Abstract
Purpose
The most common long-term complication of joint arthroplasty is aseptic loosening. The proinflammatory cytokines secreted by macrophages are involved in aseptic loosening. Recently, a novel proinflammatory cytokine IL-17C was reported to participate in inflammatory diseases by synergising with proinflammatory cytokines. However, the relationship between IL-17C and the aseptic loosening is unclear.
Methods
The tissues around aseptic loosened implants were collected during revision surgery and handled by formalin fixation and embedded in paraffin. The presence of IL-17C in the tissues around the aseptic loosened implants was investigated in 12 aseptic loosening patients using immunofluorescence.
Results
The presence of IL-17C protein in the tissues around aseptic loosened implants was detected by immunofluorescence. There are no statistical differences between optical density of IL-17C in aseptic loosening samples and in rheumatoid arthritis samples (positive control).
Conclusions
These results suggest the presence of IL-17C in aseptic loosening. Interleukin-17C was related to the inflammation of aseptic loosening, possibly by contributing to the inflammation and osteolysis in the tissues surrounding aseptic loosened implants.
Introduction
Total hip arthroplasty is an effective treatment for irreversible hip disorders, including osteoarthritis, rheumatoid arthritis, congenital hip diseases, and many other end stage joint diseases. The main long-term complication of an initially stable prosthesis is aseptic loosening [1–3]. Inflammation induced by wear particles of the prosthesis is considered to be the major cause of aseptic loosening [4, 5] with some bacterial components from wounds and intestines are suspected to contribute to the particle-induced inflammation [6–9]. This inflammation is characterised by macrophage infiltration and proinflammatory cytokine secretion, and the pattern of cytokines consists of tumour necrosis factor alpha (TNF-alpha), interleukin-1 beta (IL-1beta), and interleukin-6 (IL-6) [10–12]. After phagocytosis or the contact of the implant wear debris with the adhering bacterial components [6–9, 13–17], the nuclear transcription factor-kappa B (NF-kappa B) of the macrophage is activated, and the macrophages express the above mentioned proinflammatory cytokines in response [11, 12, 18]. These proinflammatory cytokines disrupt the local balance of osteogenesis and osteolysis through the proliferation of osteoclasts and the inhibition of osteoblasts [12, 19–21].
Interleukin-17C (IL-17C) is a recently identified member of the IL-17 superfamily [22]. Unlike other members of the IL-17 superfamily, it is secreted by a wide spectrum of cells including macrophages, rather than T cells [23–27]. IL-17C was demonstrated to have strong proinflammatory effects [26]. Normal human tissues barely express IL-17C [22]. However, the expression of IL-17C has been documented in some diseases that are usually characterised by inflammation with macrophage infiltration. For example, the expression of IL-17C has been identified in a wide spectrum of sources including CD11b+ MHC class II macrophages, CD11c+MHC class II dendritic cells, lung tissues of mouse mycoplasma pneumonia, and the paws of the mouse collagen-induced arthritis model. And IL-17C was found to contribute to the exacerbation of the inflammation by increasing TNF-alpha and IL-1beta expression [22, 26]. In humans, IL-17C has been identified to be expressed in the synovial cells of rheumatoid arthritis patients [28], in psoriatic skin [24, 29], and in cultured human keratinocytes stimulated with TNF-alpha [23]. However, little is known about the expression of IL-17C in other diseases characterised by macrophage infiltration, such as the aseptic loosening after joint arthroplasty.
We postulated that the proinflammatory cytokine IL-17C might be associated with wear debris-activated macrophages in the interface membrane surrounding aseptic loosened implants. To the best of our knowledge, no reports have previously demonstrated this. Thus, the aim of this study was to investigate the presence of IL-17C in the interface membrane surrounding the aseptic loosened implants after total joint arthroplasty, compared to the known IL-17C-positive rheumatoid synovial membrane [26, 28].
Methods
Ethics statement
The patients were fully aware of the project and gave written informed consent. The project was approved by the ethical committee of Sun Yat-sen University of Medicine.
