Summary
Mechano-electrical transduction (MeT) channels embedded in neuronal cell membranes are essential for touch and proprioception. Little is understood about the interplay between native MeT channels and membrane phospholipids, in part because few techniques are available for altering plasma membrane composition in vivo. Here, we leverage genetic dissection, chemical complementation, and optogenetics to establish that arachidonic acid (AA), an omega-6 polyunsaturated fatty acid, enhances touch sensation and mechanoelectrical transduction activity while incorporated into membrane phospholipids in C. elegans touch receptor neurons (TRNs). Because dynamic force spectroscopy reveals that AA modulates the mechanical properties of TRN plasma membranes, we propose that this PUFA is needed for MeT channel activity. These findings establish that polyunsaturated phospholipids are crucial determinants of both the biochemistry and mechanics of mechanoreceptor neurons and reinforce the idea that sensory mechanotransduction in animals relies on a cellular machine composed of both proteins and membrane lipids.
Introduction
The sensory neurons embedded in our skin use mechano-electrical transduction (MeT) channels to detect mechanical stimuli delivered by a feather’s brush, a pin’s prick, or a mobile phone’s buzz. Analogous sensory neurons in joints and muscles help to maintain balance and posture, while others innervate the aorta and regulate heart rate on a beat-by-beat basis. Most, if not all MeT channels belong to macromolecular complexes whose protein constituents have been identified only for a few mechanoreceptor cells. In C. elegans, it has been shown that members of the DEG/ENaC (MEC-4, MEC-10, and DEG-1) and TRP channel (TRP-4, also known as CeNOMPC) families are pore-forming subunits of MeT channels in vivo (Arnadottir et al., 2011; Geffeney et al., 2011; Kang et al., 2010; O’Hagan et al., 2005). Additional MeT channel subunits have been identified in fruit flies and mice: NOMPC is thought to be a pore-forming subunit in fly mechanoreceptor neurons (Gong et al., 2013; Yan et al., 2013; Zhang et al., 2013); Piezo proteins are thought to function as MeT channels in body mechanoreceptor neurons and in red blood cells (Bae et al., 2013; Coste et al., 2010; Kim et al., 2013); TMHS and TMC proteins are required for mechanotransduction by inner ear hair cells in mice (Pan et al., 2013; Xiong et al., 2012). Thus, while many of the proteins needed to form MeT channels in mechanoreceptor cells have been identified, very little is known about how the composition of the membrane modulates MeT channel function.
One of the many advantages of studying touch sensitivity in C. elegans touch receptor neurons (TRNs) is that they generate electrical signals in response to cuticle deflection and transmit signals mainly via electrical synapses onto interneurons that control locomotion (Chalfie and Sulston, 1981; Chalfie et al., 1985; Goodman, 2006). At least five genes encode membrane proteins required to form the MeT channel complex in the TRNs. Four are mec (mechanosensory abnormal) genes that can mutate to disrupt gentle touch sensation and mutations in the fifth disrupt both touch sensation and locomotion (Chalfie and Sulston, 1981; Zhang et al., 2004): mec-4, mec-10, mec-2, mec-6, and unc-24. These genes encode two DEG/ENaC ion channel subunits (MEC-4 and MEC-10), two stomatin homologs (MEC-2 and UNC-24), and a paraoxonase-like protein (MEC-6) (Arnadottir and Chalfie, 2010). Both MEC-2 and MEC-6 are thought to interact with cholesterol (Brown et al., 2008; Chelur et al., 2002; Huber et al., 2006). Indeed, MEC-2 binds cholesterol and its function as an auxiliary channel subunit depends on this ability (Brown et al., 2008; Huber et al., 2006). Therefore, it is likely that membrane composition plays an important role in modulating the response of TRNs to mechanical stimuli.
When incorporated into phospholipids, polyunsaturated fatty acids (PUFAs) confer fluidity and flexibility to membranes (Rawicz et al., 2000; Rajamoorthi et al., 2005); consequently, they could modulate membrane protein function. Free PUFAs have been shown to modulate voltage-gated and mechanosensitive ion channels in heterologous cells (Balleza et al., 2010; Kim, 1992; Maingret et al., 2000). Genetic dissection in C. elegans shows that sensory and motor neurons depend on PUFAs for their function (Kahn-Kirby et al., 2004; Lesa et al., 2003). However, the physiological role of this modulation remains to be determined. Unlike mammals, worms can synthesize PUFAs de novo from acetyl-CoA using a series of fatty acid desaturase and elongase enzymes, known as FAT and ELO proteins, respectively (Wallis et al., 2002; Watts and Browse, 2002). Mutants defective in fat and elo genes have altered PUFA content (Watts and Browse, 2002), and grow into normal adults with mild phenotypes, except for fat-2 mutants. We exploited this knowledge to ask whether PUFAs are needed for touch sensation and the normal function of the C. elegans TRNs.
Here, we demonstrate that disrupting AA content or its incorporation into phospholipids impairs TRN-dependent behavioral responses, thereby identifying membrane phospholipids containing AA as critical for touch sensitivity. Arachidonic acid is likely synthesized within TRNs in vivo, since we show that enzymes needed for its synthesis are expressed in TRNs. We used an optogenetic approach to show that the defect in touch sensation likely reflects a loss of mechanotransduction rather than lack of excitability or downstream signaling. Finally, we found that the membrane viscoelastic properties of TRNs lacking C20 PUFAs are altered (i.e., membrane bending and viscosity), yielding less flexible membranes than wild type, as determined by atomic force microscopy (AFM) based single-tether extrusion. Our findings demonstrate that when AA is part of membrane phospholipids it modulates the mechanical properties of C. elegans TRN membranes and is crucial for touch sensation.
Results
Arachidonic Acid is Required for Touch Sensation
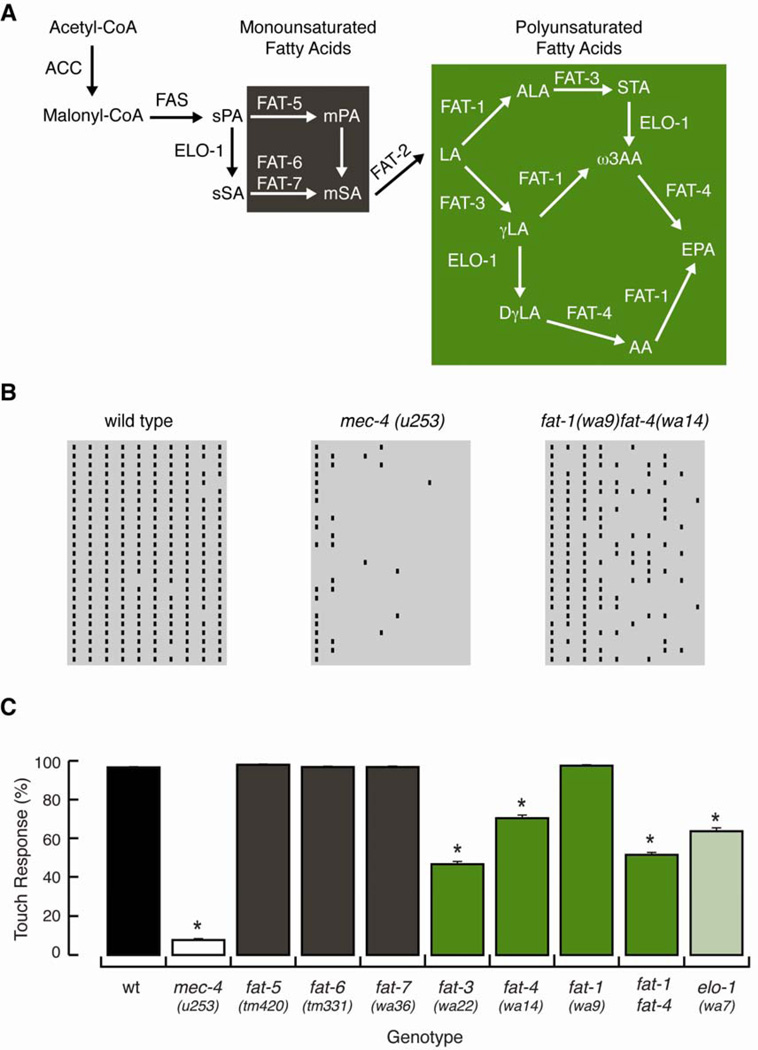
We used a behavioral test to investigate the contribution of lipids to touch sensitivity, exploiting the well-characterized enzymatic cascade (Figure 1A) that leads to the production of C20 PUFAs in C. elegans (Watts, 2009; Watts and Browse, 2002). Touch sensitivity was measured with a ten-trial touch assay that consisted of stroking an eyebrow hair across the body of young adults, alternating between the anterior and posterior part of the worm (Hart, 2006). Trials that elicited a pause or avoidance were considered positive. Figure 1B shows raster plots for wild type worms, mec-4(u253) mutants that lack functional MeT channels, and for fat-1(wa9)fat-4(wa14) double mutants, while Figure 1C shows the average number of positive trials (of ten) as a function of genotype. We used this ten-trial touch assay because it allowed us to identify modest defects that could not have been detected in three-trial touch assays used previously (Kahn-Kirby et al., 2004). This is because loss of touch-evoked avoidance was evident only after the third stimulus (Figure 1B, 1C). The defects found in fat mutants differ from the habituation evident in wild-type worms after nine or ten trials (Hart, 2006).
