Abstract
Copper is essential for aerobic life, but many aspects of its cellular uptake and distribution remain to be fully elucidated. A genome-wide screen for copper homeostasis genes in Drosophila melanogaster identified the SNARE gene Syntaxin 5 (Syx5) as playing an important role in copper regulation; flies heterozygous for a null mutation in Syx5 display increased tolerance to high dietary copper. The phenotype is shown here to be due to a decrease in copper accumulation, a mechanism also observed in both Drosophila and human cell lines. Studies in adult Drosophila tissue suggest that very low levels of Syx5 result in neuronal defects and lethality, and increased levels also generate neuronal defects. In contrast, mild suppression generates a phenotype typical of copper-deficiency in viable, fertile flies and is exacerbated by co-suppression of the copper uptake gene Ctr1A. Reduced copper uptake appears to be due to reduced levels at the plasma membrane of the copper uptake transporter, Ctr1. Thus Syx5 plays an essential role in copper homeostasis and is a candidate gene for copper-related disease in humans.
Introduction
Copper (Cu) is essential to aerobic organisms as a cofactor in diverse metabolic processes including cellular respiration and proliferation; formation of connective tissue, melanin and neurotransmitters; antioxidant defence; cell signalling; and angiogenesis [1], [2]. Although several proteins involved in copper homeostasis have been well characterized, numerous aspects of the cellular distribution remain to be fully elucidated.
Ctr1 is the major copper uptake protein in mammalian cells, and is thought to form a trimer containing a pore at the plasma membrane through which copper can pass [3], [4]. Chaperones Atox1, CCS, Cox17, Sco1 and Sco2 are required to deliver copper to copper-dependent enzymes in various subcellular compartments. Atox1, CCS and Cox17 may receive their copper either directly via protein–protein interaction with Ctr1 or indirectly via an intermediate such as glutathione or metallothionein [5], and Sco1 has been shown to receive copper from Cox17 [6]. At the trans-Golgi network (TGN), copper is transferred from Atox1 directly to the transmembrane copper-translocating P-type ATPases ATP7A (MNK) and ATP7B (WND) for transport to enzymes of the secretory pathway [7]. However, under conditions of excess cellular copper, ATP7A and ATP7B traffic towards the plasma membrane where they facilitate copper efflux [8].
Due to the varied metabolic processes for which copper is required, there are a wide variety of copper-related diseases with diverse phenotypes. For example, Menkes disease is caused by impaired ATP7A-mediated transport of dietary copper from the polarised gut epithelial cells, resulting in systemic copper deficiency and Wilson disease is caused by impaired ATP7B-mediated transport of copper from the liver resulting in copper toxicosis [reviewed in 9]. However, not all copper-related diseases have been associated with a candidate gene [10]. Copper levels and copper metabolism proteins have been implicated in gene expression, tumour cell metastasis and resistance to anti-neoplastic drugs and copper chelators have shown promise in the treatment of cancer [reviewed in 2]. Copper dyshomeostasis in the brain is associated with Alzheimer's disease and copper ionophores have shown encouraging results in clinical trials [11]. The further characterisation of genes involved in copper homeostasis is therefore required to provide additional candidate genes and support our understanding of the mechanisms underlying a range of copper-related diseases [2].
The vinegar fly Drosophila melanogaster has recently proven a useful model for characterizing the role of copper homeostasis genes. Drosophila has orthologues of all major copper homeostasis proteins and several studies have demonstrated the high level of functional conservation with humans [12]–[15]. In Drosophila, two homologous proteins, Ctr1A and Ctr1B, fulfil the function of mammalian Ctr1. Ctr1A is constitutively expressed [13], [16] and is required for baseline copper uptake while Ctr1B is induced in the midgut by dietary copper limitation and is needed to boost absorption [13], [17]. DmATP7 is the sole Drosophila orthologue of mammalian copper transporting ATPases, ATP7A and ATP7B [15].
A genetic screen was performed in Drosophila to further illuminate our understanding of copper homeostasis mechanisms [18]. This resulted in the identification of the SNARE (soluble NSF attachment protein receptor) gene Syntaxin 5 (Syx5) as playing an important role in copper regulation in the fly; flies heterozygous for a null mutation in Syx5 display significantly increased tolerance to high levels of dietary copper.
SNAREs are involved in fusion of vesicles to target membranes and are therefore central to intracellular trafficking [19]. Syx5 is localised to the trans-Golgi network (TGN) for docking of vesicles including the COPI type [20] and is required for endosome to TGN transport of Shiga toxin and the endogenous cargo protein mannose 6-phosphate receptor [21]. There is also evidence for Syx5–mediated transport between endoplasmic reticulum (ER) and Golgi [22]. Drosophila Syx5 has recently been shown to play a role in translocation of proteins to the apical membrane and is also required for Golgi reassembly following cell division [23]. This signifies a degree of functional conservation of mammalian and Drosophila Syx5. Given the known roles of mammalian Syx5 in both anterograde and retrograde intracellular trafficking, Syx5 represents an excellent candidate for involvement in uptake or intracellular distribution of copper. The current study presents evidence that Syx5 is vital for efficient copper uptake in insect and mammalian cells as well as in vivo in Drosophila.
Results
Syx5+/− heterozygotes have high tolerance to dietary copper
Increased Drosophila copper tolerance had been previously mapped to a single locus on Chromosome 2 encoding Syx5 [18]. To confirm the correct locus had been identified, Syx5AR113/CyO Drosophila were screened for copper tolerance (Fig. 1). The Syx5AR113 allele encodes a functionally null, truncated peptide which is homozygous lethal [23]. The wild-type strain Armenia, the eye-colour mutant w1118 and the mapping strain Df(2L)r10, cn1/CyO, with a deletion spanning Syx5, were included as controls. The offspring from crosses of Syx5AR113/CyO×Armenia, Df(2L)r10, cn1/CyO×Armenia, and a double-balancer stock (IF/CyO)×Armenia were also screened to confirm copper tolerance segregated with the Syx5 mutations.
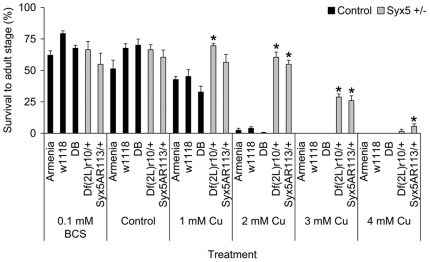
Figure 1. Syx5 +/− Drosophila show increased dietary copper tolerance.
‘Armenia’ and ‘w1118’ are control strains with normal copper homeostasis mechanisms. ‘DB’ is a double balancer strain containing the CyO balancer chromosome present in the Syx5+/− mutants. ‘Df(2L)r10’ is an original mapping strain and ‘Syx5AR113’ is a specific Syx5 null allele. Values are mean with s.e.m. Both Syx5 heterozygous strains show increased copper tolerance compared to the three controls. *Significant difference from Armenia, determined by a Mann-Whitney test (P<0.05). There was no difference in survival between w1118 and Syx5AR113/+ flies on concentrations of BCS up to 1 mM (F = 0.778, P = 0.610).
Both Syx5AR113/CyO and Df(2L)r10, cn1/CyO had significantly higher survival to the adult stage than Armenia, w1118 or IF/CyO when reared on a copper-supplemented diet, but no difference in survival was observed on basal media or the copper chelator BCS (Figure 1). χ2 analysis of the crosses revealed that the increased copper tolerance segregates with both the Df(2L)r10 deletion (χ2 = 26.385, P<0.001 on 1 mM Cu) and the Syx5A113 allele (χ2 = 18.615, P<0.001), but not with any balancer or wild-type chromosomes (Table S1). This clearly demonstrates that increased copper tolerance in Syx5+/− heterozygotes is associated with a 50% reduction in Syx5 levels compared to wild-type Drosophila. The Syx5AR113/CyO strain was used to investigate how Syx5 mediates this copper tolerance. This strain shows no viability or fertility defects (Figure S1) indicating that the copper-related phenotypes demonstrated here are not due to a non-specific reduction in fitness.
