NADPH oxidases (NOX) catalyze the production of superoxides, a type of reactive oxygen species (ROS). The dramatic induction of ROS production by human NOX2 in activated blood phagocytic cells and its role in promoting pathogen killing has long motivated research in this area (Babior et al., 2002). In plants, the NOX homologs have been named respiratory burst oxidase homologs (Rboh) and they are also involved in ROS production in response to pathogens (Sagi and Fluhr, 2001; Torres et al., 2002). However, the discovery of new types of animal NOX genes and new functions for plant Rboh genes underlines diverse roles for NOX-generated ROS in eukaryotic cell biology, including animal and plant defense, development, hormone biosynthesis, and cellular signal transduction (Foreman et al., 2003; Kwak et al., 2003; Lambeth, 2004; Sagi et al., 2004; Torres et al., 2005). This Update will focus on recent advances in our understanding of intrinsic molecular properties of Rboh as they are related to their function in plants.
STRUCTURAL SIMILARITIES IN NOX-LIKE ENZYMES
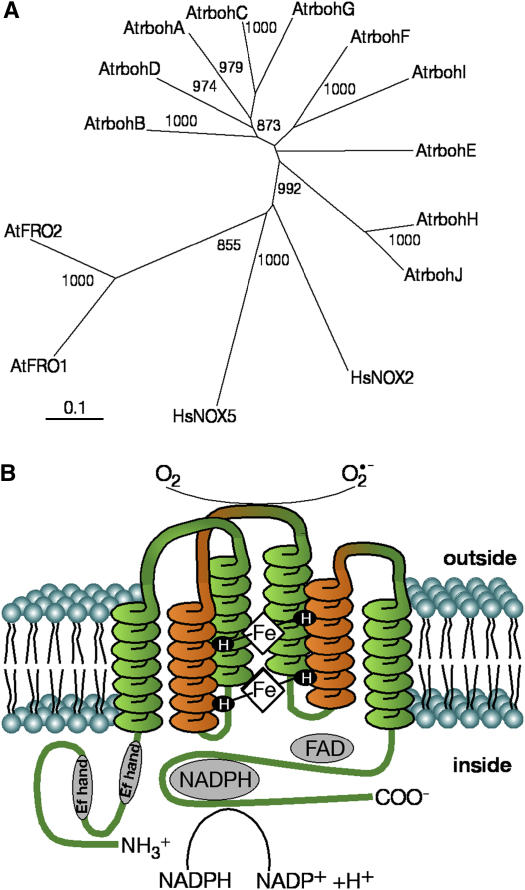
NOX homologs in the plant and animal kingdoms contain cytosolic FAD- and NADPH-binding domains and six conserved transmembrane helices. The third and fifth bind two heme groups through four critical His residues. The heme groups are required for transfer of electrons across the membrane to oxygen, the extracellular (EC) acceptor, to generate the superoxide radical (Torres et al., 1998; Lambeth, 2004). Their presence in animals, plants, and filamentous fungi indicates a common ancient unicellular origin, although they are conspicuously absent in Saccharomyces and Candida (Lara-Ortiz et al., 2003).
All seven human NOX members contain the core transmembrane part, and some include additional N-terminal diversification of calcium-binding elongation factor (EF) hands and EF hands together with a peroxidase-like subdomain. The latter type, called DUOX, is unique in producing both superoxide and hydrogen peroxide (H2O2) products (Ameziane-El-Hassani et al., 2005). In contrast, the Arabidopsis (Arabidopsis thaliana) genome contains 10 members of basically similar structures, with EF hands at the N terminus. Closely related, but still different from the animal NOX, are the Arabidopsis ferric-chelate reductases (Fig. 1A; AtFRO) and their yeast (Saccharomyces cerevisiae) counterparts, FRE1 and FRP1, which belong to a superfamily of flavocytochromes that transport electrons across membranes (Robinson et al., 1999; Staiger, 2002). AtFRO are found in roots and participate in the release of insoluble iron from FeIII oxide hydrates by their reduction to the soluble transport-ready Fe2+ form.
Figure 1.
