Abstract
Post-transcriptional regulation of RNA stability and localization underlies a wide array of developmental processes, such as axon guidance and epithelial morphogenesis. In Drosophila, ectopic expression of the classically Golgi peripheral protein dGRASP at the plasma membrane is achieved through its mRNA targeting at key developmental time-points, in a process critical to follicular epithelium integrity. However, the trans-acting factors that tightly regulate the spatio-temporal dynamics of dgrasp are unknown. Using an in silico approach, we identified two putative HOW Response Elements (HRE1 and HRE2) within the dgrasp open reading frame for binding to Held Out Wings (HOW), a member of the Signal Transduction and Activation of RNA family of RNA-binding proteins. Using RNA immunoprecipitations, we confirmed this by showing that the short cytoplasmic isoform of HOW binds directly to dgrasp HRE1. Furthermore, HOW loss of function in vivo leads to a significant decrease in dgrasp mRNA levels. We demonstrate that HRE1 protects dgrasp mRNA from cytoplasmic degradation, but does not mediate its targeting. We propose that this binding event promotes the formation of ribonucleoprotein particles that ensure dgrasp stability during transport to the basal plasma membrane, thus enabling the local translation of dgrasp for its roles at non-Golgi locations.
INTRODUCTION
Tissue and cell physiology is governed by gene expression that consists of a large spectrum of processes that allow for the versatility and adaptability of an organism. Gene expression is regulated at multiple levels. One mode of regulation is at the post-transcriptional level, where mRNA levels are fine-tuned by appropriate degradation or stabilization. The latter is critical for localized mRNAs that need to be translationally repressed but stabilized until they reach their target site. RNA-binding proteins (RBPs) are known to regulate the overall stability of mRNA as they can either recruit RNA degradation machinery or protect RNA from degradation (1–3).
Traditionally Drosophila melanogaster represents a strong model for elucidating post-transcriptional regulation events (4–6). Studies of the Drosophila egg-chambers, aiming to understand gene regulation by RNA metabolism (such as localization, stability and translation) have led to the characterization of a large number of key factors implicated in these processes (7–9), as well as shed light to their importance for correct development, specification and homeostasis of this tissue (10–12). The integrity of the follicular epithelium covering the Drosophila oocyte is also thought to depend upon a number of transcripts that, at specific stages of development, become localized in the proximity of the baso-lateral plasma membrane of the follicle cells, in a region that we name the ‘open zone of contact’ (open ZOC) (13,14). One of these transcripts is dgrasp that encodes a protein known to be associated peripherally to the Golgi membrane, with many roles in the Golgi architecture and dynamics (15). Recently, this protein has also been shown to mediate two types of unconventional secretion (16). This includes the Golgi bypass of alpha PS1 integrin subunit (αPS1) at stage 10B of Drosophila oogenesis, during which it is transported to the open ZOC in a manner that deviates from the classical pathway (ER > Golgi > PM) (13).
One of the striking features of dgrasp mRNA in the follicular epithelium is the tight window of expression. Beginning at stage 10A of oogenesis, dgrasp mRNA appears as cytoplasmic foci that we interpret to be ribonucleoprotein particles (RNPs) that are then targeted to the open ZOC at stage 10B (14). There, dgrasp mRNA appears to be locally translated (13). To understand the post-transcriptional regulation and dynamics of dgrasp mRNA, we aimed to identify RBPs involved in its metabolism.
The Signal Transduction and Activation of RNA (STAR) family is one of the most evolutionarily conserved groups of RBPs across the animal kingdom and controls a wide range of developmental events by regulating alternative splicing and translational repression of specific mRNA targets. In mammals, for instance, the most-studied member of the STAR family, Sam68, has an active role during spermatogenesis where it modulates alternative splicing in male germ cells (17). Its evolutionarily related counterpart Quaking (QKI) regulates myelination by Schwann cells and oligodendrocytes by controlling the splicing of myelin-associated glycoprotein (MAG) and other myelin targets (18–20). Interestingly, QKI also regulates muscle fibre maturation in zebrafish by stabilizing Gli2a transcripts (21). In Caenorhabditis elegans, the STAR protein GLD-1 is a translational repressor and promotes germ cell differentiation (22,23) and ASD-2 is required for alternative splicing (24).
The Drosophila member of the STAR family Held Out Wings (HOW), like its mammalian orthologue QKI, is highly expressed in muscles, tendons (25–28) and glial cells (29,30), where it plays an essential role in controlling the mRNA levels of an array of target genes during development (31). HOW exerts various functions on its target RNAs: it facilitates the alternative splicing of stripe A, a transcription factor essential for tendon cell maturation and mediates specific splicing of the transcript encoding for the septate junction protein Neurexin IV, thereby controlling glial cell maturation. It also functions by reducing mRNA levels of various targets. For example, during gastrulation, HOW-dependent down-regulation of cdc25/string, which encodes for a cell cycle promoting phosphatase, is essential to inhibit cell division in invaginating mesodermal cell (32).
The hallmark of STAR proteins is the presence of a single extended maxi-KH RNA-binding domain. Structurally, HOW, like two other STAR proteins QKI and ASD2, contains a single maxi-KH RNA-binding motif that is flanked by two additional conserved N- and C-terminal sequences, named the QUA1 and QUA2 domains, respectively (28).
Interestingly, the HOW gene has three alternative splice variants, which are named according to their length, HOW(Long), HOW(Medium) and HOW(Short). The expression of HOW(L) begins at early embryonic stages, while HOW(S) is expressed in later stages of differentiated tissues. HOW(M) was recently predicted by Flybase and its expression remains to be characterized. HOW(S) and HOW(L) are almost identical in their amino acid sequence, except that HOW(S) lacks the HPYQR signal peptide essential for nuclear retention. Consequently, HOW(S) predominantly localizes to the cytoplasm whereas HOW(L) is mostly found in the nucleus (33,34).
Here, we perform a computer-based comparative analysis to identify putative RBPs that interact with and regulate dgrasp mRNA. We propose that dgrasp is a novel target of HOW(S) and that this interaction is critical for dgrasp mRNA stabilization.
MATERIALS AND METHODS
In silico prediction of putative RBP-binding sites
RNA-Binding Protein DataBase (RBPDB) developed by (35) is a web-free database of RNA-binding experiments (http://rbpdb.ccbr.utoronto.ca/) that provides a scanning tool to identify RBP-binding sites in the given query sequences. It contains binding data on 272 RBPs, including 71 that have motifs in position weight matrix format (PWM) and 36 sets of sequences of in vivo-bound transcripts from immunoprecipitation experiments.
Briefly, the strategy pursued for the RBP prediction consists in independently running orthologous sequences of the RNA of interest through RBPDB to identify sites matching the known RBP-binding sites annotated in the database. Only putative sites with relative scores greater than the set threshold of 70% were included. RBPDB outputs for each RNA sequence were then exported in an excel file for comparison. This was followed by manual curation that removed RBPs sites not conserved in all the species included in the analysis.
For the Pitx2 3′UTRs in silico analysis, the orthologous sequences of five vertebrate species (human, mouse, chicken, zebrafish and xenopus) were considered. For dgrasp, we used the open reading frame (ORF) and the 3′UTR of orthologous sequences of six Drosophila species (D. melanogaster, D. simulans, D. sechellia, D. ananassae, D. erecta and D. yakuba). For Neurexin IV, sequences of a portion of intron 3 of 11 different Drosophila species (D. melanogaster, D. simulans, D. sechellia, D. ananassae, D. erecta, D. yakuba, D. pseudobscura, D. persimilis, D. willistoni, D. mojavensis, D. virilis) were used. The genomic intervals of intron 3 used as inputs for analysis are: D. melanogaster 5163–5232 nt; D. simulans 5111–5180 nt; D. sechellia 5120–5189 nt; D. erecta 5058–5127 nt; D. ananassae 4599–4662 nt; D. yakuba 5145–5214 nt; D. pseudobscura 4985–5055 nt; D. persimilis 4966–5031 nt; D. virilis 923–996 nt; D. mojavensis 5467–5542 nt; D. grimshawi 5399–5470 nt. Sequence interval numbering is with respect to the translational start site (ATG).
RNA structure prediction with RNApromo
For prediction of RNA structures, we used the ‘Segal tool’ RNApromo (http://genie.weizmann.ac.il/pubs/rnamotifs08/rnamotifs08_predict.html). This tool predicts a structural motif common to a set of given RNA sequences. The folding algorithm used in the Segal method is the ‘Vienna package’ and the default parameters that we used for the prediction are the following: Number of motifs to predict, 3; Stem size flexibility, 3; Loop size flexibility, 1; Background distribution, Binomia-short motifs.
Specifically, the prediction of dgrasp HRE1 and HRE2 structures was performed using sequences from six Drosophila species (D. melanogaster, D. simulans, D. sechellia, D. erecta, D. ananassae and D. yakuba). For the prediction of HRE1, the RNA intervals entered into RNApromo structure are: D. melanogaster 862–894 nt; D. simulans 876–909 nt; D. sechellia 877–909 nt; D. erecta 871–903 nt; D. ananassae 874–906 nt and D. yakuba 871–903 nt. For the prediction of HRE2, the RNA interval used are: D. melanogaster 989–1030 nt; D. simulans 1004–1045 nt; D. sechellia 1004–1045 nt; D. erecta 993–1033 nt; D. ananassae 1068–1107 nt and D. yakuba 992–1033 nt. Note that sequence interval coordinates are with respect to the translational start site (ATG).
We also use the Vienna RNAalifold WebServer (http://rna.tbi.univie.ac.at/) to predict the secondary structures of HRE1 and HRE2 motifs. For the prediction of each HRE motif using this method, a multiple sequence alignment of the six Drosophila sequences was obtained with ClustalW2 (with the same genetic intervals as for the RNApromo analysis) and used as input in the RNAalifold WebServer. Default settings of the web pipetline RNAalifold were used. As for the RNApromo analysis, the prediction of HRE1 and HRE2 structures was performed using sequences from six Drosophila species (D. melanogaster, D. simulans, D. sechellia, D. erecta, D. ananassae and D. yakuba). For the prediction of HRE1 and HRE2 structures, the same RNA sequence intervals used in RNApromo were entered.
Fly strains
Drosophila melanogaster stocks were raised on standard cornmeal-yeast agar medium at 25°C unless otherwise stated. OregonR line was used as the wild-type reference strain and is referred to as WT. The other lines used (coming from the Bloomington stock centre http://flystocks.bio.indiana.edu/) unless otherwise stated) are: w1118; HOWstru-3R-3, FRT82B/TM6B, Tb; hs::Gal4/Gla, Bc, Elp; c355::Gal4; pr pwn hs::flp/CyO; +/Ki ry; UAS-HOW(S)-3HA and UAS-HOW(L)-3HA (unpublished data, Talila Volk), w; FRT82B ubi::GFP/TM3, Sb. The additional Drosophila species D. simulans, D. sechellia, D. erecta, D. yakuba, D. ananassae were obtained from the Ehime-fly [the Drosophila stocks of Ehime University, Japan (http://kyotofly.kit.jp/cgi-bin/ehime/index.cgi)].
