Abstract
Aims: Oxidative burst is one of the earliest biochemical events in the inflammatory activation of microglia. Here, we investigated the potential role of methionine sulfoxide reductase A (MsrA), a key antioxidant enzyme, in the control of microglia-mediated neuroinflammation. Results: MsrA was detected in rat microglia and its expression was upregulated on microglial activation. Silencing of MsrA exacerbated lipopolysaccharide (LPS)-induced activation of microglia and the production of inflammatory markers, indicating that MsrA may function as an endogenous protective mechanism for limiting uncontrolled neuroinflammation. Application of exogenous MsrA by transducing Tat-rMsrA fusion protein into microglia attenuated LPS-induced neuroinflammatory events, which was indicated by an increased Iba1 (a specific microglial marker) expression and the secretion of pro-inflammatory cytokines, and this attenuation was accompanied by inhibiting multiple signaling pathways such as p38 and ERK mitogen-activated protein kinases (MAPKs) and nuclear factor kappaB (NF-κB). These effects were due to MsrA-mediated reactive oxygen species (ROS) elimination, which may be derived from a catalytic effect of MsrA on the reaction of methionine with ROS. Furthermore, the transduction of Tat-rMsrA fusion protein suppressed the activation of microglia and the expression of pro-inflammatory factors in a rat model of neuroinflammation in vivo. Innovation: This study provides the first direct evidence for the biological significance of MsrA in microglia-mediated neuroinflammation. Conclusion: Our data provide a profound insight into the role of endogenous antioxidative defense systems such as MsrA in the control of microglial function. Antioxid. Redox Signal. 22, 832–847.
Introduction
Microglia, the resident macrophage in the central nervous system (CNS), are the major component that contributes to the immediate response to injury, pathogen infection, or toxic insult. Although microglial activation may be beneficial to “an attack” in the brain by removing cell debris and pathogens (19), overactivation of microglia causes excessive inflammatory responses (16), which are associated with numerous neurologic disorders, including ischemic stroke (63), CNS infections (11), and neurodegenerative diseases (38). Thus, the dissection of mechanisms that selectively shut off the pathways involved in microglial overactivation become critical in controlling neurological diseases.
Innovation.
We demonstrate the link between methionine sulfoxide reductase A (MsrA) and the functions of microglia for the first time. We provide experimental evidence in vitro as well as in vivo, pointing to the crucial role of microglial MsrA in the anti-inflammatory actions under lipopolysaccharide-stimulated conditions. In addition, this study provides the first experimental evidence that MsrA facilitates the reaction of methionine with reactive oxygen species, which may afford the antioxidant effect that is involved in the inhibition of microglia-mediated neuroinflammation.
Microglial overactivation and reactive oxygen species (ROS) are closely interlinked. It is widely accepted that activated microglia are the major source of ROS in the CNS (3, 54). Activated microglia produce high levels of superoxide radicals and hydrogen peroxide via activating NADPH oxidase (NOX) (3, 47). Moreover, ROS may be an early signal triggering the induction of cytokines and participating in inflammatory signaling (8, 48), by acting as second messengers that are capable of modulating gene expression via activating kinase signaling, including mitogen-activated protein kinases (MAPKs) (62, 75). As a downstream signaling molecule of MAPKs, nuclear factor kappaB (NF-κB) is particularly sensitive to ROS, and it is central to the acquisition of pro-inflammatory phenotype (29). Thus, ROS/MAPKs/NF-κB signaling pathways may play a pivotal role in the switch of microglia from surveillance to an over-activated state (9, 23, 49, 61).
Microglia are also equipped with sufficient antioxidative defence mechanisms to avoid ROS-mediated cellular damage (14, 34). Although their good antioxidative potential has been revealed (20), there is much less evidence related to their participation in neuroinflammation. ROS cause reversible molecular changes and control activity of key proteins that regulate neuroinflammation, such as apoptosis signal-regulating kinase 1 (ASK1) (50), dual-specificity phosphatase 3 (DUSP3) (66), and NF-κB (39). Thus, endogenous antioxidants may apply brake on uncontrolled neuroinflammatory responses. Activated microglia produce high levels of superoxide radicals and the main antioxidant enzyme that is known to scavenge superoxide radical is Cu, Zn-superoxide dismutase-1 (SOD-1). It has been well documented that SOD-1 reduces lipopolysaccharide (LPS)-induced superoxide production, with concurrent increases in hydrogen peroxide, and it exerts a significant inhibition on microglia-mediated inflammatory events (8, 12, 31). Although the oxidative potential of hydrogen peroxide is much lower than superoxide radical, it also acts as a second messenger to mediate intracellular redox-sensitive signal transduction and enhance neuroinflammation (15, 21, 58). Therefore, other antioxidative defense mechanisms to control microglia status may exist.
Cyclic oxidation/reduction of methionine (Met) in proteins is an important process in scavenging ROS and preventing ROS from attack on key sites of proteins (37, 55). A variety of ROS react with Met residues to form methionine sulfoxide (MetO), followed by MetO reductases (Msrs)-mediated reduction back to Met. As a key member of Msrs, MetO reductase A (MsrA) repairs oxidative damage of proteins and protects against oxidative stress in many types of cells, including neuronal cells (5, 42, 44, 72, 73). Nonetheless, the role of MsrA in neuroinflammation, particularly in the function of microglia, remains virtually unknown. The principal function of MsrA in the antioxidation is to maintain the effective content of protein-bound Met by reducing MetO timely. Recently, it has been demonstrated that MsrA also serves as a stereospecific methionine oxidase to promote oxidation of protein-bound Met (32, 33), suggesting that MsrA may facilitate the antioxidation of Met (69). In this context, we demonstrated that MsrA was functionally expressed in microglia and the MsrA-catalytic antioxidation attenuated inflammatory activation of microglia in vitro and neuroinflammation in vivo. We found that endogenous MsrA system is crucial in the limitation of uncontrolled neuroinflammation.
Results
MsrA is expressed in the resting and activated microglia both in vitro and in vivo
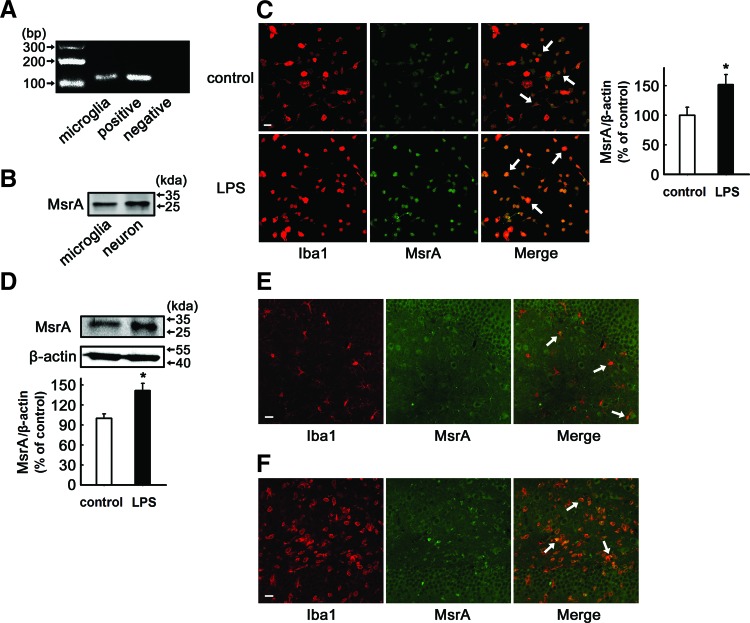
To determine whether MsrA is present in microglia, the total RNA was extracted from primary cultured rat microglia for reverse transcription-polymerase chain reaction analysis. By using specific primers for MsrA, the unique product with predicted size of 116 bp was yielded, similar to the positive control (plasmid pcDNA3.1-rMsrA) (Fig. 1A). The expression of MsrA protein in cultured rat microglia was further detected by immunoblotting. Using neuron as a positive control, the bands in Figure 1B showed the presence of MsrA protein in primary cultured rat microglia. The expression of various molecules in microglia are known to be changed after external stimuli (56), and we therefore asked whether the MsrA expression can vary with microglial activation. To address this question, primary microglial cultures were treated with LPS (100 ng/ml) for 24 h, and the MsrA immunoreactivity was examined. Immunocytochemical (Fig. 1C) and Western blotting analysis (Fig. 1D) revealed that LPS treatment increased the endogenous expression of MsrA by 151%±17% (n=6, p<0.05 vs. control) and 142%±11% (n=5, p<0.05 vs. control), respectively. Furthermore, in situ brain slices (DG area from the hippocampus) also showed the co-expression of MsrA in microglia. In the normal brain tissue, the levels of MsrA in most microglial cells are relatively low, and it seemed that MsrA colocalized with only a part of Iba1-positive cells (Fig. 1E). Under LPS-stimulated condition, most of the Iba1-positive microglial cells in situ strongly expressed MsrA (Fig. 1F), indicating that MsrA expression in the microglia is induced by environmental stimulus.
FIG. 1.
