Abstract
Heat-shock protein 60 (Hsp60) is a highly conserved stress protein which has chaperone functions in prokaryotes and mammalian cells. Hsp60 is associated with the mitochondria and the plasma membrane through phosphorylation by protein kinase A, and is incorporated into lipid membranes as a protein-folding chaperone. Its diverse intracellular chaperone functions include the secretion of proteins where it maintains the conformation of precursors and facilitates their translocation through the plasma membrane. We report here that Hsp60 is concentrated in apoptotic membrane blebs and translocates to the surface of cells undergoing apoptosis. Hsp60 is also enriched in platelets derived from terminally differentiated megakaryocytes and expressed at the surface of senescent platelets. Furthermore, the exposure of monocytic U937 cells to Hsp60 enhanced their phagocytic activity. Our results suggests that externalized Hsp60 in apoptotic cells and senescent platelets influences events subsequent to apoptosis, such as the clearance of apoptotic cells by phagocytes.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0534-0) contains supplementary material, which is available to authorized users.
Keywords: Hsp60, Apoptosis, Translocation, Platelets, Phagocytosis
Introduction
Programmed cell death [1] involves an array of highly conserved cellular mechanisms that result in loss of cellular function and provide cues for cellular removal by phagocytes [2, 3]. The critical importance of apoptosis in cell deletion during embryogenesis [4, 5], organ development, resolution of inflammation [6], degenerative diseases and cancers has been revealed over the past two decades. The influence of apoptosis in tumour biology is complex, and possibly affects susceptibility to chemotherapy [7] as well as cell proliferation [8]. The intracellular events occurring during apoptosis include cytoplasmic shrinkage, chromatin condensation, nuclear fragmentation, membrane blebbing and packaging of cellular contents into apoptotic bodies [9, 10]. Many of these morphological changes associated with apoptosis result from the actions of caspases on cellular substrates [11, 12].
We report here that heat-shock protein 60 (Hsp60) [13], which is normally localized in the cytoplasm, is translocated to the cell surface early during the apoptotic process. Emerging evidence suggests Hsp60 has diverse intracellular functions, playing an important role in regulation of multiple innate and acquired immunological functions [14, 15]. Hsp is a molecular chaperone associated with the mitochondria [16] and the plasma membrane through phosphorylation by protein kinase A [17]. Hsp60 influences the secretion of proteins by maintaining the conformation of precursors that facilitates translocation through the plasma membrane [18, 19]. In addition, Hsp60 may be incorporated into the lipid membranes as a protein-folding chaperone [20].
We identified a new monoclonal antibody developed by our group [21] that recognises Hsp60 and confirmed the presence of Hsp60 on the plasma membranes of cells of apoptotic tumour cell lines of diverse lineages. In addition, studies using MEG-01, a human megakaryocytic cell line, revealed the presence of Hsp60 on the surface of senescent platelets. Importantly, surface expression of Hsp60 was shown to influence the phagocytic activity, reflecting the potential for Hsp60 to influence a wide range of important physiological processes.
Materials and methods
Cell culture and reagents
Human cell lines were maintained in standard media supplemented with 10% fetal calf serum (FCS, Invitrogen) and 2 mM l-glutamine (GibcoBrl) at 37°C in humidified air containing 5% carbon dioxide. The following haematogenous human cell lines were used: THP-1 monocytic cell line derived from acute monocytic leukaemia, MEG-01 megakaryocytic cell line derived from chronic myelogenous leukaemia and U937 monocytic cell line derived from histiocytic lymphoma. THP-1, MEG-01 and U937 cells were maintained in RPMI-1640 (Invitrogen and Sigma). The hepatocellular carcinoma-derived HUH-7 cell line was maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen). Primary antibodies employed included BOB78 (IgM mAb) developed by our group [21], anti-human Hsp60 (Stressgen) and anti-human calreticulin (Sigma), and secondary antibodies included antibodies conjugated with FITC and TRITC (DAKO), and antibodies conjugated with phycoerythrin (PE, Serotec). Other fluorescent reagents included propidium iodide (PI, Sigma), PE-conjugated annexin V (BD Biosciences), and TO-PRO3 nuclear stain (Cambridge Bioscience). Camptothecin, monensin, and cycloheximide were purchased from Sigma for induction of apoptosis. Protein chemistry reagents including bovine serum albumin (BSA) and protein A Sepharose beads for immunoprecipitation were from Sigma unless otherwise indicated.
Immunoprecipitation with BOB78 antibody
Cells were washed with ice-cold phosphate-buffered saline (PBS), pelleted, and lysed by adding TENT lysis buffer (10 mM Tris-HCl, 5 mM EDTA, 150 mM NaCl, 1% Triton-X 100, and protease inhibitors), and incubated for 30 min at 4°C. The mixture was then centrifuged at 12,000 g for 10 min to separate the precipitates. Protein concentration was assessed using the Bio-Rad protein assay according to the manufacturer’s recommendations. To avoid nonspecific binding, 100 μl of lysate (containing about 500 μg protein) was incubated with 10 μl of protein A Sepharose beads for 1 h on ice. BOB78 mAb was conjugated to protein A Sepharose beads via the bridging antibody by first incubating the beads with rabbit anti-mouse IgM (10 μg, DAKO) for 2 h at 4°C followed by 20 μl of beads conjugated to the bridging antibody BOB78 IgM antibody for 2 h at 4°C. Finally, the BOB78 IgM-immune complexes were then incubated with cell lysate overnight at 4°C for immunoprecipitation. The beads were then collected by centrifuging at 350 g for 30 s, washed three times in lysis buffer containing protease inhibitors, each wash being performed with agitation for 10 min on a rotating platform. The immune complexes were released from the beads by incubation with 20 μl of 5× loading buffer comprising 10% sodium dodecyl sulphate (SDS) and 5% 2-beta-mercaptoethanol and boiling at 95°C for 5 min, and were then separated by a 12% SDS polyacrylamide gel electrophoresis as described below.
Western blotting
Denatured protein immunoprecipitates were loaded onto a 12% SDS-polyacrylamide gel at 15 μg per lane. One half of the gel was stained with colloidal Coomassie Blue (Gelcode, Pierce) and the other half was transferred onto a nitrocellulose membrane (BioRad) and blocked with 5% milk/Tris-buffered saline (TBS) for 20 min at room temperature (RT) followed by incubation with primary antibodies in 0.025% Tween 20/5% milk/TBS overnight at 4°C (BOB78, 1 μg/ml; or recombinant anti-human Hsp60, 1 μg/ml; Stressgen). The membrane was then washed, incubated with peroxidase-conjugated secondary antibodies and developed by enhanced chemiluminescence (ECL, Amersham Pharmacia Biotech).
