Abstract
Acetaminophen (APAP) glucuronidation is thought to occur mainly by UDP-glucuronosyltransferases (UGT) in the UGT1A family. Interindividual variation in APAP glucuronidation is attributed in part to polymorphisms in UGT1As. However, evidence suggests that UGT2B15 may also be important. We evaluated, in a controlled feeding trial, whether APAP conjugation differed by UGT1A6 and UGT2B15 genotypes and whether supplementation of known dietary inducers of UGT (crucifers, soy, and citrus) modulated APAP glucuronidation compared with a diet devoid of fruits and vegetables (F&V). Healthy adults (n = 66) received 1000 mg of APAP orally on days 7 and 14 of each 2-week feeding period and collected saliva and urine over 12 h. Urinary recovery of the percentage of the APAP dose as free APAP was higher (P = 0.02), and the percentage as APAP glucuronide (APAPG) was lower (P = 0.004) in women. The percentage of APAP was higher among UGT1A6*1/*1 genotypes, relative to *1/*2 and *2/*2 genotypes (P = 0.045). For UGT2B15, the percentage of APAPG decreased (P < 0.0001) and that of APAP sulfate increased (P = 0.002) in an allelic dose-dependent manner across genotypes from *1/*1 to *2/*2. There was a significant diet × UGT2B15 genotype interaction for the APAPG ratio (APAPG/total metabolites × 100) (P = 0.03), with *1/*1 genotypes having an approximately 2-fold higher F&V to basal diet difference in response compared with *1/*2 and *2/*2 genotypes. Salivary APAP maximum concentration (Cmax) was significantly higher in women (P = 0.0003), with F&V (P = 0.003), and among UGT1A6*2/*2 and UGT2B15*1/*2 genotypes (P = 0.02 and 0.002, respectively). APAP half-life was longer in UGT2B15*2/*2 genotypes with F&V (P = 0.009). APAP glucuronidation was significantly influenced by the UGT2B15*2 polymorphism, supporting a role in vivo for UGT2B15 in APAP glucuronidation, whereas the contribution of UGT1A6*2 was modest. Selected F&V known to affect UGT activity led to greater glucuronidation and less sulfation.
Introduction
Acetaminophen (APAP; paracetamol) is extensively conjugated with glucuronic acid and sulfate before renal excretion. APAP glucuronidation is thought to occur mainly by UDP-glucuronosyltransferases (UGT) in the UGT1A family, especially UGT1A1, 1A6, and 1A9 in the liver (Court et al., 2001) and UGT1A10 in the gut (Tukey and Strassburg, 2001). However, despite the lack of 1A activity in the Gunn rat, residual APAP glucuronidation occurs in the hepatocytes from these animals, suggesting that there is APAP-glucuronidating capacity among UGT2B enzymes also (Kessler et al., 2002). UGT2B7 and 2B15 have been shown to have some APAP-conjugating activity (Court et al., 2001; Kostrubsky et al., 2005), and additional data suggest that UGT2B15 may be a more important player in APAP conjugation than previously thought (Mutlib et al., 2006).
The reported human interindividual variation in APAP glucuronidation is attributed, in part, to polymorphisms in several UGT1As, although the studies to date are not conclusive. The UGT1A1*28 polymorphism is the genetic basis for benign unconjugated hyperbilirubinemia associated with reduced hepatic UGT conjugation of bilirubin (Gilbert syndrome) (Burchell, 2003). Individuals with Gilbert syndrome are reported to have altered APAP metabolism in some studies (De Morais et al., 1992; Esteban and Perez-Mateo, 1993) but not in others (Ullrich et al., 1987). Rauchschwalbe et al. (2004) also reported no difference in urinary APAP/APAP glucuronide ratios by the UGT1A1*28 genotype but suggested that other polymorphisms in linkage disequilibrium with this variant allele (e.g., UGT1A6*2) may be important. Liver tissue samples homozygous for UGT1A6*2 (characterized by amino acid substitutions T181A, R184S, and S7A) have exhibited a higher rate of α-naphthol and ρ-nitrophenol glucuronidation relative to tissue samples with the other genotypes, whereas recombinant coexpressed UGT1A6*1/*2 allozymes were associated with the lowest enzyme activity (Nagar et al., 2004). These findings are limited though, because ρ-nitrophenol is fairly nonselective for UGT1A6, and results were not replicated in another study, either with ρ-nitrophenol or serotonin, a probe specific for UGT1A6 (Krishnaswamy et al., 2005a). However, the same group subsequently reported greater glucuronidation of acetaminophen by human liver microsomes expressing UGT1A6*2/*2 compared with *1/*1 (Krishnaswamy et al., 2005b). The UGT2B15 Asp85Tyr (UGT2B15*1/*2) polymorphism has also been shown to affect conjugation of several drugs, including that of S-oxazepam and lorazepam (Court et al., 2002; Chung et al., 2005), but the effect on APAP has not been evaluated.
Interindividual variation in UGT activity may also derive from differences in dietary exposures. Bioactive food components in plants (e.g., phytochemicals such as isoflavones, flavonoids, coumarins, monoterpenes, allyl sulfides, isothiocyanates, and those found in green-tea extract) increase hepatic UGT activity in rodents (for review, see Saracino and Lampe, 2007), and several lines of evidence support an effect of diet on UGT activity in humans (Pantuck et al., 1984; Navarro et al., 2009). Thus, both dietary habits and genetic polymorphisms in UGTs may affect the toxicity of xenobiotics and the potency of drugs and steroid hormones by altering the amounts and activities of UGTs in the liver and other tissues. To date, the combined effects of certain exposures (e.g., chemopreventive agents or diet) and UGT polymorphisms on glucuronidation have received little attention; however, several studies suggest that these interactions may play a role in disease risk and treatment response (Harvard, 1973; Bigler et al., 2001; Nowell et al., 2005).
The aims of this study were to test 1) whether APAP conjugation differed by UGT1A6 and UGT2B15 genotypes, 2) whether supplementation of a controlled diet with specific fruits and vegetables (F&V) modulated APAP glucuronidation in humans, and 3) whether the response of APAP glucuronidation to fruit and vegetable supplementation differed by these UGT genotypes. To minimize interindividual variation, we carried out these aims in the context of a randomized, controlled, crossover feeding trial in healthy men and women and monitored UGT activities by measuring salivary acetaminophen pharmacokinetics and urinary glucuronyl- and sulfo-conjugates of APAP.
Materials and Methods
Participants.
Healthy, nonsmoking men and women, aged 20 to 40 years, were recruited for the feeding study as described previously (Chang et al., 2007). Exclusion criteria were medical history of gastrointestinal, hepatic, or renal disorders; current or planned pregnancy; lactation; weight loss or gain greater than 4.5 kg within the past 2 months; major changes in eating habits within the past year; antibiotic use within the past 3 months; body mass index >30 or <18 kg/m2; exercise regimens that require or result in significant short-term dietary changes; current use of prescription or over-the-counter medications (including oral contraceptives); known allergies to acetaminophen, aspirin, and any foods used in the feeding trial; regular exposure to passive smoke; occupational exposure to smoke or organic solvents; food dislikes that would preclude participation in the feeding trial; alcohol intake of greater than 2 drinks/day (720 ml of beer, 240 ml of wine, or 90 ml of hard liquor); and no interest in participating in a controlled feeding trial.
Prospective participants were genotyped for UGT1A1*28, UGT1A6*2, and UGT2B15*2 and those with the desired UGT genotypes and normal serum alanine aminotransferase concentrations (5–42 U/l) were invited to participate in the feeding study. The institutional review board at the Fred Hutchinson Cancer Research Center (FHCRC) approved the study, and informed, written consent was obtained from all participants.
