Abstract
Roots of Arabidopsis thaliana exhibit stable diurnal growth profiles that are controlled by the circadian clock. Here we describe the effects of mutations in leaf starch metabolism on the diurnal root growth characteristics of Arabidopsis thaliana. High temporal and spatial resolution video imaging was performed to quantify the growth kinetics of Arabidopsis wild-type as well as pgm, sex1, mex1, dpe1 and dpe2 starch metabolism mutants grown in three different photoperiods. As a result, root growth patterns of all genotypes displayed characteristic modifications in their diurnal kinetics that were also affected by the photoperiod. To further investigate the role of starch derived substrate deficiency on root growth, the effect of 0.05% extracellular sucrose was studied in 12 h-12 h light-dark cycles.
Key words: diurnal root growth kinetics, dpe1, dpe2, mex1, pgm, sex1, starch metabolism, video imaging
Root growth of Arabidopsis thaliana is highly rhythmic with respect to the time of the day.1–3 In general, root growth rates increase at night while most of the light period is characterized by declining elongation rates. Since a slow oscillation in root growth rate with a periodicity of approximately 24 h persists in free running conditions it was demonstrated that the circadian clock mediates these daily fluctuations.1 Root growth at night is fueled by the degradation of starch within the leaves. Thus, a correspondence between the time taken to degrade starch reserves and the length of the night is important to optimize growth in C-limiting conditions. Gibon et al. observed a strong correlation between the rate of starch degradation and the relative growth rate when Arabidopsis Col-0 was grown in a range of different photoperiods.4 Therefore, to avoid periods of C starvation at the end of the night the circadian clock was postulated to function as a timer that adjusts degradation of starch to the prevailing length of the night.1,5
Root growth strongly depends on the supply of sucrose from the leaves. To investigate the effects of substrate depletion on root elongation at night, 12-day-old seedlings of Col-0, pgm and sex1 growing in a 16 h photoperiod were previously investigated by digital time resolved video imaging.1 As a result, the diel growth response was strongly modified in pgm and sex1 as compared to the wild-type. Both mutants showed a pronounced inhibition of growth during the night and a gradual recovery of growth during the light period. To substantiate these findings, we here report on the root elongation patterns of additional mutants in starch metabolism, e.g., mex1, dpe1 and dpe2 detected at different photoperiods and elevated external sucrose supply.
Diurnal Growth Profiles of Starch Metabolism Mutants in Short Days
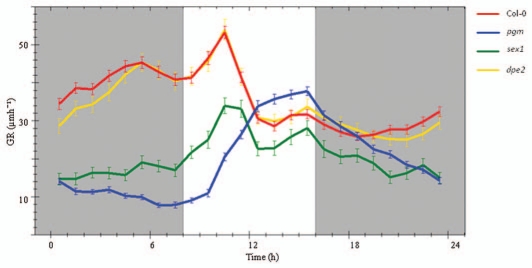
We already reported on the diurnal growth profiles of Arabidopsis wild-type roots under short day photoperiods (8 h light/16 h dark).1 In parallel, diurnal growth profiles of wild-type Col-0 seedlings displayed a strong increase in growth rate immediately after light on that was followed by a gradual decline during most parts of the light period (Fig. 1). A similar pattern in growth rate was detected in the dpe2 mutant (Fig. 1). However, due to the mutation in the disproportionating enzyme growth rates were reduced in this mutant between 20 p.m. and 4 a.m. in comparison to the wild-type (Fig. 1). This reduction in root elongation rate of the mutant indicates a deficiency in the supply of sucrose to the root at night. In accordance with measurements performed under long day conditions root growth rates of the pgm and sex1 mutants when exposed to short day photoperiods declined strongly during the dark period (Fig. 1).1 A time delay of 2 h was required to accelerate growth again in the roots of pgm after the induction of light (Fig. 1). Due to the absence of starch in the pgm mutant severe carbon starvation symptoms developed in the dark period that gave rise to delayed recovery of root growth upon illumination.
Figure 1.
Root extension pattern of Col-0 (n = 24), pgm (n = 6), sex1 (n = 8), dpe2 (n = 13) seedlings in 8 h photoperiods. Seedlings were 21 days old and maintained at a day night temperature of 21°c. Measurements were performed on six consecutive days and averaged. GR: growth rate. Growth rates of Arabidopsis roots were recorded as described previously in reference 10–12.
Modulation of Root Elongation Kinetics by 0.05% Extracellular Sucrose in Equal Day/Night Length
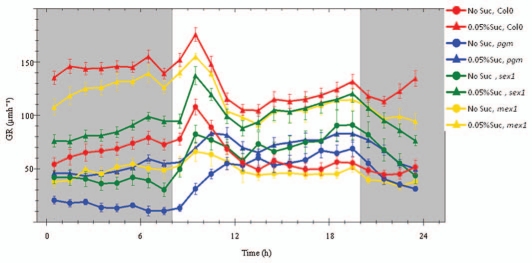
To further investigate the impact of the photoperiod and the sucrose supply for root expansion growth we exposed wild-type and mutant seedlings to 12 h/12 h photoperiods (equal nights; Fig. 2). Due to extension of the photoperiod absolute growth rates of Col-0 seedlings increased as described previously.1 The delayed acceleration of root growth that emerged in the pgm mutants upon light on was almost abolished in equal photoperiods (Fig. 2). Increase in root elongation rates of mex1 in the dark period occurred with a 1 h delay compared to the wild-type, probably indicating that sucrose supply to the roots is modified in the mutant.
