Abstract
Background
Nkx2.7 is the tinman-related gene, as well as orthologs of Nkx2.5 and Nkx-2.3. Nkx2.7 and Nkx2.5 express in zebrafish heart fields of lateral plate mesoderm. The temporal and spatial expression patterns of Nkx2.7 are similar to those of Nkx2.5, but their functions during cardiogenesis remain unclear.
Methodology/Principal Findings
Here, Nkx2.7 is demonstrated to compensate for Nkx2.5 loss of function and play a predominant role in the lateral development of the heart, including normal cardiac looping and chamber formation. Knocking down Nkx2.5 showed that heart development was normal from 24 to 72 hpf. However, when knocking down either Nkx2.7 or Nkx2.5 together with Nkx2.7, it appeared that the heart failed to undergo looping and showed defective chambers, although embryos developed normally before the early heart tube stage. Decreased ventricular myocardium proliferation and defective myocardial differentiation appeared to result from late-stage up-regulation of bmp4, versican, tbx5 and tbx20, which were all expressed normally in hearts at an early stage. We also found that tbx5 and tbx20 were modulated by Nkx2.7 through the heart maturation stage because an inducible overexpression of Nkx2.7 in the heart caused down-regulation of tbx5 and tbx20. Although heart defects were induced by overexpression of an injection of 150-pg Nkx2.5 or 5-pg Nkx2.7 mRNA, either Nkx2.5 or Nkx2.7 mRNA rescued the defects induced by Nkx2.7-morpholino(MO) and Nkx2.5-MO with Nkx2.7-MO.
Conclusions and Significance
Therefore, we conclude that redundant activities of Nkx2.5 and Nkx2.7 are required for cardiac morphogenesis, but that Nkx2.7 plays a more critical function, specifically indicated by the gain-of-function and loss-of- function experiments where Nkx2.7 is observed to regulate the expressions of tbx5 and tbx20 through the maturation stage.
Introduction
The heart is the first organ to form and function in the vertebrate embryos. The functional heart is formed after 72 hours post-fertilization (hpf). During heart developmental processes, cell fate determination, specification, differentiation and migration are all involved. In view of this complexity, investigators have used model systems, including Drosophila, Xenopus, zebrafish, chicken, and mouse, in an attempt to understand the molecular regulatory network of cardiogenesis [1]–[4]. These efforts have produced two key findings important to the hypothesis of this paper: 1) that the members of the homeobox gene family are important for heart development [5] and 2) that the NK-class of homeodomain proteins plays key roles in the establishment of myogenic lineages [6]–[11].
Among the homeobox genes, the vertebrate homologs of tinman have been cloned in many species and are highly conserved in the homeodomain structures and expression patterns. For example, Drosophila tinman is involved in the specification of the heart primordial cells. More specifically, in tinman null mutants, the heart-like dorsal vessel is absent, indicating that tinman is a critical factor in Drosophila heart-like formation [6]. The mouse tinman homolog is Nkx2.5, and Nkx2.5-knockout mice exhibit the formation of a primitive heart tube, which expresses most cardiac markers with the exception of mlc2v. The mlc2v marker, however, displays a thinning ventricular myocardium and, therefore, cannot undergo heart looping [7]. Unlike Drosophila tinman, mouse Nkx2.5 is required for murine normal heart morphogenesis, but it is not essential for the cell fate determination of heart precursors. This suggests that there may be an Nkx homolog that compensates for the loss of function of Nkx2.5 in mouse embryos. The most likely homolog of mouse Nkx2.5 is mouse Nkx2.6 which shares identical expression patterns at pharyngeal endoderm and at heart with Nkx2.5 [12]. Targeted disruption of Nkx2.6 does not cause any abnormality, either in the pharynx or in the heart [13]. In Nkx2.5−/−Nkx2.6−/− double knockout mice, the development of pharynx is totally abolished. Therefore, Tanaka et al. (2001) demonstrated that Nkx2.5 can compensate for the function of Nkx2.6 in the pharyngeal endoderm [14].
Many tinman (Nkx2.3, 2.5, 2.6, 2.7, 2.8) homologs of chicken, Xenopus and zebrafish have been isolated [10], [15]–[21]. The example cited above, mouse Nkx2.5/ Nkx2.6, suggests a redundant activity for loss of a single Nkx homolog function. In chick, cNkx2.8 expression partially overlaps with cNkx2.5 and cNkx2.3. This is seen in the onset of cNkx2.8, which appears at heart primordia and retains its expression until primary heart tube formation. After heart tube looping, however, cNkx2.8 is no longer expressed, and cNkx2.3 initiates expression at this stage and continues to express until adulthood [22]. Overexpression of cNkx2.8 can transactivate a minimal promoter containing the cis-acting element for Nkx2.5 binding. In addition, cNkx-2.8 and serum response factor can co-activate a minimal cardiac α-actin promoter like cNkx2.5 [21]. Still, there is no direct evidence to demonstrate that cNkx2.8 can compensate for cNkx2.5 loss of function in early development of chick heart.
In Xenopus, both XNkx2.3 and XNkx2.5 are expressed in cardiac mesoderm and pharyngeal endoderm [23]. Injection of XNkx2.3 or XNkx2.5 mRNA causes an abnormally enlarged heart [9]. Although the XNkx2.5 and XNkx2.3 dominant-negative engrailed-fusion repressor shows a reduced number of heart cells, co-injection of both EnHD constructs appears to result in an even more serious decrease of heart cells. This suggests that XNkx2.5 and XNkx2.3 act redundantly during heart formation [24].
In zebrafish, Nkx2.7 is the tinman-related gene, as well as orthologs of Nkx2.5 and Nkx2.3. The transcription of zebrafish Nkx2.7 is earlier than that of Nkx2.5 in cardiac mesoderm, and Nkx2.7 is also expressed at pharyngeal endodermal precursors. The temporal and spatial expression patterns of Nkx2.7 are similar to those of Nkx2.5 [19]. While knockdown of Nkx2.5 in zebrafish embryos displays no obvious defects in heart, overexpression of Nkx2.5 did show an enlarged heart and even caused dorsoventral axial defects [10]. Since it is not known which stages of cardiac development or which pathways are affected by the Nkx family of homologs, it may be necessary to knock down more than one of these genes to discover their dual functions. Meanwhile, the precise role played by tinman homolog genes in vertebrate cardiac development remains to be clarified, particularly the dual roles played by Nkx2.5 and Nkx2.7 in the cardiogenesis of zebrafish embryos. In this study, we clearly demonstrate that, while Nkx2.5 and Nkx2.7 play redundant roles in the differentiation of cardiomyocytes, Nkx2.7 has a more critical function, specifically indicated by the gain-of-function and loss-of-function experiments where Nkx2.7 is observed to regulate the expressions of tbx5 and tbx20 through the heart tube stage.
Results
Nkx2.5 and Nkx2.7 double knockdown results in serious zebrafish embryo heart defects
We designed Nkx2.5- and Nkx2.7-MO to study whether Nkx2.5 or Nkx2.7 is required for heart development of zebrafish. Heart-specific-GFP transgenic line Tg (cmlc2::GFP) was used to monitor the cardiac morphology in zebrafish embryos. By fluorescence microscope, we observed that embryos injected with 10, 12, and 14 ng of Nkx2.5-MO developed normally from 24 to 72 hpf, although we occasionally observed a small percentage of injected embryos which had a slight degree of pericardial edema. This was also observed in the wild-type embryos and in the embryos injected with a high concentration of control MO (Table 1 and Fig. 1A). In contrast, embryos injected with Nkx2.7-MO at the same concentrations totally failed to complete heart looping during 30 to 72 hpf (Table 1 and Fig. 1B). Moreover, double knockdown of Nkx2.5 and Nkx2.7 (Nkx2.5/2.7-MO) by injection of 8 ng Nkx2.5-MO combined with 8 ng of Nkx2.7-MO into embryos displayed a shrunken ventricle and an expanded atrium with an incomplete heart looping. After 72 hpf, many symptoms of heart defects appeared in both Nkx2.7 and Nkx2.5/2.7 morphants, including regurgitation of blood, arrhythmia, string-like heart and pericardial edema (data not shown). The rates of defective heart occurrence in the Nkx2.7 and the Nkx2.5/2.7 morphants were dose-dependent (Table 1). Comparing the cardiac defects among Nkx2.5 morphants, Nkx2.7 morphants and Nkx2.5/2.7 morphants, we observed that the Nkx2.5 morphants did not have any obvious heart defects, while the Nkx2.7 morphants exhibited an unlooping defect with a low percentage of shrunken ventricles. The Nkx2.5/2.7 morphants displayed not only the unlooping defect, but also shrunken ventricles (Table 1).
