Abstract
Translational read-through of the UGA stop codon is an evolutionarily conserved feature that most prominently represents the basis of selenoprotein biosynthesis. It requires a specific cis-acting stem loop control element, termed SECIS, which is located in the 3′-untranslated region of eukaryotic selenoprotein mRNAs. In a search for novel factors underlying the SECIS-directed UGA read-through process, we identified an evolutionary conserved GTPase-activating protein, termed GAPsec. We show that the activity of the Drosophila GAPsec (dGAPsec) is necessary to support SECIS-dependent UGA read-through activity in flies and the mouse homolog mGAPsec in mice tissue culture cells. However, selenoprotein biosynthesis is not impaired in flies that lack dGAPsec activity. The results indicate that GAPsec is part of a novel SECIS-dependent translational read-through system that does not involve selenocysteine incorporation.—Hirosawa-Takamori, M., Ossipov, D., Novoselov, S.V., Turanov, A.A., Zhang, Y., Gladyshev, V.N., Krol, A., Vorbrüggen, G., Jäckle, H. A novel stem loop control element-dependent UGA read-through system without translational selenocysteine incorporation in Drosophila.
Keywords: conserved, GAP factor, eEFsec, GAPsec mutant, selenoprotein, biosynthesis
Translational control of gene expression enables cells to respond rapidly to environmental cues. This process can be controlled by regulating translational initiation (1, 2), transient masking, and polyadenylation of mRNAs as well as through small RNA molecules (3,4,5). Another mechanism of translational control is through stop codon suppression or read-through (6, 7), which also presents the evolutionarily conserved basis of selenoprotein biosynthesis. Selenoprotein biosynthesis is based on recoding of the UGA stop codon as the codon for the amino acid selenocysteine (Sec), which is incorporated into growing polypeptide chains during translational elongation (8,9,10). In bacteria, this process results in UGA read-through and translation of downstream sequences. It depends on a stem loop structure next to the UGA codon, which serves as template for the binding of SelB, an EF-Tu-like elongation factor, and Sec-tRNA (sec-tRNA) (11).
The principle and components required for UGA read-through activity by Sec incorporation are conserved in evolution (11). In both prokaryotes and eukaryotes, sec-tRNA production depends on production of the selenium donor compound monoselenophosphate (MSP) from selenide and ATP, a reaction catalyzed by the enzyme selenophosphate synthetase (SPS). MSP is used to synthesize Sec from seryl-tRNA, which recognizes UGA stop codons and causes incorporation of Sec into the elongating polypeptide (11). This process involves protein-RNA interactions mediated by Sec-specific elongation factors, SelB in prokaryotes or its homolog eEFsec in eukaryotes, which interacts with sec-tRNA and a stem loop element, termed Sec insertion sequence (SECIS). In prokaryotes, SECIS elements reside immediately downstream of the UGA, whereas in archaea and eukaryotes, the corresponding SECIS elements reside in the 3′-untranslated regions of the selenoprotein coding mRNA (reviewed in refs. 1, 8, 10,11,12). In eukaryotes, the EF-Tu-like SelB function is split between eEFsec and SECIS-binding protein 2 (SBP2) (12,13,14), two components that are conserved between mammals and invertebrates such as Drosophila melanogaster (15, 16).
Here we report on a novel component of the Drosophila UGA read-through system, an evolutionarily conserved GTPase-activating protein, termed GAPsec. To distinguish between GAPsec in Drosophila (d) and mouse (m), we refer to the homologous GAPsec proteins as dGAPsec and mGAPsec, respectively. We show that GAPsec supports SECIS-dependent UGA read-through both in Drosophila and mammalian cells. However, selenoprotein synthesis is not impaired in flies, which lack the dGAPsec protein as determined by labeling the flies lacking dGAPsec with the radioisotope 75Se. Furthermore, the GAPsec protein is not restricted to selenoprotein-containing eukaryotes. The results indicate that GAPsec is part of a novel and evolutionarily conserved SECIS-dependent translational UGA read-through mechanism which, however, does not involve Sec incorporation.
