Abstract
Apoptosis mediated by endoplasmic reticulum (ER) stress plays a crucial role in several neurovascular disorders, including ischemia/reperfusion injury (I/R injury). Previous in vitro and in vivo studies have suggested that following I/R injury, ER stress is vital for mediating CCAT-enhancer-binding protein homologous protein (CHOP) and caspase-12-dependent apoptosis. However, its modulation in the presence of stem cells and the underlying mechanism of cytoprotection remains elusive. In vivo studies from our lab have reported that post-stroke endovascular administration of stem cells renders neuroprotection and regulates apoptosis mediated by ER stress. In the current study, a more robust in vitro validation has been undertaken to decipher the mechanism of stem cell-mediated cytoprotection. Results from our study have shown that oxygen–glucose deprivation/reoxygenation (OGD/R) potentiated ER stress and apoptosis in the pheochromocytoma 12 (PC12) cell line as evident by the increase of protein kinase R (PKR)-like ER kinase (p-PERK), p-Eukaryotic initiation factor 2α subunit (EIF2α), activation transcription factor 4 (ATF4), CHOP, and caspase 12 expressions. Following the co-culture of PC12 cells with MSCs, ER stress was significantly reduced, possibly via modulating the brain-derived neurotrophic factor (BDNF) signaling. Furthermore, inhibition of BDNF by inhibitor K252a abolished the protective effects of BDNF secreted by MSCs following OGD/R. Our study suggests that inhibition of ER stress-associated apoptotic pathway with MSCs co-culture following OGD/R may help to alleviate cellular injury and further substantiate the use of stem cells as a therapeutic modality toward neuroprotection following hypoxic injury or stroke in clinical settings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12192-022-01319-4.
Keywords: Mesenchymal stem cells, PC12 cells, ER stress, Apoptosis
Introduction
Ischemia/reperfusion (I/R) injury takes place in both neurovascular and cardiovascular diseases leading to cellular dysfunction (Bi et al. 2013; Kalogeris et al. 2012). The pathophysiology of I/R injury is intricate and multifaceted, culminating in excessive production of inflammatory mediators and reactive oxygen species (ROS), pushing cells toward apoptosis (Turer and Hill 2010; Vemula et al. 2014). The endoplasmic reticulum (ER) is one of the important organelles that play a critical role in secreting various proteins involved in membrane protein folding, translocation, and post-translational modifications (Sano and Reed 2013; Schwarz and Blower 2016). ER stress leads to the impairment of its function resulting in the accumulation of unfolded proteins that induces apoptosis (Rausch and Sertil 2015). Hypoxic injury in cells or stroke in animal models triggers various biochemical alterations, including elevated oxidative, nitrosative, and ER stress that contributes toward apoptosis (Kaur et al. 2021). ER stress markers like protein kinase R (PKR)-like ER kinase (p-PERK), p-Eukaryotic initiation factor 2α subunit (EIF2α), activation transcription factor 4 (ATF4), CCAT enhancer-binding protein homologous protein (CHOP), and caspase 12 are also elevated following I/R injury (Zhao et al. 2018). As ER stress is one of the key components of the pro-apoptotic signaling pathway, attenuating ER stress may be one of the strategies to render neuroprotection (Rozpedek et al. 2017). Stem cell therapy is promising, and many studies have enumerated the benefits of stem cells for stroke intervention (Chrostek et al. 2019). Studies from our lab have also shown that post-stroke stem cell endovascular therapy protects neurons from apoptosis induced by ER stress through modulation of BDNF/TrkB signaling and reduces perifocal vasogenic edema by regulating the PKCδ/MMP9/AQP4 pathway (Kaur et al. 2021; Datta et al. 2022). Our other studies have also revealed that MSCs mediate sirtuin-1 (SIRT-1) regulation of inflammasome signaling to render neuroprotection (Sarmah et al. 2022).
Stem cell therapy is emerging as a promising modality making it an important candidate for stroke intervention and enhancing post-stroke recovery (Stonesifer et al. 2017). Mesenchymal stem cells derived from bone marrow (BM MSCs) were found to be more efficacious, having higher self-renewal ability and the capability to protect the damaged cells (Han et al. 2019; Li et al. 2019, 2021). MSCs exert their protective action via paracrine signaling actions that include the release of several trophic factors, chemokines, cytokines, and extracellular matrix protein into the neighboring environment, acting as immunomodulators, angiogenic factors, antioxidants, trophic factors, anti-apoptotic factors, and cellular chemotaxis-inducers (Baraniak and McDevitt 2010; Cunningham et al. 2018; Fan et al. 2020; Hofer and Tuan 2016; Shabbir et al. 2010). Among neurotrophic factors, brain-derived neurotrophic factor (BDNF) holds great potential for promoting neuronal survival, decreasing apoptosis, reducing oxidative stress, and enhancing angiogenesis (Bathina and Das 2015; Cunningham et al. 2018; Jeong et al. 2014). Recent research has also shown that BDNF‐overexpressing MSCs mediate increased neuronal protection and enhanced functional repair and recovery (Cunningham et al. 2018; Scheper et al. 2019). Furthermore, our previous study reported that post-stroke IA MSCs treatment renders neuroprotection and can modulate apoptosis mediated by ER stress via the BDNF/TrkB signaling pathway (Kaur et al. 2021). Therefore, the present study investigates the MSCs-BDNF-mediated cytoprotection against OGD/R injury-induced oxidative and ER stress-mediated apoptosis in PC12 cells. To confirm the involvement of BDNF in MSCs-mediated cytoprotection, an inhibition study has been performed using, K252a, a non-selective Trk inhibitor (Gao et al. 2015; Jiang et al. 2015). However, K252a also inhibits TrkA and TrkC receptors, which is a major limitation of this study (Ogura et al. 2014). Nevertheless, as there are limited studies on the cytoprotective action of stem cells on hypoxic cells, our study aims to confirm the MSCs-BDNF mediated cytoprotection and related underlying mechanisms, to robustly validate the role of stem cells on hypoxic cells in alleviating ER stress mimicking cerebral ischemia in vitro.
Materials and methods
Reagents and chemicals
All chemicals and reagents were procured from Abcam and Sigma Aldrich unless specified otherwise.