Copper tolerance is associated with reduced copper levels in Syx5+/− Drosophila
Pupal metal content was measured to ascertain copper accumulation throughout the larval feeding stage, as this is most relevant to the increased tolerance of dietary copper (Figure 2). Syx5+/− heterozygotes accumulate less copper than wild-type on both basal and copper-supplemented diets. This strongly suggests that the increased copper tolerance of Syx5+/− heterozygotes is due to reduced copper levels relative to wild-type flies although we cannot rule out alternative explanations for the copper tolerance phenotype with reduced copper content being an indirect consequence. Interestingly, they also accumulate more zinc than wild-type on a zinc-supplemented diet, but tolerance to excess zinc is unaffected (Figure S2). No other metals were significantly affected.
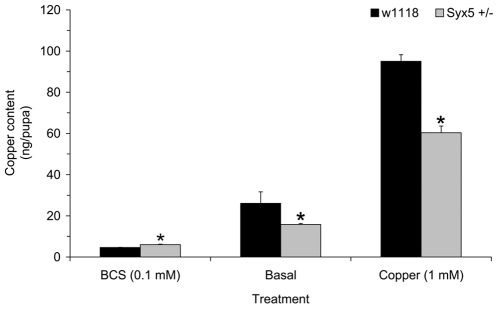
Figure 2. Copper accumulation in Syx5 +/− Drosophila.
Copper content was measured by ICP-AES in wild-type and Syx5+/− Drosophila reared to the pupal stage on copper chelator (100 µM BCS), basal media or 1 mM copper. Values are mean metal content per pupa with s.e.m. from five replicates of 50 pupae. Syx5+/− larvae accumulate less copper on both basal and copper-supplemented media. *Significant difference from wild-type, determined by a Mann-Whitney test (P<0.05).
The induction of metallothionein genes has been used previously as a proxy marker for copper excess, and Ctr1B induction as a marker for copper deficiency [15]. These genes were examined in Syx5+/− larvae raised under basal and copper supplemented conditions (Figure S3). Higher expression of Ctr1B in Syx5+/− larvae compared to wild-type (3.2±0.7 times wild-type, independent samples T-Test, P<0.05) is consistent with copper deficiency under basal conditions [17], and is alleviated by copper-supplementation (1.1±0.1 times wild-type). Copper-supplemented media stimulated the normal metallothionein (copper sequestration) response, raising expression to similar levels in both Syx5+/− and wild-type larvae. MtnC was the only metallothionein to show lower expression compared to wild-type (0.1±0.0, P<0.05). These results are consistent with direct measurements of copper content (Figure 2) which show that Syx5+/− larvae are capable of accumulating copper on supplemented media, even though the levels do not reach those of wild-type. Higher concentrations (2–4 mM copper) result in a copper load sufficient to increase Syx5+/− mortality, despite the high tolerance compared to wild-type (Figure 1). Together these data indicate that flies with 50% wild-type levels of functional Syx5 accumulate excess copper, but do so less efficiently than wild-type flies.
Tissue-specific reduction in Syx5 generates a typical copper deficiency phenotype
The GAL4-UAS system in Drosophila can be used to manipulate target gene expression in individual tissues by using tissue-specific GAL4 drivers to either ectopically express the gene of interest or inhibit it by RNA interference (RNAi) [24], [25]. RNAi lines specific to Syx5 were used to suppress Syx5 activity. Targeted suppression of Syx5 in the developing eye (Gmr-GAL4), nervous system (Elav-GAL4) or midgut (Mex-GAL4) resulted in larval or pupal lethality, probably due to the essential role of this gene in intracellular trafficking and Golgi reassembly following cell division [23].
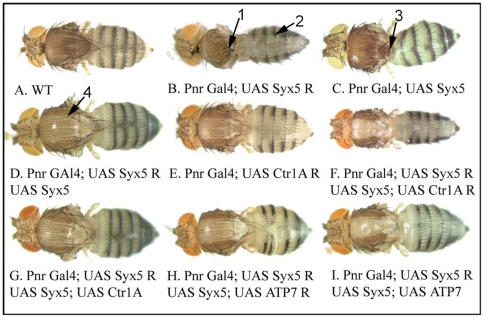
Suppression of Syx5 in the Pannier domain (Pnr-GAL4), a band down the centre of the developing thorax and abdomen, is also normally lethal. However rare survivors raised at 18°C show abdominal hypopigmentation phenotypes typical of copper deficiency [Figure 3B], [ 15,26] and complete loss of the central thorax. In contrast, over-expression of Syx5 in the same domain resulted in reduced scutellum and bristles but no change in pigmentation (Figure 3C). This milder phenotype is not necessarily related to copper transport as bristles are a mechanosensory structure, so their loss can reflect neuronal defects [27].
Figure 3. Suppression of Syx5 in adult Drosophila cuticle results in hypopigmentation typical of copper deficiency.
Gene expression/ suppression was driven in a dorsal stripe down the adult thorax/abdomen using the Pannier-GAL4 driver. A control fly is shown (A). Syx5 suppression under Pnr-GAL4 is normally lethal. Rare survivors reared at 18°C (B) show loss of dorsal thorax (arrow 1) and strong abdominal hypopigmentation (arrow 2). Syx5 over-expression results in reduced bristles and scutellum (C, arrow 3). Syx5 suppression can be rescued to a mild hypomorph by concurrent Syx5 over-expression (D), which shows hypopigmentation (arrow 4) similar to that seen for Ctr1A suppression (E). The mild hypomorph is exacerbated by Ctr1A co-suppression (F) but not rescued by Ctr1A over-expression (G). DmATP7 suppression (H) and over-expression (I) phenotypes are unaffected by addition of the Syx5 hypomorph combination.
Combining the Syx5 suppression and over-expression transgenes in the same fly results in a moderate hypopigmentation of the thorax (Figure 3D) similar to that seen in the moderate copper deficiency caused by Ctr1A suppression [Figure 3E, 26]. This is most likely a hypomorphic Syx5 phenotype where the combination of RNAi and over-expression results in an intermediate level of Syx5 transcript causing a partial loss of function.
To investigate genetic interactions between Syx5 and the copper homeostasis machinery, DmATP7 and Ctr1A levels were manipulated together with Syx5 suppression. Over-expressing or suppressing either Ctr1A (copper uptake) or DmATP7 (copper efflux) was unable to rescue the lethality caused by strong Syx5 suppression. Together with the Syx5 hypomorph combination (Figure 3D), Ctr1A suppression is additive (Figure 3F); the hypopigmentation phenotype is more severe and there is bristle loss that is not observed for either Ctr1A suppression (Figure 3E) or in the Syx5 (Figure 3D) hypomorph alone. In contrast Ctr1A over-expression, which would normally increase copper levels, does not rescue the Syx5 hypomorph (Figure 3G). The Syx5 hypomorph has no effect on the phenotype caused by either DmATP7 suppression (Figure 3H) or over-expression (Figure 3I).
These studies demonstrate that strong suppression of Syx5 causes lethality that cannot be rescued by the manipulation of major copper transporters which mediate uptake and efflux, indicating that intracellular trafficking pathways additional to those involved in copper homeostasis are disrupted. In contrast, mild suppression of Syx5 leads to a typical copper deficiency phenotype in the adult thorax and abdomen that is exacerbated by suppression of Ctr1A and cannot be rescued by Ctr1A over-expression. The copper deficiency phenotype is consistent with a reduction in copper levels found in Syx5+/− heterozygous flies (Figure 2, Figure S3) and a disruption to copper transport (Figure 3E).