MATERIALS AND METHODS
Fly stocks and genetics
Flies used to examine UGA read-through were described previously (15). PBac{PB}CG5978c05919 flies were obtained from the Bloomington stock collection (http://flystocks. bio.indiana.edu/). mGAPsec was identified by in silico analysis and cloned from mouse NIH 3T3 fibroblast RNA. dGAPsecR138K and mGAPsecR129K were generated by mutagenesis (QuickChange; Stratagene, La Jolla, CA, USA) using LD24460 cDNA (Drosophila Genomics Resource Center, http://dgrc.cgb.indiana.edu/). cDNAs were cloned into pUAST vectors (KpnI site) and used for P element transformation. For overexpression in 3T3 cells, mGAPsec, mGAPsecR129K, and meEFsec were cloned into the KpnI site of pcDNA3.1 (for details, see ref. 15).
Yeast two-hybrid screen, protein expression, and glutathione S-transferase (GST) pulldown assays
Drosophila eEFsec DNA was polymerase chain reaction-amplified and cloned into the Matchmaker III vector pGBKT7 (Clontech Laboratories Inc., Palo Alto, CA, USA) (17) to be used as bait for the yeast two-hybrid screen in conjunction with a cDNA library prepared from poly(A)+ mRNA of 0- to 21-h-old Drosophila embryos (Clontech). All steps, including all controls, were performed as described (18).
For GST-dGAPsec expression, the open reading frame DNAs were cloned into the pEGKT vector (5′-BamHI/3′-HindIII sites). Fusion protein was produced in Escherichia coli BL21Codon Plus DE3 (Stratagene). Pellets were taken up in lysis buffer (25 mM HEPES-KOH, pH 7.6; 500 mM NaCl; 0.1 mM EDTA, pH 8.0; 12.5 mM MgCl2; 0.1% Nonidet P-40; and 10% glycerol) containing EDTA-free complete protease inhibitors (Roche Diagnostics, Mannheim, Germany) and were squeezed through a French press. After centrifugation (10,000 g for 30 min), the GST fusion proteins were purified on glutathione-Sepharose 4B (Amersham Biosciences Europe GmbH, Freiburg, Germany). In vitro 35S-labeled (Pro-mix; Amersham Biosciences) eEFsec was produced by in vitro transcriptions/translation (TNT System; Promega GmbH, Mannheim, Germany) and used for GST pulldowns (18).
Cell transfections and expression analyses
NIH 3T3 cells were transfected and assayed as described (15). Developmental Northern blot analysis (17) was performed with labeled dGAPSec antisense RNA probes (in vitro transcription of LD24460 cDNA, Strip-EZ RNA kit; Ambion, Austin, TX, USA). Rabbit anti-dGAPsec antibodies were generated against peptide VHSKGEHGRRLHERV (positions 158–172) (Eurogentec, Seriang, Belgium). Western blots prepared from cytoplasmic female extracts were stained with anti-dGAPsec antibodies (1:1000 dilution) and were visualized by horseradish peroxidase (HRP) -conjugated anti-rabbit immunoglobulin G (IgG; 1:10,000 dilution; Sigma-Aldrich Chemie GmbH, Munich, Germany) using SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL, USA). As a control, the blot was reprobed with anti-tubulin antibodies (E7, 1:5000 dilution; DSHB, Iowa City, IA, USA) and HRP-conjugated anti-mouse IgG (secondary antibodies; 1:10,000 dilution; Sigma-Aldrich Chemie GmbH).
Enzymatic luciferase assay
Luciferase activity was assayed as published previously (15). In brief, cell lysates and homogenates of embryos were prepared in Reporter Lysis Buffer (Promega). LacZ activities (and the LacZ reference; Sigma-Aldrich Chemie GmbH) were determined from 100-μl samples diluted with 500 μl of assay buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgCl2, and 12.5 mM β-mercaptoethanol) and 100 μl of substrate (4 mg/ml o-nitrophenyl β-d-galactopyranoside in 60 mM Na2HPO4, 40 mM NaH2PO4). Reactions were stopped with 500 μl of 1 M NaCO3, and luciferase activity was determined in a PharMingen luminometer (405 nm) after injection of 100 μl of Luciferase Assay Reagent (Promega).