Study Design.
The feeding study was conducted between April 2002 and May 2005. We recruited participants to maintain ratios of *1/*1, *1/*2, or *1/*3 and *2/*2 of UGT1A6 as 2:2:1 and the ratio of *1/*1, *1/*2, and *2/*2 of UGT2B15 as 1:2:1. Participants were randomized, blocked on sex and UGT1A1 and UGT1A6 genotype, using a crossover study design, with each participant receiving two experimental diets, a basal diet and a basal diet supplemented with fruits and vegetables, in an assigned, random order.
The basal, low-phytochemical diet was devoid of fruits, vegetables, whole grains, and herbs and spices. The F&V diet included components of the basal diet supplemented with cruciferous vegetables (broccoli, cabbage, and daikon radish sprouts), soy foods (soy milk, veggies slices, tofu, and roasted soy nuts), and citrus fruits (grapefruit and orange juices, orange/grapefruit segments, and dried orange peel). We chose plant foods that were shown in previous animal and human studies to induce UGT genotypes (Saracino and Lampe, 2007). The amount of fruits and vegetables provided to the participants was at levels equivalent to approximately 10 servings daily; however, we dosed on the basis of participant's body weight (i.e., per 5 kg of body weight); details are provided elsewhere (Chang et al., 2007). The amount of foods in the basal diet was also adjusted to accommodate the added fruits and vegetables, such that both diets provided the same amount of energy and a similar percentage of energy from carbohydrate (56%), protein (16%), and fat (28%). Each diet was consumed for 14 days with at least a 2-week washout period between the diet periods. Participants were instructed to consume only the food and beverages provided to them during both diet periods, maintain their usual physical activity, and not use any type of medication. Dinner was served at the FHCRC Prevention Center Human Nutrition Laboratory dining room under staff supervision, and food for the following day's morning and midday meals and snacks was distributed at that time. Based on 24-h urinary analysis of total isothiocyanate and isoflavone excretion, and daily food check-off forms, participant compliance to the study diet was excellent with consumption of nonstudy food items on fewer than 1% of the study days (Chang et al., 2007).
Several studies suggest that drug glucuronidation varies over the menstrual cycle (Cordaro et al., 1993; Tanaka, 1999). Although women did not all start the study at the same time in their menstrual cycles, the study was designed to facilitate collecting samples during each feeding period at approximately the same point in the cycle; i.e., we used a 14-day study period followed by a 14-day washout, such that the second feeding period started after 28 days. Women were also asked to keep menstrual cycle diaries; these data were used to determine whether sample collection during the two treatments differed by phase of menstrual cycle.
Acetaminophen Test.
On the evenings of day 7 and day 14 of each feeding period, participants completed the APAP test, which consisted of consuming 1000 mg of acetaminophen (two 500-mg Tylenol caplets; McNeil-PPC, Inc., Fort Washington, PA), collecting all urine for the subsequent 12 h and collecting saliva samples at prescribed times. On the days of the APAP test, participants were instructed to consume their dinner meal no later than 5:00 PM and not to eat or drink any study food, except water, until 3 h after taking the APAP. At approximately 7:00 PM (or at least 2 h after the dinner meal), participants collected their first saliva sample and emptied their bladders. Then they took the APAP and collected all urine for the next 12 h and collected saliva over the next 4 h (every 15 min in hour 1, every 30 min in hours 2 and 3, and at the end of hour 4) and at hour 12. Participants recorded the test activities (e.g., times they stopped eating and drinking, took the APAP, collected saliva, and voided the last urine sample) and reported any deviations from the protocol. Urine and saliva samples were stored at 4°C until delivery the following morning. In the laboratory, total urine volume and pH were measured, and the sample was aliquoted into cryovials and stored at −80°C. Saliva samples were centrifuged and filtered and stored at −80°C.
Salivary Acetaminophen Pharmacokinetics.
Salivary APAP was measured on the Cobas Mira Analyzer (Roche Diagnostics, Indianapolis, IN) using a Stanbio Acetaminophen LiquiColor reagent kit (Stanbio Laboratory, Boerne, TX). The assay was standardized using the APAP standard supplied with the reagents. Before analysis, the frozen saliva was thawed, mixed well, and filtered through a 0.45-μm filter to remove particulates. The intra-assay CV was measured at three different concentrations of salivary APAP and was 15.9, 5.4, and 4.3% at concentrations of 3.3, 10, and 16 μg/ml, respectively. The interassay CV was 6% at 15.7 μg/ml. Recovery of APAP added to filtered saliva was 98, 100, and 110% at concentrations of 100, 30, and 10 μg/ml, respectively. Linearity of APAP spiked into saliva was verified over a range of 2 to 100 μg/ml (r2 = 0.99997). Aqueous standards were linear over a range of 2 to 300 μg/ml (r2 = 0.9999). A subset of saliva samples were also run by HPLC with excellent correlation between assay methods (r = 0.988; data not shown).
The pharmacokinetic parameters of salivary APAP were determined using STATA software (version 7.0; StataCorp LP, College Station, TX). The parameters calculated included maximum concentration (Cmax) (milligrams per liter), time of maximum concentration (Tmax) (minutes), elimination rate, half-life (minutes), the AUC0–Tmax (milligrams per minute per liter), extension of the standard time versus concentration curve from the maximum observed time to infinity using linear regression of log concentration (AUC0–∞) (milligrams per minute per liter), and clearance (l/h).
The fitpoints used in the model to calculate the AUC0–∞ were individually determined. For participants with two peaks in the pharmacokinetic plot, the later peak was chosen to determine the fitpoint. The data-handling strategy during modeling included the following: missing time points were deleted; if the last time point collected was higher than the previous one, the higher data point was deleted; data values that were zero were deleted; if the last 4 data points leveled off, the last three time points were deleted; if the last time point was less than or equal to the baseline value, it was deleted because the modeling would produce a negative value; if the baseline value was larger than mean values + 2 S.D., the value was replaced with that of the last collection time point.
HPLC Analysis of Acetaminophen in Urine.
Urine samples were analyzed for APAP, acetaminophen glucuronide (APAPG), and acetaminophen sulfate (APAPS) by HPLC using a model 1100 series HPLC system with an Agilent Zorbax Extend C18 column (3.5 μm, 4.6 × 100 mm; Agilent Technologies, Santa Clara, CA). The mobile phase was 5% acetonitrile with 0.35% trifluoroacetic acid (solvent A) and 95% 0.05 M sodium acetate buffer (solvent B). An isocratic elution at a flow rate of 0.8 ml/min was used with a column temperature of 30°C and a run time of 15 min/20-μl injection. The signal was recorded at 250 nm. Urine samples were thawed, centrifuged, and diluted with water before analysis. The dilution depended on the original volume of the collected urine and ranged from a 1:5 to 1:40 dilution. 1,7-Dimethyl xanthine at a concentration of 100 μg/ml was added as an internal standard to each sample and to the standards. Diluted urine samples were run in duplicate, and a standard curve was run with each set of samples. APAP and APAPG were obtained from Sigma-Aldrich (St. Louis, MO), and APAPS was a gift from McNeil Consumer Healthcare (Fort Washington, PA). Quality-control urine samples were run at the beginning and end of each run. On-column limits of quantification for the various analytes were as follows: APAP, 1 μg/ml; APAPS, 5 μg/ml; and APAPG, 20 μg/ml. Results in the diluted sample that fell below this limit were assigned a concentration halfway between zero and the limits of quantification. Intra-assay CVs were 0.6, 1.7, and 1.0% for APAP, APAPS, and APAPG, respectively. Interassay CVs were 8.9, 10.1, and 7.8% for APAP, APAPS, and APAPG, respectively, at concentrations of 2.14, 27.5, and 50.8 μg/ml.