Figure 1. Arachidonic and eicosapentanoic acids are required for touch sensitivity.
(A) Fatty acid synthesis in C. elegans, adapted from (Watts, 2009). Brown and green boxes denote the enzymatic cascade needed to synthesize monounsaturated and polyunsaturated fatty acids, respectively. LA: Linolenic acid (18:2n-6), ALA: α-linolenic acid (18:3n-3), STA: stearidonic acid (18:4n-3), ω-3AA: ω-3 AA (20:4n-3), EPA: eicosapentanoic acid (20:5n-3), γLA: γ-linolenoic acid (18:3n-6), DγLA: dihomolinolenic acid (20:3n-6), and AA: arachidonic acid (20:4n-6).
(B) Raster plots displaying the response to gentle-touch of cohorts of 25 worms. Bars indicate trials that elicited reversals or pauses. Columns and rows represent trials and worms, respectively.
(C) Touch response in wild type (wt), mec-4, and fat mutants. At least 75 animals were tested blind to genotype. Bars are mean ± SEM. *Values significantly different from wt; Kruskall-Wallis and Dunn's Multiple Comparisons tests, p < 0.001. See also Figure S1.
With this survey, we identified four mutants with moderate, but reproducible, defects in touch sensation (Figure 1C): elo-1, fat-3, fat-4, and fat-1fat-4. ELO-1 is required to elongate fatty acids and has a critical role in the synthesis of C20 PUFAs (Figure 1A). Its touch impairment suggests that C20 PUFAs may be critical for normal touch sensitivity (Figure 1C). Consistent with this idea, fat-3 mutants, which lack the desaturase activity required to synthesize both C18 and C20 PUFAs (Figure 1A, green box), are also partially touch-insensitive. However, fat-3 mutants also have developmental and neuromuscular junction defects (Lesa et al., 2003; Watts et al., 2003) that complicate interpretation. As a result, fat-3 mutants were not analyzed further.
The fat-4 gene also encodes a desaturase. FAT-4 acts on C20 PUFAs to produce AA and EPA, the largest PUFAs in C. elegans (green box, Figure1A and 2A) and, as a result, fat-4 mutants lack AA and EPA. They also contain two-fold more of the upstream fatty acid, ω-3 AA, than wild type (Kahn-Kirby et al., 2004). As shown in Figure 1C, the touch response of fat-4 mutants is approximately one-third that of wild-type worms. This decrease in touch sensitivity could be due to the loss of AA and EPA or to the accumulation of ω-3 AA. A central role for ω-3 fatty acids (ALA, STA, ω-3AA, EPA) seems unlikely, however, since fat-1 mutants cannot synthesize these fatty acids (green box, Figure 1A) but have wild-type touch sensitivity (Figure 1C). However, fat-1 has approximately twenty times more AA than wild type (Kahn-Kirby et al., 2004), which could potentially compensate for the loss of ω-3 fatty acids. The fat-1fat-4 double mutant has no gross defects in development or locomotion and lacks the ability to synthesize both AA and ω-3 fatty acids, including EPA (Kahn-Kirby et al., 2004), providing an additional tool for testing whether or not AA is essential. Indeed, the touch response of fat-1fat-4 double mutants was half that of wild type (Figure 1C). Collectively, these results establish that mutants lacking C20 PUFAs have defects in touch sensation and implicate AA as a key fatty acid required for normal touch sensation.
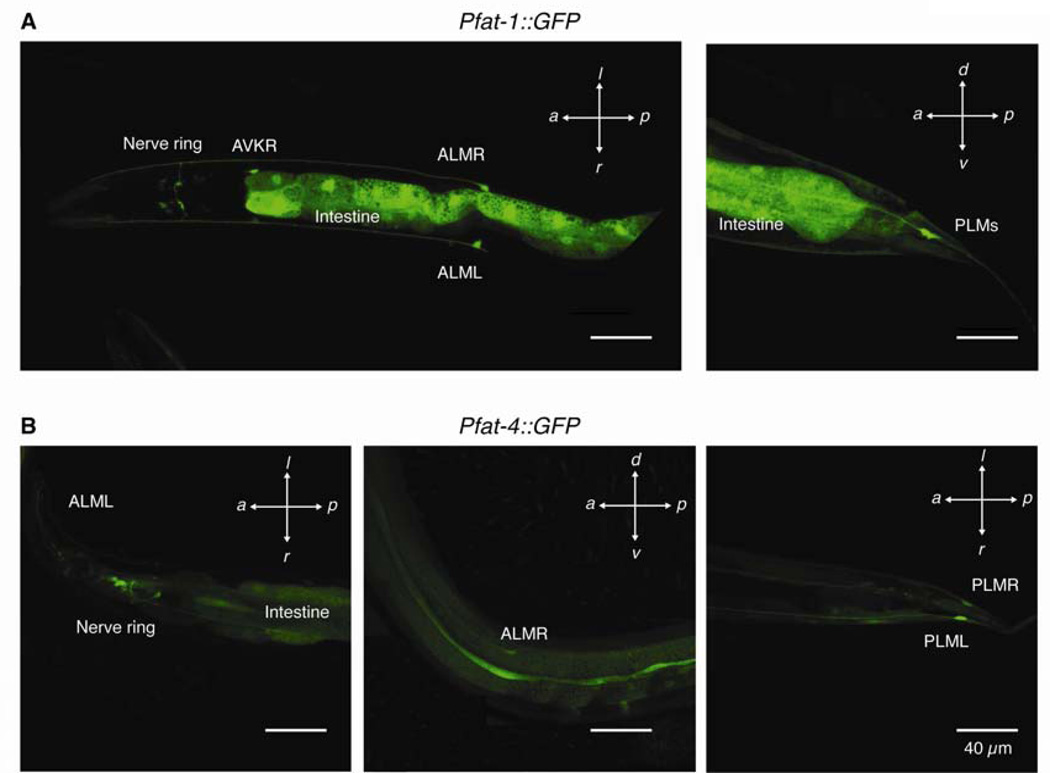
Figure 3. Touch receptor neurons express fat-1 and fat-4.
Worms express GFP under the control of promoters for fat-1 (A) and fat-4 (B) in many cells including the TRNs: ALM (L/R), PLM (L/R), and the nerve ring. Scale bars are 40 µm in all panels.
As with any genetic dissection of an enzymatic cascade, however, loss of desaturases and elongases results not only in the loss of downstream products, but also significant changes in the levels of upstream substrates (Kahn-Kirby, et al. 2004). Because this complicates interpretation, we sought other ways to manipulate fatty acid content and settled on growth temperature and chemical complementation of fat mutants as effective methods. Prior work has shown that worms grown at 15°C have less AA, more EPA, and more of the upstream substrate, linolenic acid, compared to worms grown at 20 or 25°C (Tanaka et al., 1996). When we tested the effect of growth temperature on touch sensitivity in wild type worms, however, we found no significant effect. In contrast, fat-1fat-4 mutants grown at 15°C were significantly less touch sensitive than those grown at 20 or 25°C (Figure S1). Thus, wild type worms, but not fat-1fat-4 mutants, maintain constant touch sensitivity across a 10-degree range in growth temperature. This result suggests that the ability to synthesize C20 PUFAs is needed for touch sensation and for proper thermal homeostasis of this behavior.
