GTR Home > GTR Help > Submission > Submission Overview
The GTR Submission Process
OMB NO: 0925-0651
EXPIRATION DATE: 01/31/2025
This document is an overview of the methods by which information can be registered in the GTR. Detailed information is provided in specific pages that can be accessed from the navigation menu above.
You can access a webinar about registering with GTR which will help you determine a submission strategy for your laboratory here.
Table of contents
- What constitutes a test in the GTR?
- What types of tests are currently accepted?
- What data should be collected before submitting?
- Submission scenarios
- How do I get started?
- Who is authorized to submit?
- Overview of the steps to register labs and tests
- Conventions used in the interactive submission interface
- Your submission home page
- Adding tests
- How to manage your records in the GTR
- How are my data used by NCBI?
- GTR API Submissions
What constitutes a test in the GTR?
A clinical test in the GTR is defined as the equivalent of a laboratory order code. The definition of a clinical genetic test in the GTR may be found here.
A research test in the GTR is defined as a test that is performed for the purpose of contributing to generalizable knowledge or for a laboratory to generate data in order to make technical improvements to a test.
What types of tests are currently accepted?
GTR is now accepting registration of clinical and research tests. Tests may be registered for Mendelian disorders, somatic conditions (such as cancer), complex tests and arrays, and pharmacogenetic tests, as well as for microbe tests that affect human health and disease. These tests may include multiple methods and may include multiple major method categories such as biochemical, cytogenetic, and molecular tests.
GTR is not currently accepting registration of direct-to-consumer tests.
Tests that are general and not specific to a particular condition, set of conditions or genes, or chromosomal structures are categorized by GTR as Laboratory services and will be entered in the laboratory record. One such example is Preimplantation Genetic Diagnosis (PGD). Whole exome sequencing and whole genome sequencing are considered to be tests in GTR, but are not yet in scope as fully described tests. These may now be entered as Laboratory services, with the opportunity to provide information in a comment box, and at a later phase they will be registered as tests.
Tests which are performed entirely at an outside lab/facility (send-outs) should not be registered in GTR. 'Outside' means a lab/facility that is not covered by your lab's CLIA certification, even if both labs have the same parent organization.
What data should be collected before submitting?
The list of data elements that may be submitted is provided in GTR's Data Dictionary (pdf or Word. These elements are categorized as minimal, recommended, and optional. Minimal fields are required, and submission will fail if not supplied. Recommended elements are not required, and can be skipped in the submission process. The web interface will, however, report 'not provided' for blank recommended fields. Optional fields are not required, and can be skipped in the submission process. The web interface will not display optional fields left blank.
Submission scenarios
Registration in GTR is divided into two major categories:
-
Laboratory registration
- Laboratory and personnel data that are applicable to all tests are entered once.
- All laboratory registrations are performed through the interactive web-based submission user interface.
- The 'Default parameters' tab enables the entry of information that is shared by most tests offered by the lab. Information supplied in this tab will pre-populate test records for later review and editing, potentially saving keystrokes.
-
Test registration
- Submission of test-specific information, where a clinical test is defined as the equivalent of an order code and a research test is either a component of a research study or a test undergoing technical improvements.
Scenarios for test submissions
-
Completely interactive:
The submitter enters and reviews information via web-based forms. New clinical and research tests can be created by any lab registered in GTR. -
Automatic submission using the GTR test submission template with complete set of fields (for clinical tests only):
The submitter downloads a test submission template file from their lab overview page or or from the FTP site which supports entry of all test fields. The submitter completes the spreadsheet for each test the lab wishes to register. Once successfully uploaded, the tests are automatically registered. Read more about this mode of submission for clinical genetic tests and clinical microbe tests. -
Semi-automatic submission using the GTR test submission template with minimal set of fields (for clinical genetic tests only):
The submitter downloads a test submission template file from their lab overview page or from the FTP site which supports entry of all required test fields and certain recommended fields.