Figure 2.
Modulation in the diurnal root extension pattern of Col-0, pgm, sex1 and mex1 due to 0.05% sucrose. 24 h extension kinetics of Col-0 (n = 10), pgm (n = 5), sex1 (n = 6) and mex1 (n = 2) growing in no sucrose were compared with seedlings growing in 0.05% extracellular sucrose (n = 10, n = 8, n = 5 and n = 3, respectively) in 12 h photoperiods. Values represent averages of three consecutive days plus SE.
Inclusion of 0.05% sucrose in the growth medium further increased the absolute root extension rates of wild-type Col-0 and the starch metabolism mutants pgm, sex1, mex1 (Fig. 2). Col-0 seedlings supplied with extracellular sucrose exhibited a shorter growth inhibition period after transition to darkness which was followed by 4 h of strong increase in extension rate (Fig. 2). The growth rate stayed almost constant during the last part of the night. This phase resembled the growth behaviour of WS wild-type seedlings grown in the absence of sucrose.1 Addition of 0.05% of sucrose to the growth medium of pgm seedlings induced a major change in the diurnal response (Fig. 2). The strong and progressive inhibition of growth during the dark period was significantly repressed. The kinetics during the light period also became more similar to those of the wild type with a peak 3 h after illumination. In contrast to absence of sucrose the maximum elongation rate occurred between 10 and 11 am.
The sex1 mutant also grew marginally different in the presence of 0.05% extra-cellular sucrose than in absence. While the strong inhibition of growth during the first 4 h after dusk was similar to the control seedlings, throughout the rest of the night we detected a slight recovery of growth. Upon light exposure, elongation rate decreased for an hour. In the second hour of illumination a strong increase in growth rate emerged which was followed by a 3 h decrease.
In general, root growth kinetics of mex1 seedlings closely resemble that of the wild-type (Fig. 2). It had been reported that due to the mutation in the chloroplast envelope maltose transporter (MEX) gene, the predominant route for carbohydrate export from chloroplasts at night, the maltose levels in leaves of this mutant are at least 40 times as high as those of wildtype.6 Starch levels are elevated and the rates of both synthesis and degradation are reduced. Levels of sucrose and hexoses are greatly elevated during the day but fall to levels comparable to those of wild-type leaves at night.6 However, the existence of a glucose transporter in the chloroplast envelope potentially allows the export of glucose to the cytosol, thus providing substrate for root elongation at night.7
Diurnal Growth Pattern of Starch Mutants under Long Day Conditions
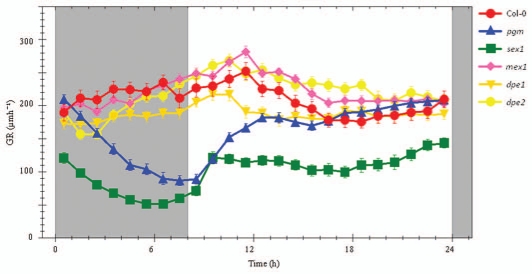
Root growth kinetics of several Arabidopsis mutants compromised in starch metabolism as, pgm, sex1, mex1, dpe1 and dpe2 were compared to wild-type under long day conditions. Col-0 exhibited the typical pattern described before. The pattern of pgm and sex1 resemble those described in Yazdanbakhsh et al.1 Root growth kinetics obtained from mex1 and dpe1 seedlings resembled Col-0 wild-type (Fig. 3). However, small deviations were detected. mex1 showed slightly decreased elongation rates after dusk for about 5 h and marginally increased rates in the light. Elongation of dpe1 proceeded at reduced rates in comparison to wild-type during the night and the first part of the light period. dpe2 displayed a more pronounced inhibition of elongation rate during the first 2–3 h of the dark period than the wild-type. Subsequently this decrease recovered towards the end of the night. At this time the growth kinetics of dpe2 closely resembled that of the wild type.
Figure 3.
Diurnal extension profiles of Col-0 (n = 8), pgm (n = 9), sex1 (n = 4), mex1 (n = 6), dpe1 (n = 11) and dpe2 (n = 9). Root extension of 12-day-old seedlings grown in 16 h photoperiods, T = 21°c was monitored. Values represent averages of four consecutive days plus SE.
The activities of most of the enzymes involved in starch metabolism had been reported not to be significantly different in dpe1 compared to the wild-type. Exceptions are enzyme activities of β-amylase being twice that of wild-type and starch phosphorylase which is slightly increased.8 Due to the mutation in the disproportionating enzyme, a plastidial α-1,4-glucanotransferase, during the diurnal cycle the amount of leaf starch is higher in dpe1 than in wild-type.8 However, the amounts of starch synthesized and degraded are lower in dpe1. In contrast to the wild type at periods of starch degradation, a large accumulation of malto-oligosaccharides occurs in the mutant. Close similarity in the morphological phenotype and the activities of enzymes involved in starch metabolism fit to the congruence in growth behavior of dpe1 and the wild type.