Mass spectrometry
Protein bands present on Coomassie Blue-stained gels but absent from control immunoprecipitations were excised, then eluted and digested with trypsin (Promega). Peptides from the digested bands were examined by LC–MS and MALDI-MS mass spectrometers. For matrix-assisted laser desorption isonization-time of flight (MALDI-TOF) analysis, 0.5-ml aliquots of the digests were mixed with 0.5 ml α-cyano-4-hydroxy cinnamic acid matrix on a MALDI sample plate. The samples were then analysed on a Voyager DE-STR MALDI-TOF MS (Applied Biosystems) and the processed spectra searched against the NCBI non-redundant database using Protein Prospector.
Induction of apoptosis
THP-1 cells and HUH-7 cells (1 × 106 cells/ml in RPMI) were synchronized through S-phase arrest by incubating with 4 mM thymidine for 16–18 h. The cells were released from thymidine block by washing in medium and resuspended at 1 × 106 cells/ml in medium with 10% FCS. Apoptosis was induced with 3 μM camptothecin, 25 μM monensin or 10 μg/ml cycloheximide for 6 h with 15–30% of cells found to be apoptotic by 4–6 h.
Immunocytochemistry of tumour cells
Cells were either grown in chamber slides or centrifuged onto adhesive microscopic slides, then fixed in ice-cold methanol and washed in PBS. Primary antibodies were added at 100 μl per chamber or 50–100 μl per slide, and incubated for 60 min at RT. Slides were then washed in PBS, followed by FITC- or TRITC-conjugated secondary antibodies at 1:20 and 1:50 dilution, respectively. Slides were then incubated in the dark for 60 min at RT. Nuclear counterstaining was performed with TO-PRO 3 for 5–10 min at RT and the slides were mounted with fluorescent mounting medium (DAKO).
Fixation and immunolabelling of erythrocytes
Erythrocytes were washed in PBS and resuspended in 1% BSA/PBS containing 50 μg/ml SDS. After 1 min, SDS and formalin (37% formaldehyde/PBS) were added to achieve a concentration of 1% formalin and 10 μg/ml SDS. After 90 min, the final concentration was increased to 4% formalin and cells were left to fix at RT overnight. Fixed cells were then washed in PBS and resuspended at 2 × 107 cells/ml in 5% BSA/PBS containing 0.1% sodium azide. Immunocytochemistry of fixed erythrocytes was performed as described above.
Quantitation of cellular DNA content by flow cytometry
Cells were pelleted and resuspended at 1 × 106 cells/ml in nuclear staining solution (PI in sodium citrate with 0.3% Nonidet 40), followed by an equal volume of RNAse solution (10 μg/ml RNAse A in 1.12% sodium citrate buffer) and incubated in the dark for 30 min at RT. DNA binding was detected by flow cytometry using a Coulter EPICS/XL cytometer.
Flow cytometric detection of surface and intracellular antigens
For detection of surface antigens, cells were harvested and washed in 1% BSA/PBS, then blocked with 20% rabbit serum/PBS for 20 min at RT and incubated with appropriate amounts of primary antibodies. For detection of intracellular antigens, cells were first fixed in 4% paraformaldehyde at 4°C for at least 15 min, then permeabilized with 100% methanol at 4°C for 30 min. After washing in PBS, cells were blocked in 20% rabbit serum/PBS. Primary antibodies at 1 μg/50 μl in 1% BSA/PBS were added to 0.5–1 × 106 cells and incubated for at least 30 min at RT. Cells were then washed with 1% BSA/PBS and incubated with PE or FITC-conjugated secondary antibodies at 0.5 μg/50 μl for 30 min at RT. Finally, cells were washed and resuspended in 1% paraformaldehyde/PBS for FACS analysis.
Phagocytosis assay
Carboxylate-modified 1 μm yellow–green fluorospheres (excitation 490 nm, emission 515 nm; Molecular Probes, Invitrogen) were used in the in vitro phagocytosis assay. Before use in the phagocytosis assay, 1 μl of beads were coated by incubation with 1.2 μg/μl of low-endotoxin and GroEL-free recombinant human (rh) Hsp60 protein (ESP-540, Stressgen) in PBS for 1 h on ice. Control beads were prepared by incubation with 1.2 μg/μl of BSA in PBS. U937 cells were harvested and 0.5 × 106 cells were incubated with 0.5 μl of labelled beads (1 cell to 12 beads) in 350 μl of HBSS containing 5% FBS. Phagocytosis was then carried out in the dark at 37°C with shaking at 200 rpm. Cells were harvested and spun down at 600 g for 5 min. The uningested beads were removed by washing cells with ice-cold PBS. Cells were subsequently fixed in 0.5% paraformaldehyde and phagocytosis was analysed by flow cytometric detection in the FL1 channel (BD FACSCalibur, Beckton Dickinson, NJ). The percentage of phagocytosis is expressed as percentage of cells with ingested beads, and Student’s t-test was used to test for statistical significance. The experiments were conducted at least thrice.
Results
BOB78 antibody identifies Hsp60
BOB78, a IgM mAb raised against apoptotic THP-1 cells, was shown to identify an unknown surface protein associated with apoptotic changes in human neutrophils [21]. The human megakaryocytic cell line MEG-01 and platelets derived from this cell line were found to express significant levels of the BOB78 antigen. In order to identify the antigen recognized by BOB78, immunoprecipitation was performed using Triton X-100 lysates of platelets derived from MEG-01 cells. BOB78 antibody–antigen complex was captured by protein A Sepharose beads conjugated with rabbit anti-mouse antibodies followed by denaturing protein electrophoresis. A band of approximately 67 kDa was identified specifically against the BOB78 antibody immunoprecipitate (Fig. 1a). In addition, immunoblotting with BOB78 IgM antibody confirmed that this band was specifically captured at 67 kDa (Fig. 1b). Subsequently, this band was excised and examined by mass spectrometry (MALDI-TOF) identifying the antigen immunoprecipitated by BOB78 as the molecular chaperone, Hsp60, also known as chaperonin (GenPept accession number P10809).
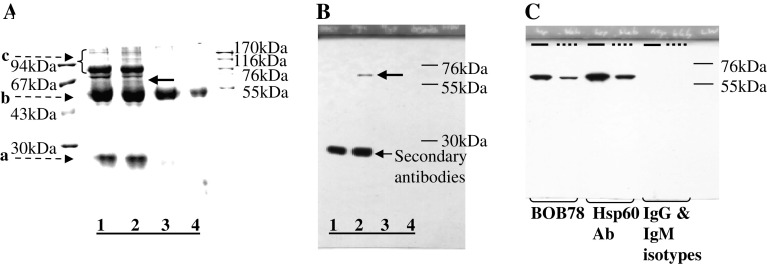
Fig. 1.
