Summary
Thisbe (Ths) and Pyramus (Pyr), two closely related Drosophila homologues of the vertebrate fibroblast growth factor (FGF) 8/17/18 subfamily, are ligands for the FGF receptor Heartless (Htl). Both ligands are required for mesoderm development, but their differential expression patterns suggest distinct functions during development. We generated single mutants and found that ths or pyr loss-of-function mutations are semi-lethal and mutants exhibit much weaker phenotypes as compared with loss of both ligands or htl. Thus, pyr and ths display partial redundancy in their requirement in embryogenesis and viability. Nevertheless, we find that pyr and ths single mutants display defects in gastrulation and mesoderm differentiation. We show that localised expression of pyr is required for normal cell protrusions and high levels of MAPK activation in migrating mesoderm cells. The results support the model that Pyr acts as an instructive cue for mesoderm migration during gastrulation. Consistent with this function, mutations in pyr affect the normal segmental number of cardioblasts. Furthermore, Pyr is essential for the specification of even-skipped-positive mesodermal precursors and Pyr and Ths are both required for the specification of a subset of somatic muscles. The results demonstrate both independent and overlapping functions of two FGF8 homologues in mesoderm morphogenesis and differentiation. We propose that the integration of Pyr and Ths function is required for robustness of Htl-dependent mesoderm spreading and differentiation, but that the functions of Pyr have become more specific, possibly representing an early stage of functional divergence after gene duplication of a common ancestor.
Keywords: Gastrulation, Mesoderm migration, Heart development, Fibroblast growth factor
INTRODUCTION
Growth factors provide extracellular signals that promote distinctive cell responses such as cell division, survival and migration, as well as cell fate decisions. Fibroblast growth factors (FGFs) play important roles in development in organisms ranging from simple metazoans to mammals (Szebenyi and Fallon, 1999). Among the various functions of FGF signalling, FGFs have been implicated in orchestrating complex developmentally controlled cell rearrangements (Ghabrial et al., 2003; Böttcher and Niehrs, 2005). In vertebrates, up to 22 different family members are known to interact with four different FGF receptors in a complex combinatorial fashion (Ornitz and Itoh, 2001; Itoh and Ornitz, 2004). Several different FGFs often act together in a non-overlapping way to promote morphogenetic events in vertebrate mesoderm development (Yang et al., 2002; Fletcher et al., 2006; Guo and Li, 2007). The complexity of FGFs and their receptors is much lower in invertebrate models such as Caenorhabdititis elegans and Drosophila melanogaster, making them particularly amenable for functional analysis of FGF signalling (Huang and Stern, 2005). Drosophila contains three FGF-encoding genes, namely branchless (bnl), thisbe (ths; FGF8-like1) and pyramus (pyr; FGF8-like2), and two FGF receptors encoded by breathless (btl) and heartless (htl) (Itoh and Ornitz, 2004). Their interactions are mutually exclusive, with Ths and Pyr acting as ligands for Htl, and Bnl acting as ligand for Btl (Wilson et al., 2005; Kadam et al., 2009). The functions of Bnl and Btl are particularly well established and present a classic example of FGF signalling providing instructive signals during organ formation (Sutherland et al., 1996; Sato and Kornberg, 2002). The Htl ligands Ths and Pyr are important for mesoderm formation, but their individual roles in this process are not well understood (Gryzik and Müller, 2004; Stathopoulos et al., 2004; Kadam et al., 2009).
FGF signalling plays an important role in the specification and morphogenesis of mesodermal tissues (Slack et al., 1996; Lewandoski et al., 1997; Leptin and Affolter, 2004; Schier and Talbot, 2005; Dormann and Weijer, 2006). Members of the FGF8/17/18 subfamily in vertebrates are directly involved in gastrulation. FGF8 is required for migration of mesoderm cells away from the primitive streak in the mouse embryo (Sun et al., 1999; Guo and Li, 2007). It has been suggested that in the chick embryo, FGF8 might exert this function by repelling post-ingression mesoderm cells away from the primitive streak, whereas FGF4 acts as an attractive cue (Yang et al., 2002). Interestingly, in mouse and Xenopus it has been shown that FGF8 is produced as different isoforms through differential splicing and that these isoforms exhibit different functions (Fletcher et al., 2006).
Signalling of the FGF8-like growth factors Pyr and Ths through Htl is essential for proper mesoderm spreading during gastrulation and for the differentiation of mesodermal lineages (Beiman et al., 1996; Gisselbrecht et al., 1996; Shishido et al., 1997; Gryzik and Müller, 2004; Stathopoulos et al., 2004). In Drosophila, the ventral part of the blastoderm epithelium invaginates, resulting in the internalisation of the mesoderm primordium (Costa et al., 1993; Leptin, 1999). Once internalised, the mesoderm cells establish contact with the basal surfaces of the ectoderm cells and undergo an epithelial-mesenchymal transition. The cells then spread out dorsolaterally to establish a monolayer by the end of gastrulation (Schumacher et al., 2004; Murray and Saint, 2007; McMahon et al., 2008). Although it is known that Ths and Pyr together are essential for this process, it has yet to be established whether they act redundantly or exhibit distinct functional requirements in mesoderm spreading.
The Ths and Pyr proteins exhibit 39% amino acid identity in their FGF core domains and share the highest degree of similarity with the core domains of the chordate FGF8/17/18 subfamily (Itoh and Ornitz, 2004). Previous studies suggested that Ths and Pyr might exert different functions in gastrulation simply because they are differentially expressed from late gastrulation onwards (Gryzik and Müller, 2004; Stathopoulos et al., 2004). In the present study, we report the individual functional characterisation of Ths and Pyr in mesoderm development using loss- and gain-of-function approaches. Our data support a model in which both FGF ligands play both overlapping and distinct roles in mesoderm morphogenesis and differentiation. Neither of the single mutations affects mesoderm spreading as severely as a deletion that eliminates both ligands, suggesting redundancy of pyr and ths function in the process of mesoderm spreading. Pyr is required for formation of cellular protrusions during dorsolateral migration of the mesoderm and for differentiation of pericardial and cardioblast lineages. Other mesodermal lineages, however, require both ligands for their consistent specification. Despite these distinct requirements of pyr and ths for mesoderm development, single mutant homozygotes survive at a low rate. We therefore conclude that the gene pair pyr ths confers robustness to Htl signalling during mesoderm development. The subtle specialisation of pyr can be considered as an early stage of functional divergence after gene duplication in a Drosophila ancestor.
MATERIALS AND METHODS
Genetics
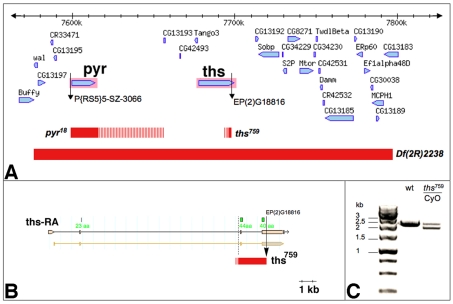
Fly stocks were maintained under standard conditions. The chromosomes utilised in this study are described in FlyBase (www.flybase.org) unless otherwise indicated. The P-element insertion EP(2)G18816 is located in the fourth exon of ths; this allele is predicted to produce a transcript encoding a C-terminally truncated protein with an intact N-terminus that includes the entire FGF core domain. Flies homozygous for thsG18816 are viable and fertile and do not exhibit any gross morphological defects (T.G. and H.-A.J.M., unpublished). We generated imprecise excisions of EP(2)G18816 and obtained two alleles containing internal deletions within the ths locus. ths141 deletes sequences corresponding to the C-terminal 12 amino acids of the highly conserved 107 amino acid FGF core domain, whereas in ths759 84 amino acids of the FGF core domain are deleted (see Fig. 1). In the case of pyr, we mobilised the transposon insertion P(2R)5-SZ-3066 and obtained an imprecise excision named pyr18, which eliminates the entire pyr gene without affecting neighbouring transcription units (Fig. 1). These data indicate that ths759 and pyr18 represent loss-of-function alleles. The generation of Df(2R)2238, removing both ths and pyr, is described by Gryzik and Müller (Gryzik and Müller, 2004). Homozygous embryos were selected with the help of balancer chromosomes marked with ftz::lacZ transgenes. w1118 flies were used as wild-type control.
