SUMMARY
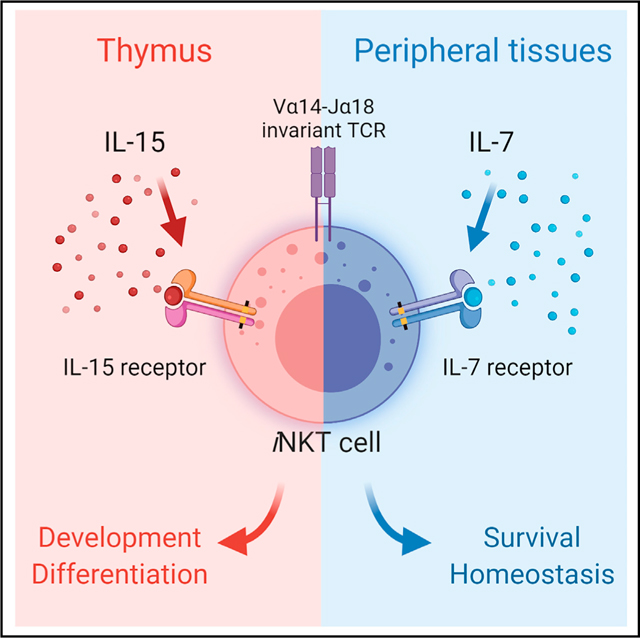
Understanding the homeostatic mechanism of invariant natural killer T (iNKT) cells is a critical issue in iNKT cell biology. Because interleukin (IL)-15 is required for the thymic generation of iNKT cells, IL-15 has also been considered necessary for the homeostasis of peripheral iNKT cells. Here, we delineated the in vivo cytokine requirement for iNKT cells, and we came to the surprising conclusion that IL-7, not IL-15, is the homeostatic cytokine for iNKT cells. Employing a series of experimental mouse models where the availability of IL-7 or IL-15 was manipulated in peripheral tissues, either by genetic tools or by adult thymectomy and cytokine pump installation, we demonstrate that the abundance of IL-7, and not IL-15, limits the size of the peripheral iNKT cell pool. These results redefine the cytokine requirement for iNKT cells and indicate competition for IL-7 between iNKT and conventional αβ T cells.
In brief
Park et al. assess the cytokine requirement for iNKT cells. While IL-15 is required for the thymic generation of iNKT cells, the survival and homeostasis of peripheral iNKT cells critically depend on the availability of IL-7 and not IL-15, revealing a dichotomy in the cytokine usage of iNKT cells.
Graphical abstract
INTRODUCTION
Natural killer (NK) T cells expressing the invariant Vα14-Jα18 T cell receptor (TCR) (iNKT cells) are CD1d-restricted immune regulatory cells that are uniquely positioned at the interface of the innate and adaptive immune systems (Godfrey and Kronenberg, 2004). As such, iNKT cells display functional characteristics and phenotypic markers of both innate NK cells and adaptive T lymphocytes (Godfrey and Kronenberg, 2004). Like T cells, iNKT cells express a somatically rearranged TCR that depends on RAG expression, and, like NK cells, iNKT cells express the NK lineage marker NK1.1 and are innate producers of pro-inflammatory cytokines (Bendelac, 1995a, b; Lantz and Bendelac, 1994). iNKT cells can recognize specific antigens and mount rapid and vigorous cytokine responses to play critical roles during microbial infection, inflammation, and in tumors (Bendelac et al., 2007; Godfrey et al., 2010).
Reflecting the shared characteristics of NK cells and T cells, the generation of iNKT cells also features a combined requirement of developmental signals that are specific to either NK cells or to T cells. Similar to conventional αβ T cells, iNKT cells are generated in the thymus from immature CD4+CD8+ double-positive (DP) thymocytes and require TCR-mediated positive selection (Bendelac, 1995b). This is in contrast to NK cells, which develop in the bone marrow and do not require a thymus (Haller et al., 1977). iNKT cells are similar to NK cells because their generation depends on interleukin (IL)-15 that is trans-presented by thymic medullary epithelial cells (Gordy et al., 2011; White et al., 2014). Such cytokine requirement is distinct from conventional T cells, which require IL-7, and not IL-15, for their development in the thymus. Thus, the generation of iNKT cells depends on a combination of cues that are characteristic for either conventional αβ T cells or NK cells.
In contrast to the generation of iNKT cells, however, the requirements for the survival and homeostasis of iNKT cells remain uncertain (Vahl et al., 2013). In particular, the role of cytokines for iNKT cell survival is incompletely understood. While conventional αβ T cells depend on IL-7, iNKT cells are proposed to mostly depend on IL-15 for their survival and homeostasis (Kennedy et al., 2000; Matsuda et al., 2002; Ranson et al., 2003a). As such, an IL-15 requirement had been documented by the paucity of liver iNKT cells of IL-15-deficient (Il15−/−) mice (Matsuda et al., 2002), and the homeostatic proliferation of adoptively transferred iNKT cells, which depends on IL-15, and not IL-7 (Matsuda et al., 2002; Ranson et al., 2003a). The induction of the transcription factor T-bet, which promotes iNKT cell maturation (Townsend et al., 2004), also depends on IL-15 (Gordy et al., 2011; Townsend et al., 2004). Altogether, IL-15, and not IL-7, is currently considered as the homeostatic cytokine for mature iNKT cells.
However, IL-15 is also required for the generation of iNKT cells in the thymus (Kennedy et al., 2000). Thus, it was confusing whether Il15−/− mice lack peripheral iNKT cells because of the failure to generate them in the thymus or because of the failure to maintain them in the periphery. Additionally, the redundancy of IL-15 with other γc family cytokines has hampered further analysis of the homeostatic requirements for iNKT cells. In particular, a role for IL-7, which is a pro-survival factor for conventional αβ T cells (Schluns et al., 2000; Tan et al., 2001), remains unclear for iNKT cell homeostasis.
In the current study, we addressed these questions using a series of in vivo mouse models. While IL-15 was clearly a critical factor for the thymic development of iNKT cells, we were surprised to find that IL-15 was dispensable for iNKT cell survival in peripheral tissues. Instead, IL-7 and not IL-15 was essential to maintain the survival and homeostasis of iNKT cells. In fact, the in vivo availability of IL-7 constrained the size of the peripheral iNKT cell pool so that transgenic overexpression or administration of recombinant IL-7 proteins dramatically increased the numbers of iNKT cells. Conversely, in a mouse model where IL-7 is abundantly expressed in the thymus but not in peripheral tissues, iNKT cells were robustly produced in the thymus but succumbed in peripheral tissues. Collectively, this study unveils a non-redundant IL-7 requirement for iNKT cell homeostasis that has broad implications for understanding iNKT cell biology and effector function.
RESULTS
A requirement for γc cytokine receptors in thymic iNKT cell development
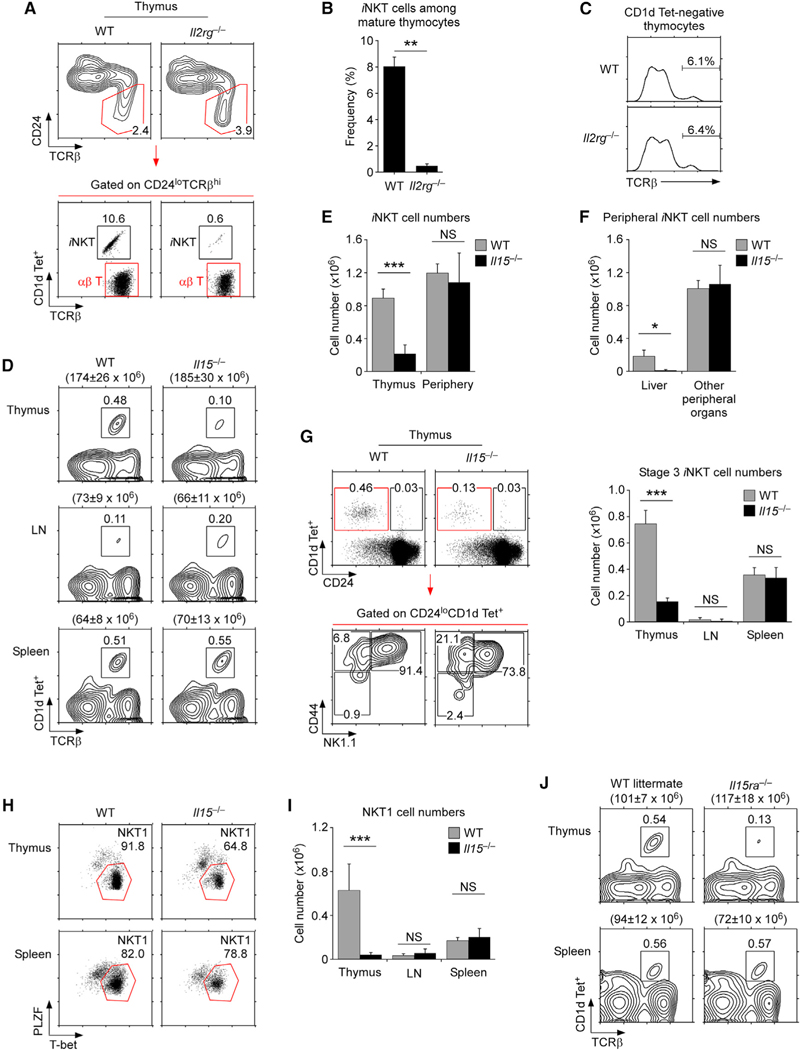
The thymus generates two distinct populations of mature (CD24loTCRβhi) αβ T cells; namely, conventional αβ T cells and iNKT cells (Bendelac et al., 2007) (Figure 1A). iNKT cells differ from conventional αβ T cells in that they are selected by glycolipid-loaded CD1d molecules instead of self-peptide/MHC complexes (Benlagha et al., 2000). In C57BL/6 (B6) mice, approximately 8% of CD24lo mature thymocytes bind to glycolipid (PBS57)-loaded CD1d tetramers (CD1d Tet+) and thus correspond to iNKT cells (Figure 1B). However, thymic iNKT cell frequencies can differ depending on the mouse strain, genetic background, age, or alterations in transcription factor expression (Constantinides and Bendelac, 2013; Esteban et al., 2003; Lee et al., 2013). Thus, iNKT cell development is controlled by a complex regulatory network, and its circuitry still needs to be deciphered. Notably, cytokines of the γc family also affect the generation of iNKT cells (McCaughtry et al., 2012). Along these lines, an in vivo requirement for γc cytokines is illustrated in the dramatic loss of mature (CD24loTCRβhi) iNKT cells in γc-deficient (Il2rg−/−) thymocytes (Figure 1A bottom and 1B). In contrast, the generation of conventional αβ T cells remained mostly unaffected in Il2rg−/− mice (Figure 1C). Thus, γc cytokines play a selective and non-redundant role in iNKT cell development in the thymus.
Figure 1. γc is required but IL-15 is dispensable for peripheral iNKT cells.
(A) iNKT cell development in Il2rg−/− thymocytes. CD24loTCRβhi mature thymocytes from WT and Il2rg−/− mice were assessed for iNKT cells by staining with anti-TCRβ and CD1d tetramers loaded with the α-GalCer analog PBS-57 (CD1d Tet+). Numbers in boxes indicate percentages of gated cells. Data are representative of five independent experiments.
(B) Frequency of thymic iNKT cells among CD24loTCRβhi mature thymocytes of WT and Il2rg−/− mice. Data show mean ± SEM of six WT and 13 Il2rg−/− mice.
(C) CD1d Tet-negative αβ TCR thymocytes in WT and Il2rg−/− mice. Numbers show percentages of TCRβhi mature T cells. Data are representative of three independent experiments.
(D) iNKT cell development and homeostasis in Il15−/− mice. iNKT cells in the indicated organs of WT and Il15−/− mice were identified by anti-TCRβ versus CD1d Tet staining. Total cell numbers are shown above each contour plot as mean ± SEM. Results are from three independent experiments with five WT and five Il15−/− mice.
(E) iNKT cell numbers in thymus and peripheral tissues of WT and Il15−/− mice. Periphery indicates the combined iNKT cells from spleen, LN, liver, lung, bone marrow, and fat tissues. Bar graph shows mean ± SEM from 15 WT and 18 Il15−/− mice.
(F) iNKT cell numbers were assessed among peripheral organs for either liver or other peripheral organs, which correspond to peripheral organs except for the liver. Bar graph shows mean ± SEM from 15 WT and 18 Il15−/− mice.
(G) iNKT cell differentiation in the thymus. Immature stage 0 and mature stage 1–3 iNKT cells in WT and Il15−/− thymocytes were identified by CD24 versus CD1d Tet staining (left, top). Stage 1–3 differentiation was determined on CD24loCD1d Tet+ thymocytes by CD44 versus NK1.1 analysis (left, bottom). Stage 3 iNKT cell numbers were determined in the indicated organs of WT and Il15−/− mice. Bar graphs show mean ± SEM from 10 WT and 13 Il15−/− mice from seven independent experiments (right).