Structure of Rboh and their phylogenetic distribution. A, Phylogenetic tree comparing Arabidopsis Atrboh with AtFRO and mammalian HsNOX2 and HsNOX5 proteins. Atrboh members are listed in Table I. Comparisons included HsNOX2 (P04839), HsNOX5 (AF317889), AtFRO1 (At1g01590), and AtFRO2 (At1g01580). Only the carboxy terminus with homology to gp91phox/NOX2 (excluding the EF hands) was used in the alignment. The phylogenetic analyses were made by neighbor-joining tree with ClustalX. The length of the horizontal lines connecting the sequences is proportional to the estimated amino acid substitutions/site between these sequences. Bootstrap values from 1,000 iterations are shown. B, Schematic diagram of Rboh structure as predicted to be located in the membrane, showing the irreversible transfer of charge from cellular NADPH to EC oxygen. Shown are the N-terminal region EF hands juxtaposed to the C-terminal end to indicate an interaction by which calcium-dependent activity is regulated.
DIVISION OF LABOR IN THE MULTIGENE Rboh FAMILY
Rboh enzymatic function is to supply ROS for physiological and developmental purposes and, in animals, a diversification in function is becoming evident. The inspection of digital northern activities in Arabidopsis gathered from recent Affymetrix microarray slides reflects analogous gene specialization (Table I). The tissue-specific division of transcript distribution falls into three basic classes; expression throughout the plant (AtrbohD and F), in the roots (Atrboh A–G, I), and in a pollen-specific manner (Atrboh H and J). The tissue-specific expression is reflected in the phylogenetic distribution shown in Figure 1A in which H and J form a small subclade. In the main clade, gene members are differentiated by their expression sensitivity to environmental inputs. The most common abiotic inducers of Atrboh transcript accumulation include conditions of anoxia/hypoxia (Branco-Price et al., 2005) and nitrogen stress, where AtrbohC to F are also induced by a variety of biotic stresses. Analysis of mutants has specifically identified AtrbohC in root hair development (Foreman et al., 2003), AtrbohD as the major constitutively active form, and AtrbohF as a biotic stress-inducible form (Torres et al., 2002). The diverse transcription patterns suggest Rboh will function in broad aspects of growth and physiological response. To what degree the detection of these transcripts reflects actual activity remains to be examined.
Table I.
Rboh tissue-specific and environmental response activities
| Rboh | Protein Code | Tissue Specificitya | Induction/Repressionb |
|---|---|---|---|
| A | At5g07390 | Root, elongation zone | Induction: hypoxia/salt stress, genotoxic, nitrogen starvation. |
| B | At1g09090 | Root, elongation zone | Induction: anoxia, hypoxia, methyl jasmonate, UVB, elevated in rbohC mutant. Repressed: ABA, cold, zeatin cycloheximide. |
| C | At5g51060 | Root, elongation zone | Induction: Botrytis cinerea, Pseudomonas syringae, Agrobacterium, ozone. Repression: cycloheximide, H2O2, 6-benzyl adenine. |
| D | At5g47910 | All plant parts | Induction: cycloheximide, anoxia, H2O2, chitin, ozone, AgNO3, methyl jasmonate, Frankliniella occidentalis, Phytophthora infestans, P. syringae. Repression: ABA, high CO2. |
| E | At1g19230 | Cell suspension, root, and seeds | Induction: Agrobacterium, nitrogen starvation, genotoxic. Repression: senescence. |
| F | At1g64060 | All plant parts | Induction: Agrobacterium, brassinolide. Repression: isoxaben. |
| G | At4g25090 | Root, elongation zone | Induction: low nitrogen, salicylic acid, Glc, Suc. |
| H | At5g60010 | Stamens, pollen | – |
| I | At4g11230 | Root, elongation zone | Induction: anoxia, cycloheximide, norflurazone. |
| J | At3g45810 | Stamens, pollen | – |
Based on data from the 2,180-microarray database compiled in GENEVESTIGATOR. Tissue signals that are significantly higher than background (P ≤ 0.06) are indicated. Experiments are summarized in https://www.genevestigator.ethz.ch (Zimmermann et al., 2004).
Induction of more than 2-fold or, where indicated, repression by 0.5-fold and above 200 in the relative signal value are indicated.
MEMBRANE LOCALIZATION OF Rboh AND COMPARTMENTALIZATION OF SUPEROXIDE PRODUCTION
Cellular fractionation of plant tissue indicates that Rboh proteins are localized into the plasmalemma membrane (Sagi and Fluhr, 2001; Simon-Plas et al., 2002). Interestingly, NtrbohD was found to be enriched in tobacco (Nicotiana tabacum) Bright-Yellow 2 cells on chemically distinct membrane microdomains, called lipid rafts, that may indicate a requirement for coupling to other membrane components (Mongrand et al., 2004). The precise submembrane distribution of Rboh is likely critical for its function, as noted in the asymmetric distribution of Rboh activity in AtrbohC-dependent ROS signaling in root hair growth (Foreman et al., 2003; Carol et al., 2005) and in Rboh involvement in xylem differentiation (Barcelo, 2005). Additionally, intracellular locations have been reported for human NOX2 and DUOX (Lambeth, 2004; Ameziane-El-Hassani et al., 2005; Murillo and Henderson, 2005), but are yet to be noted in plant Rboh biology.