Measurement of Urinary Isoflavones and Isothiocyanates.
Twenty-four-hour urine samples collected on day 14 were analyzed for isoflavones (genistein, daidzein, equol, and O-desmethylangolensin) and total isothiocyanates as dietary compliance markers of soy food and cruciferous vegetable intake, respectively (Lampe et al., 2001; Chang et al., 2007). Intra- and interassay CVs were <10% for all analytes.
Determination of UGT1A1, UGT1A6, and UGT2B15 Genotypes.
The UGT1A1*28 polymorphism consists of five to eight TA repeats in the promoter region, six TA repeats being the wild type; the others being variants. Genotyping of the UGT1A1 polymorphism (rs8175347) was completed as described previously (Lampe et al., 1999) with the exception of the use of a fluorescently tagged forward primer and analysis of amplified fragments on an ABI 3100 genetic analyzer (Applied Biosystems, Foster City, CA). The two UGT1A6 polymorphisms are missense mutations in exon I, causing a Thr to Ala substitution at amino acid 181 (T181A; rs2070959) and an Arg to Ser substitution at amino acid 184 (R184S; rs1105879) (Ciotti et al., 1997; Lampe et al., 1999). They were determined by sequencing, using polymerase chain reaction conditions described previously (Lampe et al., 1999) and BigDye version 3.2 on an ABI 3100 genetic analyzer. The UGT2B15 polymorphism (rs1902023) consists of a G-to-T point mutation, causing an Asp (*1) to Tyr (*2) substitution at amino acid 85. The polymorphism was determined by sequencing, using polymerase chain reaction conditions described previously (Lampe et al., 2000).
Statistical Analysis.
Based on the available data at the time we designed this study, our a priori hypothesis was that UGT1A6 genotypic differences would be most relevant to APAP metabolism. Thus, our primary analyses are focused on UGT1A6 genotypes. However, additional data later suggested an important role of UGT2B15 (Mutlib et al., 2006); thus, we also present the analyses in the context of the 2B15 genotypes, as we had also recruited on this basis. We evaluated the distribution of demographic variables such as age, height, weight, and body mass index by UGT1A6 genotype and sex. Using analysis of variance, we tested whether there were any differences in these demographic variables, dietary intakes, and urinary dietary biomarkers among the three UGT1A6 genotypes after adjustment for sex. Applying a linear mixed model, we tested the effect of diet, UGT1A6 genotype, sex, and the interaction between diet and UGT genotype on APAP pharmacokinetic parameters and urinary APAP metabolites. We adjusted for diet order in the model and accounted for the correlation of APAP metabolite outcomes between the two feeding periods within participants. UGT2B15 genotypes were evaluated separately using the same model. To determine the effect of phase of menstrual cycle on response to diet, the differences in APAP variables between the F&V diet versus basal diet were calculated and were compared between those women who were in the same phase of cycle versus those who were in different phases when APAP metabolism was measured. Statistical analyses were conducted using SAS (version 8.2; SAS Institute, Cary, NC), and two-sided P value for significance was set at <0.05.
Results
A total of 72 individuals were randomized into the study. Of these, 5 individuals withdrew from the study within the first 5 days of consuming the initial diet, 2 withdrew after the initial diet period, 1 withdrew during the second diet period, and 1 was noncompliant. A total of 66 participants were included in the analysis including partial data for the 3 individuals among the 8 described above who completed only the first diet period. Characteristics of study participants by UGT1A6 genotype are presented in Table 1.
TABLE 1.
Characteristics of 66 participants by UGT1A6 genotype
Data are means ± S.D. or n (%). No significant differences were seen between genotypes at P < 0.05.
|
UGT1A6*1/*1 |
UGT1A6*1/*2 |
UGT1A6*2/*2 |
||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| n | 14 | 14 | 11 | 14 | 8 | 5 |
| Age (yr) | 31.3 ± 5.8 | 28 ± 6.0 | 28.8 ± 6.0 | 30.6 ± 5.4 | 29.9 ± 5.5 | 27.8 ± 5.5 |
| Height (cm) | 178.8 ± 6.5 | 160.7 ± 8.6 | 180.2 ± 7.8 | 169.2 ± 4.7 | 174.4 ± 9.9 | 164.9 ± 6.1 |
| Weight (kg) | 76.3 ± 10.5 | 58.1 ± 9.1 | 74.8 ± 8.8 | 67.8 ± 10.3 | 74.2 ± 11.6 | 59.8 ± 4.7 |
| Body mass index (kg/m2) | 23.8 ± 2.5 | 22.5 ± 2.6 | 23.0 ± 2.2 | 23.7 ± 3.2 | 24.3 ± 2.3 | 22.0 ± 1.0 |
| Ethnicity | ||||||
| White | 10 (71) | 8 (57) | 8 (73) | 10 (71.5) | 6 (75) | 4 (80) |
| Asian | 4 (31) | 6 (43) | 1 (8) | 3 (21.5) | 1 (12.5) | 1 (20) |
| Othera | 0 | 0 | 2 (17) | 1 (7) | 1 (12.5) | 0 |
| Distribution of UGT1A1*28 genotypes | ||||||
| 6/6 | 12 (86) | 11 (79) | 2 (18) | 2 (14) | 1 (12.5) | 0 |
| 6/7 | 2 (14) | 0 | 9 (82) | 11 (79) | 1 (12.5) | 1 (20) |
| 7/7 | 0 | 3 (21) | 0 | 1 (7) | 6 (75) | 4 (80) |
| Distribution of UGT2B15*2 genotypes | ||||||
| *1/*1 | 4 (29) | 2 (14) | 2 (18) | 5 (36) | 3 (37) | 0 |
| *1/*2 | 5 (36) | 7 (50) | 7 (64) | 5 (36) | 5 (63) | 4 (80) |
| *2/*2 | 5 (36) | 5 (36) | 2 (18) | 4 (28) | 0 | 1 (20) |
Other category includes one black, one mixed race, one Pacific Islander, and one unreported.
Urinary Acetaminophen Metabolites.
We measured the amount of free APAP, APAPG, and APAPS excreted in urine over 12 h after APAP administration on days 7 and 14 of the two controlled diet periods. Data were analyzed as the molar percentage of oral APAP dose [percentage as free APAP (%APAP), percentage as APAPG (%APAPG), and percentage of APAPS (%APAPS)] and as percent molar ratios of APAP metabolites excreted [APAPG/(APAP + APAPG + APAPS) × 100 (APAPG ratio), APAPG/APAP, and APAPS/APAP].
Several of the urinary APAP excretion measures differed by sex. In women, %APAP was higher (P = 0.02), %APAPG was lower (P = 0.004), and, thus, APAPG ratio and APAPG/APAP were lower (P = 0.026 and P = 0.002, respectively; data not shown). In contrast, %APAPS and ratio of APAPS/APAP did not differ by sex. There was also a strong sex × diet interaction for %APAPG (P = 0.0045), reflecting statistically significantly higher %APAPG among women (P = 0.004) but not among men (P = 0.3) consuming the F&V diet compared with the basal diet.
