Key points
KCNE4 alters the biophysical properties and cellular localization of voltage‐gated potassium channel Kv7.4.
KCNE4 is expressed in a variety of arteries and, in mesenteric arteries, co‐localizes with Kv7.4, which is important in the control of vascular contractility.
Knockdown of KCNE4 leads to reduced Kv7.4 membrane abundance, a depolarized membrane potential and an augmented response to vasoconstrictors.
KCNE4 is a key regulator of the function and expression of Kv7.4 in vascular smooth muscle.
Abstract
The KCNE ancillary subunits (KCNE1–5) significantly alter the expression and function of voltage‐gated potassium channels; however, their role in the vasculature has yet to be determined. The present study aimed to investigate the expression and function of the KCNE4 subunit in rat mesenteric arteries and to determine whether it has a functional impact on the regulation of arterial tone by Kv7 channels. In HEK cells expressing Kv7.4, co‐expression of KCNE4 increased the membrane expression of Kv7.4 and significantly altered Kv7.4 current properties. Quantitative PCR analysis of different rat arteries found that the KCNE4 isoform predominated and proximity ligation experiments showed that KCNE4 co‐localized with Kv7.4 in mesenteric artery myocytes. Morpholino‐induced knockdown of KCNE4 depolarized mesenteric artery smooth muscle cells and resulted in their increased sensitivity to methoxamine being attenuated (mean ± SEM EC50 decreased from 5.7 ± 0.63 μm to 1.6 ± 0.23 μm), which coincided with impaired effects of Kv7 modulators. When KCNE4 expression was reduced, less Kv7.4 expression was found in the membrane of the mesenteric artery myocytes. These data show that KCNE4 is consistently expressed in a variety of arteries, and knockdown of the expression product leads to reduced Kv7.4 membrane abundance, a depolarized membrane potential and an augmented response to vasoconstrictors. The present study is the first to demonstrate an integral role of KCNE4 in regulating the function and expression of Kv7.4 in vascular smooth muscle.
Key points
KCNE4 alters the biophysical properties and cellular localization of voltage‐gated potassium channel Kv7.4.
KCNE4 is expressed in a variety of arteries and, in mesenteric arteries, co‐localizes with Kv7.4, which is important in the control of vascular contractility.
Knockdown of KCNE4 leads to reduced Kv7.4 membrane abundance, a depolarized membrane potential and an augmented response to vasoconstrictors.
KCNE4 is a key regulator of the function and expression of Kv7.4 in vascular smooth muscle.
Abbreviations
- Kv
voltage‐gated potassium channel
- PBST
PBS supplemented with 0.1% Triton X‐100
- PLA
proximity ligation assay
- QPCR
quantitative PCR
- SMDS
smooth muscle dissection solution
Introduction
KCNE4 is a member of the KCNE gene family, which consist of five isoforms (KCNE1–5). The KCNE genes encode single transmembrane domain proteins with an extracellular N‐terminus and an intracellular C‐terminus. These proteins cannot form functional ion channels themselves but, instead, function as ancillary subunits to various ion channels and regulate several properties of the channel, including their membrane trafficking, biophysical properties and pharmacology (McCrossan and Abbott, 2004; Li et al. 2006; Kanda & Abbott, 2012). In overexpression studies, KCNE4 expression was shown to differentially modulate the function of certain voltage‐gated potassium channels (Kv), particularly those encoded by KCNQ genes (Kv7.1–7.5; Grunnet et al. 2002; Strutz‐Seebohm et al. 2006; Manderfield et al. 2008; Roura‐Ferrer et al. 2009; Roura‐Ferrer et al. 2010). Expression of KCNE4 increases the current amplitude of overexpressed Kv7.4 channels (Strutz‐Seebohm et al. 2006), inhibits the Kv7.1 current (Grunnet et al. 2002) and has no effect on the Kv7.5 channel (Roura‐Ferrer et al. 2009). However, very little is known about the function of KCNE4 in native tissues. Interestingly, KCNE4 expression has been detected in various smooth muscle tissues, including arteries (Yeung et al. 2007; Zhong et al. 2010; Khanamiri et al. 2013), the uterus (McCallum et al. 2009) and the gastrointestinal tract (Jepps et al. 2009); however, the function of this protein in smooth muscle remains elusive.
It is now established that certain Kv7 family members, encoded for by the KCNQ genes, are important for regulating vascular contractility in a wide range of rodent and human blood vessels (Yeung et al. 2007; Yeung et al. 2008; Mackie et al. 2008; Joshi et al. 2009; Zhong et al. 2010; Ng et al. 2011; Jepps et al. 2011; Chadha et al. 2012; Khanamiri et al. 2013; Chadha et al. 2014; Brueggemann et al. 2014; Stott et al. 2015 a,b). KCNQ1, KCNQ4 and KCNQ5 are expressed predominantly across the vasculature (Yeung et al. 2007; Ng et al. 2011; Jepps et al. 2014) with Kv7.4 and Kv7.5 heterotetramers considered to be the dominant molecular species in vascular smooth muscle cells (Chadha et al. 2014; Brueggemann et al. 2014). Blockade of Kv7 channels produces vasoconstriction in several rodent blood vessels (Yeung et al. 2007; Joshi et al. 2009; Zhong et al. 2010; Jepps et al. 2011), as well as human visceral adipose and mesenteric arteries (Ng et al. 2011), and impairs receptor‐meditated vasorelaxations in the renal artery (Chadha et al. 2012; Stott et al. 2015 a, b), cerebral artery (Chadha et al. 2014) and coronary artery (Khanamiri et al. 2013). Moreover, attenuation of Kv7 channel function occurs in several arteries from hypertensive animals, which was associated with a considerable reduction in Kv7.4 protein expression (Jepps et al. 2011; Chadha et al. 2012; Stott et al. 2015 a). As critical regulators of vascular tone, in health and disease, it is essential that we have a comprehensive knowledge of how the Kv7 channels are regulated.
Considering the expression of KCNE4 in different smooth muscle tissues and its channel‐specific modulation of the Kv7 family members, we hypothesized that KCNE4 interacts with Kv7.4 in the vasculature to regulate its function and/or expression, thereby playing an important role in the regulation of vascular tone. In the present study, we report that KCNE4 is expressed in a range of rat arteries, and is co‐localized with Kv7.4. We also determine, using molecular interference of KCNE4 protein expression levels, a functional impact on the reactivity of mesenteric arteries, which is associated with a reduction of Kv7.4 in the membrane of vascular smooth muscle cells. These data suggest that KCNE4 expression products have a regulatory role on Kv7 channel activity in rat mesenteric arteries, ultimately impacting upon the level of arterial tone.
Methods
Ethical approval
All experiments were performed in accordance with the UK Animal (Scientific Procedures) Act 1986 or, in Denmark, conformed to the Principles of Laboratory Animal Care (National Institutes of Health, revised 1996) approved by the national ethics committee.
Animals
Male Wistar rats (Taconic, Ejby, Denmark), 12–16 weeks of age, were killed by Schedule 1 cervical dislocation, according to the European Directive 2010/63/EU.
Quantitative PCR (QPCR)
The relative expression of the KCNE1‐5 mRNA isoforms was determined in the rat thoracic aorta, renal artery and third‐order mesenteric artery by QPCR analysis, as described previously (Jepps et al. 2014). Quantitative analysis of the KCNE genes within our cDNA samples was determined using Precision‐iC SYBR green mastermix (PrimerDesign Ltd, Southampton, UK) with the CFX96 Real‐Time PCR Detection System (Bio‐Rad, Hemel Hempstead, UK). The cycling conditions were: initial activation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min, and data were collected during each cycling phase. Melt curve analysis, to ensure each primer set amplified a single specific product, completed the protocol. Quantification cycle (Cq) values were determined using CFX96 Manager, version 3.0 (Bio‐Rad). To identify the optimal reference genes required for reliable normalization of the genes of interest in our samples, we used the geNorm reference gene selection kit (PrimerDesign Ltd) (Vandersompele et al. 2002). The data were analysed with Biogazelle qbase PLUS (Biogazelle NV, Zwijnaarde, Belgium), which reports the best reference genes and the number of reference genes required for each artery. Under our experimental conditions, the optimal reference genes for the rat mesenteric artery were CANX and CYC1. The relative expression of the KCNE isoforms was calculated relative to these reference genes. All reference genes in the rat geNorm reference gene selection kit and the KCNE1‐5 assays (Table 1) were designed and optimized by PrimerDesign Ltd.
