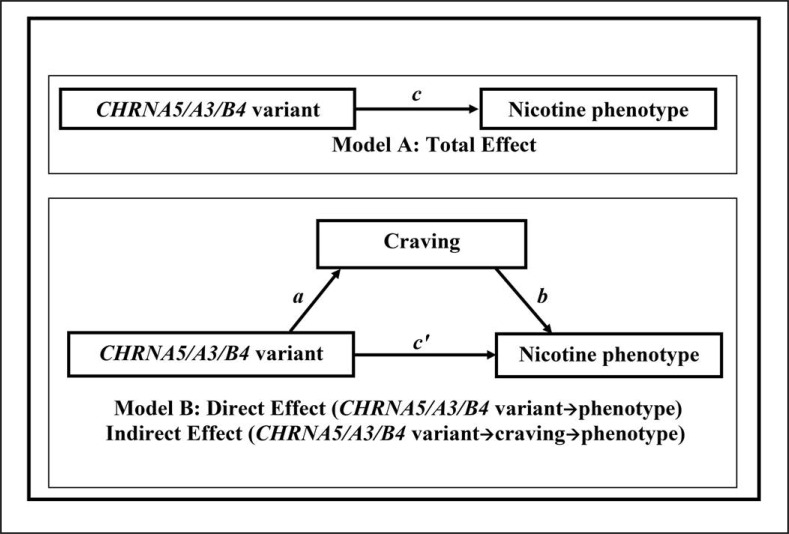
Using the SAS process macro (Hayes, 2013), a series of linear regressions were estimated, correcting for demographics, using the conceptual models shown in Figure 1, where “CHRNA5/A3/B4 variant” refers to rs3743078, rs684513, or rs578776. (No indirect effects were evaluated for rs16969968, as there was no evidence for association with craving.) First, each outcome phenotype was regressed on the CHRNA5/A3/B4 variant risk factor to estimate its total effect on CPD or FTND (Figure 1, Model A, regression coefficient c). Then, the nicotine craving scale was standardized (M = 0, SD = 1) and was regressed on the CHRNA5/A3/B4 variant (coefficient a). Last, each outcome was regressed on the CHRNA5/A3/B4 variant with standardized nicotine craving in the model (Figure 1, Model B), to estimate the effect of craving on the phenotype (coefficient b) and the direct effect of the CHRNA5/A3/B4 variant on the phenotype (coefficient c'). The indirect effect through craving was calculated as the product of coefficients a and b.
Figure 1.
Model A shows the total effect (c) of CHRNA5/A3/B4 variant (rs3743078, rs684513, or rs578776) on nicotine phenotypes, without accounting for the effect of craving. Model B shows the decomposition of the total effect into the direct effect (c') and indirect effect (a × b) through craving.
Bias-corrected 95% confidence intervals (CIs) from 10,000 bootstrap samples were produced for the indirect effect (a × b) as well as bootstrapped standard errors (Hayes, 2013), used to obtain p values. A 95% CI completely above zero indicates a significant indirect effect at the p = .05 level (Hayes, 2013). To account for the correlated variants, which represented 1.66 “variants,” and the correlated outcomes (CPD, FTND), which reflected 1.40 “phenotypes” based on matrix spectral decomposition (Nyholt, 2004b), an adjusted p value of 0.05 /(1.66 × 1.40) = 0.022 was applied. Using bootstrapping improves on tests based on the normal theory approach (e.g., the Sobel test), as the sampling distribution of indirect effects is rarely normal (Hayes, 2013). A significant indirect effect (i.e., p < .022 after correcting for correlated gene variants and outcomes) suggests that the CHRNA5/A3/B4 variant high-risk group (e.g., rs3743078 GG/CG group) is associated with higher craving scores, which in turn is associated with higher CPD or FTND scores. The values for the direct and indirect effects are the amount (averaged over the sample) by which CPD or FTND is increased in the rs3743078 GG/CG group, either directly or indirectly through craving. Last, the proportion of the effect of rs3743078 that acts through craving was calculated as (indirect effect/total effect) × 100. Results focus on rs3743078 because that variant showed the strongest evidence for indirect effects, and further analyses were carried out for rs3743078 only.
Exploratory analysis
Analysis was carried out to further explore the complex relationships between rs3743078, craving, CPD, and nicotine dependence (FTND). First, the alternate direction of an indirect effect of rs3743078 on craving through CPD was evaluated. Second, to determine if FTND items could act similarly to craving in mediating the effect of rs3743078 on FTND, an indirect effect of rs3743078 on FTND through CPD was evaluated. (Although CPD is categorized in the FTND, continuous CPD was used to maintain consistency with the other analyses reported here.) In addition, the association between rs3743078 and FTND score adjusting for each of the other FTND items was examined. (Formal indirect effects evaluation of binary FTND items was beyond the scope of this study.)
Supplementary analysis
Analysis (using STRUCTURE 2.2; Pritchard et al., 2000) of the AIMs showed no detectible population substructure within this Israeli sample (Listman et al., 2010); thus, no probabilities of subpopulation membership were calculated. When non-Jewish samples were included to establish parental populations for clustering, four subpopulations were identified (Listman et al., 2010). Two subpopulations reflected the Northern-to-Southern European cline (average contributions to present sample: 46.7% Northern, 48.7% Southern), with minor contributions from “African” (1.7%) and “Asian” (2.9%) populations. Among respondents with genotyped AIMs (N = 584), indirect effects were estimated adjusting for population substructure, by including the probabilities of subpopulation membership as continuous control variables in the regression analyses.