Patients and samples
Patients who were suffering from an episode of clinical aseptic loosening were selected from all patients in the joint department of the First Affiliated Hospital of Sun Yat-sen University and the Huangpu Joint Centre from September 2008 to September 2009. The exclusion criteria included repeat revision surgery, and the presence of rheumatoid arthritis, ankylosis spondylitis, systemic lupus erythematous, infection or any other conditions that might affect the local or systemic immune system. Twelve cases were finally included. The interface membranes surrounding the loosened parts of the implants (femoral or acetabular) were taken for biopsy during the revision operations.
Since the expression of IL-17C in rheumatoid synovium has been demonstrated [26, 28], an additional five cases of rheumatoid arthritis were included as positive controls and the synovium was taken for biopsy during the primary arthroplasty. Normal goat and mouse immunoglobulin serum fractions were used as a negative control for IL-17C and CD68 staining, respectively.
Clinical samples were immediately fixed in 30 % formalin for 24–48 hours, followed by dehydration in ethanol, clearing in xylene, embedding in paraffin, and storage at room temperature.
Immunofluorescence analysis
The immunofluorescence staining protocol was performed by referring to prior reports [30, 31]. Four-μm thick paraffin sections were cut, deparaffinised and rehydrated using routine methods. The antigen retrieval was performed by maintaining the sections at a moderate boil in a citric acid buffer solution (pH 6.0). Non-specific antigen binding sites were blocked by incubation with 10 % normal rabbit serum for 40 minutes before applying the primary antibodies (goat anti-human IL-17C, 20 μg/ml, R&D, AF1234; mouse anti-human CD68, 20 μg/ml, R&D MAB20401; 1 % normal rabbit serum). Then the mixture was incubated overnight in a moist 4 °C environment. Next, the slides were washed five times with PBS for five minutes, and the secondary antibodies were applied (rabbit anti-goat CY3-labelled, 1:100, Boster SA1076; rabbit anti-mouse FITC-labelled, 1:50, Boster). The sections were counter-stained with DAPI.
The haematoxylin and eosin staining
Five-μm paraffin sections were cut consecutively, followed by deparaffinising and rehydrating. Sections were subjected to the standard haematoxylin and eosin staining method.
Microscopy
Within 24 hours, both the immunofluorescence staining sections and the haematoxylin and eosin staining sections were examined and photographed under microscopy (Axio Imager Z1b, ZEISS). Five random high power visual fields were independently selected by two observers for each section, and the optical density was calculated.
Statistical analysis
The results are expressed as the mean and standard error (mean ± SE). Differences between groups were analysed using analysis of variance (ANOVA) with PSS 13.0. A P value of <0.05 was regarded as statistically significant.
Results
Preoperative data on clinical samples
Preoperative data of the 12 aseptic loosening cases are listed in Table 1, and the data of the five rheumatoid arthritis patients are listed in Table 2.
Table 1.
Preoperative data on revision total hip arthroplasty patients suffering of aseptic loosening
| Characteristic | Cases | Percentage (%) |
|---|---|---|
| Gender | ||
| Male | 8 | 66.7 |
| Female | 4 | 33.3 |
| Agea | ||
| ≤50 | 2 | 16.7 |
| 51 ∼ 60 | 3 | 25.0 |
| 61 ∼ 70 | 3 | 25.0 |
| 71 ∼ 80 | 1 | 25.0 |
| 81 ∼ 90 | 1 | 8.3 |
| Reasons for arthroplasty | ||
| Femur neck fracture | 5 | 41.7 |
| Femur head necrosis | 5 | 41.7 |
| Acetabulum fracture | 2 | 16.7 |
| Follow-upb | ||
| <1 | 1 | 8.3 |
| 1 ∼ 3 | 1 | 8.3 |
| 3 ∼ 5 | 2 | 16.7 |
| 5 ∼ 7 | 0 | 0 |
| 7 ∼ 9 | 4 | 33.3 |
| 9 ∼ 11 | 3 | 25.0 |
| ≥11 | 1 | 8.3 |
| Loosened parts | ||
| Cup | 2 | 16.7 |
| Stem | 1 | 8.3 |
| Both | 9 | 75.0 |
All the patients were clinically diagnosed as aseptic loosening according to the manifestation, radiology and aetiology
aAge of the patients in years
bTime intervals between the primary arthroplasty and the revision surgery in years
Table 2.
