Abstract
Wild-type stomata are spaced by intervening cells, a pattern disrupted in the Arabidopsis mutant too many mouths (tmm). To determine the mechanism of wild-type spacing and how tmm results in pattern violations, we analyzed the behavior of cells through time by using sequential dental resin impressions. Meristemoids are stomatal precursors produced by asymmetric division. We show that wild-type patterning largely results when divisions next to a preexisting stoma or precursor are oriented so that the new meristemoid is placed away. Because this placement is independent of cell lineage, these divisions may be oriented by cell–cell signaling. tmm randomizes this orientation and releases a prohibition on asymmetric division in cells at specific locations, resulting in stomatal clusters. TMM is thus necessary for two position-dependent events in leaves: the orientation of asymmetric divisions that pattern stomata, and the control of which cells will enter the stomatal pathway. In addition, our findings argue against most previous hypotheses of wild-type stomatal patterning.
INTRODUCTION
How cell types are patterned is a fundamental question in development. Pattern generation requires the selection of initials and the acquisition of specific cell fates. In plants, these events are often controlled by position rather than cell lineage (Sylvester et al., 1996; Scheres and Benfey, 1999; Schnittger et al., 1999; Lee and Schiefelbein, 2000). Positional cues, which may be transmitted by intercellular signaling (Scheres, 1997), may be especially important in plants because plant cells do not usually migrate.
In addition to their significance for gas exchange, stomata are a valuable system for studying cell patterning. They are spaced apart from each other by at least one intervening cell (Sachs, 1991), and they are distributed throughout the epidermis, an accessible and planar tissue that often contains only a few cell types. Several Arabidopsis mutations are known to disrupt their patterning, and stomatal formation involves asymmetric divisions (Larkin et al., 1997).
Divisions in which the fates of the two daughter cells differ are considered to be asymmetric (Jan and Jan, 1998). Asymmetric divisions are important in plants for generating and placing many cell types (Gallagher and Smith, 1997; Scheres and Benfey, 1999). In many dicots, such as Arabidopsis, stomata originate from a stem cell–like precursor, the meristemoid (Bünning, 1956; Zhao and Sack, 1999). The meristemoid is formed in an asymmetric division and can itself divide asymmetrically (Sachs, 1991).
Four hypotheses have been discussed for stomatal spacing in dicotyledons (Sachs, 1979, 1991; Larkin et al., 1997; Croxdale, 2000). First, the placement of the cells that form meristemoids could be regulated so that they are spaced apart from each other. Alternately, a series of asymmetric divisions could produce a complete boundary of neighbor cells of the same cell lineage as the central stoma; in this case, division placement would be generated within the cell lineage and would not require communication with surrounding cells. Or, a stoma or its precursors might prevent adjacent cells from initiating the formation of new stomata by lateral inhibition (Bünning, 1956; Korn, 1993). Finally, occasional patterning “mistakes,” such as adjacent meristemoids, could be corrected by cell divisions that are oriented by cell signaling (Sachs, 1991).
None of these hypotheses has been evaluated systematically to determine the relative contributions of each possible mechanism. Moreover, dicot stomatal precursor cells are not well defined with respect to type, frequency, distribution, and the number and placement of asymmetric divisions. In general, little is known in plants about the roles of cell signaling and asymmetric divisions in cell patterning or about the genes that control these processes (Scheres and Benfey, 1999).
Mutations at several Arabidopsis loci result in stomata in direct contact (Yang and Sack, 1995; Geisler et al., 1998; Berger and Altmann, 2000). The too many mouths (tmm) mutant displays excess stomata, many of which are arranged adjacent to each other in cotyledons and leaves. However, the functions of the TMM gene product are not well defined, especially with respect to prevention of stomatal overproduction and cluster formation.
To determine how wild-type stomata are spaced and how the tmm mutation disrupts patterning, we monitored stomatal development by making sequential dental resin impressions. We show that wild-type patterning results largely from the position-dependent orientation of asymmetric divisions occurring next to a preexisting stoma or precursor. Our results also demonstrate that tmm randomizes the orientation of these divisions and disrupts the fate of the daughter cells.
RESULTS
One-Celled Spacing Pattern
To analyze the stomatal spacing pattern, the frequencies of stomata and other epidermal cells were scored in three progressively more distant rings of cells around each stoma. Table 1 shows that the ring abutting the stoma was essentially devoid of guard cells, whereas many more would be present if chance alone determined stomatal distribution. The frequencies in the outer two rings essentially matched those in the epidermis as a whole. Thus, the stomatal spacing pattern in Arabidopsis consists of a boundary of at least one cell around each stoma, and no pattern further from the stoma is apparent as measured by the ring technique.
Table 1.
Stomatal Spacing
| % of Rings That Contained at Least One Guard Cell (Observed, Predicteda)b
|
|||
|---|---|---|---|
| Organ | Ring 1 | Ring 2 | Ring 3 |
| Cotyledon | 0.5, 42c | 73, 83 | 80, 98 |
| First leaf | 0.4, 45c | 53, 75c | 100, 99 |
See Methods for formula.
The first ring consisted of all cells adjacent to the selected stoma. The second ring included all cells in contact with and outside of the first ring, etc. A total of 300 first rings, 100 second rings, and 20 third rings were scored.
What was observed was significantly different from what was predicted (P < 0.05).
Another stomatal pattern found in members of the Brassicaceae, such as Arabidopsis, is the anisocytic arrangement of the stomatal complex. The Arabidopsis stomatal complex is here defined as consisting of a stoma plus all adjacent epidermal cells. In anisocytic complexes, the stoma is surrounded by three epidermal cells, one of which is smaller than the other two (Figure 1, top right; Landré, 1972). However, unlike the universal one-celled spacing pattern, the anisocytic arrangement in Arabidopsis leaves was present in only 40% of all stomatal complexes. The remaining ones consisted of three-celled complexes with neighbor cells of roughly equal size, or the complexes contained two or four to six neighbor cells.
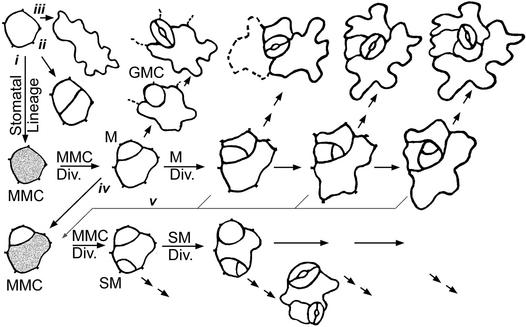
Figure 1.
Overview of Cell Types.
The stomatal lineage begins when a meristemoid mother cell (MMC; gray shading) forms and then divides asymmetrically, producing a meristemoid and a larger sister cell. Meristemoids divide asymmetrically zero to three times and then convert into guard mother cells (GMCs). The latter divide symmetrically, producing two guard cells. Smaller, less sinuous epidermal cells (upper left) become MMCs and divide asymmetrically (i), divide symmetrically (ii), or form wavy pavement cells (iii). Cells adjacent to a stoma or precursor can initiate a new stomatal lineage (iv and v), as can cells that are not adjacent. The first division of an MMC located next to a stoma or precursor is oriented so that the resulting satellite meristemoid is placed away (bottom left). Satellite meristemoids can divide zero to three times (only one division is illustrated in the bottom row). Neighbor cells that are clonally related to the stoma (see text) are drawn with solid rather than dashed lines (top row). The three stomatal complexes shown at top right are anisocytic. Div., division; M, meristemoid; SM, satellite meristemoid.