PC12 cell culture and OGD/R model
PC12 cells (ATCC, CRL-1721) were cultured in RPMI 1640 media (Invitrogen, 11,875) supplemented with 10% horse serum (Sigma, 30–2040), 5% FBS (Sigma, 30–2021), and 1% Pen/Strep (Invitrogen, 15,070) at 37 °C in 5% CO2. PC12 cells in passages 4–7 were cultured in a tissue culture flask (Falcon, USA). Rat BM MSCs (Merck) were cultured at 37 °C in 5% CO2 in MSCs expansion medium. The culture medium was changed every other day. Briefly, the culture medium was removed, and PC12 cells were washed twice using RPMI 1640 medium (without glucose). PC12 cells were kept in a hypoxia incubator having conditions 1% O2, 94% N2, and 5% CO2 at 37 °C (Don Whitley hypoxia workstation) for 6 h (Mo et al. 2016). Next, cells were cultured in RPMI 1640 medium containing 10% horse serum and 5% FBS at air/CO2 (95%/5%), the reoxygenation phase for 24 h. PC12 cells in the control group were not treated with OGD/R. Twenty-four hours after, PC12 cells were co-cultured with BMSCs using transwell chambers (Corning) in an equal ratio (1:1) to generate co-cultures with the OGD/R injured PC12 cells, and the cells treated in this manner were referred to as the hypoxia + MSCs group. For K252a treatment (a non-selective tyrosine kinase receptors (Trk) inhibitor, used as BDNF receptor TrkB inhibitor), cells were treated with 10 nm K252a (Sigma Aldrich) following OGD/R, and cells were treated with an equal amount of phosphate-buffered saline (PBS), which served as the vehicle group (Gao et al. 2015; Jiang et al. 2015).
Alamar Blue cell viability assay
5*104 PC12 cells were seeded on a 24-well plate. Following OGD/R (OGD for 6 h and reoxygenation for 24 h), media was removed, and 50 ng/ml BDNF (Gibco 10,908–010) was supplemented in serum-free media for 30 min (Sun et al. 2012). Another panel of hypoxic PC12 cells was treated with MSCs at a 1:1 ratio. Following removal of treatment (BDNF and MSCs) media, cells were treated with resazurin (Sigma life science R7017) solution. After 3 h of incubation, fluorescence was measured at an excitation/emission ratio of 560/590 nm (Eilenberger et al. 2018). Finally, cell viability (% untreated control) was represented in comparison to normal PC12 and normal MSCs (Supplementary 1).
Apoptosis assay by flow cytometry
Apoptosis assay was performed as per the manufacturer’s procedure by flow cytometry analysis using Annexin V-FITC/PI apoptosis detection kit (Life Technologies, Thermo Fischer). In brief, the cells were plated at a density of 105 cells/well in a 6-well plate (Alia et al. 2017). Treated cells were washed with cold PBS twice and resuspended in 1 × binding buffer. Annexin V 5 μl and PI 5 μl were then added to the resuspended cells and incubated for 30 min at room temperature in the dark. Apoptotic cells and necrotic cells were separated by a flow cytometer (Bio-Rad). Data were analyzed using FCS Express flow cytometry analysis software (Liu et al. 2016).
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
RNA isolation and qPCR were carried out as per the previously reported methodology (Kaur et al. 2021). KiCqStart primers (18S, BDNF, ATF4, CHOP, and caspase 12; listed primer sequence in Table 1) were used. The relative expression change of targets was calculated using the 2−ΔΔCt method were used. 18S was used as the endogenous control. Relative expression changes were calculated using the 2−ΔΔCt method (Kaur et al. 2021).
Table 1.
Primer sequences
| Primer | Sequence |
|---|---|
| BDNF | F: GGAGACGAGATTTAAGACAC |
| R: CCATAGTAAGGAAAAGGATGG | |
| ATF4 | F: AAACCTCATGGGTTCTCC |
| R: CTTTCAGGTCCATTTTCTCC | |
| Caspase 12 | F: CTTCTACCCCACATAACATTTC |
| R: AGCGTGTCATAGATACTCTC | |
| CHOP | F: GGAAACGAAGAGGAAGAATC |
| R: ATAGAACTCTGACTGGAATCTG | |
| 18S | F: 5’-ATCGGGGATTGCAATTATTC-3’ |
| R: 5’-CTCACTAAACCATCCAATCG-3’ |
Tissue lysate preparation and biochemical estimations
The total proteins in cells were extracted using RIPA buffer, and protein content was estimated using a bicinchoninic acid assay (PierceTM BCA Protein Assay Kit) as per the previously mentioned methodology (Kaur et al. 2021; Datta et al. 2022). All biochemical methods were followed as per previously reported studies (Datta et al. 2022; Kaur et al. 2021; Saraf et al. 2019). MDA levels were estimated by using the thiobarbituric acid (TBA) assay (Datta et al. 2022; Kaur et al. 2021; Pravalika et al. 2019; Saraf et al. 2019). For estimating nitrite levels, the Griess method was used (Datta et al. 2022; Kaur et al. 2021; Pravalika et al. 2019; Saraf et al. 2019). Reduced glutathione (GSH) was estimated by using DTNB assay (Datta et al. 2022; Kaur et al. 2021; Pravalika et al. 2019; Saraf et al. 2019).
Western blotting
Western blotting was performed as per the previously described methodology. A total of 30 µg cell lysates were tested for BDNF (1:5000; ab108319), eIF2α (1:1000; SAB4500729), p-eIF2α (1:500; ab32157), ATF4 (1 µg/ml; ab23760), DDIT3 (5 µg/ml; ab11419), caspase 12 (1:5000; ab62484), and beta-actin (1:5000; ab8227). ECL (chemiluminescence substrate) (Bio-Rad) was used to detect the respective protein bands, and images were digitized in a gel doc system (Bio-Rad). Beta-actin was used as a control for loading, and the values for band density were normalized to beta-actin. Data were quantified by assessing each band’s intensity with the help of ImageJ software (NIH, USA) (Datta et al. 2022; Kaur et al. 2021; Sarmah et al. 2022).
Immunofluorescence
104 PC12 cells were plated in a culture dish. Following OGD/R, the media was removed, and cells were rinsed 3 × with PBS followed by 4% paraformaldehyde (PFA) fixation for 10 min at room temperature. 0.1% Triton X-100 was used for cell permeabilization for 2 min at room temperature. Cells were blocked with 1% BSA for 30 min, followed by incubation with primary antibodies for BDNF (1:100; ab108319) and CHOP (1:500; ab11419) at 4 °C overnight. Following overnight incubation, the cells were washed 3 × with PBS and incubated with Alexa Fluor 647 (goat anti-rabbit/mouse; ab150079; ab150115; 1:1000) in the dark for 1 h at 37 °C and then washed with TBS-T three times. After washing, cells were counterstained with DAPI for 5 min at room temperature. Final images were taken by a confocal laser scanning microscope (Leica TCS SP8 Microsystem) (Kaur et al. 2021; Yang et al. 2020).