Overall, for the UGT1A6 genotype, %APAP was higher (P = 0.045) and the APAPG/APAP ratio was lower (P = 0.02) among *1/*1 relative to *1/*2 and *2/*2 individuals. For UGT2B15, %APAPG, the APAPG ratio, and APAPG/APAP decreased (P < 0.0001), and %APAPS increased (P = 0.002) in an allelic dose-dependent manner across the UGT2B15 genotypes from *1/*1 to *2/*2.
There were no overall main effects of diet on %APAP and %APAPG or APAPG/APAP ratio. However, with the F&V diet relative to the basal diet, the APAPG ratio was higher (P < 0.0001), and the %APAPS and APAPS/APAP were lower (P < 0.0001).
The effect of diet on urinary APAP measures, stratified by UGT1A6 and UGT2B15 genotypes, is presented in Tables 2 and 3, respectively. The formal interaction term for diet × genotype for UGT1A6 was not statistically significant. However, there was a statistically significant diet × UGT2B15 genotype interaction for APAPG ratio (P = 0.03). Despite having a higher APAPG ratio overall, UGT2B15*1/*1 individuals also had approximately a 2-fold higher F&V to basal diet percentage of difference in APAPG ratio response (4.6 ± 0.8) compared with the *1/*2 (2.1 ± 0.5) and *2/*2 individuals (2.2 ± 0.7), suggesting that *1/*1 individuals were more responsive to the intervention.
TABLE 2.
Salivary APAP pharmacokinetics and urinary APAP metabolite excretion by diet and UGT1A6 genotypes within diet
Data are least squares means ± S.E.
| Overall |
UGT1A6*1/*1 |
UGT1A6*1/*2 |
UGT1A6*2/*2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal | F&V | P* | Basal | F&V | Basal | F&V | Basal | F&V | |
| Salivary pharmacokinetics | |||||||||
| n | 65 | 65 | 27 | 27 | 25 | 25 | 13 | 13 | |
| Cmax (mg/l) | 16.1 ± 0.6 | 17.5 ± 0.7 | 0.003 | 17.2 ± 0.9 | 18.3 ± 1.0 | 15.1 ± 0.9 | 16.0 ± 0.9 | 16.0 ± 1.3a | 18.4 ± 1.5b |
| Clearance (1/h) | 10.0 ± 0.5 | 9.9 ± 0.5 | 0.88 | 9.3 ± 0.7‡ | 9.5 ± 0.7 | 11.9 ± 0.9† | 10.5 ± 0.8 | 9.1 ± 1.0‡ | 9.8 ± 1.1 |
| AUC (mg · min/l) | 6103 ± 270 | 6155 ± 272 | 0.88 | 6414 ± 419 | 6326 ± 408 | 5378 ± 369 | 5692 ± 387 | 6588 ± 613 | 6478 ± 611 |
| t1/2 (min) | 260 ± 15 | 282 ± 16 | 0.17 | 261 ± 22 | 281 ± 23 | 245 ± 22 | 285 ± 25 | 275 ± 33 | 279 ± 34 |
| Urinary APAP metabolite excretion | |||||||||
| n | 65 | 65 | 27 | 27 | 25 | 25 | 13 | 13 | |
| % oral does | |||||||||
| %APAP | 3.1 ± 0.12 | 3.2 ± 0.12 | 0.25 | 3.37 ± 0.16 | 3.53 ± 0.16† | 2.91 ± 0.16 | 3.00 ± 0.16‡ | 2.95 ± 0.23 | 2.98 ± 0.23‡ |
| %APAPG | 35.0 ± 0.9 | 35.8 ± 0.9 | 0.17 | 32.9 ± 1.4 | 34.7 ± 1.4 | 35.4 ± 1.4 | 36.3 ± 1.4 | 36.7 ± 2.0 | 36.6 ± 2.0 |
| %APAPS | 24.1 ± 0.9 | 22.1 ± 0.9 | <0.0001 | 25.0 ± 1.3a | 23.2 ± 1.3b | 25.0 ± 1.3a | 22.9 ± 1.3b | 22.5 ± 1.9a | 20.1 ± 1.9b |
| Metabolite ratios | |||||||||
| APAPG ratioc | 56.2 ± 1.2 | 58.9 ± 1.2 | <0.0001 | 53.5 ± 1.7a | 56.7 ± 1.7b | 56.1 ± 1.8a | 58.7 ± 1.8b | 58.9 ± 2.5a | 61.2 ± 2.5b |
| APAPG/APAP* | 11.8 ± 0.6 | 11.9 ± 0.6 | 0.83 | 10.0 ± 0.7 | 10.1 ± 0.7† | 12.7 ± 0.9 | 12.9 ± 0.9‡ | 13.2 ± 1.3 | 12.9 ± 1.3‡ |
| APAPS/APAP | 8.1 ± 0.4 | 7.1 ± 0.3 | <0.0001 | 7.5 ± 0.5a | 6.6 ± 0.4b | 8.7 ± 0.6a | 7.9 ± 0.5b | 8.1 ± 0.8a | 7.0 ± 0.7b |
Significant overall diet effect for UGT1A6 genotype (P < 0.05).
<ths>Different superscript numbers represent significant genotype differences within diet (P < 0.05).
Different superscript letters represent significant diet differences within a genotype (P < 0.05).
APAPG/(APAP + APAPG + APAPS) × 100.
TABLE 3.
Salivary APAP pharmacokinetics and urinary APAP metabolite excretion by UGT2B15 genotypes
Data are least squares means ± S.E.
|
UGT2B15*1/*1 |
UGT2B15*1/*2 |
UGT2B15*2/*2 |
||||
|---|---|---|---|---|---|---|
| Basal | F&V | Basal | F&V | Basal | F&V | |
| Salivary pharmacokinetics | ||||||
| n | 16 | 16 | 33 | 33 | 17 | 17 |
| Cmax (mg/l) | 15.9 ± 1.2 | 17.0 ± 1.3 | 15.4 ± 0.8a | 17.3 ± 0.9b | 17.8 ± 1.3 | 18.1 ± 1.3 |
| Clearance (1/h) | 10.2 ± 1.0 | 10.3 ± 1.0 | 9.3 ± 0.6 | 9.5 ± 0.6 | 12.1 ± 1.1 | 10.4 ± 1.0 |
| AUC (mg · min/l) | 5913 ± 504 | 5787 ± 493 | 6473 ± 377 | 6494 ± 373 | 5426 ± 449 | 5738 ± 482 |
| t1/2 (min) | 307 ± 31† | 282 ± 28 | 270 ± 18‡ | 290 ± 20 | 201 ± 19a,‡ | 268 ± 26b |
| Urinary APAP metabolite excretion | ||||||
| n | 16 | 16 | 33 | 33 | 17 | 17 |
| % of oral dose | ||||||
| %APAP | 3.0 ± 0.2 | 2.9 ± 0.2 | 3.0 ± 0.1 | 3.2 ± 0.1 | 3.4 ± 0.2 | 3.5 ± 0.2 |
| %APAPG | 40.2 ± 1.6† | 40.9 ± 1.6† | 35.1 ± 1.1‡ | 36.0 ± 1.1‡ | 29.3 ± 1.5§ | 30.4 ± 1.5§ |
| %APAPS* | 22.1 ± 1.6a,† | 19.0 ± 1.5b,† | 23.3 ± 1.1a,‡ | 21.6 ± 1.1b,‡ | 28.5 ± 1.5a,§ | 26.9 ± 1.5b,§ |
| Metabolite ratios | ||||||
| APAPG ratioc* | 61.3 ± 1.8a,† | 65.9 ± 1.8b,† | 57.3 ± 1.2a,‡ | 59.4 ± 1.2b,‡ | 47.9 ± 1.7a,§ | 50.1 ± 1.8b,§ |
| APAPG/APAP | 13.9 ± 1.2† | 15.5 ± 1.3† | 12.2 ± 0.7‡ | 11.7 ± 0.7‡ | 8.9 ± 0.7§ | 9.0 ± 0.7§ |
| APAPS/APAP* | 7.6 ± 0.7a | 6.8 ± 0.6b | 8.0 ± 0.5a | 6.8 ± 0.4b | 8.7 ± 0.7 | 8.0 ± 0.7 |
Significant overall diet effect for UGT2B15 genotype (P < 0.05).