For overexpression experiments of HOW(S)-3HA and HOW(L)-3HA fusion proteins in ovaries, 2-day-old hs::Gal4/+; UAS-HOW(S)-3HA/+ and hs::Gal4/+; UAS- HOW(L)-3HA/+ female progeny fattened on yeast [obtained from the cross between w; UAS-HOW(S)-3HA or w; UAS-HOW(L)-3HA and hs::Gal4/Gla, Bc, Elp, respectively] were incubated for 2 h at 37°C, and then let to recover for five additional hours at 25°C prior to dissection.
HOWstru-3R-3 homozygous mutant clones in the follicular epithelium were generated using the heat shock-inducible Flp-FRT system (36). Female progeny hs::FLP/+; HOWstru-3R-3, FRT82B/FRT82B ubi::GFP obtained from the cross of males hs::FLP/+; HOWstru-3R-3, FRT82B/Ki, ry to females hs::flip/+; FRT82B ubi::GFP/Ki, ry were collected upon eclosion and raised for 2 days in presence of yeast at 22°C. During this time, flies were exposed to two heat-shock treatments (one per day) at 37°C for 1 h prior to dissection for ovaries collection.
RNA isolation and reverse transcription
Total RNA was extracted from ovaries at different stages of development with TRIzol® Reagent (Invitrogen) in accordance with the protocol of the manufacturer. The isolated RNA was treated with RNase-free DNase I (Sigma) to eliminate possible genomic DNA contamination. RNA was reverse transcribed into cDNA with GoScript™ Reverse Transcription System (Promega) and random primers (Promega) according to manufacturer.
Immunolabelling
Ovary staining was performed as described previously in (13). Briefly, ovaries from 2-day-old virgin flies were dissected, fixed for 15 min in 4% PFA and finally permeabilized in 0.1% TritonX. Permeabilized ovaries were incubated with primary antibodies overnight at 4°C; FITC- and TRITC-conjugated secondary antibodies (Invitrogen) incubations were done for 3 h at room temperature. The following antibodies were used: rabbit anti-HOW antibody (1:300), mouse anti-α-spectrin 3A9 antibody (1:20, DSHB) and mouse anti-HA antibody 16B12 (1:50, Covance). TRITC-conjugated phalloidin (1:200, Sigma) was used for visualization of the plasma membrane in egg-chambers overexpressing HOW(S)-HA and HOW(L)-HA transgenes. Egg chambers were incubated with far-red fluorescent TO-PRO®-3 (1:500; Life Technologies) for 30 min for nuclear staining. Samples were mounted in ProLong Gold anti-fade reagent (Invitrogen) on glass slides and imaged with Leica SPE Confocal Microscope.
Fluorescent in situ hybridization
After fixation as described above, following dissection and fixation in 4% PFA, ovaries were stored in 100% methanol. Full-length dgrasp and gfp sense and antisense digoxigenin-labelled RNA probes were synthesized using DIG RNA Labelling Kit (Roche) according to the manufacturer. Fluorescent in situ hybridization (FISH) was performed as described in (13). Briefly, fixed ovaries were hybridized with a digoxigenin-labelled RNA probe overnight at 55°C. After washing, signal was developed by using an anti-digoxigenin-POD antibody (1:100, Roche) and the TSA (Tyramide Signal Amplification) Cyanine 3 System (PerkinElmer). Following tyramide reaction, samples were incubated for 1h with mouse anti-α-spectrin 3A9 antibody (1:20, DSHB) to outline the plasma membrane.
Fluorescence quantification with ImageJ
The measurement of the fluorescence intensity of dgrasp FISH in HOW−/− and HOW+/− cells was performed using images captured at identical confocal settings. Stacks of images were taken and the four slices showing the brightest labelling were used for the measurements of average pixel intensities. This was done in Image J using the line measurement tool to draw the boundaries of the HOW−/− clones. Background was estimated from a laser-off image and was subtracted. Average pixel intensities was also measured outside the clones in the surrounding areas of identical sizes (HOW+/− cells). Mean intensities for HOW−/− and HOW+/− were averaged and then statistically compared using an unpaired two-tailed Student’s t-test (n = 20).
RNA–protein co-immunoprecipitation
Ovaries from 40 females [WT or overexpressing HA-tagged HOW(L) and HOW(S)] were dissected and homogenized in RNA immunoprecipitation (RIP) lysis buffer.
Each lysate was divided into two samples; one was used for immunoprecipitation with either rabbit anti-HOW (5 μg) (37) or mouse anti-HA 16B12 (5 μg) (Covance) antibodies, and the second was used with control rabbit or mouse IgG (5 μg). The immunoprecipitation was performed using the Magna RIP™ RNA-Binding Protein Immunoprecipitation kit (Millipore) as described by the manufacturer. The IP samples and the input (10% of the lysates) were phenol–chloroform precipitated to extract the RNA. An amount of 20 ng of RNA from each fraction (input, HOW or HA IP and IgG IP fractions) was subjected to a reverse transcription (RT) reaction using random primers to generate cDNA libraries. Equivalent amounts of cDNA were then used for quantitative PCR reactions to detect the enrichment of dgrasp, Neurexin IV and shotgun mRNAs in the different immunoprecipitation.
Real-time RT–qPCR and quantitation
Real-time RT–PCR (RT-qPCR) for quantification of mRNA amount was performed as described by (38) with an MyIQ Real-time PCR systems (Bio-rad) and with iQ™ SYBR® Green Supermix (Bio-rad) used for detecting the fluorescence of amplified products. Real-time reaction was carried out as follows: pre-denaturing at 95°C for 3 min, followed by 45 cycles at 95°C for 30 s and 60°C for 1 min. Melting curves were generated for the final PCR products by decreasing the temperature to 65°C for 15 s followed by an increase in temperature to 95°C. Fluorescence was measured at 0.2°C increments. Real-time PCR MyIQ software (Bio-rad) was used to determine the amplification cycle in which product accumulation was above the threshold cycle values (CT). Real-time PCR CT values were analysed using the 2−ΔCT method (39).
In RIP experiments, the measured amount of dgrasp, Neurexin IV and shotgun mRNAs in each immunoprecipitation (IP) are presented as the relative enrichment in either HOW or HA RIP compared with IgG RIP [CT[dgrasp(HOW or HA IP)] − CT[dgrasp(IgG IP)]]. The different x-fold inductions of dgrasp, Neurexin IV and shotgun transcripts in the HOW and HA immunoprecipitations were also calculated by the 2−ΔCT method (39).
The primer sequences used for RT–qPCR reactions are the following:
| Forward | Reverse | |
|---|---|---|
| dgrasp | 5′-CACCGAAGGCTACCACGTA-3′ | 5′-TTGTCAACGTTCTGGCGGAG-3′ |
| shotgun | 5′-GACGTTTGCACCTTCAACGT-3′ | 5′-CCGCAGAATCTCGTATTCGA-3′ |
| Neurexin IV | 5′-CTCGGATGGACGCGTGATTA-3′ | 5′-GTTGAAGAGTCGGGAGGAGC-3′ |
For the quantification of dgrasp mRNA levels in single egg chambers of control and HOW−/− mutant ovaries, the housekeeping gene gpdh was analysed as internal standard. ΔCT-values were calculated as follows: CT(gpdh) − CT(dgrasp), and were normalized to gpdh levels. The dgrasp mRNA fold change in egg chambers carrying HOW−/− mutant clones was calculated by the 2−ΔCT method (39), with values normalized to gpdh and relative to control ovaries.
| Forward | Reverse | |
|---|---|---|
| dgrasp | 5′-CACCGAAGGCTACCACGTA-3′ | 5′-TTGTCAACGTTCTGGCGGAG-3′ |
| gpdh | 5′-AAATCGCGGAGCCAAGTAGT-3′ | 5′-CACGATTTTCGCTATGGCCG-3′ |
In the RNA degradation assay, upon quantification by RT–qPCR, the values obtained for each target gene (dgrasp-FL and dgrasp-ΔHRE1) were normalized against values of the endogenous housekeeping human gene GAPDH (from the Caco-2 cell extract, see below) using the 2−ΔCT method (39). Results of each condition were plotted as the percentage of synthetic dgrasp-FL and dgrasp-ΔHRE1 RNAs remaining as a function of time from the starting point T0. Sequences of primers used for the RT–qPCR are shown below.
Caco-2 cell extract preparation
Approximately 108 Caco-2 cells were washed two times with 40 ml ice-cold wash buffer (150 mM sucrose, 33 mM ammonium chloride, 7 mM potassium chloride, 4.5 mM magnesium acetate, 30 mM HEPES, pH 7.4). Cells were then resuspended in 1 ml standard reaction as described in (40) and were disrupted via passages through a thin syringe needle. After spinning for 10 min at 10 000 rpm at 4°C, the supernatant is collected and stored on ice waiting to start the in vitro mRNA degradation assay. The lysate can be stored in small aliquot at −80°C.
In vitro mRNA degradation assay
The in vitro degradation assay was adapted from (41). pCS2+ full-length dgrasp (dgrasp-FL) and ΔHRE1 mutant (dgrasp-ΔHRE1) templates were linearized with ApaI. dgrasp RNAs (dgrasp-FL and dgrasp-ΔHRE1) were synthesized using mMESSAGE mMACHINE® High Yield Capped RNA Transcription Kits (Ambion).
Prior to initiate RNA degradation reactions, 5 μl of 1 μg/μl in vitro translated HOW(S)-HA were pre-incubated with 5 μg of in vitro transcribed dgrasp mRNA at room temperature for 10 min in binding buffer [100 mM KCl, 5 mM MgCl, 10 mM HEPES, pH 7.0, 0.5% NonidetP-40, 1 mM DTT, 100 U/ml Rnasin RNase inhibitor (Promega), 2 mM vanadyl ribonucleoside complex solution and 25 μl/ml protease inhibitor cocktail] in a final volume of 40 μl. This was followed by the addition of 90 μg of Caco-2 cell extracts (for preparation of Caco-2 cell extract see section above) in presence or absence of 100 U/ml of the general RNase inhibitors (RNasin®) (Promega).
Incubations containing synthetic RNAs, HOW(S)-HA and Caco-2 cell extracts were carried at 30°C for 0, 45 and 180 min. The RNA was purified by adding one volume of TRIzol (Invitrogen) and two volumes of chloroform, and precipitated from ethanol as described by (42). cDNA libraries for each reaction were made using 500 ng of total RNA following the protocol described above, and in vitro decay of full-length WT and ΔHRE1 mutant dgrasp RNAs was assessed by RT–qPCR reactions using the following primer set
| Forward | Reserve | |
|---|---|---|
| dgrasp FL | 5′-CACCGAAGGCTACCACGTA-3′ | 5′-TTGTCAACGTTCTGGCGGAG-3′ |
| GAPDH | 5′-TGCACCACCAACTGCTTAGC-3′ | 5′-GGCATGGACTGTGGTCATGAG-3′ |
Levels of both dgrasp-FL and dgrasp-ΔHRE1 RNAs were normalized to those of endogenous human GAPDH mRNA (coming from the Caco-2 lysate) and plotted as the percentage of synthetic dgrasp-FL and dgrasp-ΔHRE1 RNAs remaining as a function of time from T0 as explained above.
