Abstract
Metastases represent the most common type of intracranial neoplasm. In women, 30% of such tumors derive from breast carcinoma. In neurosurgical cases with ambiguous cellular morphology and/or limited biopsy material, immunohistochemistry (IHC) is often performed to distinguish metastases from primary central nervous system (CNS) neoplasms. IHC for mammaglobin-A (MGA), a protein expressed in a majority of breast carcinomas, is commonly applied in this setting, but its utility for distinguishing primary CNS neoplasms from metastatic breast carcinoma is unknown; the reactivity of MGA in primary and metastatic CNS neoplasms has never been described. Here, we describe the frequency and patterns of IHC reactivity for MGA in metastatic and primary CNS neoplasms from patients with well-documented histories of breast carcinoma. Following a published protocol previously applied to non-CNS neoplasms, MGA staining of moderate to strong intensity within 5% or more of a neoplasm was considered positive. Based on these criteria, 3 of 12 (25.0%) glioblastomas, 1 of 10 (10.0%) meningiomas, and 47 of 95 (49.5%) metastases were positive. Importantly, the cytoarchitectural staining characteristics among all four MGA positive primary CNS neoplasms (cytoplasmic and nuclear) differed from those of the metastases (cytoplasmic and membranous). These findings suggest that MGA IHC staining intensity and distribution can distinguish metastases from primary CNS neoplasms (**P=0.0086) in women with a history of breast carcinoma but also indicate that cytological staining patterns must be interpreted for more accurate tumor classification.
Keywords: central nervous system, brain tumor, metastasis, breast carcinoma
INTRODUCTION
Contrast-enhancing brain masses comprise a significant proportion of adult neurosurgical cases. Although many of these cases represent high grade glioma or meningioma, approximately half represent metastasis. In women, breast carcinoma accounts for approximately 30% of brain metastases.1 Not uncommonly, such metastatic tumors are the presenting feature of the primary neoplasm. Because primary and metastatic tumors can be histologically protean, and neurosurgical biopsy often provides limited tissue for histomorphological characterization, diagnosis often rests heavily upon ancillary techniques, such as immunohistochemistry (IHC). Although some markers of breast carcinoma (e.g. gross cystic disease fluid protein-15 [GCDFP-15], estrogen receptor [ER], progesterone receptor [PR], human epidermal growth factor receptor 2 [Her-2/neu]) can be of some use in identifying metastatic breast carcinoma, these markers are often insufficiently sensitive and specific and may not adequately distinguish metastasis from glioma or meningioma.2–8 This difference is important because therapies and management differ between these tumor types (reviewed in 9,10).
Mammaglobin-A (MGA), a protein first identified as being over-expressed in breast carcinoma, is a newer marker in common use for the identification of metastatic carcinomas of breast origin.11,12 MGA has been detected by IHC and PCR in 48–95% of primary breast carcinomas13–19 and a similar frequency of MGA expression has been reported in metastatic carcinoma derived from breast.11,12 Since its emergence as a marker of breast carcinoma, MGA expression has also been described at lower frequencies in non-breast primary tumors including several types of gynecological carcinomas,13,20–23 sweat gland carcinoma,13,24 salivary gland carcinoma,13,25 pleural malignant mesothelioma,26 and various types of lung carcinomas.13,27–29 However, the expression of MGA in central nervous system (CNS) neoplasms, either metastatic or primary, is unknown. In this study we evaluate the frequency and cytoarchitectural patterns of MGA IHC in CNS neoplasms and estimate its utility for distinguishing metastases from primary CNS tumors among patients with a history of primary breast carcinoma.
MATERIALS AND METHODS
Tissue and Case Selection
Approval for the use of human subject material was granted by the Washington University Human Research Protection Office (Institutional Review Board approval number 201101716). The database of the Lauren V. Ackerman Laboratory of Surgical Pathology was searched for all available cases of an intracranial neoplasm arising in a patient with a confirmed history of breast carcinoma, spanning the years 1986 to 2012. A total of 157 surgical cases representing 153 patients were identified. All cases of breast carcinoma for which pathology or the corresponding reports were available, were of the infiltrating ductal carcinoma type. None of these patients had a known history of non-breast primary carcinoma. Of these 153 patients, formalin-fixed paraffin-embedded (FFPE) tissue blocks were available for 12 glioblastomas (WHO Grade IV), 10 meningiomas (WHO Grade I), and 95 intracranial metastatic carcinomas (moderately to poorly-differentiated). In addition, 32 cases had corresponding primary breast carcinoma FFPE blocks available for study.