The expression of methionine sulfoxide reductase A (MsrA) in resting and activated microglia. (A) MsrA transcript was detected in primary cultured rat microglia by reverse transcription-polymerase chain reaction (positive control, plasmid pcDNA3.1-rMsrA; negative control, ddH2O). (B) Western blotting indicated the existence of MsrA in primary cultured rat microglia (positive control, cortical neuron). (C, D) Primary rat microglia were stimulated with 100 ng/ml lipopolysaccharide (LPS) for 24 h. (C) Immunocytochemical analysis to detect the expression of MsrA (green) in microglia in the presence or absence of LPS (n=6). Microglial cells were stained with an anti-Iba1 (a specific microglial marker, red) antibody. The mean fluorescence intensity of MsrA was determined and expressed as a relative change in comparison with the untreated cells, which was set to 100%. (D) Western blotting analysis of MsrA expression in the presence or absence of LPS (n=5). The levels of MsrA were expressed as a relative change in comparison with the untreated cells, which was set to 100%. Data are expressed as mean±SEM, *p<0.05 versus untreated cells. (E, F) Immunolabeling of MsrA (green) and microglia (red) in hippocampus in in vivo rat brain tissues after an intrahippocampal injection of saline (E) and LPS (F) at 24 h. Arrows indicate the overlapping of MsrA with Iba1-positive microglia. Scale bars: 50 μm. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Silencing of MsrA exacerbates LPS-induced activation of microglia and the production of pro-inflammatory factors
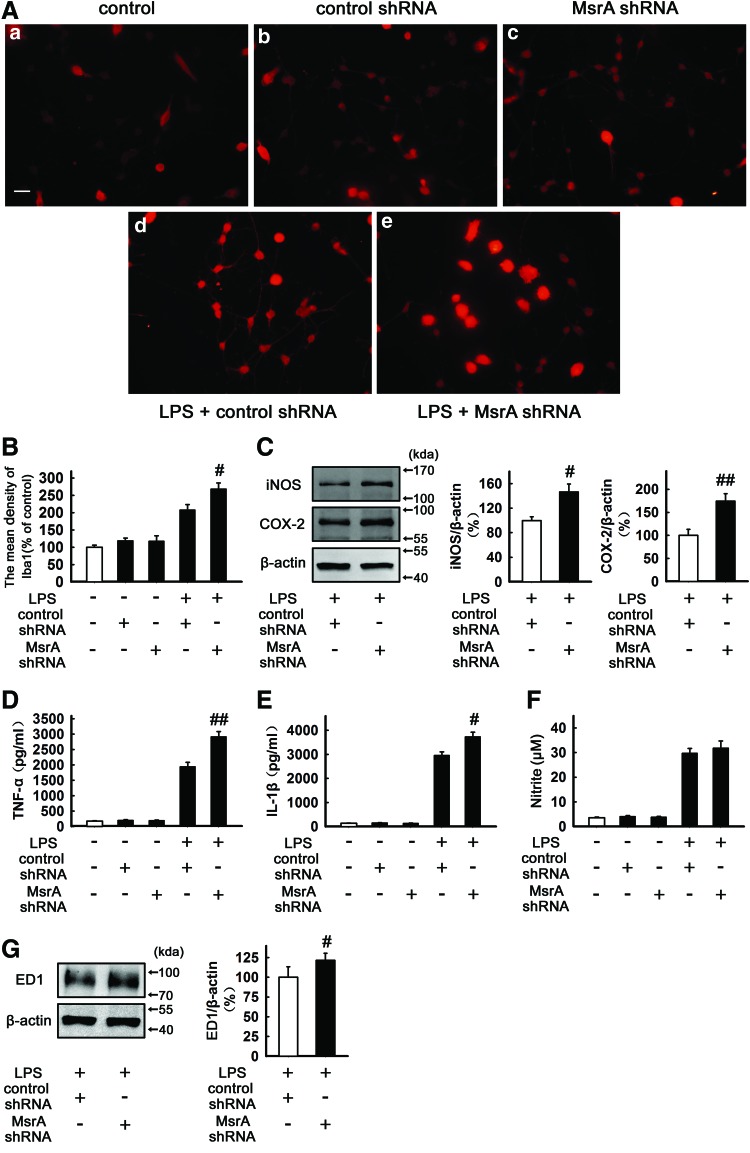
To determine the role of MsrA in the microglial status, we used lentiviral short hairpin RNAs (shRNAs) against MsrA to evaluate the role of MsrA in microglia-mediated inflammatory responses (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/ars). Microglial activation is associated with a marked increase in Iba1 expression (74). Immunofluorescence analysis showed that neither negative control shRNA (control shRNA) nor MsrA-silencing shRNA (MsrA shRNA) alone had perceptible effects on the mean density of Iba1 and microglial morphology. LPS treatment (100 ng/ml, 24 h) significantly increased the Iba1 expression. As expected, the mean density of Iba1 was significantly higher in MsrA shRNA-transfected group (268%±18%), which displayed a more “amoeboid”-activated morphology, characterized by hypertrophic bodies, with fewer, thicker, and shorter processes, when compared with control shRNA-transfected cells (208%±16%) (n=5, p<0.05 vs. LPS+control shRNA, Fig. 2A, B). Furthermore, when MsrA was silenced, LPS-induced expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) also increased by 146%±13% (n=5, p<0.05 vs. LPS+control shRNA, Fig. 2C) and 174%±16% (n=5, p<0.01 vs. LPS+control shRNA, Fig. 2C), respectively; whereas control shRNA or MsrA shRNA alone did not induce significant protein expression (Supplementary Fig. S1B). To assess whether MsrA knockdown aggravates the production of LPS-induced pro-inflammatory cytokines, tumor necrosis factor alpha (TNF-α) and IL-1β were quantified by an enzyme-linked immunosorbent assay (ELISA) in the cultured primary microglia. LPS treatment (100 ng/ml, 24 h) led to significantly increased levels of TNF-α (1947±138 pg/ml) and IL-1β (2964±139 pg/ml) in control shRNA-transfected microglial cultures, which were further enhanced by MsrA silencing (2915±165 pg/ml and 3732±188 pg/ml, respectively) (n=5, p<0.01 and p<0.05 vs. LPS+control shRNA, Fig. 2D, E). The effect of MsrA knockdown on NO production in LPS-stimulated primary microglia was also investigated. The accumulated nitrite in the culture media estimated by the Griess reaction was used as an index for NO synthesis. Although silencing of MsrA seemed to elevate LPS-induced production of nitrite, these changes were not statistically significant (n=5, Fig. 2F). Finally, we found that LPS-induced expression of ED1, a specific marker for activated microglia in LPS+MsrA shRNA group, was increased to 121%±9% of LPS+control shRNA group (n=5, p<0.05 vs. LPS+control shRNA, Fig. 2G), whereas neither control shRNA nor MsrA shRNA alone changed the ED1 expression (Supplementary Fig. S1C). Altogether, our results evidently indicate that knockdown of MsrA renders microglia more prone to activation by LPS, accompanied by higher levels of inflammatory markers.
FIG. 2.
MsrA knockdown aggravates LPS-induced microglial activation and inflammatory responses. Primary microglia were transfected with control shRNA or MsrA shRNA and incubated with 100 ng/ml LPS for 24 h. (A) Immunocytochemical analysis to evaluate the mean fluorescence density of Iba1 (n=5). Scale bars in (a–e): 50 μm. (B) The levels of Iba1 intensity were determined and expressed as a relative change in comparison with the untreated cells, which was set to 100%. (C) Primary microglia cell lysates were subjected to Western blotting for inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) (n=5). (D, E) Culture media of primary microglia were collected and subjected to (D) tumor necrosis factor alpha (TNF-α) and (E) IL-1β enzyme-linked immunosorbent assay (ELISA) (n=5). (F) NO release was measured by the Griess method (to detect nitrite, n=5). (G) The expression of ED1 was detected by Western blotting (n=5). The levels of ED-1, iNOS, and COX-2 were normalized to β-actin levels and expressed as a relative change in comparison with the LPS+control shRNA treatment. Data are expressed as mean±SEM, #p<0.05, ##p<0.01 versus LPS+control shRNA treatment. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The transduction of Tat-rMsrA fusion protein attenuates LPS-induced activation of microglia and the production of pro-inflammatory factors
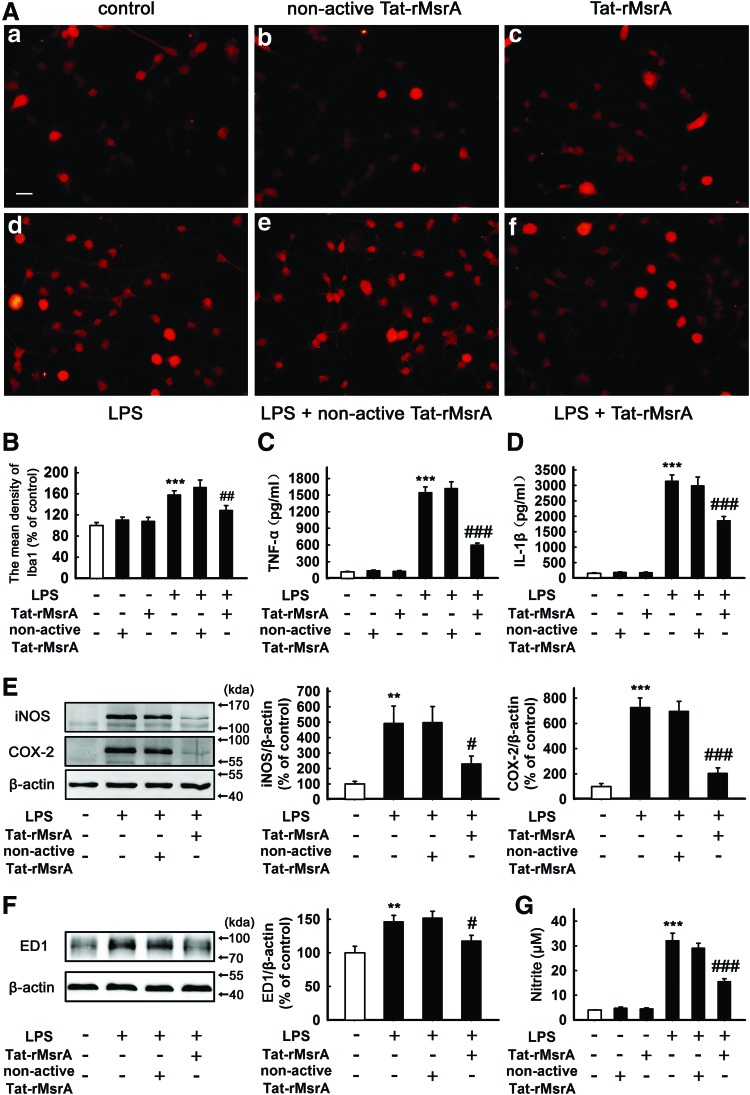
Based on the earlier observations, we speculated that MsrA may act as an endogenous regulator to limit uncontrolled inflammatory responses. To further confirm this point, we employed Tat-rMsrA fusion protein (Tat-rMsrA), a cell-permeable peptide that could be efficiently transduced into primary microglia, to increase the function of MsrA. As shown in Supplementary Figure S2A, Tat-rMsrA at 0.1, 0.5, and 1 μM increased the content of MsrA in microglia in a concentration-dependent manner (n=4, p<0.001 vs. control, Supplementary Fig. S2A). 0.5 μM Tat-rMsrA was used in the following experiments to limit anomalous Tat effects (Supplementary Figs S2B and S3A, B). Meanwhile, nonactive Tat-rMsrA that was prepared by boiling recombinant peptide at 95°C for 30 min was used as a negative control (Supplementary Fig. S2C). Pretreatment with Tat-rMsrA (0.5 μM, 1 h) attenuated LPS-mediated upregulation of Iba1 in primary microglial cells (129%±9%), when compared with the group pretreated with nonactive Tat-rMsrA (172%±14%) (n=6, p<0.01 vs. LPS+nonactive Tat-rMsrA, Fig. 3A, B). It was also found that pretreatment with Tat-rMsrA (0.5 μM, 1 h), not nonactive Tat-rMsrA, significantly inhibited LPS-induced production of TNF-α from 1546±100 pg/ml to 599±38 pg/ml, and IL-1β from 3143±196 pg/ml to 1857±139 pg/ml, respectively (n=5, p<0.001 vs. LPS+nonactive Tat-rMsrA, Fig. 3C, D). Similarly, LPS-induced expression of iNOS (493%±112%), COX-2 (727%±74%), and ED-1 (147%±9%) was obviously decreased by pretreatment with 0.5 μM Tat-rMsrA for 1 h (231%±50%, 205%±42%, and 118%±8%, respectively) (n=5, p<0.05 and p<0.001 vs. LPS+nonactive Tat-rMsrA, Fig. 3E, F). Moreover, Tat-rMsrA pretreatment (0.5 μM, 1 h) also remarkably reduced LPS-induced NO production by 46%±5% in primary microglia (n=5, p<0.001 vs. LPS+nonactive Tat-rMsrA, Fig. 3G). These results suggest that supplement of exogenous MsrA inhibits LPS-induced activation of microglia and the production of pro-inflammatory factors.