In vitro interaction assay
Full-length dgrasp RNA (FL) and dgrasp fragments (NT, CT2, CT2-ΔHRE1, CT2-ΔHRE2 and CT2-ΔHRE1-2) as well as Neurexin IV intron 3 were prepared by in vitro transcription using as templates PCR products carrying the T7 promoter on the forward primers using the MEGAscript® T7 Kit, (Ambion®).
PCR products were obtained using specific sets of primers (below). In vitro transcribed RNAs were purified with a phenol-chloroform step and precipitated in isopropanol prior to elution in nuclease-free dH2O.
| Forward | Reverse | |
|---|---|---|
| dgrasp FL | 5′-TTAATACGACTCACTATAGGGAGAAGCCACAGCATCCAT-3′ | 5′-CTCGAGGCGTTTCCAGCCTGATTCAC |
| NT | 5′-TTAATACGACTCACTATAGGGAGAAGCCACAGCATCCAT-3′ | 5′-CTCGAGGCGGGAATGCGATGCAAATAGCC-3′ |
| CT2 | 5′-TAATACGACTCACTATAGGGGACCACCGACTATTGAGCCAC-3′ | 5′-CTGTGGTGCAGAGAACATGG-3′ |
| Intron 3 | 5′-TAATACGACTCACTATAGGGGTGA | 5′-TCGAAGACGTTGTAATGGATAGAG-3′ |
| Neurexin IV | CACTGCGACTAACTAGATTGG-3′ |
CT2-ΔHRE1, CT2-ΔHRE2 and CT2-ΔHRE1-2 fragments were generated by site-directed mutagenesis using mutagenic primers (below) and CT2 primer pair (CT2F and CT2R) as flanking primers.
| Forward | Reverse | |
|---|---|---|
| HRE1mut | 5′-GAGGCTACCGATGCCTTTTTCGCTGCGCTCGAATCGC-3′ | 5′-GCGATTCGAGCGCAGCGAAAAAGGCATCGGTAGCCTC-3′ |
| HRE2mut | 5′-CCATTGCCGCCACCAGTTTTTATCTTTATACCC-3′ | 5′-GGGTATAAAGATAAAAACTGGTGGCGGCAATGG-3′ |
HOW(S)-HA was cloned into the pcDNA3.1 vector and translated in vitro using TNTR-Coupled Reticulocyte Lysate System following manufacturer instructions (Promega). Binding assays were performed by adding 5 μl (∼5 μg) of the translation mixture to 1 μg of in vitro transcribed RNA for 30 min at RT in 250 μl of binding buffer [100 mM KCl, 5 mM MgCl, 10 mM HEPES, pH 7.0, 0.5% NonidetP-40, 1 mM DTT, 100 U/ml Rnasin RNase inhibitor (Promega), 2 mM vanadyl ribonucleoside complex solution and 25 μl/ml protease inhibitor cocktail].
ProteinG magnetic beads (Invitrogen) were first washed with binding buffer [lacking glycerol and tRNA, and containing 0.5% BSA (Sigma)], and incubated with mouse anti-HA 16B12 (Covance) antibody at a concentration of 5 μg/50 μl of beads at RT for 30 min. Upon washing to remove unbound anti-HA antibody, 50 μl of the resuspended beads was added to each binding reaction overnight at 4°C.
RNA was phenol–chloroform precipitated from samples. Input RNA was used as positive control. cDNA was prepared with random primers, followed by RT–PCR with the primer combination used above for the generation of dgrasp FL, NT, CT2 and Neurexin IV intron 3.
Generation of pUAST-CT dgrasp::GFP and pUAST-CT2 dgrasp::GFP transgenic lines
GFP-tagged CT- and CT2-dgrasp fragments were generated by PCR reaction using the following primers
| Forward | Reverse | |
|---|---|---|
| CT2-dgrasp | 5′-ATGGTACCAACATGCCACCGACTATTGAGCCAC-3′ | 5′-ATGCGGCCGCTGTGGTGCAGAGAACATGG-3′ |
| CT-dgrasp | 5′-ATGGTACCAACATGATTGGCTTCGGCTATTTGC-3′ | 5′-ATGCGGCCGCTTTCCAGCCTGATTCACTG-3′ |
First, they were cloned into pRMeGFP vector using KpnI and NotI restriction sites, upstream of the GFP sequence. GFP-tagged CT- and CT2-dgrasp fragments were then sub-cloned into the pUAST vector (43) using KpnI and XbaI sites. Finally, Drosophila stable transgenic lines were generated with Bestgene (Chino Hills, CA, USA). Several independent lines were obtained for both constructs. Insertions on the third chromosome were used for all the experiments, and expression was driven by the follicular epithelium specific driver c355::Gal4 driver. 2-day-old c355::Gal4/+; +/+; UAS-CTdgrasp::GFP/+ and c355::Gal4/+; +/+; UAS-CT2::dgrasp::GFP/+ female progeny fattened on yeast at 25°C were dissected to collect ovaries.
RESULTS
Comparative in silico analysis predicts HOW response elements in dgrasp mRNA
In order to identify potential RBPs regulating dgrasp mRNA, we performed a comparative genomics-based orthologous RNA analysis using the online RBPDB (35). This strategy consists of running homologous mRNA sequences in multiple species against this database. The predicted outcome of putative RBP signatures found in one transcript is then compared with the others, and only the RBP motifs shared by all homologues are taken into consideration for further analysis (see ‘Materials and Methods’ section for further details).
To validate the sensitivity/robustness of this approach, we first ran transcripts for which RBPs have been experimentally characterized. MBNL1 interacts with the 3′UTR of the Pitx2 transcript (44). Analysis of the 3′UTR sequences of the Pitx2 transcripts from different species (human, mouse, dog, zebrafish, xenopus) using our strategy (Supplementary Figure S1A) predicts signatures for 17 putative RBPs (Supplementary Figure S1B). Notably, the all MBNL1 sites, experimentally characterized by (44), were efficiently predicted by our method (Supplementary Figure S1C), therefore validating the reliability of our approach.
Next, to identify potential RBPs regulating dgrasp mRNA, we set out to analyse the dgrasp mRNA sequences (consisting of the ORFs and 3′UTRs, but not 5′UTRs) from six different Drosophila species for which we could confidently predict the boundary of the 3′UTR: D. melanogaster, D. simulans, D. sechellia, D. ananassae, D. erecta and D. yakuba. First, we verified that dgrasp mRNA of these species showed a comparable spatio-temporal expression to D. melanogaster, i.e. with a similar initial up-regulation and formation of RNPs in the cytoplasm of the follicle cells at stage 10A followed by targeting of the dgrasp transcripts near the open ZOC at stage 10B (Supplementary Figure S2).
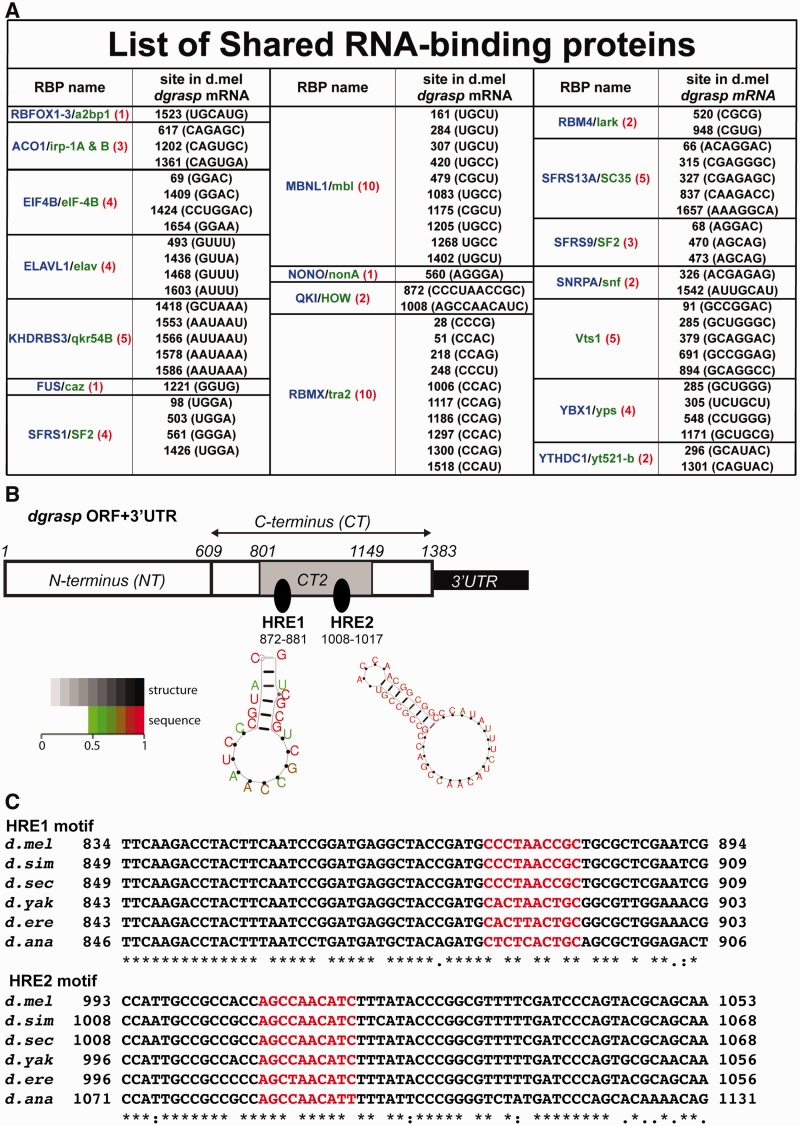
We then performed our in silico approach that led to the prediction of putative signatures for binding to 18 RBPs (Figure 1A). Two of these signatures are conserved putative binding elements for the STAR family member QKI/GLD-1/HOW (called HOW Response Elements, HRE, Figure 1B and C) and RNA secondary structure softwares (RNApromo and RNAalifold) predict that these sites are present on loops (Figure 1B and Supplementary Figure S3).
Figure 1.
Computational prediction of HREs in dgrasp mRNA ORF. (A) Outcome of the 18 predicted RBPs from the comparative RBPDB in silico analysis using dgrasp mRNA sequence (ORF and 3′UTR) from D. melanogaster, D. simulans, D. sechellia, D. ananassae, D. erecta and D. yakuba. The vertebrate RBPs are listed in blue whereas the D. melanogaster homologues are in green. The numbers of predicted sites for each RBP, with respect to the translational start site (ATG), are shown in red. (B) Cartoon representation of the two HOW Response Elements (HREs) predicted at the C-terminus of the ORF of D. melanogaster dgrasp at position 872–881 nt (HRE1) and 1008–1017 nt (HRE2) with respect to the translational start site (ATG). The structure prediction of the two HREs using the RNA secondary structure program RNA promo (Segal lab http://genie.weizmann.ac.il/pubs/rnamotifs08/rnamotifs08_predict.html) is presented. The two HRE sites are marked by arrows and are predicted to be in loops. Sequence positions in the predicted structure are colour-coded according to their probability (>0.5 by default) with scale ranging from green (low probability = 0.5) to red (high probability = 1). (C) Alignment of the coding regions of the dgrasp drosophilid orthologues. The two predicted HRE sequences are coloured in red. dgrasp coding sequences of D. melanogaster, D. simulans, D. sechellia, D. ananassae, D. erecta and D. yakuba were used for the ClustalW2-based alignment.
