Abstract
The mammalian Ku heterodimer has important roles in DNA double strand break repair, telomere maintenance, cell cycle checkpoint-arrest, tumor suppression, and cellular stress resistance. To investigate the evolutionarily conserved functions of Ku, we knocked down expression by RNA interference (RNAi) of Ku genes in C. elegans. We found that C. elegans Ku70 (CKU-70) is required for resistance to genotoxic stress, regulates cytotoxic stress responses, and influences aging. The latter effects are dependent on an IGF-1/insulin-like signaling pathway previously shown to affect life span. Reduction of CKU-70 activity amplifies the aging phenotype of long-lived insulin receptor daf-2 mutations in a daf-16-dependent manner. These observations support the view that organismal stress resistance determines life span and Ku70 modulates these effects.
Keywords: cku-70, cku-80, daf-2, daf-16, aging, stress response, DNA repair
Cytotoxic stress resistance and aging rate are strongly influenced by an insulin-like/IGF-1 (IIGF) signaling pathway in C. elegans. Mutation of an IIGF receptor encoded by daf-2 increases mean life span by 100% (1, 2). The life span extension of daf-2 mutants are suppressed by mutations in daf-16 (1, 3), which has been identified as a forkhead/winged helix family transcription factor (4, 5). This pathway influences the expression of stress and metabolic genes and thereby affects life span (6-13). The IIGF pathway phenotypically interacts with heat shock factor (HSF), the regulator of heat shock protein (HSP) expression genes (14-16). The up-regulation of heat shock proteins appears a key component to the life span extension observed in these mutations (9, 14, 15). Almost all long-lived mutants show increased resistance to stresses, including heat (17-19), UV (20), oxidative stress (6, 7), and heavy metals (10), which is likely in part to be due to elevated levels of HSPs (9, 14) and other stress induced proteins (13). For example, HSP-16, a small HSP member of the α-crystallin family of molecular chaperones (21), over-accumulates in heat shocked age-1 mutants (9) and daf-2 mutants have a constitutively elevated hsp-16 mRNA abundance (14). Introduction of extra-copies of the hsp-16 gene results in an increased daf-16-dependent, thermotolerance, and life span extension (22). Overexpression of either the gene encoding HSP-70F (23) or HSF (14, 15) also extends life span in C. elegans.
These results are consistent with the proposal that life extension results, at least in part, from elevated maintenance of protein conformation (24).
In eukaryotic cells, a complex comprising Ku and DNA-dependent protein kinase (DNA-PK) is required for DNA double stranded break (DSB) repair. Ku is a heterodimer consisting of a 70 and 80 kDa subunit (Ku70 and Ku80) that binds in a DNA sequence-independent manner to ends, nicks, gaps, and hairpins and in a sequence-dependent manner to internal sites (25). In addition to its role in DSB repair, Ku functions in telomere maintenance, cell cycle checkpoint arrest, and tumor suppression. Ku70 and Ku80 deficient mice are viable and fertile but have reduced life span and marked immunodeficient, neuronal, and growth retardation phenotypes (26, 27). The individual loss of ku70 and ku80 also reveals unique phenotypes, suggesting functions specific to each gene (26-28).
There is a growing body of evidence linking HSPs to DNA repair activity. Mouse Hsp70.1 and Hsp70.3 are required for maintenance of genomic stability in stress conditions (29). Exposure to ρ-rays induces HSP70 (30), while prior heat shock induction of HSP70 increases radioresistance (31, 32) and decreases radiation induced cell death (33). Furthermore, human HSP70 can bind and enhance the specific endonuclease activity of human apurinic/apyrimidinic endonuclease (HAP1), a key component of the base excision repair pathway (34). In parallel, Ku70 regulates stress response genes (25, 35, 36). Overexpression of Ku70 in fibroblasts results in the specific repression of HSP70 upon heat shock, while induction of the remaining suite of HSPs remain normal (36). Under normal conditions HSF is a cytoplasmic monomer. Upon heat shock, it enters the nucleus, forms homo-trimers, is differentially phosphorylated, and binds to specific promoter sites (heat shock elements, HSEs) (37). In vitro translated Ku70 interacts with HSF by binding to and displacing it from HSEs in the promoter of HSP70, suggesting that Ku70 may negatively regulate HSF binding activity in vivo (25).
