Abstract
We report a functional characterization of AtVPS45 (for vacuolar protein sorting 45), a protein from the Sec1/Munc18 family in Arabidopsis (Arabidopsis thaliana) that interacts at the trans-Golgi network (TGN) with the SYP41/SYP61/VTI12 SNARE complex. A null allele of AtVPS45 was male gametophytic lethal, whereas stable RNA interference lines with reduced AtVPS45 protein levels had stunted growth but were viable and fertile. In the silenced lines, we observed defects in vacuole formation that correlated with a reduction in cell expansion and with autophagy-related defects in nutrient turnover. Moreover, transport of vacuolar cargo with carboxy-terminal vacuolar sorting determinants was blocked in the silenced lines, suggesting that AtVPS45 functions in vesicle trafficking to the vacuole. These trafficking defects are similar to those observed in vti12 mutants, supporting a functional relationship between AtVPS45 and VTI12. Consistent with this, we found a decrease in SYP41 protein levels coupled to the silencing of AtVPS45, pointing to instability and malfunction of the SYP41/SYP61/VTI12 SNARE complex in the absence of its cognate Sec1/Munc18 regulator. Based on its localization on the TGN, we hypothesized that AtVPS45 could be involved in membrane fusion of retrograde vesicles recycling vacuolar trafficking machinery. Indeed, in the AtVPS45-silenced plants, we found a striking alteration in the subcellular fractionation pattern of vacuolar sorting receptors, which are required for sorting of carboxy-terminal vacuolar sorting determinant-containing cargo. We propose that AtVPS45 is essential for recycling of the vacuolar sorting receptors back to the TGN and that blocking this step underlies the defects in vacuolar cargo trafficking observed in the silenced lines.
Vesicle trafficking through the endomembrane system is important for plant growth, development, and responses to the environment (Surpin and Raikhel, 2004). Proteins containing a signal peptide enter the endoplasmic reticulum, are transported through the Golgi apparatus (Donohoe et al., 2007), and finally reach the trans-Golgi network (TGN), a reticular compartment at the trans face of the Golgi stack. Vesicles budding from the TGN have several possible destinations, including the plasma membrane and vacuoles. It has been proposed that at the TGN two major sorting pathways diverge to transport different sets of proteins to the vacuole (Jolliffe et al., 2005). These separate pathways could account for the presence of vacuoles with distinct functions and protein composition coexisting in certain plant cells (Hoh et al., 1995; Paris et al., 1996; Di Sansebastiano et al., 2001; Epimashko et al., 2004; Frigerio et al., 2008). The best-characterized examples of these distinct vacuoles are the storage and lytic vacuoles that play important roles in nutrient accumulation and mobilization during embryogenesis and seed germination, respectively. Moreover, even when only one vacuole is present in a particular cell, separate transport pathways may still be fully functional (Matsuoka et al., 1995; Pimpl et al., 2003; Otegui et al., 2006; Sanmartin et al., 2007; Sohn et al., 2007). However, few components involved in vacuolar trafficking have been characterized, and the existence of distinct pathways remains to be unequivocally proven (Frigerio et al., 2008; Rojo and Denecke, 2008). A key aspect to resolve is how proteins are sorted at the TGN to their respective destinations. It is widely accepted that soluble proteins require positive sorting signals in their sequence to be transported to the vacuole (Vitale and Hinz, 2005). Two main classes of vacuolar sorting determinants (VSDs) have been identified in soluble proteins. The sequence-specific VSDs (ssVSDs) are found in lytic and storage cargo, whereas the C-terminal VSDs (ctVSDs) are so far specific for storage cargo. It is thought that these VSDs are recognized by membrane-bound receptors on the TGN for their sorting into particular vesicles. Targeting of the ssVSD-containing proteins may require the VSR family of vacuolar sorting receptors (Kirsch et al., 1994; Ahmed et al., 2000; daSilva et al., 2005; Foresti et al., 2006). Sorting of storage proteins with ctVSDs also seems to be receptor mediated, and candidates for the storage protein receptor include the VSR and RMR families of receptors (Shimada et al., 2003; Park et al., 2005; J. Zouhar, A. Muñoz, and E. Rojo, unpublished data).
A fundamental event in any trafficking pathway is the fusion of a cargo vesicle with its target membrane. This membrane fusion reaction requires members of the SNARE family of proteins, which catalyze the fusion reaction itself, and of the Sec1/Munc18 (SM) family, which may regulate the fusion process (Toonen and Verhage, 2003; Hong, 2005; Jahn and Scheller, 2006). SNARE proteins are characterized by the presence of a SNARE motif consisting of a helical heptad repeat. Four SNARE helices, usually present in four individual polypeptides, form a coiled coil that is essential for membrane fusion. Three SNAREs (t-SNAREs) are present on the target organelle and one (v-SNARE) is present on the transport vesicle; interaction between the t-SNAREs and v-SNARE pull the membranes into close proximity and drive fusion between them (Whiteheart et al., 1993; Parlati et al., 2000). SNAREs may also provide specificity to fusion reactions, as distinct isoforms of SNARE proteins are present on different organelles and vesicle types (Söllner et al., 1993; McNew et al., 2000). The activity of SM proteins in membrane fusion is less clear, although in many cases it may be exerted through regulation of SNARE complex formation. However, there is no consensus on the way SM proteins interact with their corresponding SNAREs and on the effect they may have on a particular fusion reaction (Toonen and Verhage, 2003).
We have previously identified a protein complex on the TGN of Arabidopsis (Arabidopsis thaliana) that may function in fusion of recycling vesicles from the prevacuolar compartment (PVC; Bassham et al., 2000). Components of the complex include SYP41, SYP61, and VTI12 as the Q-SNAREs, possibly YKT6 as the R-SNARE, and AtVPS45 (for vacuolar protein sorting 45) as the regulatory SM protein (Bassham et al., 2000; Sanderfoot et al., 2001a; Chen et al., 2005). Genetic analyses of the SNAREs have indicated that mutants in the individual SNARE genes show differing phenotypes. A knockout mutant in SYP41 is gametophyte lethal, with defects in pollen tube growth leading to an inability to transmit the mutant genotype through the pollen (Sanderfoot et al., 2001b). By contrast, mutants in SYP61 and VTI12 are viable, although in the case of VTI12 this may be due to functional compensation by the related gene VTI11 (Surpin et al., 2003). The syp61 mutants show sensitivity to abiotic stresses (Zhu et al., 2002), but analysis of intracellular trafficking in this mutant has not been reported. The vti12 mutants have defects consistent with a role in autophagy (Surpin et al., 2003) and also function in trafficking of ctVSD-containing proteins to the vacuole (Sanmartin et al., 2007). These differences in phenotypes may indicate varying degrees of functional redundancy and multifunctionality among the TGN SNARE proteins as well as the intriguing character of the TGN (Lam et al., 2007).
Here, we examine the role of AtVPS45 in vesicle trafficking processes. Our results demonstrate that the AtVPS45 protein is essential very early in development, being necessary for pollen germination. Depleting AtVPS45 levels by RNA interference (RNAi) results in severely stunted plant growth due to reduced cell expansion that correlates with diminished vacuolar size. Our results suggest that AtVPS45 positively regulates the SYP41/SYP61/VTI12 complex activity, which may be required for recycling VSRs to the TGN to participate in additional rounds of sorting of ctVSD-containing vacuolar cargo.
