The X-ray crystal structure of the Trx-fold domain of hPDCL2 was solved at 2.70 Å resolution and resembled the Trx-fold domain of rat phosducin.
Keywords: phosducin-like protein 2, phosducin family, thioredoxin fold
Abstract
Human phosducin-like protein 2 (hPDCL2) has been identified as belonging to subgroup II of the phosducin (Pdc) family. The members of this family share an N-terminal helix domain and a C-terminal thioredoxin-fold (Trx-fold) domain. The X-ray crystal structure of the Trx-fold domain of hPDCL2 was solved at 2.70 Å resolution and resembled the Trx-fold domain of rat phosducin. Comparative structural analysis revealed the structural basis of their putative functional divergence.
1. Introduction
The thioredoxin-fold (Trx-fold) protein-structure classification (SCOP; http://scop.mrc-lmb.cam.ac.uk; Murzin et al., 1995 ▶) was characterized based on the structure of Escherichia coli Trx1 (Holmgren et al., 1975 ▶). The classic Trx fold consists of a single domain with a central five-stranded mixed β-sheet flanked by two α-helices on each side. The secondary-structure elements are arranged in the order βαβαβαββα, with β4 antiparallel to the other strands. A set of protein families containing this fold have been identified in SCOP, including Trx, glutaredoxin (Grx; Sun et al., 1998 ▶), protein disulfide isomerase (PDI; Tian et al., 2006 ▶), glutaredoxin S-transferase (Reinemer et al., 1991 ▶), calsequestrin (Wang et al., 1998 ▶) and phosducin (Pdc; Gaudet et al., 1996 ▶).
Phosducin is a 28 kDa cytosolic protein which was first found in retinal extracts (Lolley et al., 1977 ▶). It was originally identified as a modulator of heterotrimeric G-protein signalling in the retina (Schroder & Lohse, 1996 ▶). Subsequently, a series of Pdc-like proteins (PhLPs) were identified in vertebrates and lower eukaryotes that constitute the Pdc family (Blaauw et al., 2003 ▶). The members of this family share an N-terminal helix domain and a C-terminal Trx-fold domain (Gaudet et al., 1996 ▶; Stirling et al., 2007 ▶) and can be divided into three subgroups (Blaauw et al., 2003 ▶). Subgroup I includes Pdc and PhLP1, subgroup II contains PhLP2 and subgroup III contains PhLP3 (Blaauw et al., 2003 ▶; Willardson & Howlett, 2007 ▶). The proteins of subgroup I are the best characterized. Crystal structures of the Pdc–Gtβγ complex (Gaudet et al., 1996 ▶, 1999 ▶; Loew et al., 1998 ▶) showed that Pdc could block G-protein signalling by disrupting the interaction between Gtα and Gtβγ. PhLP1 contains an 11-residue sequence corresponding to the conserved Gtβγ-binding motif of Pdc in the N-terminal domain (Miles et al., 1993 ▶; Blaauw et al., 2003 ▶), which has been reported to play a similar role in regulating other G-protein βγ subunits (Loew et al., 1998 ▶) and to bind and regulate chaperonin-containing Tcp1 (CCT; Martin-Benito et al., 2004 ▶). Proteins in subgroup III have been identified as modulators of CCT and to play roles in actin and tubulin biogenesis in yeast (McLaughlin et al., 2002 ▶) and microtubule function in Caenorhabditis elegans (Stirling et al., 2007 ▶). Proteins in subgroup II were reported to be essential in Dictyostelium discoideum and yeast (Blaauw et al., 2003 ▶; Stirling et al., 2007 ▶). Moreover, recent findings have indicated that most members of the Pdc family act as cochaperones with CCT (Willardson & Howlett, 2007 ▶).
In humans, five family members, hPdc, hPhLP1, hPDCL2, hPDCL3 and hPhLP3, have been recognized to date. We determined the crystal structure of the Trx-fold domain of hPDCL2, a member of subgroup II, at 2.70 Å resolution. To date, its detailed function has not been characterized.
