Abstract
The Jun N-terminal kinase and p38 pathways, also known as stress-activated protein kinase (SAPK) pathways, are signaling conduits reiteratively used throughout the development and adult life of metazoans where they play central roles in the control of apoptosis, immune function, and environmental stress responses. We recently identified a Drosophila Ser/Thr phosphatase of the PP2C family, named Alphabet (Alph), which acts as a negative regulator of the Ras/ERK pathway. Here we show that Alph also plays an inhibitory role with respect to Drosophila SAPK signaling during development as well as under stress conditions such as oxidative or genotoxic stresses. Epistasis experiments suggest that Alph acts at a step upstream of the MAPKKs Hep and Lic. Consistent with this interpretation, biochemical experiments identify the upstream MAPKKKs Slpr, Tak1, and Wnd as putative substrates. Together with previous findings, this work identifies Alph as a general attenuator of MAPK signaling in Drosophila.
THE c-Jun N-terminal kinase (JNK) and p38 pathways are evolutionarily conserved mitogen-activated protein kinase (MAPK) signaling cascades involved in several developmental processes and homeostasis maintenance in adult organisms. Also called stress-activated protein kinase (SAPK) pathways, their misregulation often leads to neurodegenerative diseases, immune dysfunction, and cancer (Manning and Davis 2003; Zarubin and Han 2005).
SAPK pathways are composed of a three-kinase core module comprising a MAPKKK, a MAPKK, and a MAPK that transmit incoming signals through a phosphorylation cascade (Kyriakis et al. 1994). Several kinases occupy the MAPKKK position. They include mixed-lineage kinases (MLK1-4), dual-leucine zipper kinase (DLK), leucine zipper kinase (LZK), MEK kinases (MEKK1–4), apoptosis signal regulating kinase-1 (ASK1), and TGFβ-activated kinase-1 (TAK1). Upon activation, these kinases phosphorylate MAPKKs (or MKKs) specific for p38 or JNK pathways. For example, MAPKKK-mediated phosphorylation of MKK3/6 leads to p38 activation while phosphorylation of MKK4/7 promotes JNK-dependent signaling. Activated MAPKKs then phosphorylate the threonine and tyrosine residues of the so-called TEY motif situated in the activation loop of JNK or p38, thereby triggering their catalytic activation (Davis 2000; Zarubin and Han 2005). Given the variety of extracellular signals and MAPKKKs involved in SAPK activation, cells must possess mechanisms to elicit specific cellular responses. These mechanisms are currently being unraveled and appear to depend on specific scaffolds (Dhanasekaran et al. 2007), phosphatases (Owens and Keyse 2007), and components of the ubiquitin/proteasome complex (Laine and Ronai 2005) that shape signaling paths and modulate internal signaling flow. Nonetheless, the way in which these proteins work together to produce specific outputs remains largely unexplored.
SAPK pathways and their constituents have been well conserved during the evolution of metazoans, thus making genetically amenable multicellular organisms useful models for identifying and characterizing the function of novel components. For example, a number of developmental processes in Drosophila, such as embryonic dorsal closure, pupal thorax closure, and the establishment of ommatidial polarity in the developing retina, have proven to be powerful systems for deciphering the molecular events linked to JNK-dependent signaling (Noselli and Agnes 1999; Zeitlinger and Bohmann 1999; Jacinto et al. 2002). More recently, SAPK pathways were shown to be critical for stress and immune resistance in flies (Stronach and Perrimon 1999; Wang et al. 2003; Craig et al. 2004; Delaney et al. 2006). As in mammals, SAPK pathways in Drosophila can be subdivided into two branches: the Basket (Bsk; JNK homolog) pathway that uses Hemipterous (Hep; MKK7 homolog) or dMKK4 as MAPKKs and the p38 pathway that comprises two p38 isoforms (p38a and p38b) and one MAPKK named Licorne (Lic; MKK3/6 homolog). Several MAPKKKs have also been linked to these pathways in flies. They correspond to Slipper (Slpr; MLK homolog), Wallenda (Wnd; DLK/LZK homolog), Tak1, Tak1-like 1 and 2, Mekk1, and Ask1/Pk92B (Stronach 2005). Mutant alleles were isolated for most of these and genetic evidence not only showed their role in SAPK pathways, but also linked their respective activities to specific developmental events or stress responses. For example, the Drosophila MLK homolog Slpr is an essential regulator of JNK-dependent epithelial cell migration, such as those observed during embryonic dorsal closure or pupal thorax closure (Stronach and Perrimon 2002; Polaski et al. 2006). On the other hand, Tak1 is critical for the SAPK-dependent innate immune response (Vidal et al. 2001), while Mekk1 showed a clear ability to regulate p38-mediated environmental stress responses such as resistance to heat or oxidative stress (Inoue et al. 2001). Recently, loss-of-function mutations recovered in the wnd gene linked the encoded DLK/LZK homolog to JNK-dependent synaptic growth (Collins et al. 2006). Although specific roles have been attributed to MAPKKKs, redundancy has also been observed (Polaski et al. 2006).
We previously isolated mutations in a nonessential gene named alphabet (alph), which encodes a Ser/Thr phosphatase of the PP2C family closely related to mammalian PP2Cα/β isoforms (Baril and Therrien 2006). Genetic analysis demonstrated its role as a negative regulator of the Ras/ERK pathway during Drosophila eye and wing development. While its substrate(s) have not been identified, Alph is catalytically active and the recovered mutant alleles nearly abrogate phosphatase activity in vitro. Interestingly, functional characterization of PP2Cs in mammals, yeasts, and plants have identified several of their family members, including the PP2Cα/β-related proteins, as negative regulators of both JNK and p38 pathways (Saito and Tatebayashi 2004; Schweighofer et al. 2004; Lammers and Lavi 2008). Using genetic and biochemical means, we show here that Alph also negatively regulates SAPK-dependent signaling in Drosophila. Epistatic analysis suggests that Alph functions at the level of various SAPKKKs, which is consistent with the ability of Alph to regulate distinct developmental and stress-activated events mediated by SAPK signaling.
MATERIALS AND METHODS
Drosophila stocks, transgenesis, and scanning electron microscopy:
The alphXS-88, alphS-331, alphS-355 (Baril and Therrien 2006), slpr921, slpr3P5 (Stronach and Perrimon 2002), slprBS06 (Polaski et al. 2006), and hep699 (Chou and Perrimon 1996) alleles have been described previously. The bsk1, hepG0107, licG0252, pucA251.1F3, pucE69, Df(3R)Dr-rv1, and pnr-Gal4 alleles were obtained from the Bloomington Stock Center.
The pGMR-Rac1 line was kindly provided by J. Settleman (Nolan et al. 1998). The UAS-SlprWT line has previously been described in Polaski et al. (2006), whereas the psE-hepCA, psE-licCA, GMR-alph, GMR-alphS-331, and pUAS-alphRNAi lines were generated by P-element-mediated germline transformation as described in Rubin and Spradling (1982). Scanning electron microscopy was performed as described in Wassarman et al. (2000).
Plasmids and molecular biology:
The vector used for transfection experiments (pRMHA-5) is a modified version of the copper-inducible pMet vector (Therrien et al. 1998) that contains an alternate multiple cloning site. psE is a pW8-derived P-element transformation vector containing two sevenless enhancer sequences upstream of the Drosophila hsp70 promoter (Dickson et al. 1992). The pGMR vector has been described previously (Hay et al. 1994).