Fig. 1.
Genomic characterisation of single mutant alleles for ths and pyr. (A) Region of the Drosophila genome corresponding to cytological bands 48C1-48C4. Red bars indicate the extent of deletions in Df(2R)ED2238, pyr18 and ths759, as determined by PCR and RT-PCR mapping (striped bars mark regions containing breakpoints that have not been confirmed by sequencing). The pyr18 deletion extends to the insertion site of P[RS5]5-SZ-3066 proximally and excludes CG13193 distally. (B) ths759 deletes exon 3, the third intron and part of exon 4 of ths. This deletes 84 amino acids of the FGF core domain (regions that encode amino acids of the core domain are indicated in green). The distal breakpoint represents the insertion site of EP(2)G18816 and the proximal breakpoint is ∼300 bp 5′ of exon 3. (C) RT-PCR on polyA+ RNA from wild-type (wt) and ths759 heterozygous embryos showing a 2.4 kb band corresponding to the wild-type ths mRNA and an additional 1.9 kb band in ths759 heterozygotes. The 1.9 kb product was sequenced and corresponds to the deletion depicted in B.
Molecular biology
Molecular cloning was performed following standard procedures. For pyr overexpression, a pyr cDNA was cloned into the pUAST vector, which was then employed for germ line transformation using standard procedures. Antisense RNA probes were produced with ths and pyr full-length cDNAs as templates using the DIG Labeling Kit (Roche, Germany).
Immunohistology and in situ hybridisation
Embryos were fixed and stained as described (Müller, 2008). For genotyping, embryos were stained with anti-β-galactosidase antibodies to detect the marked balancer chromosome, and homozygous embryos were staged and selected under the dissecting microscope. Staging was by morphological criteria, including cephalic furrow, anterior and posterior midgut invagination and extent of germ band elongation (Campos-Ortega and Hartenstein, 1997). Selected embryos were embedded in Araldite (Durcupan, Sigma) and sectioned at 5 μm. In situ hybridisation was conducted with digoxygenin-labelled antisense RNA probes following standard protocols. The following antibodies were used: rabbit anti-Twist, mouse anti-rat CD2 (Serotec), rabbit anti-β-galactosidase (β-Gal) (Cappel), mouse anti-β-Gal (Promega), mouse anti-Eve (DSHB), rabbit anti-Mef2 (gift from K. Jagla, Clermont-Ferrand, France), mouse anti-Mhc (gift from B. Patterson, Bethesda, USA), mouse anti-Lb (Jagla et al., 1997b), mouse anti-dpERK (Sigma), and mouse anti-digoxygenin conjugated with alkaline phosphatase (Roche). For detection of horseradish peroxidase, the Vectastain ABC Kit (Vector Labs) was used. Fluorescence-conjugated secondary antibodies and antibodies conjugated with alkaline phosphatase were from Jackson ImmunoResearch (Stratech). Brightfield micrographs were taken on an Olympus Axiophot. Fluorescent imaging was performed on a widefield Deltavision Spectris (Applied Precision) or on a Leica SP2 confocal laser microscope. Images were processed with Volocity software (Improvision), ImageJ (NIH) and Photoshop CS (Adobe) on an Apple computer.
RESULTS
ths and pyr are both required for early events in mesoderm spreading
The ths and pyr genes are located in tandem within 100 kb on the right arm of the second chromosome at cytological position 48C3-4. To determine whether pyr and ths act in redundant or independent genetic pathways, we generated mutant alleles that specifically disrupt either pyr or ths gene function without affecting any neighbouring genes (Fig. 1; see Materials and methods). Expression of ths was unaffected in pyr18 mutant embryos and expression of pyr was unaffected in ths759 embryos (see Fig. S1 in the supplementary material). Genetic complementation with two transposon-induced mutations, ths02026 and pyr02915, indicate that these mutations are allelic to ths759 and pyr18, respectively, but represent weaker alleles (Kadam et al., 2009) (see Figs S2 and S3 in the supplementary material). Each of the single mutations was semi-lethal, with adult homozygotes hatching at 3.4% (n=4148 total offspring of heterozygous cross of ths759) and 0.71% (n=565 total offspring of heterozygous cross of pyr18) (see Fig. S3 in the supplementary material). Since mutations in the Htl FGF receptor are embryonic lethal, these results indicated that the presence of either FGF8-like gene can partially compensate for the loss of the other and we therefore conclude that pyr and ths have overlapping functions during embryonic development.
We first analysed the distribution of mesoderm cells in single mutants to assess whether ths and pyr play any separable roles during mesoderm spreading. Spreading occurs in several phases that can be identified in fixed embryos by immunolocalisation of Twist (Twi). After invagination, the epithelial mesoderm primordium first contacts the ectoderm cells and then undergoes an epithelial-to-mesenchymal transition (Schumacher et al., 2004; Wilson et al., 2005). Subsequently, the cells spread out to form a monolayer.
In the wild type, the mesoderm is precisely aligned along the ventral midline and spreading occurs evenly away from the midline in a highly symmetric fashion (Fig. 2A). In embryos homozygous for Df(2R)2238 (deleting both ligands), the mesoderm was not aligned along the midline, and ectoderm-mesoderm adhesion was severely disturbed. Subsequently, the cells failed to spread out dorsally (Fig. 2B). ths759 homozygotes exhibited only subtle defects in equal lateral spreading of the mesoderm (Fig. 2C). In ths759 mutants, mesoderm-ectoderm attachment was occasionally uneven along the anterior-posterior axis. This phenotype was more severe in pyr18 homozygous mutant embryos (Fig. 2C-F). At slightly later stages we observed defects in mesoderm monolayer formation in pyr18, which, however, were never observed in the wild type or in ths759 homozygotes (Fig. 2A,C-F). We conclude that ths and pyr are both required for normal cell rearrangements during early events in mesoderm spreading. The equal mesoderm collapse onto the ectoderm requires the function of both ligands, whereas proper monolayer formation depends on pyr (see below). Strikingly, in the absence of ths function, a single copy of pyr is sufficient for normal monolayer formation (Fig. 2D). Nevertheless, neither of the single mutants recapitulated the strong defects in monolayer formation that were seen in the absence of both ligands, suggesting that Pyr and Ths together promote robust spreading of the mesoderm.
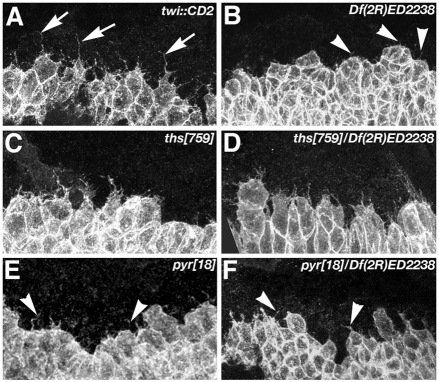
Fig. 2.
