Abstract
Objective
The aim of this study is to determine when during hematopoiesis Siglec-8 gets expressed, whether it is expressed on hematologic malignancies, and if there are other non-human species that express Siglec-8.
Methods
Siglec-8 mRNA and cell surface expression was monitored during in vitro maturation of human eosinophils and mast cells. Flow cytometry was performed on human blood and bone marrow samples, and on blood samples from dogs, baboons, and rhesus and cynomolgus monkeys.
Results
Siglec-8 is a late maturation marker. It is detectable on eosinophils and basophils from subjects with chronic eosinophilic leukemia, chronic myelogenous leukemia, and on malignant and non-malignant bone marrow mast cells, as well as the HMC-1.2 cell line. None of the Siglec-8 monoclonal antibodies tested recognized leukocytes from dogs, baboons, and rhesus and cynomolgus monkeys.
Conclusions
Siglec-8-based therapies should not target immature human leukocytes but should recognize mature and malignant eosinophils, mast cells, and basophils. So far, there is no suitable species for preclinical testing of Siglec-8 monoclonal antibodies.
Keywords: Siglec-8, eosinophils, mast cells, basophils, expression, hematologic malignancies, cell lines
Introduction
Siglec-8 is a cell surface receptor selectively expressed on eosinophils, basophils, and mast cells [1, 2]. Its N-terminal domain has lectin activity and is connected to two membrane proximal immunoglobulin-like repeats, while its cytoplasmic domain contains two tyrosine residues, including one possessing the classical structural sequences found in immunoreceptor tyrosine-based inhibitory motifs [3]. In vitro studies have shown that antibody and lectin ligand-induced engagement of Siglec-8 induces eosinophil apoptosis, whereas antibody engagement of Siglec-8 on mast cells inhibits IgE receptor-induced release of histamine and prostaglandin D2, but has no effect on cell survival [4–8].
The closest functional paralog of Siglec-8 in the mouse is Siglec-F, as there is no Siglec-8 ortholog in rodents [9, 10]. However, consistent with Siglec-8 in vitro biology, in vivo engagement of Siglec-F has selective and pronounced anti-eosinophil properties in models of eosinophilic leukemia and eosinophilic inflammation of the gastrointestinal tract and lungs [11–14]. Thus, based on available in vitro and in vivo data, Siglec-8 would appear to be an attractive target for the development of an agonistic therapeutic agent such as a monoclonal antibody. One drawback, however, in the process of developing a Siglec-8-targeting biological is an unclear path for preclinical animal testing and assessment of safety. The latter is especially relevant to bone marrow toxicity since relatively little is known about when during hematopoiesis Siglec-8 is expressed. Previous studies have demonstrated a lack of Siglec-8 expression by human CD34+ progenitors, HL60 or EOL-3 eosinophil-like cells, suggesting that surface expression of Siglec-8 is a late maturation-related event [2, 15]. This is consistent with studies of human mast cells derived from CD34+ progenitors as well as studies of mouse eosinophils, both of which suggest that Siglec-8/Siglec-F expression occurs relatively late in hematopoiesis [15, 16].
In order to directly assess differentiation-related Siglec-8 expression on maturing cells, studies were initiated employing hematopoiesis culture systems in vitro to assess the timing of Siglec-8 gene and protein expression in developing human eosinophils in vitro. Complementary studies involved the use of flow cytometry to analyze a variety of eosinophil and mast cell lines as well as blood samples from a variety of hematologic malignancies, the latter representing defects in various stages of hematopoietic maturity. Further analyses of mast cell Siglec-8 expression were performed using bone marrow samples obtained from mastocytosis and other patients. Finally, available public databases were screened for the presence of Siglec-8-related genes, which led to flow cytometric analysis of Siglec-8 expression on various non-human primates and dogs. Our findings are consistent with the notion that Siglec-8 is a late eosinophil and mast cell differentiation marker. Its expression is maintained on leukemias, and detection of Siglec-8 with available antibodies in non-human leukocytes appears to be limited to one polyclonal antibody capable of recognizing baboon eosinophils.
Methods
Antibodies
Monoclonal Siglec-8 antibodies 2C4 and 2E2 (IgG1 mouse anti-human Siglec-8) were generated using a Siglec-8–human IgG1 Fc fusion protein as previously described [2, 5]. For some experiments, another Siglec-8 monoclonal antibody was used (7C9, IgG1; Biolegend, San Diego, CA, USA) as well as an affinity-purified polyclonal sheep anti-human Siglec-8 antibody [9]. Based on information listed on the NIH Non-Human Primate Reagent Resource site (http://nhpreagents.bidmc.harvard.edu/NHP/Literature.aspx) and another from BD Biosciences (http://www.bdbiosciences.com/nvCategory.jsp?action=SELECT&form=formTree_catBean&item=744991), the following murine IgG1 monoclonal antibodies were purchased from BD Biosciences (San Jose, CA, USA) as anti-human antibodies cross-reactive with the same monkey cell surface markers: PE-conjugated anti-CD16 clone 3G8 and PE-conjugated anti-CD49d clone 9F10. To label beagle leukocytes, in addition to the Siglec-8 reagents mentioned above, the same PE-anti-CD16 antibody was used [17], while PE-conjugated anti-human CD18 clone 7C4 found to be cross-reactive to dog (mouse IgG1; Beckman-Coulter, Brea, CA, USA) and PE-conjugated anti-CD90 clone DH24A (mouse IgM anti-dog; VMRD, Inc., Pullman, WA, USA) were used.
All antibodies were used at saturating concentrations and irrelevant species- and isotype-matched control primary antibodies were used at matching concentrations.
Eosinophil and Mast Cell Differentiation from CD34+ Progenitors
CD34+ cells were purified from umbilical cord blood (CB) by MACS (Miltenyi Biotec) as previously described [18]. The procurement of de-identified umbilical cord blood for purification of CD34+ progenitors for eosinophil differentiation was conducted under an IRB-approved protocol at the University of Illinois at Chicago. The CD34+ cells were cultured in IMDM supplemented with 10% FBS and SCF (10 ng/ml), Flt3-L (10 ng/ml), and TPO (10 ng/ml) for 3 days, followed by IL-5 (25 ng/ml) only for another 18 days. Cells were collected on days 3, 6, 12, 18, and 21 of culture and total RNA and cell lysate prepared for each time point. Eosinophil differentiation was confirmed by differential cell counts using Fast Green/Neutral Red stained cytospin slides. At 21 days of culture, the cells were >95% fast-green+ eosinophils. Siglec-8 mRNA expression was determined by real-time quantitative RT-Q-PCR, and protein expression was determined by Western blotting as shown in Fig. 1.