75Se labeling of selenoproteins in vivo
Three-day-old mutant or wild-type flies were fed 2–6 days with standard fly food containing 75 μCi of 75Se sodium selenite (Na275SeO3). Protein extracts were prepared by homogenization of flies in cold radioimmunoprecipitation assay buffer (0.1% SDS, 0.5% sodium deoxycholate, and 1% Nonidet P-40 in PBS) containing phenylmethylsulfonyl fluoride (1 mM), Na3VO4 (1 mM), and aprotinin (20 mg/ml) as protease inhibitors. Proteins were resolved by SDS-PAGE, and 75Se-labeled proteins were visualized by phosphorimaging.
RESULTS
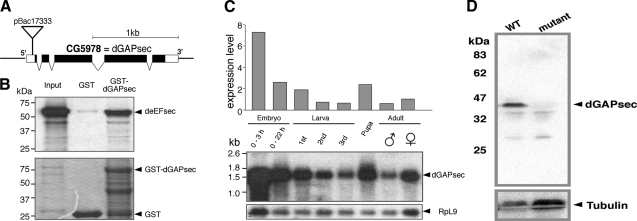
We performed a yeast two-hybrid screen to identify components of the SECIS-dependent UGA read-through system that interact with eEFsec of Drosophila. We used full-length deEFsec (17) as the bait to screen a cDNA library prepared from poly(A)+ mRNA of Drosophila embryos. In addition to the known binding partner SBP2, a novel protein was isolated. It is encoded by the annotated gene CG5978 (Figs. 1 and 2A) and shows extensive sequence homology to GAPs. In silico analyses of genomic sequences of mouse, human, and Caenorhabditis elegans revealed that the protein coding sequence is highly conserved and contains the most prominent diagnostic feature of GAPs, namely, a conserved arginine residue in the canonical GAP domain (19, 20) (Fig. 1). We called the protein Drosophila GAPsec (dGAPsec), referring to its function in SECIS-dependent read-through activity (see below). GST pulldown assays show that the recombinant GST-dGAPsec fusion protein can also bind to Drosophila eEFsec in vitro (Fig. 2B). These results establish that deEFsec and dGAPsec are capable of interacting, suggesting that dGAPsec could be associated with selenoprotein biosynthesis.
Figure 1.
Sequence comparison of Drosophila dGAPsec protein with orthologs of mouse, human and C. elegans. Conserved amino acids (black background) and GAP designating residues (red asterisks) are highlighted. Red box, essential arginine residue used to generate dominant-negative GAPsec mutations (see text). Top to bottom: dGAPsec, mGAPsec (Tbc1d13; NM_146252), human hGAPsec (Tbc1d13; NM_018201) and C. elegans ceGAPsec (F45E6.3; NM_077694).
Figure 2.
Identification of the Drosophila dGAPsec gene and lack of protein in the mutant. A) Genomic organization of dGAPsec (CG5978) as established by sequence comparison of genomic DNA (http://flybase.bio.indiana.edu/) and cDNA isolates. pBac17333, position of the pBac insertion; black bars, coding sequences; open bars, untranslated regions. The processed CG5978 transcript is 1.541 kb in length. B) Full-length GST-dGAPsec fusion protein interacts with deEFsec. GST-pulldown with in vitro translated 35S-labeled deEFsec (input) and bacterially produced GST (GST) as well as GST-dGAPsec (GST-dGAPsec). Top panel: autoradiograph of 35S-labeled protein; bottom panel: Coomassie brilliant blue-stained gel. C) Developmental profile of dGAPsec transcripts. The Northern blot was initially probed for deEFsec mRNA (17), stripped, and reprobed with a dGAPsec probe. The quantified profile is shown above the Northern blot (middle panel). Bottom panel: RpL9 transcripts served as reference for mRNA quantification. D) Anti-dGAPsec antibody staining of Western blots (WT, wild type; mutant, homozygous dGAPsec mutant flies) shows that the protein is absent in mutants. Protein loading of blots was controlled by anti-tubulin antibody staining.