2. Materials and methods
2.1. Cloning and protein expression
The gene encoding the Trx-fold domain (residues 88–209) of hPDCL2 (hPDCL2; GenBank Accession No. BC034431) was amplified by PCR from the human cDNA library (Proteintech Group Inc.). The amplified DNA was cloned into a pET29a-derived expression vector between NcoI and NotI restriction sites, which were incorporated into the sequences of the sense and antisense primers 5′-GCCCCATGGCAAATTTGGAGAATTAAG-3′ and 5′-GCCGCGGCCGCTTAGTTTTCTTCCAAATC-3′, respectively (shown in bold). The resulting construct was verified by sequencing and contained an MHHHHHHG tag at the N-terminus of the protein as a result of the cloning strategy. Escherichia coli BL21 (DE3) strain cells were transformed with this construct and cultured in 600 ml LB medium containing 10 µg ml−1 kanamycin at 310 K until the OD600 reached 0.6. Protein expression was induced by adding isopropyl β-d-1-thiogalactopyranoside (IPTG) to a final concentration of 0.2 mM for 20 h at 291 K. Cells were harvested by centrifugation at 7330g for 10 min and resuspended in cold lysis buffer (20 mM Tris–HCl pH 8.0, 50 mM NaCl). The suspension was sonicated and clarified by centrifugation at 29 000g for 25 min at 277 K.
2.2. Protein purification
The supernatant was loaded onto 2 ml Ni–NTA affinity resin (Qiagen) pre-equilibrated with lysis buffer at 289 K. After washing with 20 ml lysis buffer plus 10 mM imidazole, the target protein was eluted with lysis buffer containing 250 mM imidazole and then further purified on a HiLoad 16/60 Superdex 75 column (Amersham Biosciences) equilibrated with lysis buffer at 289 K. The fractions containing the target protein were verified using SDS–PAGE.
2.3. Crystallization and X-ray data collection
Purified protein solution (20 mM Tris–HCl pH 8.0, 50 mM NaCl) was concentrated to 8 mg ml−1 by ultrafiltration (Millipore Amicon) and treated with 4 mM dithiothreitol (DTT) for 1 h. Crystallization conditions were screened by the sitting-drop vapour-diffusion method at 291 K using Crystal Screens I and II (Hampton Research). The drops consisted of 1 µl protein solution and 1 µl reservoir solution and were equilibrated against 100 µl reservoir solution using a 48-well sitting-drop plate (XtalQuest, Beijing). After optimization, crystals suitable for X-ray diffraction grew within 3 d in a condition consisting of 28%(w/v) PEG 8000, 0.2 M ammonium sulfate. Crystals were flash-frozen in liquid nitrogen using a cryoprotectant consisting of the reservoir solution plus 25% glycerol. Diffraction data were collected to 2.70 Å resolution at 100 K using an in-house Rigaku MM007 X-ray generator (λ = 1.5418 Å) with a MAR Research 345 detector at the School of Life Sciences, University of Science and Technology of China (USTC; Hefei, People’s Republic of China). The program HKL-2000 (Otwinowski & Minor, 1997 ▶) was used for data processing and scaling and data-collection statistics are shown in Table 1 ▶. The R merge value is relatively high owing to the diffraction anisotropy of the crystal. The crystal belongs to space group P31, with a Matthews coefficient of 2.79 Å3 Da−1 and a solvent content of 55.88% (Matthews, 1968 ▶), suggesting the presence of two molecules in an asymmetric unit.
Table 1. Data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| Data collection | |
| Resolution range () | 26.142.70 (2.802.70) |
| Space group | P31 |
| Temperature (K) | 100 |
| Wavelength () | 1.5418 |
| Unit-cell parameters | |
| a () | 44.59 |
| b () | 44.59 |
| c () | 141.97 |
| () | 90.00 |
| () | 90.00 |
| () | 120.00 |
| Unique reflections | 9164 (895) |
| Completeness (%) | 93.9 (88.6) |
| Redundancy | 2.7 (2.0) |
| Mean I/(I) | 10.22 (1.53) |
| R merge † | 0.175 (0.614) |
| Refinement | |
| Resolution range () | 18.882.70 |
| R cryst ‡ | 0.236 |
| R free ‡ | 0.276 |
| Matthews coefficient (3Da1) | 2.79 |
| Solvent content (%) | 55.88 |
| Mean temperature factor (2) | 42.30 |
| R.m.s.d. bond lengths () | 0.012 |
| R.m.s.d. bond angles () | 1.221 |
| Ramachandran statistics | |
| Residues in most favoured regions (%) | 90.1 |
| Residues in additional allowed regions (%) | 9.0 |
| Residues in generously allowed regions (%) | 0.9 |
| Residues in disallowed regions (%) | 0 |
R
merge = 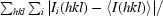
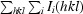 , where I(hkl) is the mean intensity of i reflections with intensities I
i(hkl) and common indices hkl.