RESULTS AND DISCUSSION
AtVPS45 Is Essential for Pollen Growth
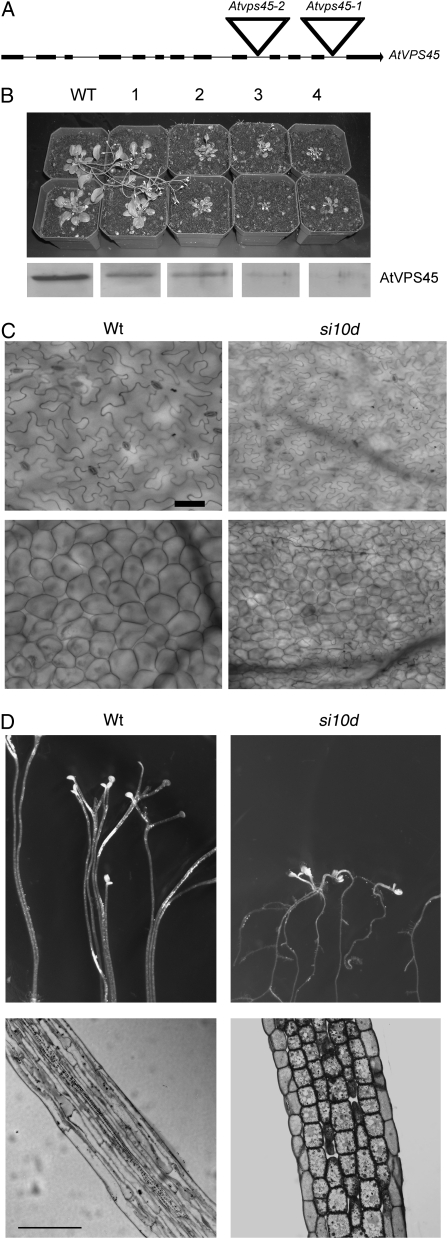
AtVPS45 is encoded by a single gene containing 13 exons on chromosome I. As an initial approach to deciphering the function of AtVPS45, a mutant with a T-DNA insertion in the AtVPS45 gene was isolated by PCR from pools of mutagenized seeds. An individual line, Atvps45-1, with a single insertion in the last intron of AtVPS45 was identified (Fig. 1A), and the insertion site was confirmed by sequencing of the PCR product. All of the plants containing the T-DNA insertion isolated from the initial screen were heterozygous for the insertion. Two plants were allowed to self-fertilize, and their progeny were analyzed by PCR for the presence of the insertion. Of 190 progeny analyzed, no plants homozygous for the insertion were identified (Table I). Moreover, we also failed to obtain homozygous mutants from a second T-DNA insertional allele, Atvps45-2, suggesting that the homozygous null mutations are lethal and that AtVPS45 is an essential gene. To confirm this, heterozygous Atvps45-1 mutant plants were transformed with an AtVPS45 cDNA driven by the native AtVPS45 promoter. Homozygous mutants could now be generated that expressed the AtVPS45 transgene, demonstrating that the lethal phenotype is due to the disruption of the AtVPS45 gene.
Figure 1.
AtVPS45 is required for cell expansion. A, Structure of the AtVPS45 gene, with boxes representing coding regions. The triangles indicate the sites of the T-DNA insertions in the Atvps45 knockout mutants. B, Individuals from the T3 generation of four independent homozygous RNAi transgenic lines are shown, with wild-type plants for comparison. Line 1 corresponds to siVPS45-1a, line 3 to siVPS45-8b, and line 4 to siVPS45-10d. Below are shown immunoblots of total protein extracts from each line probed with antibodies against AtVPS45. The size estimated for the AtVPS45 band was 66 kD. C, Cells of siVPS45-10d plants are smaller than those of wild-type plants. Wild-type or siVPS45-10d leaves were stained with chlorazol black E and observed by microscopy. The top panels show epidermal cells, and the bottom panels show mesophyll cells. Bar = 50 μm. D, Wild-type or siVPS45-10d seeds were germinated in the dark for 4 d. Longitudinal sections of hypocotyls of the etiolated seedlings were stained with toluidine blue and observed by light microscopy. Bar = 100 μm. WT or Wt, Wild type; si10d, siVPS45-10d.
Table I.
Segregation of T-DNA insertion
Progeny from two heterozygous Atvps45-1 mutant individuals were genotyped by PCR. The number of each genotype is indicated.
| Individual | VPS45/vps45 | vps45/vps45 | VPS45/VPS45 | Percentage with T-DNA |
|---|---|---|---|---|
| 1 | 47 | 0 | 47 | 50 |
| 2 | 43 | 0 | 53 | 45 |
| Total | 90 | 0 | 100 | 47 |
In the progeny from self-fertilized heterozygous mutants, an approximately 1:1 ratio of heterozygous to wild-type progeny was obtained (Table I), indicating a potential gametophyte-lethal phenotype. Similar results have been reported previously for knockout mutants in the AtVPS45-interacting t-SNAREs SYP41 and SYP42, which were shown to be required for pollen function (Sanderfoot et al., 2001b). To determine whether the Atvps45-1 mutant has a defect in the function of a gamete, reciprocal crosses of AtVPS45/Atvps45-1 heterozygous mutant plants with wild-type plants were performed and the progeny of the crosses were screened by PCR to determine their genotype. Crosses of wild-type pollen onto heterozygous mutant ovules resulted in both wild-type and heterozygous mutant progeny, indicating that the mutant allele can be transmitted via the ovule. In contrast, the reciprocal crosses of heterozygous mutant pollen onto wild-type ovules resulted in no plants containing the T-DNA insertion out of 50 progeny screened, suggesting that pollen containing the mutant allele is not viable (Table II).
Table II.
Reciprocal crosses
Progeny from reciprocal crosses between Atvps45-1 heterozygous mutants and wild-type Col-0 plants were genotyped by PCR. The number of each genotype is indicated.
| Cross | VPS45/VPS45 | VPS45/vps45 | Percentage with T-DNA |
|---|---|---|---|
| Col-0 male × vps45-1 female | 18 | 9 | 33 |
| vps45-1 male × Col-0 female | 50 | 0 | 0 |
To analyze the potential pollen defect more directly, in vitro pollen germination assays were performed. Pollen from several independent AtVPS45/Atvps45-1 heterozygous mutant or wild-type plants was plated onto pollen germination medium (Li et al., 1999) and incubated overnight to allow germination. While no obvious morphological differences could be seen between pollen from wild-type and mutant plants, on average only 46% of the pollen grains from mutant plants germinated compared with 75% of wild-type pollen (Table III). These data are consistent with a defect in Atvps45-1 mutant pollen germination, suggesting that a functional AtVPS45 gene is required for pollen growth.
Table III.