Table 1. The cardiac phenotypes of embryos injected with Nkx2.5-MO, Nkx2.7- MO and Nkx2.5/2.7-MO.
| Morpholino (ng) | Survival numbers of Embryos | Abnormal Phenotypes | |||
| Nkx2.5 | Nkx2.7 | Looping Defects | Shrinking Ventricle | pericardial edema | |
| 4 | 4 | 142/167 (85.0%) | 85(59.8%) | 75(52.8%) | — |
| 6 | 6 | 207/255 (81.2%) | 166 (80.2%) | 151(72.9%) | — |
| 8 | 8 | 183/233 (78.6%) | 177 (97.8%) | 162 (88.5%) | — |
| 0 | 10 | 115/137 (83.9%) | 56(48.7%) | 12(10.4%) | — |
| 0 | 12 | 139/177 (78.5%) | 87(62.5%) | 27(19.4%) | — |
| 0 | 14 | 183/246 (74.3%) | 143(78.1%) | 75(40.9%) | — |
| 10 | 0 | 24/188 (87.2%) | 0 | 0 | 21/164(12.8%) |
| 12 | 0 | 74/84(88.1%) | 0 | 0 | 15/76 (19.7%) |
| 14 | 0 | 82/98(83.7%) | 0 | 0 | 19/82(23.2%) |
Embryos derived from the transgenic line of Tg(cmlc2::GFP) were used, and the cardiac morphology was observed by fluorescence microscope. The major cardiac phenotype of Nkx2.5/2.7-MO morphants revealed unlooping defects and shrunken ventricle; the major phenotype for Nkx2.7-MO morphants was looping defects with a lesser rate of shrunken ventricle. The Nkx2.5-MO morphants displayed no obvious cardiac phenotype except pericardial edema. (“—“ represents no observation.) The percentage of each abnormal phenotype was counted individually.
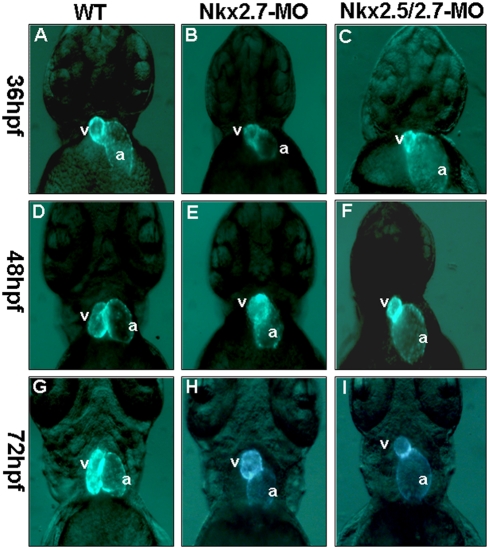
Figure 1. The defective phenotypes of zebrafish embryo heart injected with Nkx2.5-MO, Nkx2.7-MO and Nkx2.5/2.7-MO.
Eight nanograms of MO were injected into one-cell stage embryos derived from transgenic line Tg (cmlc2::GFP) to knock down the Nkx protein specifically. The embryos are shown at 36 hpf (A, B, C), 48 hpf (D, E, F), and 72 hpf (G, H, I). The heart phenotype of Nkx2.5-MO embryos was similar to that of control embryos whose ventricle is located at the right side of the atrium when embryos were observed at 36 hpf, 48 hpf and 72 hpf from the ventral view under fluorescence microscope (A, D, G). However, embryos injected with Nkx2.7-MO displayed an unlooping defect from 36 hpf to 72 hpf (B, E, H). Embryos injected with Nkx2.5/2.7-MO displayed a shrunken ventricle and an expanding atrium (C, F, I). v: ventricle; a: atrium.
To confirm that the phenotypes of morphants were specifically induced by the absence of Nkx2.5/2.7 function, we constructed plasmids of pCS2-Nkx2.5-GFP and pCS2-Nkx2.7-GFP, in which the binding sequence of MO was fused with the GFP cDNA. Unlike the GFP was expressed in the embryos (n = 173) injected 6 ng Nkx-specific MO together with 150 pg GFP mRNA, the GFP expression was not observed in the embryos (n = 155) injected 6 ng Nkx-specific MO together with 150 pg mRNA synthesized either from pCS2-Nkx2.5-GFP or from pCS2-Nkx2.7-GFP (data not shown). Embryos injected either with Nkx2.5-GFP and Nkx2.7-MO, or with Nkx2.7-GFP and Nkx2.5-MO, distinctively express GFP (data not shown). This evidence indicated that injection of Nkx2.5-MO and Nkx2.7-MO can specifically block the translation of the Nkx2.5 or Nkx2.7 mRNA, respectively. We also designed a 5-bp mismatched binding sequence for Nkx2.7 MO, termed Nkx2.7-MO-control, to serve as a control. Results showed that the Nkx2.7-MO-control-injected zebrafish embryos exhibited the wild-type-like phenotype when this control MO was injected at a range of 6.0 to 16 ng. Thus, we conclude that the defective phenotypes of morphants are induced specifically by the injection of Nkx2.5/2.7-MO.
Nkx2.5 and Nkx2.7 function redundantly in zebrafish heart development
To demonstrate the functions shared by Nkx2.5 and Nkx2.7, as well as the specificity of MO for Nkx2.5 and Nkx2.7, the Nkx2.5 mRNA or the Nkx2.7 mRNA was co-injected with MO into zebrafish embryos. Results showed that co-injection of 9 ng Nkx2.7-MO with 10 and 20 pg of Nkx2.5 mRNA in embryos decreased the occurrence of heart defects from 55% to 32% and 22%, respectively (Table 2), indicating that the defective phenotype of Nkx2.7 morphants was specifically rescued by Nkx2.5 mRNA. Co-injection of 8 ng Nkx2.5-MO plus 8 ng Nkx2.7-MO with 25 and 50 pg of Nkx2.5 mRNA in embryos decreased the occurrence of heart defects from 91% to 73% and 58%, respectively (Table 2). Furthermore, co-injection of 8 ng Nkx2.5-MO plus 8 ng Nkx2.7-MO with 0.25 and 0.5 pg of Nkx2.7 mRNA in embryos decreased the occurrence of heart defects from 92% to 61% and to 43%, respectively (Table 2), indicating that the heart defects in embryos were rescued specifically by Nkx2.5- and Nkx2.7- mRNA. Taken together, the decrease of heart defects across samples indicates that Nkx2.7 and Nkx2.5 function redundantly during the heart development of zebrafish embryos. However, since rescue experiments were performed by substantially decreasing the concentration of Nkx2.7 mRNA, we can conclude that Nkx2.7 plays a functionally more predominant role in heart formation.
Table 2. Functional redundant activities between Nkx2.5 and Nkx2.7 in zebrafish heart development.
| Morpholino (ng) | mRNA (pg) | Total (n) | Heart Defects (%) | ||
| Nkx2.5 | Nkx2.7 | Nkx2.5 | Nkx2.7 | ||
| 0 | 9 ng | 0 | 0 | 177 | 55% |
| 0 | 9 ng | 10 pg | 0 | 109 | 32% |
| 0 | 9 ng | 20 pg | 0 | 113 | 22% |
| 8 ng | 8 ng | 0 | 0 | 146 | 91% |
| 8 ng | 8 ng | 25 pg | 0 | 278 | 73% |
| 8 ng | 8 ng | 50 pg | 0 | 169 | 58% |
| 8 ng | 8 ng | 0 | 0 | 166 | 92% |
| 8 ng | 8 ng | 0 | 0.25 pg | 189 | 61% |
| 8 ng | 8 ng | 0 | 0.5 pg | 206 | 43% |
For rescue study, the Nkx2.5 mRNA was co-injected with Nkx2.7-MO into embryos derived from zebrafish transgenic line Tg(cmlc2::GFP). The percentages of heart defects were decreased compared to phenotypes which occurred in the embryos injected with Nkx2.7-MO alone. Similarly, either the Nkx2.5 or Nkx2.7 mRNA enabled embryos to be rescued from the defects as a result of the injection of Nkx2.5/2.7-MO. n: total number of embryos analyzed.