<ths>Different superscript numbers represent significant genotype differences within diet (P < 0.05).
Different superscript letters represent significant diet differences within a genotype (P < 0.05).
APAPG/(APAP + APAPG + APAPS) × 100.
Urinary APAP excretion was measured on days 7 and 14 (data not shown). Day × diet interactions were statistically significant for some measures. For %APAPG, there was not a statistically significant difference by diet on day 7, but by day 14, statistically significantly higher %APAPG was observed with the F&V compared with the basal diet (P = 0.003). Likewise, for APAPG/APAP, the ratio was lower on day 7 than on day 14 with the F&V diet (P = 0.002). In contrast, for %APAPS and APAPS/APAP ratio, the F&V diet effect was most pronounced within the 1st week of the intervention (P = 0.049 and P = 0.002, respectively), but by day 14 values were similar for both diets. The F&V effects on APAPG ratio were detected by day 7 and persisted to day 14.
We also tested whether diet effects were similar among women who were in the same menstrual cycle phase for both feeding periods (approximately 60–66% of women, depending whether day 7 or day 14 and urine or saliva data) compared with women who were not. For all outcomes, except %APAPS, no statistically significant relationships between menstrual phase difference and response to F&V supplementation were observed. The mean difference in %APAPS between the basal and F&V diets was greater among the women who were not in the same phase of cycle during both diets at day 7 (P = 0.04) but was lower with the F&V than with the basal diet in both groups of women as well as men (data not shown) and was no longer significant by day 14.
Salivary Acetaminophen Pharmacokinetics.
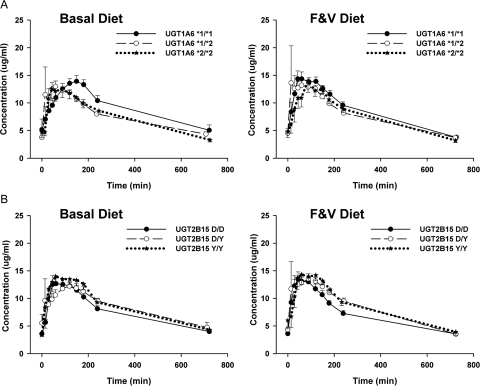
Effects of diet on salivary APAP pharmacokinetics by UGT1A6 and UGT2B15 over the course of 12 h were evaluated. Overall, the maximum concentration of salivary APAP (Cmax) was statistically significantly higher in women than in men (P = 0.0003) and was higher when participants were fed the F&V diet compared with the basal diet (P = 0.003) (Fig. 1). No sex or diet differences were observed for half-life, clearance, or area under the curve. There were no statistically significant overall effects of UGT1A6 genotype or genotype × diet interactions on Cmax; however, when stratified by UGT1A6 genotypes, the higher Cmax on the F&V diet was due predominantly to a response among individuals with the UGT1A6 *2/*2 genotype (P = 0.02) (Table 2). Likewise, when stratified by UGT2B15, the diet effect on Cmax was predominantly a response among the UGT2B15*1/*2 individuals (P = 0.002) (Table 3) with smaller, nonsignificant differences in *1/*1 and *2/*2 individuals. Comparison of day 7 and day 14 collections suggested that the differences in Cmax between diets were greater on day 7 than on day 14 (data not shown).
Fig. 1.
Cumulative salivary APAP concentrations over 12 h for 63 individuals by UGT1A6 (A) and UGT2B15 (B) genotypes on a phytochemical-free controlled diet (basal diet) and basal diet supplemented with soy foods, cruciferous vegetables, and citrus fruits (F&V diet).
Stratified analyses suggest some differences in the other pharmacokinetic parameters by genotype, although there were no consistent patterns. Clearance was higher among UGT1A6*1/*2 relative to the *1/*1 and *2/*2 individuals consuming the basal diet (P = 0.04). Furthermore, among the UGT2B15 *1/*1 relative to the *2/*2 and *1/*2 individuals, salivary APAP half-life on the basal diet was higher (P = 0.007). Response to diet also differed within genotype: APAP half-life was longer in UGT2B15*2/*2 individuals with F&V compared with the basal diet (P = 0.009), an effect that was not present in the other two UGT2B15 genotypes. As with Cmax, the diet differences were most prominent on day 7 (data not shown).
Discussion
We hypothesized that APAP glucuronidation would differ by UGT1A6 genotype, such that there would be a trend toward increasing APAPG and an increasing ratio of APAPG to free APAP in urine from individuals with UGT1A6*1/*1 to*2/*2 genotypes. Individuals with the homozygous variant have been shown to have a slower metabolism of some phenolic substrates and a faster metabolism of others, including APAP, in vitro (Ciotti et al., 1997). Increased susceptibility to APAP toxicity in cats and Gunn rats, which both lack UGT1A6 activity, lends support to the hypothesis that UGT1A6 may be important for APAP glucuronidation. In cats, UGT1A6 is a pseudogene and APAP administration results in prolonged half-life of the drug and significant formation of oxidative metabolites (Court and Greenblatt, 2000). In Gunn rats, the UGT1A6 gene is expressed but is not functional due to a frameshift mutation that inactivates all the UGT1A enzymes (Iyanagi et al., 1989). Court et al. (2001) showed, using recombinant UGTs and human liver microsomes, that although most UGTs could glucuronidate APAP, UGT1A6 was most active at low concentrations (<50 μM), whereas at higher, therapeutic concentrations to toxic concentrations (50 μM–5 mM), UGT1A9 was more active, and, at toxic concentrations, UGT1A1 contributed substantially. Our results do not support either faster or slower metabolism of APAP among individuals with a UGT1A6*2 genotype.
In the context of a controlled feeding study, we observed only a modest relationship between UGT1A6, diet, and APAP conjugation. In contrast, we detected strong associations between UGT2B15 genotype, diet, and APAP glucuronidation and sulfation. Two weeks of a controlled diet supplemented with citrus fruits, cruciferous vegetables, and soy foods, dietary sources of phytochemicals that increase UGT activity (Saracino and Lampe, 2007), had minor effects on salivary APAP pharmacokinetics but significantly increased urinary excretion of APAPG as a percentage of excreted metabolites, and decreased excretion of APAPS, particularly among carriers of the UGT2B15*1/*1 genotype.
UGT2B15 is expressed in liver, kidney, testis, mammary gland, prostate, and lung (Lévesque et al., 1997). Although considered to be a major catalyst for C19-conjugation of androgens (e.g., testosterone and dihydrotestosterone), UGT2B15 also glucuronidates a wide range of other substrates, including simple phenolic compounds, coumarins, flavonoids, anthraquinones, and drugs such as oxazepam (Chen et al., 1993; Green et al., 1994). Court et al. (2001) showed that UGT2B15 also has the capacity to conjugate APAP. The two polymorphic forms of the enzyme, expressed in HK293 cells, demonstrated similar substrate specificities and Km values; however, UGT2B15*2 had a higher Vmax than UGTB15*1 for 3-α-diol and dihydrotestosterone (Lévesque et al., 1997). Given the rather small differences in kinetics, Lévesque et al. (1997) postulated that differences in steroid hormone metabolism are unlikely to be observed in vivo; however, they suggested that this polymorphism may be contributing to individual variability in xenobiotic glucuronidation. In liver tissue, UGT2B15 D85Y(*2) was found to be a major determinant of S-oxazepam glucuronidation (Court et al., 2004) and also a major determinant of interindividual variability in pharmacokinetics and pharmacodynamics of lorazepam, a 2′-chloro-substituted oxazepam (Chung et al., 2005). As with our study, the UGT2B15*2/*2 genotype was associated with lower glucuronidation in both studies.