Fig. 1.
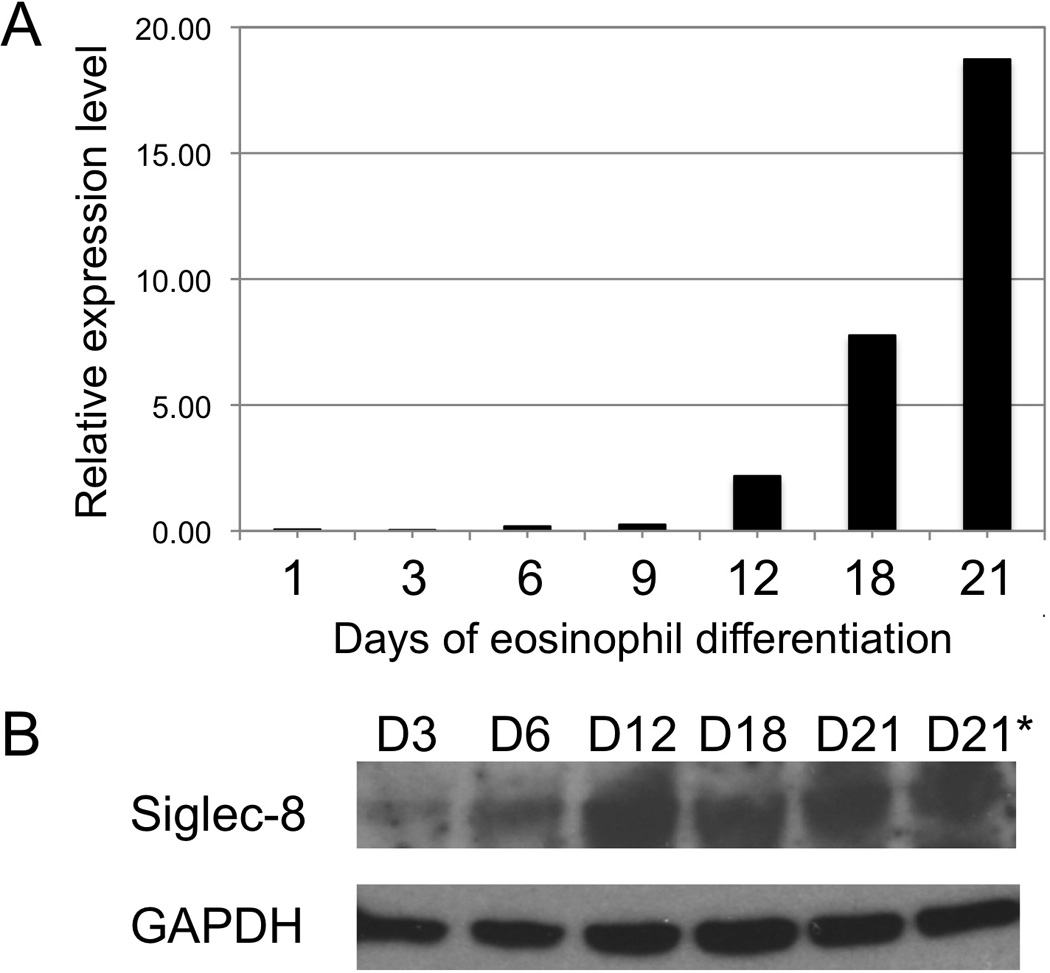
Eosinophils express Siglec-8 late in differentiation. 2.5×106 of cord blood (CB) CD34+ cells were differentiated to the eosinophil lineage in the presence of SCF, Flt3-L, IL-3, and IL-5 for 3 days, followed by IL-5 only thereafter. 5×105 cells were harvested at days 3, 6, 9, 12, 15, 18, and 21. Total RNA and cell lysate were prepared at each time point. Eosinophil differentiation was confirmed using eosinophil-specific Fast Green/Neutral Red staining. a Real-time quantitative PCR analysis of Siglec-8 mRNA expression during CD34+ cell differentiation into eosinophils. b Western blot analysis of Siglec-8 protein expression during CD34+ eosinophil differentiation using 2C4 monoclonal anti-Siglec-8 antibody. GAPDH was used as a loading control (lower panel). *The duplicate Day 21 sample is from an additional CB cultured under identical conditions
Mast cells were generated from CD133+ cord blood mononuclear cells (MNC) essentially as described [19, 20]. After 7 weeks, 70–80% of cells were mature MC as evidenced by Wright–Giemsa staining. Siglec-8 expression on mast cells was determined after 9 weeks (immature mast cell progenitors) and after 15 to 19 weeks (more mature mast cells). This study was approved by the IRB of the Medical University of Vienna. In each case, informed consent was obtained from mothers before cord blood was obtained.
Quantitative RT-PCR for Siglec-8 mRNA Expression in Maturing Eosinophils
Total RNA was extracted from cells harvested at 3-day intervals during IL-5-induced differentiation of CB CD34+ progenitors to eosinophils using TriZol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. cDNA was synthesized with random hexamer primers using a cDNA synthesis kit (Fermentas, Glen Burnie, MD, USA). Siglec-8 mRNA expression was analyzed at each time point by quantitative real-time RT-Q-PCR. A serially diluted cDNA amplicon of Siglec-8, prepared by conventional RT-PCR, was used as a standard to measure the relative expression level of Siglec-8 mRNA in each sample. RT-Q-PCR was performed using a SYBR-green-based assay in a BioRad iQ5 iCycler (BioRad, Hercules, CA, USA). The PCR primers used were as follows: forward primer 4149 CTGCAGGAAGAAATCGGCA 4167, located in Exon 6; and reverse primer 6027 AGGCCTGTTTGAGGAATCACA 6047, located in Exon 7 of the Siglec-8 gene.
Western Blotting for Siglec-8 Expression in Maturing Eosinophils
Cell lysates were prepared at 3-day intervals during IL-5-induced differentiation of CD34+ progenitors to eosinophils using RIPA lysis buffer (Roche, Mannheim, Germany) supplemented with 1× protein inhibitor cocktail (Roche, Germany) and PMSF (1 mM). Western blotting was performed as previously described [18].