In vitro pollen germination assays
Pollen from flowers of the indicated genotype was placed onto agar plates for germination. The percentage of pollen grains with a visible pollen tube was determined for at least 100 pollen grains per experiment.
| Genotype | Experiment 1 | Experiment 2 | Experiment 3 | Average |
|---|---|---|---|---|
| Col-0 | 58% | 81% | 86% | 75% |
| VPS45/vps45-1 | 29% | 50% | 60% | 46% |
AtVPS45 Is Required for Cell Expansion
The inability to generate homozygous knockout mutants for AtVPS45 precluded further functional analysis using the null mutants. Therefore, transgenic plants were generated that contain reduced amounts of the AtVPS45 protein. An RNAi construct was generated consisting of an inverted repeat of a 500-bp fragment of the AtVPS45 cDNA, with a portion of the GUS gene as a linker (Chuang and Meyerowitz, 2000). The AtVPS45 cDNA region used for silencing has no sequence homology to other SM genes or to any other gene in the Arabidopsis genome, ensuring that the silencing would be specific for AtVPS45. The RNAi construct was introduced into Arabidopsis plants, and transformants were screened for reduced protein expression by immunoblotting using AtVPS45 antibodies (Bassham and Raikhel, 1998). We obtained several independent AtVPS45-silenced (siVPS45) lines with a range of degrees of silencing (Fig. 1B). Phenotypic analysis of the RNAi lines revealed that plants with reduced AtVPS45 protein levels are severely dwarfed. The severity of the dwarf phenotype correlated well with the amount of AtVPS45 protein detected by immunoblot (Fig. 1B), suggesting that AtVPS45 is required for normal plant growth. Although the RNAi construct does not target other SM genes, we nevertheless checked the levels of the SM protein AtVPS33 as an additional control. As expected, no changes in AtVPS33 levels were observed in the transgenic lines (see Fig. 5C below). Despite their reduced size, the siVPS45 lines were fertile and produced flowers and seeds that were normal in appearance. The phenotype was stable and heritable, being still apparent at least to the T5 generation. We chose several independent transgenic lines homozygous for a single insertion of the transgene and with different extents of silencing for further experiments. The phenotypes of representative lines with strongly reduced (siVPS45-10d and siVPS45-8b) or weakly reduced (siVPS45-1a) levels of AtVPS45 protein are shown in the subsequent experiments.
Figure 5.
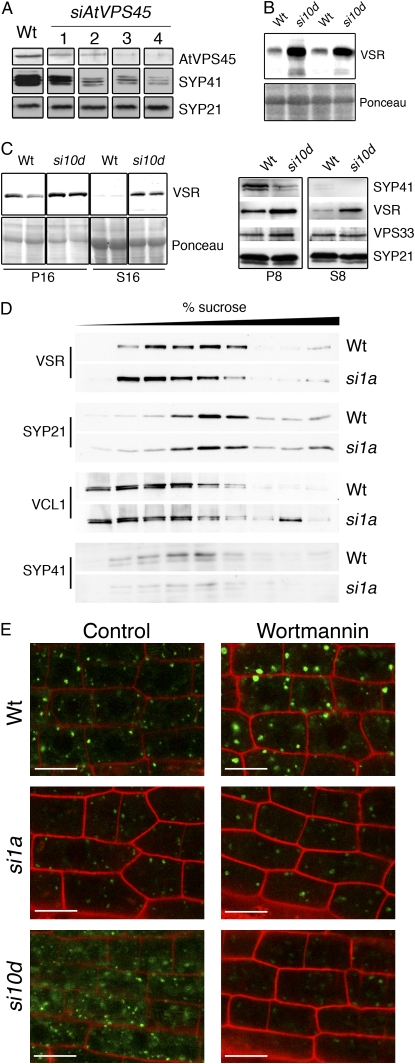
AtVPS45 is required for the stability of SYP41 and the proper subcellular distribution of VSRs. A, Protein samples from wild-type and AtVPS45-silenced lines (as in Fig. 1) were analyzed with the indicated antibodies. B, Total protein samples from rosette leaves of 4-week-old wild-type and siVPS45-10d plants were analyzed with an anti-VSR antibody. C, Left panels, Wild-type and siVPS45-10d seedlings were grown in vitro for 2 weeks in liquid Murashige and Skoog medium supplemented with 1% Suc and homogenized in 100 mm Tris, pH 8, 5 mm EDTA, and complete protease inhibitor cocktail. Pellet (P16) and soluble (S16) fractions after centrifugation at 16,000g were analyzed with an anti-VSR antibody. Right panels, Pellet (P8) and soluble (S8) fractions after centrifugation at 8,000g were analyzed with the indicated antibodies. D, Microsomes were prepared from wild-type and siVPS45-1a plants grown in liquid for 20 d, separated in a step Suc gradient as described (Bassham and Raikhel, 1998), and analyzed with the indicated antibodies. Estimated in-gel sizes of the bands detected by the antibodies: AtVPS45, 66 kD; VSR, 80 kD; SYP21, 36 kD; SYP41, 39 kD; VCL1, 100 kD; VPS33, 70 kD. E, Wild-type, siVPS45-1a, and siVPS45-10d plants transgenic for a GFP-VSR3 construct (Miao et al., 2008) were obtained by crossing to a stable transgenic GFP-VSR3 line. Roots were incubated in liquid Murashige and Skoog medium with 50 μm wortmannin (Wortmannin) or a similar amount of dimethyl sulfoxide used as a solvent (Control), and epidermal cells of the root tip were imaged 5 h later. Green signal indicates VSR3-GFP, and red signal indicates propidium iodide. Bars = 10 μm. Wt, Wild type; si10d, siVPS45-10d; si1a, siVPS45-1a.
The dwarf phenotype of siVPS45 lines could potentially be due to reduced cell size. To analyze this possibility, mature, fully expanded leaves of wild-type or siVPS45-10d plants were stained using chlorazol black to delineate the cell walls, cleared to remove chlorophyll, and observed by microscopy. Both the epidermal pavement cells (Fig. 1C, top panels) and mesophyll cells (Fig. 1C, bottom panels) were reduced in size in the siVPS45-10d leaves compared with wild-type leaves. To confirm a role of AtVPS45 in cell expansion, we analyzed the growth of hypocotyls of etiolated seedlings, which occurs solely by cell enlargement (Gendreau et al., 1997). In wild-type seedlings germinating in the dark, the hypocotyl cells elongated very rapidly, reaching a length of approximately 2 cm within a few days. In contrast, siVPS45-10d hypocotyls remained very short, reaching only 2 mm in length at most (Fig. 1D). Longitudinal sections of the hypocotyls were analyzed by light microscopy after staining with toluidine blue. Lack of cell elongation is evident in the siVPS45 seedlings, with cells remaining almost cuboid (Fig. 1D). Similar results were obtained with independent silenced lines (data not shown), demonstrating that AtVPS45 silencing causes a block in cell expansion.
