Abstract
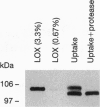
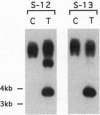
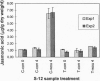
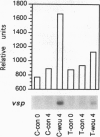
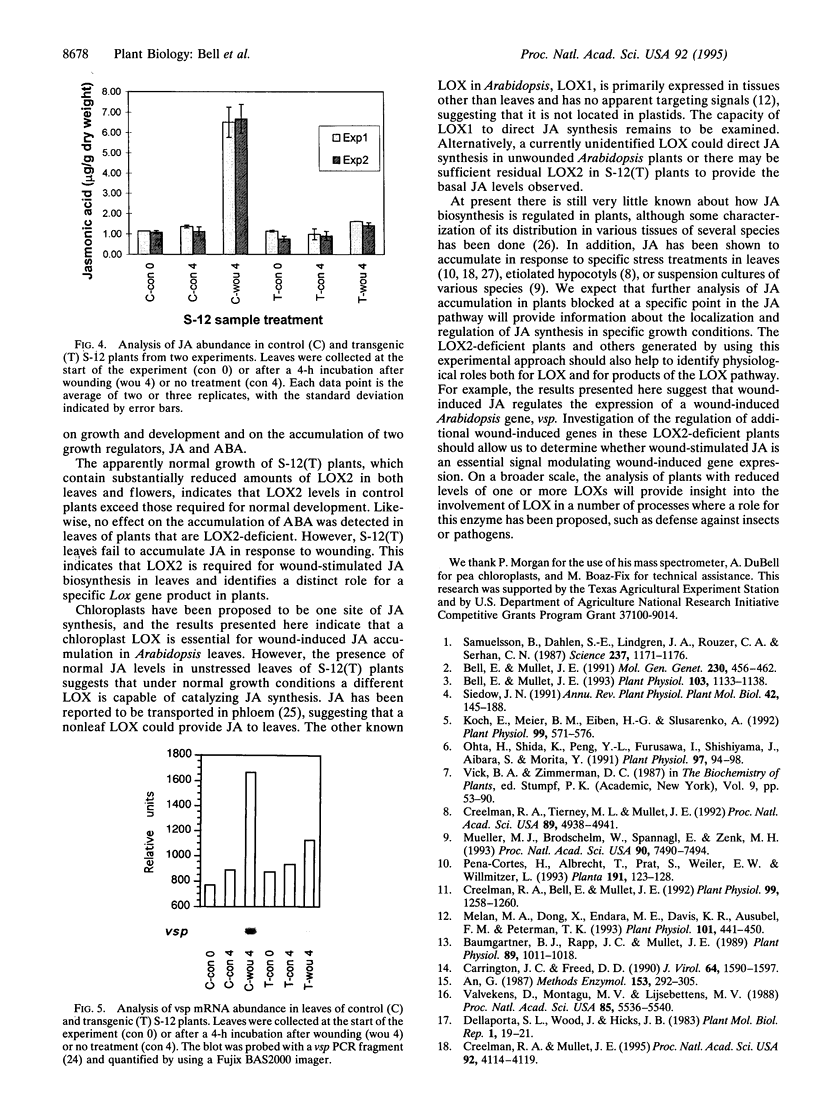
Plant lipoxygenases are thought to be involved in the biosynthesis of lipid-derived signaling molecules. The potential involvement of a specific Arabidopsis thaliana lipoxygenase isozyme, LOX2, in the biosynthesis of the plant growth regulators jasmonic acid (JA) and abscisic acid was investigated. Our characterization of LOX2 indicates that the protein is targeted to chloroplasts. The physiological role of this chloroplast lipoxygenase was analyzed in transgenic plants where cosuppression reduced LOX2 accumulation. The reduction in LOX2 levels caused no obvious changes in plant growth or in the accumulation of abscisic acid. However, the wound-induced accumulation of JA observed in control plants was absent in leaves of transgenic plants that lacked LOX2. Thus, LOX2 is required for the wound-induced synthesis of the plant growth regulator JA in leaves. We also examined the expression of a wound- and JA-inducible Arabidopsis gene, vsp, in transgenic and control plants. Leaves of transgenic plants lacking LOX2 accumulated less vsp mRNA than did control leaves in response to wounding. This result suggests that wound-induced JA (or some other LOX2-requiring component of the wound response pathway) is involved in the wound-induced regulation of this gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner B. J., Rapp J. C., Mullet J. E. Plastid transcription activity and DNA copy number increase early in barley chloroplast development. Plant Physiol. 1989 Mar;89(3):1011–1018. doi: 10.1104/pp.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E., Mullet J. E. Characterization of an Arabidopsis lipoxygenase gene responsive to methyl jasmonate and wounding. Plant Physiol. 1993 Dec;103(4):1133–1137. doi: 10.1104/pp.103.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E., Mullet J. E. Lipoxygenase gene expression is modulated in plants by water deficit, wounding, and methyl jasmonate. Mol Gen Genet. 1991 Dec;230(3):456–462. doi: 10.1007/BF00280303. [DOI] [PubMed] [Google Scholar]
- Berger S., Bell E., Sadka A., Mullet J. E. Arabidopsis thaliana Atvsp is homologous to soybean VspA and VspB, genes encoding vegetative storage protein acid phosphatases, and is regulated similarly by methyl jasmonate, wounding, sugars, light and phosphate. Plant Mol Biol. 1995 Mar;27(5):933–942. doi: 10.1007/BF00037021. [DOI] [PubMed] [Google Scholar]