Culture and Propagation of Various Cell Lines, Including EOL1, HMC-1.1, HMC-1.2, KU812, K562, HL60, KG1, KG1a, AML14.3D10, and the Dog C2 Mastocytoma Cell Line
The human mast cell lines HMC-1 was kindly provided by Dr. J. H. Butterfield (Mayo Clinic, Rochester, MN, USA). Two subclones of HMC-1 were examined: HMC-1.1, lacking KIT D816V, and HMC-1.2 cells expressing KIT D816V. The canine C2 mastocytoma cell line was kindly provided by Dr. Warren Gold (Cardiovascular Research Institute, University of California, San Francisco, CA, USA). The mast cell lines were maintained in IMDM with 10% FCS at 37°C. The human leukemic cell lines EOL1, KU812, K562, HL60, KG1, and KG1a were obtained from the DSMZ Institute (Braunschweig, Germany) and maintained in RPMI 1640 medium with 10% FCS at 37°C.
The AML14.3D10 cell line, an eosinophil-committed IL-5R+ acute myelocytic leukemia line that proliferates phenotypically as an eosinophil myelocyte/metamyelocyte, shows many characteristics of mature eosinophils [21]. The AML14 line is a less-differentiated IL-5R+ eosinophil-committed line that proliferates as an eosinophil myeloblast [21]. The AML14.3D10 and AML14 lines were maintained in culture as previously described [22, 23]. The AML14.3D10 and AML14 lines were kindly provided by Drs. Michael Baumann and Cassandra Paul (Research Service, VA Medical Center and Division of Hematology/Oncology, Wright State University, Dayton, OH, USA).
Indirect Immunofluorescence and Flow Cytometric Analysis of Cell Lines and Human Cells from Blood and Bone Marrow, Normals, and Various Diseases
Patients diagnosed with chronic myeloid leukemia (CML, n=3), acute myeloid leukemia (AML, n=3), imatinib-responsive, FIP1L1-PDGFR deletion–mutation positive chronic eosinophilic leukemia (CEL, n=2), imatinib-responsive and non-responsive FIP1L1-PDGFR deletion–mutation negative hypereosinophilic syndrome (HES, n=4), eosinophilic myelodysplastic syndrome (MDS, n=3), indolent systemic mastocytosis (ISM, n=3), and aplastic anemia (n=1) were examined. Diagnoses were established according to WHO criteria [24]. Bone marrow (BM) was obtained from the iliac crest or sternum. All donors gave written informed consent. The study was approved by the IRB of the Medical University of Vienna. Relevant clinical data on these subjects can be found in Table I.
Table I.
Patient information
| Patient # |
Sex | Age at diagnosis |
Age at analysis |
Diagnosis | Type | Cytogenetic test results |
Molecular testing results |
Therapy | Serum tryptase (ng/ml) |
White blood cell count (cells/mm3) |
Hemoglobin (g/dl) |
Platelets (per mm3) |
% Eosinophils |
% Basophils |
% Blasts (blood) |
% Blasts (bone marrow) |
Cells analyzed for Siglec-8 surface expression |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 61 | 62 | AML (refractory) | FAB M5 | 45,XX,−7,del (20)(q11)[10] 46,XX[10] | FLT3 ITD positive | None | ND | 82,130 | 10.0 | 21,000 | 0 | 0 | 77 | Not reported | CD34+ |
| 2 | Female | 52 | 53 | AML (relapsed) | FAB M2 | 46,XX[21] | FLT3 ITD negative, FLT3 D835 negative | None | ND | 20,200 | 12.7 | 128,000 | 0 | 0 | 53 | 72 | CD34+ |
| 3 | Male | 64 | 65 | AML (relapsed) | FAB M1 | 46,XY,t(14,15) (q32;q11.2) [7] 46,XY,t (7;12)(q31; q21)[5] 46, XY[9] | Multiplex PCR negative | None | 4.9 | 1,640 | 8.3 | 32,000 | 1 | 0 | 5 | 14 | CD34+ |
| 4 | Male | 59 | 61 | CEL | 46,XY[21] | FIP1L1-PDGFR alpha, del(4)(q12) positive | None | 13.9 | 8,570 | 14.1 | 184,000 | 25 | 1 | 0 | Not reported | Eosinophils, basophils | |
| 5 | Male | 51 | 51 | CEL | 46,XY[21] | FIP1L1-PDGFR alpha, del(4)(q12) positive; KIT D816V negative | None | 20.0 | 12,120 | 12.7 | 113,000 | 75 | 1 | 0 | <5 | Eosinophils, basophils | |
| 6 | Male | 48 | 49 | CML | CP | 46,XY,t(9;22) (q34;q11),t (11;22)(q13; q1?3)[19] 46, XY,[1] | BCR/ABL positive (31.747% IS) | None | ND | 34,480 | 14.0 | 145,000 | 3 | 1 | 0 | <5 | Eosinophils, basophils, CD34+ |
| 7 | Female | 22 | 22 | CML | CP | No growth | BCR/ABL positive (50.089% IS) | Hydroxyurea | ND | 230,570 | 10.4 | 720,000 | 9 | 5 | 1 | <5 | Eosinophils, basophils, CD34+ |
| 8 | Female | 66 | 66 | MDS | RAEB-2 | 47,XX,+21[3] 46,XX[22] | KIT D816V negative | None | 3.8 | 2,940 | 9.5 | 231,000 | 1 | 0 | 2 | 10 | CD34+ |
| 9 | Male | 69 | 69 | MDS/MPN | 46,XY,t(2;12) (p23;q24.1) [13] | BCR/ABL negative, JAK2 V617F negative | None | ND | 26,280 | 10.3 | 159,000 | 2 | 3 | 6 | 6 | CD34+ | |
| 10 | Male | 68 | 68 | MDS/MPN | 46,XY[9] | BCR/ABL negative, JAK2 V617F negative | None | 29 | 24,360 | 7.3 | 41,000 | 0 | 0 | 17 | 10 | CD34+ |
AML acute myelogenous leukemia, CEL chronic eosinophilic leukemia, CML chronic myelogenous leukemia, CP chronic phase, FAB French–American–British classification, IS international scale, ITD internal tandem duplication, MDS myelodysplastic syndrome, MPN myeloproliferative neoplasm, ND not done, RAEB refractory anemia with excess blasts
Heparinized BM or peripheral blood (PB) cells (106/tube) were incubated with anti-human Siglec-8 antibody 2C4 for 15 min, washed in PBS, and then incubated with PE-labeled goat anti-mouse Ig mAb (BD Biosciences). Afterwards, cells were washed twice and stained with anti-human mAbs labeled with FITC, PerCP, or APC for 15 min. Immature progenitor cells were identified as CD34+, CD45+, and CD38− or CD38+ cells. Mast cells were identified as CD117++/CD34− cells, basophils as side scatter (SSC) low/CD123++/CD34− cells, and eosinophils as SSC++/highly auto-fluorescent cells. After erythrocyte lysis (FACS-Lysing-Solution, BD Biosciences), expression of surface antigens was examined by multicolor flow cytometry on a FACSCalibur (BD Biosciences) using FlowJo software (TreeStar, Ashland, OR, USA). Antibody reactivity was controlled for by comparison to isotype-matched antibodies. Antibodies used were CD34 FITC (clone 581, BD Biosciences), CD38 APC (clone HIT2, BD Biosciences), CD45 PerCP (clone 2D1, BD Biosciences), CD117 APC (clone 104D2; Beckman Coulter, Brea, CA, USA), and CD123 APC (clone AC145; Miltenyi Biotec, Bergisch Gladbach, Germany).