The slpr (clone ID: GH26507), wnd (clone ID: LD14856), and Tak1 (clone ID: LD42274) cDNAs were obtained from the Drosophila Genomics Resource Center (DGRC) collections. The cDNAs were PCR amplified using a 5′-end oligonucleotide-containing sequence encoding a V5 epitope (GKPIPNPLLGLDST) inserted in place of the first methionine and cloned into the pRMHA-5 expression vector. The slpr cDNA obtained from DGRC had a missense mutation that changed codon Asp-314 to a tyrosine residue. This mutation has been corrected by site-directed mutagenesis. The hepCA cDNA was amplified by PCR from genomic DNA of a transgenic line containing the hep-RC cDNA that has Ser-346, Thr-350, and Ser-352 changed to Asp residues (Adachi-Yamada et al. 1999). The lic cDNA was amplified by PCR from an aliquot of the LD cDNA library (Berkeley Drosophila Genome Project) and mutagenized using the QuickChange kit (Stratagene) to replace Ser-200 and Thr-204 to Asp residues, thereby producing the licCA cDNA. The hepCA and licCA cDNAs contain a Myc epitope (AEEQKLISEEDLL) at their N terminus and were introduced into the pRMHA-5 and psE expression vectors. The alph and alphS-331 cDNAs (derived from CG1906-RB transcript), which have been described elsewhere (Baril and Therrien 2006), were transferred into pRMHA-5 and pGMR. They do not contain an epitope tag as it inactivates catalytic function (our unpublished observations). The pMET-Rac1V12 construct was generated by amplifying a DNA fragment corresponding to the Drosophila Rac1 ORF from an embryonic cDNA library. The 5′ primer encoded an amino acid change at position 12 to create a Gly-to-Val change at that position. The fragment was then subcloned into the pMet vector. The UAS-alphRNAi construct was made by introducing two copies in opposite orientation of a PCR fragment corresponding to alph exon 2 (the DNA fragment was produced using alph-1 amplicon primers shown below) in the pWIZ vector (Lee and Carthew 2003). The Act5C-Flag-Bsk construct was kindly provided by T. Ip. New cDNA inserts produced by PCR were entirely sequenced.
Double-stranded RNA production was conducted as previously described in Roy et al. (2002). alph double-stranded RNA (dsRNA) corresponding to the alph-1 amplicon reduced by >80% Alph protein levels when assayed in S2 cells (not shown). The following are the dsRNA primers used:
alph-1 amplicon (exon 2)
Top: 5′-GATAAGCCGAAAACCGCCAAG
Bottom: 5′-TGGCGATGCTCACTAGGTTAC
alph-2 amplicon (3′-UTR)
Top: 5′-GTTGCAGTCGAAACACGAAAC
Bottom: 5′-GTGTGTTCTTGTATGTTTTTG
alph-3 amplicon (exons 4 and 5)
Top: 5′-TTTTGTTGCAGACGAGATAGAGTC
Bottom: 5′-ATTTCAAAGGCTACGGCTATAATG
bsk amplicon (exons 4–7)
Top: 5′-CAGGATGTCTACCTGGTCATG
Bottom: 5′-GATTAGCTCCTTCCACTGCTC
hep amplicon (exon 2)
Top: 5′-GGAAACGGACATGAAGCTGAAG
Bottom: 5′-CCACTGTGACCTTGCCCAGG
puc amplicon (exon 4)
Top: 5′-GATCATCTCGCCCAATCTGAACTTC
Bottom: 5′-CCTCGTCAAATTGCTAGCCACATG
Embryonic lethality determination:
Embryos from slpr mutant or slpr, alph double-mutant stocks were collected overnight on grape-juice plates incubated at 25°. From each collection, ∼150 embryos were handpicked to a fresh, yeasted plate and aged at 25° for an additional 24 hr to allow larvae to hatch. Plates were then stored at 4° for at least 2 days. The unhatched (brown) embryos and unfertilized (white) eggs were counted. Embryonic lethality was calculated according to the following equation: number of unhatched (brown) embryos/(total number on a plate minus the number of unfertilized eggs) and expressed as the percentage of lethality. Three to four plates were scored per genotype.
Cuticle preparation/scoring:
Dead embryos were dechorionated in diluted bleach for 5 min and rinsed extensively in heptane. Embryos were then devitellinized in 50:50 heptane:methanol mixture with vigorous shaking. After washing several times in methanol, larvae were fixed in acetic acid: glycerin (4:1) for 30 min at 60° and then at room temperature overnight. Larvae were then mounted in Polysciences CMCP-10 mounting media: lactic acid (3:1) on a microscope slide, topped with a coverslip and placed on a 50° slide warmer overnight. Cuticle phenotypes were scored and binned as indicated. Darkfield cuticle images were captured with a SPOT camera mounted on a Nikon E800 compound microscope.
Survival assays:
alph mutant alleles were backcrossed at least six times in the w1118 background before performing survival assays. For resistance to paraquat, 3- to 5-day-old adult females were starved for 6 hr and then transferred to vials containing 0.8% low melting agarose, 10% sucrose, and 20 mm paraquat (Sigma). Dead flies were counted after 36 hr incubation. For life-span determination, flies were transferred every 2 days to fresh bottles of food. Dead flies were counted after each transfer.
UV irradiation of pupal eyes:
White prepupae were collected, immobilized laterally on a slide with double-stick tape, and allowed to mature for 24 hr in standard fly culture conditions. The pupal shell surrounding the head area was then removed and pupae were irradiated using a UV crosslinker (Stratalinker 1800) with energy set at 50 (5 mJ/cm2). After irradiation, pupae were kept in the dark until hatching. Images were acquired with a Zeiss AxioCam camera mounted on a Leica MZ FLIII stereomicroscope. To calculate the left/right (L/R) ratio, the area of each eye was outlined using Adobe-Photoshop CS3 and the number of pixels per area was determined. The L/R ratio corresponds to the area of the irradiated eye divided by the area of the non-irradiated eye of the same fly. Statistical analyses (t-test) were performed using the R software (http://www.r-project.org).
Protein analyses:
S2 cells were maintained in serum-free insect cell medium (Sigma) and transfected with Effectene (Qiagen). Protein expression was induced by adding CuSO4 (0.7 mm) to the medium 24 hr post-transfection. Sources and dilutions for antibodies are the following: α-Flag M2 mAb (1:2000; Sigma); α-V5 mAb (1:5000; Invitrogen); α-pJNK (1:2000; Cell Signaling); and α-Myc 9E10 mAb (1:3000; Santa Cruz Biotechnology). A rabbit α-Alph polyclonal antibody (1:2000) has been described previously (Baril and Therrien 2006).
Protein lysates were prepared using standard procedures. Briefly, cells were harvested 24 hr post-induction and lysed (15 min at 4°) in NP-40 lysis buffer (20 mm Tris at pH 8.0, 137 mm NaCl, 10% glycerol, 1% Igepal CA-630, 1 mm EDTA, 0.15 units/ml aprotinin, 20 μm leupeptin, and 1× Sigma phosphatase inhibitor cocktail). Cell debris were removed by centrifugation at 12,000 × g for 15 min (4°).
Quantitative immunofluorescence assays:
pMet-Rac1V12 S2 cells were seeded in 96-well plates (105 cells/well) with dsRNAs (10 μg/ml) and incubated for 4 days. Rac1V12 expression was induced by adding 0.7 m CuSO4 4 hr prior to fixation. Alternatively, cells were stimulated with LPS (50 μg/ml; Sigma) for 5 min. Next, cells were fixed in 4% paraformaldehyde/1× PBS, permeabilized in 0.2% Triton X-100/1× PBS (PBT), and blocked in 2% BSA/PBT. Cells were then stained overnight using an anti-pJNK antibody [1° antibody; 1/500; Cell Signaling (9251)], washed in PBT, and revealed using an anti-rabbit Alexa Fluor 555-conjugated secondary antibody (1/1000; Invitrogen). DAPI (0.04 μg/ml) was used to stain nuclei. An automated fluorescence microscopy system (Zeiss Axiovert) was employed to image plates at a rate of five fields per well. Image acquisition and analysis was conducted using MetaMorph (Molecular Devices) software. The cell-scoring application in MetaMorph was used to extract an average signal per cell for each field imaged. Data shown are the average signal of multiple wells over three separate experiments.