Stomatal Lineage
The dental resin technique made it possible to follow specific cells through time, to assign and quantify cell fates, and to reveal underlying commonalities. Stomatal development was found to be variable in the number, timing, location, and placement of divisions. Stomata are generated by a series of precursor cells that also produce other epidermal cell types. The relevant cell types and key events are shown in Figures 1 to 3. Figure 1 was derived from analysis of the formation of 400 stomata from 52 wild-type leaves and cotyledons. This analysis may require revision as molecular markers for specific stages and cell types become available.
Figure 3.
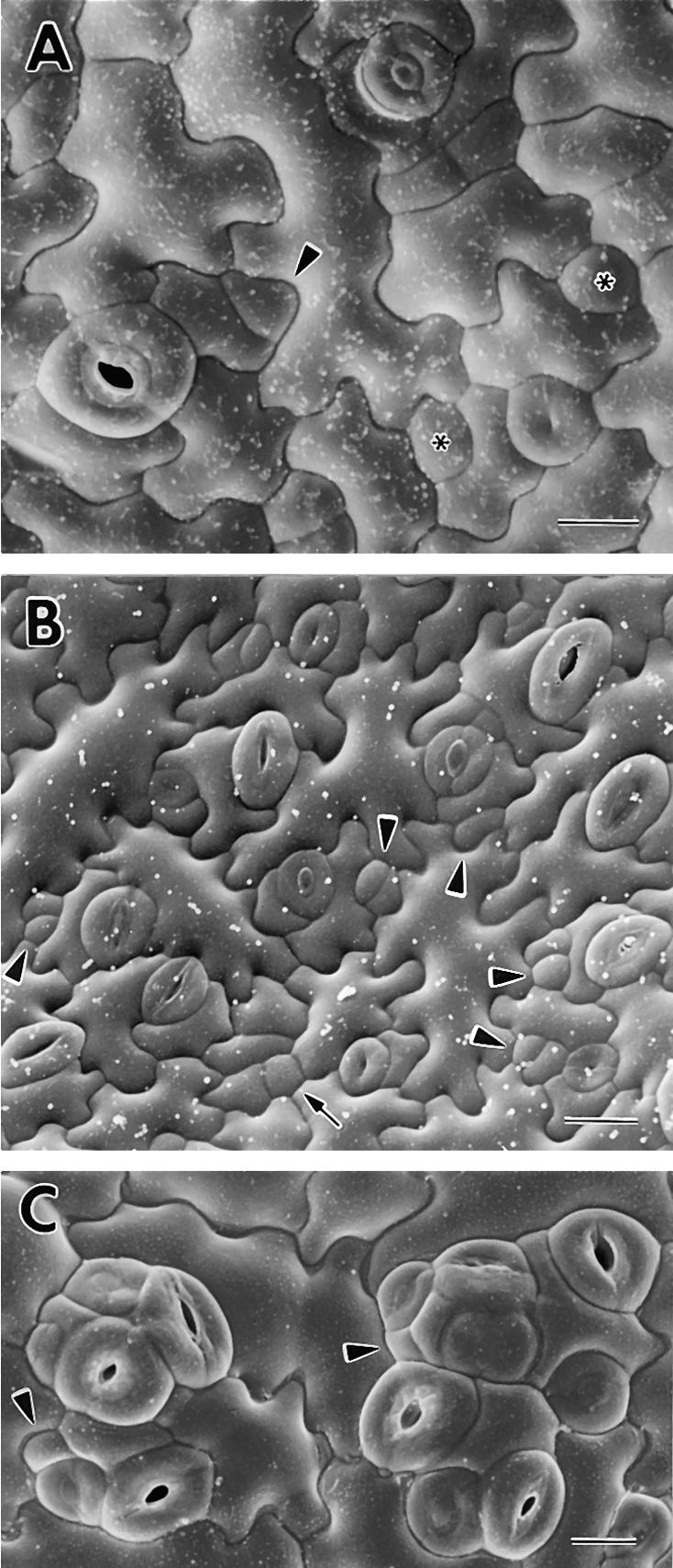
Cryoscanning Electron Microscopy of Wild-Type Patterning and the Disruption of Stomatal Spacing in tmm.
(A) Wild type. Both the satellite meristemoid (arrowhead) and the larger sister cell (at left) were derived from an asymmetric division of an MMC that was adjacent to a stoma or precursor (now a mature stoma with a pore). Two GMCs (asterisks) were each derived from a satellite meristemoid. Successive stages of pore development are shown at lower and upper right. Note that five of the six existing and future stomata shown are patterned by satellite meristemoid placement.
(B) Wild type. Arrowheads indicate satellite meristemoids, some of which have divided. Arrow shows nonsatellite meristemoid or GMC.
(C) Stomatal clusters in tmm. The arrowheads indicate incorrectly placed satellite meristemoids. Many of the stomata in the clusters are in various stages of pore formation.
 ;
;  .
.
Pavement cells make up most of the surface area of a mature epidermis. Large cells (greater than ∼400 μm2 in area) were not observed to divide and were scored as pavement cells. These cells were often shaped like jigsaw puzzle pieces with wavy margins.
Smaller, less sinuous cells displayed three different behaviors (Figure 1, i to iii, top left). Most differentiated into pavement cells; some divided symmetrically (Figure 2, cell e), and others divided asymmetrically and initiated a stomatal lineage (Figure 2, cells c and f). This suggests that at least some of the smaller cells were undifferentiated. Smaller cells are present in young as well as in expanded leaves, especially next to stomata and their precursors.
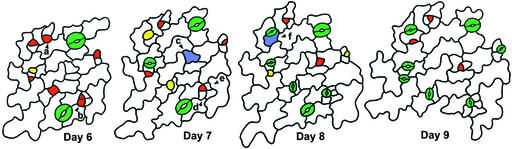
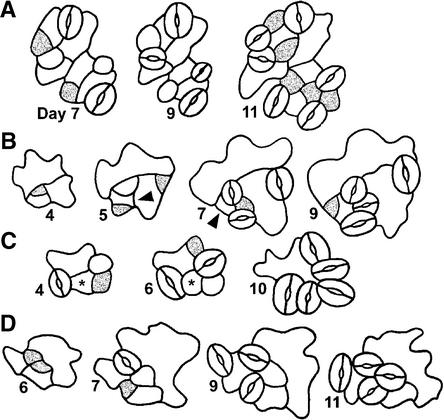
Figure 2.
Key Events in Stomatal Development Shown in a Dental Impression Series.
The abaxial epidermis of a single cotyledon is shown through time. Cells c (day 7) and f (day 8) are MMCs because they later divided asymmetrically to produce meristemoids. The division of MMC f took place next to a preexisting stoma and produced a satellite meristemoid. Both MMCs arose from smaller, less sinuous cells. One smaller cell (e; day 7) divided symmetrically by day 8. Meristemoid a (day 6) divided twice asymmetrically in an inward spiral. Two apparent meristemoids are adjacent (day 6, lower right); the upper one formed a stoma, but the lower meristemoid (b) did not progress in development (d). Blue, MMC; red, meristemoid; yellow, GMC; green, stoma.
The meristemoid mother cell (MMC) is the first type of stomatal precursor. It divides asymmetrically to produce a smaller, usually triangular meristemoid and a larger sister cell (Figure 1). Meristemoids can divide asymmetrically and eventually convert into an oval-shaped guard mother cell (GMC; Figure 3A, asterisks). The GMC divides symmetrically to produce two guard cells, which surround the stomatal pore. Thus, the stomatal lineage, which we define as those cells derived from an MMC (Figure 1), involves progressive changes in fate from MMCs to meristemoids to GMCs and then to stomata. The divisions of the MMCs located next to a stoma, meristemoid, or GMC play a key role in patterning (see below; Figure 3A). They produce satellite meristemoids, which can also divide asymmetrically (Figure 3B).