FIG. 3.
Tat-rMsrA alleviates LPS-induced microglial activation and inflammatory responses. Primary microglia enriched cultures were pretreated with Tat-rMsrA or nonactive Tat-rMsrA (0.5 μM) for 1 h and then incubated with LPS (100 ng/ml) for 24 h. (A) Immunocytochemical analysis to detect the mean density of Iba1 (n=6). Scale bars in (a–f): 50 μm. (B) The mean fluorescence intensity of Iba1 was determined, and the levels of Iba1 intensity were expressed as a relative change in comparison with the untreated cells, which was set to 100%. The amounts of (C) TNF-α and (D) IL-1β secreted were measured by ELISA (n=5). (E, F) The expression of (E) iNOS, COX-2, and (F) ED1 was evaluated by Western blotting assay (n=5). The ratios of densitometry values of iNOS, COX-2, and ED1 relative to β-actin were analyzed and normalized to the untreated cells. (G) NO release was measured using the Griess reaction to detect nitrite (n=5). Data are expressed as mean±SEM, **p<0.01, ***p<0.001 versus untreated cells, #p<0.05, ##p<0.01, and ###p<0.001 versus LPS+nonactive Tat-rMsrA treatment. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
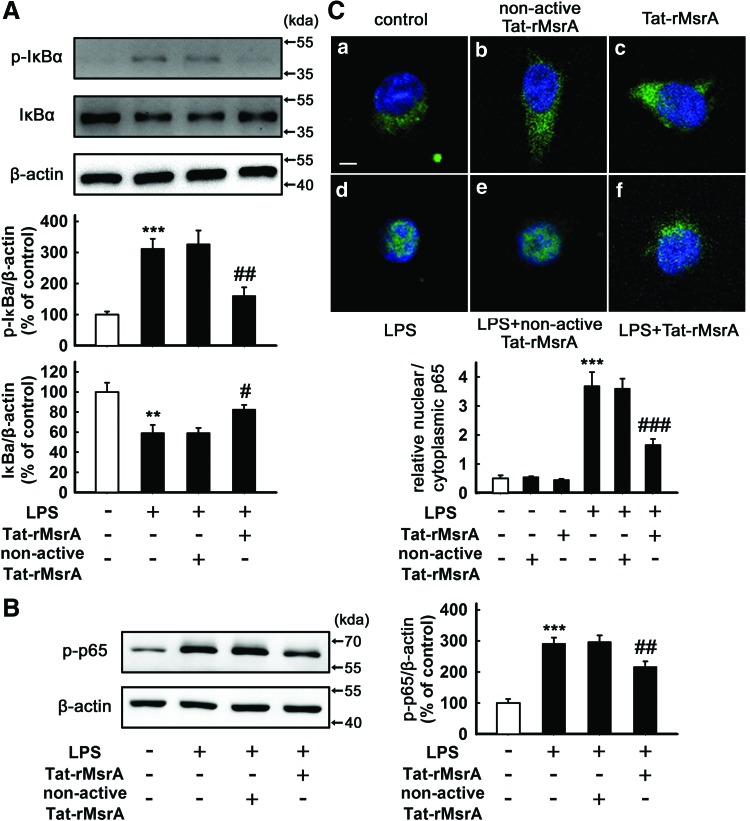
The transduction of Tat-rMsrA fusion protein inhibits LPS-induced activation of MAPKs and NF-κB signaling pathways in microglia
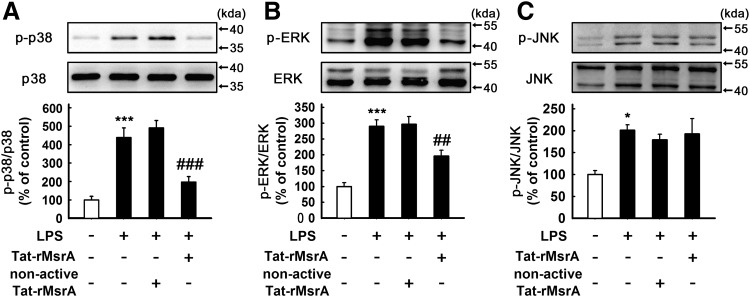
We therefore asked the underlying mechanism of the anti-inflammatory effect of MsrA. Activation of MAPKs and NF-κB signaling pathways in microglia are thought to play a pivotal role in inflammatory responses by regulating those genes encoding pro-inflammatory cytokines (22, 29). First, we investigated the effect of Tat-rMsrA on the activation of LPS-induced MAPK signaling cascades, including p38, ERK, and c-Jun N-terminal kinase (JNK). Western blotting analysis indicated that the increased phosphorylation of p38 and ERK, but not JNK was significantly decreased by pretreatment with Tat-rMsrA (0.5 μM, 1 h) in the presence of LPS (n=3–5, p<0.001 and p<0.01 vs. LPS+nonactive Tat-rMsrA, Fig. 4A–C), whereas neither Tat-rMsrA nor nonactive Tat-rMsrA alone (0.5 μM) induced a perceptible change in the expression of phosphorylated-MAPKs (Supplementary Fig. S4A–C). NF-κB is usually kept in an inactive form in the cytoplasm through binding an inhibitory protein of the IκB family, which was phosphorylated and degraded under inflammatory stimulation, allowing the phosphorylation of NF-κB p65 subunit and the translocation of NF-κB dimers to the nucleus (25). As shown in Supplementary Figure S4D and E, Tat-rMsrA or nonactive Tat-rMsrA alone (0.5 μM) had no significant effect on the expression of phosphorylated-IκBα (p-IκBα) and p-p65, two widely used indicators of NF-κB activation. Pretreatment with Tat-rMsrA (0.5 μM, 1 h) significantly reduced LPS-induced phosphorylation of IκBα and p65 by 47%±13% and 27%±4%, respectively, when compared with the nonactive Tat-rMsrA pretreatment (n=5, p<0.01 vs. LPS+nonactive Tat-rMsrA, Fig. 5A, B). Moreover, we analyzed the subcellular distribution of p65. Immunocytochemical analysis revealed that stimulation with LPS (100 ng/ml, 1 h) led to NF-κB activation in microglia, evidenced by the translocation of the p65 subunit from the cytoplasm to the nucleus. When Tat-rMsrA was pretreated (0.5 μM, 1 h), the nuclear accumulation of p65 was significantly reduced (n=5, p<0.001 vs. LPS+nonactive Tat-rMsrA, Fig. 5C). Altogether, these results demonstrate that the transduction of Tat-rMsrA fusion protein inhibits LPS-induced activation of p38, ERK, and NF-κB, suggesting that these signaling pathways play an essential role in the anti-inflammatory property of Tat-rMsrA in LPS-stimulated microglia.
FIG. 4.
Tat-rMsrA inhibits activation of p38 and ERK MAPKs in LPS-stimulated primary microglia. Primary microglia enriched cultures were pretreated with Tat-rMsrA or nonactive Tat-rMsrA (0.5 μM) for 1 h and then incubated with LPS (100 ng/ml) for 30 min. The phosphorylation of (A) p38 (n=4), (B) ERK (n=5) and (C) JNK (n=3) in the lysates was analyzed by Western blotting. The levels of phosphorylated-MAPKs were expressed as a relative change in comparison with the untreated cells, which was set to 100%. Data are expressed as mean±SEM, *p<0.05, ***p<0.001 versus untreated cells, ##p<0.01, ###p<0.001 versus LPS+nonactive Tat-rMsrA treatment.
FIG. 5.