Table 1.
KCNE assays used for QPCR experiments
| Gene | Primer sequence (+) sense, (−) antisense | GenBank accession number | Amplicon (bp) | Region spanned |
|---|---|---|---|---|
| KCNE1 | (+) 5′‐ACTCGCACGACCCTTTCA‐3′ | NM_012973 | 114 | 284–397 |
| (−) 5′‐TTTCAATGACATAGCAAGCTCTG‐3′ | ||||
| KCNE2 | (+) 5′‐TGTCATTTAAGTCCATTCCAATCAT‐3′ | NM_133603 | 116 | 769–884 |
| (−) 5′‐TGAGAAAGAAGGTTGAAAGATTTGT‐3′ | ||||
| KCNE3 | (+) 5′‐TTATGATGTCTGAGGATTGTCTTCT‐3′ | NM_022235 | 114 | 449–562 |
| (−) 5′ TGACCTAACTCTCTTACCAATTTCT‐3′ | ||||
| KCNE4 | (+) 5′‐CCCTTGAGTCCCATGTGTCT‐3′ | NM_212526 | 113 | 135–247 |
| (−) 5′‐GTAGCCCAGCATGATTCCAAT‐3′ | ||||
| KCNE5 | (+) 5′‐GTCAACGGCGTCCTGGAG‐3′ | NM_00110100 | 96 | 27–122 |
| (−) 5′‐CAGCAGCAAGCGGTTCAA‐3′ |
Mesenteric artery smooth muscle cell dispersal
Third‐order mesenteric arteries were placed in a smooth muscle dissection solution (SMDS) containing (in mmol L−1): 60 NaCl, 80 sodium glutamate, 5 KCl, 2 MgCl2, 10 glucose and 10 Hepes (pH 7.4) at 37°C for 10 min. Single myocytes were enzymatically isolated by being placed in SMDS containing BSA (1 mg ml−1; Sigma, St Louis, MO, USA), papain (0.5 mg ml−1; Sigma) and dithiothrietol (1.5 mg ml−1) at 37°C for 8–10 min. The vessels were then washed in ice‐cold SMDS before being incubated in SMDS containing 100 μmol L−1 Ca2+, BSA (1 mg ml−1) and collagenase (0.7 mg ml−1 type F and 0.4 mg ml−1 type H; Sigma) at 37°C for 8–10 min. The vessels were then washed in ice‐cold SMDS followed by gentle trituration with a fire‐polished pipette to liberate single myocytes from the digested vessels, which were kept in ice‐cold SMDS to be used within 5 h.
Immunocytochemistry
Freshly dissociated rat mesenteric artery myocytes or HEK cells were allowed to adhere to coverslips before being fixed in 4% paraformaldehyde (Sigma) in PBS for 30 min at room temperature. Blocking and permeabilization was performed by a 30 min incubation with 0.2% fish skin gelatin in PBS supplemented with 0.1% Triton X‐100 (PBST). The cells were incubated for 1 h in primary antibodies diluted in PBST. Primary antibodies were rabbit anti‐KCNE4 (dilution 1:200; HPA011420; Sigma) and mouse anti‐Kv7.4 (dilution 1:200; 73–082; Neuromab, Davis, CA, USA). Secondary antibodies were goat anti‐rabbit 488 and donkey anti‐mouse 555 (Alexa Fluor, Life Technologies, Nærum, Denmark), which were diluted in PBST and applied for 45 min. The coverslips were mounted in Prolong Gold (Life Technologies). Cells were visualized using an LSM 510 confocal microscope (Carl Zeiss, Oberkochen, Germany). Mid‐cell xy‐sections were selected and analysed using Zen software (Carl Zeiss). For each experiment, the same microscope settings and exposure times were used so that cells could be directly compared. Total cell fluorescence and intracellular fluorescence signals were quantified using Zen 2012 confocal software. Signals originating from the surface membrane were obtained by subtracting the total intracellular signal from the total cell fluorescence signal, with both normalized to their respective areas, and background fluorescence was subtracted. The surface‐associated signal was subsequently expressed as a percentage of the total cell fluorescence, as described previously (Andersen et al. 2013).
Western blots
Protein was isolated and western blots performed as described previously (Jepps et al. 2011). The primary antibodies used in the present study were rabbit anti‐KCNE4 (dilution 1:200; HPA011420; Sigma), rabbit anti‐Kv7.4 (1:200; sc‐50417; Santa Cruz Biotechnology, Santa Cruz, TX, USA) and mouse β‐Actin (dilution 1:5000; A1978; Sigma). Protein bands were visualized using fluorescently conjugated secondary antibodies raised in rabbit or mouse as appropriate (both at dilution 1:10,000; Li‐Cor Biosciences, Cambridge, UK), imaged on the Odyssey Infrared Imaging System (Li‐Cor Biosciences) and analysed with Image Studio, version 3.0 (Li‐Cor Biosciences).
Proximity ligation assay (PLA)
PLA technology is a well‐established technique for detecting proteins that reside within 40 nm (Söderberg et al. 2008; Chadha et al. 2014; Brueggemann et al. 2014) and was used to determine the co‐localization of Kv7.4, Kv7.5 with KCNE4 proteins in freshly dissociated rat mesenteric artery myocytes using the Duolink in situ (PLA) detection kit 563 (Olink, Uppsala, Sweden) in accordance with the manufacturer's instructions. Briefly, isolated third‐order mesenteric artery myocytes were fixed for 20 min in PBS containing 3% paraformaldehyde, before being permeabilized in PBS containing 0.1% Triton X‐100 for 15 min. To reduce non‐specific binding, cells were then blocked for 1 h at 37°C in Duolink blocking solution, and incubated overnight at 4°C with pairs of primary antibodies (all at dilution 1:200) in Duolink antibody diluent solution. Control experiments omitted the primary antibody. The primary antibodies employed in the present study were mouse anti‐Kv7.4 (75‐082; NeuroMab), rabbit anti‐Kv7.4 (sc‐50417; Santa Cruz Biotechnology), goat anti‐Kv7.5 (sc‐18046; Santa Cruz Biotechnology) and rabbit anti‐KCNE4 (HPA011420; Sigma). Cells were subsequently labelled with Duolink PLA anti‐rabbit PLUS and anti‐goat MINUS or anti‐rabbit PLUS and anti‐mouse MINUS probes, as appropriate, for 1 h at 37°C. The secondary antibodies of PLA PLUS and MINUS probes are attached to synthetic oligonucleotides and, if the target proteins are localized sufficiently close together (<40 nm), the oligonucleotides bound to the antibodies will hybridize and form a circular DNA strand. The hybridized oligonucleotides are then ligated for 30 min at 37°C prior to rolling circle amplification for 100 min at 37°C. Fluorescently‐labelled oligonucleotides hybridize to the rolling circle amplification product. Red punctae, indicating that two proteins are within 40 nm of each other, were visualized using an LSM 510 confocal microscope (Carl Zeiss). Mid‐cell xy‐sections were selected and the number of punctae was assessed by two independent, blinded researchers. Statistical analysis comprised one‐way ANOVA and Tukey's multiple comparisons test.
HEK cells transfection and voltage‐clamp electrophysiology
Monoclonal HEK293 cells stably expressing human Kv7.4 (the details of the generation of these cells are provided in Søgaard et al. 2000) were grown in Dulbecco's modified Eagle's medium (Substrate Department, the Panum Institute, Copenhagen, Denmark), supplemented with 10% fetal bovine serum (Th Geyer, Roskilde, Denmark) and 1% penicillin/streptomycin, and incubated at 37°C in 5% CO2. These cells were transiently transfected with human KCNE4 using siLentFectTM Lipid (Bio‐Rad, Copenhagen, Denmark) in accordance with the manufacturer's instructions. The human KCNE4 gene, obtained from IMAGE Consortium (EST 6023884), was inserted into a pXOOM vector. Kv7.4 currents were recorded from HEK cells with and without KCNE4 expression using the β‐escin (50 μmol L−1) perforated‐patch technique in voltage‐clamp mode. Cells expressing KCNE4 were identified using green fluorescent protein. Voltage‐clamp experiments were also performed in the same manner on isolated mesenteric artery myocytes that were transfected with a targeted or control KCNE4 morpholino (see below). Fire‐polished patch pipettes had a resistance of 2–4 MΩ and the internal and external solutions were the same as those used in the current‐clamp experiments on the same patch‐clamp set‐up. Currents were elicited from a holding potential of −80 mV using a step protocol with + 10 mV steps to a maximum potential of 50 mV, with 750 ms duration. For each test potential, the current amplitude was taken after 700 ms. A tail current was measured by stepping to −30 mV before stepping back to a holding potential of −80 mV.