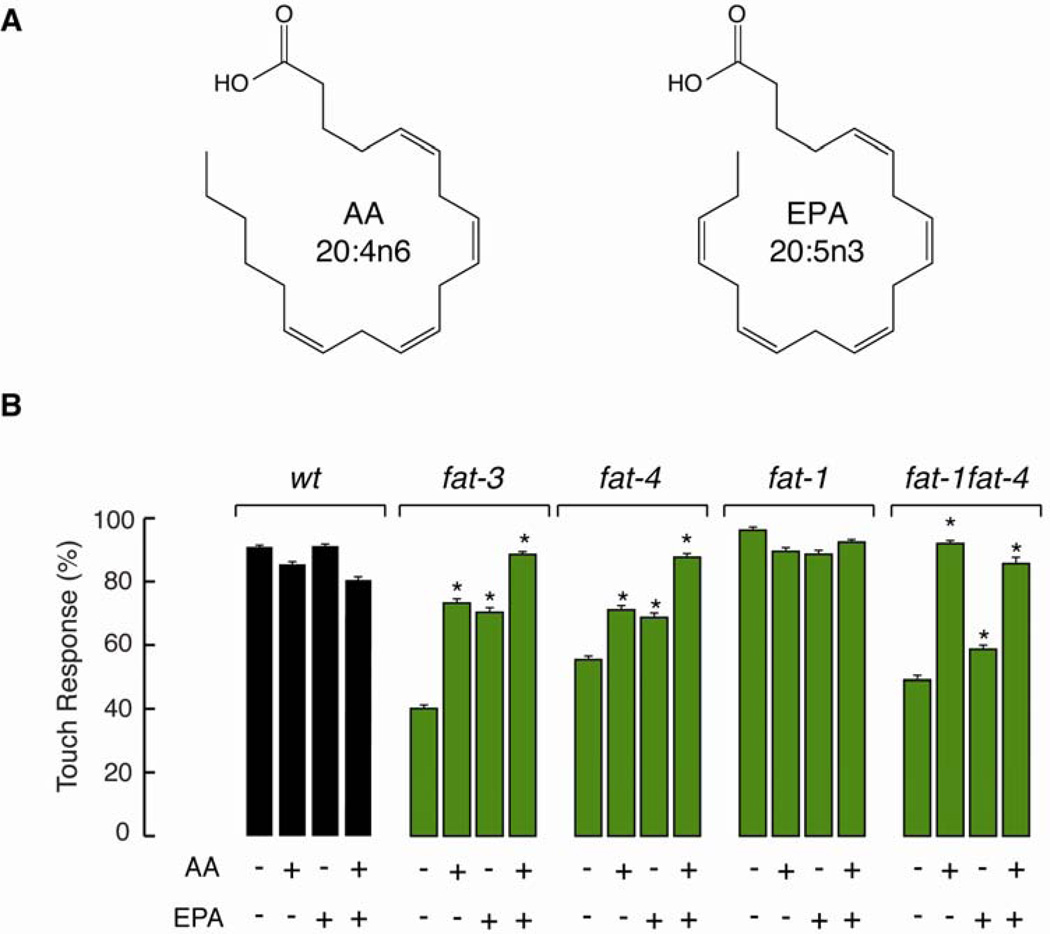
Next, we used chemical complementation of fat-1, fat-4, and fat-1fat-4 mutants to learn more about the role of AA and EPA in touch sensation. Supplementing nematode growth media (NGM) with AA, EPA, or both, leads worms to incorporate these fatty acids through their diet (Watts and Browse, 2002). This allowed us to differentiate between the absence of C20 PUFAs (AA and EPA), the accumulation of upstream fatty acids, or developmental defects. Wild type and fat-1 worms did not change their ability to respond to touch when NGM was supplemented with AA or EPA, suggesting that an excess of PUFAs does not affect touch sensitivity (Figure 2A). Complementing fat-3 and fat-4 mutants with AA or EPA improved their ability to respond to touch compared to the negative control (Figure 2B); however, only when AA and EPA were supplemented together were these worms able to respond like wild type. Taken together, these results provide additional evidence that it is the absence of AA and EPA that causes the touch defect observed in fat-3 and fat-4 worms, rather than the accumulation of upstream precursors. The double mutant fat-1fat-4, unlike the fat-4 single mutant, does not transform AA into EPA even when exogenous AA is provided (green box, Figure 1A). We found that AA, but not EPA, could recapitulate wild-type behavior in fat-1fat-4 double mutants (Figure 2B). These chemical complementation assays demonstrated that AA, but not EPA, is a crucial PUFA for the modulation of touch sensitivity.
Figure 2. Arachidonic acid rescues touch sensitivity.
(A) Chemical structures of arachidonic (AA) and eicosapentanoic acids (EPA).
(B) Touch response in fat mutants grown on NGM plates containing C20 PUFAs AA and EPA. NGM was supplemented with PUFAs as previously described (Watts et al., 2003). At least 75 animals were tested blind to genotype and treatment. Bars are mean ± SEM. *Values significantly different from experimental control; Kruskall-Wallis and Dunn's Multiple Comparisons tests, p < 0.001.
TRNs co-express fat-1 and fat-4, which are dispensable for TRN shape and MeT channel distribution, but not for TRN function
To determine whether C20 PUFAs are likely to be present in TRNs, we built fusion constructs to drive the expression of GFP from fat promoters. These experiments revealed that fat-1 and fat-4 are expressed in TRNs through larval development into adulthood (Figure 3), suggesting that AA and EPA are continuously synthesized by TRNs in situ. Similar expression patterns were observed in at least three independent transgenic lines. To test for potential defects in neuron morphology arising during development that could contribute to the poor touch sensitivity of worms lacking C20 PUFAs, we crossed fat mutants with transgenic uIs31 animals that express GFP exclusively in the TRNs (O’Hagan et al., 2005). Young adult worms that lack C20 PUFAs display GFP-labeled TRNs (Figure S2) with morphological features similar to wild type (i.e., cell body position, neurite extension and position along the body, branching). The TRN morphologies of elo-1, fat-4, and fat-1fat-4 worms were also comparable to that of wild type (data not shown). We also found that the pore-forming subunit of the MeT channel complex (MEC-4::YFP) displays a normal punctate distribution (Cueva et al., 2007) in TRNs lacking C20 PUFAs (Figure S3). Therefore, we conclude that the impaired touch response of mutants lacking C20 PUFAs does not arise from gross defects in TRN development, morphology, or from mislocalization of the MeT channel complex.
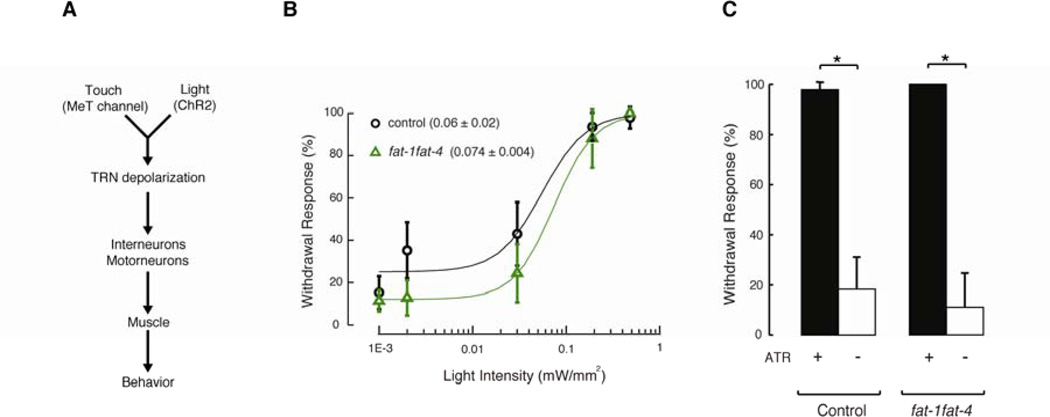
Having established that while fat-1fat-4 mutants are partially touch-insensitive, they have normal TRN morphology and distribution of MEC-4-dependent MeT channels, we next sought to determine the locus of the defect in the signaling pathway linking touch to behavior (Figure 4A). In principle, fat-1fat-4 mutants could have a defect in mechanoelectrical transduction, TRN depolarization, synaptic transmission, interneuron or motorneuron function, muscle activity, or a combination of these factors. To do this, we crossed fat mutants with transgenic worms expressing channelrhodopsin (ChR2) exclusively in the TRNs. Exogenous supplementation with all-trans-retinal (ATR) is required for ChR2-dependent blue-light stimulation in C. elegans (Nagel et al., 2005), which provides a simple way to control for non-specific effects due to optical stimulation and transgene expression. In this framework, mutants with defects downstream of mechanoelectrical transduction will exhibit defects in both touch and light-evoked behaviors, whereas mutants with defects in mechanoelectrical transduction will behave like wild-type worms when stimulated with blue-light. Accordingly, we tested the response of young adults in the presence of ATR to flashes of blue-light at different intensities, thus triggering withdrawal responses in the control and fat-1fat-4 worms (Figure 4B). We found that the midpoints of these curves were similar for control (0.06 ± 0.02 mW/mm2, mean ± sd) and fat-1fat-4 (0.074 ± 0.004 mW/mm2) worms. Figure 4C shows the results after delivering flashes at saturating light intensity (0.48 mW/mm2). This approach allowed us to compare responses to blue light and to touches delivered by the classical method (Hart et al, 2006), which delivers forces of 100 µN or more, as estimated by stroking a calibrated piezoresistive cantilever with an eyebrow hair (E.A. Mazzochette, K-W. J., M.B.G, and B.L.P. unpublished data), while behavioral responses to touch saturate near 1 µN (Petzold et al., 2013). Consistent with the idea that TRN excitability and downstream signaling are intact, we found that fat-1fat-4 mutants respond like control worms (Figure 4B–C).
Figure 4. C20 PUFA-deficient mutants respond normally to optogenetic stimulation.
(A) Schematic of the circuitry and motor neuron program downstream of TRNs.
(B) Withdrawal response elicited by illuminating young adult control and fat-1fat-4 worms expressing ChR2 exclusively in their TRNs with a 470 nm light for one second every 30 seconds at these irradiance values (0.001, 0.002, 0.03, 0.19, and 0.48 mW/mm2), in the presence of all-trans-retinal. A dose-response curve was fitted to the data. At least 10 animals were tested blind to genotype and treatment. Circles and triangles are mean ± sd.