We were prompted by the role of Ku70 in mammals as a negative regulator of HSF to study the conserved features in C. elegans. The C. elegans orthologs of mammalian Ku70 and Ku80 have been identified by the C. elegans Genome Sequencing Consortium (CAB55094 and CAA83623), and their encoding genes have been designated cku-70 and cku-80, respectively. We propose a similar mechanism of action of Ku70 in C. elegans whereby CKU-70 down-regulation will decrease resistance to genotoxic stress yet increase heat resistance. The net effect on life span is then examined.
MATERIALS AND METHODS
Strains
Worms strains N2[wild type], NL2099[rrf-3(pk1426)], CB1370[daf-2(e1370)], DR1564[daf-2(m41)], DR26[daf-16(m26)], DR1309[daf-16(m26); daf-2(e1370)], and SS104[glp-4(bn2)] were obtained from the Caenorhabditis Genetics Center. All strains were cultured on nematode growth plates (NGM) plates at 20°C unless stated otherwise.
cku-70 and cku-80 RNA interference (RNAi)
For cku-70(RNAi), an 828-bp fragment of cDNA-spanning exons 2-8 was amplified (primers 5′-ATAGCACTCTGCTTGTGCC-3′ to 5′-CCAAAATTATCTTCTCTCCACC-3′), subcloned into the L4440 vector (a gift from A. Fire, Stanford), and transformed in to HT115 (DE3) cells. For cku-80(RNAi), an 853-bp fragment of cDNA-spanning exons 2-10 was similarly cloned (primers 5′-ATTAATGCGTCAGGCTAC-3′ to 5′-CACCATCATCTACCTCATC-3′). NGM plates for RNAi were prepared as described previously (38). For all experiments L4 larvae were initially transferred onto RNAi plates, kept at 20°C overnight and allowed to lay eggs for 3-6 h. These synchronous eggs were left to develop at 20°C until reaching either L4 or adulthood and were then transferred onto fresh RNAi plates for all subsequent assays.
Methyl methane sulfonate sensitivity assay
Approximately 100 adult hermaphrodites were transferred from RNAi plates into liquid culture at 20°C, 1 × 1010 cells/ml OP50 in S-basal (39). Several hundred eggs laid over 3 h were isolated using 40 μM nylon mesh filters (Falcon®). Eggs were then exposed to various concentrations of methyl methane sulfonate (MMS), in 1 ml of S-basal, in 24-well microtiter plates for 1 h at 25°C with orbital shaking at 100 rpm. Eggs were then washed twice to remove excess MMS and plated on NGM plates. Plates were kept at 25°C for 3 days, and the number of adults was then scored.
Self-fertility assay
Approximately 15-30 hermaphrodite L4 larvae were transferred onto individual RNAi plates and allowed to develop to adulthood. These individuals were transferred to new plates every day, and their progeny was counted after development at 20°C for 2-3 days.
Thermotolerance assay
For each population, two plates of 25-30 4-day-old adult hermaphrodites were transferred onto fresh RNAi plates then shifted to 35°C. Thereafter, individuals were scored for death, lack of touch-provoked movement as described previously (18).
Life span assay
For each treatment, four populations of 25-30 L4 hermaphrodites were transferred onto fresh RNAi plates and left overnight 20°C. Day one of the life span assay commenced when these individuals were shifted to 25°C. Populations were transferred onto fresh RNAi plates daily until cessation of egg laying, and then individual worms were scored as alive or dead every 1-3 days. The day of death was determined by a lack of touch-provoked movement as described previously (17) and recorded for all individuals.
Dauer formation assay
Synchronous adults were left to lay eggs at 20°C for 3 h on RNAi plates. For wild-type and daf-2(e1370) strains, these plates were then shifted to 27°C for 3 days and then the number of adults and dauer larvae were counted. Dauer larvae were additionally scored by their resistance to 1% SDS exposure for 30 min. For daf-2(m41) populations, plates were moved to 20°C for 4 days and similarly scored.
Statistical tests
Differences in survival during life span or heat shock assays were assessed using the nonparametric Log rank test as implemented in the Prism™ software package. Differences in total fertility and mean survival after MMS exposure were examined using Student’s t-test (2-tailed).