Our decision to focus on HOW relates to the fact that homozygous mutant escapers of the weak HOW allele, HOWr17 exhibit blisters in the wings (25), a phenotype that resembles the wing phenotype reported in the dgrasp100.2 homozygous mutant escapers (13). In the absence of a reported HOW follicular epithelial mutant phenotype, the wing phenotype suggested a possible genetic interaction between these two genes, making HOW a strong candidate for post-transcriptional regulation of dgrasp mRNA.
To validate the reliability of the predicted dgrasp HREs, we used the same approach with sequences of a known HOW target, Neurexin IV (29). A number of HREs have been identified within Neurexin IV intron 3 in the proximity of the splice acceptance boundary and the interaction of HOW in this region is crucial for the generation of different Neurexin IV protein isoforms (34). Our analysis successfully led to the identification of these same HREs in the intron 3 of Neurexin IV gene (Supplementary Figure S4).
Furthermore, we used a matrix predicting binding motif for the C. elegans HOW orthologue GLD-1. GLD-1 has recently been shown to associate with hundreds of germ-line mRNAs, and these mRNAs all exhibit motifs predicted to bind GLD-1 (7 mers ± 1 or 2 variations). These data were used to build a C. elegans matrix [for description of the matrix see (45)]. Strikingly, using this same matrix, the identification of the two putative HREs in the dgrasp RNA ORF were also predicted (personal communication). Interestingly, endogenous C. elegans grasp mRNA (Y42H9AR.1) was pulled down by GLD-1 immunoprecipitation [see (45), their Supplementary Figure S1] and also displays two GLD-1 response elements in the C-terminus of the ORF (at sites 907–913 nt and 987–993 nt with respect to the TSS). Taken together, these results make HOW a strong candidate regulator of dgrasp mRNA metabolism.
HOW binds dgrasp transcript in vivo
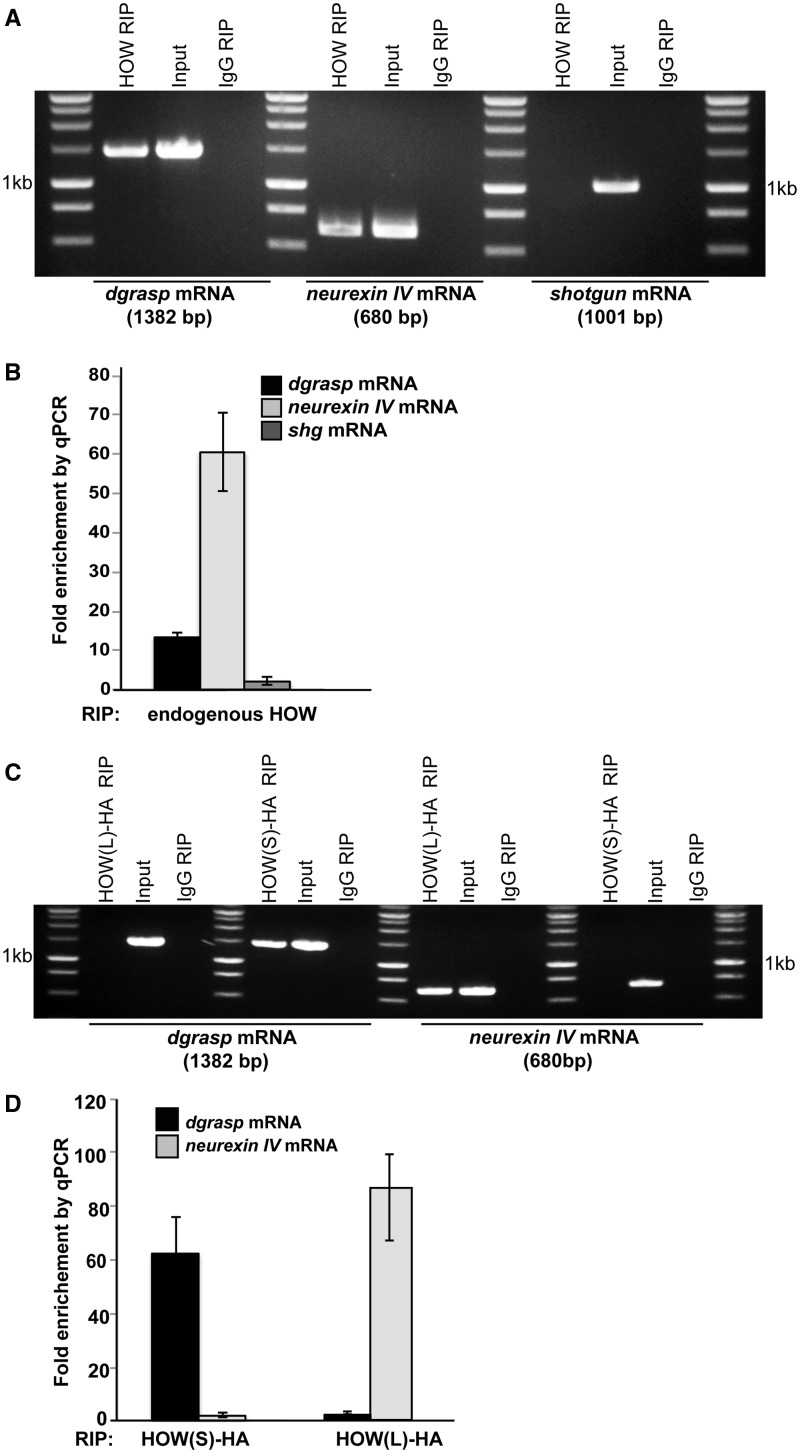
To test whether HOW binds to dgrasp mRNA in vivo in the follicular epithelium, we performed RIP assays using ovaries dissected from young WT D. melanogaster. Endogenous HOW in complex with its mRNA targets was immunoprecipitated using an anti-HOW antibody. Neurexin IV mRNA was used as a positive control since it is expressed in the follicular epithelium (13) as well as being a well-established target of HOW, while shotgun mRNA, the Drosophila DE-cadherin homologue, that has not been reported to be a HOW target was chosen as a negative control. As expected, RT–PCR from IgG RIP (negative control) did not yield amplification of Neurexin IV, dgrasp or shotgun mRNA (Figure 2A). Conversely, both Neurexin IV and dgrasp transcripts were detected in the cDNA library of the HOW RIP, whereas shotgun mRNA was not (Figure 2A). To confirm these results, we performed real-time RT–qPCR of the same RIPs. dgrasp and Neurexin IV mRNAs are significantly enriched (13.5- and 60-fold, respectively) in the HOW RIP when compared with IgG, whereas shotgun mRNA did not show any significant enrichment (Figure 2B). In summary, these results suggest that endogenous HOW binds endogenous dgrasp mRNA in the fly ovaries.
Figure 2.
Endogenous HOW binds dgrasp mRNA in Drosophila ovaries. (A) Agarose gel electrophoresis of RT-PCR reactions from RIP from WT Drosophila ovaries using anti-HOW (HOW RIP) or IgG (IgG RIP). Transcripts scored in the RIP reactions are dgrasp and Neurexin IV mRNAs; shotgun was used as a negative control. (B) Relative enrichment of endogenous dgrasp, Neurexin IV and shotgun in HOW RIP compared with IgG RIP measured by real-time RT–qPCR. Values are means ± standard deviations (SD) (error bars) (n = 3). (C) Agarose electrophoresis of PCR product from endogenous dgrasp and Neurexin IV mRNA immunoprecipitated from Drosophila ovaries overexpressing HOW(S)-HA and HOW(L)-HA using an anti-HA antibody [HOW(L)-HA RIP and HOW(S)-HA RIP] or an IgG as control (IgG RIP). Note that dgrasp mRNA binds HOW(S)-HA. (D) Relative enrichment of dgrasp and Neurexin IV in HOW(S)-HA and HOW(L)-HA RIP when compared with IgG RIP measured by real-time RT–qPCR. Values are means ± SD (error bars) (n = 3).
HOW(S) is enriched at the open ZOC
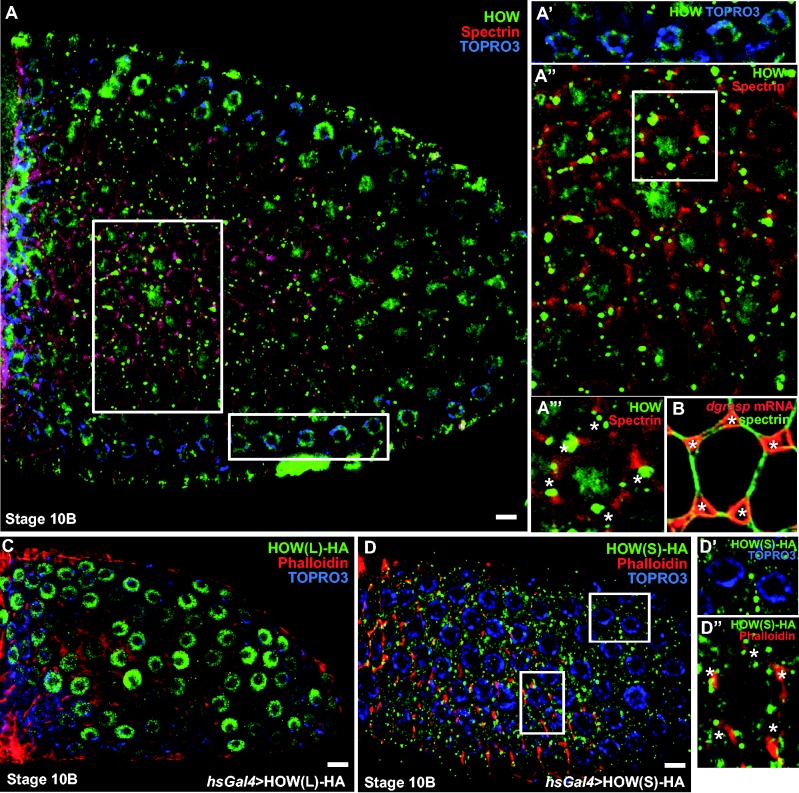
To investigate further whether HOW regulates dgrasp mRNA in the follicular epithelium, we examined the expression and localization of the endogenous HOW protein by immunofluorescence. HOW is expressed in this tissue from stage 8, and is both nuclear (arrows) and cytoplasmic (Figure 3A). The nuclear localization corresponds to HOW(L) that exhibits a nuclear localization signal (NLS) in its sequence (27), whereas the cytoplasmic pool is likely to correspond to HOW(S) (46,47). In addition, at stage 10, a pool of HOW is enriched near the baso-lateral plasma membrane, including the open ZOC (Figure 3A), in a similar location to that of dgrasp mRNA at this specific stage (Figure 3B).
Figure 3.