BOB78 antibody identifies rh-Hsp60. a, b BOB78 antigen was isolated from platelets by immunoprecipitation, and was revealed to be Hsp60 by sequencing. BOB78 antigen from the lysate of MEG-01-derived platelets was immunoprecipitated with BOB78 IgM monoclonal antibodies, complexed with rabbit anti-mouse antibodies, then captured by protein A Sepharose beads. Captured proteins were separated by SDS-PAGE and stained with Gel-Code. a Stained gel shows that BOB78 antigen was precipitated from the MEG-01 platelets lysate. There is a band specific to the reaction between BOB78 monoclonal antibody and the platelet lysate at 67 kDa (bold arrow, lane 2). This band was sent for sequencing by mass spectrometry (MALDI). Lanes 1, 3 and 4 represent control experiments: lane 1 BOB78 antibody supernatant, beads; lane 2 BOB78 antibody, platelet lysate, beads; lane 3 IgM isotype control, platelet lysate, beads; lane 4 platelet lysate, beads. Bands (broken arrows): a immunoglobulin light chain; b immunoglobulin heavy chain; c other proteins in supernatant. b Western blot probed with BOB78 antibody confirms the specificity of the BOB78 antigen band that was sequenced, as a single band at 67 kDa. The Western blot was performed on duplicate lanes corresponding to the immunoprecipitate gel in a . c The reactivity of BOB78 antibody for rh-Hsp60 protein was compared with that of commercial anti-Hsp60 monoclonal IgG. The lanes were loaded with either rh-Hsp60 (thick lines) or lysate from MEG-01 platelets (interrupted lines). The proteins were separated by SDS-PAGE and Western blotting was performed with BOB78, anti-Hsp60 monoclonal (Hsp60 Ab) and isotype control antibodies. Both the BOB78 antibody and the commercial anti-Hsp60 monoclonal antibody identify rh-Hsp60 and Hsp60 from MEG-01 platelets at 67 kDa. No band of identification is seen with the IgM and IgG isotype controls
BOB78 was subsequently demonstrated to recognize commercial preparations of rh-Hsp60 (Stressgen). Denaturing protein electrophoresis and western blotting was performed on MEG-01 platelet lysate and rh-Hsp60, followed by immunodetection with either anti-Hsp60 or BOB78 antibody. The antigen from MEG-01 lysate recognized by BOB78 and rh-Hsp60 had an identical molecular weight (Fig. 1c), thus confirming BOB78 antibody reactivity.
Surface expression of Hsp60 in haematopoietic cells undergoing apoptosis
We next studied the localization of Hsp60 during apoptosis in the THP-1 leukaemic cell line [21]. Following serum starvation, approximately one-third of the THP-1 cells showed faint PI staining and low levels of surface BOB78 staining (Fig. 2a, b). Intracellular staining revealed that Hsp60 was present in both apoptotic and viable cells (Fig. 2c). Erythrocytes were fixed, permeabilized, and stained with BOB78 and glycophorin A antibodies, with secondary detection by anti-mouse FITC-conjugated antibodies (Fig. 2d). In erythrocytes, glycophorin [22] was used as a positive control for intracellular protein detection (Fig. 2e). However, Hsp60 was absent from erythrocytes even when permeabilized (Fig. 2f). Importantly, these findings suggested that Hsp60 might be specifically translocated to the plasma membrane during apoptosis.
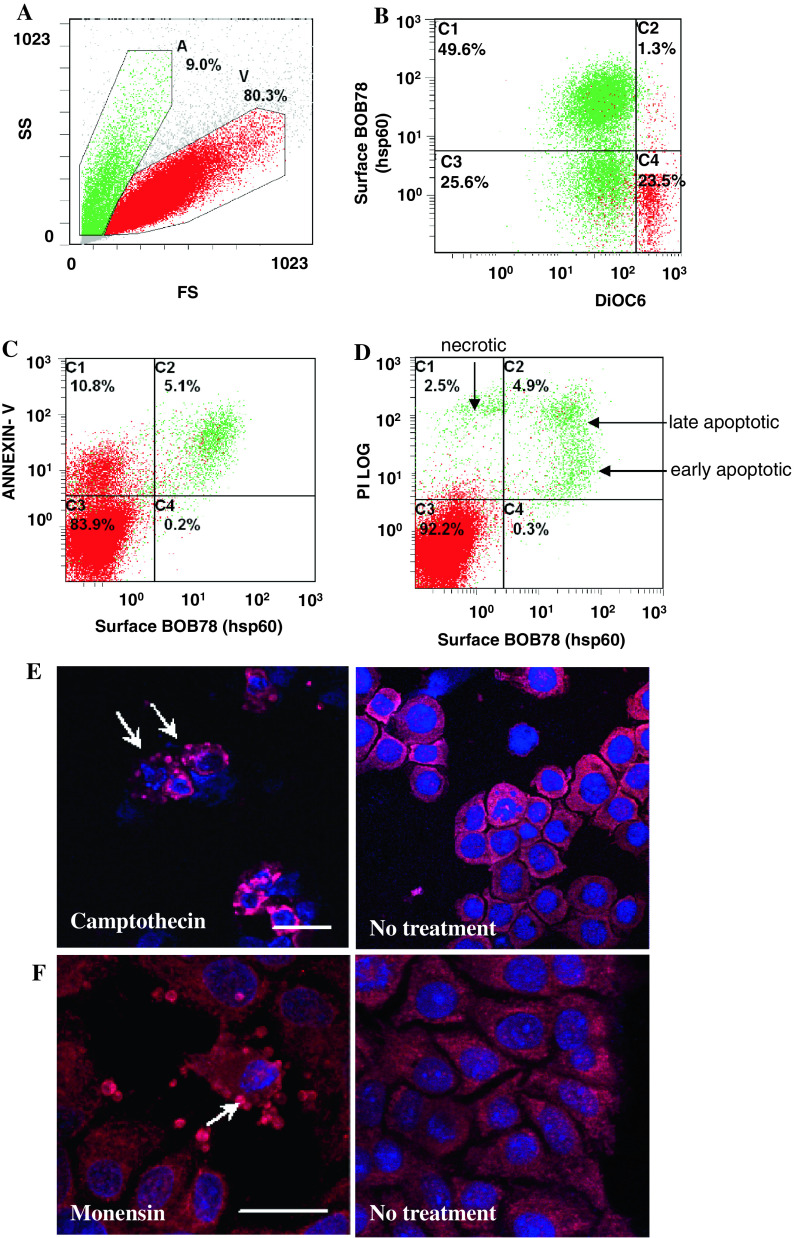
Fig. 2.
Expression profile of Hsp60 in leucocytes and erythrocytes. a Serum-starved leucocytes and monocytes were stained with BOB78 and IgM isotype control antibodies, with secondary detection by anti-mouse FITC-conjugated antibodies. Surface expression was analysed by flow cytometry. Cells were gated by size (forward scatter, FS) into dying cells (A) and viable cells (V). b Apoptotic leucocytes expressed Hsp60 on the surface. c Single peak of Hsp60 detection in permeabilized leucocytes, indicating the presence of intracellular Hsp60 in apoptotic and viable cells. d Erythrocytes were fixed, permeabilized, and stained with BOB78 and glycophorin A (control positive) antibodies, with secondary detection by anti-mouse FITC-conjugated antibodies. e Intracellular glycophorin was detectable in erythrocytes. f Hsp60 was not detected in erythrocytes
Localization of Hsp60 in plasma membrane of cells undergoing early stages of apoptosis
Next, we characterized the time course of surface expression of Hsp60 from early to late stages of apoptosis. Human THP-1 cells were treated with camptothecin, a topoisomerase I inhibitor, to induce apoptosis of cells in S and G2 phases following cell cycle arrest using thymidine. Apoptotic cells were identified by loss of mitochondrial transmembrane potential through reduced staining of DiOC6 (3,3′-dihexyloxacarbocyanine iodide) and exposure of phosphatidylserine (PtdSer) residues on the cell membrane by annexin V staining by FACS analysis. Late apoptotic and necrotic cells were identified by PI uptake, where prominent PI staining is observed due to loss of membrane integrity, whilst cells in earlier stages of membrane disintegration exhibit weaker PI staining that is almost comparable to or just above that of viable cells.