Results
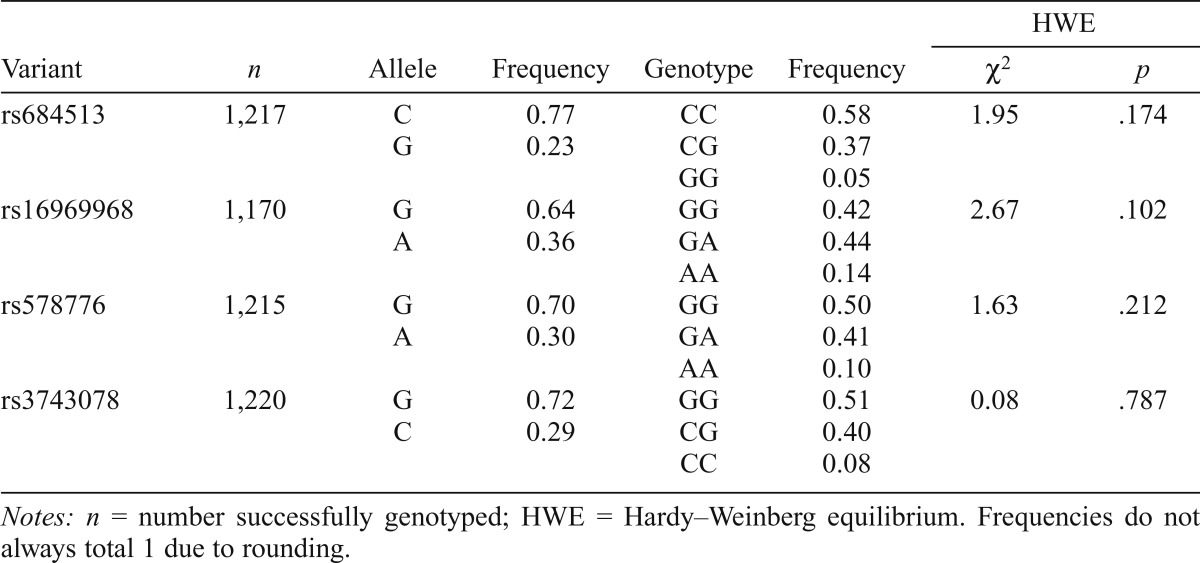
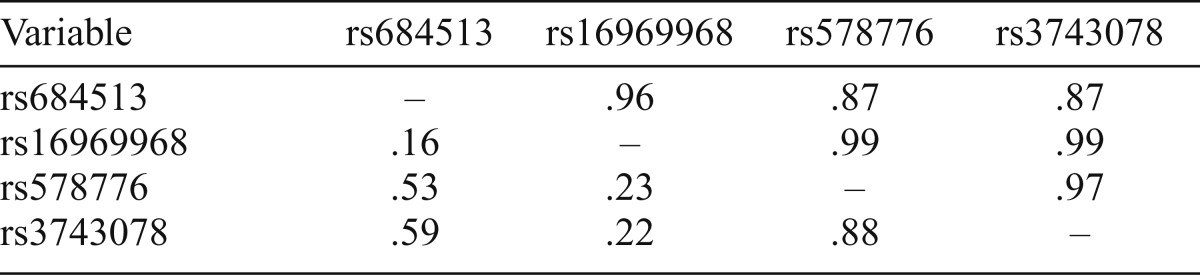
Allele and genotype frequencies for the variants for the entire sample are shown in Table 1. All distributions were consistent with HWE expectations (Table 1). All variants were in strong linkage disequilibrium based on D' (0.87–0.99; Table 2). rs684513, rs578776, and rs3743078 showed moderate to strong correlation (r2 = .53-.88), but weaker correlation with rs16969968 (r2 = .16-.23). In initial analysis, rs3743078 showed the strongest association with nicotine phenotypes; detailed findings are therefore reported for rs3743078.
Table 1.
Allele and genotype frequencies for CHRNA5/A3/B4 variants
| HWE |
|||||||
| Variant | n | Allele | Frequency | Genotype | Frequency | χ2 | p |
| rs684513 | 1,217 | C | 0.77 | CC | 0.58 | 1.95 | .174 |
| G | 0.23 | CG | 0.37 | ||||
| GG | 0.05 | ||||||
| rs16969968 | 1,170 | G | 0.64 | GG | 0.42 | 2.67 | .102 |
| A | 0.36 | GA | 0.44 | ||||
| AA | 0.14 | ||||||
| rs578776 | 1,215 | G | 0.70 | GG | 0.50 | 1.63 | .212 |
| A | 0.30 | GA | 0.41 | ||||
| AA | 0.10 | ||||||
| rs3743078 | 1,220 | G | 0.72 | GG | 0.51 | 0.08 | .787 |
| C | 0.29 | CG | 0.40 | ||||
| CC | 0.08 | ||||||
Notes: n = number successfully genotyped; HWE = Hardy–Weinberg equilibrium. Frequencies do not always total 1 due to rounding.