HOW(S) localizes near the basal plasma membrane of follicle cells at stage 10 of oogenesis. A–A″′. Immunolocalization of endogenous HOW using an anti-HOW antibody (green) in stage 10 Drosophila follicular epithelium. α-spectrin marks the cell cortex (red) and TOPRO3 marks the nucleus (blue). HOW is localized in the nucleus (see top insert, A′) as well as in the cytoplasm (A″), in particular in dots around the open ZOC (asterisks in insert bottom, A″′) at the basal side of the follicle cells. Note that the nucleolus (unstained dot in the middle of the nucleus is very large). (B) FISH localization of dgrasp mRNA (red) with respect to the open ZOC (asterisks). α-spectrin (green) marks the cell cortex. (C) Immuno-localization of HOW(L)-HA overexpressed in the follicular epithelium using the UAS-Gal4 system under the control of a heat-shock promoter. Anti-HA labelling (green) shows that HOW(L) is restricted to the nucleus at all stages in the follicular epithelium development. Phalloidin (red) stains the actin of the cell cortex and TO-PRO®-3 (blue) marks the nucleus. (D–D″) Immunolocalization of HOW(S)-HA (using an anti-HA antibody, green) overexpressed in the follicular epithelium using the UAS-Gal4 system under the control of a heat shock promoter, Phalloidin (red) stains the actin of the cell cortex and TO-PRO®-3 (blue) marks the nucleus. Inserts show that HOW(S) is absent from the nucleus (D′) whereas it is enriched around the open ZOC (asterisks, bottom insert). Scale bars: 10 µm.
To identify the HOW isoform that localizes near the plasma membrane at stage 10 of Drosophila oogenesis, both HA-tagged isoforms were independently expressed in the follicular epithelium using the UAS-GAL4 system (48). As predicted, HOW(L)-HA localized to the nucleus of the follicle cells at all stages (Figure 3C), whereas HOW(S)-HA was present in the cytoplasm as well as near the open ZOC at stage 10 (Figure 3D). This suggests that HOW(S), but not HOW(L), binds to dgrasp mRNA.
dgrasp mRNA is a direct target of HOW(S)
To test whether dgrasp is a target for HOW(S), we performed RIP on extracts from ovaries overexpressing HOW(S)-HA and HOW(L)-HA using an anti-HA antibody. dgrasp mRNA was detected in the cDNA library generated from the HOW(S)-HA IP, but not from the HOW(L)-HA or IgG IPs. Conversely, Neurexin IV mRNA was detected only from the cDNA library of the HOW(L)-HA, but not HOW(S)-HA (Figure 2C), showing the specificity of the approach. We confirmed these results by real-time RT–qPCR that shows a 62.3-fold enrichment of dgrasp mRNA in the HOW(S)-HA IP when compared with HOW(L), whereas Neurexin IV transcripts were 85.7-fold more enriched in HOW(L)-HA IP than in the HOW(S) (Figure 2D). Taken together, our results show that HOW(S) forms a complex with endogenous dgrasp mRNA.
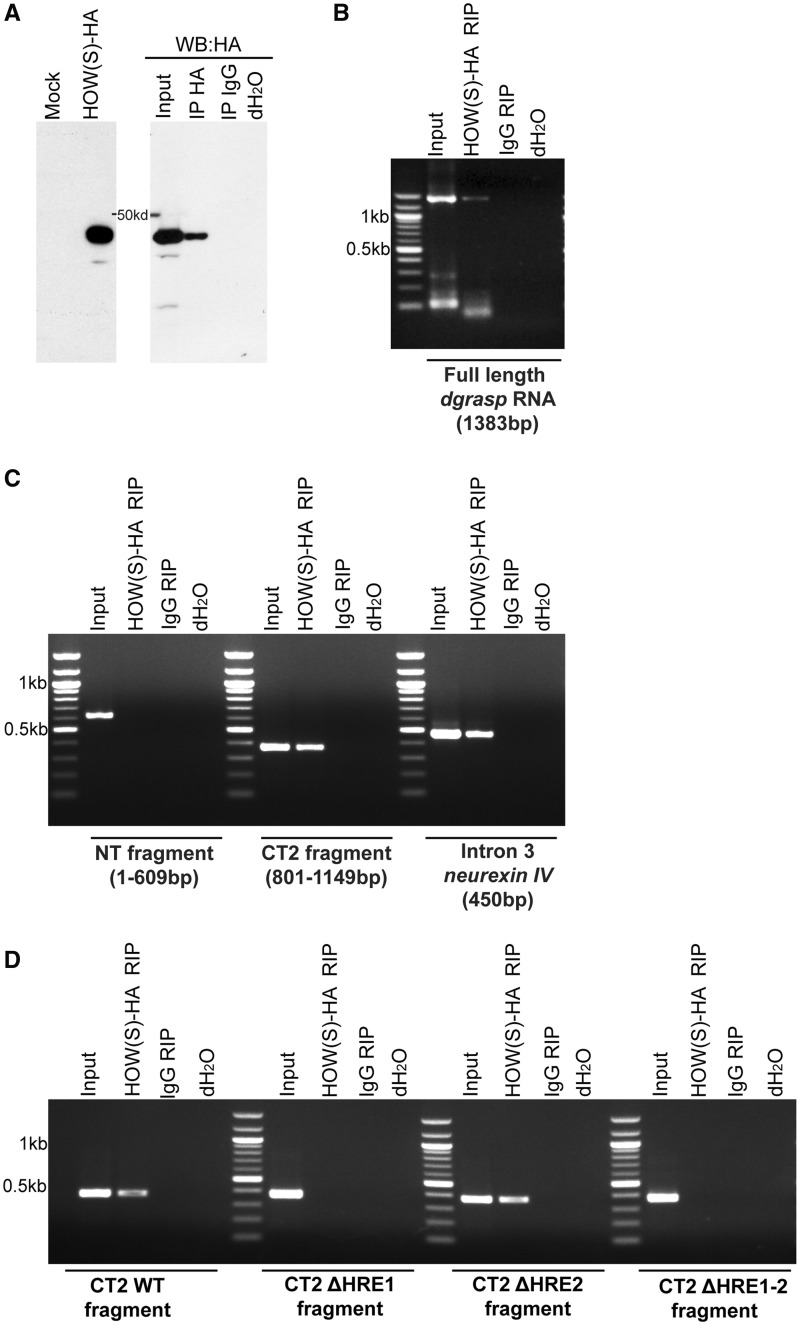
As HOW(S) contains a conserved KH RNA-binding domain, it was predicted to bind dgrasp directly. To test this, in vitro translated HOW(S)-HA was incubated in presence of in vitro transcribed full-length dgrasp RNA (Figure 4A). The protein was immunoprecipitated using an anti-HA antibody followed by RT–PCR to detect bound dgrasp. As expected, IgG RIP did not lead to dgrasp mRNA amplification, whereas HA RIP showed that, indeed, HOW(S) binds dgrasp transcript directly (Figure 4B).
Figure 4.
HOW(S) binds dgrasp HRE1. (A) Western blot detection of in vitro-translated HOW(S)-HA upon its synthesis (left panel) and after immunoprecipitation using an anti-HA (HA IP) or IgG as control (IgG IP) (right panel). (B–D) Agarose gel electrophoresis of RT–PCR reactions from in vitro RIP assays. In-vitro synthesized RNAs—full-length dgrasp RNA (B); dgrasp NT (C); dgrasp CT2 (C); Neurexin IV intron 3 (C); CT2 ΔHRE1 (D), CT2 ΔHRE2 (D) and CT2 ΔHRE1-2 fragments (D)—were incubated with in-vitro synthesized HOW(S)-HA and immunoprecipitated using an anti-HA antibody [HOW(S)HA RIP] or the IgG (IgG RIP).
Next, we asked whether the HREs that we identified (Figure 1B) were required for HOW(S) binding to dgrasp mRNA. To test this, we carried out in vitro synthesis of two dgrasp mRNA fragments, one encompassing the region where the two HREs were located (CT2), and the second corresponding to the N-terminus (NT) that did not contain any HREs (Figure 1B). Using the same approach as described in the previous paragraph, we showed that HOW(S)-HA specifically bound CT2 but not NT (Figure 4C), indicating that the HREs are likely to play a functional role in binding.
To confirm this finding and to assess which of the two predicted HREs are required for HOW(S) binding, CT2 fragment mutant isoforms, where either or both of the predicted HREs replaced by a string of Us (CT2-ΔHRE1, CT2-ΔHRE2 and CT2-ΔHRE1−2) were synthesized in vitro as above.
Using two independent RNA structure prediction programs [RNApromo (Segal lab) and RNAalifold (Vienna)], we first checked that this substitution does not disrupt the loop structure in which the HREs are located (Supplementary Figure S3C). We then tested the interaction of these fragments with purified HOW(S)-HA. As predicted, HOW(S)-HA did not interact with CT2-ΔHRE1-2, but still interacted with CT2-ΔHRE2 (Figure 4D), suggesting that only one of the HREs (HRE1, CCTAAC, 872–881nt, Figure 1B) is functional and recognized by HOW(S) for binding.
Loss of HOW expression affects dgrasp mRNA stability
To investigate the function of HOW binding to dgrasp mRNA in the follicular epithelium, we induced homozygous mutant clones carrying the strong loss of function allele HOWstru-3R-3 in this tissue (49) (see ‘Materials and Methods’ section).