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Data were analyzed by one-way ANOVA followed by Tukey’s post-test using GraphPad Prism version 5 (San Diego, CA, USA). A level of p < 0.05 was considered statistically significant ('Lenth, R.V (2006), Java Applets for power and sample size computer software, http://www.stat.uiowa.edu/~rlenth/power) (Datta et al. 2022; Kaur et al. 2021; Sarmah et al. 2022).
Results
Co-culture of PC12 cells with MSCs reduces OGD/R-induced apoptosis
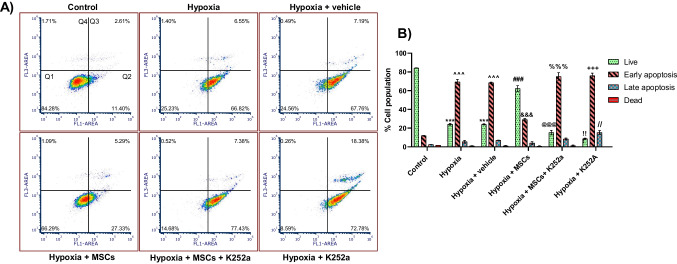
Apoptosis following OGD/R is one of the crucial pathways leading toward the process of cell death. We studied the anti-apoptotic effects of MSCs using Annexin V-FITC/PI staining assay, which allows both quantitative determination and differentiation among viable, early/late apoptotic, and necrotic cells by flow cytometry. Herein, we have found that viable cells were more in the control group. However, the hypoxia and hypoxia + vehicle group showed a higher apoptosis rate when compared to the control group. Interestingly, the hypoxia + MSCs group demonstrated a significant reduction in apoptosis as compared to the hypoxia and hypoxia + vehicle groups. Groups treated with inhibitors showed a substantial increase in apoptosis compared to the hypoxia + MSCs group. The results show that MSCs protect the damaged cells from apoptosis. However, K252a limited the anti-apoptotic effects of MSCs (Fig. 1A and B).
Fig. 1.
Effect of MSCs on OGD/R-induced apoptosis in PC12 cells was detected by flow cytometry. A Flow cytometry diagram of double-staining with Annexin V-FITC/PI. Q1: Quadrant 1 indicates healthy cells (Annexin V − /PI −), Q2: Quadrant 2 indicates early apoptotic cells (Annexin V + /PI −), Q3: Quadrant 3 indicates late apoptotic cells (Annexin V + /PI −), Q4: Quadrant 4 indicates necrotic cells (Annexin V − /PI +). B Graphical representation of the % cell population. Data are expressed as mean ± SEM and analyzed for statistical significance using two-way ANOVA with Tukey’s multiple comparison test. The cell populations of Annexin V-FITC + /PI − were calculated to represent % apoptotic cells. ***p < 0.001 vs control group live group, ^^^p < 0.001 vs control early apoptosis group, ###p < 0.001 vs hypoxia live group, &&&p < 0.001 vs hypoxia early apoptosis group, @@@p < 0.001 vs hypoxia + MSCs live group, %%%p < 0.001 vs hypoxia + MSCs early apoptosis, !!p < 0.001 vs hypoxia + MSCs live, +++p < 0.001 vs hypoxia + MSCs early apoptosis group, //p < 0.01 vs hypoxia + MSCs late apoptosis group (n = 3)
Co-culture with MSCs normalizes biochemical parameters
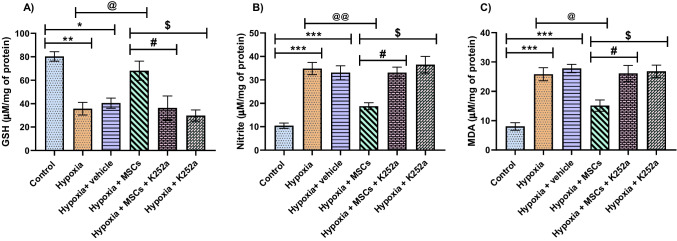
Following OGD/R, there was a significant increase in MDA and nitrite levels and a decrease in endogenous antioxidants compared to the control group. Co-culture with MSCs significantly increased the GSH levels and reduced MDA and nitrite levels. Following treatment with K252a, GSH levels were reduced, while MDA and nitrite levels were elevated in hypoxia + MSCs + K252a and hypoxia + K252a groups. These outcomes suggest that MSCs can alleviate lipid peroxidation and nitrosative stress while K252a inhibits these protective effects of MSCs (Fig. 2A–C).
Fig. 2.
Effect MSCs on biochemical parameters following OGD/R treated PC12 cells. A GSH, B Nitrite, and C MDA. Data are expressed as mean ± SEM and analyzed for statistical significance using one-way ANOVA with Tukey’s multiple comparison test, ***p < 0.001; **p < 0.01, *p < 0.05 vs control; @@p < 0.01; @p < 0.05 hypoxia group, #p < 0.05 vs hypoxia + MSCs group; $p < 0.05 vs hypoxia + MSCs (n = 3)
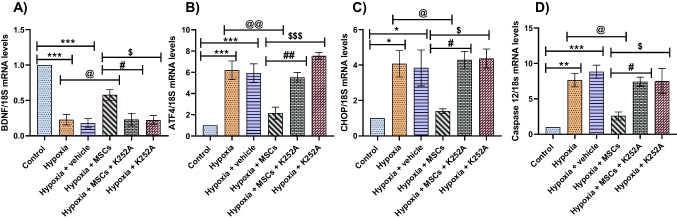
Co-culture with MSCs reduces mRNA levels of ER stress markers following OGD/R in PC12 cells
The mRNA levels of ER stress markers were significantly upregulated in the hypoxia and hypoxia + vehicle groups. Interestingly, treatment with MSCs significantly decreases the mRNA levels of these markers. Upregulation of BDNF was seen in the hypoxia + MSCs group compared to the hypoxia and hypoxia + vehicle groups. However, treatment with K252a significantly downregulated the levels of BDNF and upregulated the ER stress markers levels. The results hint that the protective effect of MSCs was inhibited by K252a (Fig. 3A–D).
Fig. 3.