Table 2.
Linkage disequilibrium between CHRNA5/A3/B4 variants among whole sample, showing D' above the diagonal and r2 below
| Variable | rs684513 | rs16969968 | rs578776 | rs3743078 |
| rs684513 | – | .96 | .87 | .87 |
| rs16969968 | .16 | – | .99 | .99 |
| rs578776 | .53 | .23 | – | .97 |
| rs3743078 | .59 | .22 | .88 | – |
Among lifetime smokers, 92.9% (n = 615) were in the rs3743078 GG/CG (high risk) group (Table 3), and mean scores were as follows: craving scale, 3.74 (SD = 2.54); CPD, 23.7 (SD = 15.8); and FTND, 3.40 (SD = 2.46).
Total effects of rs3743078
Variant rs3743078 was significantly associated with each of the three phenotypes tested. On average, the GG/CG group scored 1.16 higher on the craving scale (p = .0025), smoked 6.31 more CPD (p = .0057), and scored 1.12 points higher on the FTND (p = .0024) (Table 3). For CPD and FTND, these values correspond to the total effect shown in Model A (c in Figure 1). Craving was associated with increased CPD (regression coefficient b = 3.10, 95% CI [2.71, 3.49], p < .0001) and increased FTND (b = 0.64, 95% CI [0.58, 0.69], p <.0001).
Decomposition of the rs3743078 effect
With craving included (Figure 1, Model B), the total effect of rs3743078 on CPD was decomposed into a direct effect (c') and an indirect effect through craving (a × b). On average, participants with rs3743078 genotypes GG/CG smoked 6.31 more CPD than participants with the CC genotype, with an increase of 3.56 cigarettes (95% CI [1.22, 5.85], p = .0026) because of the indirect effect of GG/CG through craving, and the remaining increase of 2.75 cigarettes (95% CI [-1.11, 6.61], p = .16) independent of craving (direct effect). Of the rs3743078 effect on CPD, 56.4% was indirect through craving. Similarly, participants in the rs3743078 GG/CG group scored on average 1.12 points higher on the FTND than those in the CC group, with a 0.73 point increase (95% CI [0.25, 1.20], p = .0024) because of the indirect effect through craving, and the remaining 0.38 point increase (95% CI [-0.16, 0.93], p = .17) independent of craving. Of the rs3743078 effect on the FTND score, 65.2% was indirect through craving.
Exploratory analysis
Additional analyses explored the relationships between rs3743078, craving, CPD, and nicotine dependence (FTND). The alternate model of rs3743078 risk associated with increased CPD that was associated with increased craving was supported, with indirect effects (0.55, 95% CI [0.19, 0.88], p = .001) through CPD for rs3743078 and craving. A significant indirect effect through CPD was observed for rs3743078 and FTND (0.79, 95% CI [0.27, 1.26], p = .002). Other FTND items (e.g., time to first cigarette in the morning, difficulty refraining from smoking) were also associated with rs3743078 and behaved similarly to the craving scale; that is, when each item was included in the regression analysis, the association of rs3743078 and the FTND score was weaker (Table 4).
Supplementary analysis
Although there was no detectible population substructure in this Israeli sample alone, when other samples were included in the structure analysis, four subpopulations were identified. When adjusting for population substructure, the total effects of rs3743078 on CPD (4.89, 95% CI [0.13, 9.65], p = .044) and FTND (0.77, 95% CI [0.01, 1.54], p = .048) were reduced, whereas the effect of rs3743078 on craving remained similar (1.08, 95% CI [0.27, 1.89], p = .009). Thus, the indirect effects through craving were significant, even when adjusting for possible population stratification: CPD, indirect effect = 3.30 (95% CI [0.77, 5.70], p = .009); FTND, indirect effect = 0.69 (95% CI [0.16, 1.17], p = .008).
Additional variants: rs684513, rs578776, and rs16969968
Weaker association results (total effects) were seen for the two variants correlated with rs3743078. The rs684513 CC/CG group (prevalence = 95.6%; n = 632) was nominally associated with higher craving (p = .014) and FTND (p = .020), but not with CPD (Table 5); associations were not significant after adjusting for multiple testing. The rs578776 GG/GA group (prevalence = 91.5%; n = 602) was only nominally associated with higher craving (p = .048) and FTND (p = .045) (Table 6). All total effects for rs684513 or rs578776 were nonsignificant when adjusted for rs3743078, and results were not stronger with rs684513/rs3743078/rs578776 haplotypes, since association was driven by rs3743078. Variant rs684513 showed indirect effects through craving for CPD (3.69, 95% CI [0.41, 6.75], p = .023) and FTND (0.76, 95% CI [0.09, 1.37], p = .021), withp values at the .022 cutoff, whereas rs578776 showed indirect effects that were not significant for CPD (2.17, 95% CI [-.24, 4.52], p = .073) and FTND (0.44, 95% CI [-0.06, 0.91], p = .078). No associations between rs16969968 and nicotine phenotypes were observed (Table 7).