Mesoderm spreading defects in ths and pyr single mutants. (A-F) Drosophila embryos of the indicated genotypes stained with anti-Twi (top two rows of each panel) or anti-dpERK (bottom row, detects MAPK) shown as whole-mount (top row) or in cross-section (between 40% and 60% egg length). Stages 7 (left column of each panel), 8 (middle) and 9 (right) are shown. (A) Wild type. Note activation of MAPK at mesoderm-ectoderm contacts and at the mesoderm leading edge (arrows). (B) In embryos lacking both pyr and ths function, mesoderm cells fail to establish initial contact, activate MAPK (arrowheads) or spread to a monolayer. (C) In ths759 homozygotes initial contact is normal, but unequal spreading can be observed at stage 8 (arrowheads). No major changes in monolayer formation were observed and MAPK activation was normal (arrows). (D) Embryos lacking ths function and one copy of pyr [ths759/Df(2R)ED2238] exhibit similar defects to ths759 homozygotes. (E) Pyramus is required for equal dorsal spreading and monolayer formation (arrowheads). MAPK activation is strongly reduced in the absence of pyr function (arrows). (F) Similar defects are observed in pyr18/Df(2R)ED2238 embryos. Note the strong reduction of dpERK staining in leading edge cells (arrows). (G-I) pyr and ths are expressed in distinct domains during mesoderm spreading. In situ hybridisation of stage 8 embryos with antisense probes against ths (G) and pyr (H,I). (G,H) Cross-sections. (I) Fluorescent detection of pyr RNA (red) in an embryo expressing twi::CD2 (green). The right-hand panel shows a reconstructed cross-section. pyr expression is restricted to dorsal ectoderm (arrows in H, arrowheads in I) and ventral ectodermal patches (arrowheads in H).
One response of mesoderm cells to the Htl signal is the activation of the Ras-Raf-MAPK pathway, resulting in phosphorylation of MAPK (Rolled - FlyBase) that can be measured with an antibody against double-phosphorylated ERK (dpERK) (Gabay et al., 1997). In embryos lacking FGF8-like ligands, no MAPK activation is observed in mesoderm cells at any stage during spreading (Gryzik and Müller, 2004) (Fig. 2B). In early stages during collapse and initial dorsolateral spreading, pyr18 mutants exhibited strongly reduced activation of MAPK, whereas activation appeared normal in ths759 mutants (Fig. 2C,E). Embryos with only one copy of pyr or one copy of ths exhibited reduced MAPK activation in early spreading, consistent with a requirement for each of the ligands in Htl activation. However, during monolayer formation, activation of MAPK in the dorsal-most mesodermal cells was strictly dependent on Pyr activity. In ths759 homozygous embryos, MAPK was activated in dorsal mesoderm cells as in the wild type (Fig. 2C,E). Thus, pyr is involved in normal mesoderm monolayer formation and is required for normal levels of MAPK activation. Interestingly, pyr expression at this stage was mainly localised in the dorsal ectoderm (Fig. 2G-I). These results indicate that the dorsal expression domain of pyr is required for high levels of MAPK activation during dorsolateral migration of the mesoderm.
Pyr is involved in protrusion formation during dorsolateral mesoderm migration
After ectoderm-mesoderm attachment, mesoderm cells migrate dorsolaterally. This dorsolateral migration is associated with the formation of cellular protrusions at the dorsal edge of the mesoderm aggregate (Schumacher et al., 2004) (Fig. 3A). Protrusive activity of mesoderm cells coincides with dynamic changes of pyr expression towards an accumulation at the dorsal edge of the ectoderm (Fig. 2G-I). Thus, an attractive model suggests that localised expression of pyr provides a directional cue for the mesoderm cells, instructing them to migrate in a dorsolateral direction. To assess whether loss of either FGF8-like ligand affects the formation of dorsal edge protrusions, we used the twi::CD2 transgene as a marker for cellular protrusions (Dunin-Borkowski and Brown, 1995). In homozygous Df(2R)ED2238 embryos, mesoderm cells did not spread out dorsally and the cells failed to form dorsal edge protrusions (Fig. 3B). In ths759 homozygous or hemizygous mutants, dorsal edge cells formed leading edge protrusions similar to those of the wild type (Fig. 3C,D). By contrast, in pyr18 homozygous and hemizygous embryos, cellular protrusions at the dorsolateral edge of the mesoderm were strongly reduced (Fig. 3E,F). Interestingly, short filopodial protrusions were still present, even in embryos lacking both ligands (Fig. 3B,E,F). Thus, mesoderm cells possess a capacity to form filopodia even in the absence of FGF signalling. This might enable the cells to sense the environment for positional cues or substrates upon which they can extend and change their shape. The phenotype in pyr, but not ths, single mutant embryos strongly suggests that Pyr provides a signal that allows dorsal mesoderm cells to form long directional cell protrusions, which might reflect their migration in a dorsolateral direction.
Fig. 3.
Morphology of dorsal edge cells during mesoderm migration in FGF8-like ligand mutants. Cell shape at the dorsal edge of the mesoderm during dorsolateral migration visualised using twi::CD2. In wild type (twi::CD2), dorsal edge cells form thin and long protrusions (A, arrows). Only short, filopodial protrusions (arrowheads) are present in embryos lacking pyr gene function (B,E,F). In ths759 homozygous (C) or hemizygous (D) embryos, long protrusions are present similar to those in the wild type. Note that cells at the dorsal edge in E,F fail to extend along the dorsal ventral axis (dorsal is up and ventral is down).
pyr is required for dorsal mesoderm differentiation
Despite defects in equal collapse of the mesoderm to the ectoderm in embryos lacking a single ligand, monolayer formation was not affected in ths759 homozygotes and only subtly affected in pyr18 homozygotes (Fig. 2D,F). We therefore asked whether the defects in mesoderm spreading in pyr18 homozygotes translate into mesoderm differentiation defects in any way. To assess various mesoderm lineages, we analysed expression of Myocyte enhancing factor 2 (Mef2) as a marker for somatic, visceral and cardiac muscle precursors, Myosin heavy chain (Mhc) for somatic muscles and eve and Ladybird genes (lbe and lbl, referred to collectively as lb because the antibody recognises both gene products) as markers for specific cardiac and somatic muscle lineages (Frasch et al., 1987; Jagla et al., 1997a; Jagla et al., 1998).
As mesoderm spreading is a prerequisite for dorsal mesoderm differentiation, we first looked at derivatives of the dorsal mesoderm. Late embryos lacking the Htl receptor or both ligands show a total lack of Mef2-positive muscle cells in this region (Fig. 4A-F) (Michelson et al., 1998). Embryos lacking pyr also showed disrupted dorsal vessel and dorsal somatic muscle development, even though these defects were less severe than in embryos lacking both ligands (Fig. 4J-L). In some cases, both cardioblasts and dorsal muscles were lacking in the same hemisegment (Fig. 4K,M), whereas in other cases a lack of cardioblasts did not correlate with a corresponding lack of dorsal muscles in the same hemisegment (Fig. 4L,N,O). These differences in the expressivity of the phenotype might originate from defects in dorsolateral migration during gastrulation or reflect requirements for pyr for the specification of specific muscle lineages (see below).