Effect of MSCs treatment on the mRNA levels of various ER stress markers following I/R injury. A BDNF. B ATF4. C CHOP. D Caspase 12. Data are expressed as mean ± SEM and analyzed for statistical significance using one-way ANOVA with Tukey’s multiple comparison test, *p < 0.05, **p < 0.01, ***p < 0.001 vs control; @p < 0.05, @@p < 0.01 vs hypoxia group; #p < 0.05, ##p < 0.01 vs hypoxia + MSCs group; $p < 0.05, $$$p < 0.001 vs hypoxia + MSCs (n = 3)
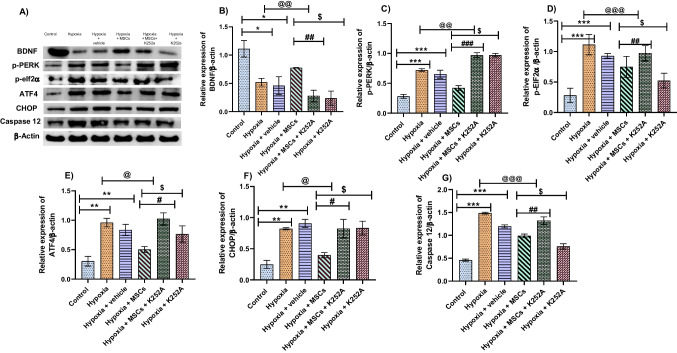
Co-culture with MSCs reduces protein levels of ER stress markers following OGD/R in PC12 cells
Protein levels of ER stress markers (p-PERK, p-EIF2α, CHOP, ATF4, and caspase 12) in hypoxia and hypoxia + vehicle groups were upregulated as compared to the control group. Co-culture with MSCs significantly decreased these markers’ expression, respectively, compared to the control group. However, protein levels of BDNF were found to be increased following MSCs treatment compared to the hypoxia and hypoxia + vehicle group. K252a treatment upregulated the levels of ER stress proteins, and no significant difference was observed between the hypoxia + MSCs + K252a and hypoxia + K252a group. Also, BDNF levels were found to be downregulated in K252a treated groups. These results hint that K252a treatment limited the protective effects of MSCs in the hypoxia group. These results show that MSCs provide a protective effect following OGD/R by decreasing ER stress via BDNF signaling (Fig. 4A–G).
Fig. 4.
Effect of MSCs treatment on the protein expression of various ER stress markers following I/R injury. A Representative western blot images. B BDNF. C p-PERK. D p-eIF2α. E ATF4. F CHOP. G Caspase 12. Data are expressed as mean ± SEM and analyzed for statistical significance using one-way ANOVA with Tukey’s multiple comparison test, *p < 0.05, **p < 0.01, ***p < 0.001 vs control; @p < 0.05, @@p < 0.01, @@@p < 0.001 vs hypoxia group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs hypoxia + MSCs group; $p < 0.05 vs hypoxia + MSCs (n = 3)
Co-culture with MSCs increases BDNF levels following OGD/R in PC12 cells
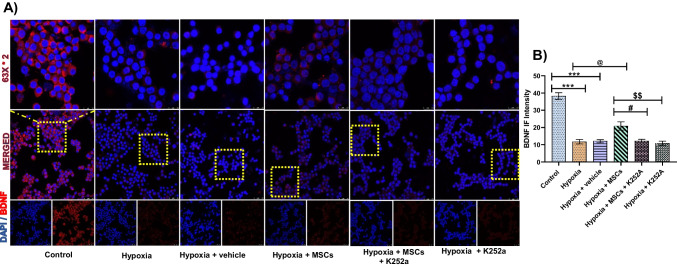
In our study, the expression of BDNF significantly reduced following OGD/R. Interestingly, MSCs treatment significantly increases the levels of BDNF compared to hypoxia and hypoxia + vehicle group. However, K252a treatment significantly reduces the expression of BDNF in hypoxia + MSCs + K252a and hypoxia + K252a group. However, no significant difference was observed between these two groups. Therefore, these results indicate that MSCs modulate the apoptosis-mediated ER stress by regulating the BDNF signaling (Fig. 5).
Fig. 5.
Co-culture with MSCs increases BDNF levels in OGD/R treated PC12 cells. A Representative images of BDNF immunofluorescence staining (nucleus stained blue with DAPI and BDNF stained red). B Statistical results of BDNF immunofluorescence intensity (IF intensity). Data are expressed as mean ± SEM and analyzed for statistical significance using one-way ANOVA with Tukey’s test, ***p < 0.001 vs control; @@p < 0.01 vs hypoxia and hypoxia + vehicle group; ##p < 0.01 vs hypoxia + MSCs group, $$p < 0.01 vs hypoxia + MSCs group. Magnification 63 × ; scale bar 10 µm (n = 3)
BDNF significantly increases cell viability following OGD/R in PC12 cells
The viability of PC12 cells following OGD/R was significantly increased by the BDNF treatment as compared to OGD/R PC12 cells. While co-culture with MSCs has also increased cell viability compared to OGD/R treated PC12 cells, the difference between BDNF treated group and MSCs treated group per se was non-significant. However, an additive effect has been observed following a combined treatment of BDNF and MSCs as compared to OGD/R treated PC12 cells, BDNF treated group and MSCs treated group (Supplementary file 1).
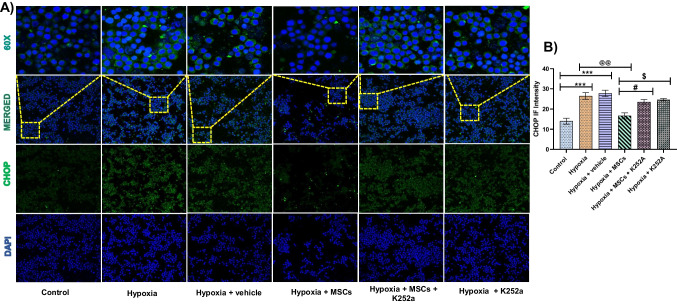
Co-culture with MSCs decreases CHOP levels following OGD/R in PC12 cells
Following co-culture with MSCs significantly reduced the expression of CHOP, indicating that MSCs were able to protect the damaged cell from ER stress-induced apoptosis. However, treatment with K252a significantly increased the expression of CHOP following OGD/R, indicating that the protective action of MSCs was inhibited by blocking BDNF signaling (Fig. 6).
Fig. 6.
