Abstract
Toll-like receptor-4 (TLR4) is a sentinel pathogen recognition receptor with a pivotal role in inflammation, tissue injury, diabetes and its complications. The aim of the study was to examine the contribution of TLR4 expression and activation to the prolonged inflammation observed in diabetic wounds. Diabetes was induced in male C57BL/6J and TLR4 knockout (KO) mice using streptozotocin (STZ) with matching non-diabetic mice as control. After 2 weeks of persistent hyperglycemia in the mice, full-thickness excision wounds were made on the backs aseptically. Total RNA and protein were subjected to real-time PCR and western blot analyses. Wound sizes were measured using digital planimetry. TLR4 mRNA and protein expression increased significantly in wounds of diabetic mice compared with non-diabetic mice (P<0.05). IL-6, TNF-α concentration and nuclear factor-κB (NF-κB) activation were increased in diabetic wounds compared to non-diabetic wounds and knockout of TLR4 alleviates wound healing and decreases inflammation in diabetic TLR4 KO mice. Collectively, our findings show that increased TLR4 mRNA and protein expression and activation contribute to the prolonged inflammation in the diabetic wounds and that absence of TLR4 may result in decreased inflammation and improved wound healing.
Keywords: TLR4, Inflammation, Diabetes Mellitus, Wound healing
Introduction
Around 348 million people worldwide and 36 million in the United States are affected by diabetes mellitus (DM) and 40–60% of these patients develop foot wounds, which account for more than 20% of all hospitalizations equating to one amputation every 30 s (1–3). Impaired wound healing derives from many pathophysiological derangements and among them is the imbalance in pro-/anti-inflammatory mediators thought to result in persistent uncontrolled inflammation (4,5). Recent data suggests that toll-like receptors (TLRs: predominant innate immune receptors) present on immunomodulatory cells are the key initiators of this inflammatory response facilitating the recognition of microbial, viral, and endogenous components and interacting with ligands generated at sites of injury (6,7). Among the TLRs, TLR4 plays a critical role in the pathogenesis of insulin resistance and inflammation in DM, under both experimental and clinical conditions (8,9). Published studies show that TLR4 expression, activation, and signaling are significantly increased in monocytes of DM patients, non-obese diabetic (NOD), and db/db mice (8). We have shown that knockout of TLR4 in diabetic mice attenuates inflammation as manifest by circulating cytokine/chemokines and macrophage biomarkers of inflammation (10). However, it is not known if TLR4 expression and activation contributes to the prolonged inflammation and impaired healing seen in DM. Thus, we examined TLR4 expression and activity in streptozotocin (STZ) induced diabetic mice with excisional full thickness wounds to determine its role using TLR4 knockout (TLR4−/−) mice.
Materials and Methods
Animals and Full thickness wound model
TLR4 knock out (TLR4−/−; male; 10 week age) and the C57BL/6J genetic background strain mice (WT) were maintained in the vivarium as previously described (10). All the mice were housed in pathogen-free filtered air rooms, were fed sterile standard chow/water ad libitum, and kept in individual barrier cages with daily autoclaved bedding changes. DM was induced using STZ as described previously (10, 11). Two weeks after persistent hyperglycemia, two full thickness cutaneous wounds (6mm size) were inflicted on mice in sterile conditions as reported (11). Non-DM mice with wounds served as controls. The wounds were digitally imaged to determine the size of the surface area on day 0 and day 10 (11). All the animal protocols were approved by the IACUC, University of California at Davis. Serum glucose levels were determined using a glucose hexokinase assay (Sigma) (11).