AtVPS45-Silenced Lines Show Defects in Vacuole Formation and Function
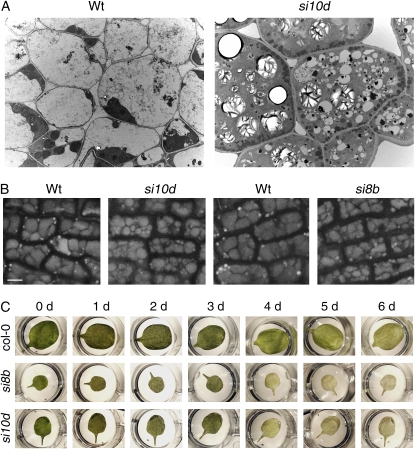
AtVPS45 is a member of the SM family of proteins that regulate SNARE-mediated membrane fusion. Thus, the developmental alterations of siVPS45 lines are probably a consequence of primary defects in vesicular trafficking. We analyzed the subcellular morphology of etiolated seedling cells to determine if AtVPS45 silencing affects the size or shape of endomembrane compartments. In wild-type seedlings, the cells have large central vacuoles, containing granular or fibrous material and occupying the majority of the cell volume, with a thin layer of dense cytoplasm surrounding them. In siVPS45-10d seedlings, the cells lack large central vacuoles and instead are full of many small vacuoles or vesicles, which often lack visible contents (Fig. 2A). Cell expansion in plants is mainly due to vacuole enlargement, and thus defective vacuole biogenesis is the likely cause of the reduced cell size in the silenced lines. To substantiate these results, we analyzed whether vacuole formation was altered in other tissues. In seeds, protein storage vacuoles (PSVs) can be directly visualized due to their intrinsic autofluorescence (Sanmartin et al., 2007). The PSVs in mature wild-type embryos were large, irregularly shaped, and compressed against each other, filling the whole cell volume (Fig. 2B). PSVs in siVPS45 embryos were smaller and more numerous, rounded, and with intervening spaces between them, indicating that AtVPS45 is required for normal PSV biogenesis. The changes in PSV morphology and distribution are similar to those observed in the enhanced vti12 mutant (Sanmartin et al., 2007), supporting a common function of AtVPS45 and VTI12 in trafficking to these compartments.
Figure 2.
The AtVPS45-silenced lines have vacuolar defects. A, Cross sections of hypocotyls from 4-d-old etiolated wild-type or siVPS45-10d seedlings were analyzed by electron microscopy. B, The protein storage vacuoles of epidermal cells from embryos of wild-type, siVPS45-10d, and siVPS45-8b seeds were imaged by confocal microscopy. Bar = 5 μm. C, The first and second true leaves from 24-d-old plants grown on soil were excised, placed on water in 24-well plates, incubated in the dark, and imaged at the indicated days after detaching. Wt, Wild type; si10d, siVPS45-10d; si8b, siVPS45-8b.
In addition to smaller vacuoles, numerous starch grains and lipid droplets are maintained in the silenced lines during germination (Fig. 2A), suggesting that AtVPS45 is required for their turnover, which may involve autophagy into the vacuole (Toyooka et al., 2001; Poxleitner et al., 2006). This is consistent with evidence implicating VTI12, an interacting partner of AtVPS45, in autophagy (Surpin et al., 2003). However, by staining autophagosomes with the fluorescent dye monodansylcadaverine (Contento et al., 2005), we did not find any differences in their cytosolic accumulation in siVPS45 lines or vti12 mutants compared with the wild-type plants (A.L. Contento and D.C. Bassham, unpublished data). This suggests that VTI12 and AtVPS45 are not involved in autophagosome formation but may still function at a later stage of autophagy, possibly during the breakdown of autophagosomes. To gain additional evidence that AtVPS45-silenced plants are defective in nutrient turnover, we analyzed their response to starvation conditions that induce autophagy. Detached leaves incubated in the dark activate autophagy and eventually senesce. A common phenotype of mutants affected in nutrient turnover is an earlier senescence in this detached leaf assay (Surpin et al., 2003; Xiong et al., 2005). We observed a clear acceleration of senescence in siVPS45 lines relative to wild-type lines (compare the total chlorosis after 4 d in leaves from the silenced lines with the presence of chlorophyll in wild-type leaves after 6 d; Fig. 2C), similar to that reported for vti12 mutants (Surpin et al., 2003), supporting a role for AtVPS45 in nutrient recycling.
We conclude from these results that AtVPS45 is likely involved in vesicle trafficking to the vacuole and that reduced levels of this protein result in defective vacuole formation and function.
AtVPS45 Participates in Trafficking of ctVSD-Containing Cargo to the Vacuole
In addition to a function in the starvation response (Surpin et al., 2003) and in PSV biogenesis (Sanmartin et al., 2007), VTI12 participates in trafficking of ctVSD-containing vacuolar cargo (Sanmartin et al., 2007), so we hypothesized that AtVPS45 would also share this function.
We have previously developed an assay for detecting missorting of vacuolar cargo via morphological changes in transgenic plants expressing VAC2. The VAC2 transgene codes for the CLAVATA3 protein fused to the ctVSD from barley (Hordeum vulgare) lectin and is expressed in Arabidopsis under the control of the constitutive 35S promoter. VAC2 is localized in the vacuole in wild-type plants, where it has no activity. When trafficking is blocked or saturated, VAC2 is secreted to the apoplasm, where it negatively regulates stem cell proliferation in shoot apical meristems (Sanmartin et al., 2007). This VAC2-secretion assay is semiquantitative, as different degrees of secretion cause a graded range of phenotypes, ranging from termination of a few of the floral meristems before carpel formation in weak secretors to plants in which the meristems are terminated prior to the production of any shoot or flower organs in strong secretors. To test if AtVPS45 is also involved in the transport of VAC2, we crossed the RNAi lines and the Atvps45-2 mutant with two transgenic lines homozygous for the VAC2 marker, L1 and L2 (Sanmartin et al., 2007). As a control, we also crossed the wild-type ecotype Columbia (Col-0) with L1 and L2. Similar results were obtained for the crosses with L1 and L2, and only the former are shown. In the F1 population resulting from the cross of siVPS45 lines with L1 and L2, we observed already a weak meristem phenotype indicative of VAC2 secretion. The primary meristem of the F1 plants from the cross of siVPS45-10d with L1 or L2 was terminated before bolting, but secondary meristems produced shoots in which a few flowers lacked carpels (Fig. 3A). In the F1 population of the cross of siVPS45-1a with L1 and L2, there was no termination of any of the shoot apical meristems, but some of the flowers lacked carpels. In the F2 population from the cross siAtVPS45-1a by L1 or L2, the silenced plants genotyped as VAC2/VAC2 showed termination of the primary meristem and bolted only from secondary meristems (Fig. 3A). In those inflorescences, many flowers developed without carpels. In the F2 population from the cross siVPS45-10d by L1 or L2, we recovered AtVPS45-silenced plants that had all the meristems terminated prior to bolting or that eventually bolted from lateral meristems but had inflorescence shoots terminated before producing flowers (Fig. 3B). Consequently, no offspring from these plants was available to determine the VAC2 zygosity. However, we assumed them to correspond to the homozygous VAC2 plants, as similar phenotypes were not found in the F1 generation or in any of the other crosses made. Notably, we did not observe any meristem termination phenotypes in the F1 or F2 generation from the crosses between Col-0 or the Atvps45-2 mutant and the L1 or L2 line. These results show that VAC2 secretion, measured by its effect in meristem proliferation, is directly related to the degree of AtVPS45 silencing. Hemizygous T-DNA mutant plants do not show a meristem phenotype, suggesting that a single copy of the gene is sufficient for efficient vacuolar targeting of VAC2. Only when the expression was further lowered through the RNAi approach could we observe the VAC2 secretion.
Figure 3.