Divisions in the lineage are important not just for creating the stomatal complex but also for producing the majority of cells in the foliar epidermis. Each asymmetric division results in a larger sister cell that rejoins the pool of nonstomatal cells. Stomatal lineages produce ∼67% of all pavement cells in cotyledons and 48% in leaves. Guard cells constitute ∼40 and 30% of all epidermal cells in cotyledons and leaves, respectively. Thus, stomatal lineages generate 65 to 82% of all epidermal cells.
Most Stomatal Complexes Include Nonclonal Neighbor Cells
All stomatal lineages originate by the asymmetric division of an MMC, but the number of subsequent asymmetric divisions varies from zero to three (Figure 1). When the meristemoid divides two or more times (34% of all meristemoids), all the neighbor cells usually derive from the same MMC and thus are clonally related to the central stoma. When the meristemoid divides only once (46%) or not at all (20%), at least one neighbor cell in the complex is not related clonally, meaning that a neighbor cell was not derived from the MMC that produced the adjacent stoma. Thus, two-thirds of all stomatal complexes contain at least one nonclonal neighbor cell. This indicates that the majority of stomata are not spaced by a series of stereotyped divisions in a cell lineage.
Asymmetry in Size and Fate of Daughter Cells
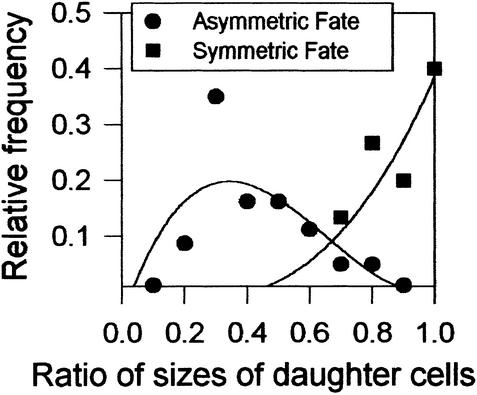
Some divisions of smaller, less sinuous cells are symmetric in fate because they ultimately produce two pavement cells (Figure 1, top left; Figure 2, cell e). Divisions of MMCs and meristemoids are asymmetric in cell fate: One cell becomes a meristemoid, whereas the other follows the three possible fates of smaller, less sinuous epidermal cells. Symmetric and asymmetric divisions were compared to determine whether daughter cell size correlates with fate. As Figure 4 shows, daughter cells from asymmetric divisions were unequal in cell size, and meristemoids were, on average, one-third as large as their sister cells. In contrast, divisions that were symmetric in fate produced daughter cells that were much closer in size. Thus, asymmetric divisions are asymmetric in both cell fate and size.
Figure 4.
Asymmetric Divisions: Unequal in Both Cell Size and Fate.
The ratio of daughter cell size in divisions with an asymmetric fate (MMCs; circles) and with a symmetric fate (divisions of smaller cells that produced two pavement cells; squares). No divisions that were asymmetric in fate had daughter cells of equal size, and no divisions that were symmetric in fate had daughter cells differing in size by >30%. Measurements of daughter cell size were made from dental resin sequences of 300 divisions that were known to be asymmetric in fate and of 100 that were symmetric in fate. Curves represent best fit.
Unpatterned Placement of MMCs
One way that stomatal patterning could be established would be to space the initial precursor cells so that no stomata can form in contact. For example, some mechanism might control where MMCs arise. A second possibility is that stomata or their precursors could laterally inhibit adjacent cells from acquiring an MMC fate. To address these possibilities, two categories of MMCs were analyzed separately, those located next to stomata or precursors and those that were not. The placement of the latter appeared random. Figures 5A and 5B show that MMCs can occur adjacent to each other, an event that occurred 54% of the time in leaves and 27% in cotyledons. These frequencies did not differ statistically from those predicted based on chance. Thus, there is no prohibition against MMCs forming in contact.
Figure 5.
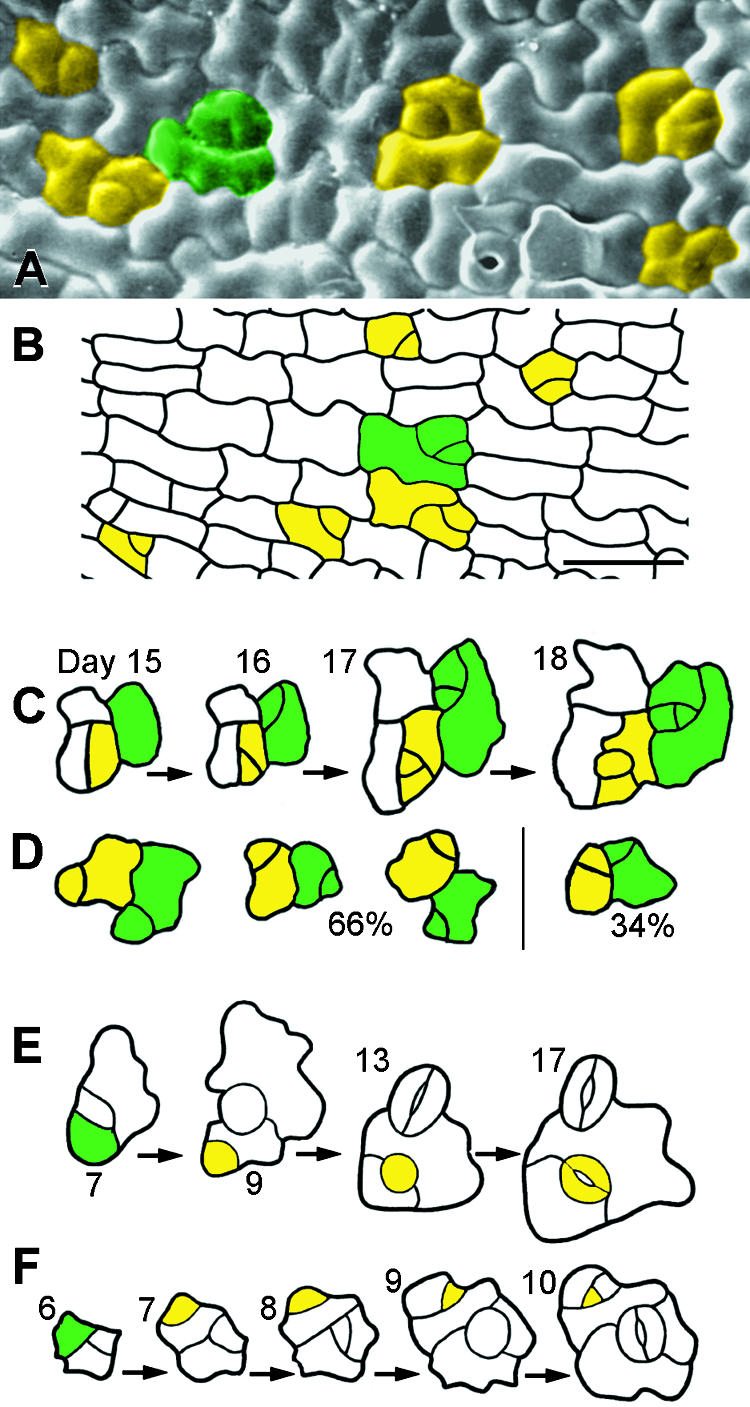
Placement and Divisions of MMCs.
(A) Scanning electron microscopy of a dental impression from a wild-type cotyledon (4 days after germination), showing that some MMCs are not spaced apart. Each group of cells delimited by color fill came from a single MMC, as deduced from other replicas in the series.
(B) As in (A) except that MMC lineages were deduced from cell wall positions. Shown is a tracing from a fixed cotyledon (12 hr after germination).