Discussion
This study presents novel findings that the rs3743078 variant in the CHRNA5/A3/B4 gene cluster shows indirect effects on nicotine consumption (CPD) and FTND score through craving. We showed that having at least one copy of the major allele (genotypes GG/CG) of rs3743078 is associated with increased nicotine craving, CPD, and FTND scores. Decomposition of these effects suggests that the rs3743078 variant is associated with nicotine craving, which in turn is associated with CPD and FTND scores.
Results from the present study are consistent with a large literature showing that the rs578776/rs3743078-tagged locus is related to CPD and FTND (Bierut et al., 2008; Chen et al., 2009; Greenbaum et al., 2009; Saccone et al., 2007, 2009a, 2009b, 2010; Stevens et al., 2008). Previous studies assumed an additive model (Chen et al., 2009; Greenbaum et al., 2009; Saccone et al., 2009a, 2009b, 2010; Stevens et al., 2008). However, our data fit a recessive model, with similar phenotype values in respondents with one or two copies of the major allele and lower phenotype values in respondents homozygous for the minor (protective) allele.
No previous study analyzed the association of the rs578776/rs3743078 locus with craving. One study showed an association of three related craving measures with CHRNA5/A3/B4 variant rs16969968 (Chen et al., 2012), which is not consistent with our results showing no association. Another study showed an association of a craving scale with joint haplotypes of variants rs16969968 and rs578776, but only among early-onset smokers (Baker et al., 2009). Thus, evidence overall is growing that craving is associated with variants in CHRNA5/A3/B4. However, the extent to which inconsistent results are attributable to differences in craving measures or sample characteristics remains unclear. Further studies using multiple measures of craving across different populations can help elucidate the relationship of craving to CHRNA5/A3/B4 variants, increasing our understanding of its biological underpinnings.
Results indicating that the rs3743078 effect acts through craving support the sensitization-homeostasis model (DiFranza & Wellman, 2005) and other models (e.g., Benowitz, 2010) of craving as central to nicotine use and disorders. Among lifetime smokers, various factors generate craving, such as situational cues, stressful life events, tolerance, or withdrawal (DiFranza & Wellman, 2005). Craving leads to smoking, providing nicotine to the brain, where nicotine binds to receptors and activates neuronal pathways that determine nicotine effects on the smoker. These effects include inhibiting craving, which reduces smoking, until factors generate craving again (DiFranza & Wellman, 2005). Thus, receptor variants (based on CHRN gene variation) that affect aspects of nicotine-receptor biology, such as strength or duration of nicotine’s binding or activation of neuronal cascades (Greenbaum & Lerer, 2009), could also affect craving, subsequently modulating smoking frequency and nicotine dependence.
Although these data support the model of rs3743078 associated with craving, which is associated with consumption/dependence, directionality cannot be determined in cross-sectional data. While the rs3743078 genotype precedes craving and CPD or FTND, the relationships between craving, cigarette consumption, and FTND are complex. Although studies support craving motivating smoking (DiFranza & Wellman, 2005; Doubeni et al., 2010), there is probably a reciprocal relationship, with increased smoking influencing increased craving and increased craving influencing increased smoking. The alternate model of an indirect effect through CPD on craving was also supported. Nevertheless, evidence suggests that controlling craving is key to successfully reducing smoking or quitting (American Cancer Society, 2014; National Health Service, 2012); considering craving the mediator provides a more useful model from the public health perspective. In addition, craving is not the only mediator of the relationship between rs3743078 and nicotine dependence. For example, there was evidence for an indirect effect of rs3743078 on FTND through CPD. Furthermore, inclusion of other FTND items (e.g., time to first cigarette, difficulty in refraining from smoking) results in a weaker association between rs3743078 and FTND score, suggesting possible mediation. Again, the craving mediator model has more utility, since controlling craving could influence consumption, the time to first cigarette, and the difficulty in refraining from smoking, thus reducing FTND score (nicotine dependence). Because mediation analysis in cross-sectional data can be imprecise (Maxwell & Cole, 2007), once support is provided for a specific model, longitudinal studies designed to establish causality should determine the complex relationships between rs3743078, craving, cigarette smoking, and nicotine dependence.
Based on these results indicating that a CHRN variant may influence nicotine phenotypes indirectly via craving, statistical and biological studies can be designed to further our understanding of craving and nicotine use disorders. Functional molecular studies should investigate the mechanism by which variation in rs3743078 (or in correlated variants) affects nicotine outcomes. Identifying additional loci at CHRNA5/A3/B4 or other CHRN genes that similarly affect nicotine phenotypes would increase our understanding of CHRN gene effects. Nicotine binding leads to other neuronal changes, such as activation of the dopamine-mediated reward pathway, hypothesized to play a strong role in nicotine dependence (Rose, 2007). Determining how this and other possible paths to nicotine disorders (e.g., the calming effect of smoking; Rose, 2007) are related to CHRN gene variation and the craving pathway will help elucidate the complex ways in which nicotine disorders can develop and aid in designing tailored interventions.