Apoptotic cells with reduced mitochondrial transmembrane potential (DiOC6-dim) were gated into two distinct populations based on surface Hsp60 expression (Fig. 3a, b). A significant population of DiOC6-dim cells were positive for Hsp60. A proportion of cells stained positively for both Hsp60 and annexin V but stained weakly for PI, indicating that these cells exhibited prominent surface staining for Hsp60 during early apoptosis (Fig. 3c). Furthermore, although Hsp60 was expressed on the surface of apoptotic cells, it was absent from PI-negative viable cells (Fig. 3d). Finally, surface Hsp60 expression was maintained in late apoptotic cells (PI high+), confirming that it is associated with the apoptotic plasma membrane.
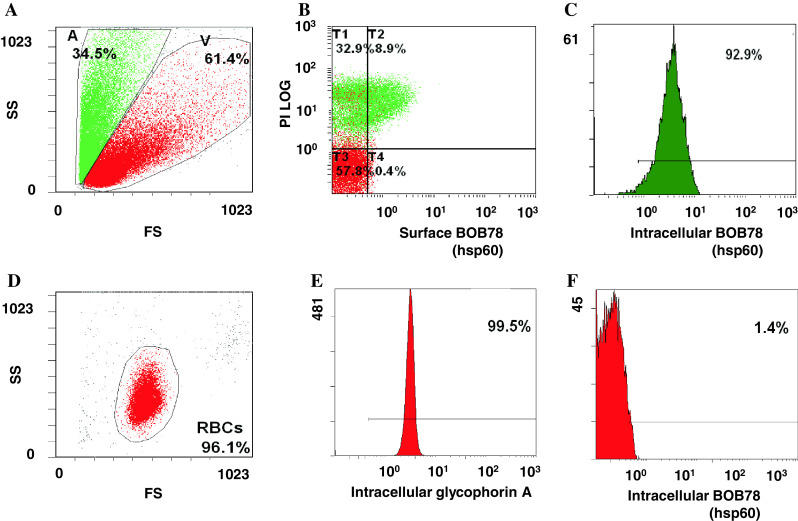
Fig. 3.
Hsp60 is expressed on the surface of cells from early to late apoptosis. Human leukaemic THP-1 cells were treated with 3 μM camptothecin for 6 h and analysed by flow cytometry. The cells were costained with BOB78 antibody (detected with anti-mouse FITC) and PI, DiOC6 or annexin V. PtdSer exposure on the cell membrane was detected by staining with PE-conjugated annexin V. a Camptothecin-treated THP-1 cells were separated by size by flow cytometry. Apoptotic cells (green, A) are smaller than viable cells (red, V). b Hsp60 was detected using BOB78 antibody by PE-conjugated anti-mouse secondary antibody. A significant proportion of cells which have lost the mitochondrial transmembrane potential, reflected by reduced DiOC6 green fluorescence, express Hsp60 on the surface. Surface expression of Hsp60 is therefore an event which follows loss of mitochondrial transmembrane potential, a key event in the initiation of apoptosis. c Hsp60 is coexpressed with PtdSer on the surface of cell membranes, indicating that Hsp60 surface exposure occurs early in apoptosis. d PI staining was performed to assess membrane permeability. Apoptotic cells express Hsp60 on the surface. Viable cells [59] exclude PI and do not express Hsp60. Apoptotic cells, early or late, express Hsp60. Hsp60 expression in necrotic cells is low as these cells lose intracellular Hsp60 expression on membrane lysis. e, f Hsp60 localizes predominantly to the membranes and blebs (arrows) of (e) apoptotic THP-1 cells treated with 3 μM camptothecin for 6 h (bar 15 μm) and (f) human hepatoma HUH-7 cells treated with 25 μM monensin for 6 h (bar 25 μm). Untreated live cells do not exhibit these Hsp60-rich blebs. Nuclear staining was effected with TOPRO-3 (blue fluorescence). Naked nuclear material probably results from lysis of late apoptotic or necrotic cells
Hsp60 is enriched in the blebs of apoptotic cells
The cellular localization of Hsp60 in camptothecin-treated apoptotic THP-1 cells counterstained with nuclear antigen TO-PRO 3 was examined by confocal microscopy. Interestingly, Hsp60 was found to be highly expressed in the blebs of apoptotic cells showing typical nuclear fragmentation, with only minor expression in the cytoplasm (Fig. 3e). The translocation of Hsp60 from cytoplasm to the membrane blebs of apoptotic cells was also examined in cells of the hepatic epithelial tumour cell line HUH-7 treated with monensin at 25 μM or cycloheximide at 10 μg/ml. Consistent with findings in camptothecin-treated THP-1 cells, confocal microscopy revealed that Hsp60 was localized in membrane blebs (Fig. 3f), suggesting that translocation of Hsp60 to membrane blebs is a general feature of different apoptotic stimuli in diverse cell types.
Hsp60 is enriched in platelets shed from MEG-01 cells
We next examined whether externalization of Hsp60 was associated with other cellular events that resemble apoptosis, such as terminal differentiation of megakaryocytes [23]. Human megakaryocytic MEG-01 cells in culture constitutively undergo terminal differentiation with concomitant production of platelets [23, 24]. MEG-01 cells were maintained under standard culture conditions and the platelets produced were shed into the culture medium. MEG-01 cells and platelets derived from them were stained for Hsp60 and FACS analysis confirmed surface localization of Hsp60 in differentiating MEG-01 cells, with minimal expression on undifferentiated viable cells (Fig. 4a, b). Surface Hsp60 expression was reduced in MEG-01 cells undergoing late stages of differentiation or apoptosis (PI high+, Fig. 4c). In addition, a proportion of platelets derived from MEG-01 cells were positive for annexin V (Fig. 4d, e), and most likely represented senescent platelets which are positive for PtdSer [25]. A similar population of platelets with strong surface expression of Hsp60 was observed, indicating Hsp60 surfaces in ageing platelets (Fig. 4f, g).
Fig. 4.