Real time RT-PCR, Western Blotting, and ELISA
Total RNA and protein were isolated from snap frozen wound tissues and used for real time PCR (TLR4 and 18s mRNA using commercial sequence-specific primers and probes purchased from SA Biosciences, Gaithersburg, MD, USA), Western blot (TLR4 [Imgenix, USA] and β-actin [Santa Cruz, USA]), and ELISA (IL-6 and TNF-α, R&D systems) assays as previously reported (10,11). Nuclear proteins were isolated from wound tissues and NF-κB transcription factor activation was determined as reported earlier (Active Motif, Carlsbad, CA, USA) (11). Briefly, 5 μg of nuclear extract were added to each NF-κB consensus-binding site oligonucleotide-precoated well. A primary antibody specific for an epitope (p65) on the bound and active form of the transcription factor was then added, followed by subsequent incubation with secondary antibody. Color was developed with the use of developing solution and stopped. Absorbance was read at 450nm and normalized to milligram tissue protein. The intra- and inter-assay coefficient of variation for these assays was <7–10% (11).
Immunohistochemistry
Wounds were bisected for immunohistochemistry (IHC) analysis as reported previously (11). Briefly, tissues were collected, fixed, and processed by IHC staining protocol (11). The primary antibody for TLR4 (1:100 dilution) and MD2 (1:100) were raised in mouse and rabbit, respectively. Appropriate secondary antibodies (alexa 594, alexa 484), and IgG isotype controls were used (11). DAPI (1 μg/ml) was used to visualize nucleus. Images were taken using Nikon Eclipse E400 fluorescent microscope (11).
Statistical Analyses
Data are presented as mean ± SD. We used two-tailed t-tests or ANOVA with appropriate post hoc analyses when we compared more than 2 groups. P<0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism software (10).
Results
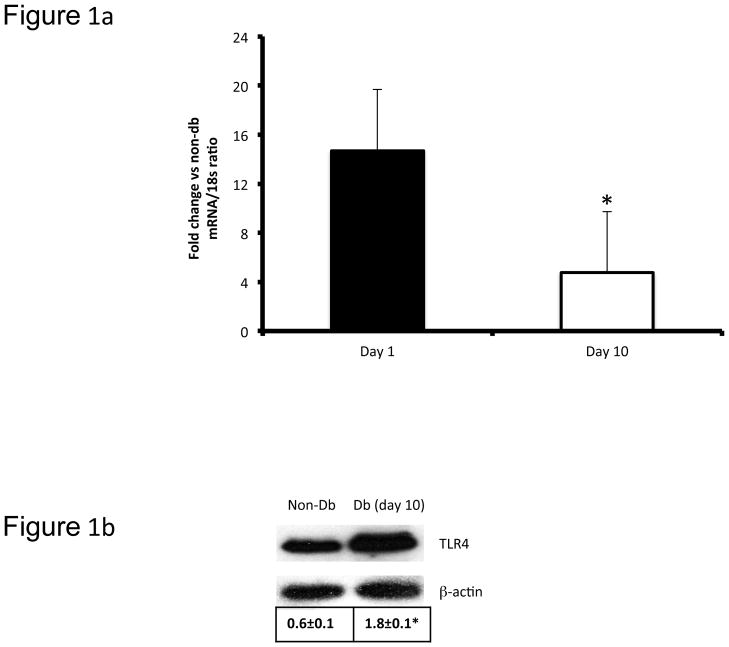
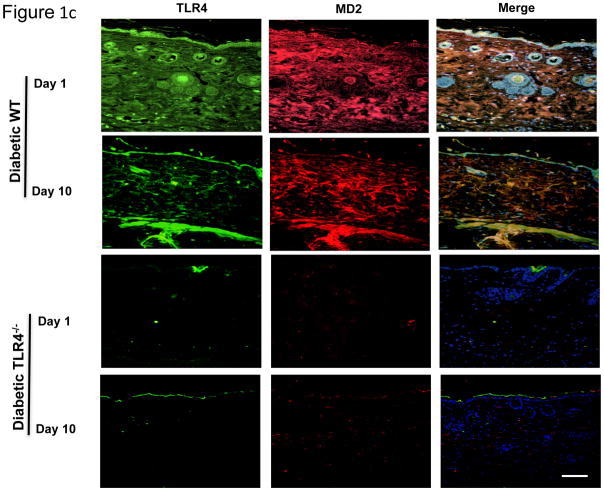
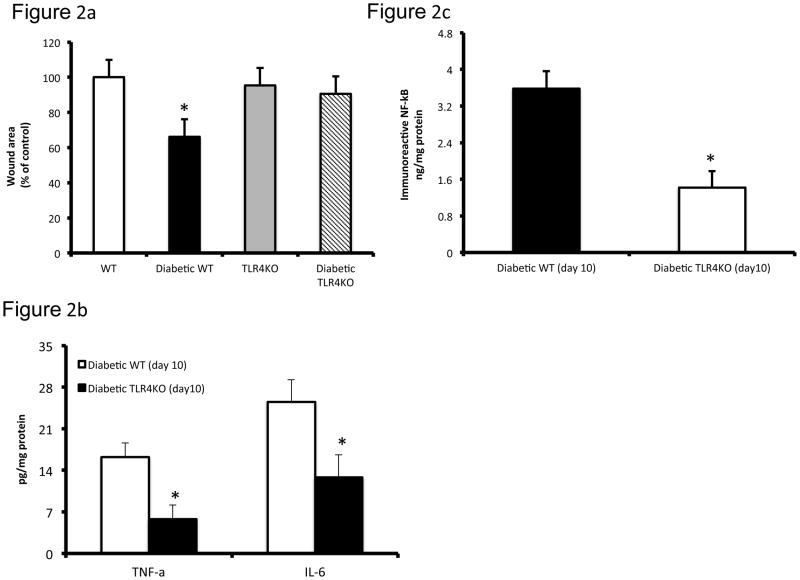