(H) NKT1 cells were identified by intracellular promyelocytic leukemia zinc finger (PLZF) protein versus T-bet staining in the thymus and spleen of WT and Il15−/− mice. Dot plots are representative of four independent experiments with a total of five WT and five Il15−/− mice.
(I) NKT1 cell numbers in the indicated lymphoid organs of WT and Il15−/− mice. Bar graph shows the mean ± SEM from four WT and four Il15−/− mice from four independent experiments.
(J) iNKT cell development and homeostasis in Il15ra−/− mice. iNKT cells in thymus and spleen of Il15ra−/− and WT littermate control mice were identified by anti-TCRβ versus CD1d Tet staining. Total cell numbers are shown above each contour plot as the mean ± SEM. Results are from three independent experiments with five WT and six Il15ra−/− mice.
IL-15 is required for the generation, but not for the survival, of iNKT cells
The γc cytokine family consists of six members, and IL-15 is a γc cytokine that is considered to be essential for iNKT cell development (Kennedy et al., 2000; Ranson et al., 2003a). We confirmed an IL-15 requirement by assessing the iNKT cell population in thymocytes of IL-15-deficient (Il15−/−) mice. Both the frequencies and numbers of Il15−/− thymic iNKT cells were dramatically reduced compared with those of wild-type (WT) iNKT cells, affirming IL-15 as a γc cytokine required for the thymic generation of iNKT cells (Figure 1D top and 1E left; Figure S1A).
IL-15 has also been considered necessary for iNKT cell survival and homeostasis in peripheral tissues (Gordy et al., 2011; Kennedy et al., 2000; Lodolce et al., 1998; Matsuda et al., 2002; Ranson et al., 2003a). Thus, we expected that the iNKT cells in peripheral tissues of Il15−/− mice would be substantially reduced. Surprisingly, however, the iNKT cell frequencies in the lymph nodes (LNs) and spleens of Il15−/− mice did not decrease (Figures 1D and S1A). Moreover, the total iNKT cell numbers pooled from multiple peripheral organs of Il15−/− mice (i.e., spleen, LN, liver, lung, bone marrow and fat) did not show significant differences to those of WT mice (Figures 1E and S1B). We confirmed such selective loss in thymic but not peripheral iNKT cells also in Il15−/− and littermate control mice (Figure S2), effectively excluding any potential differences in genetic background, age, or sex for these findings. Thus, contrary to the prevailing view (Kennedy et al., 2000; Ranson et al., 2003a), iNKT cell numbers in peripheral tissues were maintained independently of IL-15, and IL-15 appeared to be dispensable for the peripheral homeostasis of iNKT cells. Of note, we found that iNKT cells in the liver were an exception so that liver iNKT cell numbers were consistently diminished in Il15−/− mice (Figures 1F and S2C). However, such decrease in iNKT cell numbers was limited to the liver, and we did not find reduced iNKT cell numbers in any other peripheral organs (Figures 1F, S1B, S2B, and S2C). It is currently not clear to us why liver iNKT cells are uniquely sensitive to the loss of IL-15, but we aim to address this in future studies. Collectively, these results document that IL-15 is required for the thymic development but not for the maintenance of iNKT cells, and that iNKT cells can survive by IL-15-independent homeostatic mechanisms in peripheral tissues.
iNKT cells are not homogeneous; rather, they comprise different subsets that are developmentally distinct and functionally diverse (Coquet et al., 2008; Lee et al., 2013; Michel et al., 2007; Pellicci et al., 2002; Terashima et al., 2008). Thus, we considered it important to examine if IL-15 is dispensable for the homeostasis of all iNKT cells or only a select subset of iNKT cells. Conventionally, mature iNKT cells have been divided into three distinct developmental stages based on their expression of CD44 and NK1.1 (Benlagha et al., 2002, 2005; Pellicci et al., 2002). Stage 1 iNKT cells are CD44−NK1.1−, followed by stage 2 iNKT cells, which are CD44+NK1.1−, and the most differentiated stage 3 iNKT cells are CD44+NK1.1+. The differentiation of stage 2 into stage 3 iNKT cells depends on IL-15 (Gordy et al., 2011), which in turn is necessary to upregulate expression of T-bet, a key transcription factor for terminal iNKT cell maturation (Townsend et al., 2004). In the thymus of C57BL/6 mice, the stage 3 subset comprises the vast majority (~90%) of iNKT cells (Benlagha et al., 2002). Consequently, the dramatic loss of thymic iNKT cells in Il15−/− mice is primarily due to decreases in the frequency and number of stage 3 iNKT cells (Figure 1G). In contrast, the number of stage 3 iNKT cells in peripheral lymphoid organs did not differ between Il15−/− and WT mice (Figure 1G right), indicating that peripheral stage 3 iNKT cells can either accumulate or arise independently of IL-15. Along these lines, stage 1 and stage 2 iNKT cell numbers in the periphery also did not differ between Il15−/− and WT mice (Figure S3A), indicating that IL-15 is dispensable for all stages of iNKT cells in peripheral tissues.
Recently, an alternative classification of iNKT cells has been put forward that places iNKT cells into three distinct effector subsets, namely, NKT1, NKT2, and NKT17 (Lee et al., 2013). NKT1 cells are characterized by high-level expression of the transcription factor T-bet, and they largely overlap with iNKT cells that are conventionally defined as stage 3 cells. In accordance with a role for IL-15 in T-bet expression (Gordy et al., 2011), NKT1 cells were dramatically reduced in the thymus of Il15−/− mice (Figures 1H and 1I). Importantly, however, NKT1 cell numbers in peripheral lymphoid organs remained comparable with those of WT mice (Figure 1I), demonstrating that IL-15 is required for the thymic generation but not for the peripheral maintenance of NKT1 cells. IL-15 was also dispensable for the maintenance of other iNKT subsets, as we did not find differences in NKT2 and NKT17 cell numbers of Il15−/− and WT mice (Figure S3B).
Because the IL-15-independent maintenance of iNKT cells was such a striking observation, we next wished to corroborate the validity of these results by confirming the lack of IL-15 activity in peripheral tissues of Il15−/− mice. NK cells are highly dependent on IL-15 (Ranson et al., 2003b). Consistent with their IL-15 requirement, we found a complete lack of NK cells in Il15−/− mice, confirming the IL-15 deficiency in these mice and documenting that iNKT cells are indeed maintained without IL-15 (Figure S4). We further wished to exclude potential contributions of the microbiota and/or facility-specific effects on iNKT cell homeostasis, so we set up co-housing experiments of WT and Il15−/− mice. Here, we placed C57BL/6 WT mice with age- and sex-matched Il15−/− mice into the same cage for 5 weeks, and then analyzed the frequencies and numbers of iNKT cells in the thymus, LN, and spleen in co-housed mice (Figures S5 and S6). Notably, the thymic iNKT cell numbers remained dramatically diminished in Il15−/− mice, while the frequencies, numbers, and phenotypic features of LN and spleen iNKT cells were still comparable between WT and Il15−/− mice (Figures S5 and S6). Thus, we consider it unlikely that the microbiota or other environmental conditions would contribute to the IL-15-independent maintenance of peripheral iNKT cells. Lastly, to pinpoint that iNKT cell homeostasis is independent not only of IL-15 but of IL-15 signaling in general, we assessed the generation and maintenance of iNKT cells in IL-15Ra-deficient (Il15ra−/−) mice. IL-15 signaling requires the transpresentation of the cytokine by the IL-15Ra receptor (Lodolce et al., 1998). Consequently, Il15ra−/− mice are impaired in IL-15 signaling, and we expected to find the same deficit in thymic iNKT cell generation but intact homeostasis of peripheral iNKT cells as in Il15−/− mice. This was indeed the case, as we observed a profound decrease in thymic iNKT cell numbers but no significant differences in the frequency, number, and phenotype of peripheral iNKT cells that included the LN, spleen, bone marrow, liver, and lung (Figures 1J, S7, and S8). Collectively, these data demonstrate an in vivo requirement for a γc cytokine in peripheral iNKT cell homeostasis that, surprisingly, is not IL-15, as has been previously thought.
IL-7 receptor is highly abundant on iNKT cells
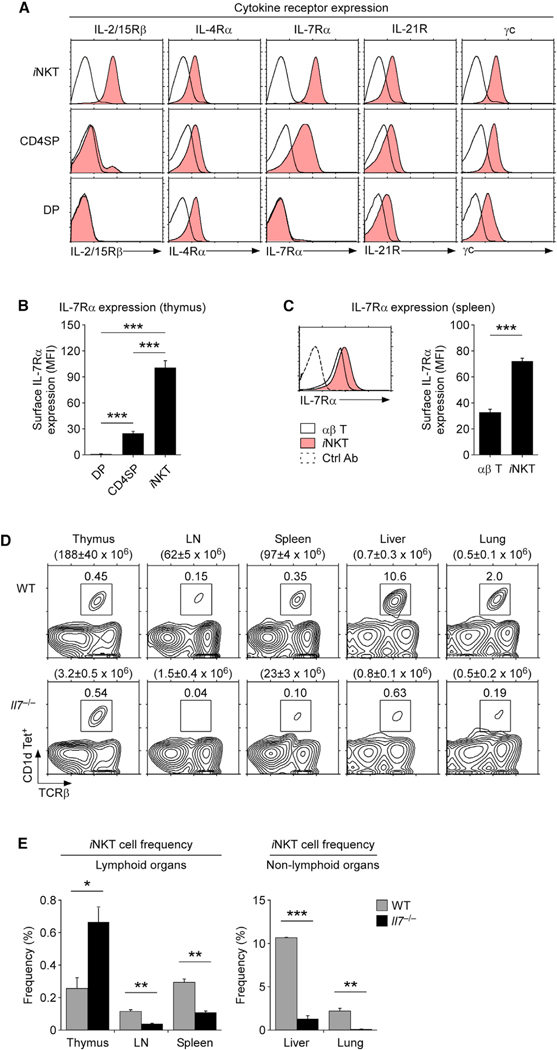
To further examine which γc cytokine is required for iNKT cell maintenance, we assessed the expression of γc family cytokine receptors on iNKT cells. Consistent with their requirement for IL-15 signaling (Carson et al., 1994), the IL-2/IL-15 receptor β chain (IL-2/15Rβ) was abundantly expressed on iNKT cells, which differed from CD4 single-positive (SP) thymocytes or immature DP cells where IL-2/15Rβ was mostly absent (Figure 2A) (Gordy et al., 2011; Kennedy et al., 2000; Matsuda et al., 2002). iNKT cells additionally expressed other γc family cytokine receptors, specifically the IL-4Ra, the IL-7Rα, and the IL-21R (Figure 2A). Thus, iNKT cells are equipped to respond to various γc cytokines, and potentially any of these cytokines could act redundantly to IL-15 to provide survival.
Figure 2. IL-7 is critical for the maintenance of iNKT cells in peripheral tissues.
(A) Expression of γc family cytokine receptors (shaded histograms) on iNKT, CD4SP, and DP thymocytes of WT mice. Control antibody staining is shown in open histograms. Results are representative of four independent experiments.
(B) Surface IL-7Rα expression on iNKT, CD4SP, and DP thymocytes from WT mice. Mean fluorescence intensity (MFI) values are the summary of three independent experiments (mean ± SEM).
(C) Surface IL-7Rα levels on spleen αβ T cells and iNKT cells. MFI values are the summary of three independent experiments (mean ± SEM).
(D) iNKT cells in the indicated tissues and organs of WT and Il7−/− mice. Total cell numbers are shown above contour plots as the mean ± SEM. Data are representative of two independent experiments with a total of four WT and three Il7−/− mice.
(E) Frequencies of iNKT cells in lymphoid (left) and non-lymphoid tissues (right) of WT and Il7−/− mice. Bar graphs show the summary of two independent experiments with a total of four WT and three Il7−/− mice.