Nkx2.5 was overexpressed in the Nkx2.7-MO-injected embryos
In the wild-type embryos, Nkx2.5 was expressed predominantly in the ventricle and weakly in the atrium at 48 hpf (Fig. 2A), limiting ventricle expression when embryos developed at 72 hpf (Fig. 2C). Unlike the Nkx2.5 expression in the wild-type, Nkx2.7-knockdown morphants retained robust Nkx2.5 expression in ventricle (Figs. 2B and 2D). Because Nkx2.7 was knocked down and Nkx2.5 was overexpressed, these results also support the hypothesis that Nkx2.5 and Nkx2.7 play similar roles in cardiac morphogenesis in zebrafish and that, moreover, in the absence of one, the other can compensate for the loss of function.
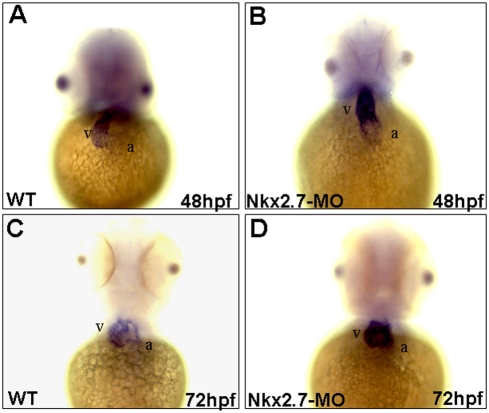
Figure 2. Nkx2.5 was overexpressed in the Nkx2.7-knockdown embryos.
Nine nanograms of Nkx2.7-MO were injected into one-cell stage of embryos. Nkx2.5 was expressed predominantly in ventricle (v) and weakly in atrium (a) of wild-type (WT) embryos at 48 hpf (A), but only minimally and weakly in ventricle at 72 hpf (C). However, the Nkx2.7-MO-injected embryos retained robust Nkx2.5 expression in ventricle both at 48 and 72 hpf (B and D). The embryonic stages were as indicated, and embryos were observed ventrally. v: ventricle; a: atrium.
The ventricle became smaller and consisted of a single layer in the Nkx2.5 and Nkx2.7 double knockdown embryos
In addition to monitoring cardiac morphology by using heart-tagged GFP zebrafish line Tg(cmlc2::GFP), we used vmhc as a ventricle-specific probe to determine the ventricular cell fate. Compared to the wild-type, the ventricle of the Nkx2.5 and Nkx2.7 double knockdown embryos became smaller when they were observed at 72 hpf. However, the expression level of vmhc in the heart of the Nkx2.5/2.7- MO-injected embryos was similar to that of wild type (Figs. 3A and 3B). It is worth noting that neither WT nor Nkx2.5/2.7 morphant embryos expressed vmhc in the head muscle until 72 hpf. Therefore, the Nkx2.5/2.7-MO morphants did not appear less developed than WT embryos. This evidence demonstrates that, although the ventricular fate is properly determined in the Nkx2.5/2.7-MO-injected embryos, their ventricular morphogenesis is abnormal.
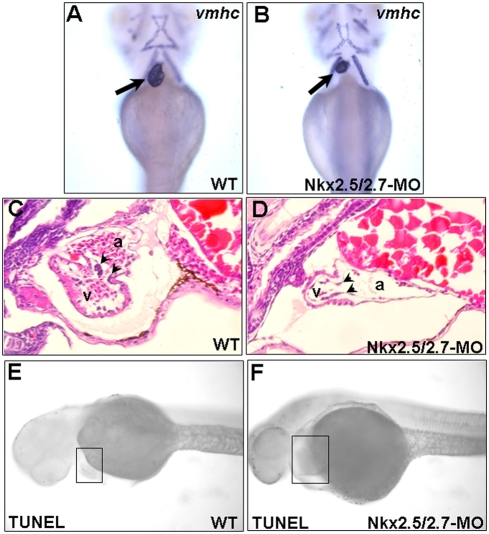
Figure 3. Ventricle becomes smaller and consists of a single layer in the Nkx2.5 and Nkx2.7 double knockdown morphants (Nkx2.5/2.7-MO).
Ventricular myosin heavy chain (vmhc) was used as a probe to detect the ventricle morphology in (A) wild-type (WT) and (B) Nkx2.5/2.7-MO embryos at 72 hpf. The ventricle of Nkx2.5/2.7-MO embryos was smaller than that of WT embryos (indicated by arrows). Hematoxylin and eosin staining showed that ventricular myocardium of WT was two or more cell layers in thickness. However, only one cell layer was retained in the ventricular myocardium of the Nkx2.5/2.7-MO embryos. In addition, compared to wild-type embryos, the endocardium of Nkx2.5/2.7-MO-injected embryos did not form endocardial cushion (indicated by arrowheads in C and D). Like WT embryos, TUNEL assay did not display the increase of TUNEL-positive cells in the heart region of Nkx2.5/2.7-MO embryos at 40 hpf (indicated by boxes in E and F). Embryos were observed ventrally (A–B) or laterally (C–F). v: ventricle; a: atrium.
In order to understand why the ventricle of Nkx2.5/2.7-MO-injected embryos is smaller than that of wild-type embryos, we performed a histological examination of the 72-hpf embryos. Compared to the wild-type (Fig. 3C), a thinning layer of myocardium with fewer cell numbers in the ventricle was observed in the Nkx2.5/2.7 morphants (Fig. 3D). Additionally, the endocardium was not observed to form an endocardial cushion in these morphants (Fig. 3D). Finally, the TUNEL assay demonstrated that the decrease of myocardium cell numbers in the ventricle did not result from cell death, but rather from defective proliferation of ventricular myocardium, which led to a smaller heart with a thinning layer (Figs. 3E and 3F).
Heart deterioration occurring in the Nkx2.5/2.7 morphants results from defective myocardial differentiation
The expressions of tbx5 and hand2 in the cardiac precursor regions were normal in the Nkx2.5/2.7-MO-injected embryos at 12 hpf (Figs. 4A and 4C vs. 4B and 4D). These two markers were present in the bilateral regions of the lateral plate mesoderm and showed the correct anterioposterior localization with the same expression level. Thus, the early cardiac marker genes were transcribed normally in the Nkx2.5/2.7 morphants.
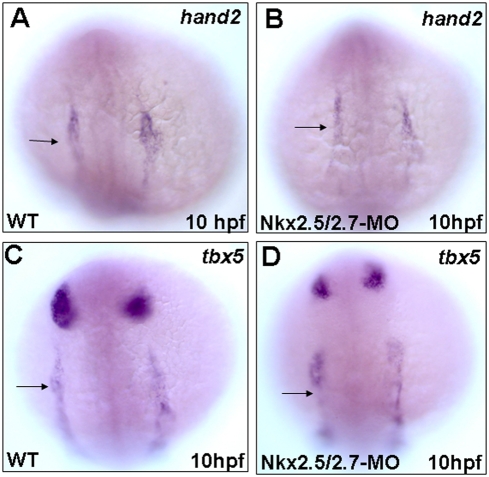
Figure 4. Early cardiac markers express normally in the Nkx2.5 and Nkx2.7 double knockdown morphants (Nkx2.5/2.7-MO).
Whole mount in situ hybridization showed that the expression patterns of hand2 and tbx5, lateral plate mesoderm markers, were similar between wild-type (WT) and Nkx2.5/2.7-MO embryos at 10 hpf (A vs. B; C vs. D, respectively). Arrows: heart field.