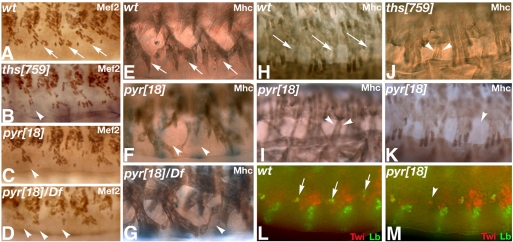
Fig. 4.
Defects in somatic and cardiac muscles in FGF8-like mutant Drosophila embryos. Embryos at stage 12 (left column), stage 13 or 14 (middle) and stage 16 (right) were fixed and stained for Mef2. (A-C) Wild-type pattern of cardiac and somatic muscle cell nuclei. (D-F) The pattern is severely disrupted in Df(2R)ED2238 homozygotes. Mef2-positive nuclei cluster in the ventral region (D) and the number of cardiac precursors is strongly reduced (E,F). (G-I) ths759 hemizygous embryos exhibit normal Mef2 expression. (J-O) pyr18 homozygous (J-L) and hemizygous (M-O) embryos exhibit missing nuclei in the cardiac (arrows) and somatic (arrowheads) muscle domains. The cardioblast defect is more severe in hemizygous embryos than in pyr homozygotes (compare O with L). Defects in dorsal somatic muscles and cardioblasts often occurred at the same anterior-posterior positions (M,K,L,O, arrows).
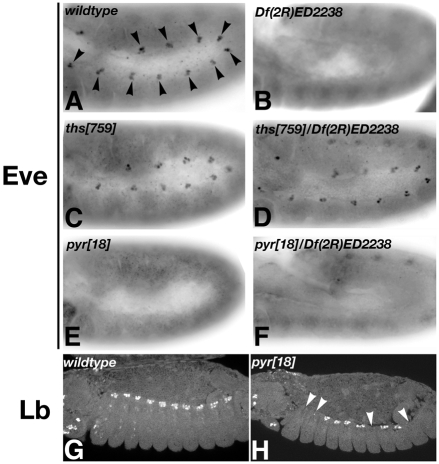
In wild-type embryos, Eve is expressed in segmental clusters in the dorsal mesoderm from stage 10; these Eve-expressing cells will form a subset of pericardial cells and the founder cell for muscle DA1 (Fig. 5A). This expression was always absent in pyr18 homozygous mutants (Fig. 5E,F), suggesting that the development of these cell types is disrupted at an early stage. Consistent with a lack of major defects in mesoderm spreading, ths was found to be largely dispensable for dorsal mesoderm development including the specification of Eve-expressing dorsal mesoderm cells (Fig. 4G-I; Fig. 5C,D). Strikingly, a single copy of the pyr gene was able to support all of the functions of FGF signalling during specification of cardioblasts, dorsal muscles and dorsal Eve-positive clusters in the mesoderm (Fig. 4G-I; Fig. 5D).
Fig. 5.
Requirement of FGF8-like ligands for dorsal mesoderm differentiation. Drosophila embryos at stage 10 (A-F) or stage 13/14 (G,H) were fixed and stained with antibodies against Eve (A-F) or Lb (G,H). (A) Wild-type expression of Eve in 11 hemisegmental cell clusters (arrowheads). (B) In Df(2R)ED2238 homozygotes, Eve expression is absent. (C,D) ths759 homozygous (C) or hemizygous (D) embryos show normal Eve expression. (E,F) pyr18 homozygous (E) and hemizygous (F) embryos lack Eve-positive dorsal mesodermal precursors. (G) Wild-type cardiac Lb expression. (H) Several, but not all, cardiac Lb clusters are missing in pyr18 homozygous embryos (arrowheads).
Formation of all dorsal mesodermal derivatives requires Dpp signalling from the dorsal ectoderm, which maintains tinman (tin) expression and regulates several other factors important in determining cell fates (Frasch, 1995). As mesoderm spreading is abnormal in pyr18 homozygotes, it is possible that a delay in cells reaching dorsal positions might prevent them from receiving inductive signals required for Eve expression. If this were the case, we would expect that neighbouring cell populations, which also require inductive signals, should be affected to a similar degree when FGF signalling is compromised. Indeed, we found that the induction of another marker of dorsal mesoderm derivatives, Lb, is affected in dorsal mesoderm cells adjacent to the Eve-positive clusters (Fig. 5G,H). However, in contrast to the lack of mesodermal Eve expression, the majority of hemisegments in pyr18 homozygotes expressed Lb (7/8 embryos have at least one cluster missing; Fig. 5G,H). Because Lb expression requires Tin, which in turn is dependent on Dpp from the dorsal ectoderm, this result implies that any delay in migration in the absence of pyr might indeed have an effect on patterning downstream of Dpp (Jagla et al., 1997a). With the exception of the requirement for Eve expression, mutations in pyr impinge on all dorsal mesodermal lineages in a random manner, consistent with variable and subtle defects in mesoderm spreading in pyr mutants.
ths and pyr are required for ventral and lateral muscle differentiation
The complete loss of Eve-positive dorsal mesoderm lineages in pyr18 homozygotes suggests that FGF signalling has specific functions in the early specification of certain mesoderm derivatives. This is supported by the occurrence of defects in tissues arising from the ventral and lateral mesoderm, which might not require dorsolateral migration to receive patterning signals from the ectoderm. In late embryos, we observed a range of somatic muscle defects consistent with the participation of FGF signalling in specific cell fate decisions (Fig. 6). Wild-type embryos develop a stereotyped arrangement of ∼30 body wall muscles in each hemisegment, with variations in the anterior and posterior regions of the embryo (Bate, 1990). Each muscle arises from a single founder myoblast, specified at a defined segmental position through the influence of ectodermal signals on the expression of mesodermal factors (Baylies et al., 1998). It was noted previously that expression of dominant-negative Htl blocks formation of ventral oblique (VO) muscles and that an intact ventral nerve chord is required for their formation (Beiman et al., 1996; Michelson et al., 1998; Schulz and Gajewski, 1999). This implied that FGF ligands expressed in the ventral neuroblasts might be required for founder specification for these muscles. Pyr and Ths are both expressed in a subset of neuroblasts (see Fig. S4 in the supplementary material) and we found that ventral oblique muscles VO4, VO5 and VO6 are affected in pyr18 and ths759 homozygous mutants (Fig. 6A-G). Defects in pyr18 were more frequent when the gene dosage of ths was also reduced (Fig. 6C,D; Table 1).
Fig. 6.
Requirement of FGF8-like ligands for the differentiation of specific subgroups of somatic muscles. (A-D) Ventral somatic muscle nuclei (stained for Mef2) in late stage Drosophila embryos. (A) Wild type (wt). Nuclei of ventral oblique (VO) muscles 4-6 are indicated by arrows. VO muscle nuclei are missing in a few hemisegments of ths759 homozygotes (B) and in several hemisegments of pyr18 homozygous (C) and hemizygous (D) embryos (arrowheads) (see also Table 1). (E-K) Myosin heavy chain (Mhc) staining showing somatic muscles in stage 17 embryos. (E-G) Ventrolateral views showing ventral muscles. One or more of VO muscles 4-6 (arrows in E, wild type) are missing in several hemisegments of pyr18 homozygous (F) or hemizygous (G) embryos (arrowheads). (H-K) Lateral views. Segment border muscles (SBMs) are indicated by arrows in the wild type (H). SBM duplications are observed in both pyr18 (I) and ths759 (J) homozygous mutants (arrowheads), and hemisegments lacking an SBM are also observed in pyr18 homozygotes (arrowhead in K). (L) Wild-type stage 10 embryo showing Lb expression (green) in the ectoderm and mesoderm. Arrows indicate segmental expression of Lb in the lateral mesoderm. (M) Lb expression in a pyr18 embryo showing absence of Lb-positive mesoderm cells in one hemisegment (arrowhead).