Among γc cytokines, we found IL-7 of particular interest because the proprietary receptor for IL-7 (i.e., IL-7Rα), was highly expressed on iNKT cells in both the thymus and the spleen (Figures 2B and 2C). In fact, iNKT cells showed the largest abundance of IL-7Rα among all thymocyte subsets and peripheral αβ T cells (Figures 2B and 2C). Thus, we sought to examine a role of IL-7 for iNKT cells. IL-7 is essential for T cell development in the thymus so thymopoiesis is severely compromised in IL-7-deficient (Il7−/−) mice (von Freeden-Jeffry et al., 1995). Both total thymocyte and iNKT cell numbers were dramatically reduced in Il7−/− mice, but the frequency of thymic iNKT cells did not decrease in the absence of IL-7 (Figure 2D), suggesting that there is no selective requirement for IL-7 for iNKT cell generation. In fact, there was a significant increase in thymic iNKT cell frequencies in Il7−/− mice (Figure 2E), indicating that iNKT cells are refractory to the lack of IL-7, presumably because they utilize IL-15 in the thymus. In peripheral tissues, however, iNKT cells were highly susceptible to the lack of IL-7, as reflected by the reductions in both the frequencies and numbers of iNKT cells in lymphoid and non-lymphoid organs of Il7−/− mice (Figures 2D, 2E, and S9A). In fact, the cell numbers of all three iNKT subsets, i.e., NKT1, NKT2, and NKT17, were reduced in Il7−/− mice, indicating a common requirement for IL-7 in the homeostasis of all iNKT subsets (Figure S9B). Such an IL-7 requirement was specific to iNKT cells because the numbers of NK cells, which rely on IL-15, were mostly unaffected in Il7−/− mice (Figure S9C). These results document an IL-7 requirement for iNKT cell survival in peripheral tissues, and they further suggest that IL-7 is the γc cytokine required for iNKT cell homeostasis.
An in vivo IL-7 requirement for the maintenance of peripheral iNKT cells
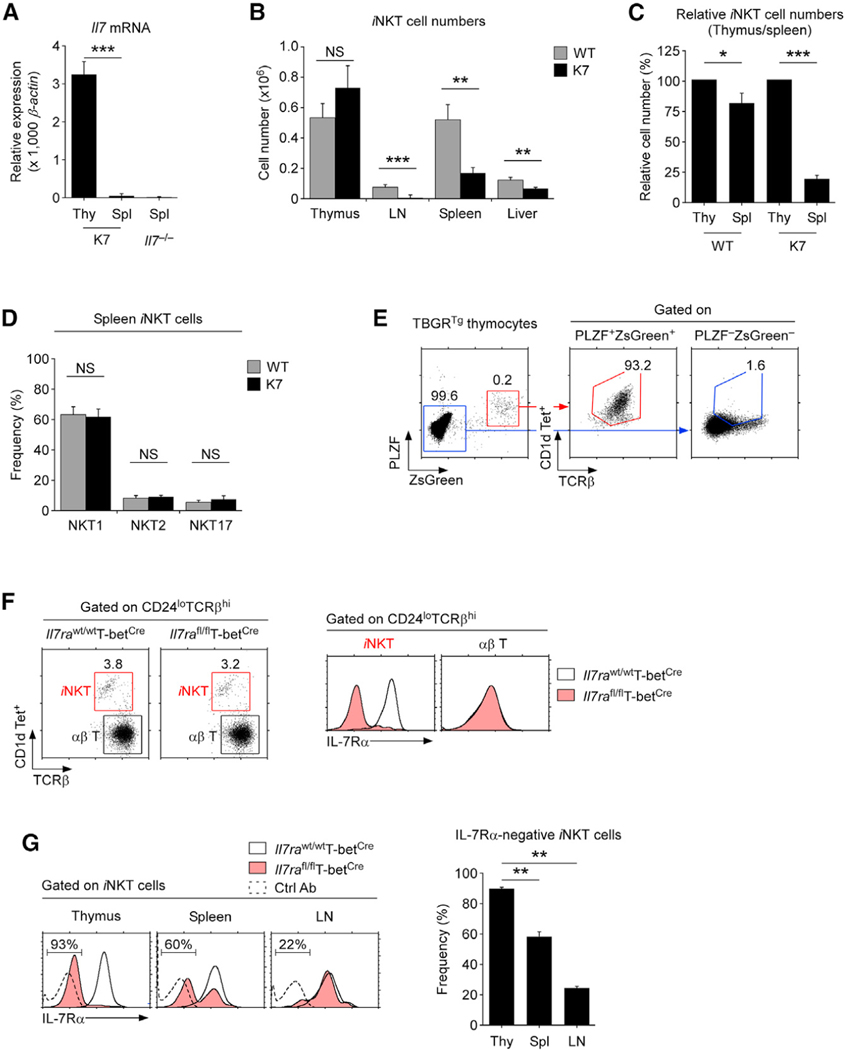
To further clarify the significance of IL-7 for peripheral iNKT cells, we employed an animal model where IL-7 deficiency is limited to peripheral tissues. K7 mice are genetically engineered to express a thymocyte-specific IL-7 transgene on an Il7−/− background (Kim et al., 2012). Consequently, K7 mice express Il7 mRNA in the thymus but not in peripheral tissues (Figure 3A). In agreement with robust IL-7 expression in the thymus, iNKT cell generation remained intact, and the frequency and number of thymic iNKT cells in K7 mice did not significantly differ from those of WT mice (Figures 3B and S10A). In contrast, the lack of IL-7 in peripheral tissues severely reduced the size of the iNKT cell pool, and iNKT cell numbers were dramatically reduced in both lymphoid and non-lymphoid tissues, including the liver (Figures 3B and S10A). These results indicated that IL-7 is critical for the homeostatic maintenance of iNKT cells in the periphery.
Figure 3. iNKT cell generation and survival in K7 mice.
(A) Quantification of IL-7 mRNA in the thymus (Thy) and spleen (Spl) of K7 mice. Il7 mRNA expression was assessed from thymus and spleen tissues of K7 mice by quantitative real-time PCR. Spleen mRNA from Il7−/− mice was used as a negative control. Data are the summary of three independent experiments.
(B) iNKT cell numbers in the indicated organs of WT and K7 mice. Bar graph shows iNKT cell numbers as the mean ± SEM from six WT and seven K7 mice from three independent experiments.
(C) Relative iNKT cell numbers in the thymus and spleen of WT and K7 mice where thymic iNKT cell numbers of each mouse strains were set to 100%. Data are the summary of 13 independent experiments with a total of 17 WT and 18 K7 mice.
(D) Subset composition of splenic iNKT cells in WT and K7 mice. The frequency of each subset was determined among spleen iNKT cells of WT and K7 mice by intracellular staining for PLZF, RORγt, and T-bet. Data show summary of six independent experiments with seven WT and eight K7 mice.
(E) Cellular identity of ZsGreen+ cells among thymocytes of TBGRTg mice. Total thymocytes of TBGRTg mice were stained for PLZF and plotted against ZsGreen reporter expression (left). The frequency of CD1dTet+ iNKT cells was then determined in PLZF+ZsGreen+ versus PLZF–ZsGreen– thymocytes (right).
(F) IL-7Rα deletion in mature iNKT and conventional abT cells of Il7rafl/flT-betCre thymocytes. iNKT and conventional αβ T cells were identified among CD24loTCRβhi thymocytes of Il7rafl/flT-betCre and control Il7rawt/wtT-betCre mice and assessed for surface IL-7Rα expression. Results are representative of four independent experiments.
(G) Surface IL-7Rα expression on iNKT cells of Il7rafl/flT-betCre mice. iNKT cells were identified in the thymus, spleen, and LN of Il7rafl/flT-betCre and littermate control Il7rawt/wtT-betCre mice and assessed for IL-7Rα expression (left). Bar graph shows the frequency of IL-7Rα+ iNKT cells of the indicated mice (right). Results are from four independent experiments with a total of six Il7rawt/wtT-betCre and nine Il7rafl/flT-betCre mice.
An IL-7 requirement for iNKT cell maintenance was further documented when we assessed the numbers of splenic iNKT cells relative to those of thymic iNKT cells. Compared with WT mice, the ratio of peripheral to thymic iNKT cells was dramatically reduced in K7 mice (Figure 3C) but did not alter their composition as assessed for each iNKT stage (Figure 3D). These results affirm a non-redundant role for IL-7 in maintaining all stages of iNKT cells in the periphery. Because IL-7 induces expression of the anti-apoptotic factor Bcl-2 (Rathmell et al.,2001), and because K7 iNKT cells contained significantly lower amounts of Bcl-2 than WT iNKT cells (Figure S10B), the loss of iNKT cells in K7 mice is most likely due to increased apoptosis. Thus, IL-7 is a critical survival factor for all peripheral iNKT cells. Finally, because the thymic output of iNKT cells affects the size of the peripheral iNKT cell pool (den Braber et al., 2012), we analyzed iNKT cell numbers in aged K7 mice (>6 months), where the thymic iNKT cell output was significantly reduced (Figure S10C). In aged K7 mice, peripheral iNKT cell numbers were still dramatically decreased (Figure S10C), demonstrating that IL-7 availability governs the homeostasis of peripheral iNKT cells independently of thymic output or aging.
Lastly, we considered it important to demonstrate a cell-intrinsic requirement for IL-7 signaling in iNKT cell survival. To this end, we utilized IL-7Rα floxed mice where IL-7 signaling can be conditionally terminated by IL-7Rα deletion using Cre recombinase expression (McCaughtry et al., 2012). For the selective IL-7Rα deletion in iNKT cells, we employed T-bet-Cre-expressing mice (Haddad et al., 2013), because we found T-bet being exclusively expressed in iNKT cells and not in other thymocytes (Figure 3E). Consequently, the T-bet-Cre-driven deletion of IL-7Rα in Il7rafl/flT-betCre mice was specific to iNKT cells, permitting the testing of iNKT cell-intrinsic roles of IL-7 signaling (Figure 3F). In fact, over 90% of all thymic iNKT cells had deleted IL-7Rα in Il7rafl/flT-betCre mice, indicating that IL-7Rα expression is mostly dispensable for the generation of iNKT cells (Figures 3F and 3G). On the other hand, the frequency of IL-7Rα-negative iNKT cells dramatically decreased in peripheral tissues where IL-7 signaling is required for their survival (Figure 3G). Thus, IL-7R signaling is clearly a survival requirement for peripheral iNKT cells so that iNKT cells that have escaped IL-7Rα deletion prevailed and populated the peripheral iNKT compartment of Il7rawt/wtT-betCre mice. Altogether, these results reaffirm a cell-intrinsic requirement for IL-7 in the homeostatic maintenance of iNKT cells.
STAT5 activation is required for iNKT cell survival
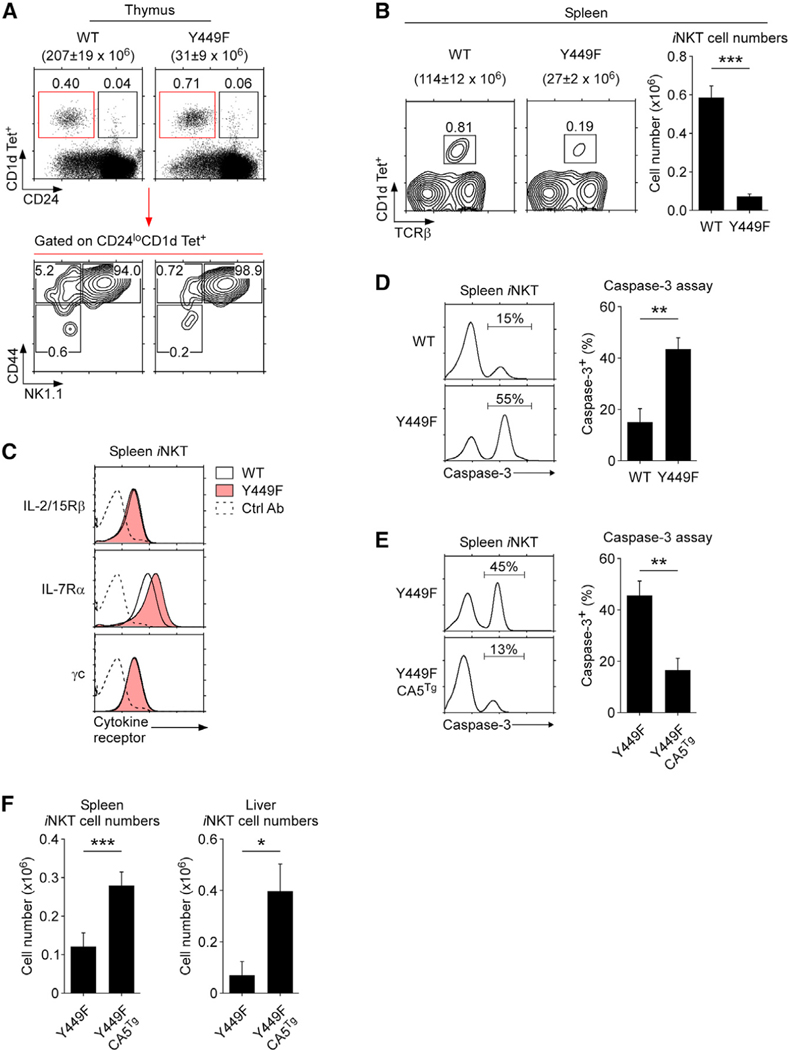
To understand the molecular basis of IL-7 requirement in iNKT cells, we next examined the effects of altering IL-7 receptor signaling in iNKT cells. IL-7 signaling triggers tyrosine phosphorylation at residue 449 (Y449) of the IL-7Rα cytoplasmic tail (Foxwell et al., 1995). Engineering Tyr449 to Phe449 (Y449F) disables IL-7-induced STAT5 recruitment and phosphorylation (Venkitaraman and Cowling, 1994) so that IL-7Rα Y449F knockin mice are impaired in IL-7-induced STAT5 phosphorylation (Osborne et al., 2007). While the lack of IL-7-induced STAT5 phosphorylation did not reduce the frequency or affect the differentiation of thymic iNKT cells (Figure 4A), both the frequencies and numbers of peripheral spleen iNKT cells were markedly diminished (Figure 4B). These results indicate that IL-7-induced STAT5 phosphorylation is dispensable for the generation of thymic iNKT cells but critical for the homeostasis of peripheral iNKT cells. Notably, the loss of peripheral iNKT cells was not due to impaired cytokine receptor expression because IL-2/15Rβ and γc expression levels were identical, and IL-7Rα expression was even higher on Y449F iNKT cells than on WT iNKT cells (Figure 4C).