Expression profile and analysis of the dGAPsec mutant
Northern blot analysis (Fig. 2C) and whole-mount in situ hybridization with a dGAPsec probe revealed that the developmental expression profiles of dGAPsec and eEFsec (17) are similar. To study dGAPsec function, we used a mutant that contains a piggy Bac (pBac) insertion (PBac{PB}CG5978c05919) in the 5′-untranslated region of the CG5978 transcript (Fig. 2A). To test whether this mutant affects dGAPsec expression, we generated anti-dGAPsec antibodies and used them for Western blot analysis. Figure 2D shows that dGAPsec cannot be detected in the mutant flies. The production of dGAPsec could be restored on the precise excision of the pBac insertion element (data not shown), indicating that the pBac insertion had caused a loss-of-function mutant that lacks dGAPsec. Such flies develop into normal-looking fertile adults. Thus, dGAPsec has no vital and morphological distinct functions during the life cycle of the fly.
We tested whether the absence of dGAPsec affects UGA read-through activity as has been observed earlier with eEFsec mutants (15, 17). We used wild-type and dGAPsec mutant flies that carry a lacZ/UGA/luciferase reporter transgene (15, 17). This reporter contains the β-galactosidase and luciferase open reading frames, which are separated by an UGA stop codon and a functional SECIS element in the 3′-untranslated region. As a control, the same reporter lacking the SECIS element (15, 17) was used. In this test system, ubiquitous expression of β-galactosidase serves as an internal control for transcriptional and translational activity of the transgene, whereas luciferase activity indicates read-through translation (15, 17, 21).
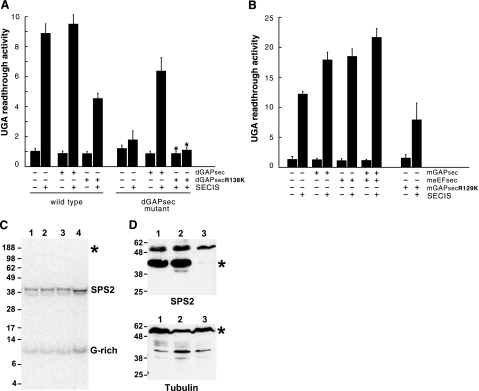
SECIS-dependent luciferase activity was observed in transgenic wild-type flies, but not in transgenic dGAPsec mutants (Fig. 3A). On dGAPsec expression from a cDNA-containing transgene under the control of the Actin5C promoter (15, 17), SECIS-dependent UGA read-through activity could be restored in dGAPsec mutants (Fig. 3A). Thus, the SECIS-dependent UGA read-through is dependent on dGAPsec.
Figure 3.
Loss of GAPsec activity affects SECIS-dependent UGA read-through in Drosophila and mouse but not selenocysteine incorporation. A) Lack of SECIS-dependent read-through in dGAPsec mutant flies. Activities of the lacZ/UGA/luciferase reporter gene (RG) with or without the SECIS element (SECIS) in wild-type and dGAPsec mutants after dGAPsec and dGAPsecR138K overexpression, respectively (details in the text). “UGA read-through activity” refers to fold increase of luciferase activity over the basis level in cells containing the reporter gene without SECIS sequences (15, 17, 21). Note that SECIS-dependent UGA read-through is impaired in dGAPsec mutants and in wild-type flies expressing dGAPsecR138K. Note also that the lack of read-through in dGAPsec mutants can be rescued by dGAPsec but not by dGAPsecR138K expression. B) UGA read-through in transfected mouse NIH 3T3 cells. Bars represent means ± sd from 6 independent experiments; for rescue experiments, mean values and limits of 2 independent experiments are shown (∗). meEFsec (B) served as the response control of the assay system (details in text). +, presence of indicated activity; −, absence of indicated activity. C) Selenoprotein synthesis of wild-type and dGAPsec mutant flies after 75Se labeling. Numbers on the left of the SDS-PAGE gel refer to sizes (kDa) of marker proteins. Lane 1: wild-type flies expressing the RG containing the SECIS-element; lane 2, wild-type flies that express only β-galactosidase (RG lacks the SECIS element); lane 3, dGAPsec mutant flies without RG; and lane 4, dGAPsec mutant flies that express only β-galactosidase (RG contains the SECIS element). Note selenoprotein synthesis (SPS2 and G-rich) in all lanes and the absence of 75Se label in the β-galactosidase/luciferase fusion protein (position indicated by ∗), showing that the fusion protein (15, 17, 21) lacks 75Se incorporation (details in text). D) Western blot containing proteins of wild-type flies (lane 1) and dGAPsec (lane 2) and deEFsec mutants (lane 3) using anti-SPS2 antibodies (top panel) and anti-tubulin antiserum (bottom panel). Numbers on the left refer to sizes (kDa) of marker proteins. ∗, positions of SPS2 and tubulin (loading control).