The AtVPS45-silenced plants secrete the VAC2 cargo. A, Plants from the F1 generation of crosses between a line homozygous for VAC2 (L1) and siVPS45-1a or siVPS45-10d display terminated flower and shoot meristems. B, Plants from the F2 generation of crosses between siVPS45-1a or siVPS45-10d and the L1 line. The plants shown were homozygous for VAC2 and either wild type (Wt) or AtVPS45 silenced (si1a and si10d). Arrows indicate flowers without carpels, and arrowheads indicate terminated shoot meristems.
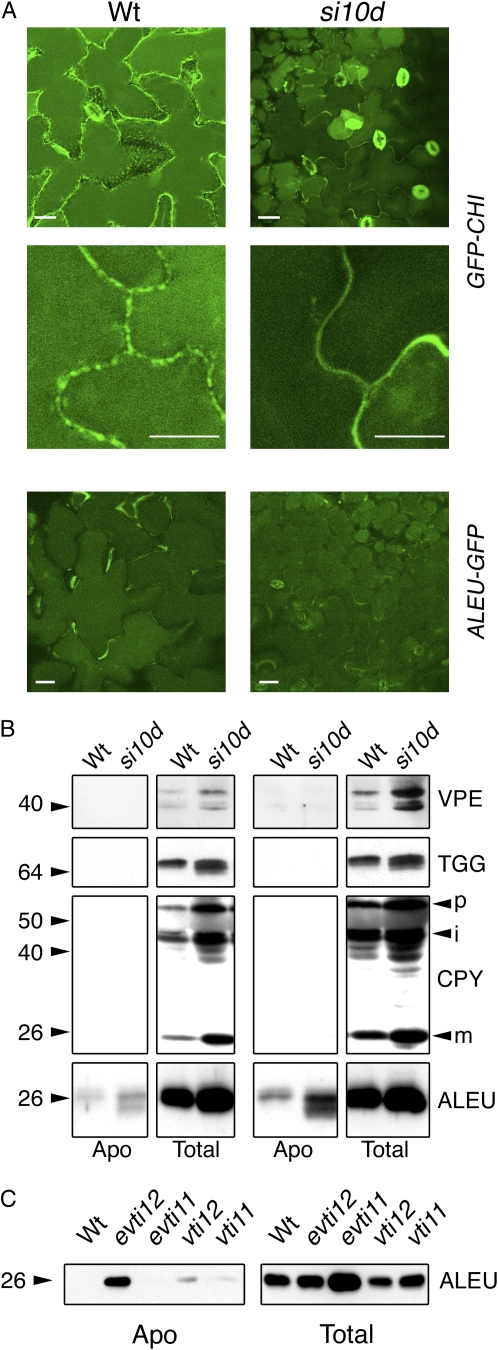
Subsequently, we studied the role of AtVPS45 in trafficking of the well-characterized ctVSD-containing vacuolar marker GFP-CHI and the ssVSD-containing vacuolar marker ALEU-GFP. The GFP-CHI protein consists of GFP carrying a signal peptide fused to the ctVSD from tobacco (Nicotiana tabacum) chitinase (Di Sansebastiano et al., 1998), while the ALEU-GFP marker consists of the N-terminal part of barley aleurain, containing the signal peptide and the ssVSD, fused to GFP (Di Sansebastiano et al., 2001). There were two noticeable changes in the GFP-CHI fluorescence pattern in the silenced lines. First, a general decrease in diffuse fluorescence, which corresponds to the vacuole-localized GFP (Sanmartin et al., 2007), was observed in the AtVPS45-silenced plants (Fig. 4A, top panels), indicating that less fusion protein was reaching the vacuole. Second, the GFP signal was found delineating the cell boundaries in those same plants, suggesting that GFP-CHI was secreted (Fig. 4A, middle panels). As a comparison, in the wild-type plants, the cell boundaries were not marked by GFP and instead a punctate pattern was observed at the cell periphery that corresponded to GFP localized in the cortical endoplasmic reticulum. In contrast to these results, we did not observe changes in the pattern of ALEU-GFP fluorescence in the silenced plants (Fig. 4A, bottom panels). We conclude from these data that AtVPS45 is a limiting factor required for trafficking of GFP-CHI to the vacuole and is either dispensable or required at lower levels for vacuolar trafficking of ALEU-GFP. These results support a role for AtVPS45 in the transport of cargo containing ctVSDs.
Figure 4.
The AtVPS45-silenced plants secrete ctVSD-containing cargo. A, Lines expressing GFP-CHI and ALEU-GFP were crossed with wild-type and siVPS45-10d plants, and the adaxial side of leaves from F1 plants was analyzed by confocal microscopy. Bars = 10 μm. B, Samples of apoplastic fluids (Apo) collected from rosette leaves of wild-type and siVPS45-10d plants were analyzed with the indicated antibodies. Total proteins (Total) from leaves of the same plants were analyzed to determine the expression levels of the proteins. The precursor (p), intermediate (i), and mature (m) forms of CPY are marked. C, The apoplastic fluids and total proteins from rosette leaves of the indicated mutants were analyzed with anti-AtAleurain antibodies (ALEU). evti11, enhanced vti11; evti12, enhanced vti12 (Sanmartin et al., 2007). Positions of molecular mass markers (in kD) are shown at left in B and C. Wt, Wild type; si10d, siVPS45-10d.
To determine whether AtVPS45 silencing provokes the secretion of endogenous vacuolar proteins, we isolated extracellular fluid from rosette leaves of soil-grown plants and analyzed the protein profiles with antibodies against AtVPEγ, AtCPY, AtAleurain, and the myrosinase TGG2. As shown in Figure 4B, we could not detect secretion of the lytic cargo AtCPY, AtVPEγ, or TGG2 in siVPS45-10d plants, indicating that these proteins do not require AtVPS45 for their transport to the vacuole. Interestingly, we detected AtAleurain in the extracellular fluids from siVPS45-10d plants. AtAleurain, similar to barley aleurain, contains both a putative ssVSD and a ctVSD that could act as dual vacuole-targeting signals (Hinz et al., 2007). Importantly, trafficking of the ALEU-GFP construct, which has the GFP fused at the C terminus of barley aleurain, masking the putative ctVSD, is not altered in siVPS45 plants, suggesting that AtVPS45 is required for ctVSD-dependent sorting of AtAleurain. Similarly, AtAleurain but not ALEU-GFP is secreted in enhanced vti12 plants (Fig. 4C; Sanmartin et al., 2007) and vsr mutants (J. Zouhar, A. Muñoz, and E. Rojo, unpublished data), which are also affected specifically in trafficking of ctVSD-containing proteins.
Taken together, these data suggest that AtVPS45 silencing does not lead to general secretion of endogenous vacuolar proteins but specifically affects trafficking of the ctVSD-containing proteins like VAC2, GFP-CHI, and AtAleurain.
AtVPS45 Silencing Destabilizes SYP41 and Redistributes Vacuolar Sorting Receptors
Our results indicate a common function of AtVPS45 and VTI12 in trafficking of ctVSD-containing cargo, in nutrient recycling in the vacuole, and in PSV biogenesis, consistent with the observed interaction between AtVPS45 and a SNARE complex containing VTI12, SYP41, and SYP61 (Bassham et al., 2000). In accordance with these results, we found that the levels of SYP41 were reduced in parallel to the levels of AtVPS45 in the RNAi-silenced lines (Fig. 5A). Importantly, the SYP41 mRNA is present at similar levels in wild-type and silenced lines (data not shown), suggesting that the SYP41 protein is unstable in the absence of its interacting partner. This is consistent with data obtained in yeast, where Tlg2p, a putative ortholog of SYP41, is unstable in a vps45 mutant (Bryant and James, 2001). As a control, we analyzed the accumulation of SYP21, a Q-SNARE that is localized in the PVC, where it forms a complex with SYP51 and VTI11 but not with AtVPS45 (Bassham et al., 2000). The levels of SYP21 remain unchanged in the silenced lines (Fig. 5A), demonstrating that AtVPS45 silencing destabilizes specifically SYP41.