Preoperative data on rheumatoid arthritis patients
| Gender | Age (years) | Samplesa | ESR | CRP | RF |
|---|---|---|---|---|---|
| Female | 73 | Left knee | 89 | 19 | 62 |
| Female | 76 | Right knee | 47 | 16 | 70 |
| Female | 52 | Left knee | 90 | 30 | 75 |
| Female | 56 | Right knee | 60 | 23 | 57 |
| Female | 51 | Left knee | 96 | 14 | 91 |
ESR preoperative erythrocyte sedimentation rate (mm/h), CRP preoperative C-reactive protein levels (mg/l), RF preoperative rheumatoid factor (U/ml)
All the patients were clinically diagnosed as rheumatoid arthritis according to the manifestation, radiology and pathology
aLocation where synovial membranes were dissected as clinical samples
Histology
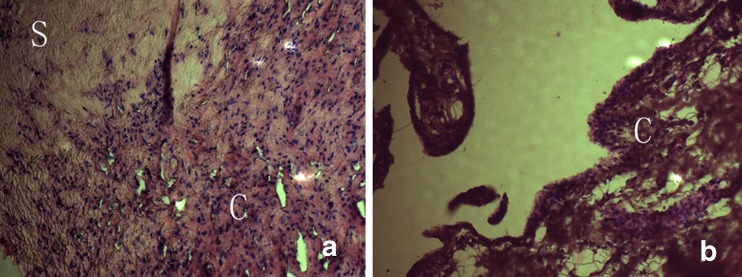
In aseptic loosening samples, a great amount of monocyte/macrophages could be viewed in the section, infiltrating the connective tissue stroma (Fig. 1a). In other areas of the section, loose or tight connective tissues were less infiltrated with proinflammatory cells. In the rheumatoid arthritis samples, even more monocytes were infiltrating the rheumatoid synovial membranes compared to the aseptic loosening samples (Fig. 1b).
Fig. 1.
Histology of tissues around aseptic loosened implants and rheumatoid synovial membrane, H&E staining. a Aseptic loosening tissues. Inflammatory cell infiltrations are accompanied by dense connective tissues (scars). b Rheumatoid synovial membrane. Loose connective tissue stroma is infiltrated with inflammatory cells. S scar, C cell infiltration
IL-17C immunofluorescence
Because of the high expression of IL-17C in the synovium of rheumatoid arthritis identified previously, we chose the rheumatoid arthritis clinical samples as the positive control [26, 28]. Compared to the negative control group, the statistically greater staining in the rheumatoid synovial membrane confirmed the previous reports [26, 28] and proved the rheumatoid synovial membrane was a good positive control. The very mild staining in the negative control and the statistically lower presence of IL-17C compared to the rheumatoid synovial membrane suggested a valid negative control group.