Morpholino transfections
We knocked‐down specific proteins in whole blood vessels using morpholino oligos targeting either KCNE4 or Kv7.4. The KCNE4 morpholino was designed to block translation initiation in the cytosol by targeting the 5′ untranslated region through the first 25 bases of coding sequence. The Kv7.4 morpholino was designed to modify pre‐mRNA splicing in the nucleus by targeting splice junctions. Both of these morpholino strategies can result in reduced protein expression of the intended target. Compared to other knockdown systems employed, such as small‐inteferring RNA, morpholinos have excellent anti‐sense properties as a result of the increased complementarity with their target RNAs and they are also free of the widespread off‐target expression modulation often observed in other knockdown systems (Summerton 1999; 2007) Each targeted morpholino is also provided with a specific control morpholino that has five bases altered from the targeted sequence, which offers increased assurance that the effects observed are not the result of off‐target modulation. In addition, morpholinos are completely stable and do not induce immune responses (Summerton 1999; 2007).
Morpholino oligonucleotides (Gene Tools Inc., Philomath, OR, USA) were mixed with Lipofectamine 2000 (Life Technologies) in Opti‐MEM and left at room temperature for 2 h. The Opti‐MEM mix was then added to Dulbecco's modified Eagle's medium containing Hepes, glutamax and NaHCO3 (Substrate Department, the Panum Institute), and the third‐order mesenteric arteries were placed in this solution at 37°C for 36 h. The morpholinos used in the present study were a KCNE4‐targeted morpholino and a Kv7.4‐targeted morpholino, with their respective control morpholino oligonucleotides. Successful knockdown of the protein with the targeted morpholino was assessed by western blotting and compared with the respective control morpholino.
Myography
Third‐order mesenteric artery segments (∼2 mm) were mounted in a myograph (Danish Myo Technology, Aarhus, Denmark) containing physiological salt solution, maintained at 37°C and aerated with 95 % O2/5 % CO2 for isometric tension recording. The composition of the physiological salt solution in the chambers comprised (in mmol L−1) 125 NaCl, 4.6 KCl, 2.5 CaCl2, 25.4 NaHCO3, 1 NaH2PO4, 0.6 MgSO4 and 10 glucose. The vessels were allowed to equilibrate for 30 min before undergoing a passive force normalization procedure (Mulvany and Halpern 1977). Sequentially increasing concentrations of methoxamine (0.1–30 μmol L−1; Sigma) were applied to segments of rat mesenteric artery, which were then washed out. Subsequently, either linopirdine (3 or 10 μmol L−1; Sigma) or HMR1556 (1 μmol L−1; Sanofi‐Aventis, Paris, France) was added before increasing concentrations of methoxamine were applied as before. The Kv7 activator, S‐1, was applied after preconstriction with 10 μmol L−1 methoxamine.
Current‐clamp electrophysiology
Membrane potential recordings were made using the β‐escin (50 μmol L−1; Sigma) perforated‐patch technique in current‐clamp mode. Fire‐polished patch pipettes had a resistance of 6–7 MΩ when filled with a pipette solution comprising (mmol L−1): 110 K+ gluconate, 30 KCl, 0.5 MgCl2, 5 Hepes and 0.1 EGTA (pH 7.3). The external solution contained (mmol L−1): 145 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 Hepes and 10 glucose. All experiments were performed at room temperature. The membrane potential for each cell was averaged over a 60 s recording period, 2 min after recording was started. The electrical signals were recorded using an Axopatch 700B patch‐clamp amplifier (Molecular Devices, Sunnydale, CA, USA) and generated and digitized using a Digidata 1322A hosted by a PC running pClamp, version 10.4 (Molecular Devices, Sunnydale, CA, USA).
Statistical analysis
All statistical analysis was performed using Prism, version 5 (GraphPad Software Inc., San Diego, CA, USA). Different statistical tests were performed throughout the present study, as appropriate, and are defined in the Results section for each data set analysed, as appropriate.
Results
KCNE4 alters Kv7.4 activity
Initial experiments aimed to consolidate previous findings (Struz‐Seebohm et al. 2006) showing that KCNE4 enhanced Kv7.4 channel activity. Figure 1 shows that co‐expression of KCNE4 in HEK cells stably expressing Kv7.4 changed the currents significantly. This was manifest as an increase in current amplitude at potentials equal to or exceeding −10 mV, a pronounced leftward shift in the voltage for half‐maximal activation by −13.81 ± 3.5 mV and an increased rate of activation (Fig. 1).
Figure 1. Whole‐cell patch‐clamp recordings on HEK cells stably expressing Kv7.4, transfected with either an empty vector or KCNE4 .
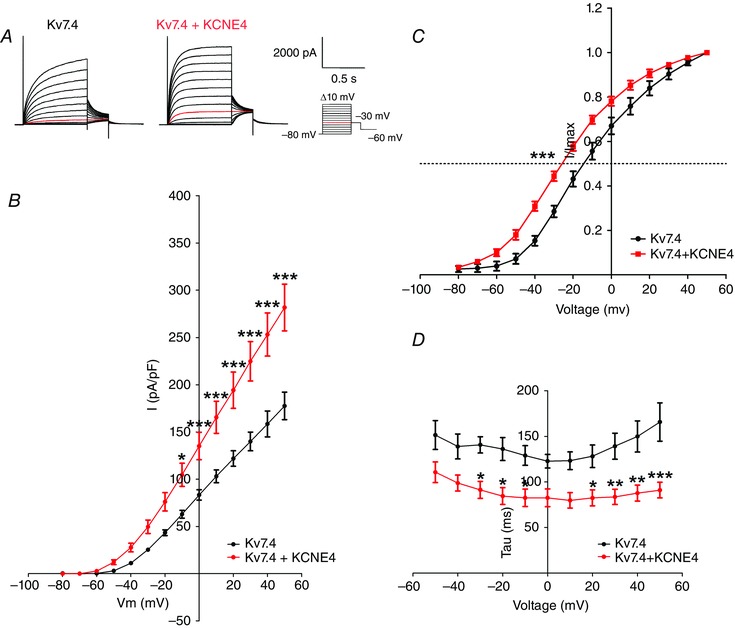
A, representative recordings of the Kv7.4 and Kv7.4/KCNE4 currents. Currents were elicited by the voltage protocol shown. The current elicited by a −30 mV test potential is indicated in red. B, current–voltage relationship of Kv7.4 (black; n = 19) and Kv7.4/KCNE4 (red; n = 9). Normalized current values are plotted against test the potentials and were obtained from steady‐state peak current amplitudes in response to depolarizing pulses from a holding potential of −80 mV. Values are normalized to the cell capacitance. A two‐way ANOVA followed by a Bonferroni post hoc test was performed. *P < 0.05; ***P < 0.001. C, comparison of the voltage dependence of activation in Kv7.4 (black; n = 7) and Kv7.4/KCNE4 (red; n = 13) expressing HEK cells. The current–voltage relation was obtained by plotting the normalized tail current amplitude at −30 mV against the preceding step potential. The tail currents were normalized against the maximal tail current measured in each experiment and the half‐activation potential was calculated using a Boltzmann fit. The mean half‐activation potential was determined for Kv7.4 and Kv7.4/KCNE4 currents and compared using an unpaired t test. ***P < 0.001. D, channel activation kinetics comparing Kv7.4 and Kv7.4/KCNE4 currents. The activation kinetics were fitted to a single exponential function. The time constants are shown as a function of the step potential. A two‐way ANOVA followed by a Bonferroni post hoc test was performed. *P < 0.05, **P < 0.01 and ***P < 0.001.
Expression of KCNE4 in the vasculature
QPCR was performed to assess the relative mRNA expression of the different KCNE isoforms in the rat arteries. Figure 2 A shows that relatively high levels of KCNE4 mRNA were consistently observed in the third‐order mesenteric arteries and that KCNE1 was never present. We also observed relatively high mRNA expression levels of KCNE4 in the thoracic aorta, renal and cerebral arteries (data not shown).