These results provide further in vivo evidence for a role of AtVPS45 in regulating the activity of the VTI12/SYP41/SYP61 SNARE complex. This SNARE complex is localized on the TGN (Bassham et al., 2000; Sanderfoot et al., 2001a), where it colocalizes with VSRs, which have to be recycled from the PVC to the TGN to ensure full vacuolar sorting capacity (daSilva et al., 2005). Moreover, recent results show that VSRs are the sorting receptors for the same ctVSD-containing proteins that are mislocalized in the siVPS45 lines (J. Zouhar, A. Muñoz, and E. Rojo, unpublished data). We thus hypothesized that defects in trafficking of ctVSD-containing vacuolar cargo in the siVPS45 plants could be due to perturbed recycling of the VSR proteins to the TGN. Consistent with a relationship between AtVPS45 function and VSR homeostasis, we observed increased levels of the VSR proteins in independent siVPS45 lines (Fig. 5B; data not shown). This implies that the trafficking defects in siVPS45 lines cannot be ascribed to a decrease in VSR levels, but they could be due to alterations in VSR localization and/or activity. Mislocalization of the sorting receptors may reduce their turnover, leading to increased VSR protein levels. In this regard, mutants in VPS29 and VPS35, components of the retromer complex that is likely involved in VSR recycling from the PVC (Oliviusson et al., 2006), also show increased accumulation of VSR protein levels (Yamazaki et al., 2008).
Direct evidence of altered localization was obtained by cell fractionation studies that showed that a larger fraction of VSRs remained in the soluble fraction after low-speed centrifugations in extracts from silenced lines than from wild-type plants (Fig. 5C). In contrast, markers for the TGN (SYP41) and the PVC (SYP21), which are the organelles through which VSRs cycle in wild-type plants, did not change in fractionation pattern, indicating that there was no change in the sedimentation properties of those organelles and implying that the VSRs were partially localizing to a different compartment/vesicle in the silenced lines. Similar changes in cell fractionation of VSRs were obtained in samples from siVPS45-8b plants (data not shown), indicating that AtVPS45 silencing was causing the alteration of VSR localization. To increase the resolution of cell fractionation, we separated microsomes in step Suc gradients and compared the distribution of VSRs with that of known markers of endomembrane compartments. To exclude differences due to gross cellular and developmental alterations, we used for these experiments the siVPS45-1a plants that are macroscopically similar to the wild-type plants but still show protein-trafficking defects (Figs. 1B and 3A). As shown in Figure 5D, in the silenced lines, there was a shift of VSRs toward lighter membrane compartments and a concomitant reduction of VSR levels in the fractions corresponding to TGN and PVC (Fig. 5D, lane 5), indicating that VSRs are displaced from these compartments.
To check for mislocalization of VSRs in planta, we analyzed the subcellular localization of a GFP-VSR3 marker driven by the constitutive 35S promoter, which at steady state labels mainly the PVC (Miao et al., 2008). As reported in that study, this construct gives a typical punctate signal in Arabidopsis wild-type cells. Moreover, treatment with wortmannin induces the formation of large vacuolated PVCs that are intensely labeled by this protein (Fig. 5E). In the siVPS45-1a line, we also observed punctate GFP labeling in untreated plants and in vacuolated PVCs in wortmannin-treated plants, but in these the GFP signal appeared lower than in wild-type plants. This was even more evident in the siVPS45-10d line, where the signal in the vacuolated PVCs was very low. In untreated siVPS45-10d roots, GFP-VSR3 was found in punctate compartments but also as a more diffusely distributed signal (Fig. 5E).
We hypothesize from these analyses that AtVPS45 silencing may block recycling from the PVC to the TGN and displace VSRs to other compartments/vesicles. This in turn causes a depletion of VSRs from the PVC-TGN recycling route, resulting in the backup and missorting of the vacuolar cargo. The wortmannin-induced PVC enlargement is thought to occur in part through PVC-TGN heterotypic fusions (Lam et al., 2007). A reduced GFP-VSR3 signal in a wortmannin-induced TGN-PVC hybrid compartment would be consistent with the depletion of VSRs in the PVC-TGN recycling route in the silenced lines due to disruption of the TGN SNARE complex.
MATERIALS AND METHODS
Antibodies
The antibodies used in this work were described previously: anti-AtVPS45 (Bassham and Raikhel, 1998), anti-SYP41 (Oliviusson et al., 2006), anti-SYP21 (da Silva Conceicao et al., 1997), anti-TGG2 3D7 monoclonal antibody (Andreasson et al., 2001), anti-AtAleurain and anti-VSR (Ahmed et al., 2000), and anti-AtCPY and anti-VPE (Rojo et al., 2003).
Identification of the Atvps45-1 Mutant
Pools of T-DNA mutant Arabidopsis (Arabidopsis thaliana) seeds obtained from the Arabidopsis Functional Genomics Center were screened for mutations in the AtVPS45 gene (At1g77140) with the assistance of Mendel Biotechnology as described (Sanderfoot et al., 2001b). Insertions were amplified by PCR using nested gene-specific primers and primers corresponding to the T-DNA ends (see Table IV for primer sequences). Products were sequenced to confirm the insertion sites, and individual plants containing an insertion in the AtVPS45 gene were identified from the pools (named Atvps45-1). The T-DNA insertion in the Atvps45-1 mutant confers resistance to kanamycin. Segregation of the T-DNA was analyzed by growth on 50 mg L−1 kanamycin and by PCR using the above primers
Table IV.
Primers used
| Experiment | Primer | Sequence |
|---|---|---|
| Atvps45-1 | Forward | AGAACGGTCCAATTAAACTGTACCACGA |
| Forward nested | TCTCTGGTGGGGTAATCATCATCATGTA | |
| T-DNA | CGCACTCAAGTCTTTCATCTACGGCAATGACC | |
| T-DNA nested | GGGGATTATTTTGTGACGCCGATTACATACGG | |
| Atvps45-2 | Forward | GTCTCCCAAGTACAAACCAG |
| Reverse | TCACCAGTGCGCTTCTCCAT | |
| T-DNA | GCAATCAGCTGTTGCCCGTCTCACTGGTG | |
| AtVPS45 complementation | Forward | GCAATTGAATCATAATAAGCTTAG |
| Reverse | GGAGAGCTTTCAGTCCGTCTAAA | |
| AtVPS45 RNAi | F-EcoRV | AGCGGATATCTGGGGATATTGGAATGAACATC |
| F-EcoRI | AGCGGAATTCTGGGGATATTGGAATGAACATC | |
| R-XbaI | TCGCTCTAGAGCCATGTTACGTGCTATATTCAAA | |
| R-BamHI | ACGCGGATCCGCCATGTTACGTGCTATATTCAAA |
Complementation of the Atvps45-1 Mutant Phenotype
The AtVPS45 promoter and the first two introns and exons of the AtVPS45 gene were amplified by PCR (see Table IV for primer sequences), and the products were digested using HindIII (introduced in the forward primer) and PvuI (present within the gene sequence). This genomic fragment was subcloned along with a PvuI/BamHI fragment of the AtVPS45 cDNA into HindIII/BglII sites of the binary vector pCAMBIA1300MCS to recreate the AtVPS45 coding sequence driven by its endogenous promoter. The construct was introduced into the Atvps45-1 heterozygous mutant by Agrobacterium tumefaciens-mediated transformation using the floral dip method (Clough and Bent, 1998). Transformants were selected by growth on hygromycin at 50 mg L−1, and genotypes were determined by PCR amplification of the T-DNA insertion and transgene.