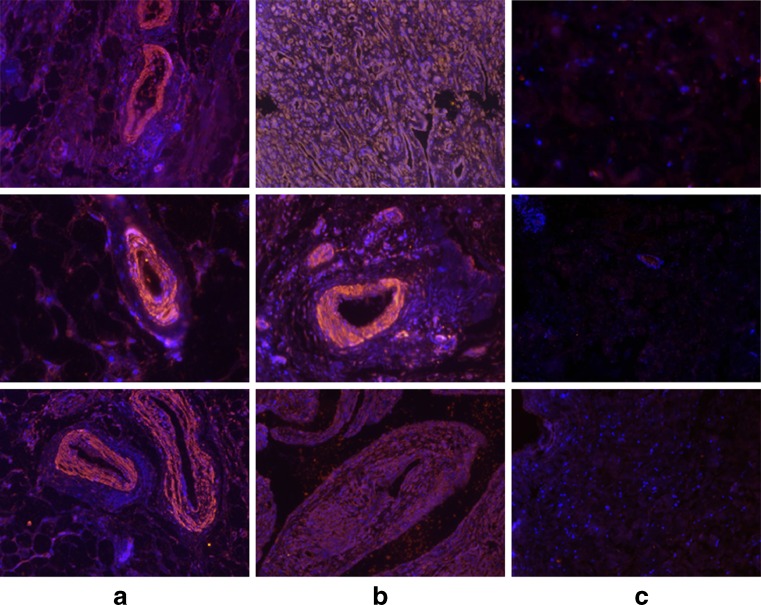
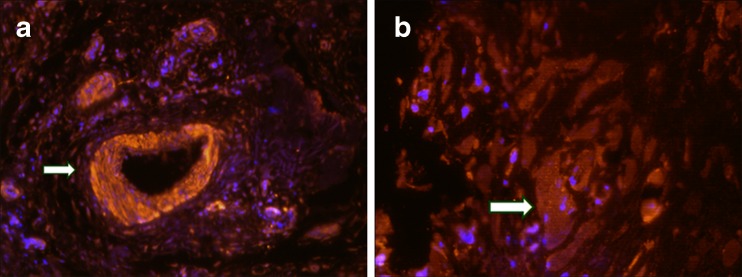
The presence of IL-17C was present in the cytoplasm of aseptic loosening samples as well as in the synovium of rheumatoid arthritis, compared to the negative control group (Fig. 2a, b and c). The “vascular sleeve” structures were significantly stained (Fig. 3a), suggesting the association of IL-17C with inflammation and vessel responses. The multinucleate giant cells scattered in the section were also stained, which were supposed to be fused by macrophages (Fig. 3b).
Fig. 2.
Presence of IL-17C in tissues surrounding aseptic loosened implants. Immunofluorescence of human IL-17C was performed on the samples of the aseptic loosening, rheumatoid arthritis and the negative control groups. The anti-human IL-17C antibody was labelled with CY3 (red). The cell nucleus was counter stained with Dapi (blue). Each column contains three different cases suffering from aseptic loosening, rheumatoid arthritis or in the negative control groups. a Samples were the rheumatoid synoviums taken from three different cases. The mean optical density is 755.29, 864.20 and 741.36 from top to bottom. b The samples were the interface membranes surrounding the aseptic loosened implants taken from three different cases. The mean optical density is 953.92, 623.14 and 855.03 from top to bottom. c The samples were negative controls from three different cases. The mean optical density is 272.96, 281.35 and 352.32 from top to bottom
Fig. 3.
Vessels and multinucleate giant cells significantly stained with anti-IL-17C. Immunofluorescence of human IL-17C in the interface membranes surrounding the aseptic loosened implants. The anti-human IL-17C antibody was labeled with CY3 (red). The cell nucleus was counter stained with Dapi (blue). a The “vascular sleeve” structures were also significantly stained (white arrow), suggesting the association of IL-17C with inflammation and vessel responses. b The multinucleate giant cells scattered in the section were also stained (white arrow). These gigantic cells, with several cell nuclei arrayed around the cell membrane to form a “chaplet”, were stained with anti-human IL-17C
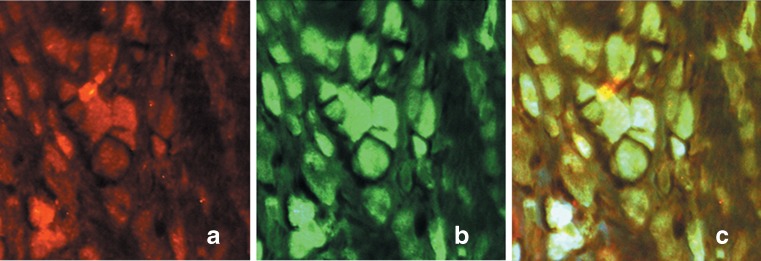
To further investigate the source of IL-17C in the tissues around aseptic loosened implants, we examined the colocalisation of IL-17C with CD68 using immunofluorescence double staining. The results suggested that 55.32 ± 10.11 % of CD68+ macrophages were colocalised with IL-17C, implying IL-17C was expressed by macrophages (Fig. 4a, b and c).