Immunohistochemistry and Scoring
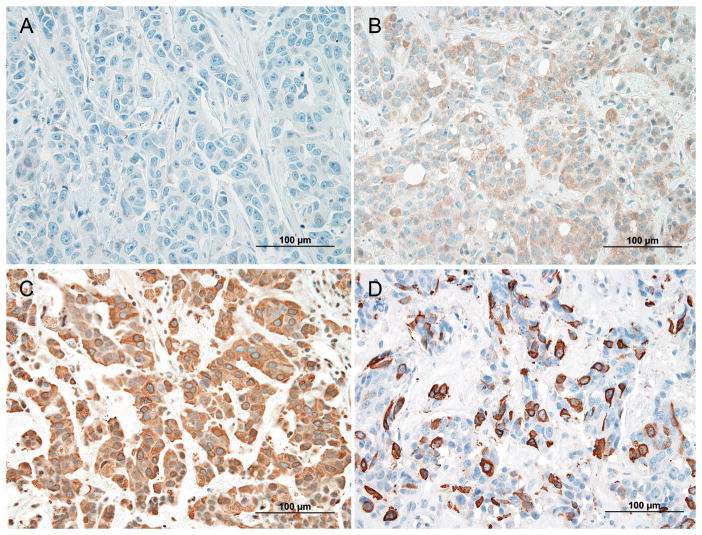
For all cases, FFPE tissue slides were prepared and immunohistochemistry (IHC) was performed by the CAP/CLIA-certified Anatomic and Molecular Pathology core IHC lab within the Department of Pathology and Immunology at the Washington University School of Medicine. The primary antibody used was the pre-diluted anti-mammaglobin-A antibody clone 31A5 (Catalog #760-4263, Cell Marque, Rocklin, CA, USA). Staining was performed utilizing a Benchmark Ultra instrument (Ventana Medical Systems Inc., Tucson, AZ, USA). Parameters for the Benchmark ultra included antigen retrieval procedure (36 minutes of ULTRA CC1 mild, 1mM EDTA pH 8.0), primary antibody incubation (37°C for 24 minutes), ancillary washing procedures (Ultrawash), and detection system (Universal Ultraview DAB detection kit, catalog #760-500). All IHC slides were de-identified, randomly coded, and blindly reviewed independently by both authors (PJC and RJP). Mammaglobin staining was scored and interpreted according to the criteria described by Sasaki, et al.18 Intensity of staining was scored as follows: 0 (none), 1+ (weak), 2+ (moderate), and 3+ (strong) (Fig. 1). Tumors were binned into one of the following three groups by the proportion of immunoreactive tumor cells: <5%, 5–90%, and >90%. Cases with 2+ or 3+ intensity appearing in 5% or more of the tumor cells were considered positive. Cases that received discrepant scores were reevaluated jointly by both authors for a consensus score.
FIGURE 1.
Intensity scoring of mammaglobin-A immunohistochemistry. A, No staining (0). B, Weak staining (1+). C, Moderate staining (2+). D, Strong staining (3+). Panel A is breast tissue and panels B–D are intracranial metastatic carcinoma.