Syx5 suppression reduces copper uptake at the cellular level
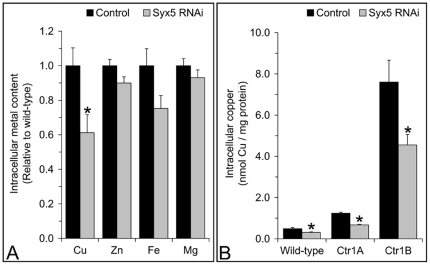
Copper accumulation was studied in cultured cells to further examine the role of Syx5 in cellular copper homeostasis. Suppression of Syx5 in the Drosophila S2 embryonic cell line resulted in reduced copper accumulation (Figure 4A), consistent with the reduction seen in pupae (Figure 2). No other metals were significantly affected. In particular, in contrast to the whole animal inductively coupled plasma atomic emission spectrometry (ICP) results, zinc content was not altered by Syx5 suppression in cells. A reduction in copper levels appears to be the key cellular Syx5 suppression phenotype and was therefore examined further.
Figure 4. Syx5 suppression in Drosophila S2 cells decreases copper accumulation.
Metal accumulation was measured by ICP-AES in control (black) and Syx5 (grey) RNAi suppression cells grown in basal media (A). Syx5 gene expression was suppressed to 19–36% of wild-type levels. Values are mean with s.e.m of eight replicates over two experiments, normalized against control cells. Mean copper accumulation during a 24 h exposure to 2 µM Cu was measured using 64Cu in S2 cell lines stably over-expressing Ctr1A, Ctr1B or an empty vector control and normalized to total cellular protein (B). Error bars are s.e.m. from nine replicates over three experiments. Syx5 gene expression levels relative to wild-type were 18–41% (control cells), 25-41% (Ctr1A) and 23-31% (Ctr1B). Copper accumulation is reduced by Syx5 suppression (A) even when Ctr1A or Ctr1B is over-expressed (B): suppression of Syx5 reduces copper levels to 50–70% of wild-type in all cell lines. *Significant difference between control and Syx5 suppression cells, determined by an independent samples T-Test (P<0.05).
To investigate how Syx5 might affect copper homeostasis, the gene was suppressed in cells with stable over-expression of Ctr1A or Ctr1B (Figure 4B). While total copper accumulation was higher in cells over-expressing either copper uptake gene, the relative efficiency of accumulation was decreased to a similar extent in control and over-expression cell lines when Syx5 was suppressed. This is consistent with data from Syx5+/− heterozygote flies, which show they are able to accumulate excess copper, but do so less efficiently than wild-type flies (Figures 1–2, Figure S3).
The effect of human Syx5 suppression was also investigated in two human fibroblast cell lines (Figure 5 and Figure S4). GM2069 cells have wild-type copper transport mechanisms. Me32a cells were derived from a Menkes disease patient and have a deletion in the ATP7A gene that introduces a premature stop codon [28]. These cells hyper-accumulate copper as the truncated ATP7A protein is unable to facilitate efflux of excess copper and the ATP7B efflux protein is not expressed.
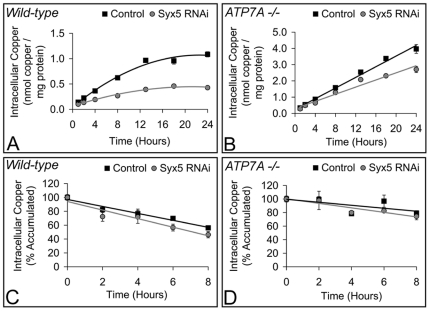
Figure 5. Syx5 suppression in human cells decreases copper accumulation but does not affect rate of turnover.
Wild-type (GM2069; A) and ATP7A deficient (Me32a; B) human cell lines were exposed to control (squares) or Syx5 (circles) siRNA for 48 h. Syx5 gene expression levels relative to wild-type were 22–46% for GM2069, and 23n45% for Me32a cells. Copper accumulation was then measured with 64Cu following 1–24 h exposure to 2 µM copper. Values are mean with s.e.m. of six replicates over two experiments. Non-linear regression analysis demonstrated copper accumulation in GM2069 cells (A) was significantly reduced following suppression of Syx5 (F = 108.0, P<0.0001). Linear regression analysis demonstrated copper accumulation was also significantly reduced in Me32a cells (B) following suppression of Syx5 (F = 44.1, P<0.0001). Rate of copper turnover of the radioisotope 64Cu was measured in wild-type (GM2069; C) and ATP7A deficient (Me32a; D) human cell lines. Cells were treated with control (squares) or Syx5 (circles) siRNA and exposed to 2 µM copper for 24 h, then returned to basal media for 2–8 h. Data are expressed as a percentage of copper accumulation at Time 0 and expressed as mean with s.e.m. of nine replicates over three experiments. Linear regression analysis shows that the rate of copper turnover was not significantly altered by Syx5 suppression.
Copper accumulation was determined at time intervals from 1 to 24 h (Figure 5A–B). Suppression of Syx5 resulted in a significant reduction in copper accumulation in both cell lines, with the difference most evident in GM2069 cells between 13 and 24 h when copper levels plateau. These results are consistent with those in S2 cells exposed to copper following suppression of Syx5 (Figure 4). Suppression of Syx5 does not significantly affect the rate of copper uptake in GM2069 cells up to 1 h (Figure S4A) or the short term copper uptake kinetics (Figure S5B–C). The reduced copper accumulation in Me32a cells (Figure 5B) indicates that functional ATP7A is not required for the Syx5 suppression phenotype. This suggests that the copper deficiency phenotype is associated with copper uptake rather than efflux, consistent with gene interaction studies in Drosophila (Figures 3 and 4B).
To exclude the possibility that Syx5 suppression stimulates an ATP7A-independent efflux mechanism, cells were allowed to accumulate copper and copper retention was examined when cells were returned to basal media (Figure 5C–D). Although less total copper accumulated following suppression of the gene (Figure 5A–B), there was no effect on the rate of copper turnover (Figure 5C–D) indicating increased copper efflux is unlikely to be responsible for the reduction in copper accumulation. Taken together these results demonstrate that suppression of Syx5 reduces copper uptake efficiency when cells are exposed to copper in the micromolar range. Reduced copper accumulation is evident after 1 h and the greatest difference occurs when copper levels have reached a steady state.
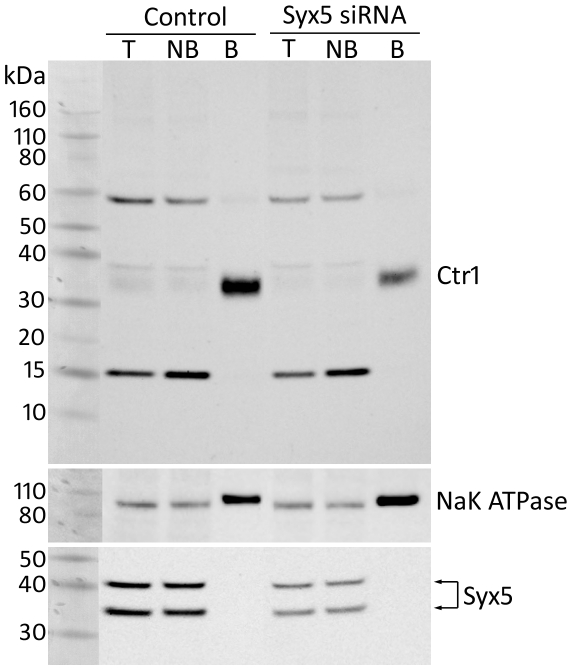
The localisation of human Ctr1 (hCtr1) was examined in human embryonic kidney (HEK293, ATCC cell line CRL-1573) cells following Syx5 suppression using biotinylation to detect myc-tagged hCtr1 at the cell surface (Figure 6). A biotinylated protein of approximately 35 kDa was detected at the cell surface, comparable to the reported size of monomeric myc-tagged hCtr1 in HEK293 cells [29]. Syx5 suppression reduced the amount of hCtr1 detected at the plasma membrane whilst a control membrane transporter, NaK-ATPase was not affected. Densitometry analysis revealed that, relative to NaK-ATPase, hCtr1 levels at the cell surface were reduced to 20% of that in control cells, consistent with the finding that copper uptake is reduced.