There was significant elevation in glucose levels (513±19 vs 101±10mg/dL; P<0.05) in diabetic mice. Since we have previously detailed relevant biomarkers of systemic inflammation and macrophage biology in these TLR4−/− diabetic mice (10), in this study, we focused on the pathobiology of diabetic wounds. We examined TLR4 mRNA and protein expression using real time RT-PCR and Western blot in day 10 wound tissues (Figure 1a, 1b). Both TLR4 mRNA and protein expressions were significantly increased in diabetic wounds compared to the non-diabetic wounds on day 10 (Figure 1a, 1b). TLR4 activation requires an accessory protein, myeloid differentiation factor 2 (MD2). Immunoflourescent staining of TLR4 (green) and MD2 (red) showed intense signal on days 1 and 10 in wound bed and wound edges in diabetic mice (Figure 1c) suggesting persistent TLR4 activation in the diabetic wounds. MD2 staining in the diabetic TLR4−/− mouse wound tissue expression was virtually absent on both days 1 and 10. Also, we did not observe appreciable signal in wounds of non-diabetic WT or TLR4−/− mice (data not shown). Next, we tested the effects of knockout of TLR4 on wound healing in diabetic mice since we have shown that TLR4 and its critical accessory protein is upregulated in diabetic wounds. Thus, we determined the wound closure in all four groups of mice at day 10 and expressed as the percent of wound area at day 0. Figure 2a depicts significantly improved wound closure in diabetic TLR4−/− mice compared with diabetic wild type (WT) mice, suggesting that absence of TLR4-mediated inflammation improves wound closure in diabetic mice. There were no differences observed in wound closure between non-diabetic WT or TLR4−/− mice (Figure 2a). To determine the functional significance of increased TLR4 expression in diabetic wounds, we measured the local IL-6 and TNF-α levels, both are accepted biomediators of TLR4 signaling (10). Figure 2b shows significantly increased concentrations of IL-6 and TNF-α in diabetic wound tissue compared with diabetic TLR4−/− mice at day 10 (P<0.005). Diabetic WT wounds had significantly increased nuclear NF-κB activity compared with non-diabetic wounds on day 10 (data not shown). However, compared with diabetic WT mice, there was a significant decrease in NF-κB activity in the diabetic TLR4−/− mice (Figure 2c; P<0.001).