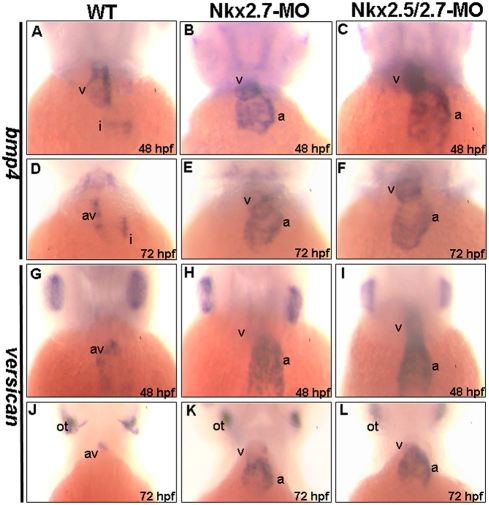
We also observed myocardial differentiation markers, such as bmp4, versican, tbx5, and tbx20. BMP4 is one of the TGF-β superfamily proteins and is involved in valve development during the cardiac maturation stage. A ventricle-enriched expression of bmp4 was the same between wild-type and Nkx-deficient embryos from 31 to 33 hpf (data not shown). Moreover, in wild-type embryos, bmp4 was found to express in the ventricle and inflow tract at 48 hpf (Fig. 5A), and then it was expressed exclusively in the atrioventricular junction at 72 hpf (Fig. 5D). However, unlike the dynamic expression of bmp4 in the wild-type embryos, bmp4 in the Nkx2.7 and in the Nkx2.5/2.7 morphants failed to change its predominant ventricular and atrial expression pattern (Figs. 5B and 5E; 5C and 5F). Similarly, versican was expressed broadly in the atrium and weakly in the ventricle of the wild-type embryos at 33 hpf. This atrium-enriched expression of versican was the same between wild-type and Nkx-deficient embryos (data not shown). After 36 hpf, the versican expression of wild-type was restricted to the AV boundary (Figs. 5G and 5J). However, Nkx2.7 and the Nkx2.5/2.7 morphants still expressed a high level versican in the atrium of the heart from 48 to 72 hpf (Figs. 5H and 5K; 5I and 5L). Nevertheless, we noticed that the versican was expressed in otoliths of both the wild-type embryos and the Nkx-deficient embryos by 72 hpf (Figs. 5J, 5K and 5L), indicating that the development of Nkx-deficient embryos was not delayed and that the defects caused by Nkx-MO were specific in the heart, not in otoliths (Figs. 5J, 5K and 5L).
Figure 5. Abnormal cardiac differentiation occurred in the Nkx2.7-knockdown zebrafish embryos.
The expressions of bmp4 (A–F) and versican (G–L) in hearts were compared between wild-type (WT) (A, D, G, J), Nkx2.7-MO- (B, E, H, K) and Nkx2.5/2.7-MO- injected embryos (C, F, I, L) at 48 (A–C, G–I) and 72 hpf (D–F, J–L). In WT embryos, bmp4 was expressed in the ventricle and inflow tract at 48 hpf (A), and then bmp4 was restricted in its expression at the AV boundary at 72 hpf (D). However, in the Nkx2.7-MO (B, E) and Nkx2.5/2.7-MO (C, F) embryos, bmp4 was still expressed predominantly in the ventricle and atrium from 48 to 72 hpf. Similarly, in WT embryos, the versican expression was more predominant in ventricle than in atrium, at about 31 to 33 hpf, and then versican was confined in its expression at the AV boundary after 33 hpf (G, J). In contrast, in the Nkx2.7-MO (H, K) and Nkx2.5/2.7-MO (I, L) embryos, the versican was significantly expressed in the atrium and ventricle. In addition, the versican expression pattern in otoliths remained unchanged (J, K, and L). All images are ventral views, anterior to the top. a: atrium; v: ventricle; i: inflow tract; av: atrioventricular boundary; ot: otoliths.
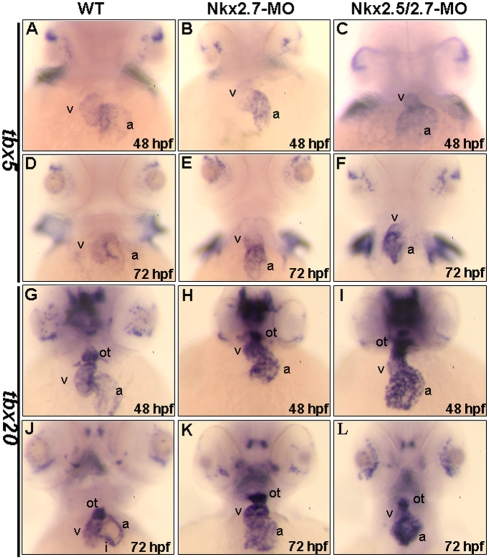
In the wild-type embryos, tbx5 expression was slightly greater in the atrium than the ventricle at 26 hpf, which was consistent with what Garrity (2002) reported [25]. Then, the gradient expression of tbx5 was changed from atrium-rich expression to ventricle-rich expression at 48 hpf (Figs. 6A and 6D). However, in the Nkx2.7 and Nkx2.5/2.7 morphants, the tbx5 expression remained atrium-rich at 48 hpf (Figs. 6B and 6C), and even beyond 48 hpf (Figs. 6E and 6F). In an overview of the whole heart, the expression level of tbx5 was greater in morphants than the wild-type embryos. Although the dynamic change of tbx5 expression pattern from atrium to ventricle in the wild-type at later stages did not occur in the Nkx2.7 or the Nkx2.5/2.7 morphants, the expression level of tbx5 in pectoral fin bud of morphants was the same as wild-type embryos.
Figure 6. Chamber maturation was affected in the Nkx2.7-knockdown zebrafish embryos.
The expressions of tbx5 (A–F) and tbx20 (G–L) in hearts were compared between wild-type (WT) (A, D, G, J), Nkx2.7-MO- (B, E, H, K) and Nkx2.5/2.7-MO- injected embryos (C, F, I, L) at 48 (A–C, G–I) and 72 hpf (D–F, J–L). In WT embryos, tbx5 was expressed strongly in ventricle, but weakly in atrium at 48 hpf and beyond (A, D). However, in the Nkx2.7-MO (B, E) and Nkx2.5/2.7-MO (C, F) embryos, tbx5 retained its strong expression in the heart, although the expression pattern was gradually changed from ventricle-enriched expression to atrium-enriched expression from 48 hpf to 72 hpf (B and E; C and F). In WT embryos, tbx20 expression was similar to the expression gradient of tbx5 in the heart at 48 hpf, and the tbx20 expression was stronger than that of tbx5. In addition, tbx20 expression was also detected in outflow tract at 48 hpf (G), and tbx20 expression was restricted to outflow tract and inflow tract by 72 hpf (J). However, in Nkx2.7-MO (H, K) and Nkx2.5/2.7-MO (I, L) embryos, tbx20 was expressed strongly in the heart and outflow tract from 48 to 72 hpf. All images are ventral views, anterior to the top. v: ventricle; a: atrium. ot: outflow tract; i: inflow tract.
We also detected another myocardial differentiation marker, tbx20, which was expressed during cardiac development. In the wild-type, this marker was expressed more predominantly in the ventricle than in the atrium at 48 hpf (Fig. 6G). The expression of tbx20 was confined to the outflow tract, AV canal and inflow tract at 72 hpf (Fig. 6J); however, its expression in the Nkx2.7 and the Nkx2.5/2.7 morphants maintained a strong signal in the ventricle and atrium of the heart (Figs. 6H and 6K; 6I and 6L). While this behavior was unlike the tbx20 expression patterns in the wild-type embryos, the expression level of tbx20 in the outflow tract of morphants was, nevertheless, the same as wild-type embryos. Still, we noticed that, like wild-type embryos, the expression level of tbx20 in tegmentum of the Nkx2.7 and Nkx2.5/2.7 morphants started to gradually decrease from 48 to 72 hpf (Figs. 6J, 6K and 6L), indicating that, although all these morphants developed to later stages without delay, they also exhibited specific heart defects.