Few studies have evaluated the effects of diet on glucuronidation in humans and on APAP conjugation in particular. Pantuck et al. (1984) showed that 500 g of cruciferous vegetables (cabbage and Brussels sprouts) consumed for 10 days stimulated formation of APAP glucuronyl conjugates and increased the APAPG/APAP ratio. Gwilt et al. (1994) found that supplementation of habitual diet with garlic extract (equivalent to 6–7 cloves/day) resulted in a small, but significant, increase in area under the plasma APAP glucuronide curve after 3 months, but no change in other glucuronide parameters. We recently reported that a controlled diet containing either 5 or 10 servings a day of cruciferous vegetables resulted in statistically significantly lower bilirubin concentrations, a surrogate marker for UTG1A1 activity (Navarro et al., 2009), but APAP glucuronidation was not directly evaluated. In an observational study, ∼30% lower bilirubin concentrations were observed among women with the highest intake of citrus consumption (Saracino et al., 2009). Induction of UGT by a number of phytochemicals in rodents and cell culture systems has been well documented (Saracino and Lampe, 2007). Most evidence suggests that up-regulation of phase 2 enzymes by these phytochemicals occurs through interaction with the cytoplasmic-anchoring protein Kelch-like ECH-associated protein 1 and the transcription factor erythroid-derived related factor 2-like 2, via the antioxidant response element (Dinkova-Kostova et al., 2005).
Soy protein and soy isoflavones have been shown to increase hepatic UGT activity in rats and mice (Staack and Jeffery, 1994; Appelt and Reicks, 1997). As for endogenous estrogens, the soy isoflavones, daidzein and genistein, and their metabolites are substrates for UGTs. In humans, glucuronides are the major isoflavone conjugates accounting for approximately 95% of total isoflavones, with sulfates and free isoflavones accounting for the remaining 5% (Adlercreutz, 1993). Furthermore, some UGTs are induced by estrogens (Mackenzie et al., 2010), and it is likely that isoflavones, as weak estrogens, induce various isoforms of these enzymes via a similar mechanism, although they also act through other signaling pathways (Saracino and Lampe, 2007).
Consistent with other studies reporting sex differences (Miners et al., 1983), we observed greater APAP glucuronidation among men, whereas there were no sex-related differences in sulfation. Court et al. (2001) also reported higher APAP glucuronidation in male human liver microsomes, and later work showed higher S-oxazepam glucuronidation by UGT2B15 in male compared with female livers (Court et al., 2004). Differences in drug metabolism have been attributed to hormonal changes during the menstrual cycle, percentage of body fat, glomerular filtration rate, and differences in biotransformation enzyme activity (Anderson, 2008). Women were much more responsive to the F&V diet, excreting a greater percentage of glucuronides after consumption of a diet rich in soy, crucifers, and citrus for 2 weeks. Because fruit and vegetables were provided on the basis of body weight, greater consumption of dietary bioactive compounds by women relative to men is unlikely to be responsible for this difference. Although we designed the study to accommodate women's menstrual cycles (i.e., we used a 14-day intervention and 14-day washout such that the second period would start 28 days after the first), logistics did not always allow for this level of control. In addition, both the controlled diet and interventions in women often alter menstrual cycle length themselves. However, we did not see any significant differences in glucuronidation on the basis of menstrual phase. The observation of greater responsiveness to diet among women may be explained by higher basal glucuronidation activity in men.
A major strength of this study is the design. We recruited on the basis of genotype, evaluated the relationship between genotype and phenotype on the background of a controlled diet, and tested the effects of adding 10 servings of plant foods to a basal diet devoid of phytochemicals (e.g., whole grains, fruits, vegetables, herbs, and spices). However, there are some possible limitations. Although larger than the previous controlled feeding studies designed to evaluate effects of diet on glucuronidation, the sample size is relatively small for detecting genotype-diet interactions. Our results suggest that certain UGT genotypes may be more responsive to a F&V intervention than others, but the data do not provide conclusive evidence regarding differences in these interactions. In addition, we did not evaluate polymorphisms in all UGTs known to glucuronidate APAP, including UGT1A1 and UGT1A9. Separate analyses were not performed on UGT1A1*28, because this polymorphism has been shown to be in high linkage disequilibrium with UGT1A6*2 and would probably not be informative. UGT1A9 has the capacity to glucuronidate APAP (Court et al., 2001), and work by Ohno and Nakajin (2009) in various tissues from healthy human donors showed higher abundance of UGT1A9 mRNA compared with UGT1A6, both in the liver and overall. Although it is not known whether these mRNA levels correlate with actual protein concentrations, these data suggest an important role for UGT1A9 in APAP conjugation. Two variants in the promoter region of the UGT1A9 gene have been shown to alter glucuronidation activity. T275A and C2152T are associated with increased enzyme activity; however, given that they have an allelic prevalence of ∼12 and 8%, respectively (Sanchez-Fructuoso et al., 2009), our study was not sufficiently powered to evaluate this polymorphism in our current sample. Studies enriching for these variants or a larger sample size is needed to evaluate their impact on APAP glucuronidation. Furthermore, we cannot rule out the possibility that the observed effects are the result of dietary influence on phase 3 excretion of the various APAP metabolites.
Saliva APAP concentrations have been shown to correlate well with plasma concentrations of free APAP (Adithan and Thangam, 1982; al-Obaidy et al., 1995; Hahn et al., 2000), making this a convenient, noninvasive method of conducting pharmacokinetic studies in larger-scale studies. Although there is fairly good agreement between these measures, there are several factors that may limit its utility, including differences in salivary flow rate, individual collection, and residual oral drug. In the present study, a gap in sampling between 4 and 12 h after dosing, may have resulted in inaccurate elimination phase estimates. Our results suggest that testing genotype effects using more conventional pharmacokinetic approaches are warranted.
Finally, the differences in APAP glucuronidation between genotypes in response to diet are small. It is not clear whether these minor differences affect disease risk or response to therapy, but, given the role of UGT2B15 in conjugation and subsequent elimination of potentially toxic xenobiotics, these results may have implications for altering carcinogen metabolism through dietary intervention. Although most of the foods included in our study protocol are commonly consumed, soy foods, orange peel, and radish sprouts are not. Furthermore, these foods may not be consumed in the combined quantities given in this study.
In conclusion, we showed that APAP glucuronidation is significantly influenced by the UGT2B15*2 polymorphism, whereas UGT1A6*2 contributed only modestly. The genotype effects of UGT2B15*2 also support, in vivo, a role for UGT2B15 in APAP glucuronidation. In addition, we showed that a basal diet supplemented with selected fruits and vegetables known to affect UGT activity can alter APAP metabolism in the direction of greater glucuronidation and less sulfate formation.
Acknowledgments
We thank the FHCRC Prevention Center Clinic staff and Kara Breymeyer and her staff in the Human Nutrition Laboratory for their dedication to and support of the study participants.