RESULTS
cku-70 and cku-80 effects on MMS sensitivity
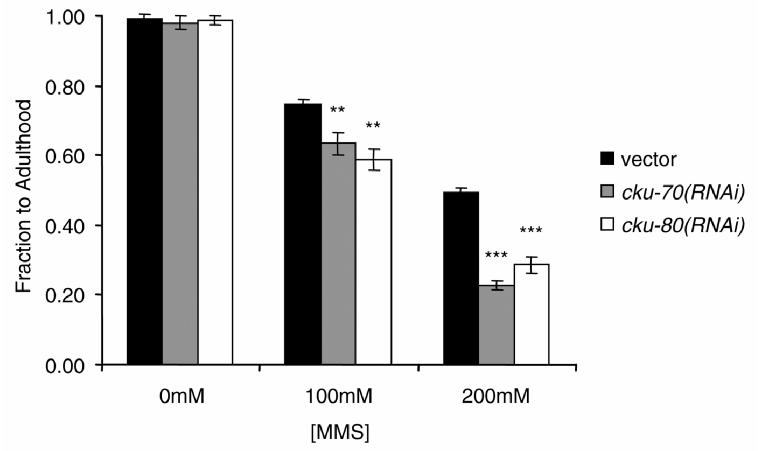
We hypothesized that knocking down Ku functions in C. elegans would render worms sensitive to DNA damage. MMS is a DNA-alkylating agent and known mutagen (40). Exposure to MMS resulted in an increasing proportion of eggs that failed to develop in a dose dependent manner(Fig. 1). Furthermore, reduction of either CKU-70 or CKU-80 activity via RNAi significantly increased this sensitivity, consistent with their roles in DNA repair processes. Comparison of wild type and cku-80(ok861), a C. elegans Gene Knockout Project strain carrying an ∼1300 bp deletion, also revealed a significant increase in sensitivity (data not shown). Similar experiments after exposure of eggs to various doses of UV found no effect of either cku-70(RNAi) or cku-80(RNAi) (data not shown). These results are consistent with CKU-70 and -80 having no role in repair of UV-induced DNA lesions.
Figure 1.
Effects of cku-70 and cku-80(RNAi) on MMS sensitivity of progeny from parents treated with either cku-70(RNAi) or cku-80(RNAi). **P < 0.01, ***P < 0.001.
cku-70 and cku-80 effects on fertility
A moderate reduction of DNA repair processes may be expected to lead to impaired genomic stability. Somewhat surprising is that overall fertility was not significantly reduced after either cku-70(RNAi) or cku-80(RNAi) (Table 1). Commencement of egg laying was also not affected by cku-70(RNAi), suggesting no change in developmental rate (data not shown).
Table 1.
Fertility effect of cku-70 and cku-80
| Treatment | Mean total offspring ± SE | n |
|---|---|---|
| vector | 294 ±6 | 28 |
| cku-70(RNAi) | 282 ±6 ns | 27 |
| cku-80(RNAi) | 287 ±13 ns | 13 |
ns: not significant.
cku-70 and cku-80 effects on thermotolerance
Altered thermotolerance would be expected if CKU-70 were a negative regulator of stress protein induction. Wild-type thermotolerance was significantly increased after cku-70(RNAi) (Table 2). However, this increase was found to be daf-16 dependent, such that daf-2(e1370) thermotolerance was also significantly increased but not in either daf-16(m26) or daf-16(m26);daf-2(e1370) genetic backgrounds. No effect on thermotolerance was observed after cku-80(RNAi) (Table 2) or between wild-type and cku-80(ok861) genotypes (data not shown). This demonstrates that CKU-70 and CKU-80 also have distinct functions in C. elegans.
Table 2.
Effects of cku-70 and cku-80 on adult thermotoleranc
| Mean survival in minutes ± SE at 35°C |
||||
|---|---|---|---|---|
| Genotype | Vector | n | cku-70(RNAi) | n |
| Wild type | 489 ±13 | 56 | 531 ±18* | 54 |
| daf-2(e1370) | 820 ±8 | 48 | 916 ±9** | 46 |
| daf-16(m26) | 496 ±10 | 50 | 469 ±11ns | 50 |
| daf-16(m26); daf-2(e1370) | 543 ±14 | 51 | 522 ±14ns | 52 |
| Mean survival in minutes ±SE at 35°C |
||||
|---|---|---|---|---|
| Genotype | Vector | n | cku-80(RNAi) | n |
| wildtype | 413 ±13 | 52 | 401 ±18 ns | 54 |
P < 0.05
P < 0.01.