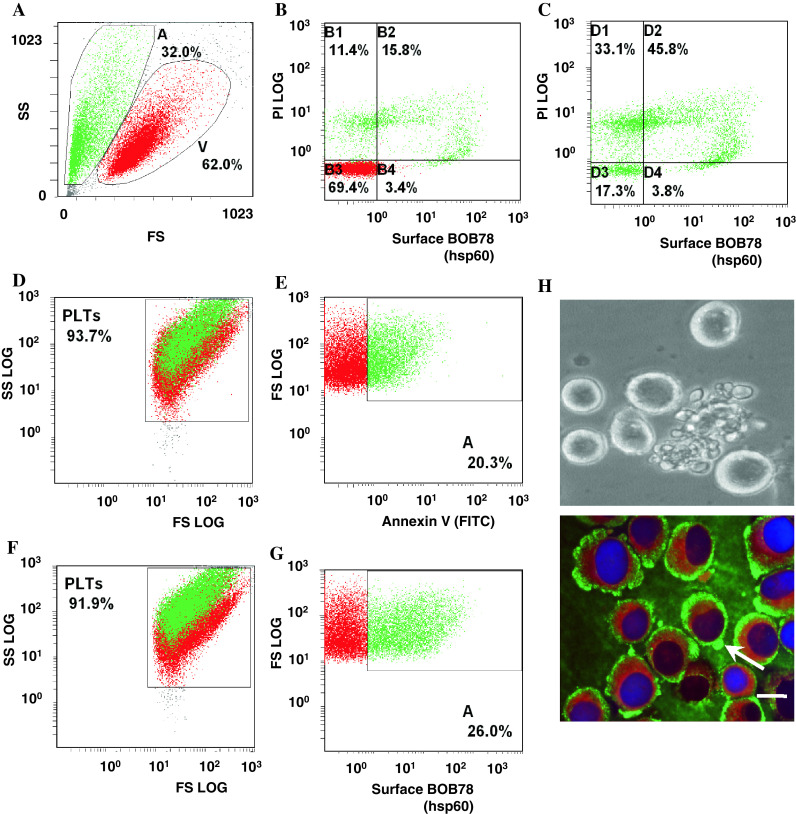
Detection of Hsp60 on the surface of differentiating megakaryocytes and senescent platelets. a MEG-01 cells were pooled from several days under standard culture conditions and analysed for Hsp60 expression with FITC-conjugated secondary antibodies and flow cytometry. Viability (V) of the cells was verified through exclusion of PI. b Flow cytometry confirms surface expression of Hsp60 in differentiating MEG-01 cells, but minimal surface expression on viable cells. Differentiating MEG-01 cells which stain weakly for PI exhibit strong expression of Hsp60 on the surface. c In late stages of differentiation and apoptosis, MEG-1 cells have scant cytoplasm. Coincident with strong PI staining of their nuclei, surface expression of Hsp60 is reduced. d, e Senescent platelets produced by standard cultures of MEG-01 stain for FITC-conjugated annexin V, indicating PtdSer exposure on the platelet surfaces. f, g Senescent platelets (which stain for annexin V) express Hsp60 on the surface. Hsp60 surface expression and PtdSer externalization are thus common events in platelet ageing and apoptosis. h Terminal differentiation of human megakaryocytic MEG-01 cells culminates in release of platelets through protrusions in the cell membrane, which resemble apoptotic membrane blebbing (bright-field image). Hsp60 (FITC-conjugated antibody secondary detection) and calreticulin (TRITC) appear to be segregated to different intracellular locations. Hsp60 is distributed to the outer cortex of the cytoplasm and membrane blebs (arrow), whilst calreticulin is limited to the inner cytoplasm. (bar 10 μm)
Hsp60 is concentrated in platelets budding off from differentiating MEG-01 cells and does not colocalize with calreticulin
Shedding of platelets from megakaryocytes is highly reminiscent of blebbing found in apoptotic cells (Fig. 4h). Calreticulin is a molecular chaperone which surfaces during apoptosis [26]. We performed double immunolabelling for calreticulin and Hsp60 in differentiating MEG-01 cells. Confocal microscopy revealed that Hsp60 was present within the cytoplasm of MEG-01 cells, and concentrated at focal areas in the peripheral cytoplasm. Calreticulin was present in the inner cytoplasm, while Hsp60 assumed a more peripheral distribution.
Hsp60 enhances phagocytosis in monocytic U937 cells
To investigate whether the presence of Hsp60 on the surface of cells would affect their clearance by phagocytosis, we used FluoSpheres, polystyrene with yellow–green fluorescence, in an in vitro assay for phagocytosis. The beads were coated with low-endotoxin rh-Hsp60 that is free of the E. coli Hsp60 contaminant GroEL and has been used in studies on immunological responses [27–29], and control beads were coated with BSA. Cells of the monocytic cell line U937 were incubated with the fluorescent beads and the proportion of cells that had ingested the beads was determined by flow cytometric detection of green fluorescence in the FL1 channel. U937 cells were incubated with beads that had been pretreated with recombinant Hsp60 (Fig. 5a) or BSA (Fig. 5b), or incubated with untreated beads (Fig. 5c). Background fluorescence of cells in the absence of beads is shown in Fig. 5d. U937 cells that were exposed to Hsp60-coated microspheres showed significantly enhanced phagocytosis compared to controls (44% vs. 13%, p < 0.05, Fig. 5e). This suggests that the surface exposure of Hsp60 in cells undergoing apoptosis may facilitate their recognition and subsequent clearance by enhancing phagocytic activity.
Fig. 5.