We note study limitations. First, recall for nicotine phenotypes may be less precise among former than current smokers. However, the effect decomposition (mediation) results were unchanged when adjusted for current smoking status, suggesting that recall issues in former smokers did not affect results. Second, the study omitted an FTND question with poor psychometric properties (“Which cigarette would you hate most to give up”). However, omitting this may actually have strengthened the results by improving the overall psychometric properties of the scale; research in other samples is needed to determine this. Third, we assumed normally distributed outcomes, as regression analysis is generally considered highly robust to deviations from normality in the errors unless the sample is very small (Hayes, 2013). Furthermore, the skew and kurtosis of the regression errors (residuals) are all less than 3, within the range considered acceptable to satisfy the normality assumption (Kline, 2010). Fourth, the cross-sectional design limits the ability to determine the directionality of these associations, although the directions modeled in this study are based on a theoretical model (DiFranza & Wellman, 2005) and supported by the results. Longitudinal studies are warranted to determine the exact temporal relationships between rs3743078, craving, and cigarette consumption or nicotine dependence. Fifth, replication in larger samples, specifically with more individuals in the rs3743078 CC genotype group (low risk), should be conducted to further explore the indirect effects of rs3743078 via craving and provide more precise effect estimates. Last, variables that are affected by rs3743078 and that influence the relationship between craving and consumption or dependence (e.g., response to smoking, including a calming effect; withdrawal) may exist. More complex models incorporating such variables should be developed and examined in the future.
The lack of observed association between rs16969968 and nicotine phenotypes in this sample was inconsistent with most previous studies but consistent with others. Although rs16969968 is considered robustly associated with CPD based on meta-analyses in populations of European origin (Furberg et al., 2010; Liu et al., 2010; Saccone et al., 2010), some of the individual data sets within the meta-analyses did not show a significant association between rs16969968 and CPD (e.g., Figure 1 in Saccone et al., 2010). Although previous studies showed an association between rs16969968 and FTND (reviewed in Ware et al., 2012), other studies did not find evidence for this association (Sherva et al., 2010; Wessel et al., 2010); evidence for this association is less robust than for CPD. As additional evidence accumulates from diverse populations with different genetic backgrounds and motivations for smoking, the relationship between rs16969968 and smoking phenotypes may become better understood.
Furthermore, genetic studies in Jewish samples can provide unique information because such samples have different frequencies for some genetic variants than populations of European origin (Behar et al., 2010; Guha et al., 2012; Ostrer & Skorecki, 2013). For example, differences were observed in the prevalence of alleles protective against alcoholism (e.g., ADH1B*2; Hasin et al., 2002; Neumark et al., 1998) and in the prevalence of risk alleles associated with type 1 diabetes and Alzheimer’s disease (Guha et al., 2012). Analysis in diverse populations can indicate the robustness of genetic associations or can suggest more complex relationships. This Jewish sample has CHRNA5/A3/B4 variant allele frequencies similar to other populations of European descent and similar linkage disequilibrium structure, with high D between the rs16969968 and rs3743078 loci, but low r2 (Greenbaum & Lerer, 2009; Saccone et al., 2007, 2009a, 2009b; Sherva et al., 2010). These similarities suggest that CHRNA5/A3/B4 allele prevalence and genetic architecture are not the main reasons underlying differences between other studies and our results (i.e., recessive model for rs3743078 and no overall association with rs16969968). In addition, the evidence for overall association between rs3743078 and CPD or FTND was weaker when adjusting for potential population substructure that was only identified when including non-Israeli samples. This most likely does not indicate spurious associations, given the previous studies providing strong evidence for association of rs3743078 and nicotine-related phenotypes. Rather, the weaker evidence might suggest the presence of other genetic factors that are related to the unique Jewish genetic background (and accounted for in the adjustment for population substructure) and are associated with both the CHRNA5/A3/B4 locus and nicotine-related phenotypes. These issues indicate the need for further studies to identify other genetic or environmental factors that act together with CHRNA5/A3/B4 variants to influence nicotine phenotypes.
Conclusions
In conclusion, we examined the role of craving in the relationship between a genetic risk factor (rs3743078 in CHRNA5/A3/B4) and two nicotine phenotypes. This is the first study to provide evidence for indirect effects of rs3743078 on consumption and dependence through craving. This study has implications for further studies of the CHRNA5/A3/B4 rs3743078 locus and these phenotypes because it suggests that information is lost by not including craving in these models, and it provides the basis for constructing more complex models incorporating other genetic loci or phenotypic variables. Furthermore, these results provide support for models positing that craving is central to nicotine disorders and may have utility as a target of therapeutic interventions, setting the stage for longitudinal studies to confirm these findings. Understanding how genetic risk factors potentially influence phenotypes provides important insight into the etiology of nicotine behaviors and disorders, potentially leading to better interventions.
Footnotes
This research was funded by National Institutes of Health Grants R01AA013654, R01DA018652, K05AA014223 (to Deborah Hasin), K23DA016743 (to Efrat Aharonovich), R01AA11330 (to Joel Gelernter), and T32MH13043 (to Jacquelyn L. Meyers), and the New York State Psychiatric Institute (to Deborah Hasin and Melanie M. Wall).
References
- American Cancer Society. 2014 Quitting smoking: Help for cravings and tough situations. Retrieved from http://www.cancer.org/healthy/stayawayfromtobacco/quitting-smoking-help-for-cravings-and-tough-situations.