cku-70 and cku-80 effects on life span
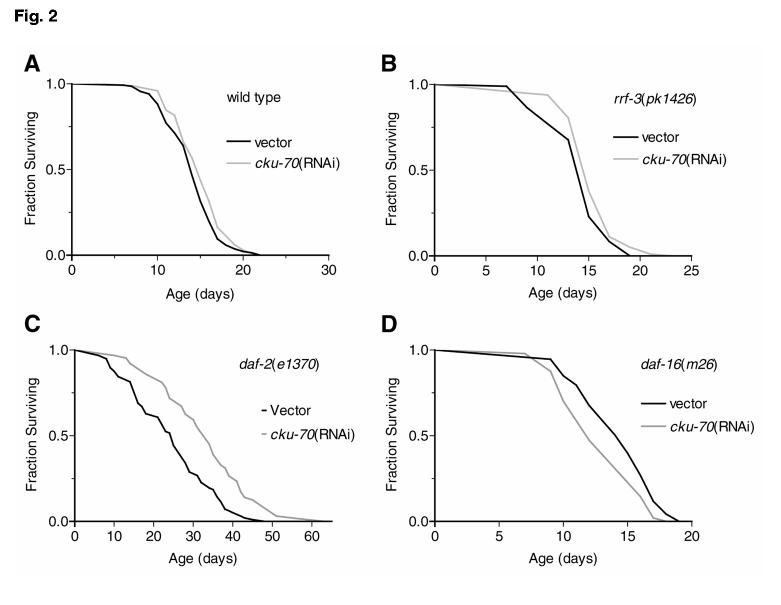
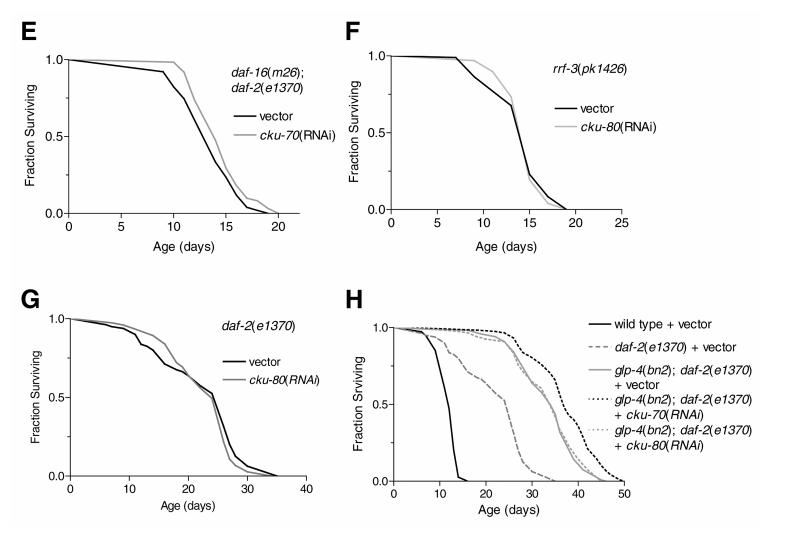
Reduction of cku-70 activity did not alter wild-type life span (Fig. 2). However, using rrf-a 3(pk1426) worms that show increased sensitivity to RNAi (41) when compared with wild type, we observed a slight but significant increase (mean increase ∼14%) in life span after cku-70(RNAi). Further examination of cku-70(RNAi) revealed that life span of daf-2(e1370) was also significantly increased (mean increase ∼35%), while daf-16(m26) life span was marginally but significantly decreased (mean decrease ∼11%). In the double mutant, daf-16(m26);daf-2(e1370) no life span difference was detected. In contrast, cku-80(RNAi) did not alter wild-type, rrf-3(pk1426), or daf-2(e1370) life span. Similarly, wild type and cku-80(ok861) did not differ in life span (data not shown).
Figure 2.
Effects of cku-70 and cku-80 on adult hermaphrodite life span (days) at 25°C. A) For wild-type worms cku-70(RNAi) did not significantly change life span; mean life span ± SE was 14.1 ± 0.3 days (vector: n=136) and 15.0 ±0.3 [cku-70(RNAi): n=98], replicate experiments r = 2. B) rrf-3(pk1426) mean life span was slightly increased (P<0.01), 14.2 ± 0.3 (vector: n=96) and 15.5 ± 0.3 [cku-70(RNAi): n=98], r = 2. C) daf-2(e1370) mean life span was significantly increased (P<0.0001), 24.2 ± 1.1 (vector: n=97) and 32.6 ± 1.4 [cku-70(RNAi): n=64], r = 3. D) daf-16(m26) mean life span was significantly decreased (P<0.0001), 14.3 ± 0.3 (vector: n=93) and 12.8 ± 0.3 [cku-70(RNAi): n=97], r = 3.E) daf-16(m26);daf-2(e1370) mean life span was not significantly changed 13.5 ±0.4 (vector: n=51) and 14.5 ± 0.3 [cku-70(RNAi): n=61], r =2. F) In rrf-3(pk1426) cku-80(RNAi) had no effect on life span 14.2 ± 0.3 (vector: n=96) and 14.7 ±0.2 (cku-80(RNAi): n=98), r = 2. G) daf-2(e1370) mean life span was not affected by cku-80(RNAi), 22.1 ± 0.8 (vector: n=80) and 22.5 ± 10.7 [cku-80(RNAi): n=75], r = 2. H) glp-4(bn2);daf-2(e1370) life span was significantly increased (P<0.0001) compared with daf-2(e1370) alone, 33.2 ± 0.6 (vector: n=119) vs. 22.1 ± 0.8 (vector: n=80). glp-4(bn2);daf-2(e1370) life span was slightly increased (P<0.0001) by cku-70(RNAi), 37 ± 0.7 [cku-70(RNAi): n=117] but unchanged by cku-80(RNAi), 33.1 ± 0.7 [cku-80(RNAi): n=119], r = 2.