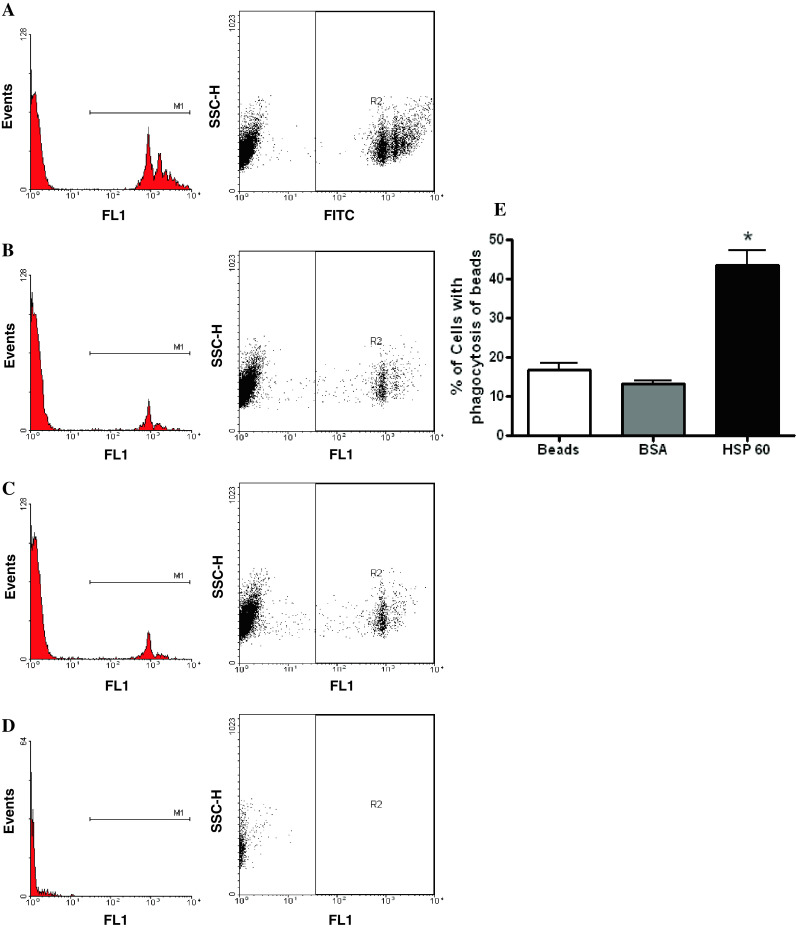
Hsp60 enhances phagocytosis by monocytic U937 cells. The proportion of U937 cells that had ingested green fluorescent microspheres was determined by flow cytometric detection in the FL1 channel after incubation with beads that were either pretreated with recombinant Hsp60 (a) or BSA (b), or with untreated beads (c). d Background fluorescence of cells in the absence of beads. e A significant increase in phagocytic activity is detected in U937 cells exposed to beads which have been pre-treated with Hsp60 (p < 0.05). Means with standard deviations are: 16.9 ± 2.7 (beads only), 13.4 ± 1.2 (BSA), 43.6 ± 6.3 (Hsp60). The figure represents data from three separate experiments
Discussion
In our earlier studies the monoclonal antibody BOB78 was shown to identify an unknown protein associated with apoptotic changes in neutrophils [21]. We have now identified the antigen recognized by BOB78 as Hsp60 (chaperonin). Hsp60 is a multilineage antigen which is conserved among normal and cancer cell lines. Our results suggest that a cytoplasmic pool of Hsp60 is present in nonapoptotic cells, consistent with the findings of previous studies [30]. We have now further extended our understanding of the localization of Hsp60 with respect to apoptosis and apoptotic-like events, such as terminal differentiation of megakaryocytes, and platelet senescence. Our findings demonstrate that Hsp60 translocates from the cytoplasm to the cell membrane during apoptosis and is highly concentrated in the apoptotic membrane blebs. Surface expression of Hsp60 coincides with externalization of PtdSer, suggesting that Hsp60 may play a role in the clearance of apoptotic bodies by phagocytes [31]. This is supported by the observations that Hsp60 receptors on monocytes and macrophages are involved in endocytic uptake [32, 33]. More recently, specific epitopes derived from Hsp60 have been shown to bind to the surfaces of macrophages [34]. We observed distinct localization of Hsp60 and calreticulin, another molecular chaperone which stimulates phagocytosis of apoptotic cells [26], suggesting that Hsp60 may function differently from calreticulin. In further support of a role for externalized Hsp60 in apoptotic clearance, we demonstrated that exposure of monocytic cells to Hsp60 enhances their phagocytic activity.
Megakaryocytic differentiation was used as a cellular model for membrane remodelling events that show some parallels with constitutive apoptosis, a process which involves the release of platelets with mitochondrial leakage of cytochrome c and activation of caspases [35]. Platelet senescence is also associated with loss of mitochondrial transmembrane potential and PtdSer exposure [36, 37]. Membrane changes in platelet senescence may prime them for uptake by macrophages [38]. Interestingly, Hsp60 is present in platelets budding from the parent megakaryocyte MEG-01 cells, and also appears on the membrane surface during senescence.
There is evidence that suggests Hsp60 has regulatory functions in apoptosis. In megakaryocytes, Hsp60 expression increases during the executive phase of apoptosis induced by diosgenin treatment, coinciding with the cleavage and activation of caspase-3 [39]. Hsp60 has been found to localize to both the mitochondria and the cytoplasm in other cell types including leukaemic cells and myocytes [27, 40], and possibly interacts with procaspase 3 and Hsp10 in a preapoptotic complex within the mitochondria [27]. This complex dissociates when procaspase 3 is activated in apoptosis, with the release of Hsp60 into the cytosol. In vitro assays using recombinant Hsp60 in cytosolic fractions suggest that Hsp60 can enhance the activation of procaspase-3 in the presence of cytochrome c. As activated caspase-3 is a crucial effector of apoptosis, it is suggested that cytosolic Hsp60 may therefore play a proapoptotic role in the apoptotic cascade. However, the roles of Hsp60 in apoptosis are complex and may also involve antiapoptotic mechanisms—cytosolic Hsp60 appears to protect cardiac myocytes against apoptosis, possibly by blocking the activities of bax and bak [41].
The disposal of apoptotic cells by the immune system has generated much interest, as it has implications for the mechanisms underlying diseases such as autoimmune diseases. The interface between apoptotic cells and phagocytes is likely to involve several interactions that attenuate the inflammatory response [42]. These may include mechanisms which facilitate phagocytosis [43, 44], direct antigen processing and tolerance induction [45], and reduce proinflammatory cytokine production [46]. Many different surface molecules have been implicated in the specific interaction between apoptotic cells and phagocytes. A number of soluble factors bind to apoptotic cells to facilitate uptake by phagocytes, including beta-2 glycoprotein, complement proteins such as C1q and C3b, pentraxin 3 (PTX3), milk fat globule-epidermal growth factor 8 (MFG-E8), and growth arrest-specific factor 6 (Gas6) [47]. Apoptotic cell recognition and uptake by macrophages is then mediated by different surface receptor classes including lectin-like receptors [48], scavenger receptors such as CD36 [49], thrombospondin [49, 50], complement receptors CR3 and CR4 [51], complement C1q [52], PtdSer receptors [53, 54], and Mer receptor tyrosine kinase [55, 56]. Defective clearance of apoptotic bodies may permit the adaptive immune system to be activated by cellular antigens which would normally have been removed in a silent manner by phagocytes [57], thereby contributing to the development of autoimmune diseases. However, the surface exposure of Hsp60 has also been suggested to be proinflammatory. Surface translocation of Hsp60 has been observed in injured cardiac myocytes and correlates with apoptosis associated with heart failure in humans and rats [40]. Increased plasma concentrations of Hsp60 and antibodies to Hsp60 are also detected in the rat heart failure model, indicating that the surface exposure of Hsp60 in injured myocytes may be immunogenic. This possibly contributes to a proinflammatory state by abnormally activating the immune system, leading to further myocyte destruction. Interestingly, the heat shock proteins Hsp70 and Hsp90 have also been observed to translocate to the plasma membrane of dying cells, and are involved in stimulation of antigen-presenting cells [58]. In this respect, the roles of Hsp60 in the clearance of apoptotic bodies or platelets by phagocytes, as well as the immunological consequences of phagocytic uptake triggered by Hsp60, merit closer inspection.