Table 1.
Defects in somatic muscles (VO and SBM) in FGF mutants
|
Percentage of embryos showing muscle defects in each genotype
|
||||
|---|---|---|---|---|
| Muscle affected | ths759/CyO | ths759/CyO × Df/CyO | pyr18/CyO | pyr18/CyO × Df/CyO |
| VO | 7.8 (28) | 15 (26) | 16 (19) | 21.4 (42) |
| SBM (duplication) | 34.8 (23) | 24 (25) | 29.6 (37) | 3.6 (28) |
| SBM (absence) | 0 (23) | 16 (25) | 22.2 (37) | 14.3 (28) |
Quantification of muscle defects in stage 16/17 embryos stained with antibodies to Mhc (as depicted in Fig. 6). Because selection of homozygous embryos using a marked balancer was not possible at this stage, muscle defects were scored in all embryos from a heterozygous cross as indicated. n, number of embryos analysed. Df, Df(2R)ED2238; VO, ventral oblique muscles 4-6; SBM, segmental border muscle.
In addition to loss of specific muscles, we also observed muscle duplications in embryos lacking the ligands of Htl. The most frequent duplication involved the segment border muscle (SBM) (Fig. 6H-K). ths and pyr single mutants exhibited duplications at a similar penetrance. In addition, only pyr mutants also showed occasional loss of this muscle (Table 1). The lineage of the SBM founder is also marked by expression of Lb (Jagla et al., 1998). Indeed, Lb staining in pyr mutant embryos revealed the absence of SBM founder cells in some segments (Fig. 6L,M; 5/10 embryos had at least one cluster missing). These results demonstrate that the formation of the SBM is affected at an early stage. More rarely, we also observed loss or duplications of other muscles (e.g. VA3 duplication; I.B.N.C. and H.-A.J.M., unpublished), suggesting that FGF signalling plays a supportive role in other cell fate decisions as well.
In summary, specific somatic muscle lineages are affected by the absence of the FGF ligands Pyr and Ths. These defects are not confined to the dorsal mesoderm, implying that the phenotypes are not simply consequences of defects in dorsolateral spreading of the mesoderm. Rather, our results indicate that muscle development in multiple lineages depends on activation by FGF signalling during mesoderm differentiation. Some pyr mutant phenotypes appear more severe, such as the loss of VO4-6 and SBM, suggesting that pyr function is more limiting than that of ths for the development of certain muscles. However, the robust, correct specification of these lineages requires both of the FGF8-like ligands.
Localised activation of Htl by Pyr is important for mesoderm spreading and differentiation
The data suggest that Pyr plays multiple roles: during gastrulation it is required for normal mesoderm spreading, and later it is required for mesoderm differentiation. If these functions rely upon a spatially restricted expression pattern of pyr, its ectopic expression in the mesoderm should abolish localised information and affect mesoderm development. Such gain-of-function analyses also have the potential to reveal whether Pyr is able to induce certain cellular behaviours, such as migration, division and differentiation.
Ectopic expression of Pyr in mesoderm cells resulted in defects in both spreading and mesoderm differentiation (Fig. 7A-L). In contrast to pyr18 homozygous mutant embryos, monolayer formation was severely affected upon overexpression of Pyr. Unlike expression of constitutively active forms of Htl, overexpression of Pyr resulted in a massive activation of MAPK in all mesoderm cells (Fig. 7E,F) (Wilson et al., 2005). The defects in generation of the mesoderm monolayer correlated with a defect in the formation of cellular protrusions at the dorsal edge during migration (Fig. 7M,N). Together, these data demonstrate that non-localised overactivation of the Htl pathway by Pyr results in severe migration defects and supports the idea that Pyr provides spatially restricted cues for dorsolateral migration of mesoderm cells.
Fig. 7.
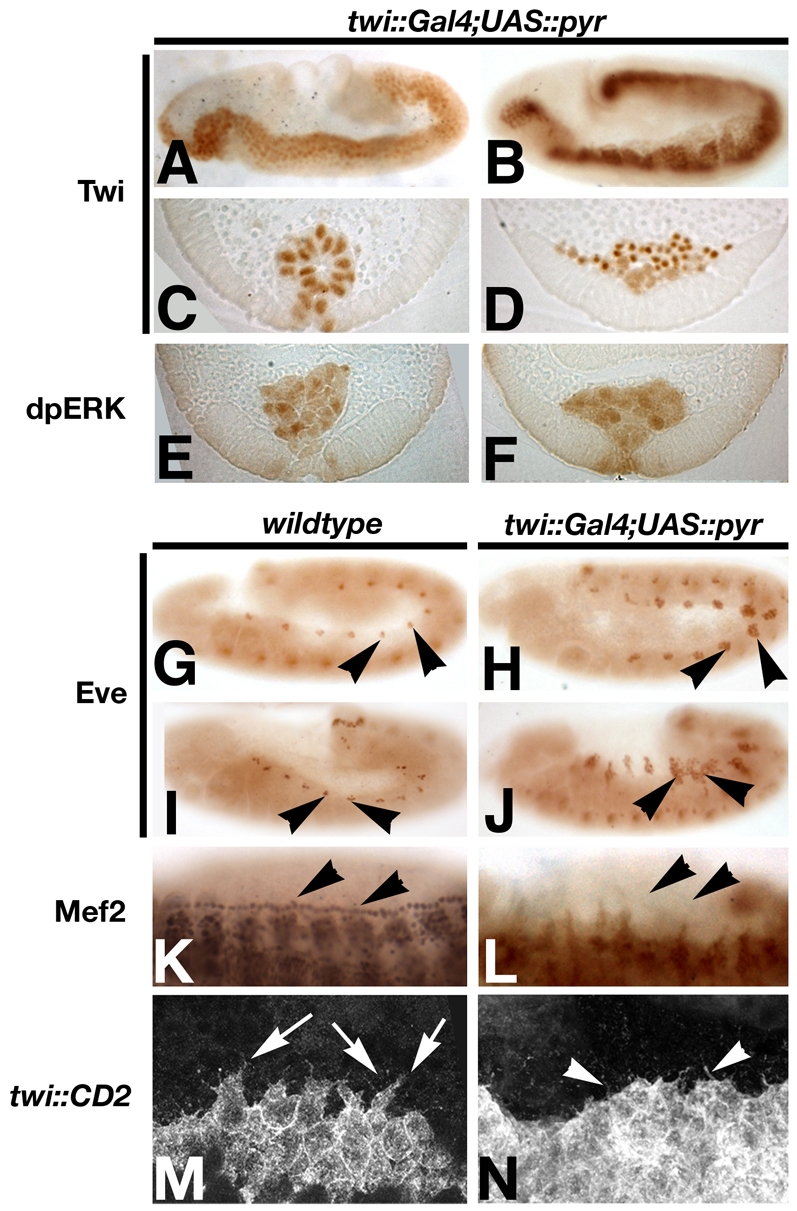
Mesoderm defects after uniform activation of Htl by Pyr in mesoderm cells. (A-F,H,J,L,N) Pyr was overexpressed in the mesoderm using a twi::Gal4 driver and UAS::Pyr-HA embryos were fixed and stained with the antibodies indicated. (G,I,K,M) Wild-type embryos for comparison. Mesoderm spreading defects in stage 7 (A,C) and stage 9 (B,D) embryos. All mesoderm cells exhibit high levels of MAPK activation at stages 7 (E) and 9 (F). Overexpression of Pyr results in large ectopic Eve-positive clusters at stages 10 and 12 (H and J, respectively, arrowheads; compare with G and I). Cardiac precursors are absent at stage 13/14 (compare K with L, arrowheads). Protrusions at the dorsal edge of the migrating mesoderm (stage 8, arrows in M) are strongly reduced (N). Note that only short filopodia-like protrusions are present (N, arrowheads).