Statistical Methodology
Statistical analyses between groups were performed utilizing two-tailed Fisher’s exact test using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
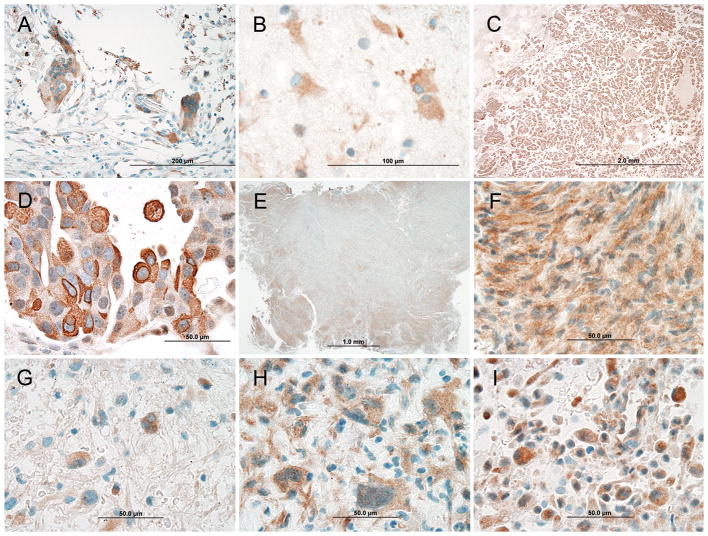
Mammaglobin-A (MGA) intensity scores (represented in Fig. 1) and staining distribution within the different tumor types are summarized in Table 1. All tumor types showed variable amounts of non-specific weak (1+) diffuse MGA staining in tumor cells and in stromal/parenchymal cells. The reason for this staining is unclear but may represent a non-specific IgG isotype reaction to the tissue. Some stromal cells, including histiocytic multinucleated giant cells, and some reactive astrocytes showed weak (1+) to moderate (2+) staining (Fig. 2A, B). Strong (3+) staining, observed only in carcinoma (Fig. 2C, D), was sufficient to discriminate metastasis from glioblastoma and meningioma; these primary CNS tumors exhibited maximal intensity of 2+ (moderate). However, only a minority of metastatic tumors showed 3+ intensity (Table 1). Furthermore, not uncommonly, 3+ intensity was observed in only a small minority of tumor cells. The more inclusive criteria, described by Sasaki, et al.,18 allow cases with only 2+ intensity to be considered positive. This approach yielded positive MGA scores in 15 of 32 (46.9%) primary breast carcinomas, 47 of 95 (49.5%) intracranial metastatic carcinomas, 3 of 12 (25.0%) glioblastomas, and 1 of 10 (10.0%) meningiomas (Table 1). Thus, the proportion of metastatic carcinomas that were positive for MGA and the proportion of primary central nervous system (CNS) neoplasms (glioblastomas and meningiomas) that were positive for MGA differed significantly (**P=0.0086). Those of meningiomas and metastatic carcinoma also differed (*P=0.0200), but those of glioblastoma and metastatic carcinoma did not (P=0.1336).
TABLE 1.
Mammaglobin-A immunohistochemical staining distribution and intensities. Cases considered ‘positive’ by established intensity and proportionality scoring have been shaded gray.
| Tumor | Distribution | Intensity | |||
|---|---|---|---|---|---|
| 0 (none) | 1+ (weak) | 2+ (moderate) | 3+ (strong) | ||
| Glioblastoma (n=12) | <5% | - | 3/12 (25.0%) | 0/12 (0.0%) | 0/12 (0.0%) |
| 5–90% | - | 2/12 (16.7%) | 3/12 (25.0%) | 0/12 (0.0%) | |
| >90% | 4/12 (33.3%) | 0/12 (0.0%) | 0/12 (0.0%) | 0/12 (0.0%) | |
|
| |||||
| Meningioma (n=10) | <5% | - | 4/10 (40.0%) | 0/10 (0.0%) | 0/10 (0.0%) |
| 5–90% | - | 2/10 (20.0%) | 1/10 (10.0%) | 0/10 (0.0%) | |
| >90% | 3/10 (30.0%) | 0/10 (0.0%) | 0/10 (0.0%) | 0/10 (0.0%) | |
|
| |||||
| Metastatic Carcinoma, Brain (n=95) | <5% | - | 10/95 (10.5%) | 11/95 (11.6%) | 7/95 (7.4%) |
| 5–90% | - | 17/95 (17.9%) | 22/95 (23.2%) | 17/95 (17.9%) | |
| >90% | 2/95 (2.1%) | 0/95 (0.0%) | 8/95 (8.4%) | 1/95 (1.0%) | |
|
| |||||
| Primary Carcinoma, Breast (n=32) | <5% | - | 3/32 (9.4%) | 5/32 (15.6%) | 3/32 (9.4%) |
| 5–90% | - | 4/32 (12.5%) | 5/32 (15.6%) | 8/32 (25.0%) | |
| >90% | 2/32 (6.3%) | 0/32 (0.0%) | 2/32 (6.3%) | 0/32 (0.0%) | |
FIGURE 2.