- Baker T. B., Weiss R. B., Bolt D., von Niederhausern A., Fiore M. C., Dunn D. M., Cannon D. S. Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine & Tobacco Research. 2009;11:785–796. doi: 10.1093/ntr/ntp064. doi:10.1093/ntr/ntp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Epel O., Haviv-Messika A., Tamir D., Nitzan-Kaluski D., Green M. Multiethnic differences in smoking in Israel: Pooled analysis from three national surveys. European Journal of Public Health. 2004;14:384–389. doi: 10.1093/eurpub/14.4.384. doi:10.1093/eurpub/14.4.384. [DOI] [PubMed] [Google Scholar]
- Behar D. M., Yunusbayev B., Metspalu M., Metspalu E., Rosset S., Parik J., Villems R. The genome-wide structure of the Jewish people. Nature. 2010;466:238–242. doi: 10.1038/nature09103. doi:10.1038/nature09103. [DOI] [PubMed] [Google Scholar]
- Benowitz N. L. Nicotine addiction. The New England Journal of Medicine. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. doi:10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut L. J., Stitzel J. A., Wang J. C., Hinrichs A. L., Grucza R. A., Xuei X., Goate A. M. Variants in nicotinic receptors and risk for nicotine dependence. American Journal of Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. doi:10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy S. J., Victor J. C., Diemert L. M. Origin and use of the 100 cigarette criterion in tobacco surveys. Tobacco Control. 2009;18:317–323. doi: 10.1136/tc.2008.027276. doi:10.1136/tc.2008.027276. [DOI] [PubMed] [Google Scholar]
- Broms U., Wedenoja J., Largeau M. R., Korhonen T., Pitkäniemi J., Keskitalo-Vuokko K., Loukola A. Analysis of detailed phenotype profiles reveals CHRNA5-CHRNA3-CHRNB4 gene cluster association with several nicotine dependence traits. Nicotine & Tobacco Research. 2012;14:720–733. doi: 10.1093/ntr/ntr283. doi:10.1093/ntr/ntr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C. S., Matise T. C., North K. E., Haiman C. A., Fesinmeyer M. D., Buyske S., Kooperberg C. L. the PAGE Consortium. Generalization and dilution of association results from European GWAS in populations of non-European ancestry: The PAGE study. PLoS Biology. 2013;11(9):e1001661. doi: 10.1371/journal.pbio.1001661. doi:10.1371/journal.pbio.1001661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrol H., Niezborala M., Chastan E., Montastruc J. L., Mullet E. A study of the psychometric properties of the Fagerström Test for Nicotine Dependence. Addictive Behaviors. 2003;28:1441–1445. doi: 10.1016/s0306-4603(02)00236-8. doi:10.1016/S0306-4603(02)00236-8. [DOI] [PubMed] [Google Scholar]
- Chen L.-S., Baker T. B., Grucza R., Wang J. C., Johnson E. O., Breslau N., Bierut L. J. Dissection of the phenotypic and genotypic associations with nicotinic dependence. Nicotine & Tobacco Research. 2012;14:425–433. doi: 10.1093/ntr/ntr231. doi:10.1093/ntr/ntr231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.-S., Johnson E. O., Breslau N., Hatsukami D., Saccone N. L., Grucza R. A., Bierut L. J. Interplay of genetic risk factors and parent monitoring in risk for nicotine dependence. Addiction. 2009;104:1731–1740. doi: 10.1111/j.1360-0443.2009.02697.x. doi:10.1111/j.1360-0443.2009.02697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza J. R., Wellman R. J. A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: Integrating the clinical and basic science literature. Nicotine & Tobacco Research. 2005;7:9–26. doi: 10.1080/14622200412331328538. doi:10.1080/14622200412331328538. [DOI] [PubMed] [Google Scholar]
- Doubeni C. A., Reed G., DiFranza J. R. Early course of nicotine dependence in adolescent smokers. Pediatrics. 2010;125:1127–1133. doi: 10.1542/peds.2009-0238. doi:10.1542/peds.2009-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J. F., Duc T. V., Perneger T. V. Validity of the Fagerström Test for Nicotine Dependence and of the Heaviness of Smoking Index among relatively light smokers. Addiction. 1999;94:269–281. doi: 10.1046/j.1360-0443.1999.94226910.x. doi:10.1046/j.1360-0443.1999.94226910.x. [DOI] [PubMed] [Google Scholar]