Figure 6. Syx5 suppression reduces the amount of hCtr1 at the plasma membrane of Hek293 cells.
Hek293 cells stably expressing Ctr1-myc were treated with scrambled negative control or Syx5 siRNA. Cell surface proteins were isolated following biotinylation using biotin-streptavidin precipitation prior to western immuno-blotting. Ctr1was detected with an anti-c-myc antibody. Syx5 knockdown was confirmed with an anti-Syx5 antibody and anti-NaK ATPase was used as control. Total lysate (T), non-biotinylated (NB), and biotinylated (B) fractions are shown. Protein bands were quantified with densitometry. Two species of Syx5 were detected and Syx5 knockdown reduced the amount of this protein in total cell lysate to approximately 33% of control siRNA treated cells. An hCtr1 monomer of approximately 35 kDa was detected in the biotinylated fraction. Densitometric analysis of hCtr1 protein intensity relative to NaK ATPase, revealed that Syx5 knockdown reduced the about of hCtr1 at the cell surface to approximately 20% of control siRNA treated cells.
Western blot densitometric analysis showed that Syx5 levels were reduced to 24–33% of wild-type in the human cell lines (Figures 6 and S5). This is milder suppression than in cases where Golgi fragmentation has been reported [21], [30] and minimal disruption to the Golgi and early endosomes was observed here (Figure S6). This is consistent with results from Drosophila in suggesting that copper homeostasis phenotypes are observed in otherwise viable, fertile flies when Syx5 levels are only mildly reduced. Greater reductions cause additional cellular disturbances and a range of phenotypes not necessarily related to copper homeostasis.
Discussion
Roles for mammalian Syx5 in both anterograde and retrograde vesicular transport have been well characterized and severe Syx5 reduction causes fragmentation and dispersal of the Golgi [21], [30]. Similarly, the Drosophila Syx5 orthologue is required for Golgi reassembly following cell division and for translocation of proteins to the apical membrane [23]. Complete loss of Syx5 activity leads to early larval lethality in the fly [23], as does the strong targeted RNAi suppression achieved by most GAL4 drivers tested for this study.
In contrast, results presented here have shown that a 50% reduction in Syx5 levels in Drosophila leads to significantly increased tolerance to high dietary copper with no adverse impact on viability or fertility. Drosophila copper tolerance can be altered by manipulating levels of known copper uptake proteins or metallothioneins directly or via suppression of the metallothionein transcription factor, MTF-1 [12], [13], [31]. However, the present study implicates for the first time a gene encoding components of vesicular trafficking in copper tolerance. Direct measurement of copper levels and analysis of copper-induced gene expression showed that this increased tolerance is associated with a reduction in copper accumulation. Consistent with this, moderate suppression of Syx5 specifically in the adult thorax generated a typical copper-deficiency phenotype.
Reduced Syx5 levels also resulted in elevated zinc accumulation in the fly but, unlike the copper phenotype, this did not affect zinc sensitivity. A possible explanation is that, with reduced copper levels, more metallothionein is available to sequester excess zinc and the flies are able to absorb more without detrimental effects. It is notable that this change in zinc accumulation was seen in Drosophila pupae only, not in cell lines, and could therefore be a systemic response to reduced copper accumulation.
The study of copper uptake and retention in cultured cells confirmed that the in vivo copper deficiency phenotype is due to a reduction in the efficiency of copper accumulation. Importantly, consistent results were seen in both Drosophila and human cell lines, suggesting that Syx5 plays an evolutionarily conserved role in cellular copper uptake. In human cell lines, we observed no detectible effect on copper uptake kinetics over 10 minutes, but rather a gradual reduction in copper accumulation over a period of hours. Copper turnover was unaffected by suppression of Syx5 and copper levels differed most at steady state levels.
Consistent with the finding that Syx5 suppression affects the copper uptake pathway, hCtr1 levels at the plasma membrane were reduced to 20% of control when Syx5 was suppressed in human cells. Previous studies have found that flies lacking Drosophila Syx5 have impaired transport of proteins to the apical membrane of epithelial cells [23]. Due to barely detectable Ctr1 levels in the whole lysate, it cannot be determined whether overall Ctr1 levels are reduced in these cells. Given the known role of Syx5 in anterograde vesicle transport and apical protein targeting, the most likely explanation is that Syx5 is required for localization of Ctr1 to the plasma membrane. However alternative explanations such as reduced synthesis or stability of Ctr1 cannot be ruled out. Thus it appears that loss of Syx5 alters Ctr1 function, thus inhibiting cellular copper uptake. This leads to a systemic copper deficiency in vivo.
Although mild dispersion of the Golgi was seen in Syx5 suppression cells and it has been shown previously that the loss of Syx5 can cause severe cellular and fertility defects [23], the impairment to copper uptake observed here occurred in the presence of sufficient Syx5 that flies show normal viability and fertility compared to wild type flies. Since the copper-tolerant Syx5+/− heterozygotes examined here are otherwise healthy, this raises the possibility that mild loss of Syx5 function may be important in copper-related disease in humans. This may be particularly relevant to conditions such as cancer and Alzheimer's disease, where subtle changes in cellular copper regulation may influence progression of the disease [1], [2], [11]. Indeed, it has been found that defects in components of the trafficking machinery can lead to a specific disease phenotype [32], although this has not previously been documented for copper homeostasis. In the case of Alzheimer's disease adapter proteins can affect Abeta40 and Abeta42 production by altering residence time of amyloid precursor protein in particular compartments including the plasma membrane [33].
We have presented evidence for a key role of Syx5 in cellular copper uptake, indicating that it plays a significant role in copper homeostasis. The finding that mild loss of Syx5 function significantly influences intracellular copper levels in the absence of other obvious phenotypes at the whole organism level provides a novel candidate for etiology of diseases resulting from copper dyshomeostasis [e.g. 2], [10].
Materials and Methods
Drosophila stocks and maintenance
All Drosophila strains were maintained on standard medium at 25°C. Armenia, Arm60 (European Drosophila Stock Centre, Umeå Sweden). w1118 (BL3605, Bloomington Stock Centre). ‘Df(2L)r10’, Df(2L)r10, cn1/CyO (BL1491). Syx5AR113/CyO, also referred to as Syx5+/− (BL3645) [23]. ‘Double balancer’, w; IF/CyO; MKRS/TM6b, Tb (gift from G. Hime, University of Melbourne). Gmr-GAL4, P{Gmr-GAL4.w−}2 (BL9146). Mex-GAL4 [34]. Pnr-GAL4, P{GawB}pnrMD237 (BL3039). ‘UAS-Syx5’, RNAi transformants 3857 and 3859 specific for Syx5 yielded the same results, and data shown are for 3857 (Vienna Drosophila RNAi Center).
Drosophila mortality screen
Standard medium was supplemented with 100–1000 µM of the copper chelator bathocuproinedisulfonic acid (BCS; Sigma) or 1–4 mM Cu (CuSO4.5H2O; Merck) as specified in the text. Survival to the adult stage was measured for five replicates of 50 first instar larvae per condition. Male Syx5AR113/CyO, Df(2L)r10/CyO or IF/CyO mutants were crossed to female Armenia and visible Chromosome 2 markers were used to monitor segregation of Syx5AR113, Df(2L)r10 and CyO. Offspring from the replicates were pooled for χ2 analysis.