Figure 2. Expression of KCNE4 in the rat mesenteric artery .
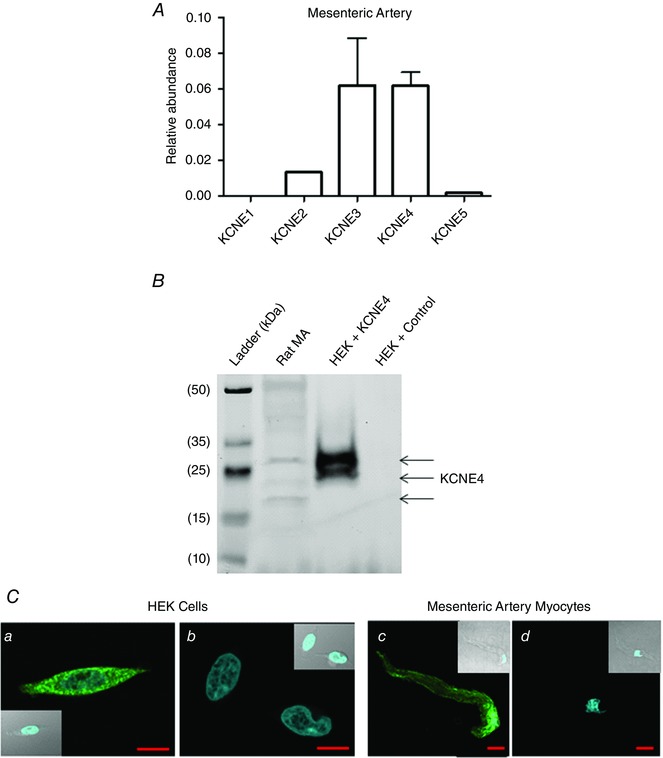
A, QPCR analysis of the relative abundance of KCNE mRNA in the rat mesenteric artery. The relative abundance of each gene was calculated using the 2−ΔCq method (n = 3). B, representative western blot of KCNE4 bands (indicated by arrows) in HEK cell lysate with and without KCNE4 protein expression, and native rat mesenteric artery protein lysate. C, representative fluorescence and transmitted light (insert) confocal mid‐cell xy‐sections of HEK cells transfected with (a) KCNE4 or (b) an empty vector and both stained for KCNE4 (green), as well as freshly isolated mesenteric artery myocytes probed with (c) a primary antibody for KCNE4 (green) or (d) a no primary control. The nuclei are stained with 4′,6‐diamidino‐2‐phenylindole (blue). Scale bars = 10 μm.
Expression of KCNE4 at a protein level in smooth muscle cells has never been defined; therefore, we performed western blots and immunocytochemistry on mesenteric arteries to determine whether KCNE4 was expressed at a protein level. Preliminary experiments were undertaken on HEK cells transfected with either KCNE4 or an empty vector (Fig. 2 B) to validate the antibody. In HEK cells overexpressing KCNE4, three KCNE4 bands were detected, representing the different glycosylation states of the protein, as reported previously (Levy et al. 2008), which were not detected in the lysates from empty vector transfected HEK cells (Fig. 2 B). Interrogation of the mesenteric artery protein lysates with the KCNE4 antibody yielded two bands corresponding to the heterologously expressed protein and a lower molecular weight band, consistent with previous studies conducted in rat heart and kidney lysates (Levy et al. 2008), which identified the bands as showing the different glycosylated states of the KCNE4 protein (Fig. 2 B). Immunostaining also showed that KCNE4 was detected throughout HEK cells transfected with KCNE4 but not with an empty vector. Immunostaining of third‐order mesenteric artery myocytes showed that the KCNE4 protein was present throughout smooth muscle cells with increased abundance in a plasma membrane‐like localization (Fig. 2 C).
KCNE4 co‐localizes with vascular Kv7 channels
KCNE4 subunits functionally regulate Kv7 channels in over‐expression systems but there is little information on this interaction in native cells including vascular smooth muscle. We therefore employed the PLA to determine whether KCNE4 proteins co‐localized with Kv7.4 and Kv7.5 proteins that constitute the functional channels in vascular smooth muscle. PLA was performed with antibodies specific for Kv7.4, Kv7.5 and KCNE4 in rat mesenteric myocytes. To validate the technique, we performed PLA experiments showing that Kv7.4 and Kv7.5 co‐localized in vascular myocytes (Chadha et al. 2014; Brueggemann et al. 2014). As reported previously, when Kv7.4 and Kv7.5 antibody combinations were used, we recorded a significant number of punctae (44.9 ± 7.3; n = 13) consistent with the Kv7 channels in mesenteric arteries existing as a Kv7.4/7.5 heteromer (Fig. 3). As shown by the representative cells in Fig. 3, PLA punctae were readily detected when the KCNE4 antibody was combined with Kv7.4 or Kv7.5. Because PLA signals are only generated if proteins reside within 40 nm of each other, these findings suggest that, in isolated mesenteric artery myocytes, the Kv7 channels are a Kv7.4/7.5 heteromer that co‐assembles with KCNE4.
Figure 3. In situ PLA detection of Kv7.4, Kv7.5 and KCNE4 protein interactions in rat mesenteric artery myocytes .
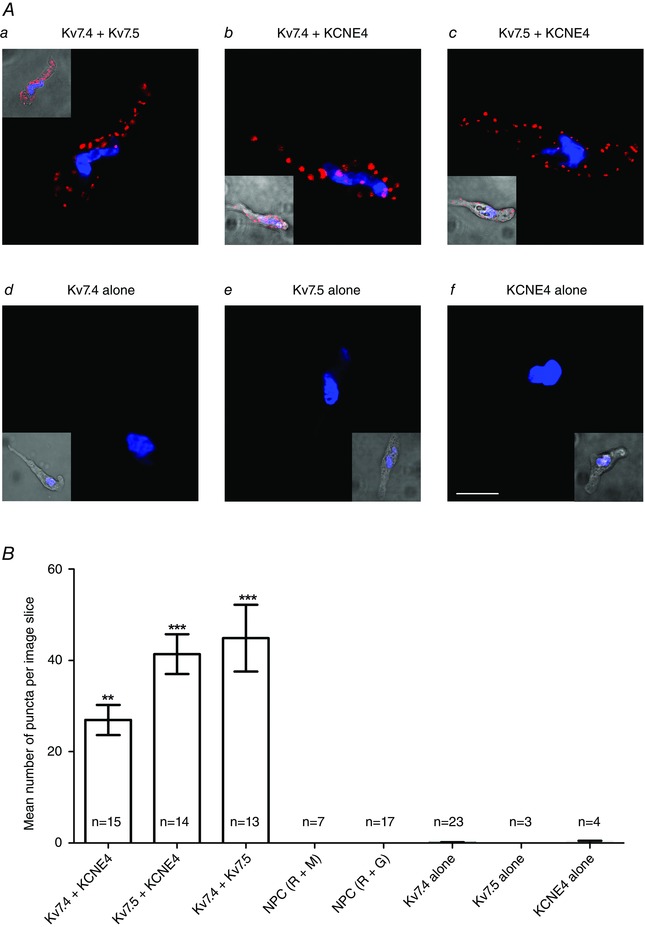
Aa–Af, representative fluorescence and transmitted light (insert) confocal mid‐cell xy‐sections of mesenteric artery myocytes probed with primary antibody combinations for KCNE4, Kv7.4 or Kv7.5 together with appropriate PLA probes. Red punctae indicate target proteins are in close proximity (<40 nm). Nuclei are shown in blue as defined by 4′,6‐diamidino‐2‐phenylindole. B, quantification of the mean ± SEM number of PLA signals per mid‐cell xy‐section for each antibody combination and a no primary control (NPC). **P < 0.01 for Kv7.4 + KCNE4 vs. NPC with anti‐rabbit (R) and anti‐mouse (M) PLA probes and ***P < 0.001 for Kv7.5 + KCNE4 or Kv7.4 + Kv7.5 vs. NPC with anti‐rabbit (R) and anti‐goat (G) PLA probes, according to a one‐way ANOVA, Tukey's multiple comparisons test. Scale bar = 10 μm.