In conclusion, our findings demonstrate that the externalization of Hsp60 at the plasma membrane is closely associated with apoptosis and constitutive cell death in a variety of cells, but not necrosis. We have shown that Hsp60 appears on the cell surface during apoptosis and apoptosis-like events such as megakaryocyte differentiation and platelet senescence. Hsp60 is expressed at the cell surface during early apoptotic events and persists until membrane integrity is lost in late apoptosis. It remains to be seen if functional links exist between Hsp60 surface expression and key apoptotic events such as caspase activation and PtdSer exposure. Intriguingly, exposure to Hsp60 enhances the phagocytic activity of monocytic cells. The externalization of Hsp60 in apoptotic cells and its ability to stimulate phagocytosis imply that Hsp60 has potentially important roles in apoptotic clearance and may influence the development of immune-mediated diseases and immune surveillance in cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Mass spectrometry analysis identifies Hsp60 as the antigen recognised by BOB78 antibody. Protein bands immunoprecipitated with BOB78 antibody were digested with trypsin, and peptides from the digestions were examined by LC-MS and MALDI-MS mass spectrometers. The samples were analysed on a Voyager DE-STR MALDI-TOF MS (Applied Biosystems) and the processed spectra searched against the NCBI non-redundant database such as SwissProt using Protein Prospector.
Acknowledgments
This work was funded by the National Medical Research Council (NMRC) of Singapore and the Singapore Totalisator Board. We thank Dr. Yaw Chyn Lim, Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, for her critical appraisal of sections of our work.
Footnotes
Yaw Chong Goh and Celestial T. Yap are co-authors, and Yaw Chong Goh and James A. Ross are co-corresponding authors.
Contributor Information
Yaw Chong Goh, Phone: +65-6321-4051, FAX: +65-6220-9323, Email: goh.yaw.chong@sgh.com.sg.
James A. Ross, Phone: +44-131-2426520, Email: j.a.ross@ed.ac.uk
References
- 1.Al-Daccak R, Mooney N, Charron D. MHC class II signaling in antigen-presenting cells. Curr Opin Immunol. 2004;16:108–113. doi: 10.1016/j.coi.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/S0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 3.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nat R, Voiculescu B, Stanciu C, Vidulescu C, Cergan R, Badiu C, Popescu LM. Apoptosis in human embryo development: 2. Cerebellum. J Cell Mol Med. 2001;5:179–187. doi: 10.1111/j.1582-4934.2001.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matwee C, Betts DH, King WA. Apoptosis in the early bovine embryo. Zygote. 2000;8:57–68. doi: 10.1017/S0967199400000836. [DOI] [PubMed] [Google Scholar]
- 6.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viktorsson K, Lewensohn R, Zhivotovsky B. Apoptotic pathways and therapy resistance in human malignancies. Adv Cancer Res. 2005;94:143–196. doi: 10.1016/S0065-230X(05)94004-9. [DOI] [PubMed] [Google Scholar]
- 8.Arends MJ, McGregor AH, Wyllie AH. Apoptosis is inversely related to necrosis and determines net growth in tumors bearing constitutively expressed myc, ras, and HPV oncogenes. Am J Pathol. 1994;144:1045–1057. [PMC free article] [PubMed] [Google Scholar]
- 9.Wyllie AH, Beattie GJ, Hargreaves AD. Chromatin changes in apoptosis. Histochem J. 1981;13:681–692. doi: 10.1007/BF01002719. [DOI] [PubMed] [Google Scholar]
- 10.Morris RG, Hargreaves AD, Duvall E, Wyllie AH. Hormone-induced cell death. 2. Surface changes in thymocytes undergoing apoptosis. Am J Pathol. 1984;115:426–436. [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu T, Pommier Y. Camptothecin-induced apoptosis in p53-null human leukemia HL60 cells and their isolated nuclei: effects of the protease inhibitors Z-VAD-fmk and dichloroisocoumarin suggest an involvement of both caspases and serine proteases. Leukemia. 1997;11:1238–1244. doi: 10.1038/sj.leu.2400734. [DOI] [PubMed] [Google Scholar]
- 13.Billack B, Heck DE, Mariano TM, Gardner CR, Sur R, Laskin DL, Laskin JD. Induction of cyclooxygenase-2 by heat shock protein 60 in macrophages and endothelial cells. Am J Physiol Cell Physiol. 2002;283:C1267–C1277. doi: 10.1152/ajpcell.00609.2001. [DOI] [PubMed] [Google Scholar]
- 14.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Cohen-Sfady M, Nussbaum G, Pevsner-Fischer M, Mor F, Carmi P, Zanin-Zhorov A, Lider O, Cohen IR. Heat shock protein 60 activates B cells via the TLR4-MyD88 pathway. J Immunol. 2005;175:3594–3602. doi: 10.4049/jimmunol.175.6.3594. [DOI] [PubMed] [Google Scholar]
- 16.Kreisel W, Hildebrandt H, Schiltz E, Kohler G, Spamer C, Dietz C, Mossner W, Heilmann C. Immuno-gold electron microscopical detection of heat shock protein 60 (hsp60) in mitochondria of rat hepatocytes and myocardiocytes. Acta Histochem. 1994;96:51–62. doi: 10.1016/S0065-1281(11)80009-7. [DOI] [PubMed] [Google Scholar]
- 17.Khan IU, Wallin R, Gupta RS, Kammer GM. Protein kinase A-catalyzed phosphorylation of heat shock protein 60 chaperone regulates its attachment to histone 2B in the T lymphocyte plasma membrane. Proc Natl Acad Sci U S A. 1998;95:10425–10430. doi: 10.1073/pnas.95.18.10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lecker S, Lill R, Ziegelhoffer T, Georgopoulos C, Bassford PJ, Jr, Kumamoto CA, Wickner W. Three pure chaperone proteins of Escherichia coli – SecB, trigger factor and GroEL – form soluble complexes with precursor proteins in vitro. EMBO J. 1989;8:2703–2709. doi: 10.1002/j.1460-2075.1989.tb08411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusukawa N, Yura T, Ueguchi C, Akiyama Y, Ito K. Effects of mutations in heat-shock genes groES and groEL on protein export in Escherichia coli. EMBO J. 1989;8:3517–3521. doi: 10.1002/j.1460-2075.1989.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torok Z, Horvath I, Goloubinoff P, Kovacs E, Glatz A, Balogh G, Vigh L. Evidence for a lipochaperonin: association of active protein-folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc Natl Acad Sci U S A. 1997;94:2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart SP, Ross JA, Ross K, Haslett C, Dransfield I. Molecular characterization of the surface of apoptotic neutrophils: implications for functional downregulation and recognition by phagocytes. Cell Death Differ. 2000;7:493–503. doi: 10.1038/sj.cdd.4400680. [DOI] [PubMed] [Google Scholar]
- 22.Southcott MJ, Tanner MJ, Anstee DJ. The expression of human blood group antigens during erythropoiesis in a cell culture system. Blood. 1999;93:4425–4435. [PubMed] [Google Scholar]
- 23.Zauli G, Vitale M, Falcieri E, Gibellini D, Bassini A, Celeghini C, Columbaro M, Capitani S. In vitro senescence and apoptotic cell death of human megakaryocytes. Blood. 1997;90:2234–2243. [PubMed] [Google Scholar]