ROS can function as cellular second messengers that are likely to modulate many different proteins, leading to a variety of responses (Mori and Schroeder, 2004). However, an enzymatic dismutation step must first take place to produce from the superoxide (O2−) the more stable H2O2 derivative that is required for a viable long-range cell-to-cell signal or for passing membranes (Allan and Fluhr, 1997). Thus, the implications for vectorial transfer of charge and EC superoxide accumulation are important to understand the complexity of Rboh biology (Fig. 1B). How is the superoxide product dismutated to give rise to the H2O2 intercellular signal? In humans, an EC-superoxide dismutase (SOD) is thought to play an important role in maintenance of EC matrix biology (Petersen et al., 2004), but in plants only a paucity of evidence supports EC-SOD activity. Although secretory motifs are absent for known plant SODs, a high pI-SOD isoform (hipI-SOD) showed an EC developmental buildup in the secondary cell wall of Zinnia elegans and Pinus sylvestris (Karpinska et al., 2001; Karlsson et al., 2005). Similarly, the nectar of the tobacco flower contains large amounts of H2O2 supplied in part by tandem action of Rboh and an EC germin-like SOD protein called Nectarin I (Carter and Thornburg, 2004). Whether other germin-like genes function in the capacity of EC-SOD is unknown.
The superoxide product is membrane impermeable in animals due to its negative charge in ambient conditions of pH (pKa of superoxide is 4.8; e.g. blood pH is 7.4). However, under conditions of exceptionally low pH, the superoxide can be protonated and, as such, has been shown to functionally cross yeast membrane compartments (Wallace et al., 2004). In plants, the physiological range of EC pH is 5, in which 16% of the superoxide would be in the membrane-permeable hydroperoxyl (HO2·) form. Thus, the external pH status could moderate the compartmentalization of superoxides produced by Rboh outside the membrane and perhaps enable the participation of cytoplasmic SOD in the catalysis of H2O2 formation.
DIRECT CONTROL OF Rboh ACTIVITY BY CALCIUM
NOX2 requires cytosolic protein components that are essential for its activation (Lambeth, 2004). In contrast, plant Rboh is stimulated directly by Ca2+, likely mediated by the N-terminal extension containing EF-hand calcium-binding motifs (Sagi and Fluhr, 2001). The mammalian NOX5 containing N-terminal EF-hand motifs is expressed in lymphoid organs and testis and generates superoxide in response to physiological intracellular Ca2+ bursts (Banfi et al., 2004). Indeed, Ca2+ binding induced conformational change of NOX5, leading to enzyme activation through N- and C-terminal intramolecular interaction. Interestingly, although NOX2 is not stimulated directly by Ca2+, it can be stimulated by the EF-hands-containing myeloid-related proteins MRP8 and MRP14 in a cytosolic effector-independent manner (Berthier et al., 2003). Moreover, in human monocytes, the assembly and activation of NOX2 in the NOX enzyme complex is regulated by calcium and protein kinase C-dependent phosphorylation (Cathcart, 2004). Taken together, stimulation by calcium is emerging as an inherently conserved trait of NOX and Rboh enzymes.
ROS PRODUCTION AND CALCIUM SIGNALING
In planta, cytosolic Ca2+ spiking can be seen to precede NOX activation as part of elicitor-induced defense responses (Nurnberger and Scheel, 2001; Zhao et al., 2005). For example, in tobacco cells, elicitors induce dynamic cytosolic Ca2+ spiking from a resting level of 50 to 100 nm to 1 to 5 μm in 2 to 5 min (Lecourieux et al., 2002). Thus, it is possible that calcium directly initiates Rboh activation. However, ROS production from the initial Ca2+-dependent activation of a NOX subsequently triggers a larger Ca2+ influx (Pugin et al., 1997; Pei et al., 2000; Kadota et al., 2004). In this scenario, ROS functions as a cellular second messenger activating Ca2+-permeable channels in a redox-controlled manner (Mori and Schroeder, 2004). AtrbohC was implicated in ROS-dependent activation of Ca2+ channels during root hair growth (Foreman et al., 2003) and AtrbohD and AtrbohF in abscisic acid (ABA)-induced activation of Ca2+ channels in guard cells (Kwak et al., 2003), suggesting the existence of a reiterated ROS to a calcium signal transduction module. If Ca2+ is involved in Rboh activation as well as serving as a target for the Rboh product, a potential self-amplifying loop will be formed. Similar, but longer, timescale activation loops were suggested in a mitogen-activated protein kinase cascade and H2O2-dependent increase of Rboh mRNA levels in Nicotiana benthamiana (Yoshioka et al., 2003). Presumably, runaway activation of Rboh can be tempered by cellular mechanisms for rapid calcium removal, substrate (NADPH) depletion (Hunt et al., 2004), or depletion of the superoxide product by interaction of superoxide with nitric oxide and other scavenging systems (Delledonne et al., 2001). The interplay of ROS and calcium offers a nexus for the fascinating and daunting prospect of signaling cross-talk (Bowler and Fluhr, 2000).