The submitter completes the spreadsheet for each test the lab wishes to register. The submitter uses the interactive submission interface to review each record seeded by the spreadsheet, enters information as desired for any recommended and optional fields not supported by the spreadsheet, and submits each test. Read more about this mode of submission here. -
Completely automatic:
GTR now supports submission of clinical tests, both clinical genetic tests and clinical microbe tests, via a RESTful (REpresentational State Transfer) API (Application Programming Interface). Data submission and the status response are in JSON (JavaScript Object Notation) data exchange format. The documentation includes the submission and response schemas, as well as helpful information to guide submitters. Contact GTR staff to create a service account and get started.
How do I get started?
The GTR help site includes multiple documents about registering laboratories and tests, with some specific and distinctive aspects of the submission/update process. The navigation menu at the top of the page provides menus from which to select the page specific to your topic of choice. Details about each tab are provided in the table below.
Laboratory information must be entered first. New laboratories have a wait period of 2-3 business days for GTR staff to review the laboratory record. Test information laboratory can be entered only after the laboratory has been registered in the GTR.
To determine whether your Web browser is supported to run NCBI web applications including data submission, use this link: Browser Advice for NCBI Web Pages
Who is authorized to submit?
Initially one person per lab can submit lab and test data to GTR.
We have enabled submissions from multiple individuals from a single lab in a function called Groups. Please contact us if this is a feature you would like to use.
Overview of the steps to register labs and tests
- Go to the GTR Submission user interface
- Login to the GTR submission site
- Create a lab record: log in to the submission interface and proceed through the steps below, then click the Add a new lab button. Once you submit your lab, you will receive an email informing you your information was submitted. GTR staff will review the information and must approve your lab before you can register your tests. This process may take 2-3 business days. We may contact the lab if more information is needed. When your lab is approved, you will receive a notification email informing you that you can register your tests.
- Once you have an active laboratory record, create new test records, review the information and submit the records interactively.
- If you have many clinical tests to register, you can consider submitting data to GTR in bulk via the full test submission spreadsheet.
Conventions used in the interactive submission interface
- Orange asterisk (*): minimal field, the submission will fail if the data are not supplied
- ?(
 ) icon: hover over icon to display an instruction tip
) icon: hover over icon to display an instruction tip - Information will display on the public site exactly as it is entered, unless otherwise noted. Therefore, please note carefully the recommended format for fields and be careful to spell out information the way you want the public to see it.
- Phone numbers require a specific format (e.g. US phone numbers should be entered as 123-456-7890 ext. 1234).
- URLs (websites) must begin with the protocol (e.g. http://, https://, or ftp://).
Your submission home page
When you have established your account, you will be presented with your GTR Submission home page. This page supports the functions of adding, deleting and editing data. When you have completed a submission, it also displays what you have submitted and the status of each submission. For example, when you login to the GTR submission interface for the first time and accept the code of conduct, this is what you will see. Note the link to these help documents, and the address to contact GTR staff by email.
Your name anchors the link to your profile page. That profile page also has a submissions tab where you can review your submissions.
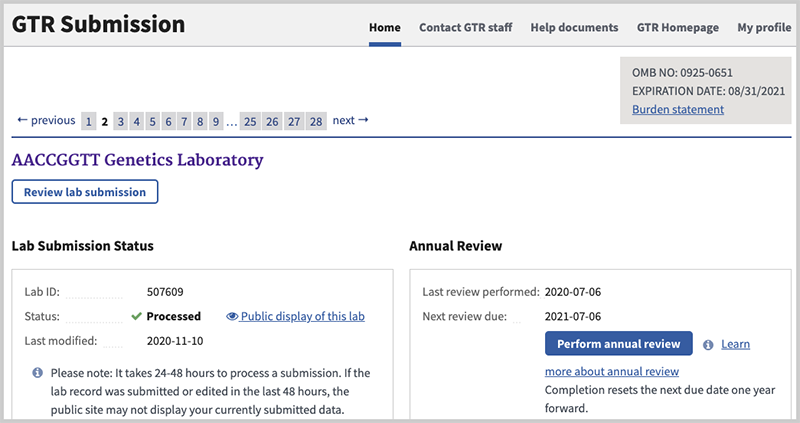
Once you have started to process some information, the page will provide a summary, separated by laboratory, of the submission you have initiated. You can click on the link (Review lab submission or Edit this lab, the latter if the lab record has not been submitted) to view / edit the lab record. Please contact GTR staff if you need to delete your laboratory from the GTR.