- 24.Clarke MC, Savill J, Jones DB, Noble BS, Brown SB. Compartmentalized megakaryocyte death generates functional platelets committed to caspase-independent death. J Cell Biol. 2003;160:577–587. doi: 10.1083/jcb.200210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira J, Palomo I, Ocqueteau M, Soto M, Aranda E, Mezzano D. Platelet aging in vivo is associated with loss of membrane phospholipid asymmetry. Thromb Haemost. 1999;82:1318–1321. [PubMed] [Google Scholar]
- 26.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. 1999;18:2040–2048. doi: 10.1093/emboj/18.8.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao B, Tsan MF. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor alpha from murine macrophages. J Biol Chem. 2003;278:22523–22529. doi: 10.1074/jbc.M303161200. [DOI] [PubMed] [Google Scholar]
- 29.Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J Immunol. 2004;173:6319–6326. doi: 10.4049/jimmunol.173.10.6319. [DOI] [PubMed] [Google Scholar]
- 30.Itoh H, Kobayashi R, Wakui H, Komatsuda A, Ohtani H, Miura AB, Otaka M, Masamune O, Andoh H, Koyama K, et al. Mammalian 60-kDa stress protein (chaperonin homolog). Identification, biochemical properties, and localization. J Biol Chem. 1995;270:13429–13435. doi: 10.1074/jbc.270.22.13429. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann PR, de Cathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Habich C, Baumgart K, Kolb H, Burkart V. The receptor for heat shock protein 60 on macrophages is saturable, specific, and distinct from receptors for other heat shock proteins. J Immunol. 2002;168:569–576. doi: 10.4049/jimmunol.168.2.569. [DOI] [PubMed] [Google Scholar]
- 33.Lipsker D, Ziylan U, Spehner D, Proamer F, Bausinger H, Jeannin P, Salamero J, Bohbot A, Cazenave JP, Drillien R, Delneste Y, Hanau D, de la Salle H. Heat shock proteins 70 and 60 share common receptors which are expressed on human monocyte-derived but not epidermal dendritic cells. Eur J Immunol. 2002;32:322–332. doi: 10.1002/1521-4141(200202)32:2<322::AID-IMMU322>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Habich C, Kempe K, Gomez FJ, Lillicrap M, Gaston H, van der Zee R, Kolb H, Burkart V. Heat shock protein 60: identification of specific epitopes for binding to primary macrophages. FEBS Lett. 2006;580:115–120. doi: 10.1016/j.febslet.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 35.De Botton S, Sabri S, Daugas E, Zermati Y, Guidotti JE, Hermine O, Kroemer G, Vainchenker W, Debili N. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100:1310–1317. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- 36.Pereira J, Soto M, Palomo I, Ocqueteau M, Coetzee LM, Astudillo S, Aranda E, Mezzano D. Platelet aging in vivo is associated with activation of apoptotic pathways: studies in a model of suppressed thrombopoiesis in dogs. Thromb Haemost. 2002;87:905–909. [PubMed] [Google Scholar]
- 37.Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, Iciek LA, Morgan SJ, Nasarre MC, Nelson R, Preusser LC, Reinhart GA, Smith ML, Rosenberg SH, Elmore SW, Tse C. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–951. doi: 10.1038/sj.cdd.4402072. [DOI] [PubMed] [Google Scholar]
- 38.Brown SB, Clarke MC, Magowan L, Sanderson H, Savill J. Constitutive death of platelets leading to scavenger receptor-mediated phagocytosis. A caspase-independent cell clearance program. J Biol Chem. 2000;275:5987–5996. doi: 10.1074/jbc.275.8.5987. [DOI] [PubMed] [Google Scholar]
- 39.Cailleteau C, Liagre B, Beneytout JL. A proteomic approach to the identification of molecular targets in subsequent apoptosis of HEL cells after diosgenin-induced megakaryocytic differentiation. J Cell Biochem. 2009;107:785–796. doi: 10.1002/jcb.22176. [DOI] [PubMed] [Google Scholar]
- 40.Lin L, Kim SC, Wang Y, Gupta S, Davis B, Simon SI, Torre-Amione G, Knowlton AA. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H2238–H2247. doi: 10.1152/ajpheart.00740.2007. [DOI] [PubMed] [Google Scholar]
- 41.Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation. 2002;105:2899–2904. doi: 10.1161/01.CIR.0000019403.35847.23. [DOI] [PubMed] [Google Scholar]
- 42.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol. 2001;11:R795–R805. doi: 10.1016/S0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 43.Gershov D, Kim S, Brot N, Elkon KB. C-reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamanaka M, Eda S, Beppu M. Carbohydrate chains and phosphatidylserine successively work as signals for apoptotic cell removal. Biochem Biophys Res Commun. 2005;328:273–280. doi: 10.1016/j.bbrc.2004.12.171. [DOI] [PubMed] [Google Scholar]
- 45.Fonteneau JF, Kavanagh DG, Lirvall M, Sanders C, Cover TL, Bhardwaj N, Larsson M. Characterization of the MHC class I cross-presentation pathway for cell-associated antigens by human dendritic cells. Blood. 2003;102:4448–4455. doi: 10.1182/blood-2003-06-1801. [DOI] [PubMed] [Google Scholar]
- 46.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trouw LA, Blom AM, Gasque P. Role of complement and complement regulators in the removal of apoptotic cells. Mol Immunol. 2008;45:1199–1207. doi: 10.1016/j.molimm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Hall SE, Savill JS, Henson PM, Haslett C. Apoptotic neutrophils are phagocytosed by fibroblasts with participation of the fibroblast vitronectin receptor and involvement of a mannose/fucose-specific lectin. J Immunol. 1994;153:3218–3227. [PubMed] [Google Scholar]
- 49.Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mevorach D. Opsonization of apoptotic cells. Implications for uptake and autoimmunity. Ann N Y Acad Sci. 2000;926:226–235. doi: 10.1111/j.1749-6632.2000.tb05615.x. [DOI] [PubMed] [Google Scholar]
- 51.Takizawa F, Tsuji S, Nagasawa S. Enhancement of macrophage phagocytosis upon iC3b deposition on apoptotic cells. FEBS Lett. 1996;397:269–272. doi: 10.1016/S0014-5793(96)01197-0. [DOI] [PubMed] [Google Scholar]
- 52.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169:3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 53.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 54.Park D, Tosello-Trampont A, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 55.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 56.Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178:5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 57.Cocca BA, Cline AM, Radic MZ. Blebs and apoptotic bodies are B cell autoantigens. J Immunol. 2002;169:159–166. doi: 10.4049/jimmunol.169.1.159. [DOI] [PubMed] [Google Scholar]
- 58.Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15:3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- 59.Rosado JA, Lopez JJ, Gomez-Arteta E, Redondo PC, Salido GM, Pariente JA. Early caspase-3 activation independent of apoptosis is required for cellular function. J Cell Physiol. 2006;209:142–152. doi: 10.1002/jcp.20715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.