Indirect Immunofluorescence and Flow Cytometric Analysis of Blood Samples from Baboon, Cynomolgus Monkeys, Rhesus Monkeys, and Beagles
Six milliliters of heparinized venous blood was obtained from normal baboons (n=3), cynomolgus monkeys (n=3), rhesus monkeys (n=3), and beagles (n=2) (Bioreclamation, Westbury, NY, USA), and shipped cold overnight for analysis within 18 h. Monkey cells were labeled for 30 min at 4°C with saturating concentrations of Siglec-8 or control antibodies and FITC-conjugated secondary antibody [polyclonal FITC-goat anti mouse IgG F(ab′)2 heavy and light chain (Invitrogen) or polyclonal FITC anti-sheep IgG (Abcam, Cambridge, MA, USA)]. After blocking of any open binding sites on the secondary antibodies with excess mouse IgG (Sigma-Aldrich, St. Louis, MO, USA), cells were incubated for 30 min at 4°C with saturating concentrations of PE-conjugated anti-CD16 (labels neutrophils and not eosinophils), PE-conjugated anti-CD49d (labels eosinophils and other leukocytes but not neutrophils), or control IgG1 antibodies. After fixation, flow cytometric analysis of at least 5,000 cells was performed on each sample using a FACSCalibur flow cytometer. Dog blood cells were labeled in an identical fashion except that PE-anti-CD18 (pan-leukocyte positive control) and PE-anti-CD90 (labels eosinophils and not neutrophils) were used.
Results
Previous studies involving several eosinophil-like cell lines (HL60 and EOL3 treated with sodium butyrate to differentiate them to a more eosinophil-like phenotype) failed to detect Siglec-8 expression, suggesting that Siglec-8 was a terminal differentiation marker for eosinophils [2]. To more directly determine when during eosinophil differentiation Siglec-8 expression occurs, in vitro culture systems were used to generate human eosinophils from CD34+ precursors. Developing eosinophils do not express mRNA or protein for Siglec-8 until day 6, with maximum expression occurring by day 21 (Fig. 1). The Siglec-8 mRNA and protein expression occurs in a similar time-frame as the initial expression of mRNA encoding secondary granule proteins such as MBP1 between 3 and 4 days [25] and the first appearance of eosinophilic myelocytes containing fast-green+ and eosin+ secondary granules on days 6–9 of culture under identical conditions [18]. Also, this time-frame resembles what was reported for the kinetics of Siglec-8 expression in CD34+-derived human mast cells [15].
Next, to determine whether various immature leukemic progenitors (from patients with AML, MDS, or CML) and mast cell-like and eosinophil-like cell lines express Siglec-8, immunofluorescence and flow cytometric analyses were performed on a range of samples and cells. As shown in Table II, none of the samples tested expressed Siglec-8, with the exception of HMC-1 cells, especially the HMC-1.2 subline. HMC-1.2 cells are derived from a patient with mast cell leukemia. The cell line is clearly of mast cell origin, and in contrast to HMC-1.1 cells (a subclone of HMC-1), HMC-1.2 cells express the KIT mutant D816V that may induce some differentiation [26].
Table II.
Expression of Siglec-8 on leukemic progenitors in AML, MDS, CML, as well as on various human hematopoietic cell lines
| Type of cells (number of samples) | Percent positive |
|---|---|
| CD34+/CD38− leukemic progenitor cells | |
| AML FAB types: M1, M2, M5 (n=1 each) | <5 |
| MDS FAB type: MDS/MPN (n=2), RAEB-2 (n=1) | <5 |
| CML (n=2) | <5 |
| CD34+/CD38+ leukemic progenitor cells | |
| AML FAB types: M1, M2, M5 (n=1 each) | <5 |
| MDS FAB type: MDS/MPN (n=2), RAEB-2 (n=1) | <5 |
| CML (n=2) | <5 |
| Cell linesa | |
| EOL-1 (n=5) | <5 |
| HMC-1.1 (n=4) | 10–20 |
| HMC-1.2 (n=5) | >90 |
| KU812 (n=3) | <5 |
| K562 (n=2) | <5 |
| HL60 (n=3) | <5 |
| KG-1 (n=5) | <5 |
| KG1a (n=1) | <5 |
| AML14 | 0b |
| AML14.3D10 | 0b |
EOL-1 is an eosinophilic leukemia line; HMC-1.1 is a subclone of the HMC-1 mast cell line; HMC-1.2 is a line derived from a patient with KIT mutant D816V mast cell leukemia; KU812 is a basophilic precursor line; K562 is an erythroleukemia line; HL60 is a promyelocytic line that can differentiate into neutrophils, eosinophils, and basophils; KG-1 is an erythroleukemia line that differentiates into granulocyte and macrophage-like cells; KG1a is a line derived from KG-1 that is less mature and differentiates more poorly; AML14 is a myeloblast line; AML14.3D10 is an eosinophil myelocyte line
Siglec-8 mRNA expression was detected in both eosinophil-committed AML14 (not shown) and AML14.3D10, but we did not detect Siglec-8 protein expression by either Western blotting or FACS using both mouse monoclonal and sheep polyclonal antibodies to Siglec-8
To determine if expression of Siglec-8 was lost on eosinophils and basophils from selected hematologic malignancies, immunofluorescence and flow cytometry were performed on samples from subjects with HES, CEL, or CML. In every sample tested (see Table III), Siglec-8 surface expression was detectable and at levels similar to those found on normal cells (data not shown). When similar testing of culture-derived or bone marrow mast cells from subjects with ISM or aplastic anemia for Siglec-8 expression was performed (Table IV), readily detectable levels were seen in all cases except for early precursors of culture-derived mast cells, as was reported previously [15]. Collectively, these data support the concepts that Siglec-8 is a late differentiation marker that is retained on eosinophils, basophils, and mast cells, even in hematologic malignancies, and could be a relevant target for these cells in these diseases.