Once a lab has submitted test information, you can view your tests using the test management page.
Adding tests
Detailed instructions are shown on the following pages:
- Using the submission wizard
- Using the spreadsheet templates
- Genetic tests (clinical only)
- All clinical fields spreadsheet submission; once the spreadsheet is uploaded, test submission is automatic; we encourage submitters to use this spreadsheet
- Minimal clinical fields spreadsheet submission; note that this method is semiautomated; the submitter will need to submit each test manually using the submission wizard once the spreadsheet has been uploaded and processed.
- Microbe tests (clinical only)
- Genetic tests (clinical only)
How to manage your records in the GTR
See detailed instructions on how to update, delete, copy and edit GTR records.
How are my data used by NCBI?
The data you submit will be displayed publicly in GTR and may be reflected in other NCBI resources such as MedGen and ClinVar. Exceptions to submitted information that will be displayed publicly, such as private comments to GTR staff and private contact information are marked in the submission user interface and in the help documentation. Please do not submit any data that you consider to be proprietary.
When your submission includes information about the alleles you test for a disorder, your data will be accessioned in ClinVar as well (http://www.ncbi.nlm.nih.gov/clinvar). We therefore encourage you to provide information about the clinical significance of each allele. By combining efforts, GTR and ClinVar hope to improve access to information about medically important variation.
GTR API Submissions
For fully automated submission of GTR clinical tests, both genetic tests and microbe tests, see GTR Submission API.
Login to the Submission Portal
OMB NO: 0925-0651
EXPIRATION DATE: 01/31/2025
This document describes how to establish access to submit data to NCBI, specifically GTR. You are responsible for maintaining the integrity and security of your data by following the instructions here.
Submission of data to GTR entails following these steps:
- Connect to the GTR submission site which is located here: https://submit.ncbi.nlm.nih.gov/subs/gtr
- Login
- Update your profile (Important and required!)
- Read and accept the Code of Conduct
- Do not share login credentials between individuals
Connect to the GTR submission site
The GTR submission site is part of NCBI's unified submission interface: https://submit.ncbi.nlm.nih.gov/subs/gtr/. The tools in this interface are being established for all submissions to NCBI. When you connect to this site, it should look like this. Note the link to the login interface at the upper right.
Logging in
Login to the Submission Portal using NIH login or third-party login credentials. You can also use your My NCBI account credentials to log in but there are upcoming changes to My NCBI accounts. See the NCBI Account Login Changes FAQs.
Update your profile
Most likely you will be using NCBI's submission interface for the first time. If so, your screen will look like this:
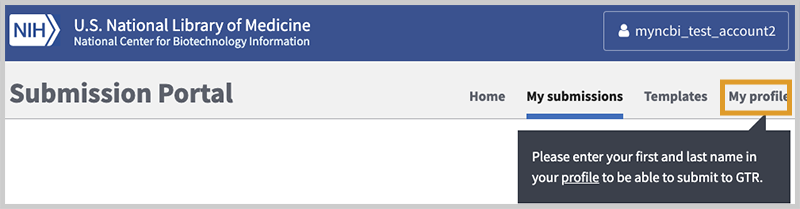
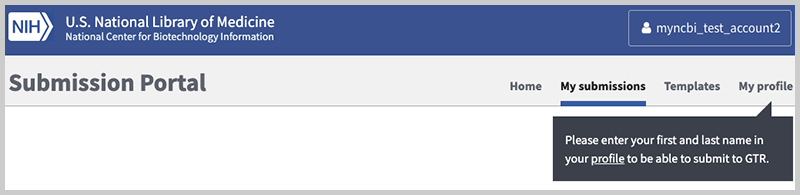
Once logged in, new users must provide contact information so that we know who you are and can contact you easily if a problem arises in your submission. Please click on the 'My profile' link and carefully follow the instructions in the next image.