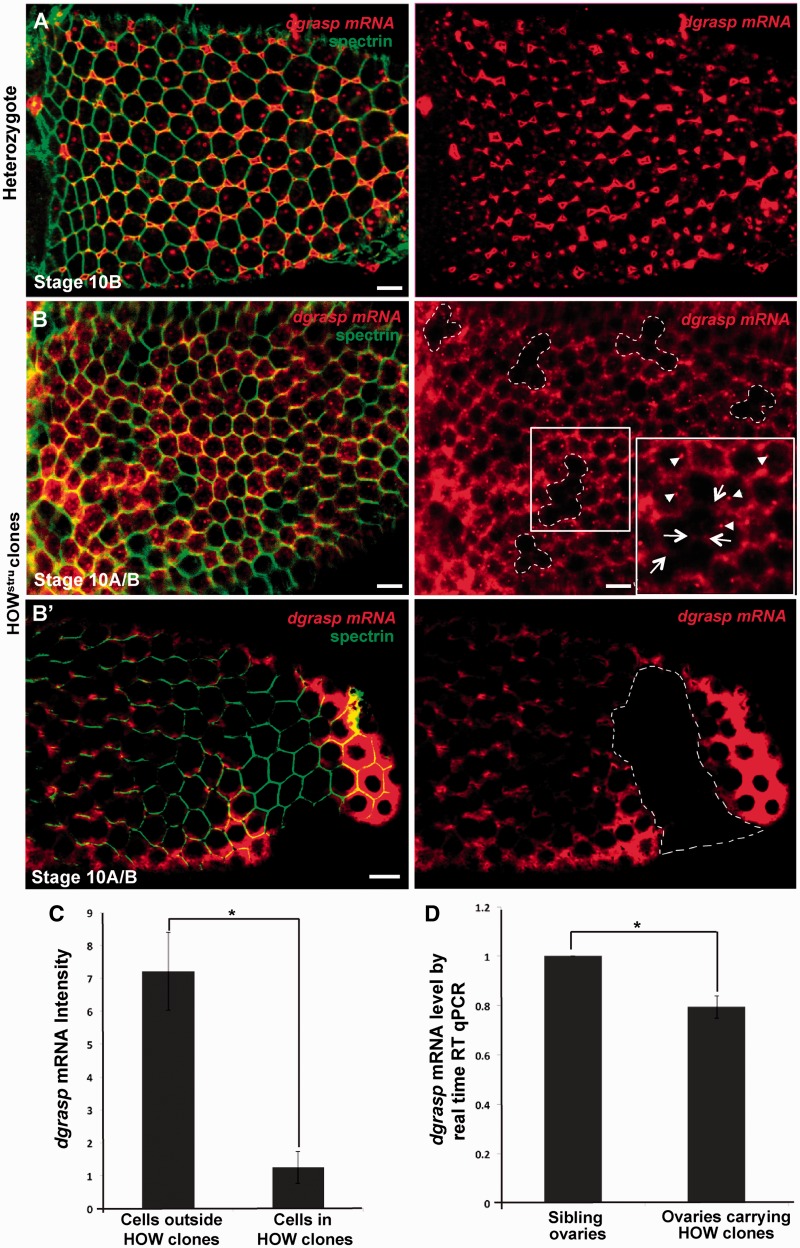
In control ovaries (siblings of the same background but not expressing the flippase) as well as in the heterozygous tissue surrounding the HOWstru-3R-3 mutant clones, the expression of dgrasp and its localization is comparable to WT and with clear targeting to the open ZOC (Figure 5A and B) as well as cytoplasmic foci corresponding to RNPs (arrowheads in Figure 5B, insert) (13). In HOWstru-3R-3 mutant clones, however, the levels of dgrasp mRNA are reduced ∼7-fold when compared with the neighbouring heterozygote cells (Figure 5B, C). We confirmed this reduction by real-time RT–qPCR on single stage 10 egg chambers. In those in which HOW mutant clones were induced, dgrasp levels were reduced by 21% when compared with control egg chambers (Figure 5D; n = 6).
Figure 5.
HOW regulates the stability of dgrasp mRNA. (A–B′) En face view of the FISH localization of dgrasp mRNA (red) in the follicular epithelium at stage 10B of siblings non-expressing the flippase (n = 7 ovaries) (A), and these displaying HOWstru-3R-3 clones (outlined with dashed lines, n = 13 ovaries). The plasma membrane is outlined by immunolabelling with α-spectrin (green). (C) Quantification by imageJ of dgrasp FISH intensity in the HOW clones and the neighbouring cells. Bars indicate standard error. Fold change of dgrasp mRNA expression between control and HOWstru-3R-3 mutants is significant (n = 20, *P < 0.001). (D) Real-time RT–qPCR analysis of dgrasp mRNA levels in HOWstru-3R-3 mutant and control stage 10 single oocytes. Bars indicate standard error. The change in dgrasp mRNA levels between control and HOWstru-3R-3 mutants is significant *P < 0.001. Scale bars: 10 µm.
The loss of dgrasp mRNA in HOWstru-3R-3 clones suggests that HOW is required for dgrasp mRNA stability, either for the formation of RNPs protecting the RNA against degradation, or for its targeting to the open ZOC. Close examination of the HOWstru-3R-3 clones showed that dgrasp RNPs were completely absent in the HOWstru-3R-3 clones and that when a faint dgrasp mRNA pool was observed, it was targeted to the plasma membrane (arrows in Figure 5B, insert). This suggests that HOW might be required for RNP formation leading, in turn, to dgrasp stabilization.
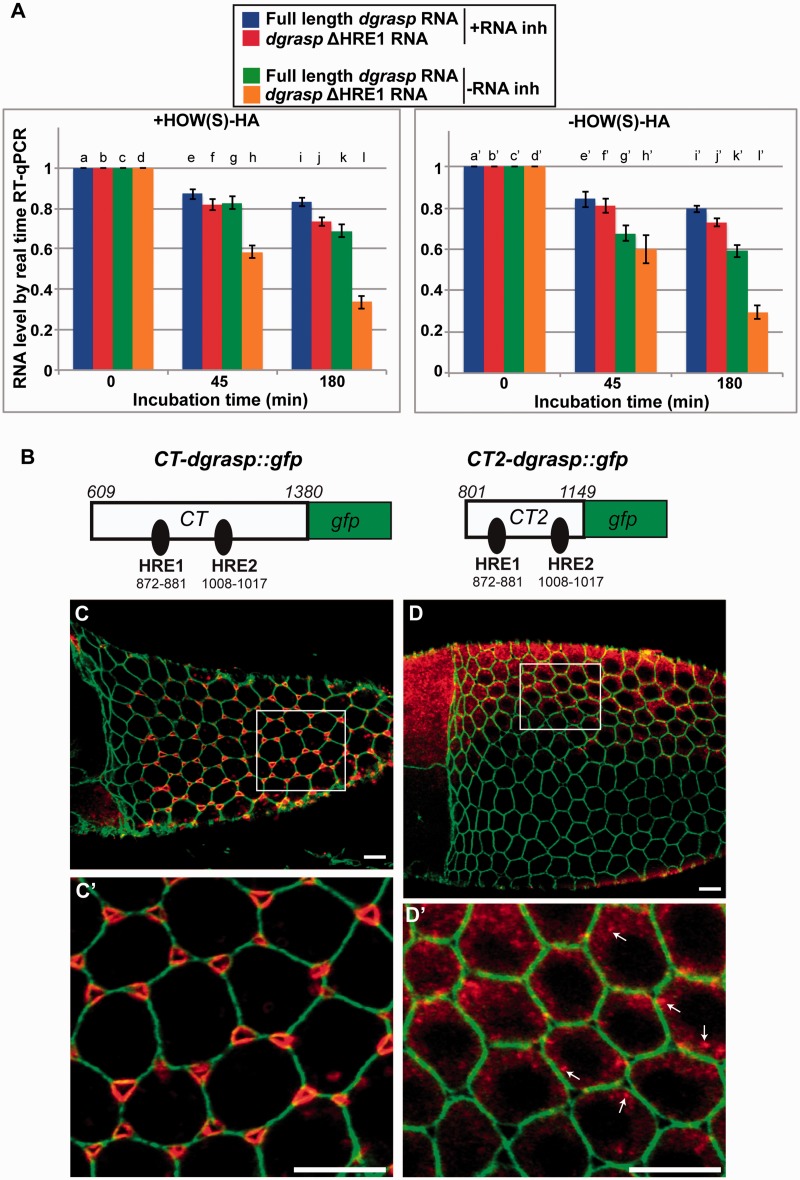
To test this hypothesis, we designed an in vitro degradation assay to compare the stability of full-length in vitro transcribed dgrasp RNA with that of a form of dgrasp where HRE1 was mutated (dgrasp-ΔHRE1). These two RNAs were incubated with HOW(S)-HA and successively exposed to Caco-2 cell lysate extract as a source of RNases for an increasing length of time (0, 45 and 180 min), both in the presence and absence of RNase inhibitors. Real-time RT–qPCR was used to measure the amount of RNA left in each sample. As expected, in presence of RNase inhibitors, both RNAs were similarly stable overtime (Figure 6A, lanes a, b, e, f, l and j). However, in absence of RNase inhibitors, dgrasp-ΔHRE1 mRNA was degraded more rapidly than that of full-length dgrasp (63% when compared with 30% after 180 min incubation; P < 0.05 c versus k; P < 0.001 d versus l; and P < 0.001 k versus l) (Figure 6A, lanes c, d and k, l). This shows that the presence of the HRE1 is necessary for the stability of the dgrasp mRNA.
Figure 6.
HRE1 is sufficient to mediate dgrasp RNA stability but not targeting to the open ZOC. (A) Time course degradation assay of full-length dgrasp and dgrasp-ΔHRE1 RNAs measured by real-time RT–qPCR. Total RNA was isolated at the indicated times (0, 45 and 180 min). The values shown are averages ± SD of three independent experiments performed in duplicate. (B–C′) Fluorescent in situ hybridization of CT-dgrasp::gfp (B) and CT2-dgrasp::gfp (C) fragments using anti-sense GFP RNA probe (red) in the follicular epithelium of stage 10B transgenic ovaries overexpressing either of the two dgrasp mRNA fragments. Plasma membrane is outlined by immunolabelling with α-spectrin (green). B′ and C′ are insets of C and D, respectively. Note CT2-dgrasp::gfp fragment is expressed in the follicular epithelium, but does not localize to the open ZOC. Scale bars: 10 µm.
We then assessed the protective role of HOW(S) by performing the same experiment in the absence of exogenous HOW(S)-HA (Figure 6A, lane a′–l′). Both full-length dgrasp and dgrasp-ΔHRE1 RNAs showed a similar degradation rate in presence of RNase inhibitors, whereas, in the absence of inhibitors, dgrasp-ΔHRE1 RNA was again degraded much faster compared with full-length dgrasp (compare lane k′ and l′). However, full-length dgrasp RNA appeared to be degraded slightly faster at 45 and 180 min in the absence of HOW(S) than in its presence [compare lane g versus g′ (P < 0.06) and k versus k′], suggesting a role for HOW(S) in binding HRE1 in dgrasp RNA stability. However, this effect as measured in this assay is small probably because Caco-2 cell extracts act as a source of QKI, the mammalian homologue of HOW. Given the highly conserved KH domain, we argue that QKI can bind HRE1, therefore providing stability to the RNA even in the absence of exogenous HOW(S).
Last, an attractive hypothesis is that HOW, in addition to its role in dgrasp mRNA protection, is also involved in its targeting to the open ZOC. To test whether HOW binding is sufficient for dgrasp mRNA targeting, we examined the localization of the GFP-tagged dgrasp CT2 RNA fragment that contained HRE1 (and HRE2) and was shown to bind HOW(S) (Figures 4C, D and 6B), and compared it with the targeting of a fragment encoding the entire C-terminal (CT-dgrasp::GFP, Figure 6B). Whereas this latter fragment recapitulates the same dynamics and open ZOC localization as the endogenous dgrasp transcript and dgrasp-GFP full length (Figure 6C and C′) (13), CT2-dgrasp::GFP mRNA forms particles in the cytoplasm (arrows in Figure 6D and D′) but does not efficiently localize to the open ZOCs (Figure 6D and D′). This suggests that HOW binding to HRE1 is not sufficient for dgrasp targeting.
In conclusion, we demonstrate that HOW(S) directly binds to one HRE (HRE1) present in the ORF of dgrasp mRNA in the cytoplasm of follicle cells at stage 10. There, HOW mediates dgrasp mRNA stability by forming RNPs that are then transported to the open ZOC through the interaction with other transacting factors, yet to be identified.
DISCUSSION
Here, we show that in the Drosophila follicular epithelium dgrasp mRNA directly interacts with HOW, a STAR family RBP, via HRE1 situated in the C-terminus of its ORF. This element is one of two HREs predicted using the RBPDB, and confirmed using the C. elegans matrix generated by the Ciosk’ laboratory (45). That a C. elegans matrix identifies the same motifs corroborates that the sequence making up these sites is conserved throughout evolution and is consistent with the significant sequence conservation of the KH domain of the STAR proteins QKI, HOW and GLD-1.