Dominant-negative GAPsec mutations reduce the UGA read-through
GAP function depends on a conserved arginine residue in the canonical GAP activation domain (19, 20). Replacement of the arginine by lysine abolishes GAP activity and causes a dominant-negative GAP mutant (22). To test whether dGAPsec exerts GAP activity, as suggested by its molecular structure, we generated the corresponding dGAPsec mutant (arginine 138→lysine 138; Fig. 1) and expressed it in both wild-type and dGAPsec mutant flies that also contained a lacZ/UGA/luciferase reporter gene. In contrast to dGAPsec, the expression of dGAPsecR138K cannot restore the read-through activity (Fig. 3A). However, transgene-derived dGAPsecR138K expression significantly reduced SECIS-dependent read-through in wild-type flies (Fig. 3A). Thus, as generally observed with GAPs (22), the arginine to lysine replacement mutation abolishes the protein activity and causes a dominant-negative effect of the protein in flies.
We next asked whether a corresponding mutation of mammalian GAPsec also affects UGA read-through. We cloned a full-length GAPsec cDNA of mouse (mGAPsec) and generated the replacement mutation mGAPsecR129K (Fig. 1). Mouse NIH 3T3 cells bearing the lacZ/UGA/luciferase reporter gene (15, 17) were transfected with mGAPsec DNA, with meEFsec DNA, with the combination of both, and with DNA expressing mGAPsecR129K. Figure 3B shows that expression of mGAPsec and mEFsec and the coexpression of both factors enhance SECIS-dependent UGA read-through. Conversely, expression of mGAPsecR129K caused reduced read-through activity. Although this effect is less pronounced in mouse compared with Drosophila tissue culture cells, the result shows that the lysine replacement mutation has a weak dominant-negative effect on mouse UGA read-through activity as well. The results establish the fact that dGAPsec and mGAPsec exert GAP activity that affects the SECIS-dependent UGA read-through in Drosophila and mouse cells.
GAPsec is not required for endogenous selenoprotein biosynthesis
The effect of reduced or a lack of GAPsec activity on SECIS-dependent UGA read-through of bipartite reporter mRNA (Fig. 3A, B) and the physical interaction of dGAPsec and eEFsec suggest that GAPsec may have a role in selenoprotein biosynthesis, a process that requires UGA recoding for Sec incorporation. Similar to the dGAPsec mutant, an eEFsec null mutation of Drosophila does not affect the viability of the mutant flies but caused complete inhibition of SECIS-dependent read-through activity (17). In addition, the selenoprotein SPS2 was not detected in eEFsec-deficient flies (17) and in vivo labeling of eEFsec null mutant flies resulted in the lack of endogenous selenoprotein synthesis (data not shown; unpublished results).
To test whether the loss of dGAPsec affects endogenous selenoprotein synthesis, we labeled wild-type and dGAPsec mutant flies with 75Se isotope and asked whether selenoproteins are synthesized in vivo. Figure 3C shows that dGAPsec mutant flies produce endogenous selenoproteins such as SPS2 and the G-rich protein, indicating that the SECIS- and deEFsec-dependent UGA recoding underlying Sec incorporation is not impaired. Metabolic 75Se labeling of transgenic wild-type flies, known to synthesize the β-galactosidase/luciferase protein in a SECIS- and deEFsec-dependent manner as shown previously (15), did not result in a selenoprotein (Fig. 3C). Furthermore, Western blot analysis with antibodies directed against the selenoprotein SPS2 (15), a well-established selenoprotein of Drosophila (16), shows that SPS2 is expressed in wild-type flies and flies lacking dGAPsec but not in flies that lack deEFsec, an essential component of the UGA read-through system that is based on Sec incorporation (Fig. 3D). Both the 75Se labeling results indicating that SPS2 contains Sec (Fig. 3C) and Western blots showing that SPS2 is expressed in dGAPsec mutants suggest that endogenous selenoprotein synthesis is not affected by the absence of GAPsec activity. To roughly quantify the relative amounts of SPS2 production and 75Se incorporation into protein of wild-type and dGAPsec-deficient flies, we examined equal aliquots of fly extracts, which were labeled in parallel and side by side and compared the intensity of SPS2 antibody staining and 75Se labeling of the different bands, respectively. In all experiments performed, we found at least a 2-fold difference between wild-type and dGAPsec mutants. We take this semiquantitative analysis as an argument to conclude that selenoprotein production in dGAPsec mutants is higher than that in wild-type flies.