Knocking down KCNE4 depolarizes mesenteric arteries and increases the contractile reactivity
As a result of the lack of a small molecule inhibitor of KCNE4, we employed a morpholino‐induced knockdown to investigate the functional impact of this protein in the vasculature. Figure 4 A shows a western blot for KCNE4 protein in transfected vessels (representative of six experiments). The KCNE4 bands were quantified together and normalized to β‐actin expression. Transfection of third‐order mesenteric arteries with a KCNE4‐targeted morpholino produced a 60% reduction in the KCNE4 protein compared to the KCNE4 mismatched control morpholino (n = 6; P = 0.0047 according to a paired t test) (Fig. 4 A). Having confirmed that morpholinos were able to consistently penetrate into the cell and disable protein translation, current‐clamp recordings were performed on the isolated smooth muscle cells. The membrane potential in isolated mesenteric artery myocytes from KCNE4 morpholino transfected arteries was considerably more depolarized than the membrane potential in cells from control transfections (P = 0.0004 according to a Mann–Whitney test) (Fig. 4 B). In addition, smooth muscle cells from arteries transfected with the KCNE4‐targeted morpholino showed a reduced K+ current compared to cells from arteries transfected with the control morpholino (n = 4, P < 0.001 according to a two‐way ANOVA, followed by a Bonferroni post hoc test) (Fig. 4 C)
Figure 4. KCNE4 knockdown depolarises rat mesenteric artery myocytes .
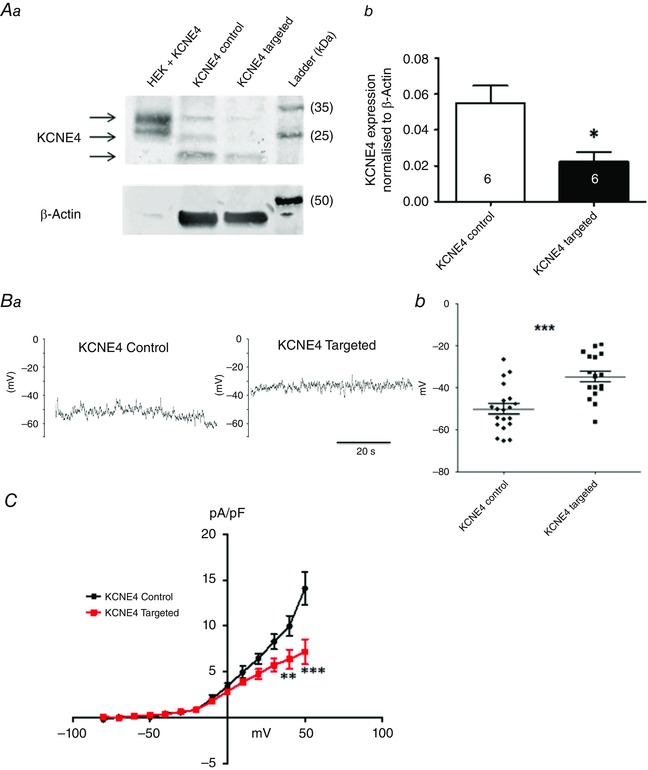
Rat mesenteric artery arteries were transfected with either a KCNE4‐targeted or the respective control morpholino. Aa, representative western blot, with KCNE4 bands indicated by arrows. Ab, mean data comparing the KCNE4 band intensity in the lysates from mesenteric arteries transfected with the targeted or control morpholino. *P < 0.05 according to a paired t test. The number of cells analysed is indicated in each bar. Representative traces (Ba) and scatter plot (Bb) showing the membrane potential from current‐clamp experiments on myocytes isolated from mesenteric arteries after transfection with the KCNE4 control morpholino (n = 21 cells from six transfections and animals), which was significantly more hyperpolarized than the membrane potential in myocytes derived from the arteries transfected with the KCNE4‐targeted morpholino (n = 17 cells from six transfections and animals; P = 0.0004 according to a Mann–Whitney test). C, current–voltage relationship comparing isolated mesenteric artery myocytes transfected with either a KCNE4‐targeted (red) or control (black) morpholino (n = 5 cells from two transfections for each group). Current values are plotted against the test potentials elicited by depolarizing steps from −80 mV to + 50 mV (10 mV increments, 750 ms duration) from a holding potential of −80 mV. A Bonferroni post hoc test was performed following a two‐way ANOVA. **P < 0.01; ***P < 0.001.
Isometric tension recordings were performed to compare the reactivity of the mesenteric resistance vessels transfected with the KCNE4 morpholino and control morpholino (Fig. 5 A). In line with the smooth muscle cells being more depolarized, mesenteric arteries with KCNE4 knocked‐down were significantly more sensitive to α1‐adrenergic stimulation than those transfected with the KCNE4 control morpholino, with the EC50 for methoxamine being reduced from 5.71 ± 0.63 μmol L−1 to 1.56 ± 0.23 μmol L−1 (n = 15; P < 0.0001 according to an unpaired t test with Welch's correction) (Fig. 5 A). For comparison, we also determined the effect of knocking down Kv7.4. Knockdown of Kv7.4 was also confirmed by western blot analysis and a decrease of 46% was found (n = 5; P = 0.0024 according to a paired t test). Knockdown of Kv7.4 also resulted in a leftward shift in the methoxamine concentration–effect curve with the EC50 decreasing from 7.16 ± 0.78 μmol L−1 in the Kv7.4 control arteries (n = 13) to 2.69 ± 0.37 μmol L−1 in the Kv7.4 knockdown arteries (n = 14; P < 0.0001 according to an unpaired t test with Welch's correction).
Figure 5. Knockdown of KCNE4 increases the contractile reactivity of rat mesenteric arteries through reduced Kv7 channel function .
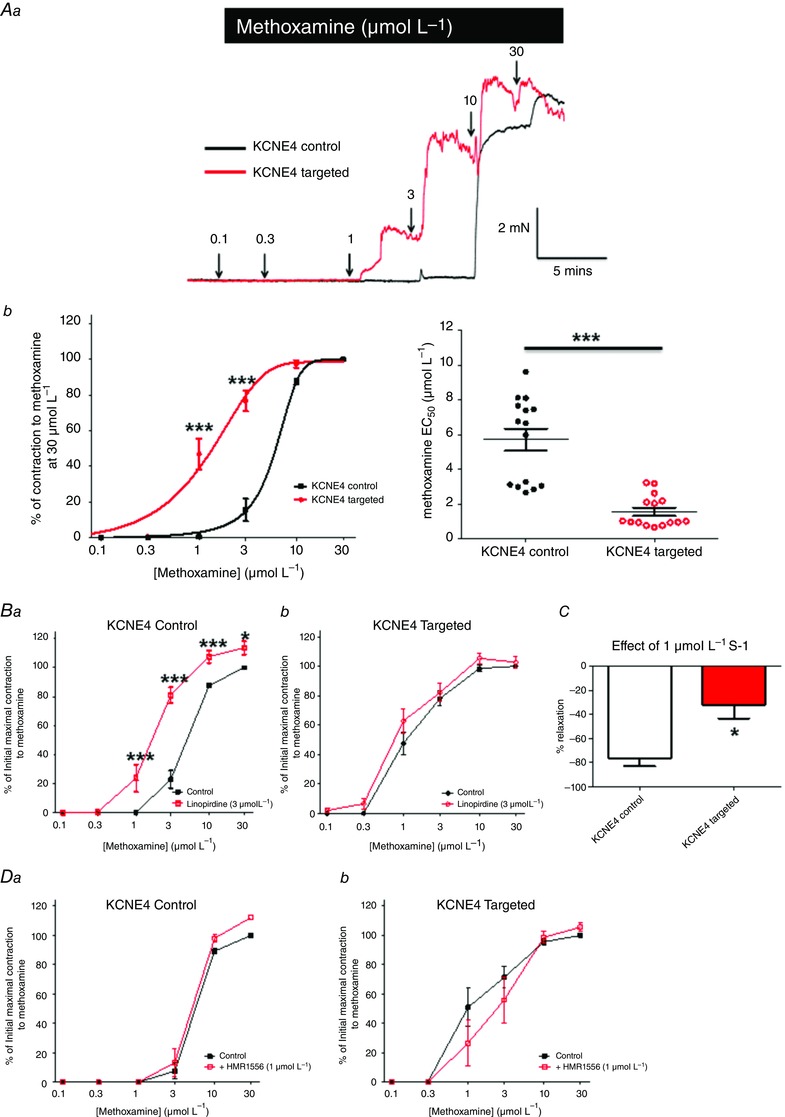
Representative traces (Aa) and mean concentration–effect curves (Ab) for methoxamine in isometric tension recordings performed on vessels transfected with either the control (black) or KCNE4‐targeted (red) morpholino. *P < 0.05 and ***P < 0.0001, respectively, according to a Bonferroni post hoc test following a two‐way ANOVA. Ac, scatter plot of the methoxamine EC50 values of each experiment. ***P < 0.0001 according to an unpaired t test with Welch's correction. The effect of 3 μmol L−1 linopirdine in vessels transfected with either the (Ba) KCNE4 control or (Bb) KCNE4‐targeted morpholino. *P < 0.05 and ***P < 0.001 according to a two‐way ANOVA followed by a Bonferroni post hoc test, respectively. C, comparison of the relaxation effect of S‐1 (1 μmol L−1) in control and targeted KCNE4 morpholino transfected vessels. *P < 0.05 according to a Mann–Whitney test. D, effect of 1 μmol L−1 HMR1556 in vessels transfected with either the (Ba) KCNE4 control or (Bb) KCNE4‐targeted morpholino. Data are the mean ± SEM.