RESULTS
alph inactivation increases SAPK-dependent signaling during Drosophila development:
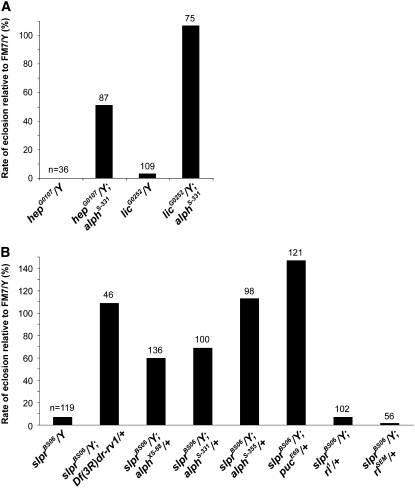
Loss-of-function alleles of hep or lic cause lethality due to early developmental defects affecting embryonic dorsal closure and anteroposterior patterning of the egg, respectively (Glise et al. 1995; Suzanne et al. 1999). To determine if Alph activity negatively influences SAPK signaling, we tested whether alph inactivation could suppress the lethality associated with hep or lic hypomorphic alleles. The hep and lic loci are located on the X chromosome and therefore the viability of mutant hemizygous males is compromised. Indeed, hepG0107/Y males die at the pupal stage, while only 3% of licG0252/Y males reach adulthood (http://flybase.bio.indiana.edu; Figure 1A). We previously showed that alph point mutants (alphXS-88, alphS-331, and alphS-355) that disrupt catalytic activity, and an insertion allele (alphPBac) that abolishes expression, are nonetheless homozygous viable with no discernible external phenotypes (Baril and Therrien 2006). Interestingly, introduction of a homozygous alphS-331 allele into either a hepG0107 or a licG0252 mutant background strongly suppressed hemizygous male lethality (Figure 1A). Although to a weaker extent, similar results were obtained when any of the four alph alleles mentioned above were tested as heterozygotes (data not shown). These findings imply that alph activity normally antagonizes hep- and lic-dependent events during development and thereby suggest that Alph acts as a negative regulator of SAPK signaling. Because the hep and lic alleles that were used are hypomorphic, it is not possible to conclude whether Alph is acting downstream or upstream of these SAPKKs.
Figure 1.—
alph mutations rescue the lethality of hep, lic, and slpr alleles. (A) The number of hepG0107/Y or licG0252/Y males was scored in the absence or in the presence of a homozygous alphS-331 allele and compared to the number of males carrying the FM7 balancer chromosome (FM7/Y) in the same cross. (B) The number of slprBS06/Y males was scored in the absence or in the presence of various heterozygous mutant alleles [or a heterozygous chromosomal deletion (Df(3R)Dr-rv1) that removes the 99A7-B11 cytological region]. Their relative numbers were also compared to males carrying the FM7 balancer chromosome in the same cross. Results are expressed as a percentage. The number of flies scored per genotype is indicated on top of each bar.
During the course of this work, we identified a chromosomal deletion on the right arm of the third chromosome (Df(3R)Dr-rv1) that dominantly restored viability to a slpr null allele (Figure 1B). The Df(3R)Dr-rv1 deficiency deletes ∼40 genes at cytological position 99A7-99B11, which includes the alph locus. Hence, we tested whether mutations in alph would have a comparable effect. Markedly, we found that heterozygous alph alleles dominantly suppressed the lethality of slprBS06 hemizygous males (Figure 1B). Similar results were obtained with a mutation in puckered (puc), which encodes a Bsk-specific dual-specificity phosphatase that acts as a negative regulator of JNK signaling (Martin-Blanco et al. 1998), whereas loss-of-function or gain-of-function alleles of the mapk/rl gene, which lies at the heart of ERK signaling, had no significant effect (Figure 1B). These results provide further genetic evidence demonstrating that JNK signaling is under a negative control by Alph and that this effect is independent of Alph's ability to negatively regulate Ras/ERK signaling.
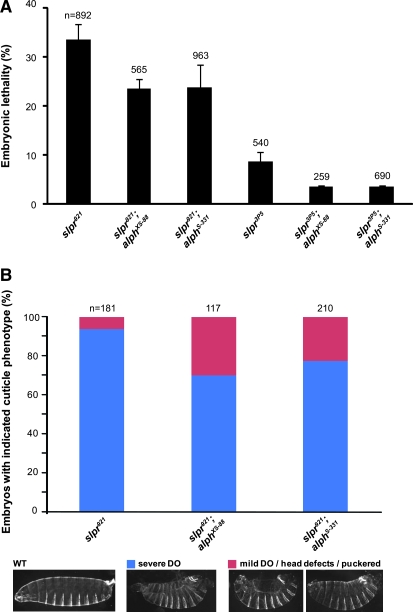
The morphogenetic processes of embryonic dorsal closure and pupal thorax closure depend on Slpr-dependent JNK signaling. Loss-of-function mutations in genes required for JNK signaling generally produce a large, dorsal anterior hole in the secreted larval cuticle that results in embryonic lethality (Jacinto et al. 2002). To determine if alph activity can be linked to a specific JNK-regulated developmental process, we looked for the ability of alph alleles to suppress the dorsal open phenotype caused by reduced Slpr-mediated JNK signaling. For this, we used two additional slpr alleles, slpr921 and slpr3P5, which, respectively, display complete and partial embryonic lethality. These two alleles are phenotypically stronger than the slprBS06 null allele whereby, unlike for the slprBS06 allele, no male escapers are observed and dead embryos exhibit a high proportion of strong dorsal open phenotype (Stronach and Perrimon 2002; Polaski et al. 2006; Figure 2B). In agreement with the results presented above, alph mutants suppressed slpr921 and slpr3P5 embryonic lethality (Figure 2A). Moreover, we found that alph alleles significantly reduced the number of dead embryos exhibiting a severe dorsal open phenotype, while increasing the proportion that either displayed a weak dorsal open phenotype or had only head defects (Figure 2B and data not shown).
Figure 2.—
alph alleles suppress the dorsal open phenotype of mutant slpr embryos. (A) Embryonic lethality expressed as the mean percentage (%) ± standard deviation is shown for two slpr alleles tested either alone or in combination with two alph alleles. The total number (n) of embryos analyzed per genotype is indicated on top of its respective bar. Statistical significance was confirmed by conducting a two-tailed Student's t-test where P-values were at least <0.007. (B) Dead embryos of the indicated genotypes were scored for dorsal open (DO) phenotypes and head defect/puckered phenotypes, which are characteristic of impaired JNK signaling. The number of severe DO (blue) embryos were then compared to those that have mild DO or minor head defects and puckering (red). The relative proportion of the two categories per genotype is displayed as a percentage. (Bottom) Representative embryonic phenotypes compared to those of wild type (WT). The total number (n) of embryos analyzed per genotype is indicated on top of their respective bar. A Student's t-test confirmed the statistical significance of the difference between slpr921 alone and slpr921;alph alleles: P < 0.025.