To determine if life span effects of cku-70(RNAi) were due to deleterious effects on the germline, we used glp-4(bn4);daf-2(e1370) double mutants that lack germ cells and have previously been shown to have extended life span beyond that of daf-2(e1370) alone (42). Here we observed a mild but reproducible increase in life span (mean increase ∼9%) after cku-70(RNAi) but no effect from cku-80(RNAi). Life span assays were conducted as either duplicate or triplicates experiments with equivalent results. Representative data are shown (Fig. 2).
cku-70 effects on Dauer formation
Dauer development phenotypes are common to genes regulating stress resistance and life span. We examined the role of CKU-70 in dauer formation. Based on the finding that cku-70(RNAi) enhanced the increased life span of daf-2, we examined the possibility that dauer formation may have also been affected. However, reduction in CKU-70 activity has no effect on dauer formation (Table 3). cku-70(RNAi) did not increase dauer formation in wild-type worms nor alter the frequency of dauer formation in daf-2(e1370) or daf-2(m41) worms. Although cku-70 functions to regulate stress response, it appears not to be involved in dauer formation.
Table 3.
Effects of cku-70 on dauer formation
| Genotype | Temperature °C | n | %Dauers* | |
|---|---|---|---|---|
| Wild type | vector | 25 | 411 | 0% |
| cku-70(RNAi) | 25 | 402 | 0% | |
| daf-2(e1370) | vector | 25 | 220 | 100% |
| cku-70(RNAi) | 25 | 193 | 100% | |
| daf-2(m41) | vector | 20 | 435 | 77% |
| cku-70(RNAi) | 20 | 425 | 73% |
Resistant to 1% SDS.
DISCUSSION
We investigated the functions of C. elegans cku-70 and cku-80, orthologs to mammalian ku-70 and ku-80, and have discovered that CKU-70 and CKU-80 are required for viability after DNA damage. We show a dose-dependent effect of the radiomimetic MMS in wild-type worms leading to decreasing egg viability, similar to previous reports (43). Reduced activity of either cku-70 or cku-80 further reduces egg viability, consistent with an impaired ability to repair DNA breaks. Thus, the C. elegans ortholog of mammalian ku-70 and ku-80 have phenotypes consistent with roles in DNA repair. Increased sensitivity to MMS has been observed for several other C. elegans DNA radiation-repair (rad) mutants (43). We found that neither cku-70 nor cku-80(RNAi) altered UV sensitivity. UV light-induced DNA damage is repaired via excision repair pathways, in which the Ku70/80 heterodimer is thought not to be involved. Surprisingly, reduction of cku-70 or cku-80 activity did not lower overall fertility and led to no obvious change to development rate. RNAi knockdown of cku-70 or cku-80 over multiple generations may be required before effects on fertility become apparent.
Reduced nematode CKU-70 activity increases thermotolerance, consistent with CKU-70 being a negative regulator of HSF-1 (25). Intrinsic thermotolerance was increased after cku-70(RNAi) in both wild-type and daf-2(e1370) backgrounds. This gain was found to be dependent on wild-type DAF-16, such that no increase was observed in daf-16(m26) or daf-16(m26);daf-2(e1370) backgrounds. DAF-16 activity is required for elevated stress resistance and life span phenotypes seen in daf-2 mutants (3, 20).
At normal growth temperature, no life span effect was observed for cku-70(RNAi) in wild-type worms. In a rrf-3(pk1426) we observe a slight increase in life span (mean increase ∼14%). Mutation in the gene rrf-3, encoding a RNA-directed RNA polymerase, has increased sensitivity to RNAi, while remaining viable and fertile (41). This suggests that the reduction of CKU-70 in the wild-type populations was suboptimal but ultimately may only produce marginal increases in mean life span. However, reduction of cku-70 activity appears to amplify the aging phenotype of IIGF mutants. Long-lived daf-2 mutations become even longer lived (mean increase ∼35%) and short-lived daf-16 mutants slightly shorter lived (mean decrease ∼11%).