In summary, we examined the expression patterns of myocardial differentiation markers, including bmp4, versican, tbx5 and tbx20, in the Nkx2.7-MO- and Nkx2.5-/2.7-MO-injected embryos. While the expression levels of these markers were the same as those at the early stage of the wild-type, we found that they became more intensive than those of the wild-type at the later stage, suggesting that myocardial differentiation is defective in the morphants. We also noticed that the hearts of these Nkx2.7- and Nkx2.5/2.7-MO-injected embryos finally deteriorated and became string-like in form without undergoing further development. Taken together, this line of evidence suggests that the late onset of myocardial differentiation results in heart defects at a later maturation stage of cardiogenesis in the Nkx-knockdown embryos.
Overexpression of Nkx2.7 or Nkx2.5 causes cardiac defects similar to those induced by Nkx-knockdown
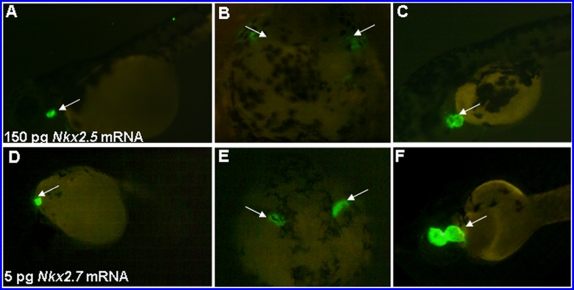
To examine whether Nkx2.7 plays roles similar to those of Nkx2.5 in cardiogenesis, we studied the effect of Nkx overexpression on heart development by injection of either Nkx2.7 or Nkx2.5 mRNA into one-celled stage embryos derived from transgenic line Tg(cmlc2::GFP), which has heart-specific GFP. By fluorescence microscopy, we observed that overexpression of 5 pg Nkx2.7 mRNA caused defective phenotypes similar to overexpression of 150 pg Nkx2.5 mRNA. These defects included a small patch of cardiac cells (64% in the 150-pg-Nkx2.5-overexpressed embryos; 28% in the 5-pg-Nkx2.7-overexpressed embryos), malposition of cardiac migration (13% in the 150-pg-Nkx2.5-overexpressed embryos; 2% in the 5-pg- Nkx2.7-overexpressed embryos), and an enlarged heart (18% in the 5-pg- Nkx2.7-overexpressed embryos) (Figs. 7A–F). Moreover, injection of Nkx2.5 mRNA in the amount of 150 pg also caused 41% of embryos (62/150) to have a dorsoventral axial defect, whereas injection of Nkx2.5 mRNA in the amount of 50 pg caused 22% of embryos (30 out of 135) to have an enlarged heart (data not shown). Based on this evidence, we suggest that Nkx2.7 carries out functions similar to those of Nkx2.5, but that Nkx2.7 appears to play a more prominent role in zebrafish heart development. This conclusion is based on the contrasting concentrations of mRNA required to generate similar heart defects; i.e., 5 pg Nkx2.7 mRNA vs. 150 pg Nkx2.5 mRNA.
Figure 7. Overexpression of Nkx2.5 and Nkx2.7 in embryos resulted in serious heart defects.
Amounts equaling 150 pg Nkx2.5 mRNA (A–C) and 5 pg Nkx2.7 mRNA (D–F) were injected individually into one-celled stage embryos derived from transgenic line Tg(cmlc2::GFP), whose hearts were specifically tagged with green fluorescent protein. We observed that there were many phenotypes of heart defects at 48 hpf resulting from the overexpression of either Nkx2.5 mRNA or Nkx2.7 mRNA. These defects included malposition of the reducing heart (A, D), bilateral heart (B, E), small heart (C) and large heart (F). Interestingly, overexpression by injection of Nkx2.7 mRNA as low as 5 pg caused an effect similar to that induced by injection 30 greater times than that of Nkx2.5 mRNA. (A, C, D, F), lateral views; (B, E), dorsal views; Arrows: green fluorescent heart.
The expressions of tbx5 and tbx20 are modulated by Nkx2.7
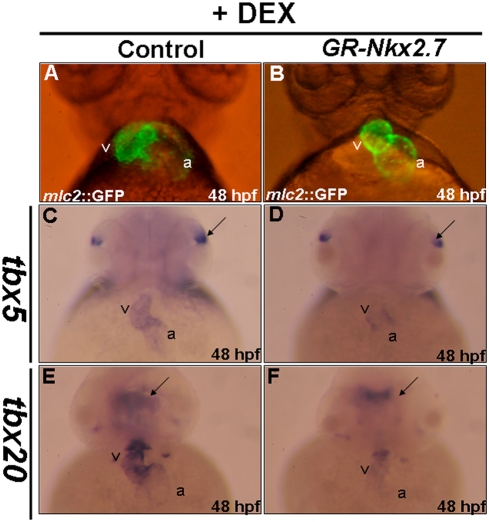
In addition to the cardiac defects occurring in the embryos that were overexpressed in either Nkx2.5 or Nkx2.7, we noticed that embryos injected with 150 pg of either Nkx2.5 or Nkx2.7 mRNA suffered a severe defect in ventralization (data not shown). However, this ventralization defect was not observed in the embryos that were treated with either Nkx2.7- or/and Nkx2.5-knockdown. This inconsistency between loss- and gain-of-function of Nkx2.5 or Nkx2.7 may result from the possibility that either Nkx2.7 or Nkx2.5 plays additional roles in zebrafish heart development before the late-gastrula period. Therefore, in order to further confirm the specific effects caused by the overexpression of Nkx2.7 in the heart after gastrulation, we constructed a dexamethasone-inducible plasmid in which the ligand-binding domain of GR was fused with the coding region of Nkx2.7. When we added dexamethasone to the 10-hpf embryos derived from the transgenic line Tg(cmlc2::GFP), these embryos were fixed at 48-hpf. Results showed that the cardiac maturation was defective, including an unlooping or shrunken heart (Fig. 8B), although the dorsoventral axis formation was not affected in the GR-Nkx2.7-produced embryos (Figs. 8D and 8F). We also noticed that, unlike wild-type embryos, the expressions of tbx5 and tbx20 in these GR-Nkx2.7-produced embryos were reduced at 48 hpf (Figs. 8D and 8F). Moreover, compared to the unchanged expression levels of tbx5 in eyes (Fig. 8C vs. 8D) and tbx20 in brain (Fig. 8E vs. 8F), the effect of Nkx2.7 overexpression on the reduced expressions of tbx5 and tbx20 was heart specific. This evidence strongly suggests that Nkx2.7 modulates the expression of tbx5 and tbx20, which, in turn, affects cardiac differentiation.
Figure 8. The expressions of tbx5 and tbx20 were modulated by Nkx2.7.
Each embryo derived from transgenic line Tg(cmlc2::GFP), whose heart was specifically tagged with green fluorescent protein, was injected with 0.15 ng of plasmid, in which Nkx2.7 mRNA was transcribed conditionally by adding dexamethasone. All embryos were treated with dexamethasone at 10 hpf and took a ventral view at 48 hpf to observe the cardiac development under the fluorescence microscope. The cardiac morphologies of the control embryos and the GR-Nkx2.7-overexpression embryos were shown. Compared to the control (A), various cardiac defects were found in around 35% of embryos from the GR-Nkx2.7-overexpression group, including unlooping and shrunken heart (B). In addition, the expressions of tbx5 (C, D) and tbx20 (E, F) were down-regulated in the GR-Nkx2.7-overexpresion embryos. However, the expression levels of tbx5 in eyes (indicated by arrows in C and D) and tbx20 in brain (indicated by arrows in E and F) remained unchanged. v: ventricle; a: atrium.
Discussion
Using two Nkx2.5- and Nkx2.7-specific MOs, we demonstrated that Nkx2.5 and Nkx2.7 are required for normal heart development. Prior to the onset of cardiac looping, cardiogenesis progresses normally in both Nkx2.7-MO and Nkx2.5/2.7-MO, indicating that Nkx is not an essential requirement for the early stage of heart development. However, at 36 hpf, when heart development reaches the looping stage, Nkx2.7 morphants showed an unlooping defect. Moreover, Nkx2.5/2.7 morphants not only displayed an unlooping defect, but also a shrunken ventricle. In addition, the expression of differentiated marker genes, including bmp4, versican, tbx5 and tbx20, was abnormal either in the ventricle or in both the ventricle and atrium (Figs. 5 and 6). Although the formation of two distinct chambers was enabled in Nkx2.7 and Nkx2.5/2.7 morphants, the hearts were not processed further, remaining, instead, a linear tube, resulting in abnormal contractions and arrhythmia.