Co-culture with MSCs reduces CHOP levels in OGD/R treated PC12 cells. A Representative images of CHOP immunofluorescence staining (nucleus stained blue with DAPI and CHOP stained green). B Statistical results of CHOP immunofluorescence intensity (IF intensity). Data are expressed as mean ± SEM and analyzed for statistical significance using one-way ANOVA with Tukey’s test, ***p < 0.001 vs control; @@p < 0.01 vs hypoxia and hypoxia + vehicle group; #p < 0.05 vs hypoxia + MSCs group, $p < 0.05 vs hypoxia + MSCs group. Magnification 20 × ; scale bar 50 and 10 µm (n = 3)
Discussion
Studies suggest that the pathophysiology of I/R injury is complex, involving multiple signaling pathways that include ROS generation, intracellular calcium overload, inflammatory response, ER stress, mitochondrial dysfunction, and autophagy (Granger and Kvietys 2015; Han et al. 2021; Kalogeris et al. 2012; Vongsfak et al. 2021). Increasing evidence also revealed that ER stress is a key phenomenon of I/R injury exacerbation that plays a crucial role in the progression of hypoxia and stroke pathologies (Cao and Kaufman 2014; Lin 2015; Lin et al. 2008; Wang et al. 2022). The present study explores how co-culturing PC12 cells with MSCs following OGD/R alleviates ER stress-induced apoptosis via BDNF signaling.
The neuronal activity requires an uninterrupted supply of oxygen and glucose. However, any interruption in this supply exacerbates the pathological consequences (Chua and Lim 2021). OGD/R injury in cells mimics the model of cerebral ischemia in vivo (Yang et al. 2020). Interestingly, OGD/R injury in the cell has made it possible to identify potential therapeutic targets and agents to explore the molecular mechanisms associated with different pathologies (Chua and Lim 2021). PC12 cells that are derived from the rodent tumor found in the adrenal medulla with catecholaminergic neuronal properties are one of the commonly used cell lines for in vitro ischemia studies and have proven to be beneficial for the initial screening of neuroprotective compounds (Chua and Lim 2021; Shi et al. 2021; Poulain et al. 2000; Westerink and Ewing 2008). Induction of ER stress in PC12 cells has been well demonstrated previously, and reduction of ER stress significantly diminished apoptosis induced by ER stress (Gao et al. 2016; Krizanova et al. 2014; Zou et al. 2009). The importance of MSCs in reducing post-stroke ER stress has been studied limitedly in the past (Jiao et al. 2020; Li et al. 2019). However, the neuroprotective activity of MSCs has been attributed to the attenuation of apoptosis toward neuroprotection (Nair et al. 2021; Zhang et al. 2019). Reports also suggest that MSCs release trophic factors that aid neuroprotection following I/R injury (Joyce et al. 2010).
Oxidative stress and ROS generation serve a key role in neuronal injury following I/R injury (Kalogeris et al. 2012). Excessive ROS production causes lipid, protein, and DNA damage leading to cellular dysfunctioning and interruption of vital cellular processes (Sharifi-Rad et al. 2020). In the present study, we have shown that following OGD/R injury, there is a significant reduction in the levels of antioxidant enzyme glutathione. As ER stress is a potent oxidative and nitrosative stress inducer, we also found a significant increase in the nitrite and malondialdehyde levels (Cao and Kaufman 2014). Interestingly, co-culture with MSCs significantly increased the levels of GSH and normalized the levels of nitrite and MDA. Therefore, the results infer that MSCs could reduce oxidative stress after OGD/R by promoting the endogenous antioxidant defense mechanism following co-culture.
Following I/R injury, ER stress triggers the activation of PERK, responsible for the phosphorylation of eIF2α, and suppresses global protein synthesis (Mei et al. 2013). Once eIF2α gets phosphorylated, it further activates ATF4 and CHOP, promoting cell death via multiple pathologies (Mei et al. 2013). Previous studies have reported that following OGD/R, there is an increase in the expression of CHOP (Sun et al. 2018). In the current study, the mRNA of different ER stress markers was found to be upregulated, which is concurrent with previous findings (Kaur et al. 2021). Studies have reported that OGD/R injury elicits persistent ER stress, thereby leading to apoptosis (Oyadomari and Mori 2004; Sun et al. 2018). Among ER stress markers, CHOP and caspase-12 are one of the key mediators of apoptosis induced by ER stress as shown in animal models of ischemic stroke (Oyadomari and Mori 2004; Szegezdi et al. 2003). Similar to previous studies, our results also demonstrated an increased expression of caspase 12 and CHOP mRNA levels following OGD/R injury. We have also observed a significant decline in the expression of these markers, indicating that MSCs were able to protect the injured cells from ER stress-induced apoptosis. At the genetic level, we observed increased mRNA levels of p-PERK, p-eIF2α, ATF4, and CHOP, while co-culture with MSCs significantly reduced the mRNA expression of these markers. Thus, we can infer that the co-culture of MSCs with PC12 could provide a neuroprotective effect following OGD/R by downregulating ER stress.
BDNF is one of the important neurotrophic factors released by MSCs, which effectively delays neuronal death, stimulates neurogenesis, and has an antioxidant effect following ischemic stroke (Liu et al. 2020; Wu et al. 2016). Supporting studies also suggest that following OGD/R, the levels of BDNF are reduced in vitro (Zhang et al. 2020). To further confirm the involvement of BDNF signaling in apoptosis mediated by ER stress following ODG/R, an inhibition study using K252a (the inhibitor of Trk receptors) was performed. K252a treatment increased ER stress protein markers expression, enhanced apoptosis, and abolished the protective effects of BDNF signaling. Taken together, results support that BDNF signaling mediates the protective effects of MSCs via a reduction in apoptosis mediated by ER stress in PC12 cells after OGD/R injury. Expression of apoptotic markers was also elevated after K252a administration, which was not altered by MSCs co-culture. These results support that MSCs co-culture modulates apoptosis mediated by ER stress through regulation of BDNF signaling. We have also observed that following OGD/R, BDNF mRNA levels were downregulated. However, following co-culture with MSCs, the levels of BDNF were upregulated. From this, we can infer that MSCs co-culture with PC12 could provide a neuroprotective effect following OGD/R by upregulating BDNF (Fig. 7). Earlier studies by Wilkins et al. have used BDNF-neutralizing antibodies to block BDNF signaling; however, several other recent studies have confirmed successful inhibition of BDNF-TrkB signaling following K252a treatment (Wilkins et al. 2009). Although K252a is a non-specific tyrosine kinase inhibitor, which is a limitation of this study, minimum protein expression of BDNF following K252a treatment confirms the blockade of the particular signaling pathway. BDNF, in the PC12 cell line, can act via p75NTR receptor, which in association with other Trk receptors may confer neuroprotection (Ogura et al. 2014; Bothwell 2019). Hence, complete blockade of all protein kinases as well as tyrosine kinase receptors was solicited. To further validate the hypothesis, we have performed an experiment to observe if commercially available purified BDNF can render protection to PC12 cells following OGD/R. As there are no significant differences in the protein sequence of mature BDNF between human and rat, we have used human recombinant purified BDNF in our study (Radka et al. 1996). We observed that BDNF treatment in PC 12 cells per se could also render cellular protection; however, the cytoprotective effect is non-significant as compared to the effect of MSCs. Moreover, we also observed an additive effect following the combination treatment of BDNF and MSCs in comparison to MSCs treatment in singlet. Earlier, Ogura et al. reported that the induction of BDNF signaling through subtoxic levels of hydrogen peroxide contributes to cellular protection in PC12 cells (Ogura et al. 2014). BDNF/TrkB pathway can also ameliorate formaldehyde-induced toxicity and corticosterone toxicity in PC12 cells (Gao et al. 2015; Jiang et al. 2015; Ogura et al. 2014). Concurrent to the reported studies, we also observed that the modulation of BDNF/TrkB pathway has cytoprotective effect on PC12 cells following OGD/R. These studies potentiates our hypothesis that MSCs may alleviate apoptosis following OGD/R via modulation of BDNF signaling toward ameliorating the ER stress.