OTHER REGULATORY MECHANISMS: ALKALINIZATION AND SMALL GTPases
Medium (or apoplast) alkalinization can precede NOX activation. It is thought to result from elicitor-induced depolarization of the plasma membrane and subsequent K+/H+ exchange followed by Ca2+ influx/Cl− efflux (Simon-Plas et al., 1997; Nurnberger and Scheel, 2001; Zhao et al., 2005). Inactivation of the NtrbohD-dependent ROS accumulation does not affect the EC pH change (Simon-Plas et al., 2002), which is attributed mainly to the activity of a plasmalemma H+-ATPase (Simon-Plas et al., 1997). Whereas Rboh activation appears to be preceded by alkalinization, a special case of concomitant EC acidification is associated with AtFRO activity. In that case, acidification of the root rhizosphere carried out by a proton-pumping system enhances local solubility of FeIII ions before reduction of the FeIII-chelate complex (Staiger, 2002). Whether pH changes preceding protein enzyme activation are common for all Rboh members is unknown.
In mammalian phagocytes, the small GTPase Rac is among the cytosolic accessory factors that activate ROS production by NOX2 (Lambeth, 2004). Despite the apparent lack of similar accessory homologs in plants, plant Rac homologs (called ROP for Rho-like proteins) appear to regulate ROS defense production most likely via NOX (Kawasaki et al., 1999; Baxter-Burrell et al., 2002; Moeder et al., 2005). Interestingly, in ozone-stimulated cell death, the concomitant activation of membrane-bound NOX is mediated through the G α-subunit of the heterotrimeric G protein (Joo et al., 2005). The role of ROP GTPases appears to be more than simple activation of Rboh, but is involved in accurate spatial emulation of ROS. A RhoGDI (SCN1/AtRhoGDI) likely controls the activity of a ROP GTPase, resulting in root hair tip-focused activation of AtrbohC (Carol et al., 2005). Without SCN1/AtRhoGDI, the Rboh activity as detected by nitroblue terazolium is spatially deregulated and spread throughout the hair cell. Asymmetric bursts of NOX activity in Z. elegans are important to pinpoint the supply of H2O2 for peroxidase-based polymerization of lignin. In this case, Rac-like GTPase protein is detected on the plasma membrane juxtaposed to the site facing developing tracheary elements (Nakanomyo et al., 2002). How GTPases and other upstream modulators of Rboh activity operate mechanistically remains to be elucidated, although their juxtaposition with Rboh on lipid rafts may facilitate their direct or indirect interaction (Mongrand et al., 2004).
THE LANGUAGE OF Rboh ROS
ROS produced by NOX have EC and intracellular ramifications. EC-ROS products are associated with direct oxidative cross-linking of cell wall components during defense (Apel and Hirt, 2004), differentiation of plant vascular tissue (Nakanomyo et al., 2002), and suberization in wounded potato (Solanum tuberosum) tubers (Razem and Bernards, 2003). Opposing depolymerization properties of ROS are likely employed in NADPH-dependent cell loosening that takes place as a prelude to cell wall expansion (Rodriguez et al., 2002). In these cases, the Rboh is meant to deliver a spatially localized product because of the rapid EC dissipation of H2O2 (Allan and Fluhr, 1997).
