GTR Home > GTR Help > Submission > Submission Overview
The GTR Submission Process
OMB NO: 0925-0651
EXPIRATION DATE: 01/31/2025
This document is an overview of the methods by which information can be registered in the GTR. Detailed information is provided in specific pages that can be accessed from the navigation menu above.
You can access a webinar about registering with GTR which will help you determine a submission strategy for your laboratory here.
Table of contents
- What constitutes a test in the GTR?
- What types of tests are currently accepted?
- What data should be collected before submitting?
- Submission scenarios
- How do I get started?
- Who is authorized to submit?
- Overview of the steps to register labs and tests
- Conventions used in the interactive submission interface
- Your submission home page
- Adding tests
- How to manage your records in the GTR
- How are my data used by NCBI?
- GTR API Submissions
What constitutes a test in the GTR?
A clinical test in the GTR is defined as the equivalent of a laboratory order code. The definition of a clinical genetic test in the GTR may be found here.
A research test in the GTR is defined as a test that is performed for the purpose of contributing to generalizable knowledge or for a laboratory to generate data in order to make technical improvements to a test.
What types of tests are currently accepted?
GTR is now accepting registration of clinical and research tests. Tests may be registered for Mendelian disorders, somatic conditions (such as cancer), complex tests and arrays, and pharmacogenetic tests, as well as for microbe tests that affect human health and disease. These tests may include multiple methods and may include multiple major method categories such as biochemical, cytogenetic, and molecular tests.
GTR is not currently accepting registration of direct-to-consumer tests.
Tests that are general and not specific to a particular condition, set of conditions or genes, or chromosomal structures are categorized by GTR as Laboratory services and will be entered in the laboratory record. One such example is Preimplantation Genetic Diagnosis (PGD). Whole exome sequencing and whole genome sequencing are considered to be tests in GTR, but are not yet in scope as fully described tests. These may now be entered as Laboratory services, with the opportunity to provide information in a comment box, and at a later phase they will be registered as tests.
Tests which are performed entirely at an outside lab/facility (send-outs) should not be registered in GTR. 'Outside' means a lab/facility that is not covered by your lab's CLIA certification, even if both labs have the same parent organization.
What data should be collected before submitting?
The list of data elements that may be submitted is provided in GTR's Data Dictionary (pdf or Word. These elements are categorized as minimal, recommended, and optional. Minimal fields are required, and submission will fail if not supplied. Recommended elements are not required, and can be skipped in the submission process. The web interface will, however, report 'not provided' for blank recommended fields. Optional fields are not required, and can be skipped in the submission process. The web interface will not display optional fields left blank.
Submission scenarios
Registration in GTR is divided into two major categories:
-
Laboratory registration
- Laboratory and personnel data that are applicable to all tests are entered once.
- All laboratory registrations are performed through the interactive web-based submission user interface.
- The 'Default parameters' tab enables the entry of information that is shared by most tests offered by the lab. Information supplied in this tab will pre-populate test records for later review and editing, potentially saving keystrokes.
-
Test registration
- Submission of test-specific information, where a clinical test is defined as the equivalent of an order code and a research test is either a component of a research study or a test undergoing technical improvements.
Scenarios for test submissions
-
Completely interactive:
The submitter enters and reviews information via web-based forms. New clinical and research tests can be created by any lab registered in GTR. -
Automatic submission using the GTR test submission template with complete set of fields (for clinical tests only):
The submitter downloads a test submission template file from their lab overview page or or from the FTP site which supports entry of all test fields. The submitter completes the spreadsheet for each test the lab wishes to register. Once successfully uploaded, the tests are automatically registered. Read more about this mode of submission for clinical genetic tests and clinical microbe tests. -
Semi-automatic submission using the GTR test submission template with minimal set of fields (for clinical genetic tests only):
The submitter downloads a test submission template file from their lab overview page or from the FTP site which supports entry of all required test fields and certain recommended fields.
The submitter completes the spreadsheet for each test the lab wishes to register. The submitter uses the interactive submission interface to review each record seeded by the spreadsheet, enters information as desired for any recommended and optional fields not supported by the spreadsheet, and submits each test. Read more about this mode of submission here. -
Completely automatic:
GTR now supports submission of clinical tests, both clinical genetic tests and clinical microbe tests, via a RESTful (REpresentational State Transfer) API (Application Programming Interface). Data submission and the status response are in JSON (JavaScript Object Notation) data exchange format. The documentation includes the submission and response schemas, as well as helpful information to guide submitters. Contact GTR staff to create a service account and get started.
How do I get started?
The GTR help site includes multiple documents about registering laboratories and tests, with some specific and distinctive aspects of the submission/update process. The navigation menu at the top of the page provides menus from which to select the page specific to your topic of choice. Details about each tab are provided in the table below.
Laboratory information must be entered first. New laboratories have a wait period of 2-3 business days for GTR staff to review the laboratory record. Test information laboratory can be entered only after the laboratory has been registered in the GTR.