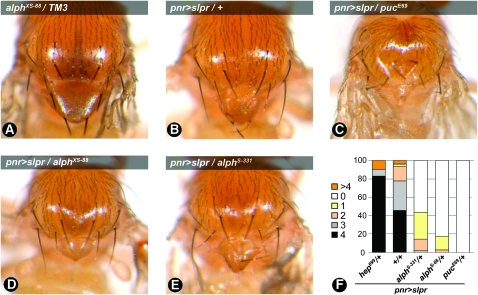
Hypomorphic mutations in components of the JNK pathway usually permit completion of embryogenesis, but can produce thorax closure (TC) defects, manifesting as a midline cleft in the thorax, during later stages of development. In contrast, hyperactivation of the pathway results in reduction of the thorax and scutellar area, often accompanied by loss of bristles (Zeitlinger and Bohmann 1999). It has been previously reported that overexpression of wild-type Slpr in dorsal developing tissues using the pannier-GAL4 (pnr-GAL4) driver resulted in a similar TC defect (Polaski et al. 2006; Figure 3: compare A and B). This phenotype depends on the strength of JNK signaling as inactivation of one copy of puc strongly enhances this phenotype, while a heterozygous hep allele suppresses it (Figure 3C and data not shown). Interestingly, we found that heterozygous alleles of alph also enhance the Slpr overexpression TC phenotype (Figure 3: compare D and E to B; note the reduction of scutellar area). To quantify the severity of the TC phenotype, the number of scutellar bristles for each genotype was determined (Figure 3F). Wild-type Slpr expression generally resulted in the loss of one or two bristles. Removing one copy of the puc gene strongly enhanced bristle loss, while a hep loss of function had the opposite effect. Although weaker, alph alleles behaved as puc and reduced the number of scutellar bristles to two or less. Taken together, these findings indicate that alph negatively regulates JNK signaling during various developmental events.
Figure 3.—
alph alleles enhance thorax closure defects generated by Slpr expression. The pannier-Gal4 (pnr-Gal4) driver was used to direct expression of wild-type slpr (pnr>slprWT) in dorsal tissues during development. Modulation of thorax defects was evaluated for each heterozygous allele. (A–E) A representative image is shown for each genotype. A wild-type thorax is shown in A. (F) Quantification of thorax closure defects by monitoring the number of scutellar bristles. Wild-type flies have four scutellar bristles while mutants with thorax closure defects generally lose a few or entirely lack these bristles. In an otherwise wild-type (+/+) background, Slpr overexpression generates a thorax closure defect that is accompanied by the loss of one to two bristles with a penetrance of ∼50%. Moreover, this genotype occasionally displays more than four bristles. This was also observed in the hep heterozygous background. The total number of flies scored for each genotype was as follows: pnr>slpr (94); pnr>slpr, hep699/+ (41); pnr>slpr, alphS-331/+ (125); pnr>slpr, alphXS-88/+ (40); pnr>slpr, pucE69/+ (6). Owing to a significantly low rate of eclosion, only six adult flies were recovered for the pnr>slpr, pucE69/+ genotype.
alph regulates SAPK signaling under various stress conditions:
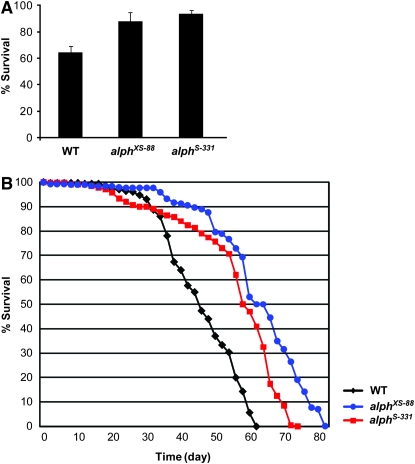
A number of studies conducted in flies have documented the protective role that JNK signaling plays under stress conditions (Stronach 2005). We sought to determine whether Alph could also modulate JNK signaling in response to stress. To address this point, we evaluated the ability of alph to alter the tolerance of flies to oxidative stress caused by the herbicide paraquat (Arking et al. 1991). Consistent with elevated JNK signaling, alphXS-88 and alphS-331 homozygous mutant backgrounds conferred increased tolerance to paraquat compared to an isogenic wild-type genotype (Figure 4A). JNK-dependent tolerance to oxidative stress has also been shown to correlate with life-span extension in flies (Wang et al. 2003). We thus verified whether alph homozygous mutant flies exhibited increased longevity. As shown in Figure 4B and summarized in Table 1, both alph homozygous alleles indeed lived significantly longer than their wild-type counterpart (increased mean and maximum life span) and performed in the same range as a heterozygous puc allele (Wang et al. 2003).
Figure 4.—
alph inactivation increases resistance to oxidative damages and life span. (A) The survival rate (%) of 3- to 5-day-old female flies fed with 20 mm paraquat was determined after 36 hr of exposure. Mean and standard deviation of three independent experiments (20 flies/vial in duplicate) are presented for each indicated genotype. Isogenic w1118 flies were used as wild-type (WT) controls. The difference observed between wild type and the two alph genotypes is statistically significant (P < 1.0E-04) according to a Student's t-test. (B) Longevity of two alph homozygous mutant alleles is shown compared to isogenic w1118 flies used as wild-type (WT) controls. For each genotype, the results correspond to the mean of two separate experiments comprising 100 and 200 males, respectively. For both assays, similar trends were observed using flies of the opposite gender (not shown).
TABLE 1.
Mean and maximum life span of alph mutant flies compared to wild type
| Mean life span (days)
|
Maximum life span (days)
|
|||
|---|---|---|---|---|
| Genotypes | Cohort 1 | Cohort 2 | Cohort 1 | Cohort 2 |
| Wild type | 41 | 48 | 54 | 60 |
| alphS-331 | 57 | 54 | 70 | 72 |
| alphXS-88 | 63 | 60 | 80 | 76 |
Cohort 1 and 2 comprised 100 and 200 males, respectively. Log-rank P-values were calculated for each cohort using the survdiff formula (Harrington and Fleming 1982) of the R software. In all cases, P = 0, which means that the P-values were smaller than the smallest value allowed by the software.
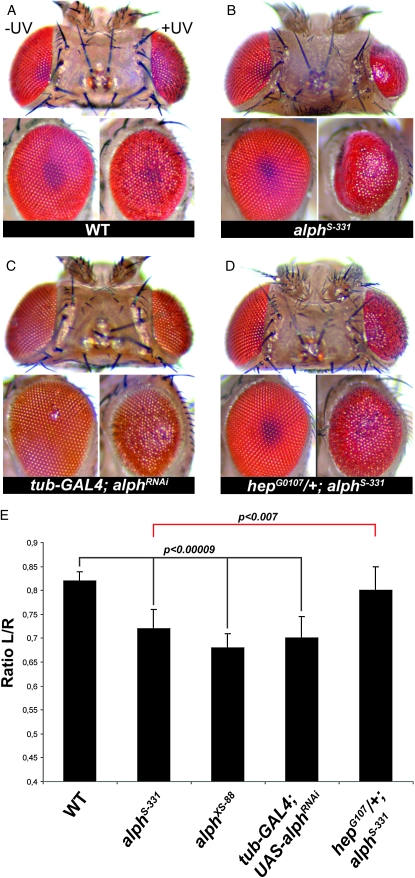
Depending on conditions and types of insult, stress-elicited JNK signaling can also have detrimental consequences as a result of apoptosis induction (Kanda and Miura 2004). A recent example identified in flies is the genotoxic stress caused by UVC irradiation during pupal eye development (Jassim et al. 2003; Luo et al. 2007). In the absence of photoreactivation-induced DNA repair, stimulation of JNK signaling by UVC at 24 hr after pupal formation promotes apoptosis, which leads to tissue loss in a dose-dependent manner. The end result is a reduction and roughening of the adult eye surface. This phenomenon is genetically amenable and a reduction of bsk gene dosage significantly reduces tissue loss following UV irradiation, whereas decreasing puc activity has the opposite effect (Luo et al. 2007). We thus used this event as an assay to verify whether alph activity was also involved in modulating JNK-dependent cell death. As shown in Figure 5, alphS-331 and alphXS-88 homozygous alleles clearly enhanced tissue loss following UVC irradiation of 24-hr pupal eyes (Figure 5, A, B, and E). A similar enhancement was observed in eyes expressing an alph RNA interference (RNAi) construct (Figure 5, C and E). The strength of the observed enhancements was comparable to the one previously reported for a puc heterozygous allele (Luo et al. 2007). Finally, consistent with the hypothesis that the alph mutant background has elevated JNK signaling, a hep heterozygous allele was found to dominantly suppress eye loss enhancement caused by alphS-331 (Figure 5, D and E). Taken together, these results provide compelling evidence that Alph modulates JNK signaling not only under normal developmental contexts, but also in response to stress.