Although the effects of KCNE4 knockdown and Kv7.4 knockdown on the methoxamine response were very similar, this does not negate an effect of KCNE4 on other cellular signals. Therefore, to consolidate an association between KCNE4 and Kv7.4, we determined whether knockdown of KCNE4 affected the ability of the Kv7 blocker linopirdine to enhance methoxamine contractions in segments of the mesenteric artery. Figure 5 B shows that application of linopirdine had no effect on the methoxamine‐induced contractions in KCNE4 knockdown vessels (n = 7), whereas linopirdine enhanced the methoxamine responses in the control vessels significantly (n = 7) (Fig. 5 B). Furthermore, the Kv7.2‐7.5 activator, S‐1 (1 μmol L−1), was significantly less effective at relaxing vessels transfected with the KCNE4‐targeted morpholino compared to the control vessels (n = 4; P = 0.02 according to a Mann–Whitney test) (Fig. 5 C). The Kv7.1‐selective blocker, HMR1556 had no significant effect on the methoxamine concentration–effect curves in either the KCNE4‐targeted or control morpholino transfected vessels (n = 5) (Fig. 5 D) and 0.1 μmol L−1 nicardipine relaxed the KCNE4‐targeted and control vessels equally (relaxations of 88.8 ± 1.7% vs. 90.3 ± 2.4%, respectively; n = 7).
Knockdown of KCNE4 reduces Kv7.4 expression in the cell membrane
To investigate whether KCNE4 expression affected the trafficking of Kv7.4 in mesenteric artery myocytes, we performed immunocytochemistry on the myocytes isolated from arteries transfected with either the KCNE4‐targeted or the control morpholinos. Line scan analysis of these cells revealed peaks for Kv7.4 at the edge of the cells transfected with the KCNE4 control morpholino, whereas the cells transfected with the KCNE4‐targeted morpholino displayed Kv7.4 staining corresponding to a more intracellular expression pattern of this protein (Fig. 6 A). Figure 6 B shows that the myocytes transfected with the KCNE4‐targeted morpholino (n = 7 from three transfections) had reduced staining for KCNE4 compared to the control morpholino (n = 5 from three transfections; P = 0.036 according to a Mann–Whitney test). Similar to the line scan analysis of these cells, we determined that KCNE4 knockdown reduced Kv7.4 abundance in or close to the cell membrane that was not observed in control morpholino treated arteries (P = 0.02 according to a Mann–Whitney test).
Figure 6. Immunostaining of mesenteric artery smooth cells transfected with either the control or KCNE4‐targeted morpholino .
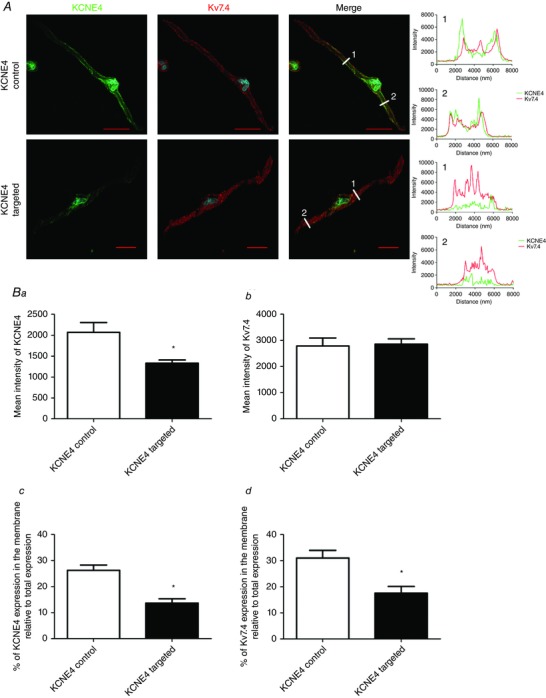
A, representative smooth muscle cells isolated and stained for KCNE4 (green) and Kv7.4 (red). 4′,6‐diamidino‐2‐phenylindole is shown in blue. Scale bars = 20 μm. Mean ± SEM intensity of (Ba) KCNE4 and (Bb) Kv7.4 staining in the smooth muscle cells comparing vessels transfected with the KCNE4 control (n = 5 from three transfections) or KCNE4‐targeted morpholino (n = 7 from three transfections). Bc and Bd, percentage of KCNE4 and Kv7.4 staining in or near the membrane of these cells relative to the total expression. Data are the mean ± SEM (n ≥ 5 cells from three transfections). *P < 0.05, according to a Mann–Whitney test.
To corroborate our findings in smooth muscle cells, we performed immunocytochemistry on HEK cells expressing either Kv7.4 alone or Kv7.4 with KCNE4 (effectively the reverse of the smooth muscle studies). Co‐expression of KCNE4 resulted in increased membrane abundance of Kv7.4 compared to those cells with Kv7.4 alone (Fig. 7). In the cells without KCNE4 expression, 13.7 ± 1.8 % of the total Kv7.4 expression was found in or near the cell membrane (n = 6) compared to 26.2 ± 2.1 % in those cells expressing KCNE4 (n = 10; P < 0.001) (Fig. 7 B). Thus, KCNE4 expression products affect the trafficking and expression of the Kv7.4 protein.
Figure 7. KCNE4 expression increases Kv7.4 membrane abundance in HEK cells .
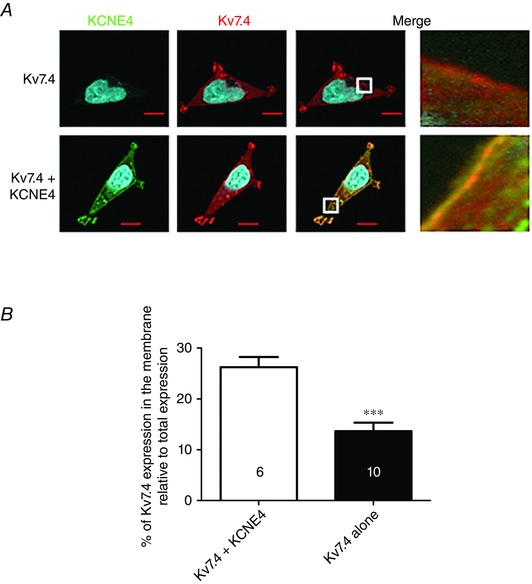
A, representative HEK cells expressing Kv7.4 (red) alone or Kv7.4 with KCNE4 (green). The white boxes in the merged panel are expanded to the right to show membrane fluorescence. B, quantification of Kv7.4 membrane‐like expression as a percentage of the total Kv7.4 expression. Data are the mean ± SEM. The number of cells analysed is indicated in each bar as taken from three separate experiments. ***P < 0.001, according to a Mann–Whitney test.
Taken together, these data suggest that KCNE4 regulates Kv7.4 and thereby has a key role in controlling the resting membrane potential in myocytes from resistance mesenteric arteries.