(C) Withdrawal response elicited by illuminating young adult worms (control and fat-1fat-4 mutants) expressing ChR2 exclusively in their TRNs with a 470 nm light for one second every 30 seconds at 0.48 mW/mm2 irradiance, in the presence or absence of all-trans-retinal. At least 10 animals were tested blind to genotype and treatment. Bars are mean ± SEM. *Values significantly different from experimental control; Kruskall-Wallis and Dunn's Multiple Comparisons tests, p < 0.001.
With this optogenetic approach, we cannot exclude the possibility that loss of C20 PUFAs affects other ion channels that may be present in the TRNs. For example, certain potassium channels are known to be potentiated by free AA (Callejo et al., 2013; Hao et al., 2013). In principle, such an effect could compensate for a partial loss of MeT channel function at saturating light intensities. Such an effect is unlikely since it would shift the light-response curve towards lower light intensities or increase the steepness of the curve. We found no evidence for either effect in fat-1fat-4 mutants. Thus, these findings imply that worms lacking C20 PUFAs have a specific defect in mechanoelectrical transduction, rather than general defects in TRN excitability, synaptic transmission or in the function of downstream interneurons, motor neurons, or muscles.
Arachidonic Acid-containing Phospholipids Modulate Touch Sensation
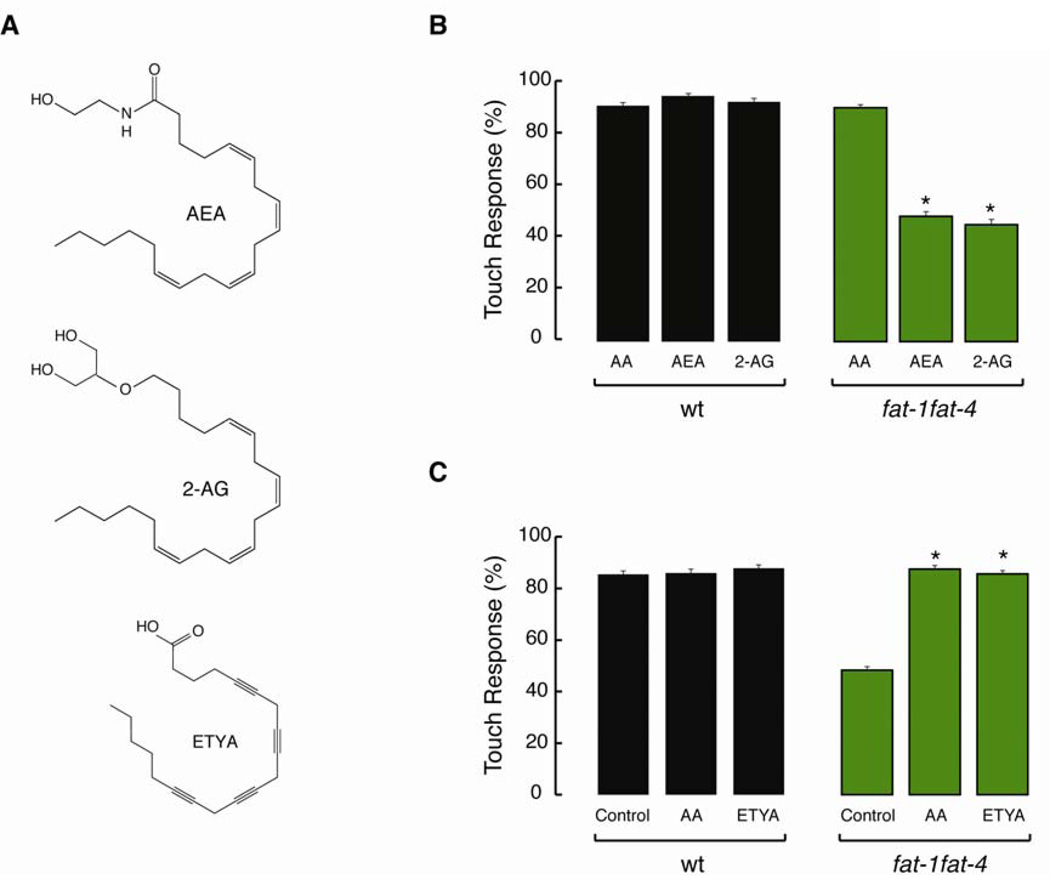
Our findings implicate AA as a key regulator of TRN mechanotransduction but do not distinguish between effects of AA itself or its metabolites, such as the endocannabinoids anandamide (AEA) and 2-archidonyl glycerol (2-AG). Recent studies show that both AEA and 2-AG are present in wild-type worms, but absent in fat-3 and fat-4 mutants (Lehtonen et al., 2011; Lucanic et al., 2011). Thus, it is possible that the touch response defect in these mutants is due to the lack of AA, AEA, or 2-AG. To identify the mechanism by which AA modulates touch sensation, we supplemented NGM with AEA, 2-AG, and eicosatetraynoic acid (ETYA), a structural analog of AA that cannot be metabolized (Figure 5A). As shown in Figure 5B, complementing with AEA or 2-AG did not reconstitute wild-type touch sensitivity. By contrast, ETYA is able to rescue the touch defect in fat-1fat-4 mutant comparable to AA (Figure 5C). Up to this point, our data supports a model where AA could exert its effect as a free fatty acid or as a constituent of membrane lipids, but not as a precursor of its metabolites.
Figure 5. Arachidonic acid, but not its metabolites are needed for touch sensitivity.
(A) Chemical structures of arachidonoyl ethanolamide (AEA), 2-Arachidonyl glycerol ether (2-AG), and eicosatetraynoic acid (ETYA).
(B) Touch response in wild type (wt) and fat-1fat-4 animals grown in the presence of AA and its endocannabinoid metabolites AEA and 2-AG. At least 75 animals were tested blind to genotype and treatment. Bars are mean ± SEM. *Values significantly different from experimental control; Kruskall-Wallis and Dunn's Multiple Comparisons tests, p < 0.001.
(C) Touch response in wild type (wt) and fat-1fat-4 animals grown in the presence of AA and its non-metabolizable analog ETYA. At least 75 animals were tested blind to genotype and treatment. Bars are mean ± SEM. *Values significantly different from experimental control; Kruskall-Wallis and Dunn's Multiple Comparisons tests, p < 0.001.
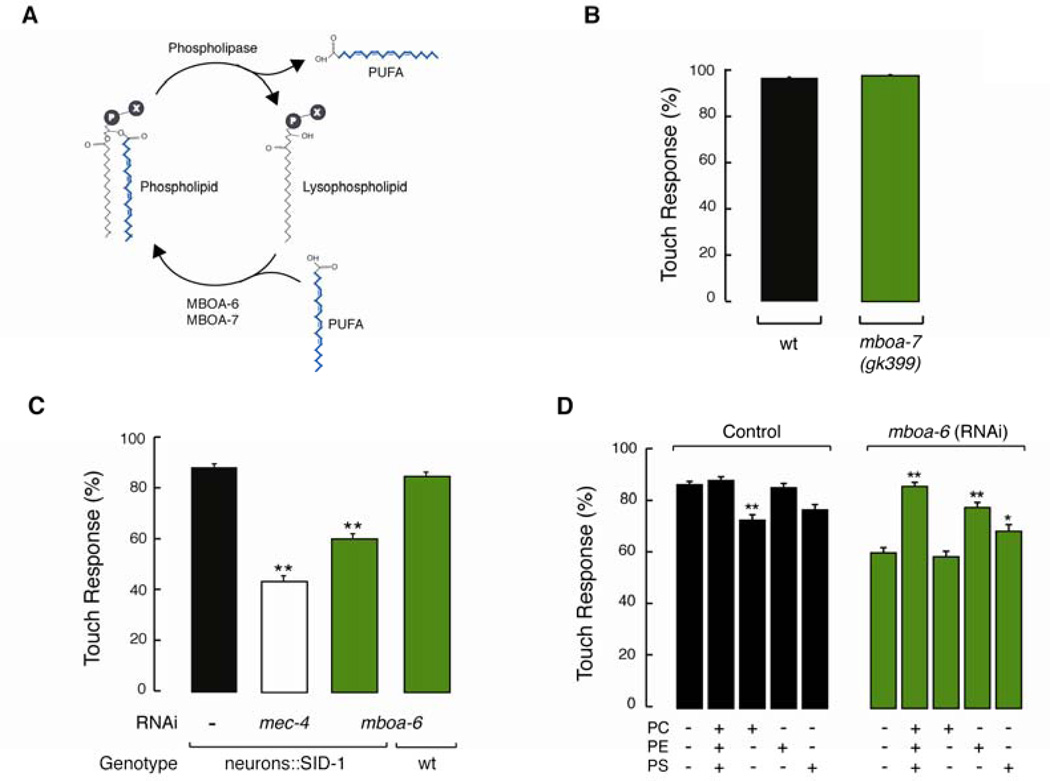
Given that AA can be incorporated into membrane phospholipids (Figure 6A), we investigated whether AA-containing phospholipids regulate the response mechanical stimuli. C. elegans has two conserved lysophospholipid transferase enzymes responsible for incorporating C20 PUFAs into the sn2 position of phospholipids: MBOA-7 links AA and EPA to phosphatidylinositol (PI) (Lee et al., 2008) and MBOA-6 links AA to phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylserine (PS) (Matsuda et al., 2008). If AA acts while incorporated into membrane lipids, then mutants deficient in the enzymes MBOA-7 and MBOA-6 could be deficient in TRN-mediated behavior. mboa-7(gk399) mutants have wild-type touch sensitivity, suggesting that AA-containing PI is not necessary for touch sensitivity (Figure 6B). Unfortunately, mboa-6 null mutants are embryonic lethal (Matsuda et al., 2008). We circumvented this severe phenotype by using RNAi to knock down mboa-6 post-embryonically (mboa-6 knock down worms have normal locomotion). Knocking down the expression of mboa-6 in wild-type worms resulted in animals fully sensitive to touch (Figure 6C), consistent with prior reports that neurons are resistant to RNAi in wild-type worms (Kamath et al., 2001; Timmons et al., 2001). To knockdown the expression of mboa-6 in neurons we used a recently engineered transgenic strain (Punc-119::SID-1) that increases RNAi sensitivity in neurons through expression of the SID-1 transmembrane protein (Calixto et al., 2010). Following this strategy, we found that knocking down mboa-6 decreased touch sensitivity by ~30%, which is ~20% more sensitive than knocking down the pore-forming subunit MEC-4 (Figure 6C). These results suggest that full expression of mboa-6 is required to achieve wild-type touch sensitivity.