This work was supported by the National Institutes of Health National Cancer Institute [Grants R01-CA92288, R01-CA112516, R25-CA94880].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.111.039149.
- APAP
- acetaminophen
- UGT
- UDP-glucuronosyltransferase
- FHCRC
- Fred Hutchinson Cancer Research Center
- F&V
- fruits and vegetables
- CV
- coefficient of variation
- HPLC
- high-performance liquid chromatography
- Cmax
- maximum concentration
- Tmax
- time of maximum concentration
- AUC
- area under the curve
- APAPG
- acetaminophen glucuronide
- APAPS
- acetaminophen sulfate
- %APAP
- percentage as free APAP
- %APAPG
- percentage as APAPG
- %APAPS
- percentage of APAPS.
Authorship Contributions
Participated in research design: S.S. Li, King, Potter, Bigler, and Lampe.
Conducted experiments: Chang, Schwarz, King, Bigler, and Lampe.
Performed data analysis: Chen, L. Li, and S.S. Li.
Wrote or contributed to the writing of the manuscript: Navarro, Chen, Chang, Potter, and Lampe.
References
- Adithan C, Thangam J. (1982) A comparative study of saliva and serum paracetamol levels using a simple spectrophotometric method. Br J Clin Pharmacol 14:107–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlercreutz H. (1993) Dietary lignans and isoflavonoid phytoestrogens, and cancer. Klin Lab 2:4–12 [Google Scholar]
- Adlercreutz H, Mousavi Y, Clark J, Höckerstedt K, Hämäläinen E, Wähälä K, Mäkelä T, Hase T. (1992) Dietary phytoestrogens and cancer: in vitro and in vivo studies. J Steroid Biochem Mol Biol 41:331–337 [DOI] [PubMed] [Google Scholar]
- al-Obaidy SS, Li Wan Po A, McKiernan PJ, Glasgow JF, Millership J. (1995) Assay of paracetamol and its metabolites in urine, plasma and saliva of children with chronic liver disease. J Pharm Biomed Anal 13:1033–1039 [DOI] [PubMed] [Google Scholar]
- Anderson GD. (2008) Gender differences in pharmacological response. Int Rev Neurobiol 83:1–10 [DOI] [PubMed] [Google Scholar]
- Appelt LC, Reicks MM. (1997) Soy feeding induces phase II enzymes in rat tissues. Nutr Cancer 28:270–275 [DOI] [PubMed] [Google Scholar]
- Bigler J, Whitton J, Lampe JW, Fosdick L, Bostick RM, Potter JD. (2001) CYP2C9 and UGT1A6 genotypes modulate the protective effect of aspirin on colon adenoma risk. Cancer Res 61:3566–3569 [PubMed] [Google Scholar]
- Burchell B. (2003) Genetic variation of human UDP-glucuronosyltransferase: implications in disease and drug glucuronidation. Am J Pharmacogenomics 3:37–52 [DOI] [PubMed] [Google Scholar]
- Chang JL, Bigler J, Schwarz Y, Li SS, Li L, King IB, Potter JD, Lampe JW. (2007) UGT1A1 polymorphism is associated with serum bilirubin concentrations in a randomized, controlled, fruit and vegetable feeding trial. J Nutr 137:890–897 [DOI] [PubMed] [Google Scholar]
- Chen F, Ritter JK, Wang MG, McBride OW, Lubet RA, Owens IS. (1993) Characterization of a cloned human dihydrotestosterone/androstanediol UDP-glucuronosyltransferase and its comparison to other steroid isoforms. Biochemistry 32:10648–10657 [DOI] [PubMed] [Google Scholar]
- Chung JY, Cho JY, Yu KS, Kim JR, Jung HR, Lim KS, Jang IJ, Shin SG. (2005) Effect of the UGT2B15 genotype on the pharmacokinetics, pharmacodynamics, and drug interactions of intravenous lorazepam in healthy volunteers. Clin Pharmacol Ther 77:486–494 [DOI] [PubMed] [Google Scholar]
- Ciotti M, Marrone A, Potter C, Owens IS. (1997) Genetic polymorphism in the human UGT1A6 (planar phenol) UDP-glucuronosyltransferase: pharmacological implications. Pharmacogenetics 7:485–495 [DOI] [PubMed] [Google Scholar]
- Cordaro JA, Morse GD, Bartos L, Gugino LJ, Maliszewski M, Colomaio R, Shelton M, O'Donnell A, Hewitt R. (1993) Zidovudine pharmacokinetics in HIV-positive women during different phases of the menstrual cycle. Pharmacotherapy 13:369–377 [PubMed] [Google Scholar]
- Court MH, Duan SX, Guillemette C, Journault K, Krishnaswamy S, Von Moltke LL, Greenblatt DJ. (2002) Stereoselective conjugation of oxazepam by human UDP-glucuronosyltransferases (UGTs): S-oxazepam is glucuronidated by UGT2B15, while R-oxazepam is glucuronidated by UGT2B7 and UGT1A9. Drug Metab Dispos 30:1257–1265 [DOI] [PubMed] [Google Scholar]
- Court MH, Duan SX, von Moltke LL, Greenblatt DJ, Patten CJ, Miners JO, Mackenzie PI. (2001) Interindividual variability in acetaminophen glucuronidation by human liver microsomes: identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J Pharmacol Exp Ther 299:998–1006 [PubMed] [Google Scholar]
- Court MH, Greenblatt DJ. (2000) Molecular genetic basis for deficient acetaminophen glucuronidation by cats: UGT1A6 is a pseudogene, and evidence for reduced diversity of expressed hepatic UGT1A isoforms. Pharmacogenetics 10:355–369 [DOI] [PubMed] [Google Scholar]
- Court MH, Hao Q, Krishnaswamy S, Bekaii-Saab T, Al-Rohaimi A, von Moltke LL, Greenblatt DJ. (2004) UDP-glucuronosyltransferase (UGT) 2B15 pharmacogenetics: UGT2B15 D85Y genotype and gender are major determinants of oxazepam glucuronidation by human liver. J Pharmacol Exp Ther 310:656–665 [DOI] [PubMed] [Google Scholar]
- de Morais SM, Uetrecht JP, Wells PG. (1992) Decreased glucuronidation and increased bioactivation of acetaminophen in Gilbert's syndrome. Gastroenterology 102:577–586 [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Wakabayashi N. (2005) Keap1, the sensor for electrophiles and oxidants that regulates the phase 2 response, is a zinc metalloprotein. Biochemistry 44:6889–6899 [DOI] [PubMed] [Google Scholar]
- Esteban A, Pérez-Mateo M. (1993) Gilbert's disease: a risk factor for paracetamol overdosage? J Hepatol 18:257–258 [DOI] [PubMed] [Google Scholar]
- Green MD, Oturu EM, Tephly TR. (1994) Stable expression of a human liver UDP-glucuronosyltransferase (UGT2B15) with activity toward steroid and xenobiotic substrates. Drug Metab Dispos 22:799–805 [PubMed] [Google Scholar]
- Gwilt PR, Lear CL, Tempero MA, Birt DD, Grandjean AC, Ruddon RW, Nagel DL. (1994) The effect of garlic extract on human metabolism of acetaminophen. Cancer Epidemiol Biomarkers Prev 3:155–160 [PubMed] [Google Scholar]
- Hahn TW, Mogensen T, Lund C, Schouenborg L, Rasmussen M. (2000) High-dose rectal and oral acetaminophen in postoperative patients—serum and saliva concentrations. Acta Anaesthesiol Scand 44:302–306 [DOI] [PubMed] [Google Scholar]