Plant Rboh also functions as intercellular signal transponders to create local ROS transients that send a message. In addition to ABA-induced guard cell closure and root hair growth, H2O2 acts as a second messenger for the induction of defense genes in response to systemin and jasmonate during wound responses (Orozco-Cardenas et al., 2001). Repressing Rboh activity altered redox-related metabolism and induced multiple pleiotropic developmental effects in addition to hindering systemic wound responses (Sagi et al., 2004). These results suggest that ROS generated by Rboh act in several hormone-signaling pathways. How will this message be interpreted specifically to modulate cell death, wound response, reaction to hypoxia, stimulation of growth, etc.? How will the cellular ROS scavenging system modify this response (Davletova et al., 2005)? In the simplest case, a differentiated cell will interpret the message from the module in a manner specific to each cell type, such as stomatal closure in guard cells or elongation in root hairs. When choices are to be made between multiple possible cellular responses, the strength, pulse length, and spatial context, as well as the interaction of ROS with other signals, are likely to play a role.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers P04839 (HsNOX2), AAG33638 (HsNOX5), NP_196356 (AtrbohA), NP_973799 (AtrbohB), AAS15724 (AtrbohC), NP_199602 (AtrbohD), NP_173357 (AtrbohE), NP_564821 (AtrbohF), NP_194239 (AtrbohG), NP_200809 (AtrbohH), NP_192862 (AtrbohI), NP_190167 (AtrbohJ), NP_171665 (AtFRO1), and NP_171664 (AtFRO2).
This work was supported in part by the Israel Science Foundation (grant no. 417/03), the Minerva Foundation, Germany, and the Weizmann-Argentina Fundacion Antorchas.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Robert Fluhr (robert.fluhr@weizmann.ac.il).
References
- Allan AC, Fluhr R (1997) Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9: 1559–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noel-Hudson MS, Francon J, et al (2005) Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem 280: 30046–30054 [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Babior BM, Lambeth JD, Nauseef W (2002) The neutrophil NADPH oxidase. Arch Biochem Biophys 397: 342–344 [DOI] [PubMed] [Google Scholar]
- Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, Cox JA (2004) Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J Biol Chem 279: 18583–18591 [DOI] [PubMed] [Google Scholar]
- Barcelo AR (2005) Xylem parenchyma cells deliver the H2O2 necessary for lignification in differentiating xylem vessels. Planta 220: 747–756 [DOI] [PubMed] [Google Scholar]
- Baxter-Burrell A, Yang ZB, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296: 2026–2028 [DOI] [PubMed] [Google Scholar]
- Berthier S, Paclet MH, Lerouge S, Roux F, Vergnaud S, Coleman AW, Morel F (2003) Changing the conformation state of cytochrome b(558) initiates NADPH oxidase activation—MRP8/MRP14 regulation. J Biol Chem 278: 25499–25508 [DOI] [PubMed] [Google Scholar]
- Bowler C, Fluhr R (2000) The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci 5: 241–246 [DOI] [PubMed] [Google Scholar]
- Branco-Price C, Kawaguchi R, Ferreira RB, Bailey-Serres J (2005) Genome-wide analysis of transcript abundance and translation in Arabidopsis seedlings subjected to oxygen deprivation. Ann Bot (Lond) 96: 647–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L (2005) A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438: 1013–1016 [DOI] [PubMed] [Google Scholar]
- Carter C, Thornburg RW (2004) Is the nectar redox cycle a floral defense against microbial attack? Trends Plant Sci 9: 320–324 [DOI] [PubMed] [Google Scholar]
- Cathcart MK (2004) Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages—contributions to atherosclerosis. Arterioscler Thromb Vasc Biol 24: 23–28 [DOI] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98: 13454–13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Hunt L, Lerner F, Ziegler M (2004) NAD—new roles in signalling and gene regulation in plants. New Phytol 163: 31–44 [DOI] [PubMed] [Google Scholar]
- Joo JH, Wang SY, Chen JG, Jones AM, Fedoroff NV (2005) Different signaling and cell death roles of heterotrimeric G protein α- and β-subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17: 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y, Goh T, Tomatsu H, Tamauchi R, Higashi K, Muto S, Kuchitsu K (2004) Cryptogein-induced initial events in tobacco BY-2 cells: pharmacological characterization of molecular relationship among cytosolic Ca2+ transients, anion efflux and production of reactive oxygen species. Plant Cell Physiol 45: 160–170 [DOI] [PubMed] [Google Scholar]
- Karlsson M, Melzer M, Prokhorenko I, Johansson T, Wingsle G (2005) Hydrogen peroxide and expression of hipI-superoxide dismutase are associated with the development of secondary cell walls in Zinnia elegans. J Exp Bot 56: 2085–2093 [DOI] [PubMed] [Google Scholar]