Figure 4. iNKT cell survival requires STAT5 activation downstream of IL-7 receptor signaling.
(A) Thymic iNKT cell development in Y449F mice. Immature (stage 0; CD24hi) and mature (stage 1–3; CD24lo) iNKT cells were identified by CD24 versus CD1d tetramer staining in WT and Y449F thymocytes (top). Stage 1–3 iNKT cells were further determined on gated CD24loCD1d Tet+ thymocytes (bottom).
(B) Splenic iNKT cells in WT and Y449F mice (left). Bar graph shows splenic iNKT cell numbers from six WT and nine Y449F mice (right).
(C) Surface cytokine receptor expression on WT and Y449F splenic iNKT cells. Data are representative of three independent experiments with one mouse each.
(D) Active caspase-3 detection in freshly isolated WT and Y449F splenic iNKT cells (left). Bar graph shows the summary of three independent experiments (right).
(E) Active caspase-3 was assessed in freshly isolated Y449F and Y449F.CA5Tg splenic iNKT cells (left). Bar graphs show the summaries of three independent experiments (right).
(F) Numbers of iNKT cells in peripheral tissues of Y449F.CA5Tg mice. iNKT cells were identified by TCRβ versus CD1d tetramer staining of whole splenocytes and CD45+ liver mononuclear cells. iNKT cell numbers are shown as the mean ± SEM from eight Y449F and four Y449F.CA5Tg mice.
To directly show that IL-7-induced pSTAT5 is required for iNKT cell survival, we next examined if peripheral Y449F iNKT cells are apoptotic. Caspase-3 activity is a prominent marker for programmed cell death (Lakhani et al., 2006). Spleen Y449F iNKT cells were indeed highly active for caspase-3, indicating that iNKT cells undergo massive apoptosis in the absence of pSTAT5 (Figure 4D). Lastly, to directly demonstrate that it is the lack of IL-7-induced pSTAT5 that led to iNKT cell death, we introduced a constitutively active STAT5b transgene (CA5Tg) into Y449F mice (Burchill et al., 2003). As expected, constitutively active STAT5 was sufficient to restore iNKT cell survival (Figure 4E) and increase the number of peripheral iNKT cells, as documented in the spleen and liver of Y449F-CA5Tg mice (Figure 4F). Altogether, these data demonstrate that iNKT cells require IL-7 receptor signaling to induce pSTAT5, which is necessary to prevent apoptosis.
Increased availability of IL-7 expands the homeostatic space for peripheral iNKT cells
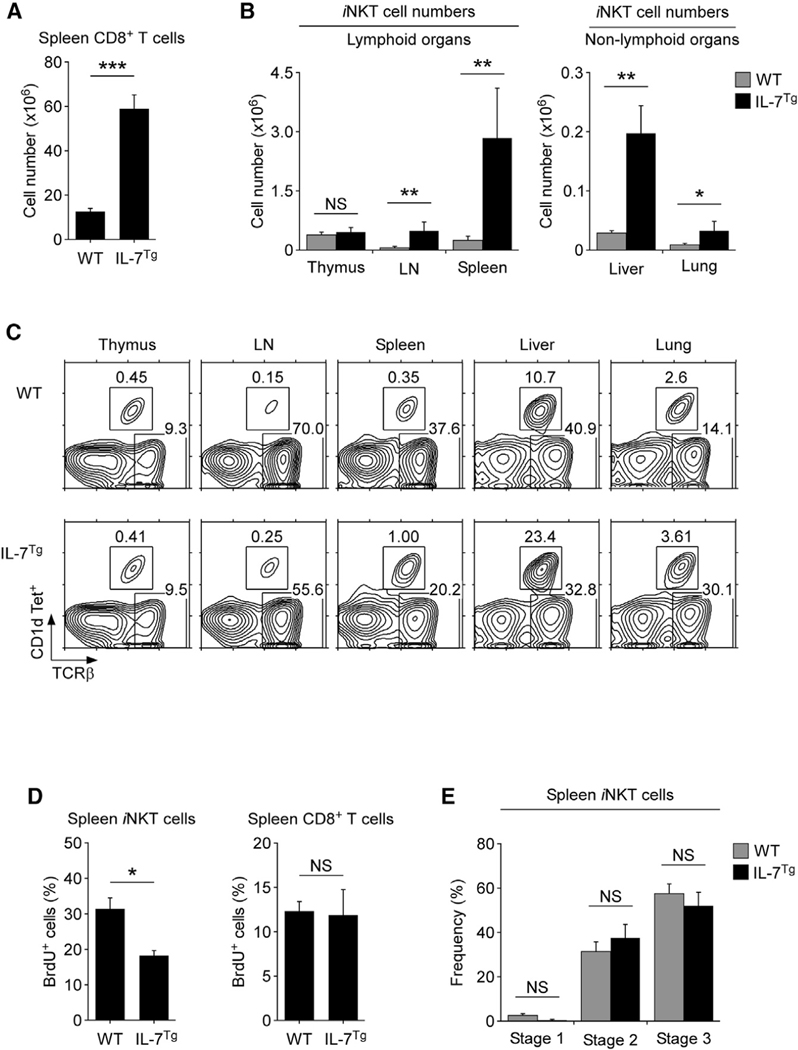
To further corroborate that IL-7 controls the homeostasis of iNKT cells, we asked if increased IL-7 availability would increase the number of peripheral iNKT cells. To this end, we employed genetically engineered mice that express MHC-II promoter-driven mouse Il7 cDNA transgenes (IL-7Tg). The MHC-II promoter is mostly active in peripheral tissue so that the IL-7 abundance is profoundly increased in the periphery but not in the thymus (Mertsching et al., 1995). Consequently, any increase in peripheral iNKT or T cell numbers is due to increased survival and not increased thymic output. As previously reported (Mertsching et al., 1995), peripheral T cell numbers were dramatically increased in IL-7Tg mice, as illustrated by the highly elevated numbers of splenic CD8+ T cells (Figure 5A). However, the numbers of NK cells, which depend on IL-15 and not on IL-7, remained mostly unaltered (Figure S11A) (Cooper et al., 2002; Prlic et al., 2003). On the other hand, IL-7 overexpression induced a dramatic increase in the frequency and number of peripheral iNKT cells (Figures 5B and 5C), concomitant with an increase in conventional αβ T cell numbers (Figure S11B). Importantly, the iNKT cell numbers in IL-7Tg mice were increased without inducing cell proliferation, as documented in bromodeoxyuridine (BrdU) incorporation assays where BrdU was administered to WT and IL-7Tg mice in their drinking water for 6 days (Figure 5D). These results support a role for IL-7 in the survival rather than proliferation of iNKT cells. Along these lines, the iNKT stage distribution in IL-7Tg mice remained comparable with that of WT mice (Figures 5E and S11B), indicating that IL-7 is of equal importance to iNKT cells of all stages. Lastly, because thymic iNKT cell numbers are not increased in IL-7Tg mice, these results permitted us to effectively exclude a scenario where increased thymic iNKT cell output would be responsible for increased iNKT cell numbers in the periphery. Therefore, these data fully align with our notion that IL-7 availability controls and constrains the size of the peripheral iNKT cell pool.
Figure 5. IL-7 overexpression expands size of the peripheral iNKT cell pool.
(A) IL-7 overexpression results in increased CD8 T cell numbers in the spleen. Bar graph shows the summary of seven WT and five IL-7Tg mice (mean ± SEM).
(B) iNKT cell numbers in lymphoid and non-lymphoid tissues of IL-7Tg mice. Bar graphs show mean ± SEM from eight WT and six IL-7Tg for lymphoid tissues and three WT and three IL-7Tg mice for non-lymphoid tissues.
(C) iNKT cell frequencies in various tissues of IL-7Tg mice. Data are representative of two independent experiments.
(D) BrdU labeling of iNKT cells and CD8 T cells in the spleen of WT and IL-7Tg mice. BrdU incorporation was assessed after 6 days of BrdU treatment in drinking water. Bar graph shows the summary of three independent experiments with total six WT and four IL-7Tg mice.
(E) iNKT cell stages in IL-7Tg mice. Bar graph shows the frequency of each stages among spleen iNKT cells in IL-7Tg and WT mice. Results show mean ± SEM from 12 WT and 10 IL-7Tg mice.
Increased IL-15 availability does not increase peripheral iNKT cell numbers
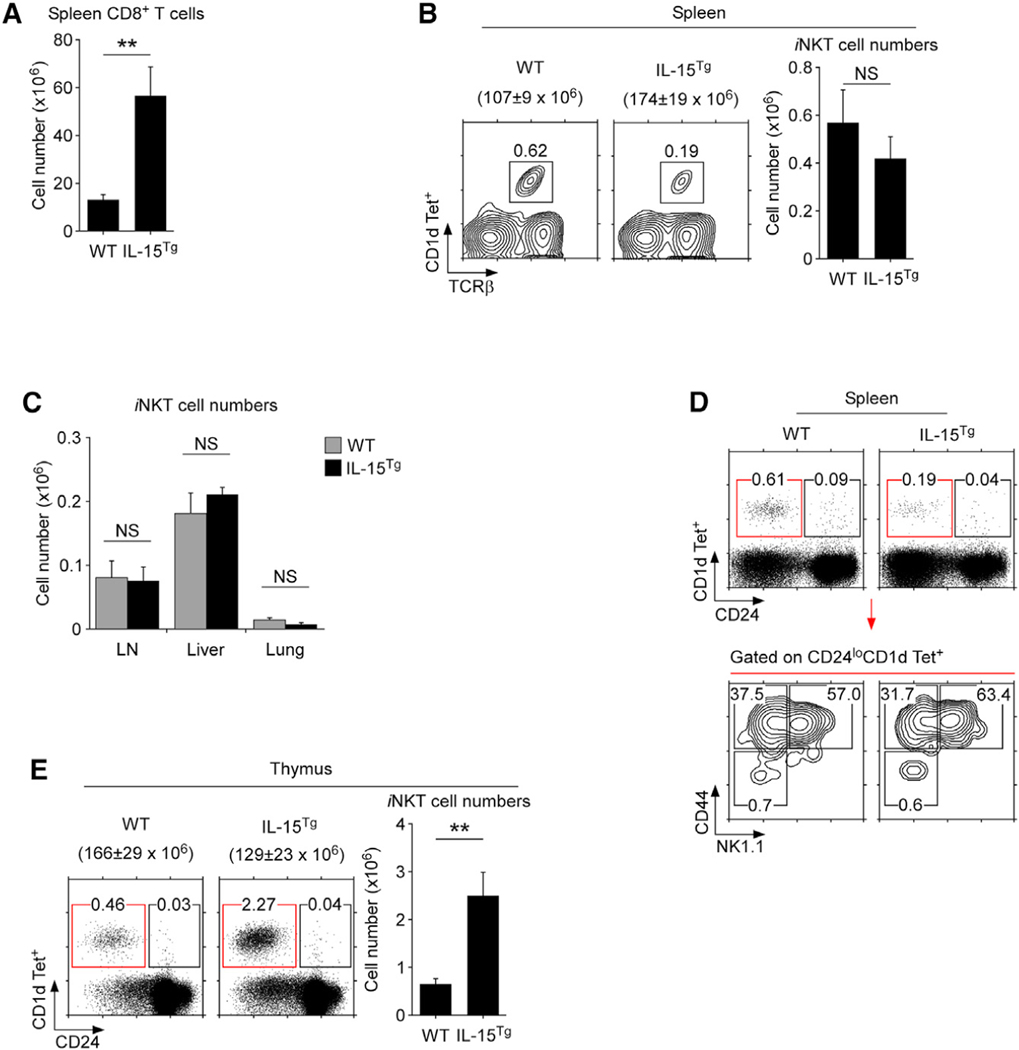
IL-15 is a homeostatic cytokine that partly overlaps with IL-7 in providing cell survival for αβ T cells (Ku et al., 2000; Park et al., 2019b; Tan et al., 2002). Accordingly, systemic overexpression of IL-15, such as in IL-15Tg mice, resulted in dramatically increased numbers of peripheral CD8 T cells (Figure 6A), and in their acquisition of a memory phenotype as previously reported (Marks-Konczalik et al., 2000) and also reaffirmed in this study (Figure S12). Surprisingly, however, the increased availability of IL-15 did not increase peripheral iNKT cell numbers (Figures 6B and 6C). The increased level of IL-15 also did not alter the stages of the peripheral iNKT cell pool (Figure 6D). These results indicated that peripheral iNKT cells are refractory to increased IL-15 availability in vivo.