This finding is consistent with the conclusion that the mechanism of SECIS-dependent UGA read-through observed with the lacZ/UGA/luciferase reporter gene, which results in SECIS-dependent β-galactosidase and luciferase activities in Drosophila and as well as in mammalian cells (15, 17, 21), is therefore not based on Sec incorporation. In fact, although the lacZ/UGA/luciferase reporter gene has been used as a reporter system to assay selenoprotein biosynthesis (15, 17, 21), the incorporation of Sec was never directly assayed by 75Se labeling as shown in Fig. 3C. Thus, whether the UGA read-through of the bipartite reporter mRNA was indeed based on recoding of UGA as the Sec codon was never critically questioned.
Collectively, the in vivo labeling experiments and Western blot analysis (Fig. 3C, D) indicate that GAPsec is specifically required for stop codon suppression, whereas eEFsec is required, as known (8,9,10), for Sec incorporation and thus recoding of the stop codon into a Sec codon during selenoprotein biosynthesis as well as for SECIS dependent read-through activity without Sec incorporation as shown here for the lacZ/UGA/luciferase reporter. These novel results indicate that the factors of the Sec incorporation machinery might have an additional role in stop codon suppression without Sec incorporation.
DISCUSSION
GAPsec is a part of an evolutionarily conserved UGA read-through system that involves the SECIS element, previously shown to mediate Sec incorporation. However, dGAPsec does not function via Sec incorporation, because selenoprotein synthesis continues in the absence of dGAPsec activity. Translation elongation factors, such as eEFsec, are G proteins that bind GTP (13). On GTP hydrolysis, they undergo a conformational switch, enabling them to continue with the next step of the translation elongation cycle (23, 24). The weak GTPase activity of these factors is enhanced several orders of magnitude by the bacterial ribosome which provides intrinsic GAP activity (24, 25). In deEFsec (17) and dGAPsec mutant flies, the UGA stop codon of LacZ/UGA/luciferase mRNA is suppressed in a SECIS-dependent manner. In deEFsec mutant flies, selenoproteins such as SPS2 fail to be expressed (17), whereas flies lacking dGAPsec appear to synthesize selenoproteins normally. Thus, the results uncover a novel UGA read-through system different from Sec incorporation. Interestingly, this read-through activity also involves the SECIS element and most likely eEFsec, two key components of selenoprotein biosynthesis. In fact, sequencing of 12 Drosophila genomes detected close to 150 conserved read-through events that are not based on Sec incorporation (26). This surprisingly high number of nonselenoproteins that are generated through a stop codon read-through supports the possibility of several read-through systems in addition to those that are identified (6, 7). Furthermore, factors such as SECIS elements and/or eEFsec could also participate in more than one UGA read-through mechanism as shown here. It remains an open question, however, whether an amino acid and which kind is incorporated in place of Sec and whether a tRNA different from sec-tRNA is involved in this process.