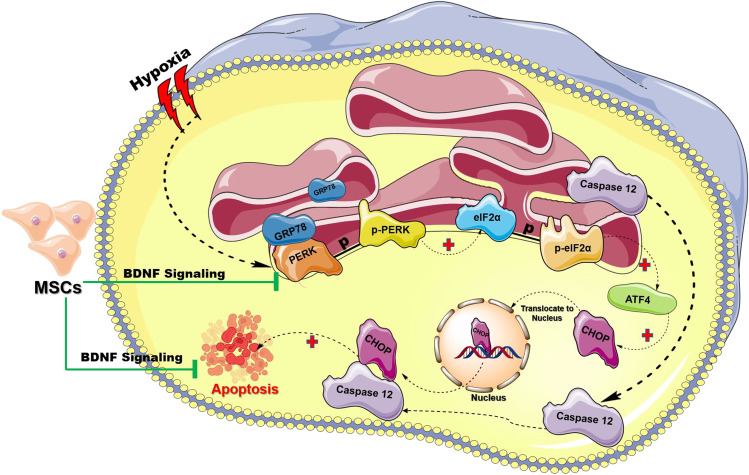
Fig. 7.
Schematic diagrammatic representation of the proposed hypothesis. Hypoxia induces misfolding of protein due to the lack of oxygen which is required for the formation of disulfide linkages and ultimately leads to endoplasmic reticulum (ER) stress. Under basal conditions, GRP78 binds to PERK and prevents its activation. However, in response to ER stress during hypoxia, GRP78 dissociates from PERK, allowing phosphorylation of PERK activation. Activated PERK further phosphorylates eIF2α resulting in a global inhibition of protein synthesis that allows the activation of ATF4, a transcription factor which then induces the expression of target genes CHOP. Once CHOP gets activated, it translocates to the nucleus and regulates the expression of genes involved in cell death (black dotted arrow). Caspase 12 is an ER-localized caspase that has been postulated to play a pivotal role in the ER stress-induced apoptosis pathway (black dotted arrow). Following hypoxia caspase 12 dissociates from ER membrane and translocate to cytosol further cleaves pro-caspase 9 and in turn activate caspase 3, thereby promoting apoptosis. We hypothesize that MSCs-mediated BDNF signaling limits the activation of ER stress and eventually preventing apoptosis to render neuroprotection (green line). (Image adapted from Servier Medical Art by Servier which is licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com/ and https://biorender.com/)
Conclusion
Our results from the study show that following co-culture with MSCs, OGD/R reduces cellular apoptosis induced by ER stress by modulation of the BDNF signaling. These outcomes provide a deeper insight into the neuroprotective mechanism conferred by MSCs post-I/R, which can help use stem cell therapy in clinical settings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors also acknowledge the Department of Pharmaceuticals, Ministry of Chemical and Fertilizers, Govt. of India and National Institute of Pharmaceutical Education and Research (NIPER) Ahmedabad, Gandhinagar, India. The authors would like to acknowledge the Indian Council of Medical Research (ICMR), New Delhi for the senior research fellowship grant of Miss Harpreet Kaur (File No. 5/3/8/16/ITR-F/2019-ITR) for the work on Stem Cell Therapy to Counteract Endoplasmic Reticulum Stress in Ischemic stroke, Servier Medical Art by Servier (licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com/) and https://biorender.com/ for graphics.
Funding
Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India. Indian Council of Medical Research (ICMR), New Delhi, File No. 5/3/8/16/ITR-F/2019-ITR, for providing a research fellowship to Ms. Harpreet Kaur.