Figure 6. Peripheral iNKT cell numbers remain unaltered in IL-15Tg mice.
(A) IL-15 overexpression increases the number of CD8 T cells. Bar graph shows the summary of splenic CD8 T cell numbers from seven WT and five IL-15Tg mice (mean ± SEM).
(B) iNKT cells in the spleens of WT and IL-15Tg mice
(left). Bar graph shows iNKT cell numbers (mean ± SEM) from eight WT and 10 IL-15Tg mice from six independent experiments (right).
(C) iNKT cell numbers in the indicated tissues of IL-15Tg mice. Bar graph shows iNKT cell numbers (mean ± SEM) from six WT and four IL-15Tg mice.
(D) iNKT cell differentiation in the spleens of WT and IL-15Tg mice. Immature and mature iNKT cells were identified by CD24 versus CD1d tetramer staining in splenocytes of WT and IL-15Tg mice. iNKT stage 1–3 were determined on gated mature (CD24loCD1d Tet+) splenocytes (bottom). Data are representative of five independent experiments.
(E) Thymic iNKT cell development in IL-15Tg mice. Dot plots show CD24 versus CD1d tetramer staining of total thymocytes in WT and IL-15Tg mice (left). Bar graph shows thymic iNKT cell numbers (mean ± SEM) from six independent experiments with a total of six WT and eight IL-15Tg mice (right).
The IL-15 transgene is driven by the human EF-1α promoter and is consequently ubiquitously expressed in all tissues, including the thymus (Marks-Konczalik et al., 2000). In marked contrast to the periphery, however, the increase in IL-15 expression dramatically boosted both the frequency and number of thymic iNKT cells (Figure 6E). These results affirm the importance of IL-15 for iNKT cell development in the thymus and show that IL-15 availability controls the thymic iNKT cell output. Collectively, these data document disparate roles of IL-15 in iNKT cell biology where IL-15 drives the thymic development of iNKT cells but is dispensable for the maintenance of the peripheral iNKT cell pool.
The availability of IL-7, not IL-15, constrains the size of the peripheral iNKT cell pool
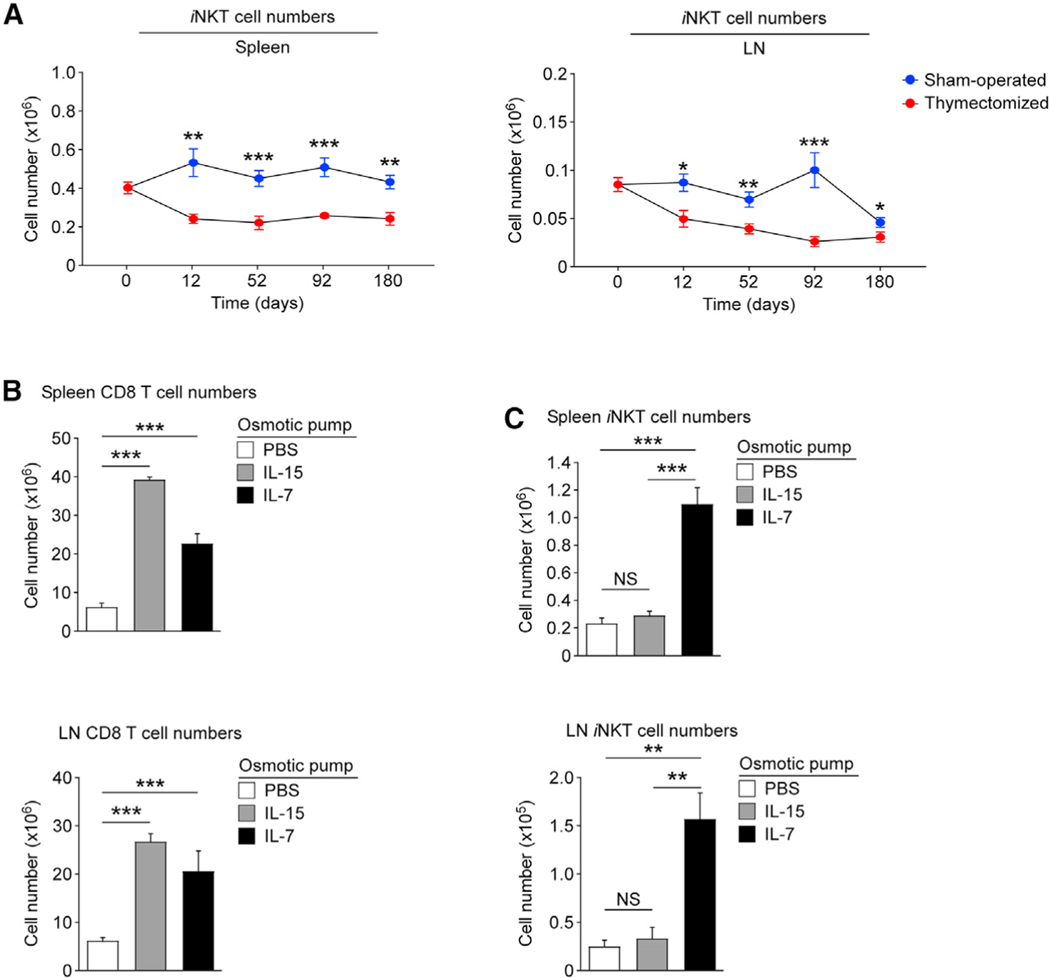
A major feature of the homeostatic mechanism is its capacity to expand the size of the existing T cell pool. A bona fide homeostatic cytokine would increase the number of iNKT cells in the absence of an influx of new iNKT cells from the thymus, and we aimed to demonstrate this for IL-7. First, we hypothesized that the size of the peripheral iNKT cell pool would rapidly shrink without the thymic contribution of new iNKT cells. However, this has not been tested, and it has been unclear to what extent iNKT cell numbers can be maintained by purely peripheral homeostatic mechanisms. To establish these parameters, we utilized WT B6 mice that were adult thymectomized at 4 weeks of age, and we monitored the number of peripheral iNKT cells with time. Compared with sham-operated mice, iNKT cell numbers in thymectomized animals rapidly declined to approximately half the numbers in the spleen and less than a third in the LN between 2 and 3 months (Figure 7A). Curiously, liver iNKT cells in thymectomized mice did not show any statistically significant loss in their numbers compared with sham-operated animals (Figure S13A).
Figure 7. Increased IL-7 availability expands the size of the peripheral iNKT cell pool.
(A) iNKT cell numbers were assessed in the spleens (left) and LNs (right) of adult thymectomized mice at the indicated days after surgery. Each data point shows the mean ± SEM of iNKT cell numbers for either sham-operated or thymectomized mice. For sham-operated mice: day 12, eight mice; day 52, 14 mice; day 92, eight mice; day 180, 10 mice. For thymectomized mice: day 0, 11 mice; day 12, eight mice; day 52, 16 mice; day 92, 12 mice; day 180, 10 mice. Mice were analyzed in three independent experiments.
(B) Splenic (top) and LN (bottom) CD8 T cell numbers in sham-operated or adult thymectomized mice where recombinant IL-15 or IL-7 was infused for 2 weeks using Alzet osmotic pumps. Bar graph shows the summary of four independent experiments with seven IL-15 pump-installed mice, seven IL-7 pump-installed mice, and five PBS control pump-installed mice.
(C) Splenic (top) and LN (bottom) iNKT cell numbers in sham-operated or adult thymectomized mice where recombinant IL-15 or IL-7 was infused for 2 weeks using Alzet osmotic pumps. Bar graph shows the summary of four independent experiments with seven IL-15 pump-installed mice, seven IL-7 pump-installed mice, and five PBS control pump-installed mice.
Thus, the liver iNKT cell pool is maintained independently of the thymic output, presumably because they comprise liver-resident non-circulating iNKT cells. For iNKT cells in other organs, however, there was a swift (12 days) loss of iNKT cells upon thymectomy, and iNKT cell numbers then stabilized at approximately 7 weeks (52 days post thymectomy; Figures 7A and S13B). These stabilized iNKT numbers were maintained at relatively constant levels for a prolonged time, up to 180 days post thymectomy (Figure 7A). Altogether, these data document that homeostatic mechanisms control the size of the peripheral iNKT cell pool.
Without new influx of thymus-derived iNKT cells, the number of peripheral iNKT cells stabilized at approximately 7 weeks after thymectomy. If the availability of the homeostatic cytokine is the constraining factor of iNKT cell numbers, we hypothesized that increasing the availability of the homeostatic cytokine would expand the size of the peripheral iNKT cell pool. To this end, we implanted cytokine-releasing osmotic pumps into thymectomized mice at day 52 post thymectomy and assessed the numbers of iNKT cells after 2 weeks. The osmotic pumps were designed to release either recombinant IL-15 or recombinant IL-7 at a constant rate over the 2-week period. The bioactivity of IL-15 and IL-7 was confirmed by the dramatically increased numbers of CD8 T cells in the spleen and LN of thymectomized mice that were implanted with the corresponding pumps (Figure 7B). Thus, conventional CD8 T cells are responsive to both IL-7 and IL-15, and these findings agree with previous reports that the survival and homeostasis of CD8 T cells are controlled by IL-7 and IL-15 (Schluns and Lefrancois, 2003; Tan et al., 2002). In marked contrast, iNKT cell numbers only increased in IL-7 and not in IL-15 pump-implanted mice (Figures 7C, S13C, and S13D). Thus, peripheral iNKT cells are refractory to the increased availability of IL-15, strongly opposing a role for IL-15 in constraining the homeostasis of iNKT cells. Instead, these results support a model where the peripheral iNKT cell pool is maintained by a homeostatic cytokine that is not IL-15, and the results from the IL-7 pump installation (Figure 7C) argue for a critical role of IL-7 in this process.
DISCUSSION
Unlike conventional αβ T cells that require IL-7 (Rochman et al., 2009), iNKT cells are considered to require IL-15 for their survival and homeostasis. Under this scenario, iNKT cells do not compete with other αβ T cells for IL-7, which is scarcely expressed in vivo and is only available in limited amounts (Hara et al., 2012; Kim et al., 2012; Park et al., 2004). Thus, the homeostasis of iNKT cells would be controlled independently of conventional αβ T cells. In marked contrast to this notion, here, we show that IL-7 is a non-redundant requirement for peripheral iNKT cells and that the availability of IL-7, not IL-15, limits the homeostasis of iNKT cells. In fact, we uncovered a dual cytokine requirement for iNKT cells, where IL-15 is necessary for the thymic development and IL-7 is necessary for the survival of iNKT cells. These data demonstrate that iNKT cells and conventional αβ T cells are subject to the same cytokine constraint for peripheral homeostasis, indicating a common mechanism of cytokine utilization for the survival of iNKT and conventional αβ T cells.
While IL-7 is a critical factor for T cells, IL-7 is not produced by T cells or by other lymphocytes. Instead, IL-7 is expressed by non-lymphoid stromal cells and at low amounts, so that IL-7 availability is limited in vivo, and αβ T cells are forced to compete for IL-7 (Hara et al., 2012; Kim et al., 2012; Park et al., 2004). As a corollary, IL-7 availability limits the size of the αβ T cell pool in peripheral tissues (Park et al., 2004). Notably, iNKT cells were considered as exempt from this constraint because they supposedly require IL-15, not IL-7, for their survival. The current study challenges this idea because we made a string of experimental observations that were inconsistent with IL-15 being the homeostatic cytokine for iNKT cells. Instead, our data indicated that the in vivo availability of IL-7 controls the homeostasis and determines the size of the peripheral iNKT cell pool. As such, increased IL-7 availability in IL-7Tg mice dramatically increased the peripheral iNKT cell numbers, but such an increase would not have been predicted if only IL-15 would control the size of the peripheral iNKT cell pool. Moreover, we found that a ubiquitously expressed IL-15 transgene increased iNKT cell numbers only in the thymus but not in the periphery, even though IL-15 was highly abundant in peripheral tissues. We know that the IL-15 transgene is expressed in the periphery because IL-15Tg mice express elevated levels of serum IL-15 and contain dramatically increased numbers of CD8 T cells in the spleen and LNs (Marks-Konczalik et al., 2000). Thus, increased IL-15 expression expands the size of the CD8 T cell pool, but it fails to increase peripheral iNKT cell numbers. These results suggest that iNKT cell survival is either independent of IL-15 or that increased IL-15 expression alone is insufficient to promote iNKT survival. In the latter case, pro-survival factors such as tonic TCR signals could be potentially limiting and constrain the size of the iNKT cell pool. However, previous studies have suggested that iNKT cell survival is likely independent of TCR engagement (McNab et al., 2005). TCR ablation in peripheral iNKT cells further demonstrated that neither the survival nor the effector function of iNKT cells depend on tonic TCR signaling (Vahl et al., 2013). In addition, the overexpression of IL-7 dramatically increased the numbers of iNKT cells without necessitating alteration in TCR signaling. Collectively, these results agree with our proposition that iNKT cell survival is independent of IL-15.