Generation of AtVPS45-RNAi Transgenic Lines
AtVPS45 gene-specific sense and antisense fragments (approximately 500 bp each) were amplified using the primers shown in Table IV, with restriction sites introduced as indicated, as described (Chuang and Meyerowitz, 2000). The fragments were cloned into the binary vector pCGN, separated by a spacer consisting of a 1-kb fragment of the GUS gene, and driven by the 35S promoter of the Cauliflower mosaic virus. The construct was introduced into Arabidopsis accession Col-0 by Agrobacterium-mediated transformation using the floral dip method (Clough and Bent, 1998). Transformants were selected by growth on 50 mg L−1 kanamycin, and homozygous plants were identified in the T2 generation by analysis of segregation of the kanamycin resistance gene.
Pollen Germination Assays
Pollen was collected from wild-type and Atvps45-1 heterozygous plants onto pollen germination medium (Li et al., 1999) and incubated overnight at room temperature to allow germination. Pollen was then scored by light microscopy for the presence or absence of a pollen tube. At least 100 pollen grains were counted per genotype in three independent experiments.
Chlorazol Black E Staining of Leaves
Leaves from wild-type and AtVPS45-RNAi plants were cleared in bleach for 30 min, followed by dehydration in an ethanol series to 95% (v/v). Chlorazol black E (1% [w/v] in 95% ethanol) was added for 3 h, and the leaves were washed several times with ethanol before mounting for microscopy.
Microscopy
For transmission electron microscopy, samples were collected and fixed with 2% (w/v) glutaraldehyde and 2% (w/v) paraformaldehyde in 0.1 m cacodylate buffer, pH 7.2, for 48 h at 4°C. Samples were rinsed three times in 0.1 m cacodylate buffer and then postfixed in 1% osmium tetroxide in 0.1 m cacodylate buffer for 1 h at room temperature. The samples were rinsed in deionized distilled water and en bloc stained with 2% (w/v) aqueous uranyl acetate for 30 min, dehydrated in a graded ethanol series, cleared with ultrapure acetone, and infiltrated and embedded using Spurr's epoxy resin (Electron Microscopy Sciences). Resin blocks were polymerized for 48 h at 65°C. Thick and ultrathin sections were made using a Reichert Ultracut S ultramicrotome (Leeds Precision Instruments). Thick (1 μm) sections were collected onto slides, stained with 1% (w/v) toluidine blue, and imaged using a Zeiss Axioplan II light microscope (Carl Zeiss). Ultrathin sections were collected onto copper grids and counterstained with 5% (w/v) uranyl acetate in deionized distilled water for 15 min followed by Sato's lead stain for 10 min. Images were captured using a JEOL 1200EX II scanning and transmission electron microscope (Japan Electron Optic Laboratories). Imaging of PSVs from dried seeds was done as described (Sanmartin et al., 2007).
Acknowledgments
We are grateful to Prof. A.M. Bones for his gift of the 3D7 monoclonal antibody against myrosinases, to Prof. L. Jiang for the GFP-VSR3 line, and to Prof. G.P. Di Sansebastiano and Prof. J.M Neuhaus for the GFP-CHI and ALEU-GFP lines. We also thank Pilar Paredes for her technical assistance, Marsha Pilgrim and Luc Adam for assistance with mutant screening, Tracey Pepper and the Iowa State University Microscopy and Nanoimaging Facility for assistance and advice on microscopy, and Tony Contento for performing the autophagy assays.
This work was supported by grants to D.C.B. from the National Science Foundation (grant no. IOB–0515998) and the Iowa State University Plant Sciences Institute, by the Spanish Ministerio de Educación y Ciencia (grant no. BIO2006–11150 to E.R.), and by a postdoctoral I3P Fellowship to J.Z. from the Consejo Superior de Investigaciones Científicas.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Diane C. Bassham (bassham@iastate.edu).
Open Access articles can be viewed online without a subscription.
References
- Ahmed SU, Rojo E, Kovaleva V, Venkataraman S, Dombrowski JE, Matsuoka K, Raikhel NV (2000) The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J Cell Biol 149 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson E, Bolt Jorgensen L, Hoglund AS, Rask L, Meijer J (2001) Different myrosinase and idioblast distribution in Arabidopsis and Brassica napus. Plant Physiol 127 1750–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Raikhel NV (1998) An Arabidopsis VPS45p homolog implicated in protein transport to the vacuole. Plant Physiol 117 407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Sanderfoot AA, Kovaleva V, Zheng H, Raikhel NV (2000) AtVPS45 complex formation at the trans-Golgi network. Mol Biol Cell 11 2251–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, James DE (2001) Vps45p stabilizes the syntaxin homologue Tlg2p and positively regulates SNARE complex formation. EMBO J 20 3380–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Shin YK, Bassham DC (2005) YKT6 is a core constituent of membrane fusion machineries at the Arabidopsis trans-Golgi network. J Mol Biol 350 92–101 [DOI] [PubMed] [Google Scholar]
- Chuang CF, Meyerowitz EM (2000) Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. Proc Natl Acad Sci USA 97 4985–4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Contento AL, Xiong Y, Bassham DC (2005) Visualization of autophagy in Arabidopsis using the fluorescent dye monodansylcadaverine and a GFP-AtATG8e fusion protein. Plant J 42 598–608 [DOI] [PubMed] [Google Scholar]
- daSilva LL, Taylor JP, Hadlington JL, Hanton SL, Snowden CJ, Fox SJ, Foresti O, Brandizzi F, Denecke J (2005) Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17 132–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Conceicao A, Marty-Mazars D, Bassham DC, Sanderfoot AA, Marty F, Raikhel NV (1997) The syntaxin homolog AtPEP12p resides on a late post-Golgi compartment in plants. Plant Cell 9 571–582 [PMC free article] [PubMed] [Google Scholar]
- Di Sansebastiano GP, Paris N, Marc-Martin S, Neuhaus JM (1998) Specific accumulation of GFP in a non-acidic vacuolar compartment via a C-terminal propeptide-mediated sorting pathway. Plant J 15 449–457 [DOI] [PubMed] [Google Scholar]
- Di Sansebastiano GP, Paris N, Marc-Martin S, Neuhaus JM (2001) Regeneration of a lytic central vacuole and of neutral peripheral vacuoles can be visualized by green fluorescent proteins targeted to either type of vacuoles. Plant Physiol 126 78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe BS, Kang BH, Staehelin LA (2007) Identification and characterization of COPIa- and COPIb-type vesicle classes associated with plant and algal Golgi. Proc Natl Acad Sci USA 104 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epimashko S, Meckel T, Fischer-Schliebs E, Luttge U, Thiel G (2004) Two functionally different vacuoles for static and dynamic purposes in one plant mesophyll leaf cell. Plant J 37 294–300 [DOI] [PubMed] [Google Scholar]