Discussion
The ion channels that determine the resting membrane potential of vascular smooth muscle cells must be carefully regulated to ensure normal vascular reactivity. It is now established that Kv7 channels are important for regulating vascular contractility in a wide range of rodent and human blood vessels, and evidence that a Kv7.4/Kv7.5 heteromeric channel dominates in isolated cerebral and mesenteric artery vascular smooth muscle cells has been generated recently (Brueggemann et al. 2011; Chadha et al. 2014; Brueggemann et al. 2014). The vascular Kv7 channels regulate vascular tone in a number of arteries and recent findings suggest that a number of physiological vasodilatations throughout the vasculature might occur via the recruitment of Kv7 channels (Chadha et al. 2012; Khanamiri et al. 2013; Chadha et al. 2014; Stott et al. 2015 a, b). However little is known about the factors that determine Kv7 channel activity in the vasculature. The results of the present study suggest that KCNE4 expression is necessary for the proper function and expression of the Kv7.4 protein and channel in resistance (third‐order) mesenteric arteries.
As shown in a previous study (Strutz‐Seebohm et al. 2006), we found that KCNE4 increased the amplitude and shifted the V ½ of activation to more negative potentials of Kv7.4 currents in stably transfected HEKs. The shift in the V ½ moved the activation of the channel into the range that would be expected if it were regulating the resting membrane potential of vascular myocytes. From QPCR expression analysis of the KCNE mRNA in different arterial beds, we identified KCNE4 transcripts to be consistently the most abundant. Similar findings have been reported in the mouse aorta (Yeung et al. 2007), rat coronary arteries (Khanamiri et al. 2013) and rat cerebral arteries (Chadha et al. 2014). Although KCNE4 can modulate Kv7.4 currents in overexpression systems, it was not known whether these subunits interacted in vascular smooth muscle cells. PLA experiments were therefore performed to identify a co‐localization of KCNE4 with Kv7.4 and Kv7.5 proteins in freshly isolated mesenteric smooth muscle cells. The PLA assay results are indicative of KCNE4 subunits co‐assembling with Kv7.4 and Kv7.5 subunits. Furthermore, a high punctae value for Kv7.4 and Kv7.5 antibody combinations infers the expression of a Kv7.4/Kv7.5 heteromer within isolated mesenteric myocytes consistent with previous findings in isolated mesenteric (Brueggemann et al. 2014) and cerebral (Chadha et al. 2014) smooth muscle cells. These data suggest that KCNE4 co‐localizes with the Kv7.4/Kv7.5 heteromeric channel in vascular smooth muscle cells. However, we have no information on the nature of this molecular interaction of KCNE4 proteins with the Kv7 channels. For the Kv7.1 channel, KCNE1‐encoded proteins interdigitate with the pore‐forming subunit in the voltage‐sensing domain (Lundby et al. 2010; Wrobel et al. 2012), leading to a stabilization of the closed conformation by directly delaying the movement of the S4–S5 linker (Van Horn et al. 2011). KCNE4 also interacts with Kv7.1, primarily via its C‐terminus (Manderfield et al. 2008, 2009), to inhibit the activity of the channel. Although our finding of KCNE4/Kv7.4 co‐localization comprises good evidence for a functional interaction, we have to apply the caveat that cell isolation can cause changes in protein expression, and PLA ideally should be performed on whole arteries.
Before the present study, nothing was known about the functional role of the KCNE ancillary subunits in the vasculature. The major problem with studying the KCNE family in native systems is that there are no pharmacological tools to act as functional probes and, accordingly, similar to Joseph et al. (2011), we used morpholino oligonucleotides to specifically knockdown KCNE4. A morpholino oligonucleotide targeted to KCNE4 decreased KCNE4 protein levels, depolarized the membrane potential of isolated mesenteric artery myocytes and enhanced the contractility of third‐order mesenteric arteries to the α1‐adrenoceptor agonist methoxamine compared to those arteries transfected with the control morpholino. This coincided with a lack of response to the Kv7 blocker linopirdine, as well as a reduced effect of the Kv7.2‐7.5 activator S‐1. One possible mechanism for this effect is that KCNE4, under normal conditions, is an important regulator of Kv7.4 abundance in the cell membrane and the associated function in vascular myocytes, which is supported by our immunocytochemical experiments on smooth muscle cells and HEK cells. Consequently, the reduction in membrane Kv7.4 combined with the altered biophysical properties would result in a lesser contribution of Kv7 channels to the membrane conductance making the vessels more depolarized, more contractile and less responsive to Kv7 pharmacological modulators. KCNE4 has been shown to inhibit Kv7.1 channel activity; however, if Kv7.1 were the predominant binding partner of KCNE4 in the vasculature, we would expect the vessels to be less contractile when KCNE4 was knocked down and for HMR1556 to have an effect in these vessels. Although we cannot completely rule out an interaction of KCNE4 with Kv7.1, the data in the present study suggest that this is unlikely. Reduced Kv7 function and Kv7.4 protein expression has been observed in the vasculature of different animal models of hypertension (Jepps et al. 2011; Chadha et al. 2012; Stott et al. 2015 a). Therefore, the identification of KCNE4 regulation of Kv7.4 in the present study may have implications in different vascular diseases, such as hypertension, possibly making KCNE4 a novel therapeutic target, which future studies will address.
As with all studies involving molecular interference, there is the possibility of ‘off target’ effects contributing to our findings, although we have strived to control for these using a mismatched control oligonucleotide. In addition, there are other caveats to the present study. First, KCNE4 may regulate other ion channels or cellular signalling in vascular smooth muscle, as well as Kv7 channels. Consequently, although we used Kv7 modulators (S‐1, linopirdine) to demonstrate an impairment of Kv7.4 by KCNE4 knockdown that was coincident with an increase in vasoconstrictor response, the changes in vascular contractility may reflect an alteration of other vasodilatory mechanisms. Second, our focus in the present study was on KCNE4 in the third‐order branches of mesenteric artery, whereas other KCNE isoforms are expressed in a variable manner in other arteries. The inter‐relationship of these proteins and the physiological roles in the vasculature are yet to be determined. Finally, because the arterial culturing/transfection technique leads to the loss of endothelial function, the present data provide no insight into the expression or role of KCNE subunits in endothelial cells. Future studies evaluating the physiological role of KCNE4 in vivo, using a genetic deletion of KCNE4, will be necessary to fully clarify the impact of this protein in the vasculature.
The findings of the present study add credence to the growing body of data suggesting that signalling proteins play a key role in vascular contractility; for example, Kv1 channels in the cerebral circulation are reliant upon PSD95 (Joseph et al. 2011) and large‐conductance calcium‐activated K+ channels are regulated by a leucine‐rich repeat containing protein 26 (LRRC26) also in cerebral arteries (Evanson et al. 2014). This present study is the first to confirm an integral role of the KCNE4‐encoded ancillary subunit in regulating the function and expression of Kv7.4 in third‐order mesenteric artery smooth muscle. KCNE4 might also complex with other channels to properly regulate the excitability of smooth muscle cells, and future experiments will aim to clarify these mechanisms.
Additional information
Competing interests
The authors have no competing interests to declare.
Author contributions
TAJ and IAG contributed to the conception of the work. TAJ, GC, PRL and IAG contributed to the study design. TAJ contributed to the acquisition and analysis of the data. TAJ, GC, PRL, SPO and IAG contributed to the interpretation of data. TAJ, GC, PRL, SPO and IAG contributed to the drafting of the manuscript. TAJ, SPO and IAG contributed to the revision of the work for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
TAJ received funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007‐2013) under REA grant agreement no. 608765 and a grant from the Lundbeck Foundation. GC was funded by a Medical Research Council grant (MR/K019074/1) awarded to IAG. IAG was additionally funded by a grant from the Lundbeck Foundation to SPO. SPO and PL were funded by the Danish National Research Foundation.
Acknowledgements
We are grateful for the work of Mr Harry Spiers with the PLA experiments. We would like to thank the Core Facility for Integrated Microscopy at the University of Copenhagen for their support.