Pyr is required for the formation of Eve-positive mesodermal precursor cells (see above). Differentiation of dorsal mesoderm derivatives was also strongly affected in embryos overexpressing Pyr. Cell numbers of Eve-positive mesoderm cells were strongly increased and Eve-positive cells were present at more ventral positions in embryos at later stages (during germ band retraction, stages 12 and 13) (Fig. 7G-J). Thus, although the enlargement of Eve-positive cell clusters suggests a shift in favour of dorsal mesoderm cell fates, these Eve-positive cells were not localised dorsally. This result demonstrates that Pyr can induce more ventral-lateral mesoderm to express eve, consistent with the idea that Pyr is a limiting factor in the induction of eve in mesodermal precursors. Since overexpression of Pyr also resulted in a defect in mesoderm cells reaching the dorsal ectoderm margin, we expected to see a lack of dorsal mesoderm in these embryos. Indeed, Pyr overexpression led to a complete lack of cardioblasts, indicating that the defects in mesoderm spreading translate into a dramatic loss of dorsal mesodermal cell fates (Fig. 7K,L). The overexpression phenotypes support the view that spatiotemporal control of Htl activation by Pyr is required for normal dorsal spreading and mesoderm differentiation, and provide evidence for an essential role of Pyr in the specification of Eve-positive mesoderm progenitor cells.
DISCUSSION
Signalling via the FGF receptor Htl is essential for mesoderm development from gastrulation onwards, but how its two ligands, Ths and Pyr, control these events is unclear. Here we present an analysis of ths and pyr single mutants and provide evidence that Pyr and Ths exhibit individual and overlapping functions in gastrulation and mesoderm differentiation.
Genetics of FGF8-like single mutants
Previously, the identification of two transposon-associated alleles, ths02026 and pyr02915, and two chromosomal deletions, Df(2R)ths238 and Df(2R)pyr36, was reported (Kadam et al., 2009). Genetic complementation analysis with pyr18 and ths759 supports the view that pyr02915 represents a loss-of-function allele, whereas ths02026 is a hypomorphic allele. However, the weaker Eve phenotype of pyr02915 compared with pyr18 indicates that pyr02915 is unlikely to be a null allele (see Figs S2 and S3 in the supplementary material). The alleles presented in this study represent loss-of-function alleles: in pyr18 the entire pyr gene is deleted, and in ths759 most of the conserved FGF core domain is deleted. In both cases, neighbouring genes remain unaffected. Df(2R)ths238 and Df(2R)pyr36 uncover ths and pyr, respectively, but also delete neighbouring genes (Kadam et al., 2009). In summary, whereas a null allele for pyr exists (pyr18), there is currently no null allele of ths available that does not simultaneously delete other genes. In ths759, 84 of the apparent 107 amino acids of the Ths FGF core domain are deleted. The FGF core domain is conserved in all FGFs, with 28 highly conserved and six identical amino acids (Ornitz and Itoh, 2001). The core domain contains amino acids important for heparin proteoglycan binding, glycosylation and FGF receptor activation (Eswarakumar et al., 2005). We previously identified nine conserved amino acids in Ths that are identical within the FGF8 subfamily, four of which are identical in all FGFs (Gryzik and Müller, 2004). In the ths759 allele, all of these nine identical amino acids are deleted. Therefore, if we exclude the formal possibility that the ths759 gene product retains activity independent of the FGF core domain, ths759 represents a functional null allele.
Functions of FGF8-like ligands in mesoderm differentiation
The complex expression patterns of htl and its two ligands, pyr and ths, in post-gastrulation stages suggested that Htl signalling functions directly in cell fate decisions during mesoderm differentiation (Shishido et al., 1993; Beiman et al., 1996; Gisselbrecht et al., 1996; Stathopoulos et al., 2004). pyr is required for Eve expression in dorsal mesoderm derivates, whereas ths is dispensable (Kadam et al., 2009) (this study). In addition, overexpression of Pyr leads to an expansion of mesodermal Eve-positive clusters in a similar fashion to experimental overactivation of the Ras1 (Ras85D) pathway (Carmena et al., 1998; Michelson et al., 1998; Liu et al., 2006). Ths exhibits similar gain-of-function effects to Pyr with respect to expansion of Eve-positive clusters in dorsal mesoderm, suggesting that Ths and Pyr have similar signalling properties (Kadam et al., 2009) (A.K. and H.-A.J.M., unpublished). However, as Eve expression is unaffected in ths single mutants, it is unlikely that Ths contributes to the expression of Eve in these cells (Kadam et al., 2009) (this work).
Expression of Eve in the precursors of the pericardial cells and DA1 muscle founders depends on the activation of several signalling pathways in a group of mesodermal pre-clusters expressing lethal of scute (Carmena et al., 1998). Wingless (Wg) and Dpp signalling define a dorsal domain of mesoderm cells that are competent to activate transcription of eve in response to localised activation of Ras1. This localised Ras1 activation is largely dependent on Htl signalling (Carmena et al., 1998). During this specification process, Pyr is expressed in segmental dorsal ectodermal patches in close proximity to the sites in the mesoderm where the dorsal Eve-positive clusters form. Whereas the effect on Eve expression is fully penetrant, the generation of other dorsal mesodermal precursors, e.g. those expressing Lb, is only mildly affected in pyr mutant embryos. Interestingly, we observed that overexpression of Pyr results in strong activation of MAPK and ectopic Eve expression in the absence of normal dorsolateral migration. These results indicate that Pyr expression causes cells to become more sensitive to Dpp and Wg signalling and thus represents a limiting factor of the signalling network that triggers specification of Eve-positive dorsal mesoderm.
With the exception of the lack of Eve-expressing mesodermal precursors, none of the other mesoderm differentiation defects in pyr single mutants occurred with similar expressivity; for instance, the defects in formation of specific somatic muscles (SBM, VO4, VO5 and VO6) were penetrant at a low expressivity as they did not occur in each segment. In addition, the defects in SBM and VO muscles were also evident in ths homozygotes and became even more severe when one copy of the ths gene was removed in a pyr homozygous background. These observations suggest overlapping functions of pyr and ths in the specification of these muscles. In summary, we conclude that both ligands are involved in the differentiation of specific subsets of muscles.