(C) Divisions in adjacent MMCs are randomly placed. A dental impression sequence shows divisions of adjacent MMCs that resulted in nonadjacent meristemoids (day 16). Green and yellow areas delimit lineages from each MMC.
(D) As in (C). Sixty-six percent of the divisions of adjacent MMCs yielded separated meristemoids but 34% produced meristemoids in contact. Drawings, which represent different positions, are each from a different dental resin series and summarize results from a sample of fifty adjacent MMCs.
(E) Dental resin series showing that satellite meristemoids are placed away from previously formed precursor cells. The asymmetric division of the MMC (green cell) is oriented so that the new meristemoid (yellow cell at day 9) is separated from the preexisting precursor cell (a meristemoid at day 7; a GMC at day 9) by an intervening sister cell.
(F) As in (E). Both meristemoids divide asymmetrically.
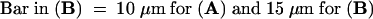 .
.
There is also no prohibition against MMCs forming next to a preexisting stoma, meristemoid, or GMC (e.g., MMC f in Figure 2). In fact, most stomata originate from MMCs in these positions (see below). Thus, the minimum one-celled spacing pattern of stomata is not generated by regulating which cells become MMCs.
Random Positioning of Asymmetric Divisions in Adjacent MMCs
We then tested whether adjacent MMCs might divide in orientations that prevent the formation of meristemoids in contact. Divisions were monitored in dental resin series to analyze the frequency with which meristemoids arose in different positions. Figures 5C and 5D show that two thirds of the time, the divisions of adjacent MMCs resulted in the separation of meristemoids. However, the frequency of adjacent meristemoids was not different from that predicted by chance. It thus appears that divisions in adjacent MMCs are not oriented relative to each other.
Asymmetric Divisions of MMCs in Contact with Stomata, Meristemoids, or GMCs Are Oriented
MMCs located next to a stoma, a GMC, or a meristemoid undergo highly oriented divisions as shown in Figures 5E and 5F. In all cases followed in the dental resin series (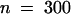 ), the asymmetric division was positioned so that the new meristemoid was placed away from the stoma or precursor (Figures 1, 2, 5E, and 5F). The placement of the satellite meristemoid ensures that the two stomata will be separated by at least one intervening cell, a sister cell to the new meristemoid. Therefore, this is a mechanism for creating the spacing pattern.
), the asymmetric division was positioned so that the new meristemoid was placed away from the stoma or precursor (Figures 1, 2, 5E, and 5F). The placement of the satellite meristemoid ensures that the two stomata will be separated by at least one intervening cell, a sister cell to the new meristemoid. Therefore, this is a mechanism for creating the spacing pattern.
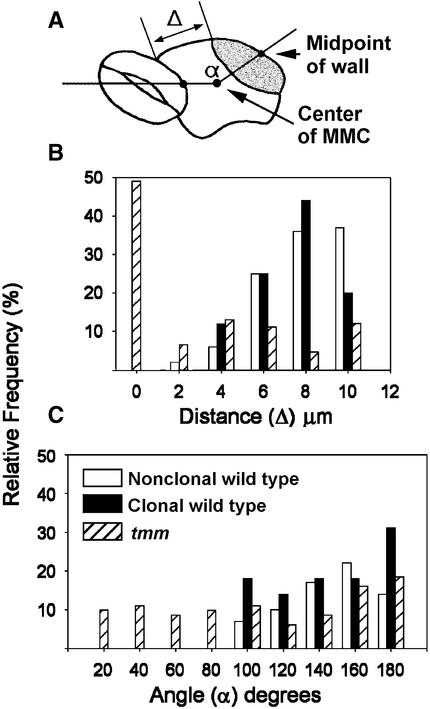
To clarify the properties defining the placement of this division, satellite meristemoid position was quantified as illustrated in Figure 6A. Figures 6B and 6C show that angles <100° or distances <4 μm were rarely observed. Distance correlated with the size of the MMC (measured soon after MMC division), but the angle did not (data not shown). This suggests that the direction rather than the distance from the preexisting stoma is primarily what is regulated.
Figure 6.
Wild-Type Satellite Meristemoids Are Correctly Placed Regardless of Cell Lineage, and Orientation Is Randomized in tmm.
(A) Measurement of angle (α) and initial distance (Δ) of satellite meristemoid from preexisting stoma, GMC, or meristemoid (see Methods).
(B) Almost all wild-type satellite meristemoids were placed at least 4 μm away regardless of whether the MMC that produced them was clonally related to the preexisting stoma or precursor. Many tmm satellite meristemoids were closer than 4 μm.
(C) Distribution of angles of tmm but not wild-type satellite meristemoids is random.
Satellite Meristemoids Are Correctly Placed Regardless of Cell Lineage
Positional cues are clearly used to orient the asymmetric division that produces satellite meristemoids. To assess whether these cues might be contained entirely within the MMC or whether they might emanate from adjacent cells, we evaluated the effect of cell lineage. As indicated above, all stomatal complexes have at least one neighbor cell that is clonally related to the preexisting stoma (or precursor), but many stomatal complexes contain at least one neighbor cell that is not clonally related. In clonally related neighbor cells, spatial information from the previous asymmetric division could be retained and used by the larger sister cell (now a neighbor cell that functions as an MMC) to orient the next asymmetric division. If so, then correctly oriented satellite meristemoids would be expected to arise only in clonally related neighbor cells, and the regulation of satellite meristemoid position would be cell autonomous. Alternately, the correct positioning of satellite meristemoids in nonclonal MMCs would support the idea that spatial cues are conveyed by cell–cell communication.
Samples of clonal and nonclonal MMCs (identified in dental resin sequences) were analyzed separately. All satellite meristemoids were positioned away from the preexisting stoma or precursor cell, regardless of cell lineage. An example of correct positioning across nonclonal cell files is shown in Figure 7. The distribution of satellite meristemoid angles and distances was equivalent regardless of lineage (Figure 6). This suggests that the orientation of this asymmetric division is regulated by extracellular spatial cues rather than by mitosis-allocated positional cues.
Figure 7.
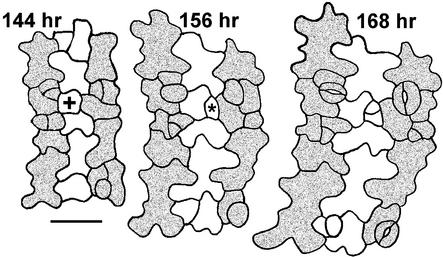
Dental Resin Sequence Showing Correct Placement of Satellite Meristemoid from Nonclonal MMC.
Shown at left is a young cotyledon (144 hr after germination) with cell files still detectable (stippling). File and cell lineage of MMC (+) differ from those of the adjacent meristemoid (left of MMC). Division results in correct placement of the satellite meristemoid (asterisk). At right, asymmetric division of the satellite meristemoid is shown. Bar = 50 μm.
Majority of Stomata Derive from Satellite Meristemoids
The above data show that placement of the satellite meristemoid generates a one-celled spacing pattern. To determine the contribution of this mechanism to stomatal patterning, we estimated the number of stomata produced by satellite meristemoids. Approximately 75% of all stomata sampled from leaves and cotyledons originated from satellite meristemoids. The remaining stomata were derived from MMCs that were not adjacent to a preexisting stoma, meristemoid, or GMC. Because the bulk of all stomata originate from satellite meristemoids in the material studied, this is a major patterning mechanism.