Figure 5.—
alph inactivation increases UV-induced retinal apoptosis in a JNK-dependent manner. (A–D) Photomicrographs of adult fly eyes (top and side views) of the indicated genotypes that either were UV irradiated (+UV, left eye) or were not (−UV, right eye) during pupal development. (E) Tissue loss following UV irradiation is quantified by determining the ratio between the eye area of the irradiated eye (left, L) to the one of the non-irradiated eye (right, R). Means ± standard deviations for the L/R ratios of at least 10 heads/genotype are depicted as bar graphs. Statistical significance was confirmed using a Student's t-test. Canton-S flies were used as wild-type (WT) controls. Identical results were obtained when using isogenic w1118 flies as controls (not shown).
alph acts upstream of hep and lic:
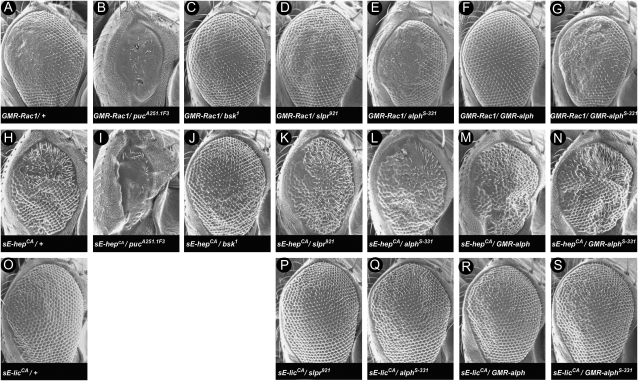
To ascertain the position of Alph relative to SAPK pathway components, we used the developing Drosophila eye as an assay system. Eye-specific expression of the small GTPase Rac1 using the pGMR driver provokes a Slpr/JNK-dependent rough eye phenotype that perturbs mainly the posterior part (Nolan et al. 1998; Stronach and Perrimon 2002; Figure 6A). The phenotype is enhanced by mutations in puc and suppressed by mutations in bsk or slpr (Stronach and Perrimon 2002; Figure 6: compare A to B, C, and D). The GMR-Rac1 rough eye phenotype is not fully penetrant and a proportion of these flies (∼20%) exhibit a relatively normal eye phenotype. However, introduction of alph alleles in the GMR-Rac1 background markedly enhanced eye roughness in a fully penetrant manner (Figure 6E; data not shown). Consistent with this genetic interaction, overexpression of wild-type Alph strongly suppressed GMR-Rac1, while a mutant version not only was devoid of suppressing activity, but also slightly enhanced the phenotype (Figure 6, compare A to F and G). Given that a wild-type version of Rac1 was used in this assay, we could not determine whether the effects of alph mutations or overexpression were taking place upstream or downstream of Rac1. However, these results provide additional evidence that alph negatively regulates SAPK signaling.
Figure 6.—
alph negative activity resides upstream of hep and lic. Shown are scanning electron micrographs of adult eyes of the following genotypes: (A) GMR-Rac1/+. (B) GMR-Rac1/+; pucA251.1F3/+. (C) GMR-Rac1/bsk1. (D) slpr921/+; GMR-Rac1/+. (E) GMR-Rac1/+; alphS-331/+. (F) GMR-Rac1/+ ; GMR-alph /+. (G) GMR-Rac1/+ ; GMR-alphS-331/+. (H) sE-hepCA /+. (I) sE-hepCA /+ ; pucA251.1F3/+. (J) sE-hepCA /bsk1. (K) slpr921/+; sE-hepCA /+. (L) sE-hepCA /+ ; alphS-331/+. (M) sE-hepCA /+ ; GMR-alph/+. (N) sE-hepCA /+; GMR-alphS-331/+. (O) sE-licCA /+. (P) slpr921/+; sE-licCA /+. (Q) sE-licCA/alphS-331. (R) sE-licCA/GMR-alph. (S) sE-licCA/GMR-alphS-331. Both sE-hepCA and sE-licCA rough eye phenotypes were not modified by overexpression of wild-type alph using the psE promoter/enhancer cassette (data not shown). Anterior is to the right.
To help refine the position of Alph with respect to specific SAPK components, we monitored the impact of alph alleles on the rough eye phenotype produced by overexpressing constitutively active hep or lic transgenes. To generate hep and lic gain-of-function alleles, we changed their activation loop Ser/Thr residues, normally phosphorylated by SAPKKKs, to phospho-mimetic residues, thereby making these kinases insensitive to upstream regulation. Expression of hepCA in the developing eye produces a phenotype sensitive to the dose of downstream components, such as bsk and puc (Figure 6: compare H to I and J), but is not affected by mutations in upstream components such as slpr (Figure 6: compare H to K). In contrast to the Rac1 eye phenotype, alph loss-of-function alleles did not modify the hepCA eye phenotype (Figure 6: compare H to L). Consistent with these results, alph overexpression also did not alter this phenotype (Figure 6: compare H to M). Therefore, these findings suggest that alph acts at a step upstream of hep. We also obtained a rough eye phenotype by overexpressing licCA during eye development (Figure 6O). As for hepCA, neither alph mutations nor alph overexpression altered this rough eye phenotype (Figures 6: compare O to Q and R). These data therefore suggest that alph activity is required at a step upstream of, or parallel to, lic.
Alph antagonizes SAPKKK-induced activation of Bsk in S2 cells:
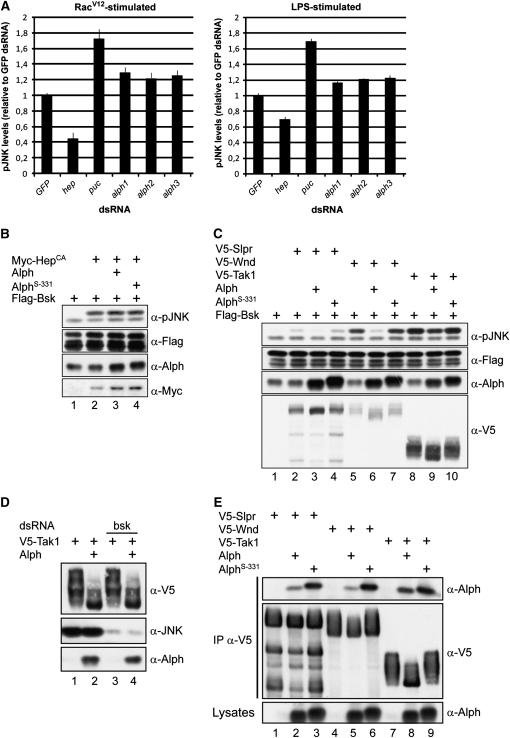
Using a quantitative immunofluorescence-based assay, we recently completed a genomewide RNAi screen in S2 cells to look for new components of the Ras/MAPK pathway (D. Ashton-Beaucage and M. Therrien, unpublished results). As expected from our previous work, an alph dsRNA was identified and confirmed as a hit that significantly enhanced RasV12-induced MAPK activation (data not shown). Using an analogous strategy, we verified whether alph depletion by RNAi could also enhance Rac1V12-induced Bsk activation. To accomplish this, we generated a stable S2 cell line that expresses Rac1V12 in a copper-inducible manner. Peak phospho-Bsk (pBsk) levels were found at 4 hr post-copper induction (not shown). Consistent with the specificity of the assay, we found that hep and puc dsRNAs, respectively, decreased and increased pBsk levels (Figure 7A). Under the same conditions, we found that three nonoverlapping alph dsRNAs behaved like puc dsRNA and, albeit modestly, significantly enhanced Rac1V12-induced pBsk levels. To verify whether this event could also be detected in conditions under which Rac1 is not overexpressed, cells were stimulated with lipopolysaccharides (LPS), a potent inducer of SAPK signaling (Park et al. 2004). Again, significant enhancement of the phospho-JNK (pJNK) level compared to control GFP dsRNA was observed upon depletion of endogenous Alph. Therefore, in agreement with the genetic results presented above, these findings provide biochemical evidence that endogenous Alph negatively regulates JNK signaling at a step upstream of Bsk.