Tat-rMsrA suppresses activation of NF-κB in LPS-stimulated primary microglia. (A, B) Primary microglia enriched cultures were pretreated with Tat-rMsrA or nonactive Tat-rMsrA (0.5 μM) for 1 h and then incubated with LPS (100 ng/ml) for 5 min. The whole-cell lysates were analyzed by Western blotting assay. The ratios of densitometry values of (A) p-IκBα, IκBα, and (B) p-p65 relative to β-actin were analyzed and normalized to the untreated cells (n=5). Data are expressed as mean±SEM. (C) Immunocytochemical assay of p65 (green) translocation from the cytoplasm to the nucleus in primary microglia (1 h post stimulus) in response to the (a) DMEM-F12; (b) nonactive Tat-rMsrA alone (0.5 μM); (c) Tat-rMsrA alone (0.5 μM); (d) LPS; (e) LPS+nonactive Tat-rMsrA pretreatment (0.5 μM, 1 h); and (f) LPS+Tat-rMsrA pretreatment (0.5 μM, 1 h) (n=5). The hoechst stained nuclei are shown in blue. Scale bars in (a-f): 10 μm. Quantification of the translocation of p65 from the cytoplasm to the nucleus is expressed as mean±SEM of the nuclear/cytoplasmic p65 fluorescence intensity. **p<0.01, ***p<0.001 versus untreated cells, #p<0.05, ##p<0.01, and ###p<0.001 versus LPS+nonactive Tat-rMsrA treatment. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
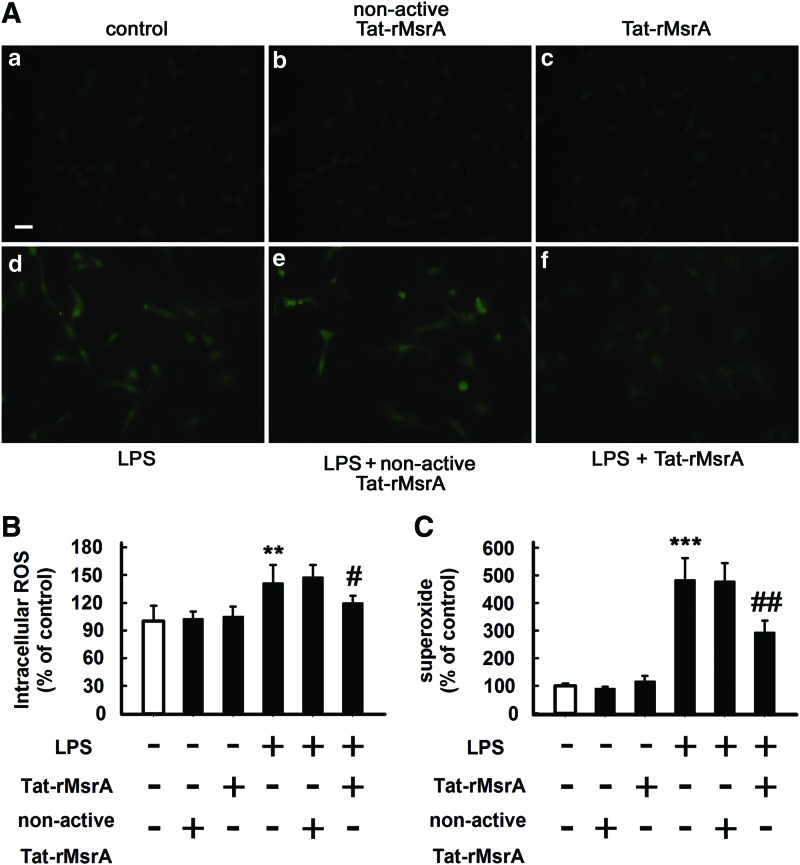
The transduction of Tat-rMsrA fusion protein alleviates oxidative stress during microglial activation
A growing number of evidence suggests that ROS can serve as signaling molecules (46, 52, 57) and are involved in the regulation of transcriptional changes through classical signaling molecules, including MAPKs and NF-κB (2, 51). Considering the role of MsrA in oxidative stress, we next examined whether the transduction of Tat-rMsrA was able to decrease LPS-induced production of ROS in primary cultured microglia. As shown in Figure 6A–C, LPS treatment (100 ng/ml, 30 min) increased the amounts of intracellular ROS (measured by H2DCFDA) and extracellular superoxide by 141%±20% (n=5, p<0.01 vs. control, Fig. 6A, B) and 483%±78% (n=5, p<0.001 vs. control, Fig. 6C), respectively, when compared with the untreated cells. Notably, pretreatment with Tat-rMsrA (0.5 μM, 1 h) reduced LPS-induced intracellular ROS production almost to the control levels (n=5, p<0.05 vs. LPS+nonactive Tat-rMsrA, Fig. 6A, B). Moreover, LPS-induced extracellular superoxide was also significantly decreased by the Tat-rMsrA pretreatment (294%±43%), in comparison to the nonactive Tat-rMsrA pretreatment (479%±67%) (n=5, p<0.01 vs. LPS+nonactive Tat-rMsrA, Fig. 6C). Thus, the transduction of Tat-rMsrA can remarkably attenuate LPS-induced oxidative stress in primary cultured microglia.
FIG. 6.
Tat-rMsrA attenuates oxidative stress during microglial activation. Primary microglia enriched cultures were pretreated with Tat-rMsrA or nonactive Tat-rMsrA (0.5 μM, 1 h) and then incubated with LPS (100 ng/ml) for 30 min. (A) The levels of intracellular ROS were detected with H2DCFDA (n=5). Scale bars in (a–f): 50 μm. (B) The fluorescence intensity of DCF was determined. (C) The release of superoxide was determined immediately by measuring the SOD-inhibitable reduction of WST-1 (n=5). Results were expressed as a percentage of control. Data are expressed as mean±SEM. **p<0.01, ***p<0.001 versus untreated cells, #p<0.05, ##p<0.01 versus LPS+nonactive Tat-rMsrA treatment. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
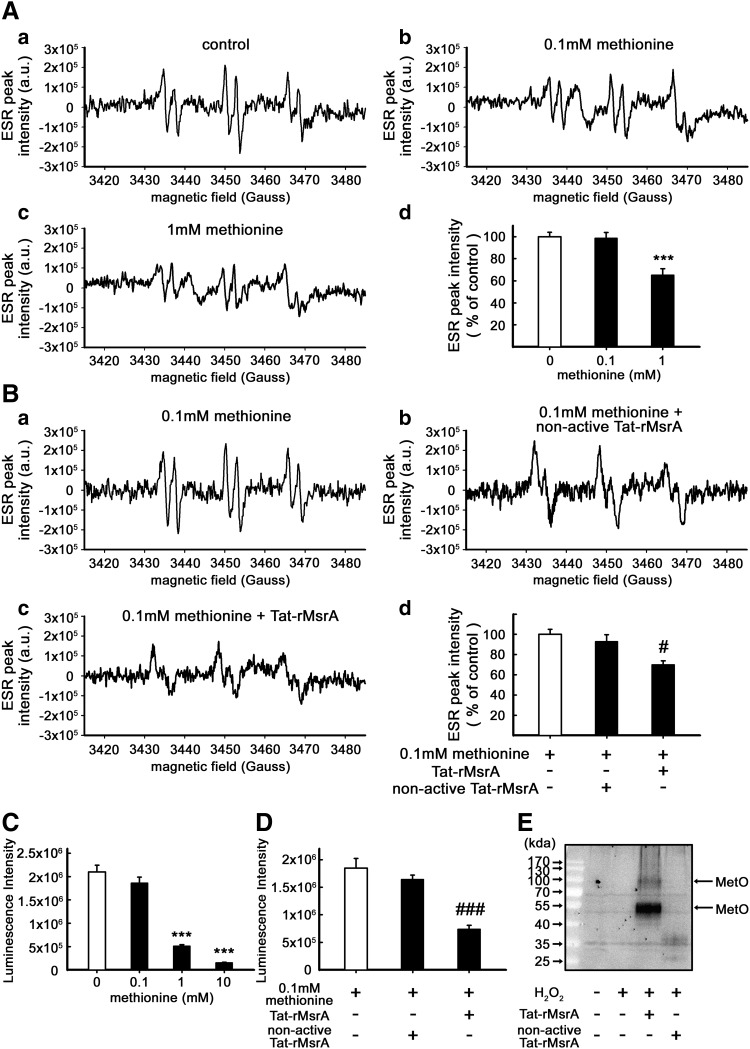
The transduction of Tat-rMsrA fusion protein increases the free radical-scavenging property of methionine via a catalytic antioxidation in vitro
As a reductase, how can MsrA scavenge LPS-induced ROS in microglia? One possible explanation is that MsrA serves as a stereospecific Met oxidase (33) and facilitates the reaction of Met with ROS. To confirm this hypothesis, we studied the antioxidant effect of Met in the presence of Tat-rMsrA or nonactive Tat-rMsrA. The hydroxyl radicals (OH•) were produced by the Fenton reaction, and the levels of OH• were evaluated by ESR spin-trapping using N-tert-butyl-α-phenylnitrone (PBN). As indicated in Figure 7A, treatment with 1 mM Met, but not 0.1 mM Met, significantly decreased the signal intensity of PBN-OH adducts (n=11, p<0.001 vs. control, Fig. 7A), which was not changed by Tat-rMsrA or nonactive Tat-rMsrA alone (1 μM) (Supplementary Fig. S5A). However, co-treatment of 0.1 mM Met and Tat-rMsrA (1 μM) decreased the PBN-OH signals by 23%±6%, when compared with the group co-treated with 0.1 mM Met and nonacitve Tat-rMsrA (n=5, p<0.05 vs. 0.1 mM Met+nonactive Tat-rMsrA, Fig. 7B), which strongly suggested that Tat-rMsrA increased Met-mediated reduction of OH•. Similar results were obtained by luminol-chemiluminescence assay. It was shown that H2O2-induced luminescence was significantly inhibited by both 1 and 10 mM Met, but not by 0.1 mM Met (n=6, p<0.001 vs. control, Fig. 7C). Therefore, we next determined the inhibitory effect of 0.1 mM Met on the luminescence intensity in the presence of Tat-rMsrA or nonactive Tat-rMsrA. Neither Tat-rMsrA nor nonactive Tat-rMsrA alone (1 μM) had a perceptible effect on the luminescence intensity (Supplementary Fig. S5B). Notably, co-treatment with 0.1 mM Met and Tat-rMsrA (1 μM), but not nonactive Tat-rMsrA, remarkably reduced the luminescence signal intensity by 54%±5% (n=5, p<0.001 vs. 0.1 mM Met+nonactive Tat-rMsrA, Fig. 7D). Furthermore, Western blotting analysis for detection of protein-bound MetO revealed that Met residues in primary cultured microglial lysates (boiled) were not oxidized to MetO in the presence of 0.5 mM H2O2 alone. However, when the primary microglial lysates (boiled) were co-treated with 0.5 mM H2O2 and Tat-rMsrA (1 μM) for 40 min, the protein-bound MetO of 100- and 50-kda protein obviously increased (n=5, Fig. 7E). Taken together, these results demonstrate that MsrA displays free radical-scavenging activity by catalyzing the oxidation reaction of ROS from Met or Met residues of proteins to MetO in vitro.