Behavior of Adjacent Meristemoids Is a Minor Spacing Mechanism
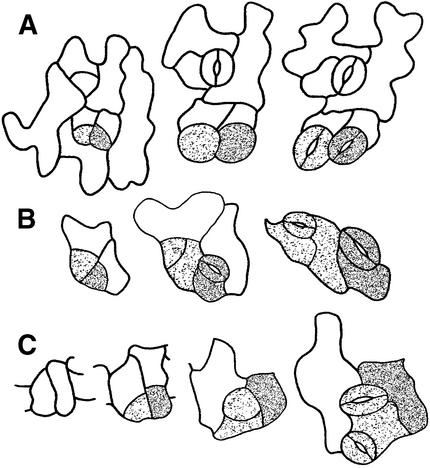
We have shown that when an MMC located next to a stoma divides, the orientation is regulated, but that when two MMCs are adjacent, their divisions are randomly placed. The latter divisions occasionally produce meristemoids in contact. Analysis of many dental resin series from cotyledons and leaves identified 20 pairs of cells that appeared to be adjacent meristemoids. Meristemoid behavior was monitored to determine whether such patterning “mistakes” were corrected. The three outcomes identified are shown in Figure 8. In one of the 20 pairs, both meristemoids converted into GMCs, producing two stomata in contact (Figure 8A). The rarity of such an uncorrected patterning mistake is consistent with our previous finding that only 0.6% of stomata are adjacent in wild-type Arabidopsis (Geisler et al., 1998). In all other pairs of adjacent meristemoids, violations of the spacing pattern were corrected by two mechanisms. In 10 of the pairs, the division of one or both meristemoids was oriented so that at least one intervening cell was produced (Figure 8B). In the nine remaining pairs, one of the adjacent meristemoids appeared to differentiate into a pavement cell (Figure 2, day 9, cell d, and Figure 8C). In contrast, isolated, nonadjacent meristemoids were never observed to differentiate into a pavement cell (n ⩾ 500). Thus, patterning mistakes (adjacent meristemoids) are corrected either by oriented asymmetric divisions or by an apparent change in the cell fate of one meristemoid. Because adjacent meristemoids constitute only ∼2% of all meristemoids, these mechanisms play only a minor role in generating the wild-type stomatal spacing pattern.
Figure 8.
Responses of Adjacent Meristemoids That Create One-Celled Spacing.
(A) On one occasion, both meristemoids were observed to convert into GMCs, producing two stomata that are in contact.
(B) Left meristemoid divides away.
(C) Right meristemoid differentiates into a pavement cell.
The lightly and darkly stippled cells denote different meristemoids and their respective derivatives.
Position-Dependent Control of Neighbor Cell Fate
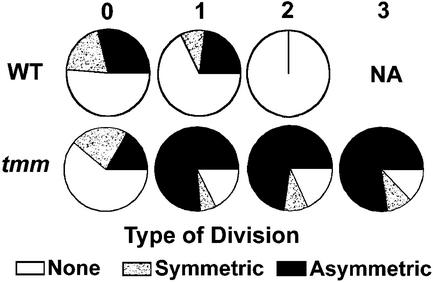
As discussed earlier, smaller, less sinuous cells displayed three different behaviors (Figure 1, upper left). Some of these cells were located next to stomata, meristemoids, or GMCs, and others were not. Dental resin series were studied to determine whether the number of adjacent stomata or precursors (or both) affects the behavior of the smaller, less sinuous cells. Figure 9 (top row) shows that the presence of one adjacent stoma or precursor slightly decreased the propensity to divide asymmetrically in comparison with cells with no such adjacent cell. Cells located next to two stomata or precursors were never observed to divide, thus indicating a position-dependent control of cell fate. This latter category included cells positioned between a satellite meristemoid and a preexisting stoma or precursor.
Figure 9.
Position-Dependent Control of Cell Fate and Disruption by tmm.
Relative frequencies with which smaller, less sinuous cells followed three different fates as a function of the number of adjacent stomata, GMCs, or meristemoids (0 to 3, singly or in combination). Wild-type (WT) cells adjacent to two stomata or precursors did not divide; they usually differentiated into pavement cells. Most tmm cells adjacent to one or more stomata or precursors divided asymmetrically regardless of the number of adjacent cells. NA, not applicable.
Overview of Position-Dependent Events and Stomatal Spacing
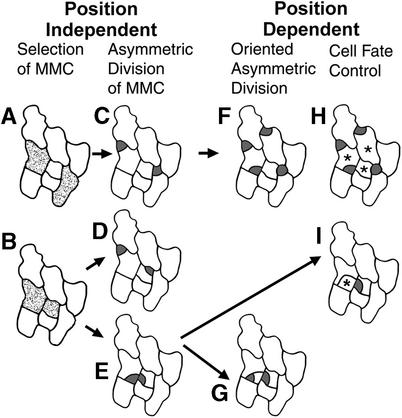
Figure 10 summarizes which developmental events are regulated by position and which contribute to the one-celled spacing pattern. The major spacing mechanism is the orientation of the asymmetric division that produces the satellite meristemoid (Figure 10F). The orientation of a second class of asymmetric divisions, those of adjacent meristemoids, also contributes to spacing, but only to a small degree (Figure 10G). Other classes of asymmetric divisions, such as those of MMCs located away from stomata, appear to be randomly oriented, even when two MMCs are in contact (Figures 10D and 10E).
Figure 10.
Overview of Position-Dependent and Position-Independent Events.
(A) and (B) The placement of the first precursor cell, the MMC, is random.
(C) The placement of asymmetric divisions of separated MMCs appears to be random.
(D) and (E) Divisions of MMCs in contact are randomly oriented and can produce meristemoids in contact.
(F) The orientation of asymmetric divisions in neighbor cells depends upon the position of the stoma or precursor.
(G) Asymmetric divisions of adjacent meristemoids can be oriented and thus space stomata.
(H) Cells next to two stomata or precursors (asterisks) follow a pavement cell fate, but this does not directly create the one-celled spacing.
(I) Adjacent meristemoids can become pavement cells (asterisk) and thus space stomata.
MMCs are shown as stippled regions in (A) and (B). Meristemoids are dark gray.
MMCs can form in cells at some positions (Figures 10A to 10C) but not in cells that contact two stomata or precursors (Figure 10H). The differentiation of an adjacent meristemoid into a pavement cell makes a minor contribution to the spacing pattern (Figure 10I).
The apparently random placement of MMCs (those not adjacent to stomata) and of their divisions results in some patterning “mistakes” (Figure 10E) as well as meristemoids that are separated from each other (Figures 10C and 10D). Three of the four events that are regulated by position contribute directly to stomatal patterning (Figures 10F, 10G, and 10I).
Orientation of Asymmetric Divisions Disrupted by tmm Mutation
In contrast to the wild type, many tmm stomata are in direct contact and are arranged in clusters (Figure 3C). Dental resin series were studied to determine how these clusters originate in tmm leaves and cotyledons. As shown in Figure 11, clusters result from several aberrations. A major defect is that many satellite meristemoids form in contact with the preexisting stomata or precursor cells (Figure 11A). Figure 3C illustrates several of these ectopic satellite meristemoids. tmm satellite meristemoids were placed at all angles with respect to the central stomata or precursors (Figure 6C), and the distribution of angles was not different statistically from a random distribution. Many satellite meristemoids were located <2 μm from the preexisting stomata or precursors (Figure 6B). This may not represent a preferred placement, because many tmm MMCs are smaller than are wild-type MMCs, and most acute angles would result in the satellite meristemoid forming very close to the preexisting stoma or precursor. Thus, the tmm mutation appears to randomize the orientation of placement of satellite meristemoids and violates the major feature of wild-type patterning.
Figure 11.
Mechanisms of tmm Stomatal Cluster Formation Shown in Dental Resin Series.
(A) Six of the seven satellite meristemoids shown (stippled) are misplaced and contact preexisting stomata or precursor cells.