Fig. 4.
Colocalisation with anti-human IL-17C and anti-human CD68. Colocalisation was performed in the tissues surrounding the aseptic loosened implants with IL-17C (CY3, red, a) and CD68 (FITC, green, b). The merged view (c) shows the colocalisation of IL-17C and CD68 in the cytoplasm, implying the expression of IL-17C by CD68+ macrophages in aseptic loosening samples. The number of IL-17C and CD68 double-positive cells and CD68 positive cells were calculated under five different high-power vision, and 55.32 % CD68 positive cells were stained with IL-17C antibody, indicating that IL-17C was related to CD68 positive macrophages
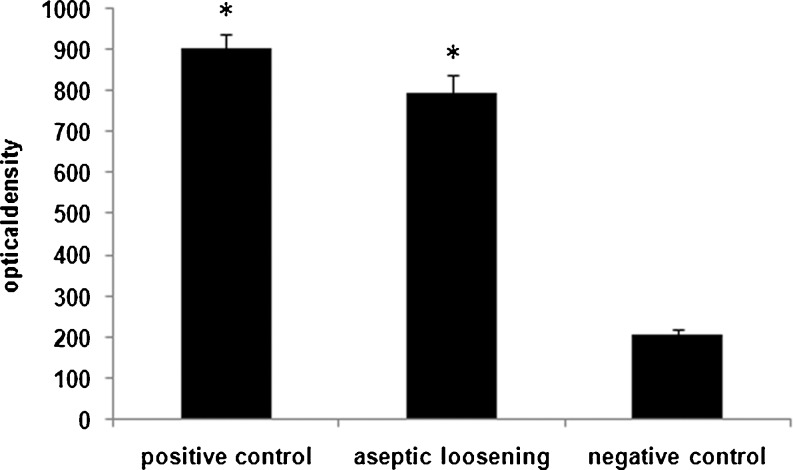
The semiquantitative analysis of the immunofluorescence revealed that the presence of IL-17C in the tissues around aseptic loosened implants was greater compared to the negative control group (in the mean optical density, 794.81 ± 41.75 vs. 206.18 ± 14.41, P < 0.001), and was similar to the positive control group (in the mean optical density, 794.81 ± 41.75 vs. 903.24 ± 33.77, P > 0.05). The presence of IL-17C in the positive control group was significantly greater compared to the negative group (in the mean optical density, 903.24 ± 33.77 vs. 206.18 ± 14.41, P < 0.001, Figs. 2 and 5).
Fig. 5.
The semiquantitative analysis for the aseptic loosening, rheumatoid arthritis and the negative control groups. Semiquantitative analysis of the immunofluorescence was performed for the samples of the aseptic loosening, rheumatoid arthritis and the negative control groups. The optical density was calculated and compared. The differences between aseptic loosening and the negative control group and between rheumatoid arthritis and the negative control group were significant (both P < 0.001), whereas the difference between aseptic loosening and rheumatoid arthritis was not significant (P > 0.05)
Discussion
IL-17C is a novel proinflammatory cytokine that is involved in many inflammatory diseases, including rheumatoid [28] and psoriatic conditions [24, 29] and conditions under the compressive force [25]. Interleukin-17C has also been proven to originate from several sources, including human keratinocytes [23], MC3T3-E1 cells [25], cartilage cells, CD3+ CD4+ cells, CD3+ CD19+ cells, CD11b+ MHC class II+ cells, CD11c+ MHC class II+ cells [26], synovial fluid mononuclear cells of rheumatoid arthritis patients [28] and the Mycoplasma pneumonia infected mouse lung tissues, but not from CD4+ T cells [26, 27].