Figure 7.—
Alph negatively regulates the JNK pathway in S2 cells. (A) Means and standard deviations of fluorescence intensities of Rac1V12- or LPS-induced pJNK levels from S2 cells treated with the indicated dsRNAs. JNK pathway activation was monitored by immunofluorescence using an anti-pJNK antibody recognizing dually phosphorylated Bsk in its activation segment. Signals were normalized to GFP dsRNA used as a negative control. The hep and puc dsRNAs were used as positive controls. Three nonoverlapping alph dsRNAs were tested and found to similarly enhance pJNK levels. Three separate experiments were conducted for each dsRNA. Statistical significance was confirmed using a Student's t-test (P < 0.001). (B–E) S2 cells were transfected with the indicated plasmid combinations. Alph proteins overexpressed in S2 cells do not contain an artificial epitope tag. Their levels were measured using anti-Alph polyclonal antibodies, which also detect endogenous Alph. As previously reported (Baril and Therrien 2006) and shown here, the AlphS-331 proteins migrate slightly more slowly than the wild-type variant. A total of 100 ng of the alph-expressing construct was used in B and C, while 500 ng was used in D and E. Lysates were directly probed with the antibodies indicated at the right to monitor protein or Bsk phosphorylation levels. In D, bsk dsRNA was added to the cells 24 hr prior to transfection. In E, the SAPKKKs were independently immunoprecipitated (IP) from NP-40 cell lysates using an anti-V5 (α-V5) antibody. AlphS-331 has a G120E change. Higher SAPKKK-binding activity was also observed for AlphXS-88 and AlphS-355 (not shown), which, respectively, have a D193V and a G173S change (Baril and Therrien 2006).
To further delineate the position at which Alph biochemical activity is required in the JNK pathway, we used a complementary approach and asked whether Alph overexpression could modulate pBsk levels induced by HepCA. Again, consistent with the genetic data, overexpression of Alph (or the S-331 mutant version) did not alter pBsk levels induced by HepCA (Figure 7B, lanes 3 and 4), thus suggesting that Alph acts upstream of Hep. Similar results were obtained when the phospho-p38a level induced by a constitutively active Licorne construct was assayed (data not shown).
Next, given that alph genetically interacts with slpr, we tested whether Alph overexpression could modulate Slpr-induced Bsk activation. Overexpression of Slpr in S2 cells is sufficient to induce its kinase activity, which in turn leads to Bsk activation (Figure 7C, lane 2). Alph co-expression decreased the ability of Slpr to induce Bsk phosphorylation, which also reduced the mobility shift of Slpr itself, whereas the S-331 mutant version was inactive (Figure 7C: compare lanes 2, 3, and 4). Thus, it appears that Alph phosphatase activity can reverse Slpr function. Previous studies conducted on PP2C phosphatases related to Alph revealed their ability to specifically dephosphorylate Tak1, which in turn inactivates IL-1-induced JNK signaling (Hanada et al. 2001; Li et al. 2003). We thus verified whether Alph could also antagonize the activity of Drosophila Tak1. We also tested the Drosophila DLK homolog, Wnd, which, like Tak1, is closely related to Slpr (Niedner et al. 2006). As shown in Figure 7C, Alph was very effective at inhibiting Wnd-mediated Bsk activation (lanes 5 and 6). It also inhibited Tak1 activity, but to a lesser degree (lanes 8 and 9). Taken together, these results support the genetic data that position alph at a step upstream of Hep and would be compatible with a model whereby Alph dephosphorylates critical phosphorylation sites on SAPKKKs.
Interestingly, Alph overexpression reduced the mobility shift observed with the three SAPKKK tested above (Figure 7C). These shifts appear to be due to phosphorylation as in vitro incubation of any of the three SAPKKKs with alkaline phosphatase eliminated their presence (data not shown). They do not appear to be caused by a MAPK-mediated feedback mechanism as shown to take place on the classical ERK module (Dougherty et al. 2005) since elimination of endogenous Bsk by RNAi did not prevent their occurrence (Figure 7D and data not shown). This result also suggests that the ability of Alph to reduce these shifts is not merely caused by downregulating SAPK activity, but that Alph directly dephosphorylates SAPKKKs. To provide support for this latter possibility, we examined whether Alph could associate with Slpr, Wnd, and Tak1. Using a co-immunoprecipitation assay, we found that Alph indeed associates with the three MAPKKK, although the interaction systematically appeared stronger with Tak1 (Figure 7E: compare lanes 2, 5, and 8). Interestingly, we also found that three point mutations encoded by our alph alleles (Baril and Therrien 2006) interacted more strongly with the three SAPKKKs than did wild-type Alph (Figure 7E and data not shown), which may reflect the fact that proper enzyme–substrate interaction has occurred, but failure to dephosphorylate particular site(s) prevented efficient substrate release. Taken together, these data strongly suggest that Alph regulates SAPK signaling at the SAPKKK level.
DISCUSSION
In this study we report that inactivation of the alph locus opposes the effects of mutations in positive components of SAPK-signaling pathways during normal development or under stress conditions. Epistatic analysis positioned its activity at a step upstream of the SAPKKs Hep and Lic. Consistent with these results, we found that Alph depletion increases pJNK levels upon stimulation by Rac1V12 or LPS, whereas its overexpression blocks JNK activation by SAPKKKs such as Slpr, Wnd, and Tak1, but not by activated SAPKK. In further support for a regulatory role for Alph at the SAPKKK level, we found that it associated with Slpr, Wnd, and Tak1 and reduced their phosphorylation status. Together, these findings identify Alph as a novel and general negative regulator of SAPK signaling in Drosophila that regulates the activity of multiple SAPKKKs. How exactly Alph modulates the action of SAPKKKs remains an open question. One obvious possibility is that Alph directly dephosphorylates specific phospho-sites on the SAPKKKs that are required for activity. Alternatively, Alph may indirectly modulate SAPKKKs by acting on an intermediate regulatory protein (e.g., another phosphatase) or through its role in a parallel pathway that negatively influences SAPKKK activity.
Intriguingly, depending on the assay performed, we noted that the alph alleles used in this study varied in their relative strength. While this remains to be demonstrated, it possibly reflects the fact that the assays depend on different effector proteins and that the distinct alph alleles affect these proteins differently. Even though the alph alleles that we used are loss-of-function alleles (Baril and Therrien 2006), they are caused by specific point mutations that may render the mutant protein incapable of dephosphorylating certain substrates, but at the same time could still dephosphorylate others, depending on the allele used. Not mutually exclusive, these point mutations may also specifically trap certain substrates and thereby prevent their dephosphorylation. Depending on the point mutation, the trapping affinity and specificity may differ. The finding that the Alph G120E mutation (derived from the alphS-331 allele) interacts significantly more strongly with Slpr and Wnd (Figure 7E) than wild-type Alph is consistent with this trapping model.