Table III.
Expression of Siglec-8 on eosinophils and basophils in peripheral blood of subjects with HES, CEL, and CML
| Type of cells (number of samples) | Percent positive |
| Eosinophils | |
| HES (n=4) | >95 |
| CEL (n=2) | >95 |
| CML (n=2) | >95 |
| Basophils | |
| HES (n=4) | Not determined |
| CEL (n=2) | >90 |
| CML (n=2) | >70 |
Table IV.
Expression of Siglec-8 on cord blood progenitor-derived mast cells and on primary bone marrow mast cells in indolent systemic mastocytosis and aplastic anemia
| Type of cells (number of samples) | Percent positive |
|---|---|
| Cultured cord blood progenitor-derived mast cells | |
| Immature, <10 weeks (n=1) | <5 |
| Mature, >15 weeks (n=2) | >70 |
| Primary bone marrow mast cells | |
| ISM (n=3) | >95 |
| Aplastic anemia (n=1) | >95 |
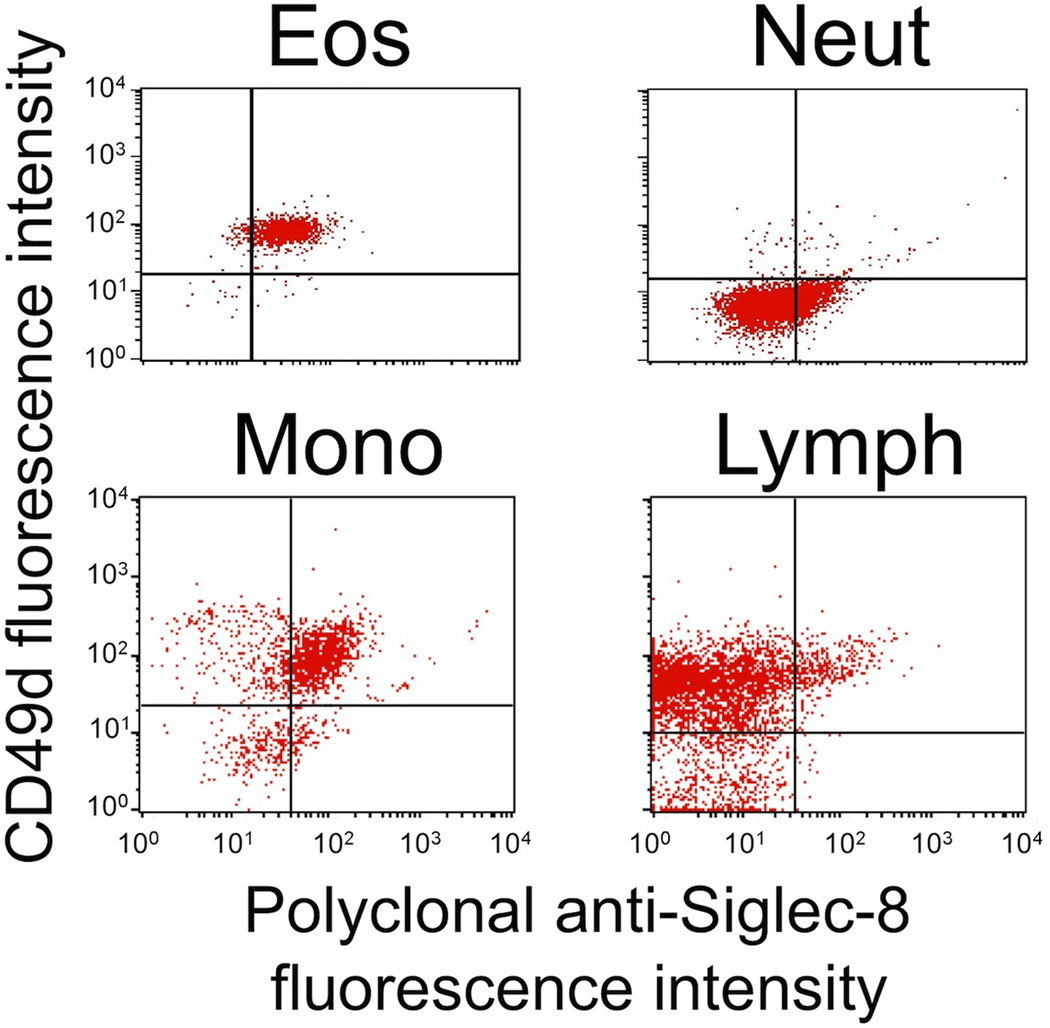
Because of recent publications suggesting that some non-human primates and dogs may express Siglec-8-like genes [27–29], in the next series of studies, both monoclonal and polyclonal antibodies recognizing Siglec-8 were tested using indirect immunofluorescence and flow cytometry for their ability to recognize leukocytes from non-human primates and dogs, as well as the dog C2 mastocytoma cell line. As shown in Fig. 2, in baboons, the one species for which genomic and genetic analyses have suggested the presence of a Siglec-8 ortholog [28], we were indeed able to demonstrate that eosinophils label with a polyclonal, affinity-purified antibody to Siglec-8. Unexpectedly, baboon monocytes also consistently labeled with this Siglec-8 polyclonal antibody, while neutrophils stained weakly and a small subset of lymphocytes also labeled. Whether this labeling truly represents Siglec-8 expression by these cells, or cross-reactivity with other siglecs, was not determined. Unfortunately, none of the Siglec-8 monoclonal antibodies (2C4, 2E2, and 7C9) labeled baboon eosinophils or any other leukocyte subset (data not shown).
Fig. 2.