To determine whether your Web browser is supported to run NCBI web applications including data submission, use this link: Browser Advice for NCBI Web Pages
Who is authorized to submit?
Initially one person per lab can submit lab and test data to GTR.
We have enabled submissions from multiple individuals from a single lab in a function called Groups. Please contact us if this is a feature you would like to use.
Overview of the steps to register labs and tests
- Go to the GTR Submission user interface
- Login to the GTR submission site
- Create a lab record: log in to the submission interface and proceed through the steps below, then click the Add a new lab button. Once you submit your lab, you will receive an email informing you your information was submitted. GTR staff will review the information and must approve your lab before you can register your tests. This process may take 2-3 business days. We may contact the lab if more information is needed. When your lab is approved, you will receive a notification email informing you that you can register your tests.
- Once you have an active laboratory record, create new test records, review the information and submit the records interactively.
- If you have many clinical tests to register, you can consider submitting data to GTR in bulk via the full test submission spreadsheet.
Conventions used in the interactive submission interface
- Orange asterisk (*): minimal field, the submission will fail if the data are not supplied
- ?(
 ) icon: hover over icon to display an instruction tip
) icon: hover over icon to display an instruction tip - Information will display on the public site exactly as it is entered, unless otherwise noted. Therefore, please note carefully the recommended format for fields and be careful to spell out information the way you want the public to see it.
- Phone numbers require a specific format (e.g. US phone numbers should be entered as 123-456-7890 ext. 1234).
- URLs (websites) must begin with the protocol (e.g. http://, https://, or ftp://).
Your submission home page
When you have established your account, you will be presented with your GTR Submission home page. This page supports the functions of adding, deleting and editing data. When you have completed a submission, it also displays what you have submitted and the status of each submission. For example, when you login to the GTR submission interface for the first time and accept the code of conduct, this is what you will see. Note the link to these help documents, and the address to contact GTR staff by email.
Your name anchors the link to your profile page. That profile page also has a submissions tab where you can review your submissions.
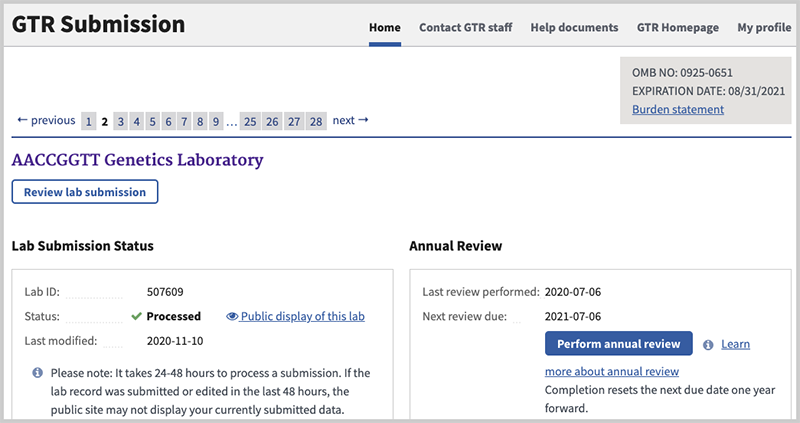
Once you have started to process some information, the page will provide a summary, separated by laboratory, of the submission you have initiated. You can click on the link (Review lab submission or Edit this lab, the latter if the lab record has not been submitted) to view / edit the lab record. Please contact GTR staff if you need to delete your laboratory from the GTR.
Once a lab has submitted test information, you can view your tests using the test management page.
Adding tests
Detailed instructions are shown on the following pages:
- Using the submission wizard
- Using the spreadsheet templates
- Genetic tests (clinical only)
- All clinical fields spreadsheet submission; once the spreadsheet is uploaded, test submission is automatic; we encourage submitters to use this spreadsheet
- Minimal clinical fields spreadsheet submission; note that this method is semiautomated; the submitter will need to submit each test manually using the submission wizard once the spreadsheet has been uploaded and processed.
- Microbe tests (clinical only)
- Genetic tests (clinical only)
How to manage your records in the GTR
See detailed instructions on how to update, delete, copy and edit GTR records.
How are my data used by NCBI?
The data you submit will be displayed publicly in GTR and may be reflected in other NCBI resources such as MedGen and ClinVar. Exceptions to submitted information that will be displayed publicly, such as private comments to GTR staff and private contact information are marked in the submission user interface and in the help documentation. Please do not submit any data that you consider to be proprietary.
When your submission includes information about the alleles you test for a disorder, your data will be accessioned in ClinVar as well (http://www.ncbi.nlm.nih.gov/clinvar). We therefore encourage you to provide information about the clinical significance of each allele. By combining efforts, GTR and ClinVar hope to improve access to information about medically important variation.
GTR API Submissions
For fully automated submission of GTR clinical tests, both genetic tests and microbe tests, see GTR Submission API.