Mammaglobin-A staining patterns of different central nervous system neoplasms. A and B, Weak (1+) to moderate (2+) intensity staining of stromal cells including multinucleated foreign body giant cells in a primary breast carcinoma and reactive astrocytes around metastatic carcinoma in the brain, respectively. C and D, Metastatic carcinoma showing diffuse architectural staining and moderate (2+) to strong (3+) cytoplasmic reactivity. E and F, Meningioma with patchy architectural staining and moderate (2+) cytoplasmic and nuclear staining. G–I, Three different glioblastomas showing moderate (2+) staining of the cytoplasm and nucleus.
Beyond the differences that could be discerned from intensity and extent of MGA immunoreactivity, these various tumor types also showed different distributions and cytoarchitectural patterns of staining. In the single MGA-positive meningioma case (Fig. 2E, F), reactivity among tumor cells was regional or patchy, preferentially staining the periphery of lobules in a pattern distinct from immunohistochemical ‘edge’ artifact; in contrast, the MGA positive cases of metastatic carcinoma showed diffuse tissue staining (Fig. 2C), sometimes with heterogeneous staining intensities of individual cells within the broader diffuse pattern (Fig. 2D). Distinctive in another manner, the three MGA positive glioblastomas all differed from the metastatic lesions in two ways, with respect to MGA staining: although both tumor classes showed cytoplasmic staining, only the glioblastomas showed nuclear staining (Fig. 2G–I) and only the metastases showed strong membrane staining (Fig. 2D). When these properties were applied as criteria to subclassify the MGA positive tumors of this study into two groups (Pattern A: cytoplasmic, nuclear, not membranous; and Pattern B: cytoplasmic, membranous, not nuclear), they distinguished cases of glioblastoma (Pattern A) and metastatic carcinomas (Pattern B) with statistical significance (***P=0.0001). All of the moderately staining glioblastomas (n=3) show pattern A and all of the moderately to strongly staining metastatic carcinomas (n=47) show pattern B. Like these MGA positive glioblastomas, the other MGA positive primary CNS tumor in this study (meningioma) also exhibited staining Pattern A (Fig. 2F). Cytoplasmic and membranous staining of MGA, as a secretoglobin family member, has been demonstrated by others in breast carcinoma and appears independent of histologic grading.15,19,30
In practice, the decision to evaluate a potential metastasis with a given IHC marker is influenced by the antigenicity of the patient’s primary tumor. To address questions of antigenic correlation between primary breast and CNS lesions within individual patients, 32 cases were included for which tissue from the primary breast carcinoma was available for MGA IHC. For 28 of these 32 cases, the corresponding CNS neoplasm was metastatic carcinoma. Among these 28 cases that allow simultaneous evaluation of primary and metastatic lesions, 50% of the primary tumors (14/28) were MGA positive. Among these 14 MGA positive breast carcinoma cases, 12 of the corresponding brain metastases were MGA positive (85.7% [12/14] concordance of MGA positivity) and 2 were considered negative. Conversely, among the 14 MGA negative breast carcinoma cases, 12 of the corresponding brain metastases were MGA negative (85.7% [12/14] concordance of MGA negativity) and 2 were considered MGA positive. The staining characteristics of primary breast carcinoma versus metastatic CNS carcinoma are summarized in Table 2. Importantly, the non-concordant samples in this study do not exhibit complete loss or de novo gain of MGA; it seems in these cases that only minor variability in staining is responsible for crossing the threshold for MGA ‘positivity.’ Indeed, even among concordant samples, as indicated in Table 2, there is similar MGA staining variability between primary and metastatic carcinomas; in these cases, the variability simply does not span the threshold that divides positive and negative staining patterns.
TABLE 2.