, where I(hkl) is the mean intensity of i reflections with intensities I
i(hkl) and common indices hkl.
R
cryst and R
free = 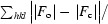
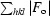 ; R
free was calculated for a 4.7% set of reflections excluded from refinement.
; R
free was calculated for a 4.7% set of reflections excluded from refinement.
2.4. Structure determination and refinement
Structure solution was achieved by molecular replacement using Phaser from the CCP4 suite (Collaborative Computational Project, Number 4, 1994 ▶) with the NMR structure of the Trx-like domain of mouse Pdc-like protein 2 (mPDCL2; PDB code 2dbc) as a search model. The programs Coot (Emsley & Cowtan, 2004 ▶) and REFMAC5 (Murshudov et al., 1997 ▶) were used to build and refine the final model, which consists of residues 88–205.
3. Result and discussion
3.1. Overall structure
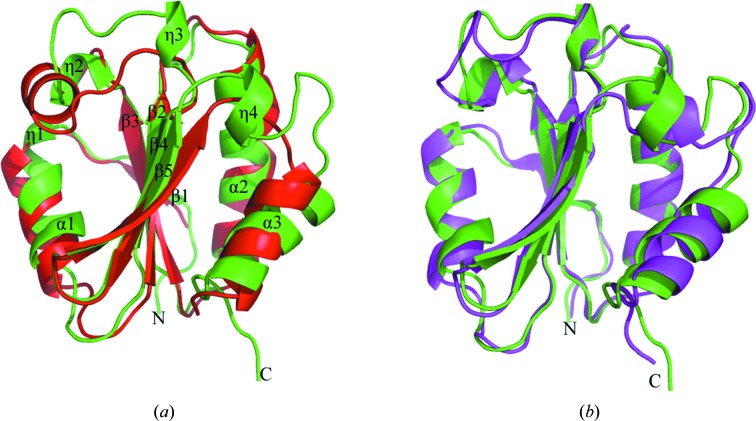
The Trx-fold domain of hPDCL2 adopts a canonical Trx fold comprising a five-stranded central β-sheet flanked by seven helices (Fig. 1 ▶). The secondary-structure elements are arranged in the order βηαβαβηηββηα, with β4 antiparallel to the other strands. The r.m.s.d. between the Cα atoms in this structure and the search model is 0.720 Å for 109 residues.
Figure 1.
Overall structure superposition of the hPDCL2 Trx-fold domain (green) with (a) hTrx1 (PDB code 1aiu; red) and (b) the Trx-fold domain of rPdc (PDB code 2trc; magenta). The secondary-structure elements are labelled sequentially in (a). The figures were prepared using PyMOL (DeLano, 2002 ▶).
3.2. Comparison of the hPDCL2 Trx-fold domain and human Trx1
Thioredoxin is a small redox-active protein with a conserved catalytic active site (-Trp-Cys-Gly-Pro-Cys-) that undergoes reversible oxidation–reduction of two thiol groups aided by Trx reductase. The Trx-fold domain of hPDCL2 has a very low sequence homology to the classic Trx fold (19% identity and 35% similarity to human Trx1) and only contains one of the two conserved Trx active-site cysteines (Fig. 2 ▶). It can be superimposed on hTrx1 with an r.m.s. deviation (r.m.s.d.) of 1.726 Å between the Cα atoms for 87 residues and is extended compared with the classic Trx fold (Fig. 1 ▶ a). Compared with the secondary-structure elements of hTrx1 (Fig. 2 ▶), helix α1 in the hPDCL2 Trx-fold domain is shorter and has an additional 310-helix η1 adjacent to its N-terminus; two 310-helices η2 and η3 with a loop between them substitute for helix α3 in hTrx1. Additionally, an extra 310-helix η4 was located between strand β5 and helix α3.
Figure 2.