Final Remarks
Based on the deviation of the growth pattern of starch mutants in different day length from the wild type, the mutants can be sorted. pgm representing the most defected pattern would be followed by sex1, dpe2, mex1 and finally dpe1 which displayed kinetics most similar to the Col-0 wild type.9 Interestingly, this order resembles the sequence of reactions in the starch metabolic pathway.9 pgm seedlings are deficient in starch accumulation. sex1 exhibits reduced rates of starch degradation suffering from a mutation in a downstream step in starch metabolism. Disproportionating enzyme (DPE1) has been shown to be required for conversion of maltotriose into larger maltooligosaccharides, which can be further attacked by amylolytic enzymes, and Glc, which is exported from the plastid.9 mex1 was shown to accumulate maltose and MEX1 encodes a novel transporter that is required for maltose export from the chloroplast.6 DPE 2 has been shown to transfer a glucosyl unit from maltose to glycogen in vitro, suggesting a novel pathway of carbohydrate metabolism in the cytosol of Arabidopsis leaves at night.8 The mutation in this gene leads to accumulation of very high levels of maltose. However, the closer similarity in growth kinetics between dpe1 and wild-type than dpe2 might reflect the existence of two parallel routes for the export of starch degradation products from the chloroplast at night: the major flux of maltose by MEX1 and a minor flux of glucose derived from disproportionation of maltotriose by DPE1, by the glucose transporter.6,9
References
- 1.Yazdanbakhsh N, Sulpice R, Graf A, Stitt M, Fisahn J. Circadian control of root elongation and C partitioning in Arabidopsis thaliana. Plant Cell Environ. 2011;34:877–894. doi: 10.1111/j-13653020.2011.02286.x. [DOI] [PubMed] [Google Scholar]
- 2.Yazdanbakhsh N, Fisahn J. Stable diurnal growth rhythms modulate root elongation of Arabidopsis thaliana. Plant Root. 2011;5:17–23. [Google Scholar]
- 3.Yazdanbakhsh N, Fisahn J. Analysis of Arabidopsis root growth kinetics with high temporal and spatial resolution. Ann Bot. 2010;105:783–791. doi: 10.1093/aob/mcq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibon Y, Pyl ET, Sulpice R, Lunn JE, Höhne M, Günther M, Stitt M. Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown very short photoperiods. Plant Cell Environ. 2009;32:859–874. doi: 10.1111/j.1365-3040.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- 5.Graf A, Schlereth A, Stitt M, Smith A. Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Nat Acad Sci USA. 2010;107:9458–9463. doi: 10.1073/pnas.0914299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niittyla T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC. A previously unkown maltose transporter essential for starch degradation in leaves. Science. 2004;303:87–89. doi: 10.1126/science.1091811. [DOI] [PubMed] [Google Scholar]
- 7.Weber A, Servaites JC, Geiger DR, Kofler H, Hille D, Gruner F, et al. Identification, purification and molecular cloning of a putative plastidic glucose translocator. Plant Cell. 2000;12:787–802. doi: 10.1105/tpc.12.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Critchley JH, Zeeman SC, Takaha T, Smith AM, Smith SM. A critical role for disproportionating enzyme in starch breakdown is revealed by a knockout mutation in Arabidopsis. Plant J. 2001;26:89–100. doi: 10.1046/j.1365-313x.2001.01012.x. [DOI] [PubMed] [Google Scholar]
- 9.Stettler M, Eicke S, Mettler T, Messerli G, Hörtensteiner S, Zeeman SC. Blocking the metabolisam of starch breakdown products in Arabidopsis leaves triggers chloroplast degradation. Mol Plant. 2009;2:1233–1246. doi: 10.1093/mp/ssp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazdanbakhsh N, Fisahn J. Investigation of plant root elongation by screening the surface of a Petri dish. In: Arabnia HR, editor. Proceedings of the 2007 international conference on image processing, computer vision and pattern recognition. CSREA press; 2007. pp. 561–566. [Google Scholar]
- 11.Yazdanbakhsh N, Fisahn J. Development of a robot-based platform applied to simultaneous root growth profiling of seedlings growing in a Petri dish. In: Aggarwal A, Yager R, Sandberg IW, editors. Proceedings of the 8th WSEAS international conference on mathematics and computers in biology and chemistry (MCBC'07) World Scientific and Engineering Academy and Society Press; 2007. pp. 69–73. ISBN: 978-960-845782-9. [Google Scholar]
- 12.Yazdanbakhsh N, Fisahn J. High throughput phenotyping of root growth dynamics, lateral root formation, root architecture and root hair development enabled by PlaRoM. Func Plant Biol. 2009;36:938–946. doi: 10.1071/FP09167. [DOI] [PubMed] [Google Scholar]