Comparison of immunohistochemical Mammaglobin-A scoring between primary breast carcinoma and intracranial metastatic breast carcinoma.
| Primary Breast Carcinoma | Metastatic CNS Carcinoma | |||||
|---|---|---|---|---|---|---|
| Case | Intensity | Proportion | MGA | Intensity | Proportion | MGA |
| 1 | 0 | N/A | − | 1+ | 5–90% | − |
| 2 | 0 | N/A | − | 2+ | <5% | − |
| 3 | 1+ | <5% | − | 1+ | <5% | − |
| 4 | 1+ | <5% | − | 3+ | <5% | − |
| 5 | 1+ | <5% | − | 1+ | 5–90% | − |
| 6 | 2+ | <5% | − | 2+ | <5% | − |
| 7 | 2+ | <5% | − | 2+ | <5% | − |
| 8 | 2+ | <5% | − | 2+ | <5% | − |
| 9 | 2+ | <5% | − | 3+ | <5% | − |
| 10 | 3+ | <5% | − | 1+ | <5% | − |
| 11* | 3+ | <5% | − | 3+ | 5–90% | + |
| 12* | 1+ | 5–90% | − | 2+ | 5–90% | + |
| 13 | 1+ | 5–90% | − | 1+ | 5–90% | − |
| 14 | 1+ | 5–90% | − | 3+ | <5% | − |
| 15* | 2+ | 5–90% | + | 3+ | <5% | − |
| 16* | 3+ | 5–90% | + | 3+ | <5% | − |
| 17 | 2+ | 5–90% | + | 3+ | 5–90% | + |
| 18 | 2+ | 5–90% | + | 3+ | >90% | + |
| 19 | 2+ | 5–90% | + | 2+ | 5–90% | + |
| 20 | 3+ | 5–90% | + | 2+ | 5–90% | + |
| 21 | 3+ | 5–90% | + | 2+ | 5–90% | + |
| 22 | 3+ | 5–90% | + | 2+ | 5–90% | + |
| 23 | 3+ | 5–90% | + | 2+ | 5–90% | + |
| 24 | 3+ | 5–90% | + | 2+ | 5–90% | + |
| 25 | 3+ | 5–90% | + | 3+ | 5–90% | + |
| 26 | 3+ | 5–90% | + | 3+ | 5–90% | + |
| 27 | 2+ | >90% | + | 2+ | >90% | + |
| 28 | 2+ | >90% | + | 2+ | >90% | + |
MGA – Mammaglobin-A
Primary carcinoma MGA positivity non-concordant from metastasis based on scoring criteria
DISCUSSION
In this study, cases were selected for history of primary breast carcinoma and subsequent enhancing brain tumor. Among these cases, nearly half of the examples of metastatic breast carcinoma showed MGA IHC staining patterns that would be defined as ‘positive’ by established criteria (Table 1).18 This frequency is consistent with previously published rates of MGA within primary and metastatic breast carcinomas appearing outside the CNS.13–19 This frequency is also significantly greater than that observed among the primary CNS tumors evaluated in this study (Table 1). Thus, for a given unknown CNS biopsy specimen from a woman with history of breast carcinoma, a ‘positive’ MGA stain would provide some support for a diagnosis of metastatic breast carcinoma. This support would be particularly strong with a 3+ staining intensity, which was observed only in breast carcinomas. However, in cases with only 2+ staining intensity, this support would be more limited; like many metastatic breast carcinomas, small subsets of glioblastomas and meningiomas show 2+ staining intensity and also meet established criteria for positivity by MGA IHC. Fortunately, even cases without 3+ staining may be properly classified as metastatic or primary on the basis of the cytoarchitectural pattern of MGA IHC staining; a cytoplasmic stain with a membranous component favors metastasis, whereas a cytoplasmic stain with a nuclear component favors primary CNS tumor (meningioma or glioblastoma). This is the first description of MGA staining in primary CNS tumors; it is not yet clear whether this immunoreactivity for MGA represents aberrant expression and unusual intracellular localization of MGA, or cross-reactivity with another protein, or an artifact of the staining process. Regardless, among the cases included in this study, this strategy of biopsy interpretation should achieve completely accurate classification of primary and metastatic CNS biopsy specimens that show ‘positive’ MGA IHC staining. Whether this approach will work in an independent set of ‘positive’ cases remains to be seen. Unfortunately, MGA IHC is less helpful for classification of biopsy specimens that are considered ‘negative.’ Only a subset of such cases shows a sufficient distribution of 2+ staining intensity to allow classification solely on the basis of cytoarchitectural staining pattern. Fortunately, neurosurgical biopsy specimens such as these are not likely to be evaluated on the basis of a single immunohistochemical stain; other information, derived from other ancillary tests such as IHC for other markers (e.g. for GFAP, cytokeratins 7 and 20, GCDFP-15, ER, PR, Her-2/Neu), and FISH for tumor-associated genetic alterations can also guide diagnosis. Nevertheless, because MGA is generally presumed to be a marker for breast carcinoma and its reactivity within primary CNS neoplasms has never been described, a ‘positive’ stain for MGA in a limited biopsy of a primary CNS neoplasm appearing in a patient with a history of breast carcinoma might lead to misdiagnosis, particularly when other ancillary stains provide negative or ambiguous results. Comparing the sensitivity and specificity of MGA to other breast carcinoma markers, it appears that MGA has a higher combined sensitivity and specificity than ER, PR, GCDFP-15, and Her-2/neu (Table 3).6,31,32
TABLE 3.