This study demonstrates for the first time that IL-17C is present in the tissues around aseptic loosened implants. Interleukin-17C has been proven to be a strong proinflammatory cytokine and contributes to exacerbation of arthritic paws of collagen-induced arthritic mice [26]. Interleukin-17C stimulates the release of TNF-alpha and IL-1beta from the monocyte cell line, THP-1 [22]. The wear debris and bacterial components-induced aseptic inflammation and proinflammatory cytokines (TNF-alpha and IL-1beta) have been shown to play an important role in the osteolysis and the aseptic loosening progression [11, 19, 32, 33]. Thus, the presence of IL-17C in the tissues around aseptic loosened implants implies a role of IL-17C in the osteolysis and progression of aseptic loosening, possibly by contributing to the enhanced expression of TNF-alpha and IL-1beta, or the direct effect of IL-17C on the osteolysis. In addition, the vascular sleeves formed by IL-17C positive cells around the small vessels imply the possible relation of IL-17C and the vessel response, suggesting a potential role of IL-17C in the inflammation of aseptic loosening.
Macrophages have been proven to be the most prevalent cells, accounting for about 70 % of all cells in tissues around aseptic loosened implants. The extensive presence of IL-17C in tissues around aseptic loosened implants indicates the possible relationship of IL-17C and macrophages. And according to our experiment, IL-17C is present in the cytoplasm, especially in the cytoplasm of multinucleate giant cells, suggesting that IL-17C is apt to be expressed by macrophages. The results of colocalisation with IL-17C and CD68 antibodies suggest that IL-17C is expressed by CD68+ macrophages in aseptic loosening samples. Previous works have also supported the view that one of the origins of IL-17C was macrophages [26].
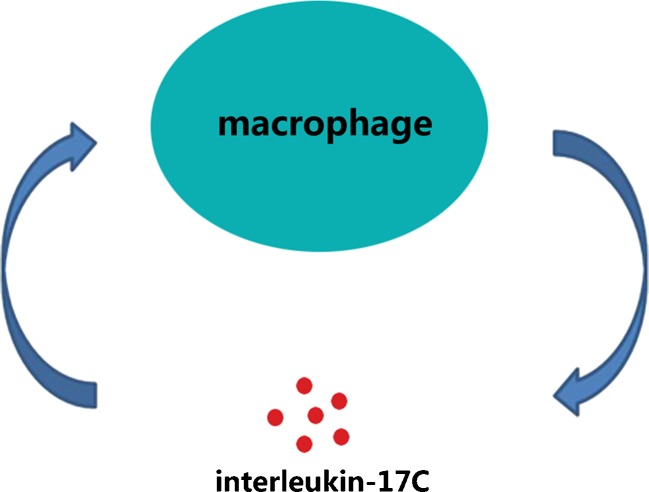
A positive feedback loop formed by the expression of IL-17C and the activation of epithelial cells has been proven to regulate the innate immune function [34] (Fig. 6). Considering macrophages acting as both the origin and the target of IL-17C, IL-17C might contribute to the regulation of inflammation and innate immune function in an autocrine manner similar to the previous report (Fig. 5) [34].
Fig. 6.