In agreement with peripheral iNKT cell numbers being unaffected by the increased abundance of IL-15, we also did not find any significant changes in peripheral iNKT cell numbers by IL-15 deficiency. iNKT cell numbers remained unaltered in most peripheral tissues of Il15−/− mice, while the numbers of CD8 memory T cells and NK cells were dramatically reduced. These results indicated that most peripheral iNKT cells are refractory to the absence of IL-15, further strengthening our model where iNKT cell homeostasis operates independently of IL-15. Curiously, liver iNKT cells presented an exception to this concept because their frequency and number were substantially reduced in Il15−/− mice. It is currently unclear to us why liver iNKT cells would be uniquely sensitive to peripheral IL-15. In fact, it was previously found that liver iNKT cells heavily rely on hepatic IL-7 for their survival (Liang et al., 2012). Thus, IL-15 and IL-7 appear to play non-redundant roles in liver iNKT cells homeostasis whereby we consider the possibility that IL-7 provides survival, but IL-15 could be critical for the recruitment or preservation of iNKT cells in hepatic tissues. Along these lines, liver iNKT cells are distinct from iNKT cells in other peripheral organs, as they depend on LFA-1-expressing liver-resident NK cells for their tissue retention (Emoto et al., 1999; Miyamoto et al., 2000). Thus, the number of iNKT cells in the liver is controlled not only by cell survival but also by cell adhesion and tissue residency. Because Il15−/− mice lack NK cells, such a scenario presents a plausible explanation for the selective paucity of iNKT cells in the liver, while total numbers of peripheral iNKT cells in Il15−/− mice can remain unchanged. Here, we also wish to point out that iNKT cells comprise a large fraction of lymphocytes in the liver but that the actual number of liver iNKT cells is diminutive (~200,000) compared with the number in the spleen (~800,000) or relative to the combined numbers of iNKT cells in all peripheral tissues (>1 million) (Godfrey et al., 2010; Gordy et al., 2011; Matsuda et al., 2002; Watarai et al., 2012). Consequently, interpreting the role of IL-15 in iNKT cells solely based on the frequency of iNKT cells and primarily relying on liver iNKT cells might result in obtaining a distorted and incomplete picture of peripheral iNKT cell homeostasis.
We acknowledge that our finding of IL-15-independent homeostasis of iNKT cells is in disagreement with studies by other groups that reported significantly reduced peripheral iNKT cell numbers of IL-15-deficient mice (Gordy et al., 2011; Matsuda et al., 2002). As a potential resolution, we wish to point out that our conclusion is based on the analysis of a large collection of peripheral organs that include LN, spleen, lung, bone marrow, and liver. These collected data points provided us with a more comprehensive picture of peripheral iNKT cell numbers than assessing iNKT cell numbers in a few selected organs. Also, we cannot exclude the possibility that environmental factors, including differences in the microbiota, could have contributed to the divergent findings of our study compared with previous reports. In fact, both the conventional gut microflora and an artificially restricted microflora can alter the generation and maintenance of iNKT cells (Wei et al., 2010), so differences in the housing conditions could account for dissimilar iNKT cell numbers between different studies.
Lastly, it remains unclear why IL-15 would not play a major role in iNKT cell maintenance but is important for iNKT cell generation in the thymus. In the same vein, it is unclear why IL-15 cannot be replaced by IL-7 to drive iNKT cell development in the thymus. IL-7 and IL-15 share the γc signaling pathway, and both cytokines induce phosphorylation of STAT5 and Akt to upregulate expression of anti-apoptotic Bcl-2 (Rochman et al., 2009). iNKT cells express high levels of IL-7Rα, and the intrathymic distributions of IL-7- and IL-15-producing thymic epithelial cells are similar and overlapping (Alves et al., 2009), so iNKT cells can bind to and encounter both IL-15 and IL-7 in the thymus (Colpitts et al., 2013; Cui et al., 2014; Sosinowski et al., 2013). However, it is IL-15 that is the major driver of thymic iNKT cell development. As a potential explanation, we postulated that only IL-15 would induce expression of iNKT cell lineage-specifying factors. IL-15 induces expression of the T-box transcription factor T-bet, which is required for iNKT cell maturation from stage 2 CD44hiNK1.1– cells into stage 3 CD44hiNK1.1+ cells (Gordy et al., 2011). Thus, IL-15 might play a non-redundant role in iNKT cell generation because it is required for upregulating the expression of T-bet.
We also acknowledge that an IL-7 requirement for iNKT cell survival is not entirely compatible with earlier reports, which proposed that IL-15, and not IL-7, is necessary for peripheral iNKT cells (Matsuda et al., 2002; Ranson et al., 2003a). How do we reconcile these differences? First, we used in vivo models of IL-7 deficiency or IL-7 overexpression that are limited to peripheral tissues to assess the IL-7 effect on peripheral iNKT cells, whereas other studies have relied on IL-7 germline deficiency or adoptive transfer models to address this question. Adoptive transfer experiments usually assess short term proliferation— and not survival—of adoptively transferred iNKT cells as an indicator for homeostasis. Importantly, homeostatic proliferation is distinct from steady-state homeostasis (Min et al., 2005; Surh and Sprent, 2008), and the effects of cytokine deficiencies on cell proliferation may not be applied to understand their effects on cell survival. Moreover, chronic lymphopenia induces profound changes in the commensal flora, which can lead to antigen-driven rapid proliferation of adoptively transferred T cells that is distinct from IL-7-driven slow homeostatic proliferation (Cho et al., 2007; Do et al., 2012; Goldrath and Bevan, 1999). Along these lines, it is interesting that adoptive transfers of Vα14+ iNKT cells into chronic lymphopenic host mice (Rag2−/−Il2rg−/−) resulted in massive proliferation of donor iNKT cells (>90% proliferation in 5 days) (Ranson et al., 2003a), whereas injection of iNKT cells into acute lymphopenic host mice (i.e., sublethally irradiated B6 mice) resulted in poor proliferation with only two or three cell divisions in 7 days (Matsuda et al., 2002). These data suggest that the host immune status can influence proliferation of adoptively transferred iNKT cells and that proliferation of adoptively transferred iNKT cells does not necessarily mirror survival and homeostasis of iNKT cells in vivo. In this regard, we think that steady-state iNKT cell numbers in peripheral IL-7-deficient K7 mice are a more reliable indicator for an IL-7 requirement because adoptive transfer and proliferation of donor cells are not required, thus directly demonstrating an IL-7 requirement for peripheral iNKT cell homeostasis.
Finally, an IL-7 requirement for iNKT cell survival is concordant with the study by Sprent and colleagues, who reported that a subset of IL-17-producing iNKT cells, i.e., NKT17 cells, depends exclusively on IL-7 for survival (Webster et al., 2014). IL-17-producing iNKT cells were identified and referred to as iNKT17 cells in the original study by Leite-de-Moraes and colleagues (Michel et al., 2007) and were then further characterized as mostly CD4–NK1.1-negative iNKT cells by Godfrey and colleagues (Coquet et al., 2008). NKT17 cells are enriched in barrier tissues such as lung, skin, and peripheral LN (Doisne et al., 2009), but their ontogeny and survival requirements remain unmapped. Because we also found NKT17 cell numbers being limited by the availability of IL-7, our data align with the proposition that NKT17 cells are maintained by IL-7 (McNab et al., 2007; Webster et al., 2014). However, our results are disparate from the Sprent study, as we found IL-7 to be critical for not only NKT17 cells but also the survival of other NK T cell subsets. Thus, the current study confirms, but also expands, the role of IL-7 in iNKT cells, and it indicates a role for IL-7 in setting the size of the peripheral iNKT cell pool.
Limitations of the study
While we have demonstrated that the peripheral iNKT cell pool is controlled by IL-7, and not by IL-15, it is evident that the generation of iNKT cells in the thymus primarily depends on IL-15. The molecular basis of such a dichotomy in cytokine requirement between thymic and peripheral iNKT cells remains unclear. Therefore, our study is limited in its scope as it has not fully addressed these issues. Nonetheless, we consider the possibility that mature iNKT cells in the thymus could be tissue resident, and thus have different cytokine requirements compared with circulating peripheral iNKT cells. Tissue adaptation of peripheral iNKT cells could represent a potential mechanism, but such issues remain to be experimentally addressed and resolved.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed and will be fulfilled by the lead contact, Jung-Hyun Park (parkhy@mail.nih.gov).
Materials availability
All reagents generated in this study are available upon request with a completed material transfer agreement.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
C57BL/6NCrl (B6) and CD45.1 congenic B6 mice were obtained from the Frederick Cancer Research and Development Center, Frederick, MD. IL-7-transgenic (IL-7Tg) mice, expressing a murine Il7 cDNA under the control of the mouse H2-Ea promoter (Mertsching et al., 1995), were purchased from the Jackson Laboratories. Adult (4 weeks) thymectomized C57BL/6 male mice were purchased from the Jackson Laboratories. γc-deficient (Il2rg−/−), IL-7-deficient (Il7−/−), IL-15-deficient (Il15−/−), IL-15Rα-deficient (Il15ra–/–), IL-7Rα Y449 knock-in (Y449F) and IL-15 transgenic (IL-15Tg) were previously described, (DiSanto et al., 1995; Kennedy et al., 2000; Lodolce et al., 1998; Marks-Konczalik et al., 2000; Osborne et al., 2007; von Freeden-Jeffry et al., 1995), and all strains were maintained on C57BL/6NCrl background. Peripheral IL-7 deficient mice (K7) were generated by introducing an lck-proximal promoter driven IL-7 transgene onto an Il7−/− background, as previously reported (Kim et al., 2012). Constitutively active STAT5b transgene (CA5Tg) mice were previously described and kindly provided by Dr. Michael Farrar (Burchill et al., 2003). IL-7Rα floxed mice (Il7rafl/fl) (McCaughtry et al., 2012) was kindly provided by Dr. Al Singer (NCI), and T-bet-Cre (Tbx21Cre) mice (Haddad et al., 2013) were obtained from the Jackson Laboratories. Il7rafl/fl and Tbx21Cre mice were intercrossed in-house to generate Il7rafl/flTbx21Cre mice. T-bet-ZsGreen reporter (TBGRTg) mice were a kind gift of Dr. Jinfang Zhu (NIAID) (Zhu et al., 2012). Animal experiments were performed with 6- to 12-week-old mice of both sexes and age-matched control mice, unless indicated otherwise. All animal experiments were approved by the NCI Animal Care and Use Committee, and all mice were cared for in accordance with NIH guidelines.
METHODS DETAILS
Flow cytometry
Cells were harvested and stained from the thymus and other organs. Data were acquired on LSR Fortessa or LSRII flow cytometers (BD Biosciences) and analyzed using software designed by the Division of Computer Research and Technology, NCI. Live cells were gated using forward scatter exclusion of dead cells stained with propidium iodide. The following antibodies were used for staining: TCRβ (H57–597), CD24 (30-F1), IL-7Rα (A7R34), NK1.1 (PK136), IL-2Rβ (TM-β1), IL-4Rα (M1), RORγt (Q31–378), T-bet (4B10), PLZF (9E12), and isotype control antibodies, all from eBioscience; CD44 (IM7), γc (4G3), CD4 (GK1.5 and RM4.5), and CD8α (53–6-7) from BD Biosciences; IL-21R (4A9) and CD45 (30-F11) from BioLegend. CD1d tetramers loaded with PBS-57 and unloaded controls were obtained from the NIH tetramer facility (Emory University, Atlanta, GA). Active caspase-3 was determined using the CaspGLOW™ fluorescein active caspase-3 staining kit (eBioscience).