Figure 6. Arachidonic acid-containing membrane phospholipids modulate touch sensitivity.
(A) Schematic representation of the phospholipid synthesis cycle, where MBOA-7 and MBOA-6 (lysophospholipid acyltransferases) act to incorporate PUFAs into membrane phospholipids (Matsuda et al., 2008) and phospholipase releases lysophospholipids and free PUFAs. Adapted from (Hishikawa et al., 2008).
(B) Touch response in mboa-7 mutants. At least 75 animals were tested blind to genotype. Bars are mean ± SEM.
(C) Touch response after knocking down the expression of mboa-6 and mec-4 with RNAi, in wild-type (wt) and transgenic worms that express sid-1 exclusively in neurons (neurons::SID-1). At least 75 animals were tested blind to genotype and treatment. Bars are mean ± SEM. **Values significantly different from experimental control; Kruskall-Wallis and Dunn's Multiple Comparisons tests, **p < 0.001. See also Figure S4.
(D) Touch response after knocking down mboa-6 in transgenic worms that express sid-1 exclusively in neurons and after supplementing NGM with the AA-containing phospholipids: PC: (18:0)(20:4) phosphatidylcholine, PE: (18:0)(20:4) phosphatidylethanolamine, PS: (18:0)(20:4) phosphatidylserine. At least 75 animals were tested blind to genotype. Bars are mean ± SEM. *Values significantly different from experimental control; Kruskall-Wallis and Dunn's Multiple Comparisons tests, **p < 0.001 and *p < 0.05.
To determine whether mboa-6 or the phospholipids it helps to synthesize are responsible for modulating touch sensitivity, we combined RNAi-mediated gene knock down and chemical complementation. First, we determined that supplementing NGM with phospholipids did not interfere with RNAi knockdown. To this end, we knocked down mec-4 in the presence of phospholipids loaded with stearic acid (18:0) and arachidonic acid (20:4) loaded at their sn1 and sn2 positions, respectively (Figure S4): (18:0)(20:4)PC, (18:0)(20:4)PE, and (18:0)(20:4)PS. This manipulation had no detectable effect on touch sensitivity, indicating that phospholipid supplementation was compatible with RNAi knockdown. Next, we sought to restore wild-type behavior in mboa-6(rnai) worms when complemented with (18:0)(20:4)PC, (18:0)(20:4)PE, and (18:0)(20:4)PS in combination (Figure 6D, left). Supplementing with (18:0)(20:4)PE, and (18:0)(20:4)PS, but not (18:0)(20:4)PC, restored touch-sensitivity to nearly wild-type levels in worms treated with mboa-6(rnai) (Figure 6D, right). The ability of (18:0)(20:4)PE, and (18:0)(20:4)PS, but not (18:0)(20:4)PC to rescue mboa-6(rnai)-induced touch defects could reflect the preferential localization of PS and PE phospholipids to the inner leaflet of the cell membranes. [PC lipids localize primarily to the outer leaflet of the plasma membrane (Fadeel and Xue, 2009; Leventis and Grinstein, 2010)]. Collectively, these results suggest that membranes with AA-containing PE and PS modulate mechanoelectrical transduction in living worms.
C20 PUFAs Regulate Membrane Mechanics in Touch Receptor Neurons
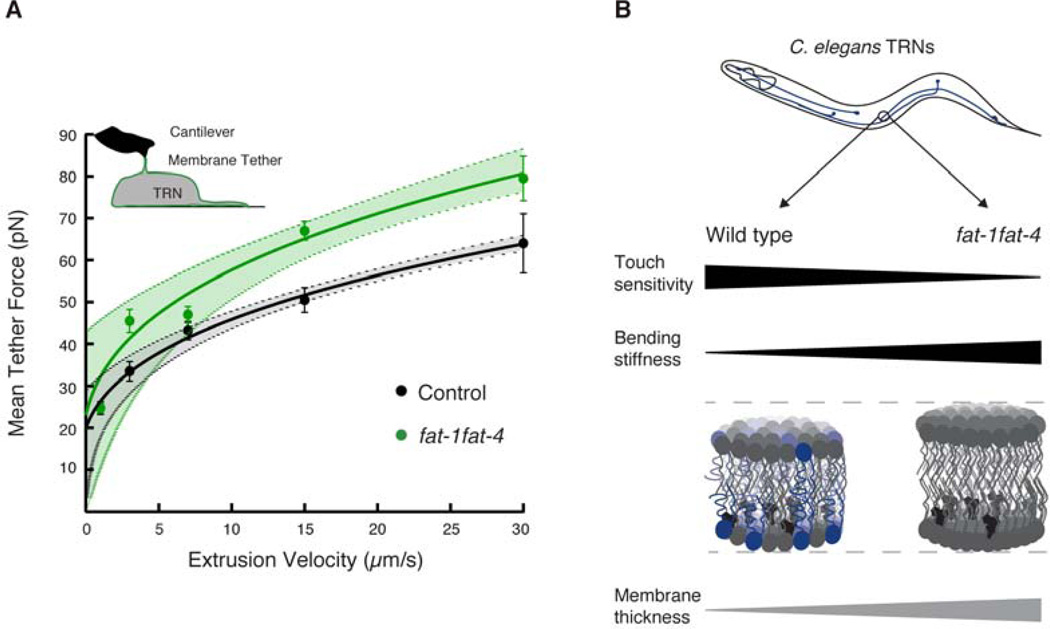
Based on the finding that C20 PUFAs modulate touch sensitivity while incorporated in membrane phospholipids, we hypothesized that simultaneous loss of fat-1 and fat-4 function and, therefore, C20 PUFAs would alter the mechanical properties of TRN membranes. To test this idea directly, we isolated cells from uIs31 control and fat-1fat-4 embryos expressing exclusively GFP in the TRNs, grew them in culture for ≥ 24 hours, and used an AFM to pull single lipid membrane nanotubes or tethers (Figure 7A, inset). To further characterize membrane mechanics, we measured tether force as a function of pulling rate and fit the resulting force-velocity curves according to a biophysical model of the relationship between the dynamics of extrusion and the mechanical properties of the membrane (Borghi and Brochard-Wyart, 2007; Brochard-Wyart et al., 2006). This procedure allowed us to extrapolate the static tether force at zero velocity (F0, the intersection between the fit and the y-axis) and to assess changes in effective membrane viscosity, bending stiffness, or the density of membrane-cytoskeleton binders (Diz-Muñoz et al., 2010). As shown in Figure 7A, the curves overlap at low velocity (p = 0.24 at 1 µm/s, Student’s t-test) and diverge as velocity increases such that larger forces are generated when pulling tethers from fat-1fat-4 mutant TRNs than from control neurons. Thus, while static force (F0) is similar in control (20 ± 3 pN, mean ± SEM) and fat-1fat-4 mutant (23 ± 8 pN) TRNs, the dynamics of tether extrusion are dramatically altered by the loss of C20 PUFAs (p < 0.05). The divergence observed at high velocities could reflect differences in membrane bending stiffness, effective viscosity, the density of binders between the membrane and the cytoskeleton, or a combination of all three factors (Borghi and Brochard-Wyart, 2007; Brochard-Wyart et al., 2006). Because the composition of the cortical cytoskeleton is likely to be similar in control and fat-1fat-4 TRNs, we propose that the differences come mainly from a change in membrane bending stiffness and effective viscosity. Collectively, these results indicate that TRN membranes lacking C20 PUFAs have different mechanical properties and are likely to be less flexible than control TRN membranes.