Transgenics
The Drosophila Syx5 open reading frame including the first intron was PCR amplified from w1118 genomic DNA, omitting the termination codon using primers: Forward, GGGGTACCATGCAAACCCGAAGACGCCT and Reverse, GCTCTAGACGACATAAAAACAACGAAG. This fragment was sub-cloned in-frame with a C-terminal myc epitope tag into the pUAST_attB vector. Embryos from the Basler laboratory φC31 strains φX-51A and φX-96E were injected by standard techniques. Microinjections utilized an Eppendorf Femtojet apparatus with Femtotips II (Eppendorf) pre-pulled glass needles. Integrants at both these attP sites were obtained. Results presented here utilized the φX-51A integrant. Adult flies were imaged with a Leica MZ6 Stereomicroscope.
Generation of myc-tagged Crt1 overexpressing HEK293 cells
The myc-tagged Ctr1 construct was generated through PCR amplification of cDNA using the forward (5′-TCATGGATCCGAAAAAATGGAACAAAAACTCATCTCAGAAGAGGATCTGGATCATTCCCACCATATGGG -3′) and reverse (5′-GGGCTCTAGAGAATTCAATGGCAATGCTCTGTGATATC -3′) oligonucleotides and by incorporation into the mammalian expression vector, pcDNA3. The forward oligonucleotide introduced sequence encoding the myc epitope in-frame immediately after the start codon and provided a flanking 5′ BamH1 endonuclease restriction site. The reverse oligonucleotide provided an EcoRI endonuclease restriction site 3′ to the stop codon. Template cDNA was isolated from human hepatoma HepG2 cells (ATCC, cell line HB-8065) using the SuperScriptTM III CellsDirect cDNA synthesis system (Invitrogen) following the manufacturer's protocol. The PCR reaction contained 1×PCR buffer, 0.2 mM of each dNTP, 2 mM MgCl2, 0.2 µM of each primer, 2.5 units Platinum Taq DNA polymerase and 3 µl of cDNA (Invitrogen). Reactions were run on an Eppendorf Epgradient S Mastercycler on the following program: one cycle of 94°C for 2min, 38 cycles of 94°C for 45s, 57°C for 60s and 72°C for 60 s, followed by one cycle of 72°C for 2 min. The resultant PCR product was digested with BamH1 and EcoR1 and cloned into pcDNA3 at the same sites. Integrity was confirmed by sequencing. Stable transfection of HEK293 cells (ATCC, cell line CRL-1573) with the myc-tagged Ctr1 construct was performed using FuGENE® HD (Roche) following the manufacturer's instructions. The cells were recovered in Dulbecco's Modified Eagle's medium (DMEM) containing 10% (v/v) FCS and transfectants were selected with 500 µg/ml G418 for 14 days.
Cell culture
Drosophila embryonic S2 cells were propagated in Serum Free Media (SFM, Invitrogen) as previously reported [14]. S2 cells maintaining stable over-expression of Ctr1A or Ctr1B were generated by co-transfecting pCoHygro with either pAcCtr1A, pAcCtr1B or pAc empty vector control using Lipofectamine2000 and propagated in Schneider's Complete Media (Invitrogen) with 10% foetal calf serum (Trace Scientific) supplemented with 300 µg/ml hygromycin-B according to the manufacturer's instructions (Invitrogen). Media was replaced with SFM for all experiments and supplemented with CuCl2 (Sigma) at the concentration specified in the text. Wild-type (GM2069) and ATP7A null (Me32a) human fibroblast cells have been described previously [28]. Cells were maintained in Eagle's basal culture medium (Thermo Scientific) supplemented with 10% foetal calf serum (Trace Scientific) at 37°C and passaged weekly. Human embryonic kidney (HEK293) cells were stably transfected with myc-tagged human Ctr1 (pcDNA3.1Ctr1-Myc). These cells were maintained in Dulbecco's modified Eagle's medium (Thermo Scientific) with 10% foetal calf serum (Trace Scientific) and 500 µg/ml Geneticin (Invitrogen) at 37°C and passaged weekly.. All experiments were conducted in growth media with 10% foetal calf serum.
RNA interference and gene expression
dsRNAi in S2 cells was conducted as previously reported [14]. dsRNA was targeted to Syx5 (cDNA bases 214–757). Control dsRNA was derived from EYFP cDNA or Adult Cuticle Protein 1 (13–558), which is not expressed in S2 cells. siRNA suppression in mammalian cells utilized Stealth RNAi duplexes (Invitrogen). 40 nM Syx5 or low GC negative control Stealth RNAi duplexes were transfected into mammalian cells with Lipofectamine2000 according to the manufacturer's instructions (Invitrogen). Cells were seeded to be 30–50% confluent on the day of transfection and growth media was replaced with Opti-MEM (Invitrogen). Opti-MEM was replaced with growth media 4–6 h after transfection 48 h before experiments. Gene suppression was confirmed using qPCR and western blot. qPCR was performed as previously described [31]. Housekeeping genes GAPDH, Actin42A and βActin were used for normalisation in Drosophila larvae, Drosophila S2 cells and mammalian cells respectively. Primer sequences are shown in Table S2.
Metal accumulation and retention
Copper accumulation was measured as previously reported [14]. Cells were incubated with ∼0.4 MBq 64Cu (Australian Radioisotopes) and non-radioactive copper at the concentrations described in the figure legends. Copper retention was measured by incubating cells with copper for 24 h, washing, and incubating for an additional 2–8 h in basal media. Radioactivity was measured with a γ-counter (1282 CompuGamma, LKB Wallac). Copper levels were normalized to total cellular protein, which was determined using BioRad protein reagent according to the manufacturer's instructions (BioRad). Total metal accumulation was measured using a Vista-AX Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-AES, Varian) in samples digested in 70% HNO3 and metal levels were measured as described previously [31]. Drosophila metal levels were measured in five replicates of 50 pupae and expressed as ng/pupa. S2 cell metal levels were normalized to total cellular protein.
Cell Surface Biotinylation and Western Blotting
Cell surface proteins were labelled with 0.5 mg/ml sulpho-NHS-SS-biotin (Thermo Scientific) and precipitated with streptavidin-agarose beads (Thermo Scientific) as previously described [35]. Protein samples were resolved on NuPAGE 4–12% Bis-Tris gels (Invitrogen) and transferred to nitrocellulose membranes for western immuno-blotting. Primary antibodies used were mouse anti-c-myc (1∶5000, Sigma), rabbit anti-Syx5 [1∶1500, 36] and mouse anti-NaK-ATPase (1∶5000, Abcam). Horseradish peroxidase coupled secondary antibodies were rabbit anti-mouse and goat anti-rabbit (1∶7000, Dako). Chemiluminescence was detected using ECL (GE Healthcare) and images were captured with a Fujifilm LAS-3000 (Fujifilm LifeScience). Densitometric analysis was conducted with Multi Gauge v2.3 (Fujifilm).
Statistics
Statistical analysis was conducted using SPSS v16 (SPSS). A one-sample Kolomogorov-Smirinov test was used to assess whether data was normally distributed. Statistical analyses are described in Figure legends. P<0.05 was deemed statistically significant.
Supporting Information
χ2 values comparing siblings from crosses to wild-type Armenia.
(0.03 MB DOC)
Quantitative PCR primer sequences.
(0.03 MB DOC)
Syx5+/− Drosophila show no viability or fertility defects. Ten individual pairs each of Syx5+/− heterozygous virgin females and w1118 males (Syx5 f×w1118 m), w1118 virgin females and Syx5+/− heterozygous males (w1118 f×Syx5 m), or w1118 virgin females and w1118 males (w1118×w1118) were maintained in vials containing standard laboratory medium which was replaced every 24 h. Fertility was measured by allowing pairs 24 h to mate, then counting eggs produced every 24 h for five days (A). Male reproductive output was measured as the egg production of females inseminated by Syx5+/− males. Twenty replicates of 50 eggs were transferred into vials containing standard laboratory medium and viability was scored as the number of adults to emerge after 20 days (B). The average number of eggs laid per female over a five day period and the average number of eggs to reach the adult state were compared among strains using a one-way ANOVA with LSD post-hoc testing. Since both Syx5−/w1118 and CyO/w1118 offspring were produced in Syx5+/− crosses, an independent samples T-Test was used to confirm there was no difference in survival between these sibling genotypes then they were pooled for comparison to the w1118 strain. The Syx5 mutation did not adversely affect fertility: there was no significant difference in the number of eggs produced from either cross compared to those produced by the w1118 control strain (Syx5+/− female×w1118 male, 306±15; w1118 female×Syx5+/− male, 241±24; w1118 female×w1118 male, 295±32; P = 0.479). There was no adverse impact on viability of eggs from Syx5+/− parents, in fact there was slightly higher survival of the w1118 female×Syx5+/− (44±1 s.e.m.) male compared to the w1118 strain (38±1; P = 0.002). Emergence from the reciprocal cross was intermediate (40±1) and not significantly different from either.