(B) Some cells between two stomata or precursors (arrowheads) divide asymmetrically.
(C) A sister cell to a satellite meristemoid becomes a GMC (asterisks) without any division.
(D) Both adjacent meristemoids (stippled) develop into stomata.
Clusters also result when adjacent meristemoids fail to divide away from each other or fail to develop into pavement cells (Figure 11D). Thus, tmm also disrupts the minor mechanisms of patterning.
Cell Fate Defects in tmm
The tmm mutation results in three cell fate defects that contribute to the formation of stomatal clusters. First, cells located adjacent to two stomata or precursors often divide asymmetrically (Figures 9 and 11B). Second, some sister cells to satellite meristemoids develop into meristemoids and GMCs without dividing (Figure 11C). Third, tmm satellite meristemoids divide fewer times than those in the wild type. The overproduction of satellite meristemoids combined with decreased spacing divisions results in stomata in contact. The overproduction of cells sometimes causes stomatal clusters to bulge out of the plane of the epidermis. Collectively, these data show that stomatal clusters result from the randomization of the orientation of asymmetric divisions that are central to wild-type patterning and from the alteration of the fates of the resulting daughter cells.
DISCUSSION
In this study, we have analyzed the behavior of cells through time to evaluate how the wild-type stomatal spacing pattern is established in Arabidopsis and how the tmm mutation produces stomatal clusters. Our findings argue against several hypotheses of wild-type patterning and instead show that spacing results largely from the orientation of one class of asymmetric divisions. The correct orientation of these divisions requires the TMM gene product and may also require cell–cell signaling.
Analysis of Patterning Hypotheses
The use of dental resin impressions has allowed us to critically evaluate existing hypotheses about how dicot stomata are spaced away from each other. According to the cell lineage hypothesis, consecutive asymmetric divisions produce both a cell boundary and the central stoma, resulting in a complex of clonally related cells (Sachs, 1991; Larkin et al., 1997). This hypothesis was supported by studies using transposon-induced sectors in which the majority (77 to 87%) of complexes exhibited staining patterns consistent with a clonal origin (Larkin et al., 1996; Serna and Fenoll, 2000). However, the large sectors analyzed originated early in leaf development from single protodermal cells. When the lineage was defined as starting with an MMC and subsequent divisions were followed by using dental resin impressions, we found that most stomata were in contact with at least one clonally unrelated cell. That is, the cell lineage hypothesis cannot account for the spacing of many stomata. Moreover, even when a stoma is entirely surrounded by clonally related neighbor cells, those cells do not provide a boundary or buffer, because many divide and produce stomata. Thus, stomatal patterning in Arabidopsis is not generated by a series of stereotyped divisions within a cell lineage.
Nor is the spacing created by lateral inhibition, as hypothesized by Bünning (1956), who suggested that cells next to stomata are prohibited from forming stomata. We found that cells next to a stoma or precursor (GMC or meristemoid) divided asymmetrically almost as often as cells that were not adjacent to any stoma or precursor. Thus, stomata do not prevent surrounding cells from becoming MMCs. In addition, there is no prohibition against two MMCs forming in contact, which indicates that lateral inhibition does not operate at this early stage and that MMC placement is not regulated.
Together, these data demonstrate that Arabidopsis stomata are not spaced by the cell lineage mechanism, by a position-dependent selection of the first precursor cell, or by lateral inhibition of stomatal initiation.
Oriented Asymmetric Divisions Create One-Celled Spacing
The major stomatal patterning mechanism is that the division of an MMC located adjacent to a stoma or precursor is regulated in a position-dependent manner. As a result, the new satellite meristemoid invariably forms away from the preexisting stoma or precursor. This event is central to patterning because ∼75% of leaf stomata derive from satellite meristemoids and because the generation of an intervening cell also spaces the preexisting stoma. Although satellite meristemoids have been documented in other dicots, the central patterning role of the divisions that produce them has not been recognized (Bünning, 1956; Landré, 1972; Sachs, 1979, 1991; Kagan et al., 1992; Serna and Fenoll, 1997).
Previous discussion of the relevance of oriented asymmetric divisions to stomatal patterning has focused on the responses of adjacent meristemoids in which one or both divide away (Sachs, 1991). We also identified Arabidopsis stomata that were spaced by this mechanism but found it to be a rare event contributing only marginally to patterning.
Asymmetric divisions in other positional contexts are not oriented. For example, as shown here, the divisions of two MMCs in contact are randomly placed. In addition, the divisions of isolated MMCs do not appear to be oriented along the leaf axis (M. Geisler, J. Nadeau, and F.D. Sack, unpublished data). Thus, Arabidopsis stomata are largely spaced by the orientation of asymmetric divisions that occur in one positional context: those next to a preexisting stoma, GMC, or meristemoid.
Specification of Asymmetric Cell Fates by Intrinsic Mechanisms
In some asymmetric divisions in different organisms, the fate of the daughter cells is established after division in response to extracellular signals (Hawkins and Garriga, 1998; Jan and Jan, 1998). In other asymmetric divisions, factors intrinsic to the mother cell specify differences in the fates of the daughter cells before cytokinesis. The asymmetric divisions that occur in the stomatal lineage appear to be of the latter type. Before cell division, a geometric asymmetry is established when the nucleus moves to one end of the MMC or meristemoid and becomes located near the site of future division, as marked by a preprophase band of microtubules (Zhao and Sack, 1999). After division, the smaller cell becomes a meristemoid, and the larger cell follows other fates (e.g., pavement cell or MMC). The selection of the smaller cell as a meristemoid occurs in divisions that take place in all positional contexts. Thus, daughter cell fate is probably specified intrinsically before division, perhaps by the asymmetric segregation of fate determinants (Fowler and Quatrano, 1997; Scheres and Benfey, 1999).
Cell Signaling and Oriented Division
In some systems, the position of cytokinesis in an asymmetric division is oriented by spatial cues within the mother cell that result in the correct placement of daughter cells. For example, in yeast budding, cortical landmarks left behind from a previous division are used to orient the next division within the cell lineage (Drubin and Nelson, 1996; Fowler and Quatrano, 1997). Because wild-type Arabidopsis satellite meristemoids were correctly oriented regardless of lineage, the plane of this division most likely is oriented by cell–cell communication rather than by spatial cues located solely within the mother cell. Thus, although the divisions of all classes of MMCs may inherently produce cells of unequal size and fate, in one class of MMCs, the placement of this polarity seems to utilize extracellular spatial cues.
Stomatal spacing in monocots results from the orientation of the first asymmetric division with respect to the leaf axis (Kennard and Cleary, 1997; Larkin et al., 1997; Hernandez et al., 1999). Whether this orientation is regulated by extracellular signals or by positional cues inherited through division, however, is not known.
Cell Signaling and Cell Fate
In addition to regulating the orientation of asymmetric divisions, cell signaling appears to regulate the fate of cells located in specific positions. Cells that were adjacent to only one stoma or precursor often divided and functioned as MMCs. In contrast, cells positioned between two stomata or precursors were prohibited from forming a new stomatal lineage. This class of cells includes sister cells to satellite meristemoids. The fate prohibition occurred regardless of whether or not the mother cell (the neighbor cell that produced the satellite meristemoid) was clonally related to the preexisting stoma or precursor. Thus, the fate prohibition may result from cell signaling, although nothing is known about the nature of the signals or when they operate. This inhibition presumably minimizes the likelihood of patterning “mistakes” by preventing the formation of new meristemoids, but it does not directly generate the one-celled spacing. The differentiation of one of two adjacent meristemoids as a pavement cell does contribute to stomatal spacing. Although this is a rare event, it identifies a second context in which cell signaling may influence cell fate during stomatal development.