FIG. 7.
Tat-rMsrA increases the free radical-scavenging property of methionine via a catalytic antioxidation in vitro. (A) ESR spectra of the spin adduct of OH• radical observed during the reaction of 0.5 mM H2O2 containing 30 mM PBN and 0.2 mM FeSO4 with 0.1 or 1 mM methionine (Met). (a) Control, containing H2O2, FeSO4, and PBS; (b) As in (a) in the presence of 0.1 mM Met; (c) As in (a) in the presence of 1 mM Met; (d) The levels of ESR peak intensity were expressed as a relative change in comparison with the untreated control, which was set to 100% (n=11). (B) The effect of 0.1 mM Met on the signal intensity of PBN-OH adducts in the presence of Tat-rMsrA or nonactive Tat-rMsrA was evaluated under the same experimental conditions (n=5). (a) Control, containing H2O2, FeSO4, PBS, and 0.1 mM Met; (b) As in (a) in the presence of nonactive Tat-rMsrA (1 μM); (c) As in (a) in the presence of Tat-rMsrA (1 μM); (d) The levels of ESR peak intensity were expressed as a relative change in comparison with the 0.1 mM Met treatment, which was set to 100%. (C) Concentration-dependent effects of Met (0.1, 1, and 10 mM) on luminol-enhanced chemiluminescence were assessed (n=6). (D) Inhibitory effect of 0.1 mM Met on luminol-enhanced chemiluminescence in the presence of Tat-rMsrA or nonactive Tat-rMsrA (1 μM) was determined (n=5). (E) Lysates of primary microglia were treated with 0.5 mM H2O2 in the presence of Tat-rMsrA or nonactive Tat-rMsrA (1 μM). The expression of methionine sulfoxide (MetO) was determined by Western blotting (n=5). Data are expressed as mean±SEM. ***p<0.001 versus control, #p<0.05, ###p<0.001 versus 0.1 mM Met+nonactive Tat-rMsrA treatment.
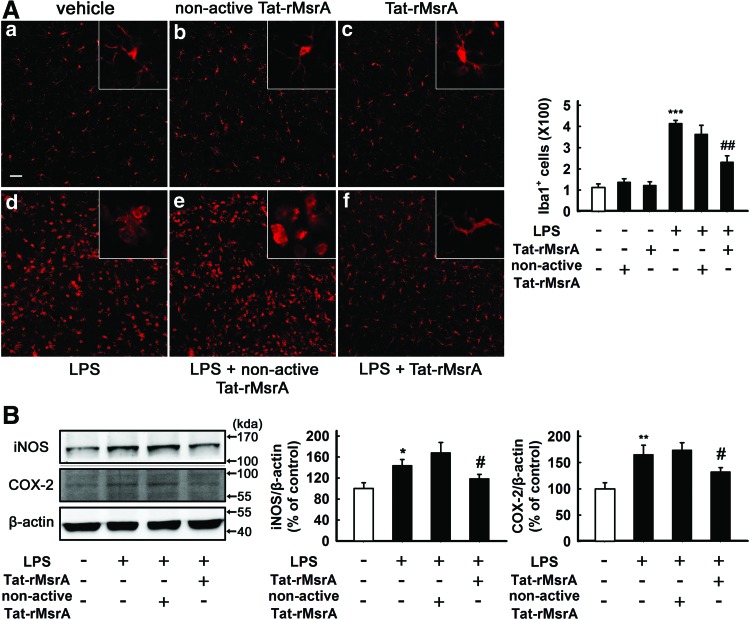
The transduction of Tat-rMsrA fusion protein inhibits LPS-induced microglial activation and neuroinflammation in vivo
It has been demonstrated that microglia are mainly distributed in the gray matter with highest concentration in the hippocampus (30). The effect of Tat-rMsrA on microglia-mediated neuroinflammation was further confirmed by using a rat model of acute neuroinflammation, in which LPS (2 μl, 5 mg/ml) was directly injected into the dentate gyrus of hippocampus (1). As shown in Figure 8A, neither Tat-rMsrA nor nonactive Tat-rMsrA alone (2 μl, 3 mg/ml) significantly altered the number and morphology of Iba1-positive microglia. Notably, at 24 h after LPS injection, microglia in the dentate gyrus displayed remarkably enhanced Iba1 immunoreactivity and an “activated” morphology with hypertrophic cell bodies, and thickened and shortened processes. Preadministration of Tat-rMsrA (2 μl, 3 mg/ml) at 24 h before LPS injection significantly reduced the number of Iba1-positive microglia, and the majority of microglial cells were reverted to the “resting” state that displayed a ramified, extensively branched morphology (n=5, p<0.01 vs. LPS+nonactive Tat-rMsrA, Fig. 8A). Furthermore, Western blotting analysis revealed that Tat-rMsrA or nonactive Tat-rMsrA alone (2 μl, 3 mg/ml) had no perceptible effect on the expression of pro-inflammatory factors such as iNOS and COX-2 (Supplementary Fig. S3C). However, LPS-induced increased levels of iNOS and COX-2 were evidently reduced by the Tat-rMsrA pretreatment (2 μl, 3 mg/ml) (119%±8% and 132%±8%, respectively), when compared with the nonactive Tat-rMsrA pretreatment (168%±20% and 174%±14%, respectively) (n=6, p<0.05 vs. LPS+nonactive Tat-rMsrA, Fig. 8B). Taken together, these observations suggest that the upregulation of MsrA expression suppresses microglial activation and the production of pro-inflammatory cytokines during LPS-induced inflammation in vivo.
FIG. 8.
Tat-rMsrA suppresses the activation of microglia and the production of pro-inflammatory factors in a rat model of acute neuroinflammation. SD rats were injected with 2 μl saline or LPS (5 mg/ml) into the dentate gyrus of hippocampus for 24 h in the presence of Tat-rMsrA or nonactive Tat-rMsrA, which were preadministrated into the hippocampus (2 μl, 3 mg/ml, 24 h before LPS injection). (A) Iba1 immunoreactivity was evaluated by immunofluorescence study, and the number of Iba1+ microglia was counted (n=5). Insets are images at high magnification. Scale bars in (a–f): 100 μm. (B) The levels of iNOS and COX-2 were normalized to β-actin levels and expressed as a relative change in comparison with the untreated slices, which was set to 100% (n=6). Data are expressed as mean±SEM, *p<0.05, **p<0.01, and ***p<0.001 versus untreated slices, #p<0.05, ##p<0.01 versus LPS+nonactive Tat-rMsrA treatment. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Discussion
In this study, we found that microglial MsrA plays an anti-inflammatory role in LPS-stimulated conditions by a mechanism involving the inhibition of LPS-induced activation of p38, ERK, and NF-κB. These effects were associated with MsrA-mediated ROS elimination in microglia, which may be derived from a catalytic effect of MsrA on the reaction of Met with ROS (Fig. 9). Our results support the notion that endogenous antioxidants may serve as a “brake” for uncontrolled neuroinflammation.
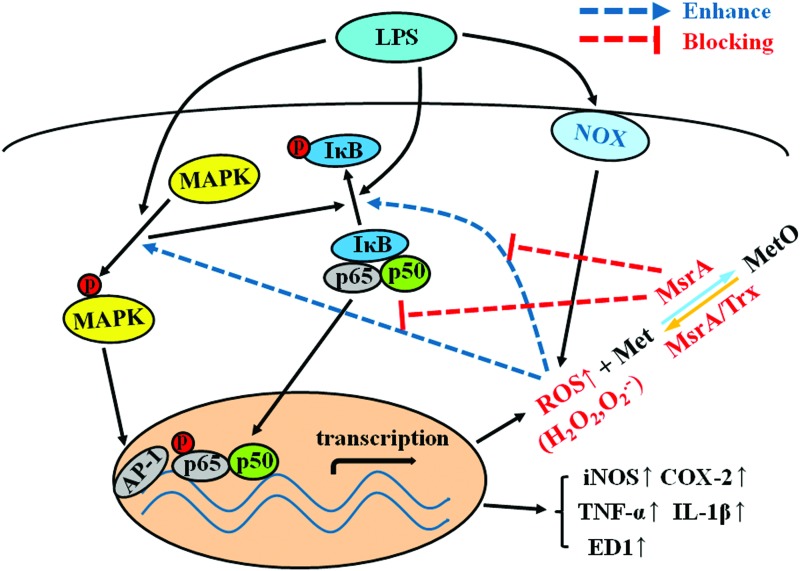
FIG. 9.