Regulation of mesoderm spreading by Pyr and Ths
Whereas htl mutants exhibit severe defects, ths and pyr single mutants exhibit weak defects in mesoderm spreading (Kadam et al., 2009) (this work). Nevertheless, we found that both ligands are required for equal attachment of the mesoderm cells on to the ectoderm after invagination. As this phenotype occurs in both single mutants, either the overall level of FGF ligand at this stage is crucial, or both of the ligands need to bind to Htl-expressing cells, or each of the FGFs exerts independent functions in this process. When the gene dosage of both of the ligands is reduced by half, early mesoderm morphogenesis was normal, excluding the possibility that the overall level of FGF plays a major role (A.K. and H.-A.J.M., unpublished). Furthermore, it was recently shown that each ligand is able to signal in the absence of the other, suggesting that Ths and Pyr do not directly cooperate in Htl activation (Kadam et al., 2009) (A.K. and H.-A.J.M., unpublished). It will be interesting to determine how each of the ligands might independently support particular aspects of early mesoderm movements.
Although both ligands are required for the early stages, only pyr mutants exhibited defects in dorsolateral migration and mesoderm monolayer formation (Kadam et al., 2009) (this work). The defects in monolayer formation observed in our mutants were only subtle, in contrast to the defects reported by Kadam et al. (Kadam et al., 2009). These discrepancies might reflect differences in the alleles used in the two studies. We did not observe monolayer defects in ths759 mutant alleles, whereas a deletion uncovering ths exhibits defects in monolayer formation (Kadam et al., 2009). This raises the possibility that domains other than the FGF core domain present in the protein encoded by the ths759 allele might exert some function in monolayer formation. We think that this is unlikely as the non-conserved C-terminal tail is dispensable for activation of Htl (A.K. and H.-A.J.M., unpublished). The deletion that was used to eliminate ths function, Df(2R)ths238, eliminates ths and ten proximal genes raising the alternative possibility that deletion of a gene (or genes) within Df(2R)ths238 contributes to the rather severe mesoderm spreading defect presented by Kadam et al. (Kadam et al., 2009). Rescue experiments using full-length genomic constructs will be informative to further characterise these ths deletion alleles.
The presently available data are consistent with a role of the localised expression of Pyr at the dorsal edge of the ectoderm in providing an instructive cue for the cells to migrate in a dorsal direction (Fig. 8). For example, Pyr expression might produce an instructive cue that promotes dorsolateral movement of the mesoderm. It has been shown previously that FGFs can exhibit characteristics of chemoattractants in other systems (Ribeiro et al., 2002; Yang et al., 2002). Although loss- and gain-of-function analyses demonstrate that pyr is required for normal protrusive activity during dorsolateral migration, monolayer formation is much less affected than in htl mutants or ligand double mutants (Kadam et al., 2009) (this work). Therefore, although Pyr might provide a directional cue, non-polarised expression of Ths alone can compensate to some extent for the absence of this putative directional cue. In this sense, the two ligands differ slightly in their requirements for mesoderm spreading, but it is the directional movement through localised expression of pyr that causes this to be a robust morphogenetic process.
Fig. 8.
Model for Ths and Pyr function in mesoderm spreading. Model for permissive and instructive functions of FGF8-like factors in Drosophila gastrulation. (A) During stages 6 and 7, both ths (blue) and pyr (red) are expressed in the ectoderm from the ventral midline (V) to the dorsal edge (D) of the neuroectoderm. We propose that both FGF8-like factors provide a permissive cue during collapse of the mesoderm (green) and attachment to the ectoderm (pink). (B) During dorsolateral migration of the mesoderm, expression of ths remains broad, whereas expression of pyr becomes restricted to the dorsal aspect of the neuroectodermal and ectodermal cells. We propose that at this stage, pyr generates an instructive cue and might act as a long-range signal.
Overlapping and distinct functions of Pyr and Ths
The FGF8-like ligands exhibit overlapping functions except for the induction of mesodermal Eve expression, the formation of the SBM and dorsolateral migration. They cooperate to provide robustness of Htl-dependent mesoderm morphogenesis and differentiation. These imperfect redundancies become obvious in the single mutant phenotypes and might reflect the fact that pyr and ths are likely to be derived from a gene duplication event in the Drosophilids (Stathopolous et al., 2004). In more basic insects, such as Anopheles gambia and Tribolium castaneum, only one FGF8-like gene exists, and this is more similar to ths and might represent a common ancestor (Beermann and Schröder, 2008). It has therefore been suggested that ths might have retained some of the ancestral functions of the Fgf8 homologue in Drosophila melanogaster. This would imply that the establishment of a localised dorsal expression domain and the hypothesised instructive role of Pyr are derived qualities. The data presented here indicate that localised expression of pyr renders mesoderm spreading more robust than in the absence of pyr expression. It would be of interest to analyse the expression and function of FGF8-like signalling in more basic insects that exhibit long germ band development and contain only one FGF8 homologue. Gene duplication has been proposed as a general mechanism in vertebrates to explain the expansion of FGF genes. Studies in dipteran species might provide insights into the evolution of the requirements for localised expression of a growth factor in directional cell movement during gastrulation.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/14/2393/DC1
Supplementary Material
We thank Kystrof Jagla, Bruce Patterson and the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA, USA) for antibodies; John James and Ryan Webster for expert technical assistance; Kate Storey and Reinhard Schröder for comments on the manuscript; and the members of the Müller laboratory for discussions. This work was funded by grants from the Deutsche Forschungsgemeinschaft (SFB590-TPB1) and a Senior Non-Clinical Research Fellowship from the Medical Research Council (MRC G0501679) to H.-A.J.M. Deposited in PMC for release after 6 months.
References
- Bate, M. (1990). The embryonic development of larval muscles in Drosophila. Development 110, 791-804. [DOI] [PubMed] [Google Scholar]
- Baylies, M. K., Bate, M. and Ruiz Gomez, M. (1998). Myogenesis: a view from Drosophila. Cell 93, 921-927. [DOI] [PubMed] [Google Scholar]
- Beermann, A. and Schröder, R. (2008). Sites of Fgf signalling and perception during embryogenesis of the beetle Tribolium castaneum. Dev. Genes Evol. 218, 153-167. [DOI] [PubMed] [Google Scholar]
- Beiman, M., Shilo, B. Z. and Volk, T. (1996). Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev. 10, 2993-3002. [DOI] [PubMed] [Google Scholar]
- Böttcher, R. T. and Niehrs, C. (2005). Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 26, 63-77. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega, J. A. and Hartenstein, V. (1997). The Embryonic Development of Drosophila melanogaster, pp. 9-102. Berlin, Germany: Springer Verlag.