Figure 1.
Figure 1a: TLR4 mRNA expression levels in diabetic mice wound tissues (6 mm) were determined using real-time PCR on day 1 and day 10 and expressed as ratio using 18s RNA as internal control. *P<0.05 compared with day 10 non-diabetic control (data not shown; n=8 mice per group).
Figure 1b: Representative Western blot showing the TLR4 protein expression in non-diabetic (non-Db) C57BL/6J and diabetic (Db) C57BL/6J mice wound tissues (6 mm). Ten days after injury, wound tissues were collected, lysed, and 20 μg protein was blotted for TLR4 and β-actin. Densitometric ratios (TRL4/β-actin) are indicated below. *P<0.05 vs day 10 non-diabetic control (n=4).
Figure 1c Co-localization of TLR4 (green) and MD2 (green) in wound tissues of diabetic WT and TLR4−/− mice on days 1 and 10 (6mm). Red fluorescence represents MD2, green fluorescence TLR4, whereas yellow represents the merge of both. White scale bar represents 1 μM; n=8 mice per group.
Figure 2.
Figure 2a Diabetic TLR4−/− mice wounds have significantly smaller wounds compared with diabetic WT mice. Wound sizes were recorded using a digital camera at days 0 and 10 after wounding as described previously (10).
Figure 2b IL-6 and TNF-α concentrations in wound tissues were determined by ELISA on day 1 and day 10. Values are normalized to mg cell protein and expressed as mean ± SD. *P<0.005 vs diabetic WT; n=8 mice per group.
Figure 2c The DNA-binding activity of nuclear NF-κB p65 in day 10 wound tissues (6 mm) was assessed by ELISA. Values are normalized to mg nuclear protein and expressed as mean ± SD. *P<0.001 vs diabetic WT; n=8 mice per group.
Discussion
Mounting evidence links inflammation as a causative factor in delayed healing and often this inflammatory response is inappropriately excessive in diabetic wounds (4, 11–13). Here, we hypothesized that TLR4 mediates inflammatory responses in cutaneous diabetic wounds and in part contributes to the delayed healing process. Major injury and trauma activate the innate immune system via increased TLRs, such as TLR4 with associated proinflammatory cytokines (IL-6, TNF-α,) in stimulated immunomodulatory cells. Besides pathogen recognition, there is an emerging concept indicating that TLR4 acts as a sensor of tissue damage or sterile inflammatory stimulation (14). TLR4 null or mutant mice are resistant to tissue injuries such as hemorrhagic shock (14). TLR4 was the first member of the pattern recognition receptor family to be characterized, identified as a critical receptor in the host defense against infection, and binds to bacterial LPS (15). TLR4 gene knockout mice are unresponsive to LPS shock and TLR4 gene mutant mice are tolerant to endotoxin treatment (15). TLR4 contributes to microvascular inflammation and leukocyte dysfunction in thermal injury (16). Using microarray analysis and PCR, increased TLR4 expression was shown in murine skin wounds at the early stages suggesting a role for TLR4 at sites of injury, and indicating that TLR4 as an important regulator of wound inflammation (17). TLR2 also plays an important role in propagating inflammation in injury and trauma (8,11,25). We recently showed that TLR2 mediated inflammation impairs cutaneous wound healing in diabetic mice and that genetic deficiency of TLR2 improves healing by decreased inflammation (11). Besides, endogenous ligands generated at sites of injury and trauma engage TLR2 and may perpetuate inflammation in skin injury, ischemia, hemorrhagic shock, and ischemia/reperfusion injuries (reviewed in 8, 25). For instance, TLR2 deficiency in injury models improving outcomes include decreased NF-κB activation, IL-6, and TNF-α levels in partial hepatectomized mice; decreased renal dysfunction and tubular damage in mice following ischemia/reperfusion injury; maintained coronary endothelial function or left ventricular function during ischemia/reperfusion injury in mice (8, 25). Thus, both TLR2 and TLR4 contribute to hyperinflammation seen in diabetic wounds and their ablation improves healing by decreasing local inflammation, in line with above studies (8,25).