We recently showed that Alph also negatively regulates Ras/MAPK signaling by acting at a step downstream of the small GTPase Ras (Baril and Therrien 2006). However, we could not rule out the possibility that Alph influenced Ras/MAPK signaling through a parallel pathway. Cross talk between MAPK pathways has been reported in multiple studies (Junttila et al. 2007) and thus it is possible that the apparent ability of Alph to modulate Erk/MAPK and SAPK pathways in flies is due to cross talk between these pathways. For example, both pathways may share a common upstream activator that is a substrate for Alph. SAPKKKs are good candidates as they require phosphorylation events for full catalytic activation and have been implicated in cross talk between SAPK and ERK signaling. For example, Mlk3 (a Slpr homolog) was shown to participate in RAF activation in various cell lines through the formation of a ternary complex involving B- and C-Raf isoforms (Chadee and Kyriakis 2004; Chadee et al. 2006). Similarly, Mekk1 was reported to participate in ERK signaling (Yujiri et al. 1998) by apparently scaffolding RAF, MEK, and ERK proteins (Karandikar et al. 2000). These observations would thus be compatible with a model whereby Alph influences Ras/MAPK signaling through its regulatory role on SAPKKKs or vice versa. However, there is currently no genetic evidence to suggest that a SAPKKK positively modulates Ras/ERK signaling in Drosophila. Alternatively, Alph may independently act on substrates dedicated to each pathway.
Interestingly, we found that Slpr, Wnd, and Tak1 overexpression in S2 cells induced MAPK activation, whereas overexpression of activated Drosophila RAF did not affect Bsk activation (data not shown). Moreover, rl/mapk loss-of-function and gain-of-function mutations do not alter slprBS06 lethality, nor does Ras/MAPK-signaling affect GMR-Rac1 eye phenotype (Figure 1 and data not shown). Thus, these data suggest that increased Ras/MAPK signaling observed in alph mutant backgrounds is not responsible for the regulatory role that Alph plays in SAPK signaling, although it cannot be ruled out that the effect of Alph on the Ras/MAPK pathway involves SAPK-signaling components.
The data presented in this study suggest that Alph antagonizes SAPK signaling in different tissues at diverse developmental stages and under various stress conditions. This wide-ranging influence is consistent with its ubiquitous expression pattern (Baril and Therrien 2006). Yet the effect of Alph appears relatively modest compared, for example, to Puckered. Evidently, one possibility to account for this moderate impact could be a functional redundancy with other phosphatases that compensate for Alph activity. More work will be required to verify this issue. Similarly, it will be interesting to determine whether Alph acts prior to signal activation to maintain SAPK phosphorylation cascades below a threshold to maximize signal inducibility or whether it acts after signal transmission to shut down signaling.
Given the role played by SAPK signaling in innate immunity, it would not be surprising that Alph also modulated SAPK-mediated events in response to pathogens. The effect of Alph depletion on LPS-induced pJNK levels supports this hypothesis (Figure 7A). We actually started addressing this possibility using oligonucleotide-based microarrays to monitor global gene expression profiles in alph mutant flies compared to wild-type flies (fold change >1.5 or <0.67 as a threshold, with a P-value <0.01; data not shown). This preliminary work identified 27 genes belonging to the GO term category “response to stimulus” (GO: 0050896) as the largest one to be significantly enriched (data not shown). This category includes mainly genes related to innate immunity and stress response. Seventy-five percent of these were actually confirmed by quantitative PCR (data not shown). These findings suggest that Alph also influences immune-response gene expression. Given that innate immunity is in part regulated by Tak1-dependent activity (Vidal et al. 2001), we also verified whether alph mutations could suppress the sensitivity of Tak1 null mutant flies to gram-negative bacterial infection. However, elimination of Alph activity in this assay had no impact (M. Lefrançois and M. Therrien, unpublished data). This negative result remains inconclusive as it is possible that the upregulation of immune genes in alph mutant backgrounds was too modest to increase resistance to gram-negative bacterial infection. Alternatively, if Alph directly acts on Tak1, eliminating Tak1 may not be suppressible by decreasing Alph dosage as there is no Tak1 activity to act upon. Finally, redundancy with other PP2C phosphatases for this particular biological event could explain the lack of genetic interaction.
Phosphatases of the PP2C family are well-known regulators of stress signaling in yeasts and plants (Schweighofer et al. 2004). Overexpression approaches have also suggested the pervasive roles played by PP2Cs in regulating stress signaling in metazoan cells, but a formal demonstration of their involvement has yet to be provided. Our work therefore represents the first genetic demonstration that a phosphatase of the PP2C family regulates stress signaling in metazoans.
Acknowledgments
We are grateful to T. Ip, J. Settleman, the Bloomington Stock Center, and the Drosophila Genome Resource Center for providing various reagents. D.A.B. is supported by a studentship from the Canadian Institute for Health Research. M.T. is a recipient of a Canada Research Chair (Tier II). This work was supported by grant no. HD045836 from the National Institutes of Health to B.S. and by a grant from the National Cancer Institute of Canada with funds from the Canadian Research Society to M.T. The Institute for Research in Immunology and Cancer is supported in part by the Canadian Center of Excellence in Commercialization and Research, by the Canada Foundation for Innovation, and by the Fonds de Recherche en Santé du Québec.
References
- Adachi-Yamada, T., K. Fujimura-Kamada, Y. Nishida and K. Matsumoto, 1999. Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature 400 166–169. [DOI] [PubMed] [Google Scholar]
- Arking, R., S. Buck, A. Berrios, S. Dwyer and G. T. Baker, III, 1991. Elevated paraquat resistance can be used as a bioassay for longevity in a genetically based long-lived strain of Drosophila. Dev. Genet. 12 362–370. [DOI] [PubMed] [Google Scholar]
- Baril, C., and M. Therrien, 2006. Alphabet, a Ser/Thr phosphatase of the protein phosphatase 2C family, negatively regulates RAS/MAPK signaling in Drosophila. Dev. Biol. 294 232–245. [DOI] [PubMed] [Google Scholar]
- Chadee, D. N., and J. M. Kyriakis, 2004. MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat. Cell Biol. 6 770–776. [DOI] [PubMed] [Google Scholar]
- Chadee, D. N., D. Xu, G. Hung, A. Andalibi, D. J. Lim et al., 2006. Mixed-lineage kinase 3 regulates B-Raf through maintenance of the B-Raf/Raf-1 complex and inhibition by the NF2 tumor suppressor protein. Proc. Natl. Acad. Sci. USA 103 4463–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, T. B., and N. Perrimon, 1996. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, C. A., Y. P. Wairkar, S. L. Johnson and A. DiAntonio, 2006. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron 51 57–69. [DOI] [PubMed] [Google Scholar]
- Craig, C. R., J. L. Fink, Y. Yagi, Y. T. Ip and R. L. Cagan, 2004. A Drosophila p38 orthologue is required for environmental stress responses. EMBO Rep. 5 1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, R. J., 2000. Signal transduction by the JNK group of MAP kinases. Cell 103 239–252. [DOI] [PubMed] [Google Scholar]