- Carmena, A., Gisselbrecht, S., Harrison, J., Jimenez, F. and Michelson, A. M. (1998). Combinatorial signaling codes for the progressive determination of cell fates in the Drosophila embryonic mesoderm. Genes Dev. 12, 3910-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, M., Sweeton, D. and Wieschaus, E. (1993). Gastrulation in Drosophila: cellular mechanisms of morphogenetic movements. In The Development of Drosophila melanogaster (ed. M. Bate and A. Martinez Arias), pp. 425-465. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Dormann, D. and Weijer, C. J. (2006). Chemotactic cell movement during Dictyostelium development and gastrulation. Curr. Opin. Genet. Dev. 16, 367-373. [DOI] [PubMed] [Google Scholar]
- Dunin-Borkowski, O. M. and Brown, N. H. (1995). Mammalian CD2 is an effective heterologous marker of the cell surface in Drosophila. Dev. Biol. 168, 689-693. [DOI] [PubMed] [Google Scholar]
- Eswarakumar, V. P., Lax, I. and Schlessinger, J. (2005). Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 16, 139-149. [DOI] [PubMed] [Google Scholar]
- Fletcher, R. B., Baker, J. C. and Harland, R. M. (2006). FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development 133, 1703-1714. [DOI] [PubMed] [Google Scholar]
- Frasch, M. (1995). Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature 374, 464-467. [DOI] [PubMed] [Google Scholar]
- Frasch, M., Hoey, T., Rushlow, C., Doyle, H. and Levine, M. (1987). Characterization and localization of the even-skipped protein of Drosophila. EMBO J. 6, 749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay, L., Seger, R. and Shilo, B. Z. (1997). MAP kinase in situ activation atlas during Drosophila embryogenesis. Development 124, 3535-3541. [DOI] [PubMed] [Google Scholar]
- Ghabrial, A., Luschnig, S., Metzstein, M. M. and Krasnow, M. A. (2003). Branching morphogenesis of the Drosophila tracheal system. Annu. Rev. Cell Dev. Biol. 19, 623-647. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht, S., Skeath, J. B., Doe, C. Q. and Michelson, A. M. (1996). heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 10, 3003-3017. [DOI] [PubMed] [Google Scholar]
- Gryzik, T. and Müller, H. A. (2004). FGF8-like1 and FGF8-like2 encode putative ligands of the FGF receptor Htl and are required for mesoderm migration in the Drosophila gastrula. Curr. Biol. 14, 659-667. [DOI] [PubMed] [Google Scholar]
- Guo, Q. and Li, J. Y. (2007). Distinct functions of the major Fgf8 spliceform, Fgf8b, before and during mouse gastrulation. Development 134, 2251-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, P. and Stern, M. J. (2005). FGF signaling in flies and worms: more and more relevant to vertebrate biology. Cytokine Growth Factor Rev. 16, 151-158. [DOI] [PubMed] [Google Scholar]
- Itoh, N. and Ornitz, D. M. (2004). Evolution of the Fgf and Fgfr gene families. Trends Genet. 20, 563-569. [DOI] [PubMed] [Google Scholar]
- Jagla, K., Frasch, M., Jagla, T., Dretzen, G., Bellard, F. and Bellard, M. (1997a). ladybird, a new component of the cardiogenic pathway in Drosophila required for diversification of heart precursors. Development 124, 3471-3479. [DOI] [PubMed] [Google Scholar]
- Jagla, K., Jagla, T., Heitzler, P., Dretzen, G., Bellard, F. and Bellard, M. (1997b). ladybird, a tandem of homeobox genes that maintain late wingless expression in terminal and dorsal epidermis of the Drosophila embryo. Development 124, 91-100. [DOI] [PubMed] [Google Scholar]
- Jagla, T., Bellard, F., Lutz, Y., Dretzen, G., Bellard, M. and Jagla, K. (1998). ladybird determines cell fate decisions during diversification of Drosophila somatic muscles. Development 125, 3699-3708. [DOI] [PubMed] [Google Scholar]
- Kadam, S., McMahon, A., Tzou, P. and Stathopoulos, A. (2009). FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation. Development 136, 739-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin, M. (1999). Gastrulation in Drosophila: the logic and the cellular mechanisms. EMBO J. 18, 3187-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin, M. and Affolter, M. (2004). Drosophila gastrulation: identification of a missing link. Curr. Biol. 14, R480-R482. [DOI] [PubMed] [Google Scholar]
- Lewandoski, M., Meyers, E. N. and Martin, G. R. (1997). Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb. Symp. Quant. Biol. 62, 159-168. [PubMed] [Google Scholar]
- Liu, J., Qian, L., Wessells, R. J., Bidet, Y., Jagla, K. and Bodmer, R. (2006). Hedgehog and RAS pathways cooperate in the anterior-posterior specification and positioning of cardiac progenitor cells. Dev. Biol. 290, 373-385. [DOI] [PubMed] [Google Scholar]
- McMahon, A., Supatto, W., Fraser, S. E. and Stathopoulos, A. (2008). Dynamic analyses of Drosophila gastrulation provide insights into collective cell migration. Science 322, 1546-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson, A. M., Gisselbrecht, S., Zhou, Y., Baek, K. H. and Buff, E. M. (1998). Dual functions of the heartless fibroblast growth factor receptor in development of the Drosophila embryonic mesoderm. Dev. Genet. 22, 212-229. [DOI] [PubMed] [Google Scholar]
- Müller, H. A. (2008). Immunolabeling of embryos. Methods Mol. Biol. 420, 207-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, M. J. and Saint, R. (2007). Photoactivatable GFP resolves Drosophila mesoderm migration behaviour. Development 134, 3975-3983. [DOI] [PubMed] [Google Scholar]
- Ornitz, D. M. and Itoh, N. (2001). Fibroblast growth factors. Genome Biol. 2, 3005.3001-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, C., Ebner, A. and Affolter, M. (2002). In vivo imaging reveals different cellular functions for FGF and Dpp signaling in tracheal branching morphogenesis. Dev. Cell 2, 677-683. [DOI] [PubMed] [Google Scholar]
- Sato, M. and Kornberg, T. B. (2002). FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev. Cell 3, 195-207. [DOI] [PubMed] [Google Scholar]
- Schier, A. F. and Talbot, W. S. (2005). Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet. 39, 561-613. [DOI] [PubMed] [Google Scholar]
- Schulz, R. A. and Gajewski, K. (1999). Ventral neuroblasts and the heartless FGF receptor are required for muscle founder cell specification in Drosophila. Oncogene 18, 6818-6823. [DOI] [PubMed] [Google Scholar]
- Schumacher, S., Gryzik, T., Tannebaum, S. and Müller, H. A. (2004). The RhoGEF Pebble is required for cell shape changes during cell migration triggered by the Drosophila FGF receptor Heartless. Development 131, 2631-2640. [DOI] [PubMed] [Google Scholar]
- Shishido, E., Higashijima, S., Emori, Y. and Saigo, K. (1993). Two FGF-receptor homologues of Drosophila: one is expressed in mesodermal primordium in early embryos. Development 117, 751-761. [DOI] [PubMed] [Google Scholar]
- Shishido, E., Ono, N., Kojima, T. and Saigo, K. (1997). Requirements of DFR1/Heartless, a mesoderm-specific Drosophila FGF-receptor, for the formation of heart, visceral and somatic muscles, and ensheathing of longitudinal axon tracts in CNS. Development 124, 2119-2128. [DOI] [PubMed] [Google Scholar]
- Slack, J. M., Isaacs, H. V., Song, J., Durbin, L. and Pownall, M. E. (1996). The role of fibroblast growth factors in early Xenopus development. Biochem. Soc. Symp. 62, 1-12. [PubMed] [Google Scholar]
- Stathopoulos, A., Tam, B., Ronshaugen, M., Frasch, M. and Levine, M. (2004). pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev. 18, 687-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X., Meyers, E. N., Lewandoski, M. and Martin, G. R. (1999). Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 13, 1834-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland, D., Samakovlis, C. and Krasnow, M. A. (1996). branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell 87, 1091-1101. [DOI] [PubMed] [Google Scholar]
- Szebenyi, G. and Fallon, J. F. (1999). Fibroblast growth factors as multifunctional signaling factors. Int. Rev. Cytol. 185, 45-106. [DOI] [PubMed] [Google Scholar]
- Wilson, R., Vogelsang, E. and Leptin, M. (2005). FGF signalling and the mechanism of mesoderm spreading in Drosophila embryos. Development 132, 491-501. [DOI] [PubMed] [Google Scholar]
- Yang, X., Dormann, D., Munsterberg, A. E. and Weijer, C. J. (2002). Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev. Cell 3, 425-437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.