Polyclonal Siglec-8 labeling of eosinophils (Eos), neutrophils (Neut), monocytes (Mono), and lymphocytes (Lymph) in baboon blood samples. Shown are results from a single experiment representative of three experiments. Eosinophils and neutrophils are shown separately because of the need to use different quad-stat settings due to differences in background autofluorescence seen with control antibodies. Note that the gating strategies for these experiments included light scatter to separate granulocytes from monocytes and lymphocytes, and then CD49d antibody labeling was used to distinguish eosinophils (positive) from neutrophils (negative). Also note that the CD49d+ monocytes represented contamination of the scatter gate with neutrophils
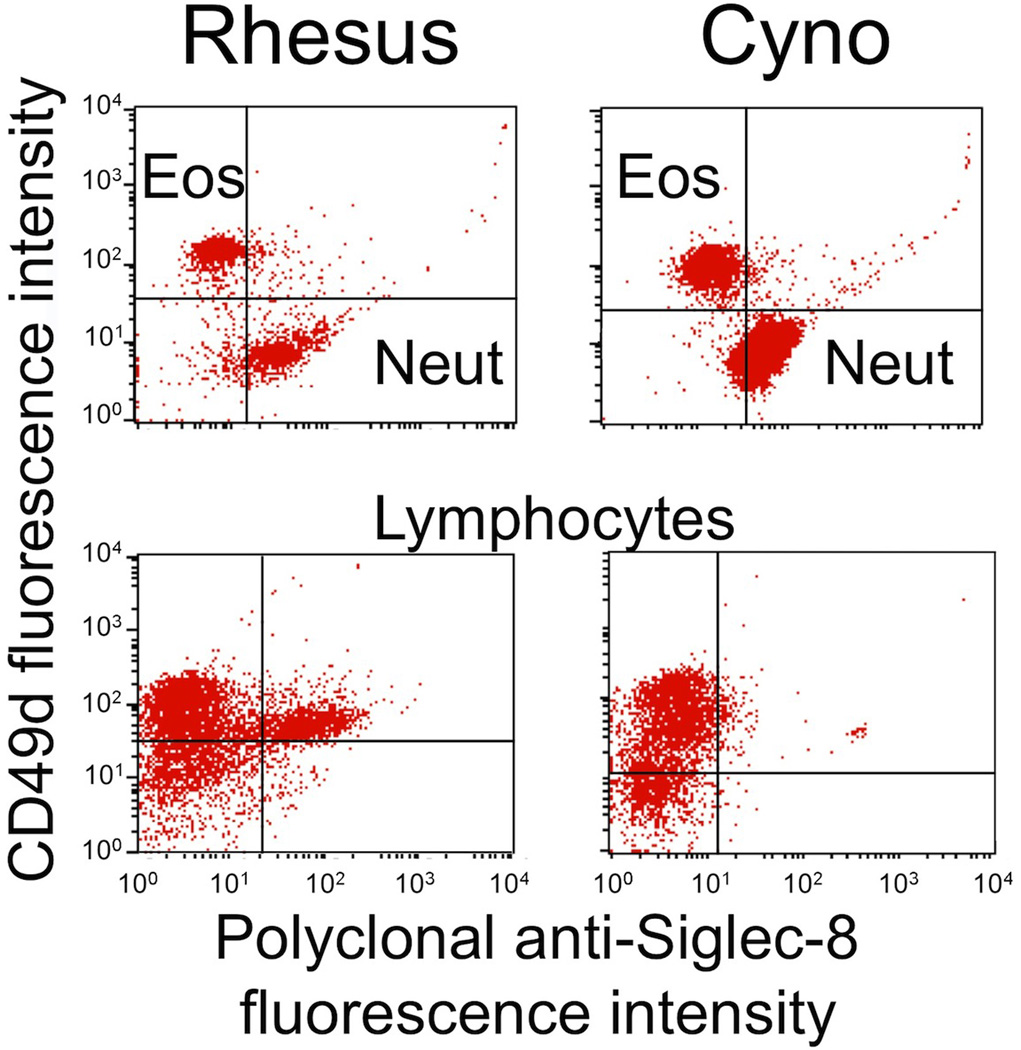
Prior genetic and genomic analyses suggested that neither rhesus nor cynomolgus monkey leukocytes would possess a Siglec-8 ortholog, so labeling with Siglec-8 antibodies was not expected. While this was indeed the case for eosinophils (CD49d+ granulocytes), we were surprised to observe that both rhesus and cynomolgus monkey neutrophils stained well with the Siglec-8 polyclonal antibody (Fig. 3). Unlike for baboon monocytes, no labeling of rhesus or cynomolgus monocytes was detected using the polyclonal antibody. A subset of rhesus lymphocytes, and a smaller subset of cynomolgus lymphocytes, also stained with Siglec-8 polyclonal antibody. As expected, no labeling of any leukocyte subset, including eosinophils, was seen with 2C4, 2E2, or 7C9 monoclonal antibodies (data not shown). Finally, despite the suggestion that dog leukocytes may possess Siglec-8-like genes, neither the polyclonal nor monoclonal Siglec-8 antibodies recognized dog leukocytes in whole blood or dog C2 mastocytoma cells (data not shown).
Fig. 3.
Polyclonal Siglec-8 labeling of eosinophils (Eos), neutrophils (Neut), monocytes (Mono), and lymphocytes (Lymph) in rhesus and cynomolgus monkey (Cyno) blood samples. Shown are results from a single experiment representative of three experiments. Note that the gating strategies for these experiments included light scatter to separate granulocytes from monocytes and lymphocytes, and then CD49d antibody labeling was used to distinguish eosinophils (positive) from neutrophils (negative)
Discussion
The goal of the present experiments was to investigate the expression of Siglec-8 on human and non-human cells in order to better define its utility as a potential target for therapies of malignancies of hematopoietic origin. Studies demonstrated that CD34+ precursor cells in humans do not express Siglec-8. Siglec-8 appears late in eosinophil and mast cell differentiation, and is maintained in CEL and chronic myelogenous leukemia on both on eosinophils and basophils. Primary bone marrow mast cells in ISM and aplastic syndromes maintain Siglec-8 expression as well. In contrast, among the wide range of cell lines tested, including those considered eosinophil-like (EOL-1), eosinophil-committed (AML14), or eosinophil-differentiated (AML14.3D10), none expressed surface Siglec-8 except for the HMC-1.2 cell line and perhaps weakly on the HMC-1.1 cell line. Of note, both of the eosinophil-committed AML14 cell lines expressed Siglec-8 mRNA transcripts identified by RT-PCR (data not shown), but no Siglec-8 protein, perhaps reflecting their hematopoietic immaturity as eosinophil myeloblasts (AML14) and myelocytes (AML14.3D10). Studies also showed that there is no non-human species in which existing Siglec-8 monoclonal antibodies recognize eosinophils, but there appears to be a molecule recognized by a polyclonal Siglec-8 antibody on baboon eosinophils and monocytes. This same polyclonal antibody also recognized neutrophils, monocytes, and some lymphocytes in rhesus and cynomolgus monkeys but not eosinophils, suggesting that it may be recognizing something other than Siglec-8. Finally, Siglec-8 antibodies also do not recognize any dog leukocytes including eosinophils.