Sensitivities and specificities of various immunohistochemical breast markers in intracranial metastatic carcinoma.
| Immunohistochemical Marker | Sensitivity | Specificity | Reference |
|---|---|---|---|
| Mammaglobin-A (MGA) | 49.5% | *82% | Our Study |
| **100% | |||
|
| |||
| Gross Cystic Disease Fluid Protein 15 (GCDFP-15) | 33% | 92% | Perry, et al; 1997 |
|
| |||
| Estrogen Receptor (ER) | 33% | 84% | Perry, et al; 1997 |
| 34% | - | Gaedcke, et al; 2007 | |
| 13.8% | - | Yonemori, et al; 2008 | |
|
| |||
| Progesterone Receptor (PR) | 87% | 28% | Perry, et al; 1997 |
| 18% | - | Gaedcke, et al; 2007 | |
| 6.9% | - | Yonemori, et al; 2008 | |
|
| |||
| Human Epidermal Growth Factor Receptor 2 (Her-2/neu) | 34% | - | Gaedcke, et al; 2007 |
| 37.9% | - | Yonemori, et al; 2008 | |
Specificity includes evaluating MGA for intensity and distribution only
Specificity incorporates cytoarchitectural patterns to intensity and distribution
The interpretation of MGA IHC staining results outlined above is relatively clear. However, the design of this study imposes some limitations regarding how its results and conclusions might be applied in general pathology practice. First, because this study involved only women with history of breast carcinoma, these results might not be applicable to men with history of breast carcinoma, or to women or men without a history of breast carcinoma. Though unlikely, it is possible that primary CNS neoplasms arising in women without a breast cancer history might exhibit a different MGA IHC pattern than was observed in this cohort. Second, the total number of primary CNS neoplasms evaluated in this study was somewhat limited by the small number of breast carcinoma patients at our institution who subsequently developed primary CNS tumors and underwent biopsy or resection. Thus, the accuracy of the estimate afforded by this study of MGA IHC staining characteristics among primary CNS neoplasms is similarly limited. Third, because this study – by necessity – involved only cases in which a surgical biopsy was performed, these results may not be generalizable to cases in which the tumor burden or distribution might prompt administration of radiation/chemotherapy without a tissue diagnosis, or that might be encountered at autopsy. Fourth, by selecting only cases with confirmed history of breast carcinoma and without another known primary carcinoma, this study was designed to include only metastatic carcinomas of breast origin; it was not designed to evaluate the utility of MGA IHC to distinguish metastatic breast carcinoma from other CNS metastases, which may warrant further investigation given the recently described lack of specificity for primary sites of carcinoma.13,20–29 Although it might be expected that the characteristics of MGA IHC staining among non-breast origin metastatic tumors to the CNS would resemble those among metastatic tumors to peripheral sites, such a result has not been empirically determined. Fifth, some of the immunostained biopsy slides were prepared from small biopsy specimens that may misrepresent the overall staining characteristics of the entire tumor; this possibility of ‘sampling error,’ though not unique to this study, might account for one or more of the dichotomously staining pairs of primary and metastatic tumors discussed above (Table 2). Finally, although slide processing and MGA IHC staining was rigidly controlled within a CLIA approved laboratory, the durations of formalin fixation and paraffin block storage prior to IHC were not uniform across samples. While it would be unlikely for a systematic bias to arise from these variables, such a possibility cannot be absolutely discounted.