- Harvard CW. (1973) Drug-induced disease. Ghana Med J 12:130–141 [PubMed] [Google Scholar]
- Iyanagi T, Watanabe T, Uchiyama Y. (1989) The 3-methylcholanthrene-inducible UDP-glucuronosyltransferase deficiency in the hyperbilirubinemic rat (Gunn rat) is caused by a −1 frameshift mutation. J Biol Chem 264:21302–21307 [PubMed] [Google Scholar]
- Kessler FK, Kessler MR, Auyeung DJ, Ritter JK. (2002) Glucuronidation of acetaminophen catalyzed by multiple rat phenol UDP-glucuronosyltransferases. Drug Metab Dispos 30:324–330 [DOI] [PubMed] [Google Scholar]
- Kostrubsky SE, Sinclair JF, Strom SC, Wood S, Urda E, Stolz DB, Wen YH, Kulkarni S, Mutlib A. (2005) Phenobarbital and phenytoin increased acetaminophen hepatotoxicity due to inhibition of UDP-glucuronosyltransferases in cultured human hepatocytes. Toxicol Sci 87:146–155 [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S, Hao Q, Al-Rohaimi A, Hesse LM, von Moltke LL, Greenblatt DJ, Court MH. (2005a) UDP glucuronosyltransferase (UGT) 1A6 pharmacogenetics: I. Identification of polymorphisms in the 5′-regulatory and exon 1 regions, and association with human liver UGT1A6 gene expression and glucuronidation. J Pharmacol Exp Ther 313:1331–1339 [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S, Hao Q, Al-Rohaimi A, Hesse LM, von Moltke LL, Greenblatt DJ, Court MH. (2005b) UDP glucuronosyltransferase (UGT) 1A6 pharmacogenetics: II. Functional impact of the three most common nonsynonymous UGT1A6 polymorphisms (S7A, T181A, and R184S). J Pharmacol Exp Ther 313:1340–1346 [DOI] [PubMed] [Google Scholar]
- Lampe JW, Bigler J, Bush AC, Potter JD. (2000) Prevalence of polymorphisms in the human UDP-glucuronosyltransferase 2B family: UGT2B4(D458E), UGT2B7(H268Y), and UGT2B15(D85Y). Cancer Epidemiol Biomarkers Prev 9:329–333 [PubMed] [Google Scholar]
- Lampe JW, Bigler J, Horner NK, Potter JD. (1999) UDP-glucuronosyltransferase (UGT1A1*28 and UGT1A6*2) polymorphisms in Caucasians and Asians: relationships to serum bilirubin concentrations. Pharmacogenetics 9:341–349 [DOI] [PubMed] [Google Scholar]
- Lampe JW, Skor HE, Li S, Wähälä K, Howald WN, Chen C. (2001) Wheat bran and soy protein feeding do not alter urinary excretion of the isoflavan equol in premenopausal women. J Nutr 131:740–744 [DOI] [PubMed] [Google Scholar]
- Lévesque E, Beaulieu M, Green MD, Tephly TR, Bélanger A, Hum DW. (1997) Isolation and characterization of UGT2B15(Y85): a UDP-glucuronosyltransferase encoded by a polymorphic gene. Pharmacogenetics 7:317–325 [DOI] [PubMed] [Google Scholar]
- Mackenzie PI, Hu DG, Gardner-Stephen DA. (2010) The regulation of UDP-glucuronosyltransferase genes by tissue-specific and ligand-activated transcription factors. Drug Metab Rev 42:99–109 [DOI] [PubMed] [Google Scholar]
- Miners JO, Attwood J, Birkett DJ. (1983) Influence of sex and oral contraceptive steroids on paracetamol metabolism. Br J Clin Pharmacol 16:503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlib AE, Goosen TC, Bauman JN, Williams JA, Kulkarni S, Kostrubsky S. (2006) Kinetics of acetaminophen glucuronidation by UDP-glucuronosyltransferases 1A1, 1A6, 1A9 and 2B15. Potential implications in acetaminophen-induced hepatotoxicity. Chem Res Toxicol 19:701–709 [DOI] [PubMed] [Google Scholar]
- Nagar S, Zalatoris JJ, Blanchard RL. (2004) Human UGT1A6 pharmacogenetics: identification of a novel SNP, characterization of allele frequencies and functional analysis of recombinant allozymes in human liver tissue and in cultured cells. Pharmacogenetics 14:487–499 [DOI] [PubMed] [Google Scholar]
- Navarro SL, Peterson S, Chen C, Makar KW, Schwarz Y, King IB, Li SS, Li L, Kestin M, Lampe JW. (2009) Cruciferous vegetable feeding alters UGT1A1 activity: diet- and genotype-dependent changes in serum bilirubin in a controlled feeding trial. Cancer Prev Res 2:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell SA, Ahn J, Rae JM, Scheys JO, Trovato A, Sweeney C, MacLeod SL, Kadlubar FF, Ambrosone CB. (2005) Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat 91:249–258 [DOI] [PubMed] [Google Scholar]
- Ohno S, Nakajin S. (2009) Determination of mRNA expression of human UDP-glucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug Metab Dispos 37:32–40 [DOI] [PubMed] [Google Scholar]
- Pantuck EJ, Pantuck CB, Anderson KE, Wattenberg LW, Conney AH, Kappas A. (1984) Effect of brussels sprouts and cabbage on drug conjugation. Clin Pharmacol Ther 35:161–169 [DOI] [PubMed] [Google Scholar]
- Rauchschwalbe SK, Zühlsdorf MT, Wensing G, Kuhlmann J.(2004) Glucuronidation of acetaminophen is independent of UGT1A1 promotor genotype. Int J Clin Pharmacol Ther 42:73–77 [DOI] [PubMed] [Google Scholar]
- Sánchez-Fructuoso AI, Maestro ML, Calvo N, Viudarreta M, Pérez-Flores I, Veganzone S, De la Orden V, Ortega D, Arroyo M, Barrientos A. (2009) The prevalence of uridine diphosphate-glucuronosyltransferase 1A9 (UGT1A9) gene promoter region single-nucleotide polymorphisms T-275A and C-2152T and its influence on mycophenolic acid pharmacokinetics in stable renal transplant patients. Transplant Proc 41:2313–2316 [DOI] [PubMed] [Google Scholar]
- Saracino MR, Bigler J, Schwarz Y, Chang JL, Li S, Li L, White E, Potter JD, Lampe JW. (2009) Citrus fruit intake is associated with lower serum bilirubin concentration among women with the UGT1A1*28 polymorphism. J Nutr 139:555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saracino MR, Lampe JW. (2007) Phytochemical regulation of UDP-glucuronosyltransferases: implications for cancer prevention. Nutr Cancer 59:121–141 [DOI] [PubMed] [Google Scholar]
- Staack EH, Jeffery EH. (1994) Effects of isoflavonoids from soy on rat hepatic drug metabolizing enzymes (Abstract). J Nutr 19:805S [Google Scholar]
- Tanaka E. (1999) Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther 24:339–346 [DOI] [PubMed] [Google Scholar]
- Tukey RH, Strassburg CP. (2001) Genetic multiplicity of the human UDP-glucuronosyltransferases and regulation in the gastrointestinal tract. Mol Pharmacol 59:405–414 [DOI] [PubMed] [Google Scholar]
- Ullrich D, Sieg A, Blume R, Bock KW, Schröter W, Bircher J. (1987) Normal pathways for glucuronidation, sulphation and oxidation of paracetamol in Gilbert's syndrome. Eur J Clin Invest 17:237–240 [DOI] [PubMed] [Google Scholar]