The exact reasons for the differences in recognition between the polyclonal and monoclonal Siglec-8 reagents are unclear. One possibility is that the polyclonal antibody recognizes the 6′-sulfated sialyl Lewis X binding epitope on Siglec-8, whereas the monoclonals did not [4]. Another possibility is simply the known ability of polyclonal antibodies to often recognize more than one epitope on a given protein. This may also explain why the polyclonal antibody unexpectedly recognized surface antigens on baboon monocytes, and on neutrophils and some lymphocytes of both rhesus and cynomolgus monkey leukocytes. Given the patterns of cellular staining and the fact that Siglec-8 is most similar in sequence to human Siglec-7 (≈75% identical) and human Siglec-9 (≈73% identical) compared to other human siglecs (≈55–30% sequence identity), we speculate that these may be what the polyclonal antibody is detecting.
By searching available online databases, Siglec-8 orthologs could not be identified in rhesus, macaque, cynomolgus macaque [from a sequence search of the Macaca mulatta (rhesus macaque) genome or Macaca fascicularis (cynomolgus macaque) sequences submitted to NCBI], mouse, or rat. This observation is consistent with other published reports [27, 28]. However, Siglec-8 orthologs are present and highly conserved in human, chimpanzee (≈98% identical to human), and orangutan (≈95% identical to human), and syntenic regions for human, chimpanzee, and baboon have been described [28]. Although a Siglec-8 ortholog has been identified in orangutan, the CD33-like siglec cluster has not been characterized.
There is precedent for the development of siglec-targeting drugs [30]. Myelotarg™ (gemtuzumab ozogamicin) was a toxin-conjugated anti-CD33 (Siglec-3) antibody available for use in the treatment of certain leukemias. It was voluntarily withdrawn from the market in 2010 due to safety and efficacy concerns. CD33, unlike Siglec-8, is expressed on immature bone marrow-derived cells, and this suggests that some of the marrow toxicity seen with CD33 would not be expected with a Siglec-8-targeting agent. While not yet in clinical trials, preclinical testing of CD22 (Siglec-2) targeting agents have shown promise in treating B cell lymphomas [31]. And a recent report suggested that siRNA knockdown of Siglec-6 in human B cells among a panel of inhibitory receptors was a particularly effective way of restoring memory B-cell proliferative responses in subjects infected with HIV, where their B cells display an “exhausted” phenotype [32]. Given the expression of Siglec-8 on eosinophils, basophils, and mast cells, potential clinical indications of a Siglec-8-targeting antibody or other agent might include asthma, allergic rhinitis, atopic dermatitis, nasal polyposis, urticaria, eosinophilic gastrointestinal disorders, Churg Strauss syndrome, hypereosinophilic syndromes, or malignancies such as systemic mastocytosis, eosinophilic leukemia, and basophilic leukemias [33].
Conclusion
These data suggest that while Siglec-8 may be a useful surface marker of mature human eosinophils, mast cells, and basophils that is retained on malignancies of hematopoietic origin, including CEL, CML, and ISM, and on eosinophils in patients with HES, there appears to be no animal species that would be suitable for preclinical testing of such monoclonal antibodies. Fortunately, administration of Siglec-8 antibodies would not be expected to have any broad detrimental effects on early stages of hematopoiesis for these lineages. However, since human Siglec-8 and mouse Siglec-F, the latter being a mouse siglec and a proposed functional paralog [3], have both evolved to recognize the same glycan ligand, namely 6′-sulfated sialyl Lewis X [9], murine cells could theoretically be used for preclinical testing of a 6′-sulfated sialyl Lewis X-based glycomimetic or targeting agent. For monoclonal antibody development, perhaps a more suitable approach for preclinical testing might be to create a transgenic mouse expressing Siglec-8 in eosinophils and mast cells.
Acknowledgments
The authors thank Warren Gold, Cassandra Paul, Michael Baumann, and Joseph Butterfield for generously providing cell lines used in this paper. We also thank Joe Chrest for assistance with cell sorting.
Supported in part by grants from the National Institutes of Health (AI41472 and AI72265 to BSB), by the Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich (SFB #018-20 to PV), the Campaign Urging Research on Eosinophilic Diseases (CURED, to SJA), and a pilot grant (to SJA) from The University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center For Research Resources. The work of the Center is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health. Dr. Bochner also received support as a Cosner Scholar in Translational Research from The Johns Hopkins University School of Medicine, and is a co-author on existing and pending Siglec-8-related patents. If Siglec-8-related products are developed in the future, Dr. Bochner may be entitled to a share of royalties received by The Johns Hopkins University on the potential sales of such products.
Footnotes
The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Contributor Information
Sherry A. Hudson, Department of Medicine, Division of Allergy and Clinical Immunology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Harald Herrmann, Department of Internal Medicine I, Division of Hematology and Hemostaseology, Medical University of Vienna, Vienna, Austria.
Jian Du, Departments of Biochemistry and Molecular Genetics, and Medicine—Section of Hematology Oncology, College of Medicine, University of Illinois at Chicago, Chicago, IL, USA.
Paul Cox, Sanofi-Aventis US, LLC, Bridgewater, NJ, USA.
El-Bdaoui Haddad, Sanofi-Aventis US, LLC, Bridgewater, NJ, USA.
Barbara Butler, Sanofi-Aventis US, LLC, Bridgewater, NJ, USA.
Paul R. Crocker, Division of Cell Biology and Immunology, Wellcome Trust Biocentre, College of Life Sciences, University of Dundee, Dundee, UK
Steven J. Ackerman, Departments of Biochemistry and Molecular Genetics, and Medicine—Section of Hematology Oncology, College of Medicine, University of Illinois at Chicago, Chicago, IL, USA
Peter Valent, Department of Internal Medicine I, Division of Hematology and Hemostaseology, Medical University of Vienna, Vienna, Austria.
Bruce S. Bochner, Email: bbochner@jhmi.edu, Department of Medicine, Division of Allergy and Clinical Immunology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Division of Allergy and Clinical Immunology, Johns Hopkins Asthma and Allergy Center, 5501 Hopkins Bayview Circle, Baltimore, MD 21224-6821, USA.