The functional HRE we characterized, is situated in the dgrasp ORF, and not in the 3′UTR as classically reported for QKI/GLD-1 targets. Interestingly, the C. elegans homologue of dgrasp mRNA (Y42H9AR.1) also displays two predicted HREs in its ORF, suggesting that this feature is conserved. RBPs binding mRNAs in the ORF (including members of the STAR family) are an emerging trend in RNA metabolism with roles in translational repression, transport, as well as stabilization (50). The study of these new targets will open new avenues in the understanding of how RNA metabolism is regulated. In this respect, a crucial step is to determine whether a functional biological bias exists between the bindings of RBPs in the ORF versus the UTRs of a transcript.
HOW, GLD-1 and QKI, have generally been considered to be either translational repressors when bound to the 5′ and 3′UTRs of their targets (45,51) or to modulate alternative splicing when bound to introns (34,52). Here, we show that loss of HOW expression in the follicular epithelium results in dgrasp degradation, in favour of a role for HOW in stabilization. This is in agreement with an emerging role for the proteins of this family in mRNA stabilization. For instance, zebrafish QKI modulates Hedgehog signalling during muscle-fibre maturation by stabilizing of Gli2a mRNA upon binding to the 3′UTR (21). Furthermore, the C. elegans GLD-1 is not only a translational repressor but has also been shown to protect its targets during their transport to specific cellular compartments, thus ensuring that they are sufficiently abundant to sustain robust local translation (50). Similarly, during oligodendrocyte differentiation, p27kip mRNA is stabilized upon the binding of QKI7 (53). Interestingly, the QRE in p27kip mRNA also resides in the ORF, as in the case of dgrasp mRNA.
As for its orthologues, HOW proteins display multiple isoforms and each one displays a different localization. These isoforms are almost identical in their amino acid sequence, and the only difference resides at the C-terminus, which results in the presence of a NLS in the HOW(L) protein (33). Consistently, our immunohistochemical data show that the longer isoform is restricted to the nucleus, whereas HOW(S) is found in the cytoplasm. A body of evidence from different studies, including this one suggests that although both HOW(L) and HOW(S) proteins are capable of recognizing and binding the same RNA sequence in vitro, their RNA substrates seem to differ (30,34,37). In this respect, we show here that the HREs in Neurexin IV intron 3 are recognized by both HOW isoforms in vitro, but only to HOW(L) in vivo. Hence, we propose the different sub-cellular distribution of these HOW isoforms might outline a separation of function and define the substrate specificity.
HOW(S) is also present in punctae near to the basal plasma membrane of the follicle cells at the stage of oogenesis at which dgrasp mRNA forms RNPs and is targeted. Accordingly, loss of HOW function leads to the sharp decrease in the amount of dgrasp mRNA and the disappearance of the RNPs. One possibility is that HOW is required for dgrasp mRNA transport to the open ZOC, perhaps by recruiting motors or adaptors, the absence of which would in turn leads to the mRNA instability. However, although HOW binding to HRE1 is sufficient to form RNPs, it is not sufficient to mediate dgrasp localization to the open ZOC. Conversely, we show that HOW binding to HRE1 provides stability to dgrasp RNA, perhaps by masking it against degradative activities.
We propose the model according to which HOW(S) binding to dgrasp HRE1 in the cytoplasm of stage 10 follicle cells regulates the stability of the dgrasp transcripts. When HOW is bound to HRE1, it may recruit a RBP complex that is bound either to the 5′ or 3′UTRs of the dgrasp mRNA. This complex may in turn prevent the recruitment of the RNA degradation machinery (de-anylation or decapping factors), by hiding signatures that are recognized by RNase complexes. This remains to be investigated.
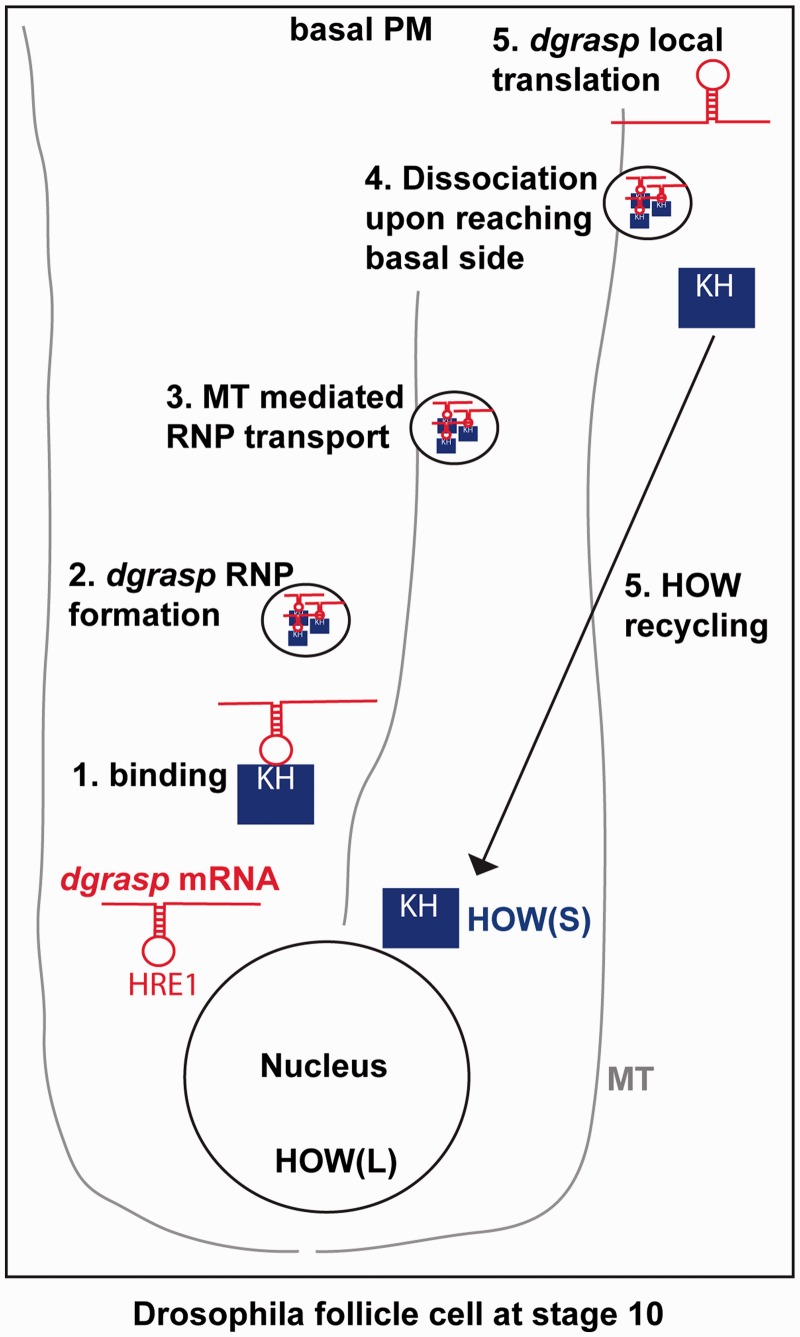
HOW(S) is then perhaps also involved (although not sufficient) in the transport of dgrasp RNPs near the basal membrane in a microtubule-dependent manner (Figure 7). Accordingly, microtubule depolymerization leads RNPs not been targeted to the open ZOC (not shown). Following dgrasp localization, HOW(S) would be released to allow dgrasp mRNA local translation. The release of HOW interaction is a necessary step as HRE1 resides in the dgrasp ORF and would therefore interfere with translation.
Figure 7.
Schematic representation of the role of HOW(S) in dgrasp metabolism. Following transcription and nuclear metabolic processes (splicing, 5′-capping and polyadenylation) in stage 10 Drosophila follicle cells. The dgrasp transcripts are exported into the cytoplasm. There, HOW(S) binds the dgrasp mRNA at the HRE1 site via its maxi-KH domain (1) to form together with other factors an RNP that protects dgrasp mRNA against degradation (2). dgrasp RNPs are then transported near the basal plasma membrane via microtubules (3). Upon reaching destination, dgrasp RNPs dissociate (4), HOW(S) is recycled back to the cytoplasm (5) whereas dgrasp mRNA is anchored and gets locally translated (5).
HOW loss of function leads to a strong disorganization of the follicular epithelium in line with a role for dGRASP in the delivery of alphaPS1 integrins (13). However, the defects are much stronger because integrins are also a target of HOW, consistent with the up-regulation of mew mRNA at the stage 10B (13). It is interesting to note that in this tissue, both the substrate and the machinery are targets of the same RBP. Whether dgrasp mRNA metabolism is also regulated by HOW in other tissues and whether HOW is required for the stability of other mRNAs (including those with HRE in the ORF) remains to be investigated.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online, including [34].
FUNDING
F.G. and G.G. were supported by ZonMW TOP subsidie [912.080.24 to C.R.]; ISF grant no. [71/12 to T.V.]. Funding for open access charge: ZonMW TOP subsidie [912.080.24], The Netherlands.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors thank the members of the Rabouille laboratory for discussion and Adam Grieve, Dominic Grun, Bernardo Blanco-Sanchez and Nerys Williams for critically reading the manuscript; Rafal Ciosk for the use of his GLD-1 matrix prior to publication; Leena Karhinen for generating the transgenic lines carrying the GFP tagged dgrasp fragments; Roberto Magliozzi for his help and suggestions on establishing the in vitro binding assay. We acknowledge the use of flybase (http://flybase.org/) and the use of Hubrecht Imaging facility (http://www.hubrecht.eu/information/imagingcenter.html) for microscopy.