- Foresti O, daSilva LL, Denecke J (2006) Overexpression of the Arabidopsis syntaxin PEP12/SYP21 inhibits transport from the prevacuolar compartment to the lytic vacuole in vivo. Plant Cell 18 2275–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, Hinz G, Robinson DG (2008) Multiple vacuoles in plant cells: rule or exception? Traffic 9 1564–1570 [DOI] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Hofte H (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz G, Colanesi S, Hillmer S, Rogers JC, Robinson DG (2007) Localization of vacuolar transport receptors and cargo proteins in the Golgi apparatus of developing Arabidopsis embryos. Traffic 8 1452–1464 [DOI] [PubMed] [Google Scholar]
- Hoh B, Hinz G, Jeong BK, Robinson DG (1995) Protein storage vacuoles form de novo during pea cotyledon development. J Cell Sci 108 299–310 [DOI] [PubMed] [Google Scholar]
- Hong W (2005) SNAREs and traffic. Biochim Biophys Acta 1744 493–517 [PubMed] [Google Scholar]
- Jahn R, Scheller RH (2006) SNAREs: engines for membrane fusion. Nat Rev Mol Cell Biol 7 631–643 [DOI] [PubMed] [Google Scholar]
- Jolliffe NA, Craddock CP, Frigerio L (2005) Pathways for protein transport to seed storage vacuoles. Biochem Soc Trans 33 1016–1018 [DOI] [PubMed] [Google Scholar]
- Kirsch T, Paris N, Butler JM, Beevers L, Rogers JC (1994) Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc Natl Acad Sci USA 91 3403–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SK, Tse YC, Robinson DG, Jiang L (2007) Tracking down the elusive early endosome. Trends Plant Sci 12 497–505 [DOI] [PubMed] [Google Scholar]
- Li H, Lin Y, Heath RM, Zhu MX, Yang Z (1999) Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11 1731–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Bassham DC, Raikhel NV, Nakamura K (1995) Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J Cell Biol 130 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Söllner TH, Rothman JE (2000) Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407 153–159 [DOI] [PubMed] [Google Scholar]
- Miao Y, Li K, Li H, Yao X, Jiang L (2008) The vacuolar transport of aleurain-GFP and 2S albumin-GFP fusions is mediated by the same pre-vacuolar compartments in tobacco BY-2 and Arabidopsis suspension cultured cells. Plant J 56 824–839 [DOI] [PubMed] [Google Scholar]
- Oliviusson P, Heinzerling O, Hillmer S, Hinz G, Tse YC, Jiang L, Robinson DG (2006) Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 18 1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui MS, Herder R, Schulze J, Jung R, Staehelin LA (2006) The proteolytic processing of seed storage proteins in Arabidopsis embryo cells starts in the multivesicular bodies. Plant Cell 18 2567–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris N, Stanley CM, Jones RL, Rogers JC (1996) Plant cells contain two functionally distinct vacuolar compartments. Cell 85 563–572 [DOI] [PubMed] [Google Scholar]
- Park M, Lee D, Lee GJ, Hwang I (2005) AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. J Cell Biol 170 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F, McNew JA, Fukuda R, Miller R, Söllner TH, Rothman JE (2000) Topological restriction of SNARE-dependent membrane fusion. Nature 407 194–198 [DOI] [PubMed] [Google Scholar]
- Pimpl P, Hanton SL, Taylor JP, Pinto-daSilva LL, Denecke J (2003) The GTPase ARF1p controls the sequence-specific vacuolar sorting route to the lytic vacuole. Plant Cell 15 1242–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poxleitner M, Rogers SW, Lacey Samuels A, Browse J, Rogers JC (2006) A role for caleosin in degradation of oil-body storage lipid during seed germination. Plant J 47 917–933 [DOI] [PubMed] [Google Scholar]
- Rojo E, Denecke J (2008) What is moving in the secretory pathway of plants? Plant Physiol 147 1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo E, Zouhar J, Carter C, Kovaleva V, Raikhel NV (2003) A unique mechanism for protein processing and degradation in Arabidopsis thaliana. Proc Natl Acad Sci USA 100 7389–7394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Kovaleva V, Bassham DC, Raikhel NV (2001. a) Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol Biol Cell 12 3733–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot AA, Pilgrim M, Adam L, Raikhel NV (2001. b) Disruption of individual members of Arabidopsis syntaxin gene families indicates each has essential functions. Plant Cell 13 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartin M, Ordonez A, Sohn EJ, Robert S, Sanchez-Serrano JJ, Surpin MA, Raikhel NV, Rojo E (2007) Divergent functions of VTI12 and VTI11 in trafficking to storage and lytic vacuoles in Arabidopsis. Proc Natl Acad Sci USA 104 3645–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I (2003) Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA 100 16095–16100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn EJ, Rojas-Pierce M, Pan S, Carter C, Serrano-Mislata A, Madueno F, Rojo E, Surpin M, Raikhel NV (2007) The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proc Natl Acad Sci USA 104 18801–18806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature 362 318–324 [DOI] [PubMed] [Google Scholar]
- Surpin M, Raikhel N (2004) Traffic jams affect plant development and signal transduction. Nat Rev Mol Cell Biol 5 100–109 [DOI] [PubMed] [Google Scholar]
- Surpin M, Zheng HJ, Morita MT, Saito C, Avila E, Blakeslee JJ, Bandyopadhyay A, Kovaleva V, Carter D, Murphy A, et al (2003) The VTI family of SNARE proteins is necessary for plant viability and mediates different protein transport pathways. Plant Cell 15 2885–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toonen RF, Verhage M (2003) Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol 13 177–186 [DOI] [PubMed] [Google Scholar]
- Toyooka K, Okamoto T, Minamikawa T (2001) Cotyledon cells of Vigna mungo seedlings use at least two distinct autophagic machineries for degradation of starch granules and cellular components. J Cell Biol 154 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Hinz G (2005) Sorting of proteins to storage vacuoles: how many mechanisms? Trends Plant Sci 10 316–323 [DOI] [PubMed] [Google Scholar]
- Whiteheart SW, Griff IC, Brunner M, Clary DO, Mayer T, Buhrow SA, Rothman JE (1993) SNAP family of NSF attachment proteins includes a brain-specific isoform. Nature 362 353–355 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Contento AL, Bassham DC (2005) AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. Plant J 42 535–546 [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Shimada T, Takahashi H, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I (2008) Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant Cell Physiol 49 142–156 [DOI] [PubMed] [Google Scholar]
- Zhu JH, Gong ZZ, Zhang CQ, Song CP, Damsz B, Inan G, Koiwa H, Zhu JK, Hasegawa PM, Bressan RA (2002) OSM1/SYP61: a syntaxin protein in Arabidopsis controls abscisic acid-mediated and non-abscisic acid-mediated responses to abiotic stress. Plant Cell 14 3009–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]