Figure 7. C20 PUFAs contribute to membrane mechanics in touch receptor neurons.
(A) AFM force spectroscopy on isolated TRNs expressing GFP. Plot of tether force vs. cantilever retraction speed. Velocities between 1 and 30 µm/s were tested. The equation f3 − f F02 = av was fitted to the data, where f is the tether force at velocity, v, and F0 is the static force, and a is the coefficient characterizing the dynamics of extrusion (Borghi and Brochard-Wyart, 2007; Brochard-Wyart et al., 2006). Circles are mean ± SEM. The dotted lines indicate the 95% confidence bands for the fit. At least 20 TRNs were tested. Inset shows a graphical representation of the tether extrusion experiment in which an AFM cantilever was coated with peanut lectin and approached onto an isolated TRN and kept in contact for 0.4 s upon retraction, a lipid tube eventually formed in 20% of all contacts.
(B) Schematic of the differences in behavior and TRN membrane mechanics in wild-type and fat-1fat-4 worms. Blue: AA-containing phospholipids, gray: phospholipids with less than four unsaturations, and black: cholesterol. Black wedges are measured quantities; the gray wedge shows a predicted difference in membrane thickness.
Discussion
Classical genetics has uncovered many of the proteins crucial to form MeT channels in C. elegans TRNs and other mechanoreceptor cells (Arnadottir and Chalfie, 2010), including mammalian auditory and vestibular hair cells (Pan et al., 2013; Xiong et al., 2012). This approach lacks the power to uncover roles for specific lipids, however, since a plethora of genes are needed for lipid synthesis and metabolism. To circumvent this difficulty, we exploited the well-characterized enzymatic cascade used by C. elegans to synthesize fatty acids, including C20 PUFAs (Watts, 2009). By surveying how mutants lacking mono- and poly-unsaturated fatty acids (fat and elo mutants) respond to gentle touch and by using chemical complementation of selected fat mutants, we established that AA is needed for touch sensation. Rather than acting as a signaling molecule or as a metabolic precursor for other signaling molecules, AA exerts its effect on touch sensation when it is incorporated into phospholipids (Figure 6).
Though highly penetrant, the touch defect seen in fat mutants is less severe than the defect present in mec-4 null mutants that lack MEC-4, an indispensible pore-forming subunit of the MeT channel complex (O’Hagan et al., 2005). Also, fat mutants responded to initial, but not later trials in the ten-trial touch assay (Figure 1B). Though the basis for this adaptation is not yet clear, it differs from the habituation seen in wild type animals which requires more stimulation, is less severe and depends primarily on the neural circuitry linking the TRNs to motor output (reviewed by Ardiel and Rankin, 2008). Both of these features of fat mutant touch sensation point toward flaws in the function of MeT channels, but not in their expression or distribution. Indeed, the distribution of MEC-4::YFP puncta was indistinguishable in fat-1fat-4 mutant and control TRNs (Figure S3).
At least two lines of additional evidence support the idea that AA acts within the TRNs to ensure proper MeT channel function. First, the TRNs have the capacity to synthesize AA since they express both of the desaturase genes (fat-1 and fat-4) needed for AA synthesis (Figures 1A, 3). Second, transgenic fat-1fat-4 mutants expressing the light-activated ChR2 protein exclusively in the TRNs have impaired touch sensation, but respond just like control transgenics to flashes of blue light (Figure 4). Thus, most, if not all signaling events downstream of the activation of MeT channels function normally in AA-deficient fat-1fat-4 mutants. While we cannot exclude compensatory, pleiotropic effects within the TRNs, the simplest model consistent with all of our data is that loss of AA-containing phospholipids compromises the ability of touch to activate MeT channels.
The biophysical properties of membrane lipids modulate integral membrane proteins, including mechanosensitive channels (Andersen and Koeppe, 2007; Brown, 2012; Phillips et al., 2009). For instance, reconstituting MscL channels in thin phospholipid bilayers containing short acyl chains stabilizes open states, whereas reconstitution in thicker phospholipid bilayers containing longer chain fatty acids stabilizes closed states (Perozo et al., 2002). Including PUFAs in phospholipid membranes also modulate the physical properties of bilayers, increasing disorder and decreasing thickness (Salmon et al., 1987). Thus, depleting native plasma membranes of PUFAs could increase membrane thickness as well as the energy required for deformation, leading to higher bending rigidity. Experimental evidence collected from synthetic phospholipids supports this idea since PUFA-containing bilayers are more deformable than ones containing saturated fatty acids (Rawicz et al., 2000; Rajamoorthi et al., 2005). Our data on native TRN membranes agree with these biophysical studies: The plasma membrane of fat-1fat-4 mutant TRNs that lack C20 PUFAs generates the force-velocity curves expected if PUFA deficiency increased membrane bending rigidity. Additionally, fat-1fat-4 worms cultivated at 15°C are less touch sensitive than those grown at 20° or 25°C, a result which suggests that the further decrease in membrane fluidity expected at cooler temperatures exacerbates the touch defect of animals lacking C20 PUFAs. Within this framework, we propose that the response of TRNs to mechanical stimuli is regulated by lipid composition and that the presence of multiple unsaturated bonds creates a distinct environment that enables the MeT channel complex function.
Current models of sensory mechanoelectrical transduction posit that mechanical energy is delivered to MeT channels either through the plasma membrane or mainly by protein tethers (Kung, 2005; Zanini and Göpfert, 2013). As the plasma membrane is a vital element of both these models, our findings cannot differentiate among them. Instead, we provide evidence that AA-containing phospholipids are required for touch sensation and suggest that these lipids are needed to regulate the sensitivity or time course of MeT channel activation. Though additional studies will be needed to test this model in detail, this study helps to determine the specialized lipid environment essential for MeT channel function.
How might variation in the composition and physical properties of the plasma membrane alter the mechanoelectrical transduction process? The picture emerging from our work is that the TRN membranes of wild-type animals include AA-containing phospholipids (PE and PS, that localize primarily to the inner leaflet) and cholesterol, and these elements are balanced to enhance and modulate touch sensitivity (Fig. 7B). From previous studies, we know that cholesterol is needed for proper function of the MEC-4 channel complex and it is recruited by the stomatin-like protein MEC-2 (Brown et al., 2008; Huber et al., 2006). Recently, Anishkin and Kung (2013) speculated that channel complexes embedded in cholesterol-rich nano-environments reorient in response to membrane stretch (strain) such that phospholipids dilute cholesterol regions, an event predicted to induce membrane thinning near the channel complex and subsequent MeT channel activation. Our results suggest that polyunsaturated acyl chains contained in PE and PS phospholipids are required to modulate the mechanoelectrical transduction process in TRN membranes. Further, they imply that touch-evoked membrane deformation and MeT channel activation would be compromised in mutants lacking C20 PUFAs in their acyl chains. Regulation of touch sensation by C20 PUFA-containing phospholipids may be conserved in mammals, including humans, since high dietary intake of PUFAs, including AA, is known to improve nerve conduction in older humans and lowers the risk of peripheral neuropathy (Lauretani et al., 2007).
Experimental Procedures
Strains
Worm culture and genetics were based on standard procedures (Brenner, 1974). Wild-type (N2) and mutant strains: BX24 fat-1(wa9) IV, BX30 fat-3(wa22) IV, BX17 fat-4(wa14) IV, BX107 fat-5(tm420) V, BX106 fat-6(tm331) IV, BX153 fat-7(wa36) V, BX52 fat-1(wa9); fat-4(wa14) IV, and BX14 elo-1(wa7) IV were obtained from the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). Null mutant TU253 mec-4(u253) X, and transgenics TU3270 (Punc-119::sid-1; Punc-119::yfp; Pmec-6::mec-6) and GN402 uIs58[Pmec-4::cfp, Pmec-4::mec-4::yfp] were provided by Martin Chalfie (Columbia University, NY, New York). Strain GN402 was crossed with BX52 and BX24 to obtain GN563 and GN564, respectively. Strain AQ2313 ljIs123 (Pmec-4::ChR2 codon optimized; Punc-122::rfp) was provided by William R. Schafer (Cambridge, UK). AQ2313 animals were also crossed with fat mutants BX30 and BX52 to obtain GN439 and GN406, respectively. Null mutant mboa-7(gk399) was provided by Hiroyuki Arai (University of Tokyo, Japan) after out-crossing it five times.
A transgenic animal that expresses GFP under the control of Pmec-17 (O’Hagan et al., 2005), TU2769 uIs31 [Pmec-17::GFP] III was crossed with fat mutant strains BX30, BX14, BX17, BX52 to obtain GN212, GN214, GN215, and GN381, respectively.