Nkx2.7, a member of the tinman family of related genes, contains the tinman-like amino terminal decapeptide, homeobox, and NK2 domain. Nkx2.7 exhibits temporal and spatial expression patterns very similar to Nkx2.5 during zebrafish cardiac development, although the onset of Nkx2.7 at the 10.5 hpf comes somewhat earlier than that of Nkx2.5. In addition, Nkx2.7 is also detected with a co-localized expression pattern at pharyngeal endoderm progenitors like Nkx2.3 [19]. These expression profiles indicate that Nkx2.7 and Nkx2.5 are closely associated in the roles they play in the context of the regulation of cardiac differentiation.
In Drosophila, tinman is essential for primary cell lineage determination and early morphogenesis. Embryos that lack tinman function do not develop any dorsal vessel or gut muscle progenitor cells [6]. Nkx2.5, the mouse homolog of tinman, is an early marker of cardiomyocyte precursors. In the knockout of Nkx2.5 by gene targeting techniques, mouse embryos are arrested at the looping stage [7]. Surprisingly, this mouse Nkx2.5-knockout embryo does not exhibit the same defective phenotype of tinman null embryos, indicating that there are other Nkx2.5 homologs to compensate for Nkx2.5 loss of function in mouse heart development. For example, Nkx2.6 compensates for Nkx2.5 functions in the pharyngeal endoderm of mouse embryos, but not in hearts [14]. Recently, mouse Nkx2.7 was found (GeneID: 108060). Thus, it is worthwhile to study whether mouse Nkx2.7 functions redundantly with mouse Nkx2.5 in a way that is similar to zebrafish Nkx2.7 and Nkx2.5. In Xenopus, Cleaver et al. (1996) [9] suggested that XNkx2.3 and XNkx2.5 play the same roles in cardiac development. In zebrafish, we demonstrate in this study that Nkx2.7 does compensate for the loss of Nkx2.5 function in cardiac morphogenesis.
Recently, Targoff et al. (2008) [26] demonstrated that Nkx2.5/Nkx2.7 morphants of zebrafish are well developed at early embryonic stage; however, morphants fail to elongate normally at heart tube stage. Thus, the authors concluded that abnormal heart tube extension will cause cardiac differentiation effects on ventricular and atrial cell numbers. In this study, we also observed similar phenotypes of Nkx2.5/Nkx2.7 morphants in that the heart tubes of Nkx2.5/Nkx2.7 morphants cannot extend further and the ventricle portion displays shorter and wider than that of wild-type embryo. However, in our study, we described the defective phenotypes and studied the functions of Nkx2.5/Nkx2.7 in more detail. To summarize, we first observed the cardiac phenotype at various developmental stages, including the period from 26 to 55 hpf when zebrafish ventricle is undergoing differentiation and proliferation. Targoff et al. (2008) [26], on the other hand, did not investigate this important period of time. Second, by taking the advantage of zebrafish transgenic line Tg(cmlc2::GFP), which possesses the heart-specific GFP signal, we were able to observe the dynamic change of ventricular morphology in live embryos. In so doing, we found that the ventricle of Nkx2.5/Nkx2.7 morphants retained the pre-mature phase during this period rather than undergoing further cardiac morphogenesis. Thus, we suggested that the differentiation and proliferation of ventricle in Nkx2.5/Nkx2.7 morphants may cease after 26 hpf, resulting in the decrease of cell numbers of ventricle at the later stages. Third, by detecting the differentiated markers, such as versican, bmp4, tbx5 and tbx20, we also found that the expression patterns of these markers in Nkx-deficient embryos were the same as those of wild type at early heart tube stage. However, the expressions of these markers in Nkx-deficient embryos were not restrained to the places where they should be confined at later cardiac differentiation stage. Based on these lines of evidence, we suggest that the decrease of ventricular myocardial proliferation and the defective myocardial differentiation both result from the up-regulation of bmp4, versican, tbx5 and tbx20 at late stage, even though these genes are all expressed normally in hearts at an early heart tube stage. Fourth, we performed a histological examination of the 72-hpf embryos and found that, compared to the wild-type, a thinning layer of myocardium with fewer cell numbers in the ventricle was observed in the Nkx2.5/2.7 morphants and that the endocardium of morphants did not form an endocardial cushion (Figs. 3C and 3D). Fifth, by using histological section of the Nkx2.5/2.7-MO-injected embryos, we found that the formation of atrioventricular valves was abnormal at 72 hpf (Fig. 3D).
We observed that the heart of Nkx2.5/2.7-MO double-knockdown morphants appeared to have a vigorous and rhythmic peristaltic contraction initially. Subsequently, however, the hearts of these double knockdown morphants failed to undergo looping morphogenesis, and the contractile function diminished progressively. Moreover, blood was regurgitated within the heart chamber after 72 hpf, and the heart became silent with cessation of blood circulation by 84 hpf. These defective phenotypes could have resulted from valve dysfunction. Interestingly, both bmp4 and versican are restricted to atrioventricular boundary of heart at the later stage of heart development [27]. Both genes are involved in cardiac valve formation and both are examined in this study. Moreover, it has been found that mutation of Apc [28] or NXT2 [29] of zebrafish showed that expression of bmp4 and versican genes are upregulated and that domains are expanded throughout the hearts. Similarly, in this study, we found that bmp4 and versican are dramatically upregulated and that the domains of expression are greatly expanded to the ventricle in the hearts of Nkx2.5/2.7 morphants. Therefore, we speculate that blood regurgitation occurring in the Nkx2.5/2.7 morphants' hearts resulted from valve dysfunction, which, in turn, causes the defective morphogenesis and function of heart.
Prall et al. (2007) [30] used cDNA microarray analysis to compare the transcripts of mouse Nkx2.5 heterozygous and null mutant embryos from E8.0 to E9.5 and found that tbx5 is upregulated two-fold greater in null embryos at E8.0-E8.5. We found that both Nkx2.7 and Nkx2.5/2.7 morphants exhibit overexpression of tbx5 and tbx20 in heart, suggesting that Nkx2.5 and Nkx2.7 may be negative modulators of tbx5 and tbx20 in zebrafish. Therefore, the finding of Prall et al. supports our present study. On the other hand, Prall et al. (2007) [30] also demonstrated that there is an Nkx2.5/Bmp2/Smad1 negative loop pathway to regulate heart precursor specification and proliferation. They pointed out that Nkx2.5 null mutants cause over-specification of cardiac progenitors and that the area of over-specification of cardiac progenitors appears to be a broad expression of early cardiac markers in the cardiac crescents. Most importantly, Prall et al. (2007) [30] further stated that impaired proliferation of secondary heart field-derived cells, which contribute to most ventricle cells, results from over-specification of cardiac progenitors. For this reason, Nkx2.5 null mutants have a thin ventricular myocardium.
However, in our study, the expression of early cardiac markers, tbx5 and hand2, do not exhibit expansion of cardiac progenitors (Figs. 4A–4D), indicating that the over-specification of cardiac progenitors does not occur. Instead, the Nkx2.5/2.7-MO-injected embryos consist of a single layer of ventricle, which does not result from over-specification of progenitors in a manner similar to mouse Nkx2.5 null mutants. At the same time, tbx5 was overexpressed in the ventricle and atrium of zebrafish (Fig. 6F). The fact that overexpression of tbx5 in the heart inhibits cardiomyocyte proliferation has been demonstrated in mice [31] and chicks [32], suggesting that the function of tbx5 acts as growth arrest signal to regulate cellular proliferation. Thus, it is reasonable to conclude that the thin-layer of heart of zebrafish Nkx2.5/2.7 morphants may result from the effect of higher expression of tbx5. Therefore, we reason that the heart defect induced by the knockdown of Nkx2.5/2.7 is more likely to be caused by the late onset of cardiac differentiation. It was also demonstrated in this study that Nkx2.7 is a modulator of tbx5 and tbx20 expression, which further supports the conclusion noted above and gives added support to the prominent role Nkx2.7 plays in cardiac morphogenesis.