REFERENCES
- 1.Glisovic T, Bachorik J, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS lett. 2008;582:1977–1986. doi: 10.1016/j.febslet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lasko P. Gene regulation at the RNA layer: RNA binding proteins in intercellular signaling networks. Sci. STKE. 2003;2003:RE6. doi: 10.1126/stke.2003.179.re6. [DOI] [PubMed] [Google Scholar]
- 3.Wu X, Brewer G. The regulation of mRNA stability in mammalian cells: 2.0. Gene. 2012;500:10–21. doi: 10.1016/j.gene.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnstone O, Lasko P. Translational regulation and RNA localization in Drosophila oocytes and embryos. Annu. Rev. Genet. 2001;35:365–406. doi: 10.1146/annurev.genet.35.102401.090756. [DOI] [PubMed] [Google Scholar]
- 5.Tadros W, Westwood JT, Lipshitz HD. The mother-to-child transition. Dev. Cell. 2007;12:847–849. doi: 10.1016/j.devcel.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136:719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tadros W, Lipshitz HD. Setting the stage for development: mRNA translation and stability during oocyte maturation and egg activation in Drosophila. Dev. Dyn. 2005;232:593–608. doi: 10.1002/dvdy.20297. [DOI] [PubMed] [Google Scholar]
- 8.Besse F, Ephrussi A. Translational control of localized mRNAs: restricting protein synthesis in space and time. Nat. Rev. Mol. Cell Biol. 2008;9:971–980. doi: 10.1038/nrm2548. [DOI] [PubMed] [Google Scholar]
- 9.Meignin C, Davis I. Transmitting the message: intracellular mRNA localization. Curr. Opi. Cell Biol. 2010;22:112–119. doi: 10.1016/j.ceb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Vanzo N, Oprins A, Xanthakis D, Ephrussi A, Rabouille C. Stimulation of endocytosis and actin dynamics by Oskar polarizes the Drosophila oocyte. Dev. Cell. 2007;12:543–555. doi: 10.1016/j.devcel.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Stitzel ML, Seydoux G. Regulation of the oocyte-to-zygote transition. Science. 2007;316:407–408. doi: 10.1126/science.1138236. [DOI] [PubMed] [Google Scholar]
- 12.Zimyanin V, Lowe N, St Johnston D. An Oskar-dependent positive feedback loop maintains the polarity of the Drosophila oocyte. Curr. Biol. 2007;17:353–359. doi: 10.1016/j.cub.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schotman H, Karhinen L, Rabouille C. dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev. Cell. 2008;14:171–182. doi: 10.1016/j.devcel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Schotman H, Karhinen L, Rabouille C. Integrins mediate their unconventional, mechanical-stress-induced secretion via RhoA and PINCH in Drosophila. J. Cell Sci. 2009;122:2662–2672. doi: 10.1242/jcs.039347. [DOI] [PubMed] [Google Scholar]
- 15.Vinke F, Grieve A, Rabouille C. The multiple facets of the Golgi reassembly stacking proteins. Biochem. J. 2011;433:423–433. doi: 10.1042/BJ20101540. [DOI] [PubMed] [Google Scholar]
- 16.Rabouille C, Malhotra V, Nickel W. Diversity in unconventional protein secretion. J. Cell Sci. 2012;125:5251–5255. doi: 10.1242/jcs.103630. [DOI] [PubMed] [Google Scholar]
- 17.Paronetto MP, Messina V, Bianchi E, Barchi M, Vogel G, Moretti C, Palombi F, Stefanini M, Geremia R, Richard S, et al. Sam68 regulates translation of target mRNAs in male germ cells, necessary for mouse spermatogenesis. J. Cell Biol. 2009;185:235–249. doi: 10.1083/jcb.200811138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki K, Zagoren JC. Quaking mouse: an ultrastructural study of the peripheral nerves. J. Neurocytol. 1977;6:71–84. doi: 10.1007/BF01175415. [DOI] [PubMed] [Google Scholar]
- 19.Ebersole T, Chen Q, Justice M, Artzt K. The quaking gene product necessary in embryogenesis and myelination combines features of RNA binding and signal transduction proteins. Nat. Genet. 1996;12:260–265. doi: 10.1038/ng0396-260. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Reed R, Grabowski P, Artzt K. Function of quaking in myelination: regulation of alternative splicing. Proc. Natl Acad. Sci. USA. 2002;99:4233–4238. doi: 10.1073/pnas.072090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobbardi R, Lambert G, Zhao J, Geisler R, Kim H, Rosa F. Fine-tuning of Hh signaling by the RNA-binding protein Quaking to control muscle development. Development. 2011;138:1783–1794. doi: 10.1242/dev.059121. [DOI] [PubMed] [Google Scholar]
- 22.Jan E, Motzny C, Graves L, Goodwin E. The STAR protein, GLD-1, is a translational regulator of sexual identity in Caenorhabditis elegans. EMBO J. 1999;18:258–269. doi: 10.1093/emboj/18.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong J, Verheyden J, Kimble J. Cyclin E and Cdk2 control GLD-1, the mitosis/meiosis decision, and germline stem cells in Caenorhabditis elegans. PLoS Genet. 2011;7:e1001348. doi: 10.1371/journal.pgen.1001348. doi:10.1371/journal.pgen.1001348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohno G, Hagiwara M, Kuroyanagi H. STAR family RNA-binding protein ASD-2 regulates developmental switching of mutually exclusive alternative splicing in vivo. Gene Dev. 2008;22:360–374. doi: 10.1101/gad.1620608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baehrecke E. who encodes a KH RNA binding protein that functions in muscle development. Development. 1997;124:1323–1332. doi: 10.1242/dev.124.7.1323. [DOI] [PubMed] [Google Scholar]
- 26.Fyrberg C, Becker J, Barthmaier P, Mahaffey J, Fyrberg E. A Drosophila muscle-specific gene related to the mouse quaking locus. Gene. 1997;197:315–323. doi: 10.1016/s0378-1119(97)00278-3. [DOI] [PubMed] [Google Scholar]
- 27.Lo P, Frasch M. A novel KH-domain protein mediates cell adhesion processes in Drosophila. Dev. Biol. 1997;190:241–256. doi: 10.1006/dbio.1997.8699. [DOI] [PubMed] [Google Scholar]
- 28.Zaffran S, Astier M, Gratecos D, Sémériva M. The held out wings (how) Drosophila gene encodes a putative RNA-binding protein involved in the control of muscular and cardiac activity. Development. 1997;124:2087–2098. doi: 10.1242/dev.124.10.2087. [DOI] [PubMed] [Google Scholar]
- 29.Edenfeld G, Volohonsky G, Krukkert K, Naffin E, Lammel U, Grimm A, Engelen D, Reuveny A, Volk T, Klambt C. The splicing factor crooked neck associates with the RNA-binding protein HOW to control glial cell maturation in Drosophila. Neuron. 2006;52:969–980. doi: 10.1016/j.neuron.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Reuveny A, Elhanany H, Volk T. Enhanced sensitivity of midline glial cells to apoptosis is achieved by HOW(L)-dependent repression of Diap1. Mech. Dev. 2009;126:30–41. doi: 10.1016/j.mod.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Volk T. Drosophila star proteins. In: Volk T, Artzt K, editors. Post-Transcriptional Regulation by STAR Proteins: Control of RNA Metabolism in Development and Disease. Springer; 2010. USA Adv. Exp. Med. Biol., 693, 93–105. [Google Scholar]
- 32.Helit N-R, Hila T-K, Volohonsky G, Volk T. Cell divisions in the Drosophila embryonic mesoderm are repressed via posttranscriptional regulation of string/cdc25 by HOW. Curr. Biol. 2005;15:295–302. doi: 10.1016/j.cub.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 33.Sette C. Post-translational Regulation of STAR Proteins and Effects on Their Biological Functions. In: Volk T, Artzt K, editors. Post-Transcriptional Regulation by STAR Proteins: Control of RNA Metabolism in Development and Disease. Springer; 2010. USA. Adv. Exp. Med. Biol., 693, 54–66. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues F, Thuma L, Klambt C. The regulation of glial-specific splicing of Neurexin IV requires HOW and Cdk12 activity. Development. 2012;139:1765–1776. doi: 10.1242/dev.074070. [DOI] [PubMed] [Google Scholar]
- 35.Cook K, Kazan H, Zuberi K, Morris Q, Hughes T. RBPDB: a database of RNA-binding specificities. Nucleic Acids Res. 2011;39:D301–D308. doi: 10.1093/nar/gkq1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golic K, Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- 37.Volohonsky G, Edenfeld G, Klambt C, Volk T. Muscle-dependent maturation of tendon cells is induced by post-transcriptional regulation of stripeA. Development. 2007;134:347–356. doi: 10.1242/dev.02735. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Ebata A, Dong Y, Rizki G, Iwata T, Lee S. Caenorhabditis elegans HCF-1 functions in longevity maintenance as a DAF-16 regulator. PLoS Biol. 2008;6 doi: 10.1371/journal.pbio.0060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Agy M, Sherbert C, Katze M. Development of an in vitro mRNA degradation assay utilizing extracts from HIV-1- and SIV-infected cells. Virology. 1996;217:158–166. doi: 10.1006/viro.1996.0103. [DOI] [PubMed] [Google Scholar]
- 41.Krikorian C, Read G. In vitro mRNA degradation system to study the virion host shutoff function of herpes simplex virus. J. Virol. 1991;65:112–122. doi: 10.1128/jvi.65.1.112-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rio D, Ares M, Hannon G, Nilsen T. Purification of RNA using TRIzol. Cold Spring Harb. Protoc. 2010;2010 doi: 10.1101/pdb.prot5439. pdb.prot5439. doi: 10.1101/pdb.prot543. [DOI] [PubMed] [Google Scholar]
- 43.Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 44.Masuda A, Andersen H, Doktor T, Okamoto T, Ito M, Andresen B, Ohno K. CUGBP1 and MBNL1 preferentially bind to 3′ UTRs and facilitate mRNA decay. Sci. Rep. 2012;2:209. doi: 10.1038/srep00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright J, Gaidatzis D, Senften M, Farley B, Westhof E, Ryder S, Ciosk R. A quantitative RNA code for mRNA target selection by the germline fate determinant GLD-1. EMBO J. 2011;30:533–545. doi: 10.1038/emboj.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.H N-R, Dorevitch N, Reuveny A, Volk T. The balance between two isoforms of the Drosophila RNA-binding protein how controls tendon cell differentiation. Mol. Cell. 1999;4:573–584. doi: 10.1016/s1097-2765(00)80208-7. [DOI] [PubMed] [Google Scholar]
- 47.H N-R, Volohonsky G, Reuveny A, R Z-B, Volk T. Two isoforms of the Drosophila RNA binding protein, how, act in opposing directions to regulate tendon cell differentiation. Dev. Cell. 2002;2:183–193. doi: 10.1016/s1534-5807(01)00118-6. [DOI] [PubMed] [Google Scholar]
- 48.Phelps CB, Brand AH. Ectopic gene expression in Drosophila using GAL4 system. Methods. 1998;14:367–379. doi: 10.1006/meth.1998.0592. [DOI] [PubMed] [Google Scholar]
- 49.Prout M, Damania Z, Soong J, Fristrom D, Fristrom J. Autosomal mutations affecting adhesion between wing surfaces in Drosophila melanogaster. Genetics. 1997;146: 275–285. doi: 10.1093/genetics/146.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheckel C, Gaidatzis D, Wright J, Ciosk R. Genome-wide analysis of GLD-1-mediated mRNA regulation suggests a role in mRNA storage. PLoS Genet. 2012;8:e1002742. doi: 10.1371/journal.pgen.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jungkamp A-C, Stoeckius M, Mecenas D, Grun D, Mastrobuoni G, Kempa S, Rajewsky N. In vivo and transcriptome-wide identification of RNA binding protein target sites. Mol. Cell. 2011;44:828–840. doi: 10.1016/j.molcel.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novikov L, Park J, Chen H, Klerman H, Jalloh A, Gamble M. QKI-mediated alternative splicing of the histone variant MacroH2A1 regulates cancer cell proliferation. Mol. Cell. Biol. 2011;31:4244–4255. doi: 10.1128/MCB.05244-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larocque D, Galarneau A, Liu H-N, Scott M, Almazan G, Richard S. Protection of p27(Kip1) mRNA by quaking RNA binding proteins promotes oligodendrocyte differentiation. Nat. Neurosci. 2005;8:27–33. doi: 10.1038/nn1359. [DOI] [PubMed] [Google Scholar]