(0.14 MB TIF)
Metal accumulation and zinc tolerance in Syx5+/− heterozygote Drosophila. Metal content was measured by ICP-AES on flies reared to the pupal stage on basal media (A). Data are mean ± s.e.m. metal content per pupa from five replicates of 50 pupae and are expressed relative to wild-type (w1118) levels. In addition to a decrease in copper accumulation (shown in detail in Figure 2 of the main text), Syx5+/− flies accumulated 1.5-fold more zinc than wild-type (A). Zinc tolerance was determined as described for copper tolerance in the main text by supplementing media with 0–8 mM zinc (ZnSO4.7H20, Ajax) (B). No significant differences in mortality were detected.
(0.37 MB TIF)
Copper-responsive gene expression in Syx5+/− Drosophila. Wild-type and Syx5+/− Drosophila were reared to third instar on basal media (A) or 1 mM copper (B) and qPCR was used to investigate Ctr1B and MtnA-D expression levels from three replicates of 50 larvae. Ctr1A has no transcriptional response to copper levels and is included as a control. Gene expression is mean relative to wild-type. Error bars are s.e.m. Under basal conditions Ctr1B is upregulated in Syx5+/− larvae, indicative of copper deficiency. Copper exposure alleviates the deficiency and leads to similar MtnA, MtnB and MtnD upregulation in Syx5+/− and wild-type larvae. An independent samples T-Test was used to determine statistical significance for differences between Syx5+/− and wild-type exceeding a two-fold magnitude, as indicated by dotted lines (*P<0.05).
(0.24 MB TIF)
Syx5 suppression in human cells does not significantly affect copper uptake kinetics. (A) Copper uptake measured over one hour in GM2069 cells treated with control (squares) or Syx5 (circles) siRNA for 48 h. 64Cu was used to measure copper accumulation in cells exposed to 2 µM copper for 5–60 minutes. Values are mean with s.e.m. of nine replicates from three experiments. There was a tendency for the rate of copper accumulation to be lower following Syx5 suppression, however linear regression analysis demonstrated that this was not significantly different. (B) GM2069 cells treated with control (squares) or Syx5 (circles) siRNA for 48 h. 64Cu was used to measure copper accumulation in cells exposed to 2–100 µM copper for 10 min. Values are mean with s.e.m. of six replicates from two independent experiments. Non-linear regression analysis demonstrated that copper uptake kinetics were not statistically significant different following knockdown of Syx5.
(0.31 MB TIF)
RNAi suppression in human cells reduces protein levels for Syx5. GM2069 and Me32a cells treated with control or Syx5 siRNA for 48 h. Western blot analysis of whole cell lysate from these cells using anti-Syx5 antibody detected two electrophoretic species as previously reported [1]. Rabbit anti-Actin 20–33 (1∶300, Sigma) was used as a loading control. The amount of both Syx5 species was reduced by Syx5 suppression in each of these cell lines: Densitometry analysis demonstrates that, relative to control cells, Syx5 protein levels were reduced to 27.7% in GM2069 and 31.1% in Me32a cells. 1. Subramaniam VN, Loh E, Hong WJ (1997) N-ethylmaleimide-sensitive factor (NSF) and alpha-soluble NSF attachment proteins (SNAP) mediate dissociation of GS28-syntaxin 5 Golgi SNAP receptors (SNARE) complex. J Biol Chem 272: 25441–25444.
(0.24 MB TIF)
Syx5 suppression does not cause substantial Golgi fragmentation or affect early endosome localization in human cells. Golgi distribution: Immunocytochemistry in GM2069 cells utilized anti-Syx5 (1∶50) and mouse anti-Golgin 97 was used as a TGN marker (1∶200, Prof. Paul Gleeson). Secondary antibodies were Alexa 488 anti-rabbit and Alexa 594 anti-mouse (1∶400, Invitrogen). DAPI (300 nM, Invitrogen) was used to detect the nucleus. Images were recorded at 100× magnification using an Olympus FluoView 1000 confocal microscope with Olympus FluoView ver1.6a software (Olympus). Images at each wavelength were captured sequentially and multi-color maximum brightness stacked images were prepared using Image J (NIH, Bethesda, MD, USA). GM2069 cells were treated with control siRNA (A–C) or Syx5 siRNA (D–F). Syx5 is shown in Green (A, D), Golgin 97 is shown in Red (B, E) and DAPI is shown in Blue. Merged images are also shown (C, F). Syx5 suppression reduced Syx5 levels but did not dramatically alter the distribution of Golgin 97 (D–F). Golgi distribution was measured using Image J and was found to be 42.5±7.2 µm2 in control and 72.2±9.7 µm2 following Syx5 suppression. Thus the Syx5 RNAi suppression achieved in this study produced a mild phenotype in comparison to the effects of extreme Syx5 inhibition found in previous studies [1], [2]. Early endosome localization: Immunocytochemistry in GM2069 cells utilized anti-Syx5 (1∶50) and mouse anti-EEA1 was used as an early endosome marker (1∶100, BD Biosciences). Secondary antibodies, DAPI staining and image analysis were conducted as described above for Golgi distribution. GM2069 cells were treated with control siRNA (G–I) or Syx5 siRNA (J–L). Syx5 is shown in Green (G, J), EEA1 is shown in Red (H, K) and DAPI is shown in Blue. Merged images are also shown (I, L). The localization of early endosomes was not affected by Syx5 suppression (J–L). 1. Amessou M, Fradagrada A, Falguieres T, Lord JM, Smith DC, Roberts LM, Lamaze C, Johannes, L (2007) Syntaxin 16 and syntaxin 5 are required for efficient retrograde transport of several exogenous and endogenous cargo proteins. J Cell Sci 120: 1457–1468. 2. Diao A, Frost L, Morohashi Y, Lowe M (2008) Coordination of Golgin Tethering and SNARE Assembly: GM130 binds Syntaxin 5 in a p115-regulated manner. J Biol Chem 283: 6957–6967.