Stable transgenic strains GN384 (40 ng/µl of Pfat-3::gfp;Punc-122::rfp) (Watts et al., 2003), GN558 (10 ng/µl of Pfat-4::gfp; Pmyo-2::mcherry; Punc-122::rfp), and GN394 (40 ng/µl of Pfat-1::gfp; Punc-12::rfp) were obtained according to standard methods (Evans, 2006) by injecting the desired DNA construct and a co-transformation marker into N2 animals. At least three independent transgenic lines were analyzed for each construct; there was no detectable difference in the expression pattern of these lines.
Behavioral assays
Gentle touch sensitivity was tested and scored as described (Hart, 2006). Briefly, ten-trial touch assays were performed by stroking a hair across the body, and scored as percent response to anterior and posterior body touch; at least 25 animals were tested in each day’s trial and results were compared across three trials. All assays were performed blind to genotype and/or treatment.
Polyunsaturated fatty acids and phospholipids supplementation
AA (5,8,11,14-eicosatetraenoic acid) and EPA (5,8,11,14,17-eicosapentaenoic acid) were obtained from Nu-Chek Prep (Elysian, MN). ETYA (5, 8, 11, 14-eicosatetraynoic acid), AEA [N-(2-hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide], and 2-AG (5Z,8Z,11Z,14Z-eicosatetraen-2-glyceryl ether) were acquired from Cayman Chemical (Ann Arbor, MI). NGM was supplemented with PUFAs as previously described (Watts et al., 2003). Briefly, PUFAs were dissolved in ethanol and 0.1% tergitol was added to NGM agar to reach, approximately, a 200 µM final concentration. Endocannabinoid AEA and 2-AG were dried with Ar gas, resuspended in DMSO, and added to NGM to reach, approximately, a 200 µM final concentration. 18:0–20:4 PC (1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphocholine), 18:0–20:4 PE (1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphoethanolamine), and 18:0–20:4 PS (1-stearoyl-2-arachidonoyl-snglycero-3-phospho-L-serine) were obtained from Avanti Polar Lipids, Inc (Alabaster, AL). Liposomes were prepared as previously described (Cortes and Perozo, 1997) and added to NGM to a final concentration of 100 µM. Plates were seeded with E. coli OP50 and kept at room temperature for two days before the addition of embryos. Worm embryos were placed on fresh supplemented plates (≤ one week old).
Imaging
For promoter-fusion experiments worms were paralyzed with polystyrene microspheres (Polysciences) and imaged with a 40×/1.25 oil immersion lens on a Leica SP5 confocal system. To evaluate TRN morphology and MeT channel distribution worms were paralyzed on 1 % agarose pads containing 150 mM of 2,3-butanedione monoxime and imaged with a 5×/0.15 air or 63×/1.4 oil immersion lens on Zeiss Axioplan 2 microscope.
Optogenetics
All-trans-retinal (ATR) was added to OP50 E. coli cultures and spread onto plates containing NGM (Nagel et al., 2005). Worm embryos were placed on fresh ATR plates (≤ one week old). Young adults were tested for withdrawal response in the presence and absence of ATR in the media. Worms were stimulated with blue-light at (0.48 mW/mm2, 470 nm) for one second, every thirty seconds, at an intensity that produces saturating responses in wild-type worms. Neither ChR2 transgenics grown in the absence of ATR (Figure 4C) nor wild-type (N2) worms (data not shown) respond to this stimulus. Behavior was analyzed off-line and defined as a worm reversal within one second of the light pulse and scored as percent in withdrawal response. All assays were performed blind to genotype and/or treatment.
RNAi feeding
NGM plates were seeded with E. coli HT115 and dsRNAi feeding was performed as previously described (Kamath et al., 2001). To knockdown mec-4 and mboa-6 expression, wild type (N2) and TU3270 adult worms were allowed to lay eggs for 2 hours on RNAi containing plates, and the resulting progeny was tested for touch sensitivity as young adults. mboa-6 RNAi was kindly provided by Hiroyuki Arai (University of Tokyo, Japan).
Primary cell culture and atomic force microscopy
TRNs were isolated as described (Strange et al., 2007). Individual TRNs were visualized by GFP. AFM experiments were carried out essentially as previously described for embryonic zebrafish cells (Krieg et al., 2008), using a BioScope Catalyst™ BioAFM (Bruker NanoAXS) mounted on a Leica inverted microscope. Briefly, Olympus Biolevers (6 mN/m) were coated with 1 mg/mL peanut lectin in MES buffer (pH 6, 50 mM) overnight and calibrated according to thermal noise method before each experiment. Individual membrane nanotubes were pulled from isolated cells after 100–900 ms contact time and 400 pN contact force. Interaction frequency was adjusted so that 20% of cell-cantilever contacts yielded an event. This strategy ensures that only single tether events were studied. Dynamic force-distance curves were analyzed using a step-fitting algorithm. The mean tether force vs. extrusion velocity was plotted; we fit to the data the equation f3 − fF02 = aν, where f is the tether force at a velocity ν; F0 is the static force, and a is the coefficient characterizing the dynamics of extrusion. This coefficient is defined as a = (2π)32κ2ηνln(Rc/Rt), where κ is the plasma membrane bending rigidity, η is the membrane surface viscosity, and Rc and Rt are the radius of the cell and tether, respectively (Borghi and Brochard-Wyart, 2007; Brochard-Wyart et al., 2006; Diz-Muñoz et al., 2010).
Supplementary Material
Highlights.
Arachidonic acid is a key fatty acid essential for response to gentle touch.
Arachidonic acid is locally synthesized in the touch receptor neurons.
AA-containing phospholipids modulate touch sensitivity.
Loss of long PUFAs increases bending stiffness of neuronal cell membranes.
Acknowledgments
The authors thank J.G. Cueva, A.L. Eastwood, Z. Liao, for technical assistance; members of the Goodman and Pruitt Labs for comments and experimental advice; J. Shaw, A. Slade, and S. Minne (Bruker NanoAXS) for their generous support and loan of the AFM; H. Arai, M. Chalfie, W. Schafer, for strains and RNAi plasmids. This work was supported by grants from the National Institutes of Health grants (NS047715, EB006745 to M.B.G) and fellowships from the American Heart Association Western States Affiliate (V.V), Human Frontier Science Program (M.K), and a Stanford Graduate Fellowship (D.L). The authors declare that they have no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen OS, Koeppe RE., II Bilayer thickness and membrane protein function: an energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- Anishkin A, Kung C. Stiffened lipid platforms at molecular force foci. Proc. Natl. Acad. Sci. USA. 2013;110:4886–4892. doi: 10.1073/pnas.1302018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardiel EL, Rankin CH. Behavioral plasticity in the C. elegans mechanosensory circuit. J. Neurogenet. 2008;22:239–255. doi: 10.1080/01677060802298509. [DOI] [PubMed] [Google Scholar]
- Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- Arnadottir J, O’Hagan R, Chen Y, Goodman MB, Chalfie M. The DEG/ENaC protein MEC-10 regulates the transduction channel complex in Caenorhabditis elegans touch receptor neurons. J. Neurosci. 2011;31:12695–12704. doi: 10.1523/JNEUROSCI.4580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae C, Gnanasambandam R, Nicolai C, Sachs F, Gottlieb PA. Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc. Natl. Acad. Sci. USA. 2013;110:E1162–E1168. doi: 10.1073/pnas.1219777110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleza D, Gómez-Lagunas F, Quinto C. Cloning and functional expression of an MscL ortholog from Rhizobium etli: characterization of a mechanosensitive channel. J. Membr. Biol. 2010;234:13–27. doi: 10.1007/s00232-010-9235-8. [DOI] [PubMed] [Google Scholar]
- Borghi N, Brochard-Wyart F. Tether extrusion from red blood cells: integral proteins unbinding from cytoskeleton. Biophys. J. 2007;93:1369–1379. doi: 10.1529/biophysj.106.087908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard-Wyart F, Borghi N, Cuvelier D, Nassoy P. Hydrodynamic narrowing of tubes extruded from cells. Proc. Natl. Acad. Sci. USA. 2006;103:7660–7663. doi: 10.1073/pnas.0602012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AL, Liao Z, Goodman MB. MEC-2 and MEC-6 in the Caenorhabditis elegans sensory mechanotransduction complex: auxiliary subunits that enable channel activity. J. Gen. Physiol. 2008;131:605–616. doi: 10.1085/jgp.200709910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MF. Curvature forces in membrane lipid–protein interactions. Biochemistry. 2012;51:9782–9795. doi: 10.1021/bi301332v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat. Methods. 2010;7:554–559. doi: 10.1038/nmeth.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejo G, Giblin JP, Gasull X. Modulation of TRESK background K+ channel by membrane stretch. PLoS ONE. 2013;8:e64471. doi: 10.1371/journal.pone.0064471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Sulston J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 1981;82:358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelur DS, Ernstrom GG, Goodman MB, Yao CA, Chen L, O Hagan R, Chalfie M. The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel. Nature. 2002;420:669–673. doi: 10.1038/nature01205. [DOI] [PubMed] [Google Scholar]