A hypothesis of a positive feedback loop between the expression of IL-17C and the activation of macrophages. The expression of IL-17C in macrophages was identified [23, 26] and further supported in this study. And the direct proinflammatory effect of IL-17C on macrophages was also identified [22, 26, 34]. Taken together, macrophages activated by wear particles and bacterial components secrete IL-17C, and IL-17C in turn enhances the activation of macrophages in an autocrine manner [34], thus forming a positive feedback loop
The shortage of the immunofluorescence study lies in the fact that the possible expression of IL-17C in non inflamed tissues around stable implants is not excluded. In conclusion, our study demonstrates the presence of IL-17C in the tissues around aseptic loosened implants, implying the involvement of a new cytokine in the inflammation of aseptic loosening.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (81171710) (http://www.nsfc.gov.cn/Portal0/default166.htm), the Industrialisation of New and High Technology Projects–Industrial Research of Guangdong Province, China (2011B010500012), and the International Science and Technology Cooperation of Science and Technology of Guangdong Province, China (2010B050300009) (http://www.gdstc.gov.cn/eng/mission.html). The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We sincerely thank Mr. Chaohong Li (Zhongshan Medical school of Sun Yat-sen University, Guangzhou, China) for his technical guidance and device support.
References
- 1.Howard JL, Kremers HM, Loechler YA, et al. Comparative survival of uncemented acetabular components following primary total hip arthroplasty. J Bone Joint Surg Am. 2011;93(17):1597–1604. doi: 10.2106/JBJS.J.00195. [DOI] [PubMed] [Google Scholar]
- 2.Della VC, Mesko NW, Quigley L, et al. Primary total hip arthroplasty with a porous-coated acetabular component. A concise follow-up, at a minimum of twenty years, of previous reports. J Bone Joint Surg Am. 2009;91(5):1130–1135. doi: 10.2106/JBJS.H.00168. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin JR, Lee KR. Total hip arthroplasty with an uncemented tapered femoral component. J Bone Joint Surg Am. 2008;90(6):1290–1296. doi: 10.2106/JBJS.G.00771. [DOI] [PubMed] [Google Scholar]
- 4.Willert HG, Bertram H, Buchhorn GH (1990) Osteolysis in alloarthroplasty of the hip. The role of ultra-high molecular weight polyethylene wear particles. Clin Orthop Relat Res (258):95–107 [PubMed]
- 5.von Knoch M, Jewison DE, Sibonga JD, et al. The effectiveness of polyethylene versus titanium particles in inducing osteolysis in vivo. J Orthop Res. 2004;22(2):237–243. doi: 10.1016/j.orthres.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Tatro JM, Taki N, Islam AS, et al. The balance between endotoxin accumulation and clearance during particle-induced osteolysis in murine calvaria. J Orthop Res. 2007;25(3):361–369. doi: 10.1002/jor.20289. [DOI] [PubMed] [Google Scholar]
- 7.Bi Y, Seabold JM, Kaar SG, Bi Y, Seabold JM, Kaar SG, et al. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001;16(11):2082–2091. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- 8.Cho DR, Shanbhag AS, Hong CY, et al. The role of adsorbed endotoxin in particle-induced stimulation of cytokine release. J Orthop Res. 2002;20(4):704–713. doi: 10.1016/S0736-0266(01)00179-6. [DOI] [PubMed] [Google Scholar]
- 9.Schwab LP, Xing Z, Hasty KA, et al. Titanium particles and surface-bound LPS activate different pathways in IC-21 macrophages. J Biomed Mater Res B Appl Biomater. 2006;79(1):66–73. doi: 10.1002/jbm.b.30512. [DOI] [PubMed] [Google Scholar]
- 10.Ren W, Li XH, Chen BD, et al. Erythromycin inhibits wear debris-induced osteoclastogenesis by modulation of murine macrophage NF-kappaB activity. J Orthop Res. 2004;22(1):21–29. doi: 10.1016/S0736-0266(03)00130-X. [DOI] [PubMed] [Google Scholar]
- 11.Gallo J, Kaminek P, Ticha V, et al. Particle disease. A comprehensive theory of periprosthetic osteolysis: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2002;146(2):21–28. doi: 10.5507/bp.2002.004. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz EM, Lu AP, Goater JJ, et al. Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J Orthop Res. 2000;18(3):472–480. doi: 10.1002/jor.1100180321. [DOI] [PubMed] [Google Scholar]