Possible mechanisms by which MsrA inhibits LPS-induced microglial activation and inflammatory responses. LPS initiates the activation of NOX and the production of ROS. ROS can function as “signaling molecules” and modulate large numbers of transcriptional changes through classical signaling molecules, including MAPKs and NF-κB, which lead to microglial overactivation and the release of proinflammatory factors. MsrA scavenges ROS through catalyzing the reaction of ROS with Met residues in proteins, thereby suppressing the self-perpetuating vicious cycle of “ROS-MAPKs-NFκB” signaling and micoglia-mediated neuroinflammation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Activated microglia are an important source for production of ROS in the CNS (3, 54, 68), and ROS also have been reported as a pathological situation that aggravates microglial response by modulating their activity (9, 23, 49, 61). Microglial cells contain sufficient antioxidative defense mechanisms to avoid ROS-mediated cellular damage (14, 20, 24, 34). However, there is much less evidence related to their participation in the function of microglia. It has been demonstrated that both SOD-1 and peroxiredoxin I inhibit microglial activation and exert significant anti-inflammatory effects (6, 10, 24, 29). Here, we found that SOD-1 rapidly transforms superoxide into hydrogen peroxide, which also acts as a second messenger to mediate signal transduction and regulate microglial status (15, 21, 58). Therefore, other synergistical antioxidant mechanisms to control neuroinflammation may exist.
A recent study has reported that peroxiredoxin I, an ROS/p38 MAPK-dependent endogenous antioxidant, inhibits NF-κB function and microglial activation (26). We found that MsrA was an inducible antioxidant under LPS-stimulated conditions in rat microglia. In the normal brain tissue, the levels of MsrA in most Iba1-positive cells are relatively low. In the cultured microglia, nearly all the Iba1-immunopositive cells expressed MsrA, which may be related with environmental stimulus during primary microglia culture, such as trypsinization or trituration. Silencing of MsrA accelerated LPS-induced changes in the mean density of Iba1 and morphology of cultured microglial cells and the production of inflammatory markers, including iNOS, COX-2, TNF-α, IL-1β, and ED-1. However, MsrA silencing had little effect on subsequent NO secretion in LPS-stimulated microglia. Under LPS-stimulated condition, most reports have demonstrated that iNOS and subsequent NO production is pro-inflammatory (4). The insignificant increase in nitrite production after MsrA knockdown under LPS-stimulated condition may be caused by the fact that MsrA silencing induced a compensatory increase in other endogenous antioxidants such as thioredoxin that can scavenge RNS. Supplement of exogenous MsrA by transducing Tat-rMsrA fusion protein attenuated LPS-induced neuroinflammatory events, including an increased expression of Iba1 and the production of pro-inflammatory cytokines, such as iNOS, COX-2, TNF-α, IL-1β, and NO. Furthermore, injection of Tat-rMsrA fusion protein suppressed microglial activation and the expression of pro-inflammatory factors in a rat model of neuroinflammation in vivo. Considering that only activated microglia cells strongly expressed COX-2 and iNOS, the reduced expression of COX-2 and iNOS is mainly due to the inhibition of microglia activation by MsrA. Our results clearly demonstrate that MsrA brakes LPS-induced uncontrolled neuroinflammation in the microglia.
There is increasing evidence that ROS serves as the messenger molecule to mediate cellular signaling pathways (46, 52, 57). In microglial cells, ROS may be an early signal triggering the induction of cytokines and participating in inflammatory signaling (8, 48). For instance, ROS control activity of key proteins that regulate the activation of MAPK signaling cascades, such as ASK1 (50) and DUSP3 (66). Furthermore, LPS-mediated ROS generation is closely associated with the activation of NF-κB (17), a redox-sensitive transcription factor that governs the production of neuroinflammatory mediators, such as TNF-α, ILs, and NOS. The results obtained in this work indicated that the exogenous supplement of MsrA by transduction peptide significantly decreased LPS-induced phosphorylation of p38 and ERK MAPKs in cultured microglial cells. In addition, Tat-rMsrA also inhibited LPS-induced phosphorylation of IκBα and subsequent translocation of p65, a key process that initiates the expression of inflammatory genes. It has been reported that methionine oxidation activates a transcription factor named HypT in bacterias and regulates gene expression in response to oxidative stress (13). Thus, the nuclear expression of MsrA may serve as a potential regulator in the control of gene expression in response to redox status. Although other mechanisms may exist, current results demonstrate that p38, ERK, and NF-κB are the major inhibitory signaling pathways managed by the transduction of exogenous MsrA.
MsrA has historically been considered a reductase, not an ROS scavenger (41, 59). In spite of its impressive cytoprotection and antioxidation (5, 42, 44, 72, 73), the precise mechanisms and fundamental chemical principles for MsrA-mediated antioxidation remain largely unclear. In this study, the transduction of exogenous MsrA was able to decrease LPS-induced production of ROS in primary microglia. How can MsrA scavenge ROS? It is generally considered that MsrA-catalytic antioxidation depends on the Met-centered redox cycle (40, 65), which is mainly due to the antioxidant activity of protein-bound Met residues. MsrA may exhibit antioxidation via maintaining an effective concentration of Met by reducing MetO timely. Interestingly, our previous study has indicated that under oxidative stress, more than 80% of protein-bound Met residues are kept in a reduced form (36), indicating that there a sufficient store of Met exists. Moreover, we have found that after inhibition of MsrA function by DMSO (27), the antioxidation of Met (1–5 mM) on CHO cells is largely abolished (69), suggesting that Met is not merely an antioxidant. Recently, it has been demonstrated that MsrA also serves as a stereospecific methionine oxidase (32, 33), which may facilitate the reaction of Met with ROS. This work provided the first experimental evidence that MsrA facilitated the reaction of Met with ROS, which may afford the antioxidant effect. In Fenton reaction system, both ESR spin-trapping and luminol-chemiluminescence assay revealed that MsrA facilitated the Met (0.1 mM)-mediated scavenging effect on hydroxyl radicals. Moreover, we found that protein-bound Met of primary microglia lysates (boiled) could not be oxidized to MetO by 0.5 mM H2O2 alone. Interestingly, when the same lysates were co-treated with 0.5 mM H2O2 and active MsrA (1 μM), the MetO levels of 100- and 50-kda protein obviously increased. Taken together, our results demonstrate that MsrA displays free radical-scavenging activity by catalyzing the reaction of ROS with Met residues.
Until now, the role of MsrA in the inflammation remains largely unknown. A recent study has elucidated that deletion of MsrA enhances renal inflammatory responses under I/R conditions (25). Our current findings demonstrate the link between MsrA and microglia-mediated neuroinflammation for the first time. It is widely accepted that microglia can be driven to adopt two phenotypes, that is, pro-inflammatory (M1) and anti-inflammatory (M2) phenotype (48). Our results revealed that MsrA prevents microglial cells from activating into M1 phenotype. An interesting question that remains open is whether MsrA promotes activation of microglia cells into M2 phenotype. The role of MsrA in microglial M2 activation requires further investigation.
Imbalance of redox homeostasis plays a key pathological role in the process of neurodegenerative diseases and neuroinflammation. To maintain redox homeostasis, vitagenes network, an emerging concept that includes a group of genes involved in preserving cellular homeostasis during stressful conditions, operates actively to preserve neuron survival and serves as an integrated mechanism (6, 7, 10, 60). Vitagenes encode for cytoprotective proteins such as heat shock proteins, the thioredoxin, and the sirtuin protein systems and their expression can be activated by oxidative stress or dietary antioxidants, such as polyphenols. Thus, MsrA may act as a new number in the vitagenes network for its cytoprotection and inducible expression during stressful conditions. Previous studies have revealed that enhancing MsrA function may be beneficial in Parkinson's disease (PD) and Alzheimer's disease (AD) via scavenging ROS and repairing methionine sulfoxide (35, 43, 64). Taken together, our results suggest that MsrA negatively controls microglia-mediated neuroinflammation, and pharmacological enhancement of MsrA function may serve as a reasonable therapeutic strategy aimed at reducing neurodegeneration in the clinic. There are at least three ways to enhance MsrA function: (i) supplement of its substrates, including L-Met and S-methyl-L-cysteine (SMLC) (64, 67); (ii) upregulation of endogenous MsrA expression (70); and (iii) transduction of exogenous MsrA using novel drug delivery systems such as cell-penetrating peptides. Their clinical applicability requires further investigation.
Materials and Methods
Animals
Adult male Sprague–Dawley (SD) rats weighing 200–250 g were obtained from the Experimental Animals of Tongji Medical College, Huazhong University of Science and Technology. The rats were housed individually on a controlled 12: 12 light cycle at a constant temperature (22°C±1°C) with free access to water and food, and allowed to acclimate a week. The use of animals for experimental procedures was conducted in accordance with the Declaration of Helsinki and with the Guide for Care and Use of Laboratory Animals as adopted and promulgated by the United National Institutes of Health. The experimental procedures were approved by the Animal Welfare Committee of Huazhong University of Science & Technology.
Microglial culture
Microglial cells were isolated from the mixed cultures of cerebrocortical cells from postnatal day 1–3 SD rats, as previously described, with some modification (53). In brief, rats were decapitated, and the cortices were removed and trypsinized with 0.125% trypsin (Amresco, Solon, OH) for 10 min at 37°C. After trituration and centrifugation at 120 g for 8 min, the cells were resuspended and plated on culture flasks, which were precoated with poly-L-lysine (Sigma-Aldrich, St. Louis, MO). The cells were then cultured in DMEM-F12 (Gibco, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT), 2 mM L-glutamine, and 100 U/ml penicillin-streptomycin (Sigma-Aldrich). After 24 h, the medium was changed into fresh DMEM-F12 and replaced every 3–4 days in vitro. The cultures were maintained at 37°C in a humidified 5% CO2 atmosphere incubator. The confluency was achieved after 9–11 days in vitro. High enriched microglial cells were isolated from the mixed culture by mild trypsinization. The mixed glial cultures were incubated with a trypsin solution (0.25% trypsin and 1 mM EDTA) diluted 1: 3 in DMEM-F12 for 30–60 min, which resulted in detachment of an upper layer of astrocytes in one piece, while the microglia remained attached to the bottom of the culture flask. The purity of microglia was confirmed by immunofluorescence with an anti-Iba1 antibody (98%) (Abcam, Cambridge, United Kingdom).