In summary, this study demonstrates that MGA IHC has some utility for distinguishing primary CNS tumors from metastatic CNS tumors in women with history of breast carcinoma: a circumstance that is often necessitated and complicated by limited neurosurgical biopsy material. Importantly, the evaluation of MGA IHC in such specimens requires careful consideration of cytoarchitectural patterns of staining in addition to intensity and distribution; a subset of primary CNS tumors that meets established criteria for ‘positive’ staining shows nuclear immunoreactivity, rather than the membranous immunoreactivity observed in metastatic breast carcinoma.
Acknowledgments
Sources of Support:
This research was funded by the Department of Pathology and Immunology, Washington University School of Medicine.
References
- 1.Preusser M, Capper D, Ilhan-Mutlu A, et al. Brain metastases: pathobiology and emerging targeted therapies. Acta Neuropathol. 2012;123:205–222. doi: 10.1007/s00401-011-0933-9. [DOI] [PubMed] [Google Scholar]
- 2.Carroll RS, Zhang J, Dashner K, et al. Steroid hormone receptors in astrocytic neoplasms. Neurosurgery. 1995;37:496–503. doi: 10.1227/00006123-199509000-00019. discussion 503–494. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Aguero G, Ondarza R, Gamboa-Dominguez A, et al. Progesterone receptor isoforms expression pattern in human astrocytomas. Brain Res Bull. 2001;56:43–48. doi: 10.1016/s0361-9230(01)00590-1. [DOI] [PubMed] [Google Scholar]
- 4.Martinez R, Marcos ML, Figueras A, et al. Estrogen and progesterone receptors in intracranial tumors. Clin Neuropharmacol. 1984;7:338–342. doi: 10.1097/00002826-198412000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Mineo JF, Bordron A, Baroncini M, et al. Low HER2-expressing glioblastomas are more often secondary to anaplastic transformation of low-grade glioma. J Neurooncol. 2007;85:281–287. doi: 10.1007/s11060-007-9424-1. [DOI] [PubMed] [Google Scholar]
- 6.Perry A, Parisi JE, Kurtin PJ. Metastatic adenocarcinoma to the brain: an immunohistochemical approach. Hum Pathol. 1997;28:938–943. doi: 10.1016/s0046-8177(97)90009-5. [DOI] [PubMed] [Google Scholar]
- 7.Brandis A, Mirzai S, Tatagiba M, et al. Immunohistochemical detection of female sex hormone receptors in meningiomas: correlation with clinical and histological features. Neurosurgery. 1993;33:212–217. doi: 10.1227/00006123-199308000-00005. discussion 217–218. [DOI] [PubMed] [Google Scholar]
- 8.Schlegel J, Ullrich B, Stumm G, et al. Expression of the c-erbB-2-encoded oncoprotein and progesterone receptor in human meningiomas. Acta Neuropathol. 1993;86:473–479. doi: 10.1007/BF00228582. [DOI] [PubMed] [Google Scholar]
- 9.Eckhardt BL, Francis PA, Parker BS, et al. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012;11:479–497. doi: 10.1038/nrd2372. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DR, Chang SM. Recent medical management of glioblastoma. Adv Exp Med Biol. 2012;746:26–40. doi: 10.1007/978-1-4614-3146-6_3. [DOI] [PubMed] [Google Scholar]
- 11.Watson MA, Fleming TP. Isolation of differentially expressed sequence tags from human breast cancer. Cancer Res. 1994;54:4598–4602. [PubMed] [Google Scholar]
- 12.Watson MA, Fleming TP. Mammaglobin, a mammary-specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer Res. 1996;56:860–865. [PubMed] [Google Scholar]
- 13.Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: an immunohistologic validation survey for sensitivity and specificity. Am J Clin Pathol. 2007;127:103–113. doi: 10.1309/TDP92PQLDE2HLEET. [DOI] [PubMed] [Google Scholar]
- 14.Fleming TP, Watson MA. Mammaglobin, a breast-specific gene, and its utility as a marker for breast cancer. Ann N Y Acad Sci. 2000;923:78–89. doi: 10.1111/j.1749-6632.2000.tb05521.x. [DOI] [PubMed] [Google Scholar]
- 15.Han JH, Kang Y, Shin HC, et al. Mammaglobin expression in lymph nodes is an important marker of metastatic breast carcinoma. Arch Pathol Lab Med. 2003;127:1330–1334. doi: 10.5858/2003-127-1330-MEILNI. [DOI] [PubMed] [Google Scholar]