- Fagerström K., Furberg H. A comparison of the Fagerström Test for Nicotine Dependence and smoking prevalence across countries. Addiction. 2008;103:841–845. doi: 10.1111/j.1360-0443.2008.02190.x. doi:10.1111/j.1360-0443.2008.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furberg H., Kim Y. J., Dackor J., Boerwinkle E., Franceschini N., Ardissino D., Sullivan P. F. the Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nature Genetics. 2010;42:441–447. doi: 10.1038/ng.571. doi:10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. F., Dawson D. A., Stinson F. S., Chou P. S., Kay W., Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): Reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug and Alcohol Dependence. 2003;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. doi:10.1016/S0376-8716(03)00070-X. [DOI] [PubMed] [Google Scholar]
- Grant B. F., Harford T. C., Dawson D. A., Chou P. S., Pickering R. P. The Alcohol Use Disorder and Associated Disabilities Interview schedule (AUDADIS): Reliability of alcohol and drug modules in a general population sample. Drug and Alcohol Dependence. 1995;39:37–44. doi: 10.1016/0376-8716(95)01134-k. doi:10.1016/0376-8716(95)01134-K. [DOI] [PubMed] [Google Scholar]
- Greenbaum L., Lerer B. Differential contribution of genetic variation in multiple brain nicotinic cholinergic receptors to nicotine dependence: recent progress and emerging open questions. Molecular Psychiatry. 2009;14:912–945. doi: 10.1038/mp.2009.59. doi:10.1038/mp.2009.59. [DOI] [PubMed] [Google Scholar]
- Greenbaum L., Rigbi A., Teltsh O., Lerer B. Role of genetic variants in the CHRNA5–CHRNA3–CHRNB4 cluster in nicotine dependence risk: importance of gene–environment interplay [Letter to the editor] Molecular Psychiatry. 2009;14:828–830. doi: 10.1038/mp.2009.25. doi:10.1038/mp.2009.25. [DOI] [PubMed] [Google Scholar]
- Guha S., Rosenfeld J. A., Malhotra A. K., Lee A. T., Gregersen P. K., Kane J. M., Lencz T. Implications for health and disease in the genetic signature of the Ashkenazi Jewish population. Genome Biology. 2012;13:R2. doi: 10.1186/gb-2012-13-1-r2. doi:10.1186/gb-2012-13-1-r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D., Aharonovich E., Liu X., Mamman Z., Matseoane K., Carr L., Li T. K. Alcohol and ADH2 in Israel: Ashkenazis, Sephardics, and recent Russian immigrants. American Journal of Psychiatry. 2002;159:1432–1434. doi: 10.1176/appi.ajp.159.8.1432. doi:10.1176/appi.ajp.159.8.1432. [DOI] [PubMed] [Google Scholar]
- Hayes A. F. Introduction to mediation, moderation, and conditional process analysis. New York, NY: Guilford Press; 2013. [Google Scholar]
- He J.-Q., Hallstrand T. S., Knight D., Chan-Yeung M., Sandford A., Tripp B., Daley D. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. Journal of Allergy and Clinical Immunology. 2009;124:222–229. doi: 10.1016/j.jaci.2009.04.018. doi:10.1016/j.jaci.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Martins S. S., Shmulewitz D., Santaella J., Wall M. M., Keyes K. M., Hasin D. S. Childhood maltreatment, stressful life events, and alcohol craving in adult drinkers. Alcoholism: Clinical and Experimental Research. 2014;38:2048–2055. doi: 10.1111/acer.12473. doi:10.1111/acer.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline P. Handbook of psychological testing. 2nd ed. London, England: Routledge; 2000. [Google Scholar]
- Kline R. B. Principles and practice of structural equation modeling. 3rd ed. New York, NY: Guilford Press; 2010. [Google Scholar]
- Listman J. B., Hasin D., Kranzler H. R., Malison R. T., Mutirangura A., Sughondhabirom A., Gelernter J. Identification of population substructure among Jews using STR markers and dependence on reference populations included. BMC Genetics. 2010;11:48. doi: 10.1186/1471-2156-11-48. doi:10.1186/1471-2156-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Z., Tozzi F., Waterworth D. M., Pillai S. G., Muglia P., Middleton L., Marchini J. the Wellcome Trust Case Control Consortium. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nature Genetics. 2010;42:436–440. doi: 10.1038/ng.572. doi:10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes H. H., Sullivan P. F., Bulik C. M., Neale M. C., Prescott C. A., Eaves L. J., Kendler K. S. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychological Medicine. 2004;34:1251–1261. doi: 10.1017/s0033291704002405. doi:10.1017/S0033291704002405. [DOI] [PubMed] [Google Scholar]
- Maxwell S. E., Cole D. A. Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods. 2007;12:23–44. doi: 10.1037/1082-989X.12.1.23. doi:10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- National Health Service. 2012 Stop smoking: Coping with cravings. Retrieved from http://www.nhs.uk/Livewell/smoking/Pages/Copingwithcravings.aspx.