Chen and Fishman (1996) [10] reported that overexpression of Nkx2.5 (100 pg) in zebrafish causes a large hyperplastic heart with normal function and chamber morphology. Moreover, injection of a higher dose (250 pg) of Nkx2.5 mRNA affected the dorsoventral axis of embryos severely. Cleaver et al. (1996) [9] also demonstrated that overexpression of Nkx2.5 and Nkx2.3 in Xenopus resulted in a hyperplastic heart. In our study, injection of 150 pg of Nkx2.5 mRNA or 5 pg of Nkx2.7 mRNA obtained results similar to Chen and Fishman (1996) [10]. Injection of 50 pg of Nkx2.5 mRNA caused 22% of embryos (30 out of 135; 30/135) to have an enlarged heart and injection of 150 pg of Nkx2.5 mRNA caused 64% of embryos (67/104) to have a reduced heart, whereas injection of 5 pg of Nkx2.7 mRNA caused 18% (32/182) and 28% (51/182) of embryos to have a reduced heart and an enlarged heart, respectively. Our data are supported by the paper published by Chen et al. (1996) [10], who demonstrated that embryos injected with 100 pg Nkx2.5 mRNA showed an enlarged heart, but embryos injected with 250 pg Nkx2.5 mRNA displayed bilateral beating heart, reduced heart or dorsal-ventral axial defects. We speculate that ectopic expression of Nkx2.5 mRNA at the one-cell stage results in nonspecific defects in zebrafish embryos. For example, there are no reports about Nkx2.5 function in dorsal-ventral axis formation. Based on this point, we suggest that Nkx2.7 carries out functions similar to those of Nkx2.5, but, because there may be many unexpected effects on embryos from overexpression of either Nkx2.5 or Nkx2.7, we performed a specific knockdown of morpholino and a GR-inducible assay to learn the exact functions of Nkx2.5 and Nkx2.7 in zebrafish development. Specifically, we note that injection of 5 pg Nkx2.7 mRNA results in a phenotype similar in defect to the injection of 150 pg Nkx2.5 mRNA (Fig. 7). Compared to the injection dosage of Nkx2.5 mRNA, we notice that only a one-thirtieth concentration of Nkx2.7 mRNA is required to rescue the defects induced by Nkx2.5 and 2.7 double knockdowns (Table 2), suggesting that Nkx2.7 has more effective function than Nkx2.5.
Finally, in the loss-of-function experiment, the ventralized embryos were not observed in the Nkx2.5/2.7-knockdown embryos. In contrast, these results were observed in the gain-of-function experiment, in which Nkx2.5 and Nkx2.7 were overexpressed. However, based on the GR-Nkx2.7 inducible experiment where Nkx2.7 is induced after gastrulation, we proved that Nkx2.7 has a specific effect on heart development, but not axis formation (Fig. 8). Therefore, we speculate that this inconsistency between gain-of-function and loss-of-function experiments may result from the fact that Nkx2.5 and Nkx2.7 are not expressed until late-gastrula stage. However, since either the overexpressed Nkx2.5 or Nkx2.7 is injected at the one-cell stage, these misexpressional genes can cause abnormal axis formation in embryogenesis. In conclusion, we find that Nkx2.7 and Nkx2.5 act as functional homologs of tinman in the zebrafish embryos. While Nkx2.5 and Nkx2.7 play redundant roles in cardiac morphogenesis, Nkx2.7 appears to have a more critical function in its effect on cardiac differentiation, as indicated by the gain-of-function and loss-of-function experiments where Nkx2.7 is observed to regulate the expressions of tbx5 and tbx20 through the heart tube stage.
Materials and Methods
Fish husbandry and observation
The wild-type AB strain [33] and the transgenic line Tg (cmlc2::GFP) [34] of zebrafish were cultured at 28.5°C. Embryos were staged by hours post fertilization (hpf) [35]. The phenotype of heart formation was observed under a fluorescent stereomicroscope, MZ FLIII (Leica). Images were captured with a Fine pix S2 pro camera (Nikon) using the Camera Shooting software.
Morpholino (MO) knockdown
The MOs designed specifically for blocking the translation of Nkx2.5 and Nkx2.7 mRNAs were TCATTTGGCTAGAGAACATTGCCAT (Nkx2.5-MO) and GTCACAGGACTCGGAAGCATCGTGC (Nkx2.7-MO), respectively. A control MO specific for Nkx2.7 was designed as GTgACAcGACTCcGAAGgATCGTcC (Nkx2.7-MO-control), in which a 5-bp mismatched sequence of Nkx2.7 is indicated in lower case. All MOs were prepared at a stock concentration of 1 mM and diluted to the desired concentration for microinjection into each embryo.
Plasmid construct
In order to further demonstrate the specificity of MO targeting, we constructed plasmids of pCS2-Nkx2.5-GFP and pCS2-Nkx2.7-GFP, in which the binding sequence of either Nkx2.5-MO or Nkx2.7-MO was fused with the GFP reporter cDNA. The NotI-cut pCS2-Nkx2.5-GFP, pCS2-Nkx2.7-GFP or pCS2-GFP served as a template to synthesize RNA using the mMessage Machine kit (Ambion). The synthesized RNA (100 pg per embryo) was co-injected with Nkx-specific MO or alone into one-celled zebrafish embryos. The appearance of GFP in the treated embryos was observed at the 24-hpf stage using green fluorescent microscopy.
In order to avoid nonspecific effect of the overexpressed Nkx2.7 in the early gastrula stage, we constructed a dexamethasone-inducible plasmid, pCS2GR-Nkx2.7, in which the human glucocorticoid receptor (GR) ligand binding domain was fused with Nkx2.7.
Whole-mount in situ hybridization and TUNEL assay
Whole-mount in situ hybridization was performed as previously described [36]. Antisense probes used in this study were as follows: versican [27]; bmp4 [37]; tbx5 [25]; tbx20 [38] and vmhc [39]. The TUNEL assay was performed as described previously [40] using The DeadEnd™ Colorimetric TUNEL System (Promega).
Rescue and overexpression experiments
Capped mRNA transcripts of Nkx2.5 and Nkx2.7 for either rescue or overexpression experiments were synthesized by SP6 in vitro transcription according to the protocol of the manufacturer (Epicentre). We synthesized the truncated Nkx2.5 and Nkx2.7 mRNAs that did not include the specific MO-target site to avoid affecting rescue efficiency. The resultant mRNAs were diluted to the desired concentration (from 150 to 250 pg/µl), and approximately 2.3 nl was microinjected into one-celled stage embryos. For the GR-induced experiment, capped mRNA of a NotI-cut CS2 template contained an insert of the human GR ligand binding domain fused with Nkx2.7. The resultant mRNAs were diluted to 2.3 nl of RNA at a concentration of 0.15 ng and were injected into one-celled embryos. When the injected embryos developed at gastrula stage (10-hpf), we immersed embryos with 10 um dexamethasone in phenylthiourea water to induce the synthesis of GR-Nkx2.7.
Histology
For paraffin section, embryos were collected at 72 hpf and fixed in 4% PFA for 16 h. Then they were decalcified, dehydrated in a graded series of ethanol, and embedded in paraffin for sectioning into a 5-µm thickness [41]. The resultant sections were rinsed in distilled water, washed with 0.01 M phosphate buffered saline, and stained with hematoxylin and eosin.