(0.84 MB TIF)
Acknowledgments
The authors are grateful to G. Hime for providing Drosophila stocks and P. Gleeson for providing antibodies used in this work.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the International Copper Association (grant DP0451599, www.copper.org), the Australian Research Council (www.arc.gov.au), and the Australian Institute of Nuclear Science and Engineering (www.ainse.edu.au). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Danks DM. Copper Deficiency in Humans. Annu Rev Nutr. 1988;8:235–257. doi: 10.1146/annurev.nu.08.070188.001315. [DOI] [PubMed] [Google Scholar]
- 2.Turski ML, Thiele DJ. New Roles for Copper Metabolism in Cell Proliferation, Signaling, and Disease. J Biol Chem. 2009;284:717–721. doi: 10.1074/jbc.R800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klomp AEM, Juijn JA, van der Gun LTM, van den Berg IET, Berger R, et al. The N-terminus of the human copper transporter 1 (hCTR1) is localized extracellularly, and interacts with itself. Biochem J. 2003;370:881–889. doi: 10.1042/BJ20021128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aller SG, Unger VM. Projection structure of the human copper transporter CTR1 at 6-A resolution reveals a compact trimer with a novel channel-like architecture. Proc Natl Acad Sci USA. 2006;103:3627–3632. doi: 10.1073/pnas.0509929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maryon EB, Molloy SA, Zimnicka AM, Kaplan JH. Copper entry into human cells: progress and unanswered questions. 5th International Biometals Symposium (Biometals 2006) Welches, OR: Springer; 2007. pp. 355–364. [DOI] [PubMed] [Google Scholar]
- 6.Horng YC, Cobine PA, Maxfield AB, Carr HS, Winge DR. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. The Journal of biological chemistry. 2004;279:35334–35340. doi: 10.1074/jbc.M404747200. [DOI] [PubMed] [Google Scholar]
- 7.Hamza I, Schaefer M, Klomp LWJ, Gitlin JD. Interaction of the copper chaperone HAH1 with the Wilson disease protein is essential for copper homeostasis. Proc Natl Acad Sci USA. 1999;96:13363–13368. doi: 10.1073/pnas.96.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, et al. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. Embo J. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- 9.de Bie P, Muller P, Wijmenga C, Klomp LW. Molecular pathogenesis of Wilson and Menkes disease: correlation of mutations with molecular defects and disease phenotypes. Journal of medical genetics. 2007;44:673–688. doi: 10.1136/jmg.2007.052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bach J-P, Kumar N, Depboylu C, Noelker C, Klockgether T, et al. Copper deficiency associated with severe neurological disorder – A genetic work-up of possible mutations in copper transport proteins. Journal of the Neurological Sciences. 291:95–97. doi: 10.1016/j.jns.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Barnham KJ, Bush AI. Metals in Alzheimer's and Parkinson's diseases. Curr Opin Chem Biol. 2008;12:222–228. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Egli D, Selvaraj A, Yepiskoposyan H, Zhang B, Hafen E, et al. Knockout of ‘metal-responsive transcription factor’ MTF-1 in Drosophila by homologous recombination reveals its central role in heavy metal homeostasis. EMBO J. 2003;22:100–108. doi: 10.1093/emboj/cdg012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H, Cadigan KM, Thiele DJ. A copper-regulated transporter required for copper acquisition, pigmentation, and specific stages of development in Drosophila melanogaster. J Biol Chem. 2003;278:48210–48218. doi: 10.1074/jbc.M309820200. [DOI] [PubMed] [Google Scholar]
- 14.Southon A, Burke R, Norgate M, Batterham P, Camakaris J. Copper homoeostasis in Drosophila melanogaster S2 cells. Biochem J. 2004;383:303–309. doi: 10.1042/BJ20040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norgate M, Lee E, Southon A, Farlow A, Batterham P, et al. Essential roles in development and pigmentation for the Drosophila copper transporter DmATP7. Mol Biol Cell. 2006;17:475–484. doi: 10.1091/mbc.E05-06-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turski ML, Thiele DJ. Drosophila Ctr1A functions as a copper transporter essential for development. J Biol Chem. 2007;282:24017–24026. doi: 10.1074/jbc.M703792200. [DOI] [PubMed] [Google Scholar]
- 17.Selvaraj A, Balamurugan K, Yepiskoposyan H, Zhou H, Egli D, et al. Metal-responsive transcription factor (MTF-1) handles both extremes, copper load and copper starvation, by activating different genes. Genes Dev. 2005;19:891–896. doi: 10.1101/gad.1301805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norgate M, Southon A, Zou SG, Zhan M, Sun Y, et al. Copper homeostasis gene discovery in Drosophila melanogaster. 5th International Biometals Symposium (Biometals 2006) Welches, OR: Springer; 2007. pp. 683–697. [DOI] [PubMed] [Google Scholar]
- 19.Chen YA, Scheller RH. Snare-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- 20.Shorter J, Beard MB, Seemann J, Dirac-Svejstrup AB, Warren G. Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J Cell Biol. 2002;157:45–62. doi: 10.1083/jcb.200112127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amessou M, Fradagrada A, Falguieres T, Lord JM, Smith DC, et al. Syntaxin 16 and syntaxin 5 are required for efficient retrograde transport of several exogenous and endogenous cargo proteins. J Cell Sci. 2007;120:1457–1468. doi: 10.1242/jcs.03436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dascher C, Matteson J, Balch WE. Syntaxin-5 regulates endoplasmic-reticulum to Golgi transport. J Biol Chem. 1994;269:29363–29366. [PubMed] [Google Scholar]
- 23.Xu H, Brill JA, Hsien J, McBride R, Boulianne GL, et al. Syntaxin 5 is required for cytokinesis and spermatid differentiation in Drosophila. Dev Biol. 2002;251:294–306. doi: 10.1006/dbio.2002.0830. [DOI] [PubMed] [Google Scholar]
- 24.Lam G, Thummel CS. Inducible expression of double-stranded RNA directs specific genetic interference in Drosophila. Curr Biol. 2000;10:957–963. doi: 10.1016/s0960-9822(00)00631-x. [DOI] [PubMed] [Google Scholar]
- 25.Brand AH, Perrimon N. Targeted gene-expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 26.Binks T, Lye J, Camakaris J, Burke R. Tissue-specific interplay between copper uptake and efflux in Drosophila. Journal of Biological Inorganic Chemistry. [DOI] [PubMed]
- 27.Norga KK, Gurganus MC, Dilda CL, Yamamoto A, Lyman RF, et al. Quantitative analysis of bristle number in Drosophila mutants identifies genes involved in neural development. Curr Biol. 2003;13:1388–1397. doi: 10.1016/s0960-9822(03)00546-3. [DOI] [PubMed] [Google Scholar]
- 28.La Fontaine S, Firth SD, Camakaris J, Englezou A, Theophilos MB, et al. Correction of the copper transport defect of Menkes patient fibroblasts by expression of the Menkes and Wilson ATPases. J Biol Chem. 1998;273:31375–31380. doi: 10.1074/jbc.273.47.31375. [DOI] [PubMed] [Google Scholar]
- 29.Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem. 2003;278:9639–9646. doi: 10.1074/jbc.M209455200. [DOI] [PubMed] [Google Scholar]
- 30.Diao A, Frost L, Morohashi Y, Lowe M. Coordination of Golgin Tethering and SNARE Assembly: GM130 binds Syntaxin 5 in a p115-regulated manner. J Biol Chem. 2008;283:6957–6967. doi: 10.1074/jbc.M708401200. [DOI] [PubMed] [Google Scholar]
- 31.Southon A, Farlow A, Norgate M, Burke R, Camakaris J. Malvolio is a copper transporter in Drosophila melanogaster. J Exp Biol. 2008;211:709–716. doi: 10.1242/jeb.014159. [DOI] [PubMed] [Google Scholar]
- 32.Stein M, Wandinger-Ness A, Roitbak T. Altered trafficking and epithelial cell polarity in disease. Trends in cell biology. 2002;12:374–381. doi: 10.1016/s0962-8924(02)02331-0. [DOI] [PubMed] [Google Scholar]
- 33.Small SA, Gandy S. Sorting through the Cell Biology of Alzheimer's Disease: Intracellular Pathways to Pathogenesis. 2006;52:15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips MD, Thomas GH. Brush border spectrin is required for early endosome recycling in Drosophila. J Cell Sci. 2006;119:1361–1370. doi: 10.1242/jcs.02839. [DOI] [PubMed] [Google Scholar]
- 35.Pase L, Voskoboinik I, Greenough M, Camakaris J. Copper stimulates trafficking of a distinct pool of the Menkes copper ATPase (ATP7A) to the plasma membrane and diverts it into a rapid recycling pool. Biochem J. 2004;378:1031–1037. doi: 10.1042/BJ20031181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramaniam VN, Loh E, Hong WJ. N-ethylmaleimide-sensitive factor (NSF) and alpha-soluble NSF attachment proteins (SNAP) mediate dissociation of GS28-syntaxin 5 Golgi SNAP receptors (SNARE) complex. J Biol Chem. 1997;272:25441–25444. doi: 10.1074/jbc.272.41.25441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
χ2 values comparing siblings from crosses to wild-type Armenia.