- Neumark Y. D., Friedlander Y., Thomasson H. R., Li T. K. Association of the ADH2*2 allele with reduced ethanol consumption in Jewish men in Israel: A pilot study. Journal of Studies on Alcohol. 1998;59:133–139. doi: 10.15288/jsa.1998.59.133. doi:10.15288/jsa.1998.59.133. [DOI] [PubMed] [Google Scholar]
- Nyholt D. R. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. American Journal of Human Genetics. 2004a;74:765–769. doi: 10.1086/383251. doi:10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholt D. Matrix Spectral Decomposition (matSpD) - estimate the equivalent number of independent variables in a correlation (r) matrix. 2004b Retrieved from http://neurogenetics.qimrberghofer.edu.au/matSpD/
- Ostrer H., Skorecki K. The population genetics of the Jewish people. Human Genetics. 2013;132:119–127. doi: 10.1007/s00439-012-1235-6. doi:10.1007/s00439-012-1235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M. E., Piasecki T. M., Federman E. B., Bolt D. M., Smith S. S., Fiore M. C., Baker T. B. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. doi:10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Pritchard J. K., Stephens M., Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. E. Multiple brain pathways and receptors underlying tobacco addiction. Biochemical Pharmacology. 2007;74:1263–1270. doi: 10.1016/j.bcp.2007.07.039. doi:10.1016/j.bcp.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Russell M. A. H., Peto J., Patel U. A. The Classification of Smoking by Factorial Structure of Motives. Journal of the Royal Statistical Society. Series A (General) 1974;137:313–346. doi:10.2307/2344953. [Google Scholar]
- Saccone N. L., Culverhouse R. C., Schwantes-An T.-H., Cannon D. S., Chen X., Cichon S., Bierut L. J. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLOS Genetics. 2010;6(8):e1001053. doi: 10.1371/journal.pgen.1001053. doi:10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone N. L., Saccone S. F., Hinrichs A. L., Stitzel J. A., Duan W., Pergadia M. L., Bierut L. J. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009a;150B:453–466. doi: 10.1002/ajmg.b.30828. doi:10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone N. L., Wang J. C., Breslau N., Johnson E. O., Hatsukami D., Saccone S. F., Bierut L. J. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Research. 2009b;69:6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. doi:10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone S. F., Hinrichs A. L., Saccone N. L., Chase G. A., Konvicka K., Madden P. A. F., Bierut L. J. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Human Molecular Genetics. 2007;16:36–49. doi: 10.1093/hmg/ddl438. doi:10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherva R., Kranzler H. R., Yu Y., Logue M. W., Poling J., Arias A. J., Gelernter J. Variation in nicotinic acetylcholine receptor genes is associated with multiple substance dependence phenotypes. Neuropsychopharmacology. 2010;35:1921–1931. doi: 10.1038/npp.2010.64. doi:10.1038/npp.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S., Waters A., Hickcox M. The nicotine dependence syndrome scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6:327–348. doi: 10.1080/1462220042000202481. doi:10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Shmulewitz D., Keyes K., Beseler C., Aharonovich E., Aivadyan C., Spivak B., Hasin D. The dimensionality of alcohol use disorders: Results from Israel. Drug and Alcohol Dependence. 2010;111:146–154. doi: 10.1016/j.drugalcdep.2010.04.002. doi:10.1016/j.drugalcdep.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmulewitz D., Keyes K. M., Wall M. M., Aharonovich E., Aivadyan C., Greenstein E., Hasin D. Nicotine dependence, abuse and craving: Dimensionality in an Israeli sample. Addiction. 2011;106:1675–1686. doi: 10.1111/j.1360-0443.2011.03484.x. doi:10.1111/j.1360-0443.2011.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmulewitz D., Wall M. M., Aharonovich E., Spivak B., Weizman A., Frisch A., Hasin D. Validity of proposed DSM-5 diagnostic criteria for nicotine use disorder: Results from 734 Israeli lifetime smokers. Psychological Medicine. 2013;43:2179–2190. doi: 10.1017/S0033291712002954. doi:10.1017/S0033291712002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulakova J. N., Hartman A. M., Liu B., Willis G. B., Augustine S. Reliability of adult self-reported smoking history: Data from the tobacco use supplement to the current population survey 2002-2003 cohort. Nicotine & Tobacco Research. 2012;14:952–960. doi: 10.1093/ntr/ntr313. doi:10.1093/ntr/ntr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens V. L., Bierut L. J., Talbot J. T., Wang J. C., Sun J., Hinrichs A. L., Calle E. E. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiology, Biomarkers & Prevention. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. doi:10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson T. E., Gudbjartsson D. F., Surakka I., Vink J. M., Amin N., Geller F., Stefansson K. the ENGAGE Consortium. Sequence variants at CHRNB3–CHRNA6 and CYP2A6 affect smoking behavior. Nature Genetics. 2010;42:448–453. doi: 10.1038/ng.573. doi:10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany S. T., Wray J. M. The clinical significance of drug craving. Annals of the New York Academy of Sciences. 2012;1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x. doi:10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt V. T., Bordeleau M., Laviolette M., Laprise C. From expression pattern to genetic association in asthma and asthma-related phenotypes. BMC Research Notes. 2012;5:630. doi: 10.1186/1756-0500-5-630. doi:10.1186/1756-0500-5-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J. J., van den Bree M., Munafò M. R. From men to mice: CHRNA5/CHRNA3, smoking behavior and disease. Nicotine & Tobacco Research. 2012;14:1291–1299. doi: 10.1093/ntr/nts106. doi:10.1093/ntr/nts106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J., McDonald S. M., Hinds D. A., Stokowski R. P., Javitz H. S., Kennemer M., Bergen A. W. Resequencing of nicotinic acetylcholine receptor genes and association of common and rare variants with the Fagerström Test for Nicotine Dependence. Neuropsychopharmacology. 2010;35:2392–2402. doi: 10.1038/npp.2010.120. doi:10.1038/npp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2011 WHO report on the global tobacco epidemic, 2011: Warning about the dangers of tobacco. Retrieved from http://whqlibdoc.who.int/publications/2011/9789240687813_eng.pdf.