TMM
Based on phenotype, the TMM gene product probably plays a central role in the asymmetric divisions that lead to stomatal formation and patterning. However, this role may not be that of a fate determinant. Loss-of-function mutations in fate determinants usually cause both daughter cells to follow the same default fate in intrinsically specified asymmetric divisions (Jan and Jan, 1998). tmm is probably a loss-of-function mutation because both alleles are recessive (Geisler et al., 1998). Although a small fraction of tmm divisions produce two daughter cells that each convert into GMCs, most tmm MMC divisions are unequal in size and fate, which suggests that the TMM gene does not encode a factor that specifies cell fate.
We have shown that tmm randomizes the orientation of divisions that produce satellite meristemoids; as a result, ectopic satellite meristemoids and stomatal clusters are formed. This suggests that TMM functions in a cell-signaling pathway that regulates placement of the division site. If TMM received or interpreted information about the position of the preexisting stoma or precursor, then tmm mutants would be blind to these extracellular cues, and satellite meristemoids would be randomly placed. A similar outcome would ensue if TMM were responsible for the generation or transmission of a signal.
Abnormal or absent cell signaling could also explain some fate defects in tmm. Cells located between two stomata or precursors that are normally prohibited from dividing frequently did divide in tmm. Thus, TMM acts as a negative regulator of stomatal production partly by preventing these cells from dividing asymmetrically and producing meristemoids. An overproduction of meristemoids in tmm might result if these cells were blind to the extracellular cues normally received or communicated by TMM.
The cell fate defects in tmm raise the possibility that TMM is also required for the correct segregation of factors that specify cell fate. Although many tmm asymmetric divisions correctly produce two cells of unequal size, the fates of these cells, especially of the larger cell, are altered. The conversion of both daughter cells into GMCs or an asymmetric division of the larger daughter cell could result if both cells inherited factors that promote stomatal formation. This outcome might ensue if the location of MMC cytokinesis were not completely aligned with the distribution of cell fate determinants. By this reasoning, TMM might operate in a signaling pathway that coordinates the placement of the division site with the polar localization of the factors that specify cell fate.
TMM may perform novel functions compared with other plant genes known to affect asymmetric divisions. The scarecrow and short-root mutations eliminate an asymmetric division in Arabidopsis roots (Scheres and Benfey, 1999). Both SCARECROW and SHORT-ROOT encode putative transcription factors necessary for the asymmetric division to take place (Helariutta et al., 2000). gemini pollen1 disrupts the fate of daughter cells produced by asymmetric division of the microspore but does not alter division orientation (Park et al., 1998). discordia misplaces asymmetric divisions in the maize epidermis but probably disrupts cell plate guidance rather than the earlier selection of the division site (Gallagher and Smith, 1999).
As does tmm, the stomatal density and distribution1 (sdd1) mutant of Arabidopsis displays stomatal clusters that develop from ectopic satellite meristemoids (Berger and Altmann, 2000). However, sdd1 clusters contain fewer stomata than do those in tmm, and sdd1 exhibits a much greater density of nonclustered stomata. SDD1 encodes a subtilisin-like serine protease that is hypothesized to be a negative regulator of meristemoid formation, perhaps by activating a proteinaceous signal molecule.
Unlike SDD1, TMM can function as either a negative or a positive regulator of MMC formation. Although tmm leaves and cotyledons have excess stomata, tmm stems lack stomata, and flower stalks display clustered to no stomata in an apical–basal gradient (Geisler et al., 1998). Whether the absence of stomata in some tmm organs indicates additional roles for this gene product, or whether the loss of the same function or functions has different consequences in different domains of the plant, remains to be seen. Determination of the molecular identity of the TMM gene product may clarify these issues, but it is already evident that TMM plays a central role in the mechanisms of stomatal spacing and initiation.
METHODS
Plant Cultivation and Fixation
The trichomeless gl1 mutant background of Arabidopsis thaliana (Columbia ecotype) was used to facilitate obtaining multiple dental resin impressions of both TMM and tmm-1 plants. Plants were grown on Promix medium (Premier Horticulture, Dorval, Canada) at room temperature (22 to 26°C) under a 12-hr photoperiod with Coolwhite (General Electric, Fairfield, CT) fluorescent lamps at an irradiance of 75–100 μmol m−2 s−1. For determinations involving fixed tissue, whole seedlings were immersed in a solution of 9% (v/v) formaldehyde, 82% ethyl alcohol, and 9% acetic acid. The methods used for cryoscanning electron microscopy were described by Yang and Sack (1995).
Dental Resin Impressions
Dental resin impressions were used to obtain developmental series of the behavior of the same epidermal cells over time. President Light Body polyvinylsiloxane dental resin (No. 4667; Coltène/Whaledent, Mahwah, NJ) was applied in a modification of the technique of Kagan et al. (1992). Fresh resin was applied to the abaxial epidermis with a pin or hair. Peels were usually taken every 12 or 24 hr for as many as 12 successive peels from one leaf or cotyledon. The series of peels started at different developmental stages for different plants. The dental resin mold was filled with either nail polish or Spurr's epoxy resin to create a cast that was examined by light microscopy. In a few cases, the casts were examined directly with a scanning electron microscope (JSM-820; JEOL, Peabody, MA). Bright-field images of nail polish casts were projected onto a video monitor at a final magnification of ×1000 to ×2000, and cell outlines were traced onto transparent acetate overlays. Data analysis was based on serial impressions from 40 wild-type cotyledons and 12 wild-type leaves and from 30 tmm cotyledons. Most impressions were of the abaxial epidermis. The identification of epidermal cell types was based on both morphology and the behavior of specific cells through time.
Resin applications sometimes damaged or killed tissue. With practice and care, however, many healthy leaves and cotyledons were recovered after even 12 impressions. These plants appeared normal in epidermal development compared with untreated plants, as judged by cell morphology and the total number of stomata and other epidermal cells.
Sampling and Calculations
To determine whether the number of stomata in each ring of cells around a stoma differed from the overall stomatal frequency, the formula 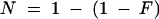 r was used, where N predicts the percentage of rings that should contain at least one guard cell, F is the frequency of guard cells as a percentage of all epidermal cells, and r is the mean number of cells in each ring. A comparable formula was used to determine the frequency with which meristemoid mother cells (MMCs) would form in contact randomly; in this case, F is the frequency of all MMCs, and r is the average number of cells in contact with MMCs. MMCs (300 total) were identified on the basis of their behavior in the dental resin series or the outline of cell walls in fixed material. All statistical significance was determined for at least P < 0.05 by using χ2 tests.
r was used, where N predicts the percentage of rings that should contain at least one guard cell, F is the frequency of guard cells as a percentage of all epidermal cells, and r is the mean number of cells in each ring. A comparable formula was used to determine the frequency with which meristemoid mother cells (MMCs) would form in contact randomly; in this case, F is the frequency of all MMCs, and r is the average number of cells in contact with MMCs. MMCs (300 total) were identified on the basis of their behavior in the dental resin series or the outline of cell walls in fixed material. All statistical significance was determined for at least P < 0.05 by using χ2 tests.
To predict the frequency with which meristemoids would randomly form in contact from the divisions of two adjacent MMCs, we analyzed tracings of 50 MMCs in contact that divided within 12 to 24 hr of each other. Most MMC divisions were observed to take place in two or three orientations. The frequencies of meristemoids randomly forming in contact were 50% (two of four possible combinations, assuming two orientations in each MMC), 22% (assuming three orientations), and 33% (assuming two orientations in one MMC and three in the other). These predictions were compared with the actual division orientations (measured from dental resin series) of 50 MMCs that had formed in contact.