An as yet unidentified germ line signal has been observed to modulate life span adult (44). Ablation of the germ line precursor cells or mutation causing absence of germ cells in adults also increased daf-2 mutant life span (42, 44). We propose that the increase in daf-2(e1370) life span by cku-70(RNAi) may be due in part to processes independent of any germ line signals. First, we observed no gross fertility deficit from RNAi knockdown of cku-70. Second, in a glp-4(bn2);daf-2(e1370) mutants, which essentially lack germ cells (45), a life span increase after cku-70(RNAi) is still reproducibly detected (mean increase ∼9%).
Reduction of cku-80 activity also resulted in increased MMS sensitivity but without thermotolerance or life span phenotypes, regardless of the genetic background. The discordance between the cku-70 and cku-80(RNAi) results suggest we have uncovered thermotolerance and life span phenotypes specific to CKU-70, rather than phenotypes derived from a CKU-70/80 heterodimer. In contrast, ku86 mutant mice (where Ku86 is the murine ortholog of human Ku80) exhibit characteristics of premature senescence and reduced life span (27). A possible explanation for this disparity is that unlike in the mouse no somatic cell proliferation takes place during nematode adulthood.
The underlying molecular nature of any CKU-70 and DAF-16 interaction remains unclear. The coordinate regulation of DNA repair/genomic stability processes and stress response has been observed previously (29, 31, 32) consistent with a direct interaction. The cumulative effect of these processes may alter rates of aging. In a screen for proteins interacting with DNA damage response proteins CKU-70 and CKU-80 exhibited reciprocal interactions (46). However, neither DAF-16 nor HSF-1 was identified as an interactor; further investigation will determine if either protein interacts with CKU-70.
Despite cku-70(RNAi) increasing sensitivity to DNA damage, it can also increase life span, suggesting that DNA damage does not play a major role in determination of C. elegans life span. However, loss of the C. elegans RecQ5 ortholog also causes sensitivity to DNA damage and results in a shortened in life span (47). Thus, understanding of the subtleties genomic stability may play in determining C. elegans aging requires further work. Interestingly, recent work shows checkpoint functions can also regulate stress responses and modulate life span (Olsen and Lithgow, unpublished data).
If CKU-70 is involved in a mechanism that coordinately regulates stress responses, DNA repair, genomic stability, and ultimately aging, what then is the endogenous role of CKU-70 in stress responses? The Ku heterodimer binds with high affinity to broken DNA ends, preventing degradation and facilitating repair (48). Breaking of DNA after a genotoxic stress may also involve chromatin damage. The HSPs would then assist in repair of the protein component of damaged chromatin. Under benign conditions, CKU-70 will have limited DNA ends to bind and so may associate, for example, with HSP70 promoters to suppress expression. After DNA breakage, the high affinity of CKU-70 for binding of DNA ends could result in titration of CKU-70 away from HSP70 promoters, which in turn would relax suppression and lead to increased HSP70 expression. Such a model is consistent with observations in cell culture, where ρ-ray exposure induces Hsp70 expression (32) and the overexpression of Ku70 followed by heat shock results in the specific lack of Hsp70 induction (35, 36).
Increased DAF-16 activity (e.g., daf-2 mutants) is thought to increase expression of stress genes, including HSPs (9, 14). Overexpression of HSF-1 itself increases life span, although in a DAF-16-dependent manner (14, 15). The combined loss of both DAF-16 and HSF-1 function does not further decrease life span compared with loss of each singularly. Thus, DAF-16 and HSF-1 function in a common pathway to control life span in the nematode. Reduction of cku-70 activity may further increase HSF-1 binding on HSP70 promoters, which in turn may increase expression of HSP70 through stress and life span. Decreased DAF-16 activity (e.g., daf-16 mutants) results in impaired stress and HSP gene induction, lowered thermotolerance, and decreased life span. The combined dysregulation of HSF-1 activity by both lowered DAF-16 and CKU-70 activity throughout an entire life span may be further detrimental.
This study describes an interesting interplay between DNA repair and aging. Processes intended to reduce the risks of tumor formation may, as a by-product, shorten maximal life span consistent with the notion that aging results from late-acting detrimental actions of genes selected for early life beneficial effects (49, 50).