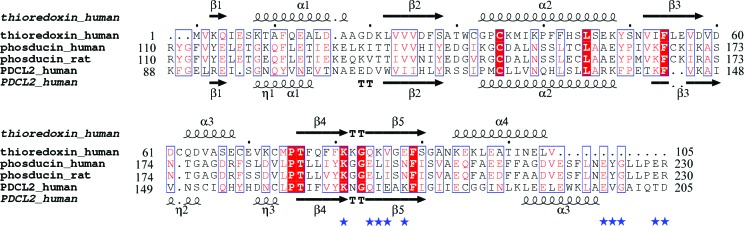
Structure-based sequence alignment. The sequences of hTrx1, hPdc, rPdc and hPDCL2 were obtained from the Swiss-Prot database (accession Nos. P10599, P20941, P20942 and Q8N4E4, respectively). The alignment region of each sequence is labelled at the N- and C-termini. The secondary structure of hTrx1 is shown at the top and that of the hPDCL2 Trx-fold domain is shown at the bottom. Alignments were performed using the programs MULTALIN (Corpet, 1988 ▶) and ESPript (Gouet et al., 2003 ▶). Conserved residues are marked in red. Conserved residues in the Trx-fold domain of rPdc that contact G protein are labelled with blue stars.
3.3. Comparison of Trx-fold domains between hPDCL2 and rPdc
The Trx-fold domain of hPDCL2 highly resembles that of rat Pdc based on a structure-homology search using the program DALI (Holm et al., 2008 ▶). The sequence identity between the Trx-fold domains of hPDCL2 and rPdc is 24% and the corresponding r.m.s.d. between the Cα atoms is 1.194 Å for 97 residues (Figs. 1 ▶ b and 2 ▶).
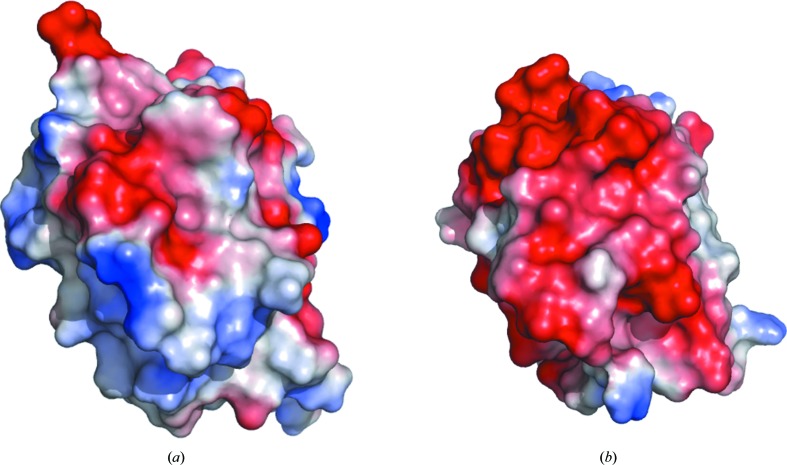
Although the Trx-fold domain of hPDCL2 is very similar to that of rPdc in overall structure, it contains some variations. Ten conserved residues in the rPdc Trx-fold domain were identified to bind G protein (Gaudet et al., 1996 ▶). However, the Trx-fold domain of hPDCL2 only contains three of these ten conserved residues (Fig. 2 ▶), which indicates that this domain might not possess the ability to interact with G protein. Furthermore, the electrostatic potential of the hPDCL2 Trx-fold domain is greatly different from that of rPdc (Fig. 3 ▶). The Trx-fold domain of rPdc is highly negatively charged and the upper surface interacts with the β-subunit of G protein, whereas the opposite surface contributes to Gβγ translocation from the membrane (Gaudet et al., 1996 ▶). The Trx-fold domain of hPDCL2 is less negatively charged than that of rPdc, especially at the surface corresponding to the membrane-interacting surface of rPdc.
Figure 3.
Electrostatic potential representations of the Trx-fold domains of (a) hPDCL2 and (b) rPdc. Figures were prepared using PyMOL (DeLano, 2002 ▶).
In conclusion, the Trx-fold domain of hPDCL2 adopts a monothiol Trx-fold like that of rPdc in three-dimensional structure, but it lacks most of the conserved G-protein-interacting residues and exhibits a clear difference in electrostatic potential when compared with rPdc. Therefore, it could be hypothesized that hPDCL2 does not act as a modulator of G-protein signalling like rPdc.
Supplementary Material
PDB reference: thioredoxin-fold domain of human phosducin-like protein 2, 3evi, r3evisf
Acknowledgments
This research was supported by projects 2006AA02A318, 2006CB910202 and 2006CB806501 from the Ministry of Science and Technology of China, grants 30470366 and 30670461 from the Chinese National Natural Science Foundation, the 100-Talent Project of the Chinese Academy of Science and a start-up fund from USTC.