Angles, cell size (paradermal area), and distance were measured by National Institutes of Health Image software from scanned tracings or from digitized microscopic images. The angle of satellite meristemoid placement was calculated from three points, the center of which was the MMC as the vertex, the midpoint of the outer wall of the satellite meristemoid, and the midpoint of the wall of the stoma (or precursor cell) facing the sister cell. For the dental resin series, the distance was measured within 8 to 12 hr after MMC division; therefore, this measurement is the initial distance of the satellite meristemoid and does not reflect subsequent cell expansion. The sample sizes for Figure 6 were 150 clonal wild-type, 16 nonclonal wild-type, and 100 tmm satellite meristemoids. Only MMCs that were adjacent to a single stoma or precursor were analyzed. The samples were entirely (tmm) or primarily (wild type) from cotyledons.
To calculate the relative sizes of two daughter cells produced by a division that is symmetric in fate, only divisions that produced pavement cells (determined from dental impressions) were sampled. To estimate the proportion of stomata that originate from MMCs that were and were not located next to stomata (or precursors), the origins of 200 stomata were determined from dental resin series of different stages of leaf and cotyledon development. To estimate the fraction of stomata produced by satellite meristemoids, stomatal lineages (113 and 120 from leaves and cotyledons, respectively) were followed in the dental resin series to determine the relative origins of stomata at representative stages of organ development.
The fates of smaller, less sinuous cells were followed in dental resin series of 397 wild-type and 88 tmm cells (Figure 9). Data collection from each series started when the smaller cell was formed in a division and ended when that cell either divided or became enlarged (greater than ∼400 μm2) and sinuous. The sample of cells adjacent to two stomata or precursors included sister cells to satellite meristemoids as well as other cells. A small fraction of the tmm cells included in the asymmetric division category converted directly into guard mother cells without division.
Acknowledgments
This research was supported by grants from the National Science Foundation (Nos. IBN-9505687 and IBN-9904826). Thanks to Lien Lai, Harald Vaessin, and Liming Zhao for helpful discussions.
References
- Berger, D., and Altmann, T. (2000). A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes Dev. 14, 1119–1131. [PMC free article] [PubMed] [Google Scholar]
- Bünning, E. (1956). General processes of differentiation. In The Growth of Leaves, F.L. Milthorpe, ed (London: Butterworths Scientific Publications), pp. 18–30.
- Croxdale, J.L. (2000). Stomatal patterning in angiosperms. Am. J. Bot. 87, 1069–1080. [PubMed] [Google Scholar]
- Drubin, D.G., and Nelson, W.J.. (1996). Origins of cell polarity. Cell 84, 335–344. [DOI] [PubMed] [Google Scholar]
- Fowler, J.E., and Quatrano, R.S. (1997). Plant cell morphogenesis: Plasma membrane interactions with the cytoskeleton and cell wall. Annu. Rev. Cell Dev. Biol. 13, 697–743. [DOI] [PubMed] [Google Scholar]
- Gallagher, K., and Smith, L.G. (1997). Asymmetric cell division and cell fate in plants. Curr. Opin. Cell Biol. 9, 842–848. [DOI] [PubMed] [Google Scholar]
- Gallagher, K., and Smith, L.G. (1999). discordia mutations specifically misorient asymmetric cell divisions during development of the maize leaf epidermis. Development 126, 4623–4633. [DOI] [PubMed] [Google Scholar]
- Geisler, M., Yang, M., and Sack, F.D. (1998). Divergent regulation of stomatal initiation and patterning in organ and suborgan regions of the Arabidopsis mutants too many mouths and four lips. Planta 205, 522–530. [DOI] [PubMed] [Google Scholar]
- Hawkins, N., and Garriga, G. (1998). Asymmetric cell division: From A to Z. Genes Dev. 12, 3625–3638. [DOI] [PubMed] [Google Scholar]
- Helariutta, Y., Fukaki, H., Wysocka-Diller, J., Nakajima, K., Jung, J., Sena, G., Hauser, M.-T., and Benfey, P.N. (2000). The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101, 555–567. [DOI] [PubMed] [Google Scholar]
- Hernandez, M.L., Passas, H.J., and Smith, L.J. (1999). Clonal analysis of epidermal patterning during maize leaf development. Dev. Biol. 216, 646–658. [DOI] [PubMed] [Google Scholar]
- Jan, Y.N., and Jan, L.Y. (1998). Asymmetric cell division. Nature 392, 775–779. [DOI] [PubMed] [Google Scholar]
- Kagan, M.L., Novoplansky, N., and Sachs, T. (1992). Variable cell lineages form the functional pea epidermis. Ann. Bot. 69, 303–312. [Google Scholar]
- Kennard, J.L., and Cleary, A.L. (1997). Pre-mitotic nuclear migration in subsidiary mother cells of Tradescantia occurs in G1 of the cell cycle and requires F-actin. Cell Motil. Cytoskeleton 36, 55–67. [DOI] [PubMed] [Google Scholar]
- Korn, R.W. (1993). Evidence in dicots for stomatal patterning by inhibition. Int. J. Plant Sci. 154, 367–377. [Google Scholar]
- Landré, P. (1972). Origine et développment des épidermes cotylédonaires et foliares de la moutarde (Sinapis alba L.). Différenciation ultrastructurale des stomates. Ann. Sci. Nat. Bot. 12, 247–322. [Google Scholar]
- Larkin, J.C., Young, N., Prigge, M., and Marks, M.D. (1996). The control of trichome spacing and number in Arabidopsis. Development 122, 997–1005. [DOI] [PubMed] [Google Scholar]
- Larkin, J.C., Marks, M.D., Nadeau, J., and Sack, F.D. (1997). Epidermal cell fate and patterning in leaves. Plant Cell 9, 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.M., and Schiefelbein, J. (2000). WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal patterning. Cell 99, 473–483. [DOI] [PubMed] [Google Scholar]
- Park, S.E., Howden, R., and Twell, D. (1998). The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development 125, 3789–3799. [DOI] [PubMed] [Google Scholar]
- Sachs, T. (1979). Cellular interactions in the development of stomatal patterns in Vinca major L. Ann. Bot. 43, 693–700. [Google Scholar]
- Sachs, T. (1991). Pattern Formation in Plant Tissues. (New York: Cambridge University Press).
- Scheres, B. (1997). Cell signaling in root development. Curr. Opin. Genet. Dev. 7, 501–506. [DOI] [PubMed] [Google Scholar]
- Scheres, B., and Benfey, P.N. (1999). Asymmetric cell division in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 505–537. [DOI] [PubMed] [Google Scholar]
- Schnittger, A., Folkers, U., Schwab, B., Jürgens, G., and Hülskamp, M. (1999). Generation of a spacing pattern: The role of TRIPTYCHON in trichome patterning in Arabidopsis. Plant Cell 11, 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna, L., and Fenoll, C. (1997). Tracing the ontogeny of stomatal clusters in Arabidopsis with molecular markers. Plant J. 12, 747–755. [DOI] [PubMed] [Google Scholar]
- Serna, L., and Fenoll, C. (2000). Stomatal development and patterning in Arabidopsis leaves. Physiol. Plant. 109, 351–358. [Google Scholar]
- Sylvester, A.W., Smith, L., and Freeling, M. (1996). Acquisition of identity in the developing leaf. Annu. Rev. Cell Dev. Biol. 12, 257–304. [DOI] [PubMed] [Google Scholar]
- Yang, M., and Sack, F.D. (1995). The too many mouths and four lips mutations affect stomatal production in Arabidopsis. Plant Cell 7, 2227–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L., and Sack, F.D. (1999). Ultrastructure of stomatal development in Arabidopsis (Brassicaceae) leaves. Am. J. Bot. 86, 929–939. [PubMed] [Google Scholar]