ACKNOWLEDGMENTS
We acknowledge the International C. elegans Gene Knockout Consortium for the cku-80(ok861) strain and A. Fire for vectors. We thank Nicole Jenkins, Al Fisher, and Patrick Kaminker for critical comments. G. McColl is supported by the Glenn Foundation/AFAR and NIH RO1AG21069. G. J. Lithgow is supported by the Ellison Medical Foundation and RO-122868.
REFERENCES
- 1.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 2.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 3.Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139:1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 5.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 6.Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanfleteren JR. Oxidative stress and ageing in Caenorhabditis elegans. Biochem. J. 1993;292:605–608. doi: 10.1042/bj2920605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- 9.Walker GA, White TM, McColl G, Jenkins NL, Babich S, Candido EP, Johnson TE, Lithgow GJ. Heat shock protein accumulation is up-regulated in a long-lived mutant of Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:B281–B287. doi: 10.1093/gerona/56.7.b281. [DOI] [PubMed] [Google Scholar]
- 10.Barsyte D, Lovejoy DA, Lithgow GJ. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. FASEB J. 2001;15:627–634. doi: 10.1096/fj.99-0966com. [DOI] [PubMed] [Google Scholar]
- 11.Yu H, Larsen PL. DAF-16-dependent and independent expression targets of DAF-2 insulin receptor-like pathway in Caenorhabditis elegans include FKBPs. J. Mol. Biol. 2001;314:1017–1028. doi: 10.1006/jmbi.2000.5210. [DOI] [PubMed] [Google Scholar]
- 12.McElwee J, Bubb K, Thomas JH. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell. 2003;2:111–121. doi: 10.1046/j.1474-9728.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 13.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the life span of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 14.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 15.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol. Biol. Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker GA, Thompson FJ, Brawley A, Scanlon T, Devaney E. Heat shock factor functions at the convergence of the stress response and developmental pathways in Caenorhabditis elegans. FASEB J. 2003;17:1960–1962. doi: 10.1096/fj.03-0164fje. [DOI] [PubMed] [Google Scholar]
- 17.Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl. Acad. Sci. USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lithgow GJ, White TM, Hinerfeld DA, Johnson TE. Thermotolerance of a long-lived mutant of Caenorhabditis elegans. J. Gerontol. 1994;49:B270–B276. doi: 10.1093/geronj/49.6.b270. [DOI] [PubMed] [Google Scholar]
- 19.Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami S, Johnson TE. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics. 1996;143:1207–1218. doi: 10.1093/genetics/143.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leroux MR, Melki R, Gordon B, Batelier G, Candido EP. Structure-function studies on small heat shock protein oligomeric assembly and interaction with unfolded polypeptides. J. Biol. Chem. 1997;272:24646–24656. doi: 10.1074/jbc.272.39.24646. [DOI] [PubMed] [Google Scholar]
- 22.Walker GA, Lithgow GJ. Life span extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama K, Fukumoto K, Murakami T, Harada S, Hosono R, Wadhwa R, Mitsui Y, Ohkuma S. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 2002;516:53–57. doi: 10.1016/s0014-5793(02)02470-5. [DOI] [PubMed] [Google Scholar]
- 24.Lithgow GJ. Invertebrate gerontology: the age mutations of Caenorhabditis elegans. Bioessays. 1996;18:809–815. doi: 10.1002/bies.950181007. [DOI] [PubMed] [Google Scholar]
- 25.Tang D, Xie Y, Zhao M, Stevenson MA, Calderwood SK. Repression of the HSP70B promoter by NFIL6, Ku70, and MAPK involves three complementary mechanisms. Biochem. Biophys. Res. Commun. 2001;280:280–285. doi: 10.1006/bbrc.2000.4118. [DOI] [PubMed] [Google Scholar]
- 26.Li GC, Ouyang H, Li X, Nagasawa H, Little JB, Chen DJ, Ling CC, Fuks Z, Cordon-Cardo C. Ku70: a candidate tumor suppressor gene for murine T cell lymphoma. Mol. Cell. 1998;2:1–8. doi: 10.1016/s1097-2765(00)80108-2. [DOI] [PubMed] [Google Scholar]