- Delaney, J. R., S. Stoven, H. Uvell, K. V. Anderson, Y. Engstrom et al., 2006. Cooperative control of Drosophila immune responses by the JNK and NF-kappaB signaling pathways. EMBO J. 25 3068–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran, D. N., K. Kashef, C. M. Lee, H. Xu and E. P. Reddy, 2007. Scaffold proteins of MAP-kinase modules. Oncogene 26 3185–3202. [DOI] [PubMed] [Google Scholar]
- Dickson, B., F. Sprenger, D. Morrison and E. Hafen, 1992. Raf functions downstream of Ras1 in the Sevenless signal transduction pathway. Nature 360 600–603. [DOI] [PubMed] [Google Scholar]
- Dougherty, M. K., J. Müller, D. A. Ritt, M. Zhou, X. Z. Zhou et al., 2005. Regulation of Raf-1 by direct feedback phosphorylation. Mol. Cell 17 215–224. [DOI] [PubMed] [Google Scholar]
- Glise, B., H. Bourbon and S. Noselli, 1995. hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell 83 451–461. [DOI] [PubMed] [Google Scholar]
- Hanada, M., J. Ninomiya-Tsuji, K. Komaki, M. Ohnishi, K. Katsura et al., 2001. Regulation of the TAK1 signaling pathway by protein phosphatase 2C. J. Biol. Chem. 276 5753–5759. [DOI] [PubMed] [Google Scholar]
- Harrington, D. P., and T. R. Fleming, 1982. A class of rank test procedures for censored survival data. Biometrika 69 553–566. [Google Scholar]
- Hay, B. A., T. Wolff and G. M. Rubin, 1994. Expression of baculovirus P35 prevents cell death in Drosophila. Development 120 2121–2129. [DOI] [PubMed] [Google Scholar]
- Inoue, H., M. Tateno, K. Fujimura-Kamada, G. Takaesu, T. Adachi-Yamada et al., 2001. A Drosophila MAPKKK, D-MEKK1, mediates stress responses through activation of p38 MAPK. EMBO J. 20 5421–5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto, A., S. Woolner and P. Martin, 2002. Dynamic analysis of dorsal closure in Drosophila: from genetics to cell biology. Dev. Cell 3 9–19. [DOI] [PubMed] [Google Scholar]
- Jassim, O. W., J. L. Fink and R. L. Cagan, 2003. Dmp53 protects the Drosophila retina during a developmentally regulated DNA damage response. EMBO J. 22 5622–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila, M. R., S. P. Li and J. Westermarck, 2007. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 22 954–965. [DOI] [PubMed] [Google Scholar]
- Kanda, H., and M. Miura, 2004. Regulatory roles of JNK in programmed cell death. J. Biochem. 136 1–6. [DOI] [PubMed] [Google Scholar]
- Karandikar, M., S. Xu and M. H. Cobb, 2000. MEKK1 binds raf-1 and the ERK2 cascade components. J. Biol. Chem. 275 40120–40127. [DOI] [PubMed] [Google Scholar]
- Kyriakis, J. M., P. Banerjee, E. Nikolakaki, T. Dai, E. A. Rubie et al., 1994. The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369 156–160. [DOI] [PubMed] [Google Scholar]
- Laine, A., and Z. Ronai, 2005. Ubiquitin chains in the ladder of MAPK signaling. Sci. STKE 2005 re5. [DOI] [PubMed] [Google Scholar]
- Lammers, T., and S. Lavi, 2008. Role of type 2C protein phosphatases in growth regulation and in cellular stress signaling. Crit. Rev. Biochem. Mol. Biol. 42 437–461. [DOI] [PubMed] [Google Scholar]
- Lee, Y. S., and R. W. Carthew, 2003. Making a better RNAi vector for Drosophila: use of intron spacers. Methods 30 322–329. [DOI] [PubMed] [Google Scholar]
- Li, M. G., K. Katsura, H. Nomiyama, K. Komaki, J. Ninomiya-Tsuji et al., 2003. Regulation of the interleukin-1-induced signaling pathways by a novel member of the protein phosphatase 2C family (PP2Cepsilon). J. Biol. Chem. 278 12013–12021. [DOI] [PubMed] [Google Scholar]
- Luo, X., O. Puig, J. Hyun, D. Bohmann and H. Jasper, 2007. Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation. EMBO J. 26 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, A. M., and R. J. Davis, 2003. Targeting JNK for therapeutic benefit: From junk to gold? Nat. Rev. Drug Discov. 2 554–565. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco, E., A. Gampel, J. Ring, K. Virdee, N. Kirov et al., 1998. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 12 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedner, R. H., O. V. Buzko, N. M. Haste, A. Taylor, M. Gribskov et al., 2006. Protein kinase resource: an integrated environment for phosphorylation research. Proteins 63 78–86. [DOI] [PubMed] [Google Scholar]
- Nolan, K. M., K. Barrett, Y. Lu, K. Q. Hu, S. Vincent et al., 1998. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 12 3337–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noselli, S., and F. Agnes, 1999. Roles of the JNK signaling pathway in Drosophila morphogenesis. Curr. Opin. Genet. Dev. 9 466–472. [DOI] [PubMed] [Google Scholar]
- Owens, D. M., and S. M. Keyse, 2007. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26 3203–3213. [DOI] [PubMed] [Google Scholar]
- Park, J. M., H. Brady, M. G. Ruocco, H. Sun, D. Williams et al., 2004. Targeting of TAK1 by the NF-kappa B protein Relish regulates the JNK-mediated immune response in Drosophila. Genes Dev. 18 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polaski, S., L. Whitney, B. W. Barker and B. Stronach, 2006. Genetic analysis of slipper/mixed lineage kinase reveals requirements in multiple Jun-N-terminal kinase-dependent morphogenetic events during Drosophila development. Genetics 174 719–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, F., G. Laberge, M. Douziech, D. Ferland-McCollough and M. Therrien, 2002. KSR is a scaffold required for activation of the ERK/MAPK module. Genes Dev. 16 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, G. M., and A. C. Spradling, 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218 348–353. [DOI] [PubMed] [Google Scholar]
- Saito, H., and K. Tatebayashi, 2004. Regulation of the osmoregulatory HOG MAPK cascade in yeast. J. Biochem. 136 267–272. [DOI] [PubMed] [Google Scholar]
- Schweighofer, A., H. Hirt and I. Meskiene, 2004. Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci. 9 236–243. [DOI] [PubMed] [Google Scholar]
- Stronach, B, 2005. Dissecting JNK signaling, one KKKinase at a time. Dev. Dyn. 232 575–584. [DOI] [PubMed] [Google Scholar]
- Stronach, B. E., and N. Perrimon, 1999. Stress signaling in Drosophila. Oncogene 18 6172–6182. [DOI] [PubMed] [Google Scholar]
- Stronach, B., and N. Perrimon, 2002. Activation of the JNK pathway during dorsal closure in Drosophila requires the mixed lineage kinase, slipper. Genes Dev. 16 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzanne, M., K. Irie, B. Glise, F. Agnes, E. Mori et al., 1999. The Drosophila p38 MAPK pathway is required during oogenesis for egg asymmetric development. Genes Dev. 13 1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrien, M., A. M. Wong and G. M. Rubin, 1998. CNK, a RAF-binding multidomain protein required for RAS signaling. Cell 95 343–353. [DOI] [PubMed] [Google Scholar]
- Vidal, S., R. S. Khush, F. Leulier, P. Tzou, M. Nakamura et al., 2001. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-kappaB-dependent innate immune responses. Genes Dev. 15 1900–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. C., D. Bohmann and H. Jasper, 2003. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev. Cell 5 811–816. [DOI] [PubMed] [Google Scholar]
- Wassarman, D. A., N. Aoyagi, L. A. Pile and E. M. Schlag, 2000. TAF250 is required for multiple developmental events in Drosophila. Proc. Natl. Acad. Sci. USA 97 1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yujiri, T., S. Sather, G. R. Fanger and G. L. Johnson, 1998. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science 282 1911–1914. [DOI] [PubMed] [Google Scholar]
- Zarubin, T., and J. Han, 2005. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15 11–18. [DOI] [PubMed] [Google Scholar]
- Zeitlinger, J., and D. Bohmann, 1999. Thorax closure in Drosophila: involvement of Fos and the JNK pathway. Development 126 3947–3956. [DOI] [PubMed] [Google Scholar]