- Cortes DM, Perozo E. Structural dynamics of the Streptomyces lividans K+ channel (SKC1): oligomeric stoichiometry and stability. Biochemistry. 1997;36:10343–10352. doi: 10.1021/bi971018y. [DOI] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva JG, Mulholland A, Goodman MB. Nanoscale organization of the MEC-4 DEG/ENaC sensory mechanotransduction channel in Caenorhabditis elegans touch receptor neurons. J. Neurosci. 2007;27:14089–14098. doi: 10.1523/JNEUROSCI.4179-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Muñoz A, Krieg M, Bergert M, Ibarlucea-Benitez I, Muller DJ, Paluch E, Heisenberg C-P. Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 2010;8:e1000544. doi: 10.1371/journal.pbio.1000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TC. Transformation and microinjection. WormBook. 2006:1–15. [Google Scholar]
- Fadeel B, Xue D. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 2009;44:264–277. doi: 10.1080/10409230903193307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffeney SL, Cueva JG, Glauser DA, Doll JC, Lee THC, Montoya M, Karania S, Garakani AM, Pruitt BL, Goodman MB. DEG/ENaC but not TRP channels are the major mechanoelectrical transduction channels in a C. elegans nociceptor. Neuron. 2011;71:845–857. doi: 10.1016/j.neuron.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Wang Q, Wang Z. NOMPC is likely a key component of Drosophila mechanotransduction channels. Eur. J. Neurosci. 2013;38:2057–2064. doi: 10.1111/ejn.12214. [DOI] [PubMed] [Google Scholar]
- Goodman MB. Mechanosensation. WormBook. 2006:1–14. doi: 10.1895/wormbook.1.62.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Padilla F, Dandonneau M, Lavebratt C, Lesage F, Noël J, Delmas P. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron. 2013;77:899–914. doi: 10.1016/j.neuron.2012.12.035. [DOI] [PubMed] [Google Scholar]
- Hart A. Behavior. WormBook. 2006 [Google Scholar]
- Hishikawa D, Shindou H, Kobayashi S, Nakanishi H, Taguchi R, Shimizu T. Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity. Proc. Natl. Acad. Sci. USA. 2008;105:2830–2835. doi: 10.1073/pnas.0712245105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TB, Schermer B, Müller RU, Höhne M, Bartram M, Calixto A, Hagmann H, Reinhardt C, Koos F, Kunzelmann K, et al. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc. Natl. Acad. Sci. USA. 2006;103:17079–17086. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn-Kirby AH, Dantzker JLM, Apicella AJ, Schafer WR, Browse J, Bargmann CI, Watts JL. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell. 2004;119:889–900. doi: 10.1016/j.cell.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2001;2 doi: 10.1186/gb-2000-2-1-research0002. RESEARCH0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZS. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. A mechanosensitive K+ channel in heart cells. Activation by arachidonic acid. J. Gen. Physiol. 1992;100:1021–1040. doi: 10.1085/jgp.100.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2013;483:209–2012. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M, Helenius J, Heisenberg C-P, Muller DJ. A bond for a lifetime: employing membrane nanotubes from living cells to determine receptor-ligand kinetics. Angew. Chem. Int. Ed. 2008;47:9775–9777. doi: 10.1002/anie.200803552. [DOI] [PubMed] [Google Scholar]
- Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436:647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Bandinelli S, Benedetta B, Cherubini A, Iorio AD, Blè A, Giacomini V, Corsi AM, Guralnik JM, Ferrucci L. Omega-6 and omega-3 fatty acids predict accelerated decline of peripheral nerve function in older persons. Eur. J. Neurol. 2007;14:801–808. doi: 10.1111/j.1468-1331.2007.01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-C, Inoue T, Imae R, Kono N, Shirae S, Matsuda S, Gengyo-Ando K, Mitani S, Arai H. Caenorhabditis elegans mboa-7, a member of the MBOAT family, is required for selective incorporation of polyunsaturated fatty acids into phosphatidylinositol. Mol. Biol. Cell. 2008;19:1174–1184. doi: 10.1091/mbc.E07-09-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen M, Storvik M, Malinen H, Hyytiä P, Lakso M, Auriola S, Wong G, Callaway JC. Determination of endocannabinoids in nematodes and human brain tissue by liquid chromatography electrospray ionization tandem mass spectrometry. J. Chromatogr. B. 2011;879:677–694. doi: 10.1016/j.jchromb.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Lesa GM, Palfreyman M, Hall DH, Clandinin MT, Rudolph C, Jorgensen EM, Schiavo G. Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. elegans. J. Cell Sci. 2003;116:4965–4975. doi: 10.1242/jcs.00918. [DOI] [PubMed] [Google Scholar]
- Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- Lucanic M, Held JM, Vantipalli MC, Klang IM, Graham JB, Gibson BW, Lithgow GJ, Gill MS. N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature. 2011;473:226–229. doi: 10.1038/nature10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Lysophospholipids open the two-pore domain mechano-gated K(+) channels TREK-1 and TRAAK. J. Biol. Chem. 2000;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Inoue T, Lee H-C, Kono N, Tanaka F, Gengyo-Ando K, Mitani S, Arai H. Member of the membrane-bound O-acyltransferase (MBOAT) family encodes a lysophospholipid acyltransferase with broad substrate specificity. Genes Cells. 2008;13:879–888. doi: 10.1111/j.1365-2443.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- Pan B, Géléoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR. TMC1 and TMC2 are componentsof the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron. 2013;79:504–515. doi: 10.1016/j.neuron.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- Petzold BC, Park S-J, Mazzochette EA, Goodman MB, Pruitt BL. MEMS-based force-clamp analysis of the role of body stiffness in C. elegans touch sensation. Integr. Biol. 2013;5:853. doi: 10.1039/c3ib20293c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459:379–385. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamoorthi K, Petrache HI, McIntosh TJ, Brown MF. Packing and viscoelasticity of polyunsaturated ω-3 and ω-6 lipid bilayers as seen by 2H NMR and X-ray diffraction. J. Am. Chem. Soc. 2005;127:1576–1588. doi: 10.1021/ja046453b. [DOI] [PubMed] [Google Scholar]
- Rawicz W, Olbrich KC, McIntosh T, Needham D, Evans E. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 2000;79:328–339. doi: 10.1016/S0006-3495(00)76295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon A, Dod SW, Williams GD, Beach JM, Brown MF. Configurational statistics of acyl chains in polyunsaturated lipid bilayers from 2H NMR. J. Am. Chem. Soc. 1987;109:2600–2609. [Google Scholar]
- Strange K, Christensen M, Morrison R. Primary culture of Caenorhabditis elegans developing embryo cells for electrophysiological, cell biological and molecular studies. Nat. Protoc. 2007;2:1003–1012. doi: 10.1038/nprot.2007.143. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ikita K, Ashida T, Motoyama Y, Yamaguchi Y, Satouchi K. Effects of growth temperature on the fatty acid composition of the free-living nematode Caenorhabditis elegans. Lipids. 1996;31:1173–1178. doi: 10.1007/BF02524292. [DOI] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Wallis JG, Watts JL, Browse J. Polyunsaturated fatty acid synthesis: what will they think of next? Trends Biochem. Sci. 2002;27:467. doi: 10.1016/s0968-0004(02)02168-0. [DOI] [PubMed] [Google Scholar]
- Watts JL. Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends Endocrinol. Metab. 2009;20:58–65. doi: 10.1016/j.tem.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JL, Browse J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2002;99:5854–5859. doi: 10.1073/pnas.092064799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JL, Phillips E, Griffing KR, Browse J. Deficiencies in C20 polyunsaturated fatty acids cause behavioral and developmental defects in Caenorhabditis elegans fat-3 mutants. Genetics. 2003;163:581–589. doi: 10.1093/genetics/163.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Grillet N, Elledge HM, Wagner TFJ, Zhao B, Johnson KR, Kazmierczak P, Müller U. TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell. 2012;151:1283–1295. doi: 10.1016/j.cell.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493:221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini D, Göpfert MC. Mechanosensation: tethered ion channels. Curr. Biol. 2013;23:R349–R351. doi: 10.1016/j.cub.2013.03.045. [DOI] [PubMed] [Google Scholar]
- Zhang S, Arnadottir J, Keller C, Caldwell GA, Yao CA, Chalfie M. MEC-2 is recruited to the putative mechanosensory complex in C. elegans touch receptor neurons through its stomatin-like domain. Curr. Biol. 2004;14:1888–1896. doi: 10.1016/j.cub.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yan Z, Jan LY, Jan YN. Sound response mediated by the TRP channels NOMPC, NANCHUNG, and INACTIVE in chordotonal organs of Drosophila larvae. Proc. Natl. Acad. Sci. USA. 2013;110:13612–13617. doi: 10.1073/pnas.1312477110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.