The findings that dGAPsec homologs are restricted to eukaryotes (Supplemental Fig. 1) and are found in selenoprotein-lacking species such as higher plants, the yeast Schizosaccharomyces pombe, and the parasite Encephalitozoon cuniculi (27) are consistent with the argument that GAPsec functions in a different context than selenoprotein synthesis. However, sequence analysis concerning the presence of eEFsec and GAPsec in organisms with sequenced genomes revealed that eEFSec is only found in genomes encoding selenoproteins, whereas GAPsec coding sequences are also found in genomes that do not encode selenoproteins and lack eEFsec coding sequences. This observation suggests that GAPsec functions as a more general GTPase-activating enzyme independent of eEFsec and may act in processes different from the one shown here. Furthermore, the reported dual mode of SECIS-dependent UGA read-through, with Sec incorporation and without, suggests different types and functions of SECIS elements in the 3′-untranslated region of mRNAs for UGA suppression, with and without the incorporation of Sec. The different read-through mechanisms, one involving SEC incorporation and the other that does not, are likely to be competing processes in vivo. This conclusion is consistent with the observation that the amount of selenoprotein is consistently higher in flies lacking dGAPsec activity. Thus, we expect competition of the Sec incorporation mechanism and the GAPsec-dependent read-through activity to provide new insight into why selenoprotein biosynthesis is such an inefficient process in eukaryotes.
Supplementary Material
Acknowledgments
We thank our colleagues in the laboratory for various contributions and critical discussions, G. Dowe for sequencing, and U. Jahns-Meyer for generating fly transformants. This work was supported by the Max-Planck-Society, the SP “Selenoproteine” of the Deutsche Forschungsgemeinschaft (G.V. and H.J.), and U.S. National Institutes of Health grant GM061603 (V.N.G.).
References
- Dever T E. Gene-specific regulation by general translation factors. Cell. 2002;108:545–556. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Richter J D, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- Mendez R, Richter J D. Translational control by CPEB: a means to the end. Nat Rev Mol Cell Biol. 2001;2:521–529. doi: 10.1038/35080081. [DOI] [PubMed] [Google Scholar]
- Pillai R S, Bhattacharyya S N, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Wilkie G S, Dickson K S, Gray N K. Regulation of mRNA translation by 5′- and 3′-UTR-binding factors. Trends Biochem Sci. 2003;28:182–188. doi: 10.1016/S0968-0004(03)00051-3. [DOI] [PubMed] [Google Scholar]
- Valle R P, Morch M D. Stop making sense: or regulation at the level of termination in eukaryotic protein synthesis. FEBS Lett. 1988;235:1–15. doi: 10.1016/0014-5793(88)81225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von der Haar T, Tuite M F. Regulated translational bypass of stop codons in yeast. Trends Microbiol. 2007;15:78–86. doi: 10.1016/j.tim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Low S C, Berry M J. Knowing when not to stop: selenocysteine incorporation in eukaryotes. Trends Biochem Sci. 1996;21:203–208. [PubMed] [Google Scholar]
- Atkins J F, Gesteland R F. The twenty-first amino acid. Nature. 2000;407:463–465. doi: 10.1038/35035189. [DOI] [PubMed] [Google Scholar]
- Driscoll D M, Copeland P R. Mechanism and regulation of selenoprotein synthesis. Annu Rev Nutr. 2003;23:17–40. doi: 10.1146/annurev.nutr.23.011702.073318. [DOI] [PubMed] [Google Scholar]
- Böck A. Biosynthesis of selenoproteins—an overview. Biofactors. 2000;11:77–78. doi: 10.1002/biof.5520110122. [DOI] [PubMed] [Google Scholar]