References
- 1.Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8: a novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–866. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 2.Kikly KK, Bochner BS, Freeman S, Tan KB, Gallagher KT, D'Alessio K, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells and basophils. J Allergy Clin Immunol. 2000;105:1093–1100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 3.Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39:317–324. doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudson SA, Bovin N, Schnaar RL, Crocker PR, Bochner BS. Eosinophil-selective binding and pro-apoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6'-sulfated sialyl Lewis X. J Pharmacol Exp Ther. 2009;330:608–612. doi: 10.1124/jpet.109.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 6.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005;336:918–924. doi: 10.1016/j.bbrc.2005.08.202. [DOI] [PubMed] [Google Scholar]
- 7.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–124. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoi H, Choi OH, Hubbard W, Lee H-S, Canning BJ, Lee HH, et al. Inhibition of FcεRI-dependent mediator release and calcium flux from human mast cells by Siglec-8 engagement. J Allergy Clin Immunol. 2008;121:499–505. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6'-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–1135. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 10.Varki A, Angata T. Siglecs—the major sub-family of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 11.Cho JY, Song DJ, Pham A, Rosenthal P, Miller M, Dayan S, et al. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 2010;11:154. doi: 10.1186/1465-9921-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song DJ, Cho JY, Miller M, Strangman W, Zhang M, Varki A, et al. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin Immunol. 2009;131:157–169. doi: 10.1016/j.clim.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, et al. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183:5333–5341. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–1163. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokoi H, Myers A, Matsumoto K, Crocker PR, Saito H, Bochner BS. Alteration and acquisition of Siglecs during in vitro maturation of CD34+ progenitors into human mast cells. Allergy. 2006;61:769–776. doi: 10.1111/j.1398-9995.2006.01133.x. [DOI] [PubMed] [Google Scholar]
- 16.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lilliehook I, Johannisson A, Hakansson L. Expression of adhesion and Fcγ-receptors on canine blood eosinophils and neutrophils studied by anti-human monoclonal antibodies. Vet Immunol Immunopathol. 1998;61:181–193. doi: 10.1016/s0165-2427(97)00154-2. [DOI] [PubMed] [Google Scholar]
- 18.Bedi R, Du J, Sharma AK, Gomes I, Ackerman SJ. Human C/EBP-epsilon activator and repressor isoforms differentially reprogram myeloid lineage commitment and differentiation. Blood. 2009;113:317–327. doi: 10.1182/blood-2008-02-139741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirkina I, Schweighoffer T, Kricek F. Inhibition of human cord blood-derived mast cell responses by anti-FcεRI mAb 15/1 versus anti-IgE omalizumab. Immunol Lett. 2007;109:120–128. doi: 10.1016/j.imlet.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Aichberger KJ, Gleixner KV, Mirkina I, Cerny-Reiterer S, Peter B, Ferenc V, et al. Identification of proapoptotic Bim as a tumor suppressor in neoplastic mast cells: role of KIT D816V and effects of various targeted drugs. Blood. 2009;114:5342–5351. doi: 10.1182/blood-2008-08-175190. [DOI] [PubMed] [Google Scholar]
- 21.Baumann MA, Paul CC. The AML14 and AML14.3D10 cell lines: a long-overdue model for the study of eosinophils and more. Stem Cells. 1998;16:16–24. doi: 10.1002/stem.160016. [DOI] [PubMed] [Google Scholar]
- 22.Du J, Stankiewicz MJ, Liu Y, Xi Q, Schmitz JE, Lekstrom-Himes JA, et al. Novel combinatorial interactions of GATA-1, PU. 1, and C/EBPepsilon isoforms regulate transcription of the gene encoding eosinophil granule major basic protein. J Biol Chem. 2002;277:43481–43494. doi: 10.1074/jbc.M204777200. [DOI] [PubMed] [Google Scholar]
- 23.Du J, Alsayed YM, Xin F, Ackerman SJ, Platanias LC. Engagement of the CrkL adapter in interleukin-5 signaling in eosinophils. J Biol Chem. 2000;275:33167–33175. doi: 10.1074/jbc.M003655200. [DOI] [PubMed] [Google Scholar]
- 24.Vardiman J, Melo J, Baccarani M, Thiele J. Chronic myelogenous leukemia, BCR/ABL1 positive. In: Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al., editors. World Health Organization (WHO) classification of tumours pathology & genetics tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 32–37. [Google Scholar]
- 25.Ellis AK, Ackerman SJ, Crawford L, Du J, Bedi R, Denburg JA. Cord blood molecular biomarkers of eosinophilopoiesis: kinetic analysis of GATA-1, MBP1 and IL-5R alpha mRNA expression. Pediatr Allergy Immunol. 2010;21:640–648. doi: 10.1111/j.1399-3038.2010.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayerhofer M, Gleixner KV, Hoelbl A, Florian S, Hoermann G, Aichberger KJ, et al. Unique effects of KIT D816V in BaF3 cells: induction of cluster formation, histamine synthesis, and early mast cell differentiation antigens. J Immunol. 2008;180:5466–5476. doi: 10.4049/jimmunol.180.8.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angata T, Hingorani R, Varki NM, Varki A. Cloning and characterization of a novel mouse Siglec, mSiglec-F: differential evolution of the mouse and human (CD33) Siglec-3-related gene clusters. J Biol Chem. 2001;276:45128–45136. doi: 10.1074/jbc.M108573200. [DOI] [PubMed] [Google Scholar]
- 28.Angata T, Margulies EH, Green ED, Varki A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci USA. 2004;101:13251–13256. doi: 10.1073/pnas.0404833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao H, de Bono B, Belov K, Wong ES, Trowsdale J, Barrow AD. Comparative genomics indicates the mammalian CD33rSiglec locus evolved by an ancient large-scale inverse duplication and suggests all Siglecs share a common ancestral region. Immunogenetics. 2009;61:401–417. doi: 10.1007/s00251-009-0372-0. [DOI] [PubMed] [Google Scholar]
- 30.O'Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci. 2009;30:240–248. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen WC, Completo GC, Sigal DS, Crocker PR, Saven A, Paulson JC. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood. 2010;115:4778–4786. doi: 10.1182/blood-2009-12-257386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kardava L, Moir S, Wang W, Ho J, Buckner CM, Posada JG, et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Invest. 2011;121:2614–2624. doi: 10.1172/JCI45685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Gunten S, Bochner BS. Expression and function of Siglec-8 in human eosinophils, basophils and mast cells. In: Pawankar R, Holgate S, Rosenwasser LJ, editors. Allergy frontiers: classification and pathomechanisms. Tokyo: Springer; 2009. pp. 297–313. [Google Scholar]