- 13.Tunney MM, Patrick S, Gorman SP, et al. Improved detection of infection in hip replacements. A currently underestimated problem. J Bone Joint Surg Br. 1998;80(4):568–572. doi: 10.1302/0301-620X.80B4.8473. [DOI] [PubMed] [Google Scholar]
- 14.Tunney MM, Patrick S, Curran MD, et al. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol. 1999;37(10):3281–3290. doi: 10.1128/jcm.37.10.3281-3290.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke MT, Roberts CP, Lee PT et al (2004) Polymerase chain reaction can detect bacterial DNA in aseptically loose total hip arthroplasties. Clin Orthop Relat Res (427):132–137 [DOI] [PubMed]
- 16.Savarino L, Baldini N, Tarabusi C, et al. Diagnosis of infection after total hip replacement. J Biomed Mater Res B Appl Biomater. 2004;70(1):139–145. doi: 10.1002/jbm.b.30030. [DOI] [PubMed] [Google Scholar]
- 17.Nalepka JL, Lee MJ, Kraay MJ, et al. Lipopolysaccharide found in aseptic loosening of patients with inflammatory arthritis. Clin Orthop Relat Res. 2006;451:229–235. doi: 10.1097/01.blo.0000224050.94248.38. [DOI] [PubMed] [Google Scholar]
- 18.Maloney WJ, James RE, Smith RL (1996) Human macrophage response to retrieved titanium alloy particles in vitro. Clin Orthop Relat Res (322):268–278 [PubMed]
- 19.Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67(2):182–188. [PubMed] [Google Scholar]
- 20.Ingham E, Green TR, Stone MH, et al. Production of TNF-alpha and bone resorbing activity by macrophages in response to different types of bone cement particles. Biomaterials. 2000;21(10):1005–1013. doi: 10.1016/S0142-9612(99)00261-6. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima Y, Sun DH, Trindade MC, et al. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. J Bone Joint Surg Am. 1999;81(5):603–615. doi: 10.2106/00004623-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Chen J, Huang A, et al. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci USA. 2000;97(2):773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansen C, Riis JL, Gedebjerg A, et al. Tumor necrosis factor alpha-mediated induction of interleukin 17C in human keratinocytes is controlled by nuclear factor kappa B. J Biol Chem. 2011;286(29):25487–25494. doi: 10.1074/jbc.M111.240671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansen C, Vinter H, Soegaard-Madsen L, et al. Preferential inhibition of the mRNA expression of p38 mitogen-activated protein kinase regulated cytokines in psoriatic skin by anti-TNFalpha therapy. Br J Dermatol. 2010;163(6):1194–1204. doi: 10.1111/j.1365-2133.2010.10036.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang F, Wang CL, Koyama Y, et al. Compressive force stimulates the gene expression of IL-17s and their receptors in MC3T3-E1 cells. Connect Tissue Res. 2010;51(5):359–369. doi: 10.3109/03008200903456942. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi Y, Fujio K, Shoda H, et al. IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. J Immunol. 2007;179(10):7128–7136. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- 27.Wu Q, Martin RJ, Rino JG, et al. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9(1):78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang SY, Kim HY. Expression of IL-17 homologs and their receptors in the synovial cells of rheumatoid arthritis patients. Mol Cells. 2005;19(2):180–184. [PubMed] [Google Scholar]
- 29.Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160(2):319–324. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- 30.Robertson D, Savage K, Reis-Filho JS, et al. Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biol. 2008;9:13. doi: 10.1186/1471-2121-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long DN, Buggs C. Microwave oven-based technique for immunofluorescent staining of paraffin-embedded tissues. J Mol Histol. 2008;39(1):1–4. doi: 10.1007/s10735-007-9093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purdue PE, Koulouvaris P, Potter HG, et al. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251–261. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- 33.Abu-Amer Y, Darwech I, Clohisy JC. Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Res Ther. 2007;9(Suppl 1):S6. doi: 10.1186/ar2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez-Carrozzi V, Sambandam A, Luis E, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12(12):1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]