- Copeland P R, Fletcher J E, Carlson B A, Hatfield D L, Driscoll D M. A novel RNA binding protein, SBP2, is required for the translation of mammalian selenoprotein mRNAs. EMBO J. 2000;19:306–314. doi: 10.1093/emboj/19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagegaltier D, Hubert N, Yamada K, Mizutani T, Carbon P, Krol A. Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation. EMBO J. 2000;19:4796–47805. doi: 10.1093/emboj/19.17.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tujebajeva R M, Copeland P R, Xu X M, Carlson B A, Harney J W, Driscoll D M, Hatfield D L, Berry M J. Decoding apparatus for eukaryotic selenocysteine insertion. EMBO Rep. 2000;1:158–163. doi: 10.1093/embo-reports/kvd033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosawa-Takamori M, Jäckle H, Vorbrüggen G. The class 2 selenophosphate synthetase gene of Drosophila contains a functional mammalian-type SECIS. EMBO Rep. 2000;1:441–446. doi: 10.1093/embo-reports/kvd087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B C, Böck A, Jäckle H, Vorbrüggen G. SelD homolog from Drosophila lacking selenide-dependent monoselenophosphate synthetase activity. J Mol Biol. 1997;274:174–180. doi: 10.1006/jmbi.1997.1371. [DOI] [PubMed] [Google Scholar]
- Hirosawa-Takamori M, Chung H R, Jäckle H. Conserved selenoprotein synthesis is not critical for oxidative stress defence and the lifespan of Drosophila. EMBO Rep. 2004;5:317–322. doi: 10.1038/sj.embor.7400097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke S, Jäckle H. Novel guanine nucleotide exchange factor GEFmeso of Drosophila melanogaster interacts with Ral and Rho GTPase Cdc42. FASEB J. 2006;20:683–691. doi: 10.1096/fj.05-5376com. [DOI] [PubMed] [Google Scholar]
- Scheffzek K, Ahmadian M R, Wiesmuller L, Kabsch W, Stege P, Schmitz F, Wittinghofer A. Structural analysis of the GAP-related domain from neurofibromin and its implications. EMBO J. 1998;17:4313–4327. doi: 10.1093/emboj/17.15.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak A, Fedorov R, Alexandrov K, Albert S, Goody R S, Gallwitz D, Scheidig A J. Crystal structure of the GAP domain of Gyp1p: first insights into interaction with Ypt/Rab proteins. EMBO J. 2000;19:5105–5113. doi: 10.1093/emboj/19.19.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmus H, Flohe L, McCarthy J E. Analysis of eukaryotic mRNA structures directing cotranslational incorporation of selenocysteine. Nucleic Acids Res. 1996;24:1195–1201. doi: 10.1093/nar/24.7.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert S, Will E, Gallwitz D. Identification of the catalytic domains and their functionally critical arginine residues of two yeast GTPase-activating proteins specific for Ypt/Rab transport GTPases. EMBO J. 1999;18:5216–5225. doi: 10.1093/emboj/18.19.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprang S R. G protein mechanisms: insights from structural analysis. Annu Rev Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- Rodnina M V, Stark H, Savelsbergh A, Wieden H J, Mohr D, Matassova N B, Peske F, Daviter T, Gualerzi C O, Wintermeyer W. GTPases mechanisms and functions of translation factors on the ribosome. Biol Chem. 2000;381:377–387. doi: 10.1515/BC.2000.050. [DOI] [PubMed] [Google Scholar]
- Wahl M C, Moller W. Structure and function of the acidic ribosomal stalk proteins. Curr Protein Pept Sci. 2002;3:93–106. doi: 10.2174/1389203023380756. [DOI] [PubMed] [Google Scholar]
- Stark A, Lin M F, Kheradpour P, Pedersen J S, Parts L, Carlson J W, Crosby M A, Rasmussen M D, Roy S, Deoras A N, Ruby J G, Brennecke J, Hodges E, Hinrichs A S, Caspi A, Paten B, Park S W, Han M V, Maeder M L, Polansky B J, Robson B E, Aerts S, van Helden J, Hassan B, Gilbert D G, Eastman D A, Rice M, Weir M, Hahn M W, Park Y, Dewey C N, Pachter L, Kent W J, Haussler D, Lai E C, Bartel D P, Hannon G J, Kaufman T C, Eisen M B, Clark A G, Smith D, Celniker S E, Gelbart W M, Kellis M, Crosby M A, Matthews B B, Schroeder A J, Gramates L S, St. Pierre S E, Roark M, Wiley K L, Jr, Kulathinal R J, Zhang P, Myrick K V, Antone J V, Gelbart W M, Carlson J W, Yu C, Park S, Wan K H, Celniker S E. Discovery of functional elements in 12 Drosophila genomes using evolutionary signatures. Nature. 2007;450:219–232. doi: 10.1038/nature06340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoselov S V, Rao M, Onoshko N V, Zhi H, Kryukov G V, Xiang Y, Weeks D P, Hatfield D L, Gladyshev V N. Selenoproteins and selenocysteine insertion system in the model plant cell system, Chlamydomonas reinhardtii. EMBO J. 2002;21:3681–3693. doi: 10.1093/emboj/cdf372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.