Data Availability
All data generated are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alia C, Spalletti C, Lai S, Panarese A, Lamola G, Bertolucci F, Vallone F, di Garbo A, Chisari C, Micera S. Neuroplastic changes following brain ischemia and their contribution to stroke recovery: novel approaches in neurorehabilitation. Front Cell Neurosci. 2017;11:76. doi: 10.3389/fncel.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11:1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi XY, He X, Zhao M, Yu XJ, Zang WJ. Role of endothelial nitric oxide synthase and vagal activity in the endothelial protection of atorvastatin in ischemia/reperfusion injury. J Cardiovasc Pharmacol. 2013;61:391–400. doi: 10.1097/FJC.0b013e318286baf3. [DOI] [PubMed] [Google Scholar]
- Bothwell M (2019) Recent advances in understanding context-dependent mechanisms controlling neurotrophin signaling and function. F1000 8 [DOI] [PMC free article] [PubMed]
- Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrostek MR, Fellows EG, Guo WL, Swanson WJ, Crane AT, Cheeran MC, Low WC, Grande AW. Efficacy of cell-based therapies for traumatic brain injuries. Brain Sci. 2019;9:270. doi: 10.3390/brainsci9100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua P, Lim WK. Optimisation of a PC12 cell-based in vitro stroke model for screening neuroprotective agents. Sci Rep. 2021;11:8096. doi: 10.1038/s41598-021-87431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CJ, Redondo-Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab. 2018;38:1276–1292. doi: 10.1177/0271678X18776802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Sarmah D, Kaur H, Chaudhary A, Mounica KL, Kalia K, Borah A, Yavagal DR, Bhattacharya P. Post-stroke impairment of the blood–brain barrier and perifocal vasogenic edema is alleviated by endovascular mesenchymal stem cell administration: modulation of the PKCδ/MMP9/AQP4-mediated pathway. Mol Neurobiol. 2022;59:2758–2775. doi: 10.1007/s12035-022-02761-2. [DOI] [PubMed] [Google Scholar]
- Eilenberger C, Kratz SRA, Rothbauer M, Ehmoser EK, Ertl P, Kupcu S. Optimized alamarBlue assay protocol for drug dose-response determination of 3D tumor spheroids. MethodsX. 2018;5:781–787. doi: 10.1016/j.mex.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77:2771–2794. doi: 10.1007/s00018-020-03454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Li W, Zou W, Zhang P, Tian Y, Xiao F, Gu H, Tang X. H2S protects PC12 cells against toxicity of corticosterone by modulation of BDNF-TrkB pathway. Acta Biochim Biophys Sin (shanghai) 2015;47:915–924. doi: 10.1093/abbs/gmv098. [DOI] [PubMed] [Google Scholar]
- Gao H, Chen Z, Fu Y, Yang X, Weng R, Wang R, Lu J, Pan M, Jin K, McElroy C, Tang B, Xia Y, Wang Q. Nur77 exacerbates PC12 cellular injury in vitro by aggravating mitochondrial impairment and endoplasmic reticulum stress. Sci Rep. 2016;6:34403. doi: 10.1038/srep34403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8:886. doi: 10.3390/cells8080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Yuan M, Guo YS, Shen XY, Gao ZK, Bi X. Mechanism of endoplasmic reticulum stress in cerebral ischemia. Front Cell Neurosci. 2021;15:704334. doi: 10.3389/fncel.2021.704334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther. 2016;7:131. doi: 10.1186/s13287-016-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong CH, Kim SM, Lim JY, Ryu CH, Jun JA, Jeun SS. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed Res Int. 2014;2014:129145. doi: 10.1155/2014/129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JM, Zhou CF, Gao SL, Tian Y, Wang CY, Wang L, Gu HF, Tang XQ. BDNF-TrkB pathway mediates neuroprotection of hydrogen sulfide against formaldehyde-induced toxicity to PC12 cells. PLoS ONE. 2015;10:e0119478. doi: 10.1371/journal.pone.0119478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Z, Liu X, Ma Y, Ge Y, Zhang Q, Liu B, Wang H. Adipose-derived stem cells protect ischemia-reperfusion and partial hepatectomy by attenuating endoplasmic reticulum stress. Front Cell Dev Biol. 2020;8:177. doi: 10.3389/fcell.2020.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Sarmah D, Veeresh P, Datta A, Kalia K, Borah A, Yavagal DR, Bhattacharya P. Endovascular stem cell therapy post stroke rescues neurons from endoplasmic reticulum stress-induced apoptosis by modulating brain-derived neurotrophic factor/tropomyosin receptor kinase B signaling. ACS Chem Neurosci. 2021;12:3745–3759. doi: 10.1021/acschemneuro.1c00506. [DOI] [PubMed] [Google Scholar]
- Krizanova O, Steliarova I, Csaderova L, Pastorek M, Hudecova S. Capsaicin induces apoptosis in PC12 cells through ER stress. Oncol Rep. 2014;31:581–588. doi: 10.3892/or.2013.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Leung JCK, Chan LYY, Yiu WH, Li Y, Lok SWY, Liu WH, Chan KW, Tse HF, Lai KN, Tang SCW. Amelioration of endoplasmic reticulum stress by mesenchymal stem cells via hepatocyte growth factor/c-Met signaling in obesity-associated kidney injury. Stem Cells Transl Med. 2019;8:898–910. doi: 10.1002/sctm.18-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Shi L, Hu B, Hong Y, Zhang H, Li X, Zhang Y. Mesenchymal stem cell-based therapy for stroke: current understanding and challenges. Front Cell Neurosci. 2021;15:628940. doi: 10.3389/fncel.2021.628940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-L. Attenuation of endoplasmic reticulum stress as a treatment strategy against ischemia/reperfusion injury. Neural Regen Res. 2015;10:1930. doi: 10.4103/1673-5374.169615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu X, Chen M, Ge Q, Shen Y, Pan S. Resveratrol protects PC12 cells against OGD/ R-induced apoptosis via the mitochondrial-mediated signaling pathway. Acta Biochim Biophys Sin (shanghai) 2016;48:342–353. doi: 10.1093/abbs/gmw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wang X, O'Connor M, Wang G, Han F. Brain-derived neurotrophic factor and its potential therapeutic role in stroke comorbidities. Neural Plast. 2020;2020:1969482. doi: 10.1155/2020/1969482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y, Thompson MD, Cohen RA, Tong X. Endoplasmic reticulum stress and related pathological processes. J Pharmacol Biomed Anal. 2013;1:1000107. [PMC free article] [PubMed] [Google Scholar]
- Nair S, Rocha-Ferreira E, Fleiss B, Nijboer CH, Gressens P, Mallard C, Hagberg H. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: role of mitochondria, inflammation, and reactive oxygen species. J Neurochem. 2021;158:59–73. doi: 10.1111/jnc.15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Sato K, Kawashima K, Kobayashi N, Imura S, Fujino K, Kawaguchi H, Nedachi T. Subtoxic levels of hydrogen peroxide induce brain-derived neurotrophic factor expression to protect PC12 cells. BMC Res Notes. 2014;7:840. doi: 10.1186/1756-0500-7-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Poulain B, Bader MF, Molgó J. In vitro physiological studies on clostridial neurotoxins. Biological models and procedures for extracellular and intracellular application of toxins. Methods Mol Biol. 2000;145:259–286. doi: 10.1385/1-59259-052-7:259. [DOI] [PubMed] [Google Scholar]