Lymphocyte isolation from non-lymphoid organs
Liver and lung mononuclear cells (MNCs) were prepared as previously described with minor modifications (Watarai et al., 2008; Zhang et al., 2005). In brief, liver tissues were pressed through a 70-μm cell strainer (BD Biosciences) and resuspended in PBS. Cell suspensions were centrifuged at 100g for 3 min, and supernatants were collected, spun down and washed again with cold PBS. Lungs were harvested after PBS perfusion, diced into pieces, and digested with collagenase IV (1 mg/ml in PBS, Life Technology) for 45 min at 37°C. Liver and lung samples underwent enrichment for lymphocytes by centrifugation in a two-step Percoll gradient (GE Life Sciences). Lymphocytes at the interphase were harvested, washed, and resuspended in cell culture media before further analysis. MNCs in fat tissues were isolated as previously described (Lynch et al., 2015). In brief, visceral fat was harvested and digested for 25 min at 37 °C with 20 ml of collagenase IV solution (1 mg/ml). After digestion, MNCs were isolated by filtration through a 40-μm nylon mesh (Fisher Scientific) and centrifugation for 5 min at 300g. All MNCs from non-lymphoid organs were identified by CD45 expression.
iNKT subset staining
In brief, iNKT cells were stained with PBS-57-loaded mouse CD1d tetramers followed by staining for other surface markers, as previously described (Park et al., 2019a). Specifically, for each analysis, 10 million thymocytes were stained with fluorochrome-conjugated CD1d tetramers in FACS buffer (0.5% BSA, 0.1% sodium azide in Ca2+- and Mg2+-free HBSS) for 20 min at 4°C. Without removing the tetramer reagents, antibodies against surface proteins were then added, and cells were incubated for an additional 30 min at 4°C. Excess reagents were then washed out with FACS buffer by centrifugation for 7 min at 1,500 rpm. Pelleted cells were then resuspended in 150 μl of 1:3 mixture of concentrate/diluent working solution of the Foxp3 Transcription Factor Staining Buffer kit (eBioscience Thermo Fisher) and 100 μl of FACS buffer and incubated at room temperature for 20 minutes. Cells were then washed twice with 1x permeabilization buffer (eBioscience Thermo Fisher), before adding antibodies for transcription factors, such as PLZF, RORγt, and T-bet. Cells were incubated at room temperature for 1 hour, before washing out excess reagents with FACS buffer followed by flow cytometry analysis.
BrdU labeling
Mice were given intraperitoneal injections of 1 mg BrdU (5-bromo-2’-deoxyuridine) dissolved in PBS (10 mg/ml; Sigma-Aldrich). Injected mice were then kept for 6 days with 1 mg/ml BrdU in their drinking water before analysis. Cell staining was performed using a BrdU flow kit according to the manufacturer’s instructions (BD Biosciences).
Quantitative real-time PCR
iNKT cells were electronically sorted from WT, Il7−/−, and K7 spleen T cells. RNA was isolated using the RNEasy kit (Qiagen) and then reverse transcribed into cDNA with the QuantiTect Reverse Transcription kit (Qiagen). QuantiTect SYBR Green (Qiagen) and an ABI PRISM 7900HT Sequence Detection System was used for quantitative RT-PCR analysis. The following primers were used for detection: Il7 (Forward: 5’-CTGATGATCAGCATCGATGAATTGG-3’; Reverse: 5’-GCAGCACGATTTAGAAAAGCAGCT T-3’), Bcl2 (Forward: 5’-GGATAACGGAGGCTGGGATGCCT-3’; Reverse: 5’-CAGAGTGATGCAGGCCCCGAC-3’), actinb (Forward: 5’-GAGAGGG AAATCGTGCGTGA-3’; Reverse: 5’-ACATCTGCTGGAAGGTGG-3’), Hprt (Forward: 5’-TCATTATGCCGAGGATTTGGA-3’; Reverse: 5’-CAGAGGGCCACAATGTGATG-3’). Gene expression values were normalized to actinb or Hprt signals in the same sample.
Alzet osmotic pump installation
Recombinant mouse IL-7 or mouse IL-15 (R&D system) was administered into thymectomized mice using ALZET osmotic pumps (DURECT) following the manufacturer’s instruction. The pumps were each set to release 5 μg/ml of cytokines per 24 hours.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data are shown as the mean ± SEM. The two-tailed Mann-Whitney U test was used to calculate P-values, where *, p<0.05; **, p< 0.01; ***, p<0.001 were considered statistically significant. NS = not significant.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| FITC anti-TCRβ (H57–597) | eBioscience Thermo Fisher | Cat# 11–5961-82, RRID:AB_465322 |
| PE-Cy7 anti-TCRβ (H57–597) | eBioscience Thermo Fisher | Cat# 25–5961-82, RRID:AB_2573506 |
| PE-Cy7 anti-CD24 (30-F1) | eBioscience Thermo Fisher | Cat# 12–0242-82, RID:AB_10853806 |
| PE anti-IL-7Rα (A7R34) | eBioscience Thermo Fisher | Cat# 12–1271-82, RRID:AB_465844 |
| eFluor660 anti-IL-7Rα (A7R34) | eBioscience Thermo Fisher | Cat# 50–1271-82, RID:AB_11219081 |
| PE anti-NK1.1 (PK136) | eBioscience Thermo Fisher | Cat# 12–5941-82, RRID:AB_466050 |
| PE anti-IL-2Rα (CD25; PC61.5) | eBioscience Thermo Fisher | Cat# 12–0251-82, RRID:AB_465607 |
| PE anti-IL-2Rβ (CD122; TM-b1) | eBioscience Thermo Fisher | Cat# 12–1222-82, RRID:AB_465836 |
| eFluor 660 anti-T-bet (eBio4B10) | eBioscience Thermo Fisher | Cat# 50–5825-82, RID:AB_10596655 |
| PE anti-IL-4Rα (CD124; mIL4R-M1) | BD Biosciences | Cat# 552509, RRID:AB_394407 |
| APC anti-CD44 (IM7) | BD Biosciences | Cat# 559250, RRID:AB_398661 |
| PE anti-γc (CD132; 4G3) | BD Biosciences | Cat# 554457, RRID:AB_395404 |
| PE-Cy7 anti-CD4 (GK1.5) | BD Biosciences | Cat# 563933, RRID:AB_2738492 |
| PE-Cy7 anti-CD4 (RM4.5) | BD Biosciences | Cat# 558107, RRID:AB_397030 |
| Alexa Fluor 647 anti-CD8α (53–6-7) | BD Biosciences | Cat# 557682, RRID:AB_396792 |
| BV786 anti-RORγt (Q31–378) | BD Biosciences | Cat# 564723, RRID:AB_2738916 |
| PE anti-IL-21R (4A9) | BioLegend | Cat# 131906, RRID:AB_1279430 |
| Pacific Blue anti-CD45 (30-F11) | BioLegend | Cat# 103126, RRID:AB_493535 |
| PE anti-PLZF (9E12) | BioLegend | Cat# 145804, RRID:AB_2561973 |
| CD1d tetramers (PBS-57 loaded) | NIH tetramer facility | Emory University, Atlanta, Georgia |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| BrdU (5-bromo-2’-deoxyuridine) | Sigma-Aldrich | Cat# B5002 |
| QuantiTect SYBR® Green PCR Kits | Qiagen | Cat# 204145 |
| RNeasy Plus Micro kit | Qiagen | Cat# 74034 |
| QuantiTect reverse transcription kit | Qiagen | Cat# 205313 |
| Collagenase IV | Thermo Fisher | Cat# 17104019 |
| Percoll | GE Life Sciences | Cat# 17089101 |
| Recombinant mouse IL-7 | R&D system | Cat# 407-ML-025/CF |
| Recombinant mouse IL-15 | R&D system | Cat# 447-ML-010/CF |
|
| ||
| Critical commercial assays | ||
|
| ||
| eBioscience Fixation/Perm diluents | eBioscience Thermo Fisher | Cat# 00–5223-56 |
| eBioscience Fixation/Perm concentrate | eBioscience Thermo Fisher | Cat# 00–5213-43 |
| Permeabilization Buffer 10x | eBioscience Thermo Fisher | Cat# 00–8333-56 |
| CaspGLOW™ fluorescein active caspase-3 staining kit | eBioscience Thermo Fisher | Cat# 88–7004-42 |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| Mouse: C57BL/6 (C57BL/6NCrl) | Charles River Laboratories | Stock# 24107773, 24107757 |
| Mouse: γc-deficient (Il2rg−/−) | The Jackson Laboratory | (DiSanto et al., 1995) Stock #003174 |
| Mouse: IL-15-deficient (Il15−/−) | In house | (Kennedy et al., 2000) |
| Mouse: IL-15Rα-deficient (Il15ra−/−) | The Jackson Laboratory | (Lodolce et al., 1998) Stock #003723 |
| Mouse: IL-7-deficient (Il7−/−) | In house | (von Freeden-Jeffry et al., 1995) |
| Mouse: K7 | In house | (Kim et al., 2012) |
| Mouse: IL7Rα floxed (Il7Rafl/fl) | Provided by Dr. A. Singer | (McCaughtry et al., 2012) |
| Mouse: T-bet-ZsGreen reporter (TBGRTg) | Provided by Dr. J. Zhu | (Zhu et al., 2012) |
| Mouse: T-betcre | The Jackson Laboratory | (Haddad et al., 2013) Stock #024507 |
| Mouse: IL-7RαY449 knock-in (Y449F) | In house | (Osborne et al., 2007) |
| Mouse: Constitutively active STAT5b transgene (CA5Tg) |
Provided by Dr. M. Farrar | (Burchill et al., 2003) |
| Mouse: IL-7-transgenic (IL-7Tg) mice | The Jackson Laboratory | (Mertsching et al., 1995) Stock# 008334 |
| Mouse: IL-15 transgenic (IL-15Tg) | In house | (Marks-Konczalik et al., 2000) |
| Mouse: Thymectomized adult mice 4 weeks (C57BL/6) | The Jackson Laboratory | Stock# 000664 |
|
| ||
| Oligonucleotides | ||
|
| ||
| qRT-PCR Il7 Forward: 5’-CTGATG ATCAGCATCGATGAATTGG-3’ |
Integrated DNA Technologies | This paper |
| qRT-PCR Il7 Reverse: 5’-GCAGC ACGATTTAGAAAAGCAGCT T-3’ |
Integrated DNA Technologies | This paper |
| qRT-PCR Bcl2 Forward: 5’-GGATA ACGGAGGCTGGGATGCCT-3’ |
Integrated DNA Technologies | This paper |
| qRT-PCR Bcl2 Reverse: 5’-CAGAG TGATGCAGGCCCCGAC-3’ |
Integrated DNA Technologies | This paper |
| qRT-PCR actinb Forward: 5’-GAGA GGGAAATCGTGCGTGA-3’ |
Integrated DNA Technologies | This paper |
| qRT-PCR actinb Reverse: 5’-ACATC TGCTGGAAGGTGG-3’ |
Integrated DNA Technologies | This paper |
| qRT-PCR Hprt Forward: 5’-TCATT ATGCCGAGGATTTGGA-3’ |
Integrated DNA Technologies | This paper |
| qRT-PCR Hprt Reverse: 5’-CAGAG GGCCACAATGTGATG-3’ |
Integrated DNA Technologies | This paper |
|
| ||
| Software and algorithms | ||
|
| ||
| GraphPad Prism 7 | GraphPad software | https://www.graphpad.com |
| FlowJo software version 10.2 | FlowJo | https://www.flowjo.com |
| Flow cytometry analysis software. Active Control 4.2.0.7 | Division of Computer Research and Technology, NIH | This paper |
|
| ||
| Other | ||
|
| ||
| Canvas X | Canvas GFX | https://www.canvasgfx.com |
| BD FACS LSRII | BD Biosciences | https://www.bdbiosciences.com |
| BD FACS LSRFortessa | BD Biosciences | https://www.bdbiosciences.com |
| ALZET® Osmotic Pumps | DURECT | Cat# Model 2002 |
Highlights.
IL-15 is required for the generation but not the survival of peripheral iNKT cells
IL-7 is a homeostatic cytokine for iNKT cells in peripheral tissues
IL-7 availability constrains the size of the peripheral iNKT cell pool
ACKNOWLEDGMENTS
We thank Drs. A. Singer and D. Kovalovsky for critical review of this manuscript. We thank members of the Park laboratory for discussion and experimental help. This work was supported by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2021.110219.