References
- Andersen MN, Krzystanek K, Petersen F, Bomholtz SH, Olesen SP, Abriel H, Jespersen T & Rasmussen HB (2013). A phosphoinositide 3‐kinase (PI3K)‐serum‐ and glucocorticoid‐inducible kinase 1 (SGK1) pathway promotes Kv7.1 channel surface expression by inhibiting Nedd4‐2 protein. J Biol Chem 288, 36841–36854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Mackie AR, Martin JL, Cribbs LL & Byron KL (2011). Diclofenac distinguishes among homomeric and heteromeric potassium channels composed of KCNQ4 and KCNQ5 subunits. Mol Pharmacol 79, 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Mackie AR, Cribbs LL, Freda J, Tripathi A, Majetschak M & Byron KL (2014). Differential protein kinase C‐dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. J Biol Chem 289, 2099–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha PS, Zunke F, Zhu HL, Davis AJ, Jepps TA, Olesen SP, Cole WC, Moffatt JD & Greenwood IA (2012). Reduced KCNQ4‐encoded voltage‐dependent potassium channel activity underlies impaired β‐adrenoceptor‐mediated relaxation of renal arteries in hypertension. Hypertension 59, 877–884. [DOI] [PubMed] [Google Scholar]
- Chadha PS, Jepps TA, Carr G, Stott JB, Zhu HL, Cole WC & Greenwood IA (2014). Contribution of Kv7.4/Kv7.5 heteromers to intrinsic and calcitonin gene‐related peptide‐induced cerebral reactivity. Arterioscler Thromb Vasc Biol 34, 887–893. [DOI] [PubMed] [Google Scholar]
- Evanson KW, Bannister JP, Leo MD & Jaggar JH (2014). LRRC26 is a functional BK channel auxiliary γ subunit in arterial smooth muscle cells. Circ Res 115, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, Rasmussen HB, Ljungstrøm T, Jorgensen NK, Olesen SP & Klaerke DA (2002). KCNE4 is an inhibitory subunit to the KCNQ1 channel. J Physiol 542, 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps TA, Greenwood IA, Moffatt JD, Sanders KM & Ohya S (2009). Molecular and functional characterization of Kv7 K+ channel in murine gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol 297, G107–G115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps TA, Chadha PS, Davis AJ, Harhun MI, Cockerill GW, Olesen SP, Hansen RS & Greenwood IA (2011). Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation 124, 602–611. [DOI] [PubMed] [Google Scholar]
- Jepps TA, Bentzen BH, Stott JB, Povstyan OV, Sivaloganathan K, Dalby‐Brown W & Greenwood IA (2014). Vasorelaxant effects of novel Kv 7.4 channel enhancers ML213 and NS15370. Br J Pharmacol 171, 4413–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph BK, Thakali KM, Pathan AR, Kang E, Rusch NJ & Rhee SW (2011). Postsynaptic density‐95 scaffolding of Shaker‐type K⁺ channels in smooth muscle cells regulates the diameter of cerebral arteries. J Physiol 589, 5143–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Sedivy V, Hodyc D, Herget J & Gurney AM (2009). KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J Pharmacol Exp Ther 329, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda VA & Abbott GW (2012). KCNE regulation of K+ channel trafficking – a sisyphean task? Front Physiol 3, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanamiri S, Soltysinska E, Jepps TA, Bentzen BH, Chadha PS, Schmitt N, Greenwood IA & Olesen SP (2013). Contribution of Kv7 channels to basal coronary flow and active response to ischemia. Hypertension 62, 1090–1097. [DOI] [PubMed] [Google Scholar]
- Levy DI, Wanderling S, Biemesderfer D & Goldstein SA (2008). MiRP3 acts as an accessory subunit with the BK potassium channel. Am J Physiol Renal Physiol 295, F380–F387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Um SY & McDonald TV (2006). Voltage‐gated potassium channels: regulation by accessory subunits. Neuroscientist 12, 199–210. [DOI] [PubMed] [Google Scholar]
- Lundby A, Tseng GN & Schmitt N (2010). Structural basis for K(V)7.1‐KCNE(x) interactions in the I(Ks) channel complex. Heart Rhythm 7, 708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie AR, Brueggemann LI, Henderson KK, Shiels AJ, Cribbs LL, Scrogin KE & Byron KL (2008). Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther 325, 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manderfield LJ & George AL Jr (2008). KCNE4 can co‐associate with the I(Ks) (KCNQ1‐KCNE1) channel complex. FEBS J 275, 1336–1349. [DOI] [PubMed] [Google Scholar]
- Manderfield LJ, Daniels MA, Vanoye CG & George AL Jr (2009). KCNE4 domains required for inhibition of KCNQ1. J Physiol 587, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum LA, Greenwood IA & Tribe RM (2009). Expression and function of K(v)7 channels in murine myometrium throughout oestrous cycle. Pflügers Arch 457, 1111–1120. [DOI] [PubMed] [Google Scholar]
- McCrossan ZA & Abbott GW (2004). The MinK‐related peptides. Neuropharmacology 47, 787–821. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ & Halpern W (1977). Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41, 19–26. [DOI] [PubMed] [Google Scholar]
- Ng FL, Davis AJ, Jepps TA, Harhun MI, Yeung SY, Wan A, Reddy M, Melville D, Nardi A, Khong TK & Greenwood IA (2011). Expression and function of the K+ channel KCNQ genes in human arteries. Br J Pharmacol 162, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura‐Ferrer M, Etxebarria A, Solé L, Oliveras A, Comes N, Villarroel A & Felipe A (2009). Functional implications of KCNE subunit expression for the Kv7.5 (KCNQ5) channel. Cell Physiol Biochem 24, 325–334. [DOI] [PubMed] [Google Scholar]
- Roura‐Ferrer M, Solé L, Oliveras A, Dahan R, Bielanska J, Villarroel A, Comes N & Felipe A (2010). Impact of KCNE subunits on KCNQ1 (Kv7.1) channel membrane surface targeting. J Cell Physiol 225, 692–700. [DOI] [PubMed] [Google Scholar]
- Söderberg O, Leuchowius KJ, Gullberg M, Jarvius M, Weibrecht I, Larsson LG & Landegren U (2008). Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods 45, 227–232. [DOI] [PubMed] [Google Scholar]
- Søgaard R, Ljungstrøm T, Pedersen KA, Olesen SP & Jensen BS (2000). KCNQ4 channels expressed in mammalian cells: functional characteristics and pharmacology. Am J Physiol Cell Physiol 280, C859–C866. [DOI] [PubMed] [Google Scholar]
- Stott JB, Barrese V, Jepps TA, Leighton EV & Greenwood IA (2015. a). Contribution of Kv7 channels to natriuretic peptide mediated vasodilation in normal and hypertensive rats. Hypertension 65, 676–682. [DOI] [PubMed] [Google Scholar]
- Stott JB, Povstyan OV, Carr G, Barrese V & Greenwood IA (2015. b). G‐protein βγ subunits are positive regulators of Kv7.4 and native vascular Kv7 channel activity. Proc Natl Acad Sci USA 112, 6497–6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz‐Seebohm N, Seebohm G, Fedorenko O, Baltaev R, Engel J, Knirsch M & Lang F (2006). Functional coassembly of KCNQ4 with KCNE‐beta‐ subunits in Xenopus oocytes. Cell Physiol Biochem 18, 57–66. [DOI] [PubMed] [Google Scholar]
- Summerton J (1999). Morpholino antisense oligomers: the case for an RNase H‐independent structural type. Biochim Biophys Acta 1489, 141–158. [DOI] [PubMed] [Google Scholar]
- Summerton J (2007). Morpholino, siRNA, and S‐DNA compared: impact of structure and mechanism of action on off‐target effects and sequence specificity. Med Chem 7, 651–660. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A & Speleman F (2002). Accurate normalization of real‐time quantitative RT‐PCR data by geometric averaging of multiple internal control genes. Genome Biol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn WD, Vanoye CG & Sanders CR (2011). Working model for the structural basis for KCNE1 modulation of the KCNQ1 potassium channel. Curr Opin Struct Biol 21, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel E, Tapken D & Seebohm G (2012). The KCNE Tango ‐ How KCNE1 Interacts with Kv7.1. Front Pharmacol 3, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung SY, Pucovský V, Moffatt JD, Saldanha L, Schwake M, Ohya S & Greenwood IA (2007). Molecular expression and pharmacological identification of a role for Kv7 channels in murine vascular reactivity. Br J Pharmacol 151, 758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung S, Schwake M, Pucovský V & Greenwood IA (2008). Bimodal effects of the Kv7 channel activator retigabine on vascular K+ currents. Br J Pharmacol 155, 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XZ, Harhun MI, Olesen SP, Ohya S, Moffatt JD, Cole WC & Greenwood IA (2010). Participation of KCNQ (Kv7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol 588, 3277–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]