Construction of rat MsrA shRNA lentiviral expression vector
A third generation of self-inactivating lentivirus vector (GeneChem, Shanghai, China) containing a CMV-driven GFP reporter and a U6 promoter upstream of the cloning sites (Age I and EcoR I) was used for cloning small hairpin RNAs (shRNAs). The target sequence for rat MsrA was 5′-AGCACGTCAGCTTTGAGGA-3′, and for control scrambled shRNA it was 5′-TTCTCCGAACGTGTCACGT-3′. Microglial cells were infected with lentivirus at a multiplicity of infection of 50 for 8 h. Then, the medium was replaced with fresh complete medium. After 72 h, cells were observed under fluorescence microscopy to confirm that more than 80% of cells were GFP positive.
Preparation of Tat-rMsrA
See details in the Supplementary Methods section.
Analysis of Tat-rMsrA activity
The activity of recombinant Tat-rMsrA was rapidly monitored by detecting both MetO-reducing activity and methyl sulfoxide-dependent oxidation of dithiothreitol (DTT) (71). The details are provided in the Supplementary Methods section.
Measurements of TNF-α and IL-1β levels by ELISA
The levels of TNF-α and IL-1β were quantified in microglial supernatants using the TNF-α and IL-1β ELISA kits (Boster, Wuhan, China) according to the manufacturer's instructions.
Nitrite quantification
The production of NO was evaluated by indirectly measuring the accumulated levels of nitrite in the culture supernatants (18), a stable metabolite of NO, using the Griess reagent (Beyotime, Shanghai, China) according to the manufacturer's instructions.
Electron spin resonance measurement
PBN was used as a free radical trapper. Electron spin resonance (ESR) signals were detected with a Bruker e-scan ESR spectrometer (Burker, Karlsruhe, Germany). As a standard of the reactant of OH• with PBN, we produced hydroxyl radical (OH•) by the Fenton reaction in the mixture of 0.5 mM H2O2 and 0.2 mM FeSO4 in the presence of 30 mM PBN. The spin traps, methionine (0.1 or 1 mM), and Tat-rMsrA/nonactive Tat-rMsrA (1 μM) were added before the Fe (II) and H2O2. Samples (20 μl) were loaded into a quartz tube, and the ESR spectra were recorded at room temperature.
The ESR microwave power was set to 4.88 mW. The modulation frequency was 9.76 GHz. The time constant was 20.48 ms. The conversion time was 20.48 ms, and a sweep time of 10.49 s was used. Each sample was scanned for a total of 20 times. A sweep width of 70 G was used for experiments with PBN. The receiver gain was set to 3.17×103. Simulation and fitting of the ESR spectra were performed using the Bruker WinEPR program.
Methionine sulfoxide assay
Detection of methionine sulfoxide was performed with the methionine sulfoxide immunoblotting kit (Cayman, Ann Arbor, MI) according to the manufacturer's protocol (28, 45). The details are provided in the Supplementary Methods section.
Measurements of intracellular ROS
See details in the Supplementary Methods section.
Superoxide assay
See details in the Supplementary Methods section.
Luminol-enhanced chemiluminescence assay
See details in the Supplementary Methods section.
Reverse transcription-polymerase chain reaction
See details in the Supplementary Methods section.
Western blotting
See details in the Supplementary Methods section.
Immunolabeling
See details in the Supplementary Methods section.
Rat model of acute neuroinflammation
LPS was injected into the dentate gyrus of hippocampus to evoke neuroinflammation in rats, as previously described, with minor modification (1). The details are provided in the Supplementary Methods section.
Statistics
All experiments were performed at least thrice with similar results. Data from experiments were analyzed with the statistical program SPSS 18.0 software (SPSS, Chicago, IL). Comparison between two groups was evaluated by an unpaired and two-sided Student's t-test. ANOVAs and post hoc tests (Fisher's LSD) were used to analyze differences in different treatment groups. Data are presented as mean±SEM. Differences between experimental conditions were considered statistically significant when p<0.05.
Supplementary Material
Abbreviations Used
- AD
Alzheimer's disease
- ASK1
apoptosis signal-regulating kinase 1
- CNS
central nervous system
- COX-2
cyclooxygenase-2
- DCF
2′, 7′-dichlorofluorescein
- DCFH
2′, 7′-dichlorodihydrofluorescein
- DUSP3
dual-specificity phosphatase 3
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-regulated kinase
- ESR
electron spin resonance
- GFP
green fluorescent protein
- H2DCFDA
2′, 7′-dichlorodihydrofluorescein diacetate
- IL-1β
interleukin-1 beta
- iNOS
inducible nitric oxide synthase
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAPKs
mitogen-activated protein kinases
- Met
methionine
- MetO
methionine sulfoxide
- MsrA
methionine sulfoxide reductase A
- Msrs
methionine sulfoxide reductases
- NF-κB
nuclear factor kappaB
- NOX
NADPH oxidase
- OH•
hydroxyl radicals
- PBN
N-tert-butyl-α-phenylnitrone
- PD
Parkinson's disease
- ROS
reactive oxygen species
- SD rats
Sprague Dawley rats
- shRNAs
short hairpin RNAs
- SOD
superoxide dismutase
- SOD-1
Cu, Zn-superoxide dismutase-1
- Tat-rMsrA
Tat-rMsrA fusion protein
- TNF-α
tumor necrosis factor alpha
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (the 973 Program, No. 2013CB531303 to Dr. J.G.C.; No. 2014CB744601 to F.W.) and the National Natural Scientific Foundation of China (NSFC, No. 81222048 to F.W.; No. 81302754 to P.F.W). It was also supported by the International Science & Technology Cooperation Program of China (No. 2011DFA32670 to J.G.C.) and PCSIRT (No. IRT13016).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ambrosini A, Louin G, Croci N, Plotkine M, and Jafarian-Tehrani M. Characterization of a rat model to study acute neuroinflammation on histopathological, biochemical and functional outcomes. J Neurosci Methods 144: 183–191, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Asehnoune K, Strassheim D, Mitra S, Kim JY, and Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol 172: 2522–2529, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Block ML, Zecca L, and Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8: 57–69, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Brown GC. and Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 41: 242–247, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Cabreiro F, Picot CR, Perichon M, Friguet B, and Petropoulos I. Overexpression of methionine sulfoxide reductases A and B2 protects MOLT-4 cells against zinc-induced oxidative stress. Antioxid Redox Signal 11: 215–225, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Calabrese V, Cornelius C, Cuzzocrea S, Iavicoli I, Rizzarelli E, and Calabrese EJ. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Aspects Med 32: 279–304, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, and Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal 13: 1763–1811, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang SC, Kao MC, Fu MT, and Lin CT. Modulation of NO and cytokines in microglial cells by Cu/Zn-superoxide dismutase. Free Radic Biol Med 31: 1084–1089, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, and Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci 28: 12039–12051, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelius C, Trovato Salinaro A, Scuto M, Fronte V, Cambria MT, Pennisi M, Bella R, Milone P, Graziano A, Crupi R, Cuzzocrea S, Pennisi G, and Calabrese V. Cellular stress response, sirtuins and UCP proteins in Alzheimer disease: role of vitagenes. Immun Ageing 10: 41, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellacasa-Lindberg I, Fuks JM, Arrighi RB, Lambert H, Wallin RP, Chambers BJ, and Barragan A. Migratory activation of primary cortical microglia upon infection with Toxoplasma gondii. Infect Immun 79: 3046–3052, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimayuga FO, Wang C, Clark JM, Dimayuga ER, Dimayuga VM, Bruce-Keller AJ. SOD1 overexpression alters ROS production and reduces neurotoxic inflammatory signaling in microglial cells. J Neuroimmunol 182: 89–99, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drazic A, Miura H, Peschek J, Le Y, Bach NC, Kriehuber T, and Winter J. Methionine oxidation activates a transcription factor in response to oxidative stress. Proc Natl Acad Sci U S A 110: 9493–9498, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal 7: 1223–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Eguchi H, Fujiwara N, Sakiyama H, Yoshihara D, and Suzuki K. Hydrogen peroxide enhances LPS-induced nitric oxide production via the expression of interferon beta in BV-2 microglial cells. Neurosci Lett 494: 29–33, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Glass CK, Saijo K, Winner B, Marchetto MC, and Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell 140: 918–934, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gloire G, Legrand-Poels S, and Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72: 1493–1505, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, and Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126: 131–138, 1982 [DOI] [PubMed] [Google Scholar]
- 19.Hanisch UK. and Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10: 1387–1394, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hirrlinger J, Gutterer JM, Kussmaul L, Hamprecht B, and Dringen R. Microglial cells in culture express a prominent glutathione system for the defense against reactive oxygen species. Dev Neurosci 22: 384–392, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Holmquist L, Stuchbury G, Steele M, and Munch G. Hydrogen peroxide is a true first messenger. J Neural Transm Suppl: 39–41, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Hwang D, Jang BC, Yu G, and Boudreau M. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide: mediation through both mitogen-activated protein kinase and NF-kappaB signaling pathways in macrophages. Biochem Pharmacol 54: 87–96, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Innamorato NG, Lastres-Becker I, and Cuadrado A. Role of microglial redox balance in modulation of neuroinflammation. Curr Opin Neurol 22: 308–314, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Kaneko YS, Ota A, Nakashima A, Mori K, Nagatsu I, and Nagatsu T. Regulation of oxidative stress in long-lived lipopolysaccharide-activated microglia. Clin Exp Pharmacol Physiol 39: 599–607, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Kim JI, Choi SH, Jung KJ, Lee E, Kim HY, and Park KM. Protective role of methionine sulfoxide reductase A against ischemia/reperfusion injury in mouse kidney and its involvement in the regulation of trans-sulfuration pathway. Antioxid Redox Signal 18: 2241–2250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SU, Park YH, Min JS, Sun HN, Han YH, Hua JM, Lee TH, Lee SR, Chang KT, Kang SW, Kim JM, Yu DY, Lee SH, and Lee DS. Peroxiredoxin I is a ROS/p38 MAPK-dependent inducible antioxidant that regulates NF-kappaB-mediated iNOS induction and microglial activation. J Neuroimmunol 259: 26–36, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Kwak GH, Choi SH, and Kim HY. Dimethyl sulfoxide elevates hydrogen peroxide-mediated cell death in Saccharomyces cerevisiae by inhibiting the antioxidant function of methionine sulfoxide reductase A. BMB Rep 43: 622–628, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Kwon TJ, Cho HJ, Kim UK, Lee E, Oh SK, Bok J, Bae YC, Yi JK, Lee JW, Ryoo ZY, Lee SH, Lee KY, and Kim HY. Methionine sulfoxide reductase B3 deficiency causes hearing loss due to stereocilia degeneration and apoptotic cell death in cochlear hair cells. Hum Mol Genet 23: 1591–1601, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1: a001651, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawson LJ, Perry VH, Dri P, and Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39: 151–170, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Lee JA, Song HY, Ju SM, Lee SJ, Seo WY, Sin DH, Goh AR, Choi SY, and Park J. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 by cell-permeable superoxide dismutase in lipopolysaccharide-stimulated BV-2 microglial cells. Mol Cells 29: 245–250, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Lim JC, Kim G, and Levine RL. Stereospecific oxidation of calmodulin by methionine sulfoxide reductase A. Free Radic Biol Med 61C: 257–264, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim JC, You Z, Kim G, and Levine RL. Methionine sulfoxide reductase A is a stereospecific methionine oxidase. Proc Natl Acad Sci U S A 108: 10472–10477, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindenau J, Noack H, Asayama K, and Wolf G. Enhanced cellular glutathione peroxidase immunoreactivity in activated astrocytes and in microglia during excitotoxin induced neurodegeneration. Glia 24: 252–256, 1998 [PubMed] [Google Scholar]
- 35.Liu F, Hindupur J, Nguyen JL, Ruf KJ, Zhu J, Schieler JL, Bonham CC, Wood KV, Davisson VJ, and Rochet JC. Methionine sulfoxide reductase A protects dopaminergic cells from Parkinson's disease-related insults. Free Radic Biol Med 45: 242–255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long LH, Wu PF, Guan XL, Zhang JQ, Jin Y, Zhang Z, Wang Y, Li YY, Chen JG, and Wang F. Determination of protein-bound methionine oxidation in the hippocampus of adult and old rats by LC-ESI-ITMS method after microwave-assisted proteolysis. Anal Bioanal Chem 399: 2267–2274, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Luo S. and Levine RL. Methionine in proteins defends against oxidative stress. FASEB J 23: 464–472, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGeer PL, Itagaki S, Boyes BE, and McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38: 1285–1291, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Midwinter RG, Cheah FC, Moskovitz J, Vissers MC, and Winterbourn CC. IkappaB is a sensitive target for oxidation by cell-permeable chloramines: inhibition of NF-kappaB activity by glycine chloramine through methionine oxidation. Biochem J 396: 71–78, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moskovitz J. Roles of methionine suldfoxide reductases in antioxidant defense, protein regulation and survival. Curr Pharm Des 11: 1451–1457, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, and Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A 98: 12920–12925, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moskovitz J, Berlett BS, Poston JM, and Stadtman ER. The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc Natl Acad Sci U S A 94: 9585–9589, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moskovitz J, Maiti P, Lopes DH, Oien DB, Attar A, Liu T, Mittal S, Hayes J, and Bitan G. Induction of methionine-sulfoxide reductases protects neurons from amyloid beta-protein insults in vitro and in vivo. Biochemistry 50: 10687–10697, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nan C, Li Y, Jean-Charles PY, Chen G, Kreymerman A, Prentice H, Weissbach H, and Huang X. Deficiency of methionine sulfoxide reductase A causes cellular dysfunction and mitochondrial damage in cardiac myocytes under physical and oxidative stresses. Biochem Biophys Res Commun 402: 608–613, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Oien DB, Canello T, Gabizon R, Gasset M, Lundquist BL, Burns JM, and Moskovitz J. Detection of oxidized methionine in selected proteins, cellular extracts and blood serums by novel anti-methionine sulfoxide antibodies. Arch Biochem Biophys 485: 35–40, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prabhakar NR, Kumar GK, Nanduri J, and Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal 9: 1397–1403, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, and Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem 279: 1415–1421, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Rojo AI, McBean G, Cindric M, Egea J, Lopez MG, Rada P, Zarkovic N, and Cuadrado A. Redox control of microglial function: molecular mechanisms and functional significance. Antioxid Redox Signal 21: 1766–1801, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosales-Corral S, Reiter RJ, Tan DX, Ortiz GG, and Lopez-Armas G. Functional aspects of redox control during neuroinflammation. Antioxid Redox Signal 13: 193–247, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, and Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17: 2596–2606, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB, Jr., Ritchie TC, Hunninghake GW, Zandi E, and Engelhardt JF. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J Biol Chem 276: 30188–30198, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Sauer H, Wartenberg M, and Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem 11: 173–186, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Saura J, Tusell JM, and Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia 44: 183–189, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Sorce S. and Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal 11: 2481–2504, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Stadtman ER, Moskovitz J, Berlett BS, and Levine RL. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol Cell Biochem 234–235: 3–9, 2002 [PubMed] [Google Scholar]
- 56.Streit WJ, Graeber MB, and Kreutzberg GW. Functional plasticity of microglia: a review. Glia 1: 301–307, 1988 [DOI] [PubMed] [Google Scholar]
- 57.Sugden PH. and Clerk A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid Redox Signal 8: 2111–2124, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Takeda H, Tomita M, Tanahashi N, Kobari M, Yokoyama M, Takao M, Ito D, and Fukuuchi Y. Hydrogen peroxide enhances phagocytic activity of ameboid microglia. Neurosci Lett 240: 5–8, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Tarrago L, Kaya A, Weerapana E, Marino SM, and Gladyshev VN. Methionine sulfoxide reductases preferentially reduce unfolded oxidized proteins and protect cells from oxidative protein unfolding. J Biol Chem 287: 24448–24459, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trovato Salinaro A, Cornelius C, Koverech G, Koverech A, Scuto M, Lodato F, Fronte V, Muccilli V, Reibaldi M, Longo A, Uva MG, and Calabrese V. Cellular stress response, redox status, and vitagenes in glaucoma: a systemic oxidant disorder linked to Alzheimer's disease. Front Pharmacol 5: 129, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turchan-Cholewo J, Dimayuga VM, Gupta S, Gorospe RM, Keller JN, and Bruce-Keller AJ. NADPH oxidase drives cytokine and neurotoxin release from microglia and macrophages in response to HIV-Tat. Antioxid Redox Signal 11: 193–204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waetzig V, Czeloth K, Hidding U, Mielke K, Kanzow M, Brecht S, Goetz M, Lucius R, Herdegen T, and Hanisch UK. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 50: 235–246, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Tang XN, and Yenari MA. The inflammatory response in stroke. J Neuroimmunol 184: 53–68, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wassef R, Haenold R, Hansel A, Brot N, Heinemann SH, and Hoshi T. Methionine sulfoxide reductase A and a dietary supplement S-methyl-L-cysteine prevent Parkinson's-like symptoms. J Neurosci 27: 12808–12816, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weissbach H, Etienne F, Hoshi T, Heinemann SH, Lowther WT, Matthews B, St John G, Nathan C, and Brot N. Peptide methionine sulfoxide reductase: structure, mechanism of action, and biological function. Arch Biochem Biophys 397: 172–178, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Wentworth CC, Alam A, Jones RM, Nusrat A, and Neish AS. Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3. J Biol Chem 286: 38448–38455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood JM, Decker H, Hartmann H, Chavan B, Rokos H, Spencer JD, Hasse S, Thornton MJ, Shalbaf M, Paus R, and Schallreuter KU. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J 23: 2065–2075, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, and Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci U S A 100: 6145–6150, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu PF, Long LH, Zeng JH, Guan XL, Zhou J, Jin Y, Ni L, Wang F, Chen JG, and Xie N. Protection of L-methionine against H2O2-induced oxidative damage in mitochondria. Food Chem Toxicol 50: 2729–2735, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Wu PF, Xie N, Zhang JJ, Guan XL, Zhou J, Long LH, Li YL, Xiong QJ, Zeng JH, Wang F, and Chen JG. Resveratrol preconditioning increases methionine sulfoxide reductases A expression and enhances resistance of human neuroblastoma cells to neurotoxins. J Nutr Biochem 24: 1070–1077, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Wu PF, Zhang Z, Guan XL, Li YL, Zeng JH, Zhang JJ, Long LH, Hu ZL, Wang F, and Chen JG. A specific and rapid colorimetric method to monitor the activity of methionine sulfoxide reductase A. Enzyme Microb Technol 53: 391–397, 2013 [DOI] [PubMed] [Google Scholar]
- 72.Yermolaieva O, Xu R, Schinstock C, Brot N, Weissbach H, Heinemann SH, and Hoshi T. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc Natl Acad Sci U S A 101: 1159–1164, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang C, Jia P, Jia Y, Weissbach H, Webster KA, Huang X, Lemanski SL, Achary M, and Lemanski LF. Methionine sulfoxide reductase A (MsrA) protects cultured mouse embryonic stem cells from H2O2-mediated oxidative stress. J Cell Biochem 111: 94–103, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang F, Shi JS, Zhou H, Wilson B, Hong JS, and Gao HM. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol Pharmacol 78: 466–477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y, Ling EA, and Dheen ST. Dexamethasone suppresses monocyte chemoattractant protein-1 production via mitogen activated protein kinase phosphatase-1 dependent inhibition of Jun N-terminal kinase and p38 mitogen-activated protein kinase in activated rat microglia. J Neurochem 102: 667–678, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.