(0.03 MB DOC)
Quantitative PCR primer sequences.
(0.03 MB DOC)
Syx5+/− Drosophila show no viability or fertility defects. Ten individual pairs each of Syx5+/− heterozygous virgin females and w1118 males (Syx5 f×w1118 m), w1118 virgin females and Syx5+/− heterozygous males (w1118 f×Syx5 m), or w1118 virgin females and w1118 males (w1118×w1118) were maintained in vials containing standard laboratory medium which was replaced every 24 h. Fertility was measured by allowing pairs 24 h to mate, then counting eggs produced every 24 h for five days (A). Male reproductive output was measured as the egg production of females inseminated by Syx5+/− males. Twenty replicates of 50 eggs were transferred into vials containing standard laboratory medium and viability was scored as the number of adults to emerge after 20 days (B). The average number of eggs laid per female over a five day period and the average number of eggs to reach the adult state were compared among strains using a one-way ANOVA with LSD post-hoc testing. Since both Syx5−/w1118 and CyO/w1118 offspring were produced in Syx5+/− crosses, an independent samples T-Test was used to confirm there was no difference in survival between these sibling genotypes then they were pooled for comparison to the w1118 strain. The Syx5 mutation did not adversely affect fertility: there was no significant difference in the number of eggs produced from either cross compared to those produced by the w1118 control strain (Syx5+/− female×w1118 male, 306±15; w1118 female×Syx5+/− male, 241±24; w1118 female×w1118 male, 295±32; P = 0.479). There was no adverse impact on viability of eggs from Syx5+/− parents, in fact there was slightly higher survival of the w1118 female×Syx5+/− (44±1 s.e.m.) male compared to the w1118 strain (38±1; P = 0.002). Emergence from the reciprocal cross was intermediate (40±1) and not significantly different from either.
(0.14 MB TIF)
Metal accumulation and zinc tolerance in Syx5+/− heterozygote Drosophila. Metal content was measured by ICP-AES on flies reared to the pupal stage on basal media (A). Data are mean ± s.e.m. metal content per pupa from five replicates of 50 pupae and are expressed relative to wild-type (w1118) levels. In addition to a decrease in copper accumulation (shown in detail in Figure 2 of the main text), Syx5+/− flies accumulated 1.5-fold more zinc than wild-type (A). Zinc tolerance was determined as described for copper tolerance in the main text by supplementing media with 0–8 mM zinc (ZnSO4.7H20, Ajax) (B). No significant differences in mortality were detected.
(0.37 MB TIF)
Copper-responsive gene expression in Syx5+/− Drosophila. Wild-type and Syx5+/− Drosophila were reared to third instar on basal media (A) or 1 mM copper (B) and qPCR was used to investigate Ctr1B and MtnA-D expression levels from three replicates of 50 larvae. Ctr1A has no transcriptional response to copper levels and is included as a control. Gene expression is mean relative to wild-type. Error bars are s.e.m. Under basal conditions Ctr1B is upregulated in Syx5+/− larvae, indicative of copper deficiency. Copper exposure alleviates the deficiency and leads to similar MtnA, MtnB and MtnD upregulation in Syx5+/− and wild-type larvae. An independent samples T-Test was used to determine statistical significance for differences between Syx5+/− and wild-type exceeding a two-fold magnitude, as indicated by dotted lines (*P<0.05).
(0.24 MB TIF)
Syx5 suppression in human cells does not significantly affect copper uptake kinetics. (A) Copper uptake measured over one hour in GM2069 cells treated with control (squares) or Syx5 (circles) siRNA for 48 h. 64Cu was used to measure copper accumulation in cells exposed to 2 µM copper for 5–60 minutes. Values are mean with s.e.m. of nine replicates from three experiments. There was a tendency for the rate of copper accumulation to be lower following Syx5 suppression, however linear regression analysis demonstrated that this was not significantly different. (B) GM2069 cells treated with control (squares) or Syx5 (circles) siRNA for 48 h. 64Cu was used to measure copper accumulation in cells exposed to 2–100 µM copper for 10 min. Values are mean with s.e.m. of six replicates from two independent experiments. Non-linear regression analysis demonstrated that copper uptake kinetics were not statistically significant different following knockdown of Syx5.
(0.31 MB TIF)
RNAi suppression in human cells reduces protein levels for Syx5. GM2069 and Me32a cells treated with control or Syx5 siRNA for 48 h. Western blot analysis of whole cell lysate from these cells using anti-Syx5 antibody detected two electrophoretic species as previously reported [1]. Rabbit anti-Actin 20–33 (1∶300, Sigma) was used as a loading control. The amount of both Syx5 species was reduced by Syx5 suppression in each of these cell lines: Densitometry analysis demonstrates that, relative to control cells, Syx5 protein levels were reduced to 27.7% in GM2069 and 31.1% in Me32a cells. 1. Subramaniam VN, Loh E, Hong WJ (1997) N-ethylmaleimide-sensitive factor (NSF) and alpha-soluble NSF attachment proteins (SNAP) mediate dissociation of GS28-syntaxin 5 Golgi SNAP receptors (SNARE) complex. J Biol Chem 272: 25441–25444.
(0.24 MB TIF)
Syx5 suppression does not cause substantial Golgi fragmentation or affect early endosome localization in human cells. Golgi distribution: Immunocytochemistry in GM2069 cells utilized anti-Syx5 (1∶50) and mouse anti-Golgin 97 was used as a TGN marker (1∶200, Prof. Paul Gleeson). Secondary antibodies were Alexa 488 anti-rabbit and Alexa 594 anti-mouse (1∶400, Invitrogen). DAPI (300 nM, Invitrogen) was used to detect the nucleus. Images were recorded at 100× magnification using an Olympus FluoView 1000 confocal microscope with Olympus FluoView ver1.6a software (Olympus). Images at each wavelength were captured sequentially and multi-color maximum brightness stacked images were prepared using Image J (NIH, Bethesda, MD, USA). GM2069 cells were treated with control siRNA (A–C) or Syx5 siRNA (D–F). Syx5 is shown in Green (A, D), Golgin 97 is shown in Red (B, E) and DAPI is shown in Blue. Merged images are also shown (C, F). Syx5 suppression reduced Syx5 levels but did not dramatically alter the distribution of Golgin 97 (D–F). Golgi distribution was measured using Image J and was found to be 42.5±7.2 µm2 in control and 72.2±9.7 µm2 following Syx5 suppression. Thus the Syx5 RNAi suppression achieved in this study produced a mild phenotype in comparison to the effects of extreme Syx5 inhibition found in previous studies [1], [2]. Early endosome localization: Immunocytochemistry in GM2069 cells utilized anti-Syx5 (1∶50) and mouse anti-EEA1 was used as an early endosome marker (1∶100, BD Biosciences). Secondary antibodies, DAPI staining and image analysis were conducted as described above for Golgi distribution. GM2069 cells were treated with control siRNA (G–I) or Syx5 siRNA (J–L). Syx5 is shown in Green (G, J), EEA1 is shown in Red (H, K) and DAPI is shown in Blue. Merged images are also shown (I, L). The localization of early endosomes was not affected by Syx5 suppression (J–L). 1. Amessou M, Fradagrada A, Falguieres T, Lord JM, Smith DC, Roberts LM, Lamaze C, Johannes, L (2007) Syntaxin 16 and syntaxin 5 are required for efficient retrograde transport of several exogenous and endogenous cargo proteins. J Cell Sci 120: 1457–1468. 2. Diao A, Frost L, Morohashi Y, Lowe M (2008) Coordination of Golgin Tethering and SNARE Assembly: GM130 binds Syntaxin 5 in a p115-regulated manner. J Biol Chem 283: 6957–6967.
(0.84 MB TIF)