Acknowledgments
We are grateful to Dr. Anna-Pavlina Haramis for providing the bmp4 and versican probes, to Dr. David Kimelman for providing the plasmid CS2-GR and tbx20 probe, to Miss Shuan Tseng (Veterinary Hospital, NTU) for helping with the paraffin section.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This paper was partially supported by the grant from National Science Council, ROC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stainier DY. Zebrafish genetics and vertebrate heart formation. Nat Rev Genet. 2001;2:39–48. doi: 10.1038/35047564. [DOI] [PubMed] [Google Scholar]
- 2.Bruneau BG. Transcriptional regulation of vertebrate cardiac morphogenesis. Circ Res. 2002;90:509–519. doi: 10.1161/01.res.0000013072.51957.b7. [DOI] [PubMed] [Google Scholar]
- 3.Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 5.McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- 6.Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118:719–729. doi: 10.1242/dev.118.3.719. [DOI] [PubMed] [Google Scholar]
- 7.Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, et al. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 8.Schultheiss TM, Xydas S, Lassar AB. Induction of avian cardiac myogenesis by anterior endoderm. Development. 1995;121:4203–4214. doi: 10.1242/dev.121.12.4203. [DOI] [PubMed] [Google Scholar]
- 9.Cleaver OB, Patterson KD, Krieg PA. Overexpression of the tinman-related genes XNkx-2.5 and XNkx-2.3 in Xenopus embryos results in myocardial hyperplasia. Development. 1996;122:3549–3556. doi: 10.1242/dev.122.11.3549. [DOI] [PubMed] [Google Scholar]
- 10.Chen JN, Fishman MC. Zebrafish tinman homolog demarcates the heart field and initiates myocardial differentiation. Development. 1996;122:3809–3816. doi: 10.1242/dev.122.12.3809. [DOI] [PubMed] [Google Scholar]
- 11.Harvey RP. NK-2 homeobox genes and heart development. Dev Biol. 1996;178:203–216. doi: 10.1006/dbio.1996.0212. [DOI] [PubMed] [Google Scholar]
- 12.Biben C, Hatzistavrou T, Harvey RP. Expression of NK-2 class homeobox gene Nkx2-6 in foregut endoderm and heart. Mech Dev. 1998;73:125–127. doi: 10.1016/s0925-4773(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Yamasaki N, Izumo S. Phenotypic characterization of the murine Nkx2.6 homeobox gene by gene targeting. Mol Cell Biol. 2000;20:2874–2879. doi: 10.1128/mcb.20.8.2874-2879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka M, Schinke M, Liao HS, Yamasaki N, Izumo S. Nkx2.5 and Nkx2.6, homologs of Drosophila tinman, are required for development of the pharynx. Mol Cell Biol. 2001;21:4391–4398. doi: 10.1128/MCB.21.13.4391-4398.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komuro I, Izumo S. Csx: a murine homeobox- containing gene specifically expressed in the developing heart. Proc Natl Acad Sci USA. 1993;90:8145–8149. doi: 10.1073/pnas.90.17.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:419–431. doi: 10.1242/dev.119.2.419. [DOI] [PubMed] [Google Scholar]
- 17.Evans SM, Yan W, Murillo MP, Ponce J, Papalopulu N. tinman, a Drosophila homeobox gene required for heart and visceral mesoderm specification, may be represented by a family of genes in vertebrates: XNkx-2.3, a second vertebrate homologue of tinman. Development. 1995;121:3889–3899. doi: 10.1242/dev.121.11.3889. [DOI] [PubMed] [Google Scholar]
- 18.Buchberger A, Pabst O, Brand T, Seidl K, Arnold HH. Chick Nkx2-3 represents a novel family member of vertebrate homologues to the Drosophila homeobox gene tinman: differential expression of cNKx-2.3 and cNkx 2-5 during heart and gut development. Mech Dev. 1996;56:151–163. doi: 10.1016/0925-4773(96)00521-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee KH, Xu Q, Breitbart RE. A new tinman-related gene, nkx2.7, anticipates the expression of nkx2.5 and nkx2.3 in zebrafish heart and pharyngeal endoderm. Dev Biol. 1996;180:722–731. doi: 10.1006/dbio.1996.0341. [DOI] [PubMed] [Google Scholar]
- 20.Pabst O, Schneider A, Brand T, Arnold HH. The mouse Nkx2-3 homeodomain gene is expressed in gut mesenchyme during pre- and postnatal mouse development. Dev Dyn. 1997;209:29–35. doi: 10.1002/(SICI)1097-0177(199705)209:1<29::AID-AJA3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Reecy JM, Yamada M, Cummings K, Sosic D, Chen CY, et al. Chicken Nkx-2.8: a novel homeobox gene expressed in early heart progenitor cells and pharyngeal pouch-2 and -3 endoderm. Dev Biol. 1997;188:295–311. doi: 10.1006/dbio.1997.8641. [DOI] [PubMed] [Google Scholar]
- 22.Brand T, Andrée B, Schneider A, Buchberger A, Arnold HH. Chicken Nkx2-8, a novel homeobox gene expressed during early heart and foregut development. Mech Dev. 1997;64:53–59. doi: 10.1016/s0925-4773(97)00044-0. [DOI] [PubMed] [Google Scholar]
- 23.Newman CS, Krieg PA. tinman-related genes expressed during heart development in Xenopus. Dev Genet. 1998;22:230–238. doi: 10.1002/(SICI)1520-6408(1998)22:3<230::AID-DVG5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Fu Y, Yan W, Mohun TJ, Evans SM. Vertebrate tinman homologues XNkx2-3 and XNkx2-5 are required for heart formation in a functionally redundant manner. Development. 1998;125:4439–4449. doi: 10.1242/dev.125.22.4439. [DOI] [PubMed] [Google Scholar]
- 25.Garrity DM, Childs S, Fishman MC. The heartstrings mutation in zebrafish causes heart/fin Tbx5 deficiency syndrome. Development. 2002;129:4635–4645. doi: 10.1242/dev.129.19.4635. [DOI] [PubMed] [Google Scholar]
- 26.Targoff KL, Schell T, Yelon D. Nkx genes regulate heart tube extension and exert differential effects on ventricular and atrial cell number. Dev Biol. 2008;322:314–321. doi: 10.1016/j.ydbio.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh EC, Stainier DY. UDP-glucose dehydrogenase required for cardiac valve formation in zebrafish. Science. 2001;293:1670–1673. doi: 10.1126/science.293.5535.1670. [DOI] [PubMed] [Google Scholar]
- 28.Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, et al. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Zhang B, Hartenstein PA, Chen JN, Lin S. NXT2 is required for embryonic heart development in zebrafish. BMC Dev Biol. 2005;24:7. doi: 10.1186/1471-213X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, et al. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–959. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughan CJ, Basson CT. Molecular determinants of atrial and ventricular septal defects and patent ductus arteriosus. Am J Med Genet. 2000;97:304–309. doi: 10.1002/1096-8628(200024)97:4<304::aid-ajmg1281>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Liberatore CM, Searcy-Schrick RD, Yutzey KE. Ventricular expression of tbx5 inhibits normal heart chamber development. Dev Biol. 2000;223:169–180. doi: 10.1006/dbio.2000.9748. [DOI] [PubMed] [Google Scholar]
- 33.Westerfield M. The zebrafish book. Eugene: University of Oregon Press; 1995. [Google Scholar]
- 34.Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev Dyn. 2003;228:30–40. doi: 10.1002/dvdy.10356. [DOI] [PubMed] [Google Scholar]
- 35.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 36.Chen YH, Lee WC, Liu CF, Tsai HJ. Molecular structure, dynamic expression, and promoter analysis of zebrafish (Danio rerio) myf-5 gene. Genesis. 2001;29:22–35. doi: 10.1002/1526-968x(200101)29:1<22::aid-gene1002>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 37.Martínez-Barberá JP, Toresson H, Da Rocha S, Krauss S. Cloning and expression of three members of the zebrafish Bmp family: Bmp2a, Bmp2b and Bmp4. Gene. 1997;198:53–59. doi: 10.1016/s0378-1119(97)00292-8. [DOI] [PubMed] [Google Scholar]
- 38.Szeto DP, Griffin KJ, Kimelman D. HrT is required for cardiovascular development in zebrafish. Development. 2002;129:5093–5101. doi: 10.1242/dev.129.21.5093. [DOI] [PubMed] [Google Scholar]
- 39.Yelon D, Horne SA, Stainier DY. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol. 1999;214:23–37. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
- 40.Lin CY, Yung RF, Lee HC, Chen WT, Chen YH, et al. Myogenic regulatory factors Myf5 and Myod function distinctly during craniofacial myogenesis of zebrafish. Dev Biol. 2006;299:594–608. doi: 10.1016/j.ydbio.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 41.Moore JL, Aros M, Steudel KG, Cheng KC. Fixation and decalcification of adult zebrafish for histological, immunocytochemical, and genotypic analysis. Biotechniques. 2002;32:296–298. doi: 10.2144/02322st03. [DOI] [PubMed] [Google Scholar]