REFERENCES
- Alves NL, Richard-Le Goff O, Huntington ND, Sousa AP, Ribeiro VS, Bordack A, Vives FL, Peduto L, Chidgey A, Cumano A, et al. (2009). Characterization of the thymic IL-7 niche in vivo. Proc. Natl. Acad. Sci. U S A 106, 1512–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A (1995a). CD1: presenting unusual antigens to unusual T lymphocytes. Science 269, 185–186. [DOI] [PubMed] [Google Scholar]
- Bendelac A (1995b). Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med 182, 2091–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, and Teyton L (2007). The biology of NKT cells. Annu. Rev. Immunol 25, 297–336. [DOI] [PubMed] [Google Scholar]
- Benlagha K, Kyin T, Beavis A, Teyton L, and Bendelac A (2002). A thymic precursor to the NKT cell lineage. Science 296, 553–555. [DOI] [PubMed] [Google Scholar]
- Benlagha K, Wei DG, Veiga J, Teyton L, and Bendelac A (2005). Characterization of the early stages of thymic NKT cell development. J. Exp. Med 202, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlagha K, Weiss A, Beavis A, Teyton L, and Bendelac A (2000). In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med 191, 1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill MA, Goetz CA, Prlic M, O’Neil JJ, Harmon IR, Bensinger SJ, Turka LA, Brennan P, Jameson SC, and Farrar MA (2003). Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J. Immunol 171, 5853–5864. [DOI] [PubMed] [Google Scholar]
- Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, and Caligiuri MA (1994). Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med 180, 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Boyman O, Kim HO, Hahm B, Rubinstein MP, Ramsey C, Kim DM, Surh CD, and Sprent J (2007). An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J. Exp. Med 204, 1787–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpitts SL, Stonier SW, Stoklasek TA, Root SH, Aguila HL, Schluns KS, and Lefrancois L (2013). Transcriptional regulation of IL-15 expression during hematopoiesis. J. Immunol 191, 3017–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides MG, and Bendelac A (2013). Transcriptional regulation of the NKT cell lineage. Curr. Opin. Immunol 25, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, and Caligiuri MA (2002). In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood 100, 3633–3638. [DOI] [PubMed] [Google Scholar]
- Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, and Godfrey DI (2008). Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1-NKT cell population. Proc. Natl. Acad. Sci. U S A 105, 11287–11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Hara T, Simmons S, Wagatsuma K, Abe A, Miyachi H, Kitano S, Ishii M, Tani-ichi S, and Ikuta K (2014). Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc. Natl. Acad. Sci. U S A 111, 1915–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, et al. (2012). Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 36, 288–297. [DOI] [PubMed] [Google Scholar]
- DiSanto JP, Muller W, Guy-Grand D, Fischer A, and Rajewsky K (1995). Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. U S A 92, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do JS, Foucras G, Kamada N, Schenk AF, Shaw M, Nunez G, Paul WE, and Min B (2012). Both exogenous commensal and endogenous self antigens stimulate T cell proliferation under lymphopenic conditions. Cel. Immunol 272, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doisne JM, Becourt C, Amniai L, Duarte N, Le Luduec JB, Eberl G, and Benlagha K (2009). Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor (gamma)t+ and respond preferentially under inflammatory conditions. J. Immunol 183, 2142–2149. [DOI] [PubMed] [Google Scholar]
- Emoto M, Mittrucker HW, Schmits R, Mak TW, and Kaufmann SH (1999). Critical role of leukocyte function-associated antigen-1 in liver accumulation of CD4+NKT cells. J. Immunol 162, 5094–5098. [PubMed] [Google Scholar]
- Esteban LM, Tsoutsman T, Jordan MA, Roach D, Poulton LD, Brooks A, Naidenko OV, Sidobre S, Godfrey DI, and Baxter AG (2003). Genetic control of NKT cell numbers maps to major diabetes and lupus loci. J. Immunol 171, 2873–2878. [DOI] [PubMed] [Google Scholar]
- Foxwell BM, Beadling C, Guschin D, Kerr I, and Cantrell D (1995). Interleukin-7 can induce the activation of Jak 1, Jak 3 and STAT 5 proteins in murine T cells. Eur. J. Immunol 25, 3041–3046. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, and Kronenberg M (2004). Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest 114, 1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, Stankovic S, and Baxter AG (2010). Raising the NKT cell family. Nat. Immunol 11, 197–206. [DOI] [PubMed] [Google Scholar]
- Goldrath AW, and Bevan MJ (1999). Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity 11, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordy LE, Bezbradica JS, Flyak AI, Spencer CT, Dunkle A, Sun J, Stanic AK, Boothby MR, He YW, Zhao Z, et al. (2011). IL-15 regulates homeostasis and terminal maturation of NKT cells. J. Immunol 187, 6335–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Lanjuin A, Madisen L, Zeng H, Murthy VN, and Uchida N (2013). Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nat. Neurosci 16, 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O, Hansson M, Kiessling R, and Wigzell H (1977). Role of non-conventional natural killer cells in resistance against syngeneic tumour cells in vivo. Nature 270, 609–611. [DOI] [PubMed] [Google Scholar]
- Hara T, Shitara S, Imai K, Miyachi H, Kitano S, Yao H, Tani-ichi S, and Ikuta K (2012). Identification of IL-7-producing cells in primary and secondary lymphoid organs using IL-7-GFP knock-in mice. J. Immunol 189, 1577–1584. [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. (2000). Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med 191, 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GY, Ligons DL, Hong C, Luckey MA, Keller HR, Tai X, Lucas PJ, Gress RE, and Park JH (2012). An in vivo IL-7 requirement for peripheral Foxp3+ regulatory T cell homeostasis. J. Immunol 188, 5859–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku CC, Murakami M, Sakamoto A, Kappler J, and Marrack P (2000). Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288, 675–678. [DOI] [PubMed] [Google Scholar]
- Lakhani SA, Masud A, Kuida K, Porter GA Jr., Booth CJ, Mehal WZ, Inayat I, and Flavell RA (2006). Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311, 847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz O, and Bendelac A (1994). An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8-T cells in mice and humans. J. Exp. Med 180, 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Holzapfel KL, Zhu J, Jameson SC, and Hogquist KA (2013). Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol 14, 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Hara T, Wagatsuma K, Zhang J, Maki K, Miyachi H, Kitano S, Yabe-Nishimura C, Tani-Ichi S, and Ikuta K (2012). Role of hepatocyte-derived IL-7 in maintenance of intrahepatic NKT cells and T cells and development of B cells in fetal liver. J. Immunol 189, 4444–4450. [DOI] [PubMed] [Google Scholar]
- Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, and Ma A (1998). IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9, 669–676. [DOI] [PubMed] [Google Scholar]
- Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, Besra G, Vomhof-Dekrey EE, Tighe M, Koay HF, et al. (2015). Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat. Immunol 16, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, and Tagaya Y (2000). IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl. Acad. Sci. U S A 97, 11445–11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, Surh CD, and Kronenberg M (2002). Homeostasis of V alpha 14i NKT cells. Nat. Immunol 3, 966–974. [DOI] [PubMed] [Google Scholar]
- McCaughtry TM, Etzensperger R, Alag A, Tai X, Kurtulus S, Park JH, Grinberg A, Love P, Feigenbaum L, Erman B, et al. (2012). Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus. J. Exp. Med 209, 2263–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab FW, Berzins SP, Pellicci DG, Kyparissoudis K, Field K, Smyth MJ, and Godfrey DI (2005). The influence of CD1d in postselection NKT cell maturation and homeostasis. J. Immunol 175, 3762–3768. [DOI] [PubMed] [Google Scholar]
- McNab FW, Pellicci DG, Field K, Besra G, Smyth MJ, Godfrey DI, and Berzins SP (2007). Peripheral NK1.1 NKT cells are mature and functionally distinct from their thymic counterparts. J. Immunol 179, 6630–6637. [DOI] [PubMed] [Google Scholar]
- Mertsching E, Burdet C, and Ceredig R (1995). IL-7 transgenic mice: analysis of the role of IL-7 in the differentiation of thymocytes in vivo and in vitro. Int. Immunol 7, 401–414. [DOI] [PubMed] [Google Scholar]
- Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, and Leite-de-Moraes MC (2007). Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med 204, 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, Yamane H, Hu-Li J, and Paul WE (2005). Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J. Immunol 174, 6039–6044. [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Emoto M, Brinkmann V, van Rooijen N, Schmits R, Kita E, and Kaufmann SH (2000). Cutting edge: contribution of NK cells to the homing of thymic CD4+NKT cells to the liver. J. Immunol 165, 1729–1732. [DOI] [PubMed] [Google Scholar]
- Osborne LC, Dhanji S, Snow JW, Priatel JJ, Ma MC, Miners MJ, Teh HS, Goldsmith MA, and Abraham N (2007). Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R alpha mutant mice. J. Exp. Med 204, 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, and Singer A (2004). Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity 21, 289–302. [DOI] [PubMed] [Google Scholar]
- Park JY, DiPalma DT, Kwon J, Fink J, and Park JH (2019a). Quantitative difference in PLZF protein expression determines iNKT lineage fate and controls innate CD8 T cell generation. Cell Rep. 27, 2548–2557.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Kwon J, Kim EY, Fink J, Kim HK, and Park JH (2019b). CD24(+) cell depletion permits effective enrichment of thymic iNKT cells while preserving their subset composition. Immune Netw. 19, e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, and Godfrey DI (2002). A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. J. Exp. Med 195, 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prlic M, Blazar BR, Farrar MA, and Jameson SC (2003). In vivo survival and homeostatic proliferation of natural killer cells. J. Exp. Med 197, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, and Di Santo JP (2003a). IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc. Natl. Acad. Sci. U S A 100, 2663–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, and Di Santo JP (2003b). IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood 101, 4887–4893. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Farkash EA, Gao W, and Thompson CB (2001). IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol 167, 6869–6876. [DOI] [PubMed] [Google Scholar]
- Rochman Y, Spolski R, and Leonard WJ (2009). New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol 9, 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, and Lefrancois L (2000). Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol 1, 426–432. [DOI] [PubMed] [Google Scholar]
- Schluns KS, and Lefrancois L (2003). Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol 3, 269–279. [DOI] [PubMed] [Google Scholar]
- Sosinowski T, White JT, Cross EW, Haluszczak C, Marrack P, Gapin L, and Kedl RM (2013). CD8alpha+ dendritic cell trans presentation of IL-15 to naive CD8+ T cells produces antigen-inexperienced T cells in the periphery with memory phenotype and function. J. Immunol 190, 1936–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, and Sprent J (2008). Homeostasis of naive and memory T cells. Immunity 29, 848–862. [DOI] [PubMed] [Google Scholar]
- Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, and Surh CD (2001). IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. U S A 98, 8732–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, and Surh CD (2002). Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med 195, 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, Watarai H, Inoue S, Sekine E, Nakagawa R, Hase K, Iwamura C, Nakajima H, Nakayama T, and Taniguchi M (2008). A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J. Exp. Med 205, 2727–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, and Glimcher LH (2004). T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity 20, 477–494. [DOI] [PubMed] [Google Scholar]
- Vahl JC, Heger K, Knies N, Hein MY, Boon L, Yagita H, Polic B, and Schmidt-Supprian M (2013). NKT cell-TCR expression activates conventional T cells in vivo, but is largely dispensable for mature NKT cell biology. PLoS Biol 11, e1001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaraman AR, and Cowling RJ (1994). Interleukin-7 induces the association of phosphatidylinositol 3-kinase with the alpha chain of the interleukin-7 receptor. Eur. J. Immunol 24, 2168–2174. [DOI] [PubMed] [Google Scholar]
- von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, and Murray R (1995). Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med 181, 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, and Taniguchi M (2008). Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat. Protoc 3, 70–78. [DOI] [PubMed] [Google Scholar]
- Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, Yoshida H, Kubo M, Kawamoto H, Koseki H, et al. (2012). Development and function of invariant natural killer T cells producing T(h)2- and T(h) 17-cytokines. PLoS Biol 10, e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KE, Kim HO, Kyparissoudis K, Corpuz TM, Pinget GV, Uldrich AP, Brink R, Belz GT, Cho JH, Godfrey DI, et al. (2014). IL-17-producing NKT cells depend exclusively on IL-7 for homeostasis and survival. Mucosal Immunol. 7, 1058–1067. [DOI] [PubMed] [Google Scholar]
- Wei B, Wingender G, Fujiwara D, Chen DY, McPherson M, Brewer S, Borneman J, Kronenberg M, and Braun J (2010). Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J. Immunol 184, 1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ, Jenkinson WE, Cowan JE, Parnell SM, Bacon A, Jones ND, Jenkinson EJ, and Anderson G (2014). An essential role for medullary thymic epithelial cells during the intrathymic development of invariant NKT cells. J. Immunol 192, 2659–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Dong Z, Zhou R, Luo D, Wei H, and Tian Z (2005). Isolation of lymphocytes and their innate immune characterizations from liver, intestine, lung and uterus. Cell. Mol. Immunol 2, 271–280. [PubMed] [Google Scholar]
- Zhu J, Jankovic D, Oler AJ, Wei G, Sharma S, Hu G, Guo L, Yagi R, Yamane H, Punkosdy G, et al. (2012). The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 37, 660–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.