- Carrington J. C., Freed D. D. Cap-independent enhancement of translation by a plant potyvirus 5' nontranslated region. J Virol. 1990 Apr;64(4):1590–1597. doi: 10.1128/jvi.64.4.1590-1597.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Bell E., Mullet J. E. Involvement of a lipoxygenase-like enzyme in abscisic Acid biosynthesis. Plant Physiol. 1992 Jul;99(3):1258–1260. doi: 10.1104/pp.99.3.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Mullet J. E. Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4114–4119. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Tierney M. L., Mullet J. E. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Zeevaart J. A. Incorporation of oxygen into abscisic Acid and phaseic Acid from molecular oxygen. Plant Physiol. 1984 May;75(1):166–169. doi: 10.1104/pp.75.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B. Inactivation of gene expression in plants as a consequence of specific sequence duplication. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3490–3496. doi: 10.1073/pnas.91.9.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch E., Meier B. M., Eiben H. G., Slusarenko A. A Lipoxygenase from Leaves of Tomato (Lycopersicon esculentum Mill.) Is Induced in Response to Plant Pathogenic Pseudomonads. Plant Physiol. 1992 Jun;99(2):571–576. doi: 10.1104/pp.99.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melan M. A., Dong X., Endara M. E., Davis K. R., Ausubel F. M., Peterman T. K. An Arabidopsis thaliana lipoxygenase gene can be induced by pathogens, abscisic acid, and methyl jasmonate. Plant Physiol. 1993 Feb;101(2):441–450. doi: 10.1104/pp.101.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M. J., Brodschelm W., Spannagl E., Zenk M. H. Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7490–7494. doi: 10.1073/pnas.90.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H., Shida K., Peng Y. L., Furusawa I., Shishiyama J., Aibara S., Morita Y. A Lipoxygenase Pathway Is Activated in Rice after Infection with the Rice Blast Fungus Magnaporthe grisea. Plant Physiol. 1991 Sep;97(1):94–98. doi: 10.1104/pp.97.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. D., Zeevaart J. A. The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7496–7499. doi: 10.1073/pnas.88.17.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987 Sep 4;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Valvekens D., Van Montagu M., Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochem Biophys Res Commun. 1983 Mar 16;111(2):470–477. doi: 10.1016/0006-291x(83)90330-3. [DOI] [PubMed] [Google Scholar]