- 16.Marchetti A, Buttitta F, Bertacca G, et al. mRNA markers of breast cancer nodal metastases: comparison between mammaglobin and carcinoembryonic antigen in 248 patients. J Pathol. 2001;195:186–190. doi: 10.1002/path.943. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien N, Maguire TM, O’Donovan N, et al. Mammaglobin a: a promising marker for breast cancer. Clin Chem. 2002;48:1362–1364. [PubMed] [Google Scholar]
- 18.Sasaki E, Tsunoda N, Hatanaka Y, et al. Breast-specific expression of MGB1/mammaglobin: an examination of 480 tumors from various organs and clinicopathological analysis of MGB1-positive breast cancers. Mod Pathol. 2007;20:208–214. doi: 10.1038/modpathol.3800731. [DOI] [PubMed] [Google Scholar]
- 19.Watson MA, Dintzis S, Darrow CM, et al. Mammaglobin expression in primary, metastatic, and occult breast cancer. Cancer Res. 1999;59:3028–3031. [PubMed] [Google Scholar]
- 20.Onuma K, Dabbs DJ, Bhargava R. Mammaglobin expression in the female genital tract: immunohistochemical analysis in benign and neoplastic endocervix and endometrium. Int J Gynecol Pathol. 2008;27:418–425. doi: 10.1097/PGP.0b013e31815d05ec. [DOI] [PubMed] [Google Scholar]
- 21.Zafrakas M, Petschke B, Donner A, et al. Expression analysis of mammaglobin A (SCGB2A2) and lipophilin B (SCGB1D2) in more than 300 human tumors and matching normal tissues reveals their co-expression in gynecologic malignancies. BMC Cancer. 2006;6:88. doi: 10.1186/1471-2407-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Spaulding B, Sienko A, et al. Mammaglobin, a valuable diagnostic marker for metastatic breast carcinoma. Int J Clin Exp Pathol. 2009;2:384–389. [PMC free article] [PubMed] [Google Scholar]
- 23.Hagemann IS, Pfeifer JD, Cao D. Mammaglobin expression in gynecologic adenocarcinomas. Hum Pathol. 2012 doi: 10.1016/j.humpath.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Rollins-Raval M, Chivukula M, Tseng GC, et al. An immunohistochemical panel to differentiate metastatic breast carcinoma to skin from primary sweat gland carcinomas with a review of the literature. Arch Pathol Lab Med. 2011;135:975–983. doi: 10.5858/2009-0445-OAR2. [DOI] [PubMed] [Google Scholar]
- 25.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 26.Carletti AM, Roncella S, Canessa PA, et al. Expression of human mammaglobin gene in pleural effusions of patients with malignant mesothelioma. Thorax. 2006;61:271. doi: 10.1136/thx.2005.049270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koga T, Horio Y, Mitsudomi T, et al. Identification of MGB1 as a marker in the differential diagnosis of lung tumors in patients with a history of breast cancer by analysis of publicly available SAGE data. J Mol Diagn. 2004;6:90–95. doi: 10.1016/S1525-1578(10)60495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sjodin A, Guo D, Sorhaug S, et al. Dysregulated secretoglobin expression in human lung cancers. Lung Cancer. 2003;41:49–56. doi: 10.1016/s0169-5002(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 29.Takeda Y, Tsuta K, Shibuki Y, et al. Analysis of expression patterns of breast cancer-specific markers (mammaglobin and gross cystic disease fluid protein 15) in lung and pleural tumors. Arch Pathol Lab Med. 2008;132:239–243. doi: 10.5858/2008-132-239-AOEPOB. [DOI] [PubMed] [Google Scholar]
- 30.Zehentner BK, Carter D. Mammaglobin: a candidate diagnostic marker for breast cancer. Clin Biochem. 2004;37:249–257. doi: 10.1016/j.clinbiochem.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Gaedcke J, Traub F, Milde S, et al. Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod Pathol. 2007;20:864–870. doi: 10.1038/modpathol.3800830. [DOI] [PubMed] [Google Scholar]
- 32.Yonemori K, Tsuta K, Shimizu C, et al. Immunohistochemical profiles of brain metastases from breast cancer. J Neurooncol. 2008;90:223–228. doi: 10.1007/s11060-008-9654-x. [DOI] [PubMed] [Google Scholar]