Besides, these critical functions in tissue injury, repair and trauma, recent studies demonstrate the contribution of TLR4 to the pro-inflammatory state of diabetes and its complications (10,18). We previously reported that TLR4 contributes to the proinflammatory state of Type 1 diabetes mellitus (T1DM) using STZ induced T1DM in WT and TLR4−/− mice (10). Diabetic TLR4−/− mice showed decreased circulating cytokines/chemokine levels (inflammation) compared to wild type diabetic mice; macrophages from diabetic TLR4−/− mice showed inhibition of MyD88 dependent and independent signaling, NF-κB activity, and pro-inflammatory mediators compared to diabetic WT mice (10). At the same time, high fat feeding in TLR4 gene deficient mice, despite greater adiposity, showed decreased adipose tissue inflammation (19). Ghanim et al demonstrated that the intake of a single high-fat, high-carbohydrate meal results in the induction of TLR4 and TLR2 receptor expression in humans (mononuclear cells) compared to several weeks of high fat diet in experimental animals, suggesting that inflammation is doubled rapidly in a high-fat and high-glucose milieu (20). We reported increased TLR2/4 expression and activity in monocytes of both T1DM and T2DM, associated with increased secretion of proinflammatory cytokines (8,21) and also showed amelioration of the proinflammatory response in TLR2/4−/− mice (10) supports our posit that TLRs especially TLR2/4 could serve as a critical cellular hub directing the release of proinflammatory mediators and exacerbating the inflammatory response. However, the role of TLR4 in diabetic wound healing has not been tested before.
Previously, we have shown that in hyperglycemic milieu, inhibition of TLR4 gene expression using gene specific siRNAs significantly abolished HG-induced inflammation as evidenced by alleviation of HG-induced cytokine /chemokine release, decreased TLR-mediated signaling and decreased NF-κB activity (22), in support of the present data. Besides, the administration of 5g/hr glucose for 4hr is sufficient to induce an increase in TLR4 expression in patients with T1DM suggesting that even mild hyperglycemia leads to the induction of TLR4 expression and insulin administration reverses this induction (23, 24). This report demonstrates the role of TLR4 in contributing to the pro-inflammatory state of diabetic wounds since TLR4 knock out was able to improve wound closure, decrease NF-κB activity, and abrogate release of proinflammatory cytokines locally under diabetic conditions. In another study, Lin et al have shown that overexpression of TLR4 in the human diabetic kidney was correlated with CD68+ cell infiltration, suggesting a possible role for TLR4 in mediating monocyte/macrophage recruitment and tubulointerstitial inflammation in diabetic nephropathy (18). The absence of this phenomenon in renal tissues of nondiabetic subjects suggested that the observed TLR4 activation was not merely a nonspecific consequence of heavy proteinuria (17). Furthermore, authors have shown renoprotective effects in diabetic TLR4−/− mice (17). Our data is consistent with these reports in diabetic TLR4−/− mice.
Collectively, these findings suggest that TLR4 may be a new molecular target to reduce the increased inflammatory burden of diabetes which appear to play a role in diabetic microvascular complications and wounds (10, 15, 19, 25). Our novel data confirms the hypothesis that TLR4 is a key element mediating persistent inflammation in the diabetic wounds. Taken together, these findings suggest that diabetes-induced TLR4 activation and inflammation is undesirable in wounds and that genetic deficiency of TLR4 significantly abrogates this state of diabetic wounds. These novel findings underscore a role for TLR4 in the diabetic wound repair process and emphasize the importance of understanding the innate immune inflammatory signaling mechanisms.