- Karpinska B, Karlsson M, Schinkel H, Streller S, Suss KH, Melzer M, Wingsle G (2001) A novel superoxide dismutase with a high isoelectric point in higher plants: expression, regulation, and protein localization. Plant Physiol 126: 1668–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Henmi K, Ono E, Hatakeyama S, Iwano M, Satoh H, Shimamoto K (1999) The small GTP-binding protein Rac is a regulator of cell death in plants. Proc Natl Acad Sci USA 96: 10922–10926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD (2004) Nox enzymes and the biology of reactive oxygen. Nature Rev Immunol 4: 181–189 [DOI] [PubMed] [Google Scholar]
- Lara-Ortiz T, Riveros-Rosas H, Aguirre J (2003) Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol Microbiol 50: 1241–1255 [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A (2002) Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 14: 2627–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeder W, Yoshioka K, Klessig DF (2005) Involvement of the small GTPase Rac in the defense responses of tobacco to pathogens. Mol Plant-Microbe Interact 18: 116–124 [DOI] [PubMed] [Google Scholar]
- Mongrand S, Morel J, Laroche J, Claverol S, Carde JP, Hartmann MA, Bonneu M, Simon-Plas F, Lessire R, Bessoule JJ (2004) Lipid rafts in higher plant cells—purification and characterization of triton X-100-insoluble microdomains from tobacco plasma membrane. J Biol Chem 279: 36277–36286 [DOI] [PubMed] [Google Scholar]
- Mori IC, Schroeder JI (2004) Reactive oxygen species activation of plant Ca2+ channels: a signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetical mechanotransduction. Plant Physiol 135: 702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo I, Henderson LM (2005) Expression of gp91(phox)/Nox2 in COS-7 cells: cellular localization of the protein and the detection of outward proton currents. Biochem J 385: 649–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanomyo I, Kost B, Chua NH, Fukuda H (2002) Preferential and asymmetrical accumulation of a Rac small GTPase mRNA in differentiating xylem cells of Zinnia elegans. Plant Cell Physiol 43: 1484–1492 [DOI] [PubMed] [Google Scholar]
- Nurnberger T, Scheel D (2001) Signal transmission in the plant immune response. Trends Plant Sci 6: 372–379 [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13: 179–191 [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Petersen SV, Oury TD, Ostergaard L, Valnickova Z, Wegrzyn J, Thogersen IB, Jacobsen C, Bowler RP, Fattman CL, Crapo JD, et al (2004) Extracellular superoxide dismutase (EC-SOD) binds to type I collagen and protects against oxidative fragmentation. J Biol Chem 279: 13705–13710 [DOI] [PubMed] [Google Scholar]
- Pugin A, Frachisse JM, Tavernier E, Bligny R, Gout E, Douce R, Guern J (1997) Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell 9: 2077–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razem FA, Bernards MA (2003) Reactive oxygen species production in association with suberization: evidence for an NADPH-dependent oxidase. J Exp Bot 54: 935–941 [DOI] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397: 694–697 [DOI] [PubMed] [Google Scholar]
- Rodriguez AA, Grunberg KA, Taleisnik EL (2002) Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol 129: 1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R (2004) Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 16: 616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91(phox) NADPH oxidase: modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126: 1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Plas F, Elmayan T, Blein JP (2002) The plasma membrane oxidase NtrbohD is responsible for AOS production in elicited tobacco cells. Plant J 31: 137–147 [DOI] [PubMed] [Google Scholar]
- Simon-Plas F, Rusterucci C, Milat ML, Humbert C, Montillet JL, Blein JP (1997) Active oxygen species production in tobacco cells elicited by cryptogein. Plant Cell Environ 20: 1573–1579 [Google Scholar]
- Staiger D (2002) Chemical strategies for iron acquisition in plants. Angew Chem Int Ed Engl 41: 2259–2264 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91(phox) homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37: 1130–1134 [DOI] [PubMed] [Google Scholar]
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JDG (1998) Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91(phox)). Plant J 14: 365–370 [DOI] [PubMed] [Google Scholar]
- Wallace MA, Liou LL, Martins J, Clement MHS, Bailey S, Longo VD, Valentine JS, Gralla EB (2004) Superoxide inhibits 4Fe-4S cluster enzymes involved in amino acid biosynthesis—cross-compartment protection by CuZn-superoxide dismutase. J Biol Chem 279: 32055–32062 [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N (2003) Nicotiana benthamiana gp91(phox) homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15: 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23: 283–333 [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136: 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]