- 27.Vogel H, Lim DS, Karsenty G, Finegold M, Hasty P. Deletion of Ku86 causes early onset of senescence in mice. Proc. Natl. Acad. Sci. USA. 1999;96:10770–10775. doi: 10.1073/pnas.96.19.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu Y, Sekiguchi J, Gao Y, Dikkes P, Frank K, Ferguson D, Hasty P, Chun J, Alt FW. Defective embryonic neurogenesis in Ku-deficient but not DNA-dependent protein kinase catalytic subunit-deficient mice. Proc. Natl. Acad. Sci. USA. 2000;97:2668–2673. doi: 10.1073/pnas.97.6.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt CR, Dix DJ, Sharma GG, Pandita RK, Gupta A, Funk M, Pandita TK. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol. Cell. Biol. 2004;24:899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sierra-Rivera E, Voorhees GJ, Freeman ML. Gamma irradiation increases hsp-70 in Chinese hamster ovary cells. Radiat. Res. 1993;135:40–45. doi: 10.2307/3578394. [DOI] [PubMed] [Google Scholar]
- 31.Park SH, Lee SJ, Chung HY, Kim TH, Cho CK, Yoo SY, Lee YS. Inducible heat-shock protein 70 is involved in the radioadaptive response. Radiat. Res. 2000;153:318–326. doi: 10.1667/0033-7587(2000)153[0318:ihspii]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Calini V, Urani C, Camatini M. Overexpression of HSP70 is induced by ionizing radiation in C3H 10T1/2 cells and protects from DNA damage. Toxicol. In Vitro. 2003;17:561–566. doi: 10.1016/s0887-2333(03)00116-4. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, Choi SA, Lee KH, Chung HY, Kim TH, Cho CK, Lee YS. Role of inducible heat shock protein 70 in radiation-induced cell death. Cell Stress Chaperones. 2001;6:273–281. doi: 10.1379/1466-1268(2001)006<0273:roihsp>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenny MK, Mendez F, Sandigursky M, Kureekattil RP, Goldman JD, Franklin WA, Bases R. Heat shock protein 70 binds to human apurinic/apyrimidinic endonuclease and stimulates endonuclease activity at abasic sites. J. Biol. Chem. 2001;276:9532–9536. doi: 10.1074/jbc.M009297200. [DOI] [PubMed] [Google Scholar]
- 35.Yang SH, Nussenzweig A, Yang WH, Kim D, Li GC. Cloning and characterization of rat Ku70: involvement of Ku autoantigen in the heat-shock response. Radiat. Res. 1996;146:603–611. [PubMed] [Google Scholar]
- 36.Yang SH, Nussenzweig A, Li L, Kim D, Ouyang H, Burgman P, Li GC. Modulation of thermal induction of hsp70 expression by Ku autoantigen or its individual subunits. Mol. Cell. Biol. 1996;16:3799–3806. doi: 10.1128/mcb.16.7.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 38.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 39.Stiernagle T. Maintenance of C. elegans. In: Hope IA, editor. C. elagans: A Practical Approach. Oxford University Press; Oxford: 1999. pp. 51–67. [Google Scholar]
- 40.Glaab WE, Tindall KR, Skopek TR. Specificity of mutations induced by methyl methanesulfonate in mismatch repair-deficient human cancer cell lines. Mutat. Res. 1999;427:67–78. doi: 10.1016/s0027-5107(99)00091-3. [DOI] [PubMed] [Google Scholar]
- 41.Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, Plasterk RH, Fire A. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- 42.McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J. Biol. Chem. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- 43.Hartman PS, Herman RK. Radiation-sensitive mutants of Caenorhabditis elegans. Genetics. 1982;102:159–178. doi: 10.1093/genetics/102.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- 45.Beanan MJ, Strome S. Characterization of a germ-line proliferation mutation in C. elegans. Development. 1992;116:755–766. doi: 10.1242/dev.116.3.755. [DOI] [PubMed] [Google Scholar]
- 46.Boulton SJ, Gartner A, Reboul J, Vaglio P, Dyson N, Hill DE, Vidal M. Combined functional genomic maps of the C. elegans DNA damage response. Science. 2002;295:127–131. doi: 10.1126/science.1065986. [DOI] [PubMed] [Google Scholar]
- 47.Jeong YS, Kang Y, Lim KH, Lee MH, Lee J, Koo HS. Deficiency of Caenorhabditis elegans RecQ5 homologue reduces life span and increases sensitivity to ionizing radiation. DNA Repair (Amst.) 2003;2:1309–1319. doi: 10.1016/j.dnarep.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- 49.Walker DW, McColl G, Jenkins NL, Harris J, Lithgow GJ. Evolution of life span in C. elegans. Nature. 2000;405:296–297. doi: 10.1038/35012693. [DOI] [PubMed] [Google Scholar]
- 50.Campisi J. Cancer and ageing: rival demons? Nat. Rev. Cancer. 2003;3:339–349. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]