Acknowledgments
We thank Dr. Peter Tobias, Scripps Research Institute, La Jolla CA for providing TLR4−/− mice. MRD thanks American Diabetes Association for a Junior Faculty Award. IJ thanks NIH (ROI HL074360) and JDRF for grant support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose): National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2. 7 million participants. Lancet. 2011;378 (9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361:1545–1551. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- 3.Sibbald RG, Woo KY. The biology of chronic foot ulcers in persons with DM. Diab Metab Res Rev. 2008;24 (1):S25–30. doi: 10.1002/dmrr.847. [DOI] [PubMed] [Google Scholar]
- 4.Acosta JB, del Barco DG, Vera DC, Savigne W, Lopez-Saura P, Nieto GG, Schultz GS. The pro-inflammatory environment in recalcitrant diabetic foot wounds. International Wound Journal. 2008;5(4):530–539. doi: 10.1111/j.1742-481X.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. Journal of Investigative Dermatology. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 6.Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006;84(9):712–25. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- 7.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279(17):17079–84. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez SR, Dasu MR. Toll-like receptors and diabetes complications: recent advances. Curr Diabetes Rev. 2012;8(6):480–8. doi: 10.2174/157339912803529887. [DOI] [PubMed] [Google Scholar]
- 9.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devaraj S, Tobias P, Jialal I. Knock-out of TLR4 attenuates the pro-inflammatory state of diabetes. Cytokine. 2011;55:441–445. doi: 10.1016/j.cyto.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Dasu MR, Thangappan R, Bourgette A, DiPietro LA, Isseroff R, Jialal I. TLR2 Expression and signaling dependent inflammation impair wound healing in diabetic mice. Laboratory Investigation. 2010;90(11):1628–36. doi: 10.1038/labinvest.2010.158. [DOI] [PubMed] [Google Scholar]
- 12.Liu R, Bal HS, Desta T, Behl Y, Graves DT. Tumor necrosis factor-α mediates diabetes-enhanced apoptosis of matrix-producing cells and impairs diabetic healing. Am J Pathol. 2006;168(3):757–64. doi: 10.2353/ajpath.2006.050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkötter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 MØ population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121(3):985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26(5):430–7. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 15.Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol. 2002;270:109–20. doi: 10.1007/978-3-642-59430-4_7. [DOI] [PubMed] [Google Scholar]
- 16.Breslin JW, Wu MH, Guo M, Reynoso R, Yuan SY. Toll-like receptor 4 contributes to microvascular inflammation and barrier dysfunction in thermal injury. Shock. 2008;29(3):349–55. doi: 10.1097/shk.0b013e3181454975. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Guo S, Ranzer MJ, Dipietro LA. Toll-like receptor 4 has an essential role in early skin wound healing. J Invest Dermatol. 2013;133(1):258–67. doi: 10.1038/jid.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS, Chan KW, Lai KN, Tang SC. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. 2012;23(1):86–102. doi: 10.1681/ASN.2010111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 20.Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32(12):2281–7. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93:578–83. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57:3090–8. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dandona P, Ghanim H, Green K, Sia CL, Abuaysheh S, Kuhadiya N, Batra M, Dhindsa S, Chaudhuri A. Insulin infusion suppresses while glucose infusion induces Toll-like receptors and high-mobility group-B1 protein expression in mononuclear cells of type 1 diabetes patients. Am J Physiol Endocrinol Metab. 2013;304(8):E810–8. doi: 10.1152/ajpendo.00566.2012. [DOI] [PubMed] [Google Scholar]
- 24.Ghanim H, Korzeniewski K, Sia CL, Abuaysheh S, Lohano T, Chaudhuri A, Dandona P. Suppressive effect of insulin infusion on chemokines and chemokine receptors. Diabetes Care. 2010;33(5):1103–8. doi: 10.2337/dc09-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jialal I, Kaur H. The Role of Toll-Like Receptors in Diabetes-Induced Inflammation. Implications for Vascular Complications. Curr Diab Rep. 2012 Feb 8; doi: 10.1007/s11892-012-0258-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]