References
- Blaauw, M., Knol, J. C., Kortholt, A., Roelofs, J., Ruchira, Postma, M., Visser, A. J. & van Haastert, P. J. (2003). EMBO J. 22, 5047–5057. [DOI] [PMC free article] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Corpet, F. (1988). Nucleic Acids Res. 16, 10881–10890. [DOI] [PMC free article] [PubMed]
- DeLano, W. L. (2002). The Pymol Molecular Graphics System. http://www.pymol.org.
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Gaudet, R., Bohm, A. & Sigler, P. B. (1996). Cell, 87, 577–588. [DOI] [PubMed]
- Gaudet, R., Savage, J. R., McLaughlin, J. N., Willardson, B. M. & Sigler, P. B. (1999). Mol. Cell, 3, 649–660. [DOI] [PubMed]
- Gouet, P., Robert, X. & Courcelle, E. (2003). Nucleic Acids Res. 31, 3320–3323. [DOI] [PMC free article] [PubMed]
- Holm, L., Kaariainen, S., Rosenstrom, P. & Schenkel, A. (2008). Bioinformatics, 10.1093/bioinformatics/btn507. [DOI] [PMC free article] [PubMed]
- Holmgren, A., Soderberg, B. O., Eklund, H. & Brändén, C. I. (1975). Proc. Natl Acad. Sci. USA, 72, 2305–2309. [DOI] [PMC free article] [PubMed]
- Loew, A., Ho, Y. K., Blundell, T. & Bax, B. (1998). Structure, 6, 1007–1019. [DOI] [PubMed]
- Lolley, R. N., Brown, B. M. & Farber, D. B. (1977). Biochem. Biophys. Res. Commun. 78, 572–578. [DOI] [PubMed]
- Martin-Benito, J., Bertrand, S., Hu, T., Ludtke, P. J., McLaughlin, J. N., Willardson, B. M., Carrascosa, J. L. & Valpuesta, J. M. (2004). Proc. Natl Acad. Sci. USA, 101, 17410–17415. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- McLaughlin, J. N., Thulin, C. D., Hart, S. J., Resing, K. A., Ahn, N. G. & Willardson, B. M. (2002). Proc. Natl Acad. Sci. USA, 99, 7962–7967. [DOI] [PMC free article] [PubMed]
- Miles, M. F., Barhite, S., Sganga, M. & Elliott, M. (1993). Proc. Natl Acad. Sci. USA, 90, 10831–10835. [DOI] [PMC free article] [PubMed]
- Murshudov, G. N., Vagin, A. A. & Dodson, E. J. (1997). Acta Cryst. D53, 240–255. [DOI] [PubMed]
- Murzin, A. G., Brenner, S. E., Hubbard, T. & Chothia, C. (1995). J. Mol. Biol. 247, 536–540. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Reinemer, P., Dirr, H. W., Ladenstein, R., Schaffer, J., Gallay, O. & Huber, R. (1991). EMBO J. 10, 1997–2005. [DOI] [PMC free article] [PubMed]
- Schroder, S. & Lohse, M. J. (1996). Proc. Natl Acad. Sci. USA, 93, 2100–2104. [DOI] [PMC free article] [PubMed]
- Stirling, P. C., Srayko, M., Takhar, K. S., Pozniakovsky, A., Hyman, A. A. & Leroux, M. R. (2007). Mol. Biol. Cell, 18, 2336–2345. [DOI] [PMC free article] [PubMed]
- Sun, C., Berardi, M. J. & Bushweller, J. H. (1998). J. Mol. Biol. 280, 687–701. [DOI] [PubMed]
- Tian, G., Xiang, S., Noiva, R., Lennarz, W. J. & Schindelin, H. (2006). Cell, 124, 61–73. [DOI] [PubMed]
- Wang, S., Trumble, W. R., Liao, H., Wesson, C. R., Dunker, A. K. & Kang, C. H. (1998). Nature Struct. Biol. 5, 476–483. [DOI] [PubMed]
- Willardson, B. M. & Howlett, A. C. (2007). Cell. Signal. 19, 2417–2427. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: thioredoxin-fold domain of human phosducin-like protein 2, 3evi, r3evisf