- Pravalika K, Sarmah D, Kaur H, Vats K, Saraf J, Wanve M, Kalia K, Borah A, Yavagal DR, Dave KR, Bhattacharya P. Trigonelline therapy confers neuroprotection by reduced glutathione mediated myeloperoxidase expression in animal model of ischemic stroke. Life Sci. 2019;216:49–58. doi: 10.1016/j.lfs.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Radka SF, Hoist PA, Fritsche M, Altar CA. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 1996;709:122–130. doi: 10.1016/0006-8993(95)01321-0. [DOI] [PubMed] [Google Scholar]
- Rausch MP, Sertil AR. A stressful microenvironment: opposing effects of the endoplasmic reticulum stress response in the suppression and enhancement of adaptive tumor immunity. Int Rev Immunol. 2015;34:104–122. doi: 10.3109/08830185.2015.1018415. [DOI] [PubMed] [Google Scholar]
- Rozpedek W, Nowak A, Pytel D, Diehl JA, Majsterek I. Molecular basis of human diseases and targeted therapy based on small-molecule inhibitors of ER stress-induced signaling pathways. Curr Mol Med. 2017;17:118–132. doi: 10.2174/1566524017666170306122643. [DOI] [PubMed] [Google Scholar]
- Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraf J, Sarmah D, Vats K, Kaur H, Pravalika K, Wanve M, Kalia K, Borah A, Dave KR, Yavagal DR, Bhattacharya P. Intra-arterial stem cell therapy modulates neuronal calcineurin and confers neuroprotection after ischemic stroke. Int J Neurosci. 2019;129:1039–1044. doi: 10.1080/00207454.2019.1633315. [DOI] [PubMed] [Google Scholar]
- Sarmah D, Datta A, Kaur H, Kalia K, Borah A, Rodriguez AM, Yavagal DR, Bhattacharya P (2022) Sirtuin-1-mediated NF-κB pathway modulation to mitigate inflammasome signaling and cellular apoptosis is one of the neuroprotective effects of intra-arterial mesenchymal stem cell therapy following ischemic stroke. Stem Cell Rev Rep 1–18 [DOI] [PubMed]
- Scheper V, Schwieger J, Hamm A, Lenarz T, Hoffmann A. BDNF-overexpressing human mesenchymal stem cells mediate increased neuronal protection in vitro. J Neurosci Res. 2019;97:1414–1429. doi: 10.1002/jnr.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz DS, Blower MD. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci. 2016;73:79–94. doi: 10.1007/s00018-015-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbir A, Zisa D, Lin H, Mastri M, Roloff G, Suzuki G, Lee T. Activation of host tissue trophic factors through JAK-STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am J Physiol Heart Circ Physiol. 2010;299:H1428–H1438. doi: 10.1152/ajpheart.00488.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, TsouhFokou PV, Azzini E, Peluso I, Prakash Mishra A, Nigam M, El Rayess Y, Beyrouthy ME, Polito L, Iriti M, Martins N, Martorell M, Docea AO, Setzer WN, Calina D, Cho WC, Sharifi-Rad J. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Chai Y, Zhang J, Chen X. Endoplasmic reticulum stress-associated neuronal death and innate immune response in neurological diseases. Front Immunol. 2021;12:794580. doi: 10.3389/fimmu.2021.794580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. 2017;158:94–131. doi: 10.1016/j.pneurobio.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Ma X, Yang H, Zhao J, Zhang J. Brain-derived neurotrophic factor prevents beta- amyloid-induced apoptosis of pheochromocytoma cells by regulating Bax/Bcl-2 expression. Neural Regen Res. 2012;7:347–351. doi: 10.3969/j.issn.1673-5374.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Dai L, Zhang H, He X, Hou F, He W, Tang S, Zhao D. Neuritin attenuates neuronal apoptosis mediated by endoplasmic reticulum stress in vitro. Neurochem Res. 2018;43:1383–1391. doi: 10.1007/s11064-018-2553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186–194. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula P, Gautam B, Abela GS, Wang DH. Myocardial ischemia/reperfusion injury: potential of TRPV1 agonists as cardioprotective agents. Cardiovasc Hematol Disord Drug Targets. 2014;14:71–78. doi: 10.2174/1871529X13666131129103759. [DOI] [PubMed] [Google Scholar]
- Vongsfak J, Pratchayasakul W, Apaijai N, Vaniyapong T, Chattipakorn N, Chattipakorn SC. The alterations in mitochondrial dynamics following cerebral ischemia/reperfusion injury. Antioxidants (basel) 2021;10:1384. doi: 10.3390/antiox10091384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu Y, Zhang X, Ye Y, Xiong X, Zhang S, Gu L, Jian Z, Wang H. Endoplasmic reticulum stress and the unfolded protein response in cerebral ischemia/reperfusion injury. Front Cell Neurosci. 2022;16:864426. doi: 10.3389/fncel.2022.864426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink RH, Ewing AG. The PC12 cell as model for neurosecretion. Acta Physiol (oxf) 2008;192:273–285. doi: 10.1111/j.1748-1716.2007.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63–70. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Wu CL, Chen SD, Yin JH, Hwang CS, Yang DI. Nuclear factor-kappaB-dependent sestrin2 induction mediates the antioxidant effects of BDNF against mitochondrial inhibition in rat cortical neurons. Mol Neurobiol. 2016;53:4126–4142. doi: 10.1007/s12035-015-9357-1. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wang L, Hu Y, Wang F. Butorphanol protects PC12 cells against OGD/R-induced inflammation and apoptosis. Mol Med Rep. 2020;22:1969–1975. doi: 10.3892/mmr.2020.11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yu S, Tuazon JP, Lee JY, Corey S, Kvederis L, Kingsbury C, Kaneko Y, Borlongan CV. Neuroprotective effects of human bone marrow mesenchymal stem cells against cerebral ischemia are mediated in part by an anti-apoptotic mechanism. Neural Regen Res. 2019;14:597–604. doi: 10.4103/1673-5374.247464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Liu F, Chen Y, Guo WC, Zhang ZH. Proprotein convertase 1/3-mediated down-regulation of brain-derived neurotrophic factor in cortical neurons induced by oxygen-glucose deprivation. Neural Regen Res. 2020;15:1066–1070. doi: 10.4103/1673-5374.270314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Wang X, Chen A, Cheng X, Zhang G, Sun J, Zhao Y, Huang Y, Zhu Y. Rhein protects against cerebral ischemic-/reperfusion-induced oxidative stress and apoptosis in rats. Int J Mol Med. 2018;41:2802–2812. doi: 10.3892/ijmm.2018.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou CG, Cao XZ, Zhao YS, Gao SY, Li SD, Liu XY, Zhang Y, Zhang KQ. The molecular mechanism of endoplasmic reticulum stress-induced apoptosis in PC-12 neuronal cells: the protective effect of insulin-like growth factor I. Endocrinology. 2009;150:277–285. doi: 10.1210/en.2008-0794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated are available from the corresponding author upon reasonable request.