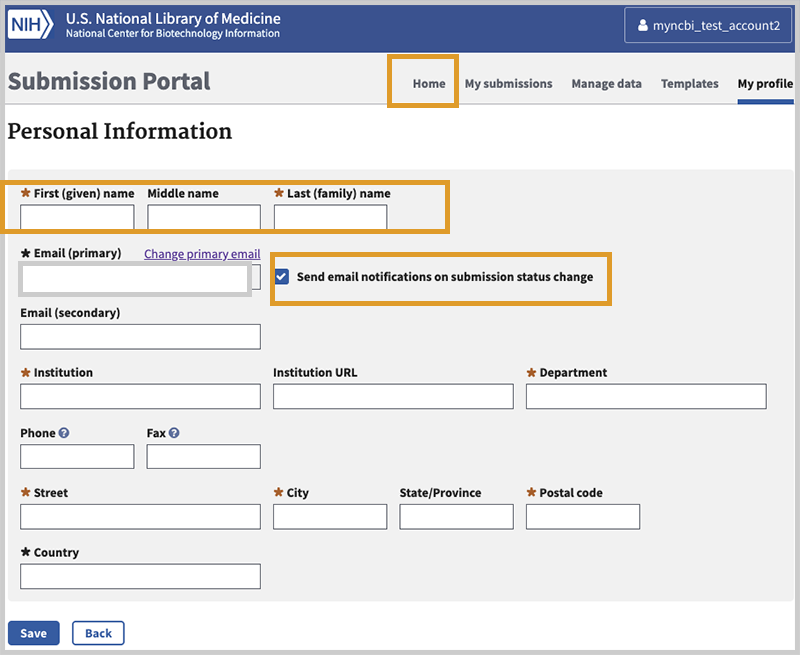
Complete your profile.
You will be presented with a form like the one above. Please note the following:
- When you update your profile, you must submit your first and last name. Completing this information as part of your laboratory submission does not fulfill the requirement to enter it here.
- We highly recommend you leave this box checked - Send email notifications on submission status change - so you will be notified when your submission is processed. You may enter other contact details, but be advised that as a data submitter we will be asking for those data again when you process your laboratory's data.
NOTE: Country field must be completed. For U.S., State must be provided. - Click Save.
- If you are not automatically taken to a new page, click the Home tab at the top of the page or the gray area labelled Submission Portal in the upper left-hand corner.
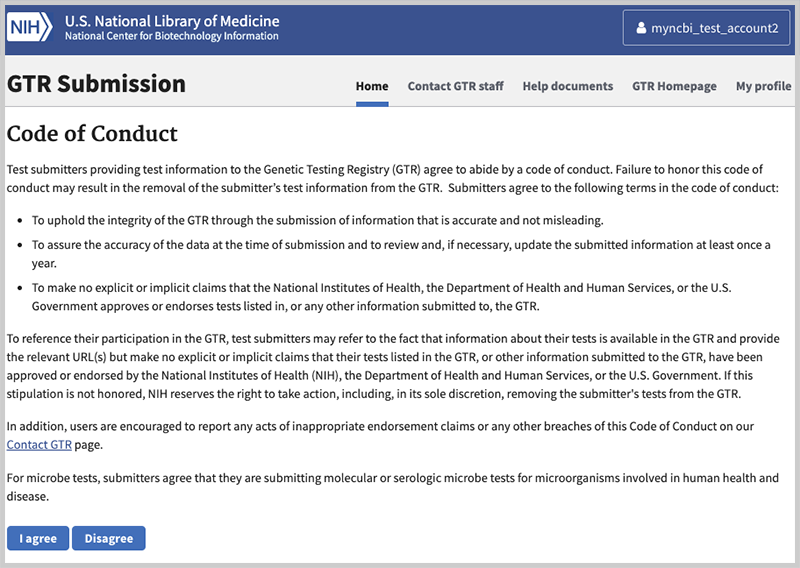
After completing the login process you will be presented with the Code of Conduct.
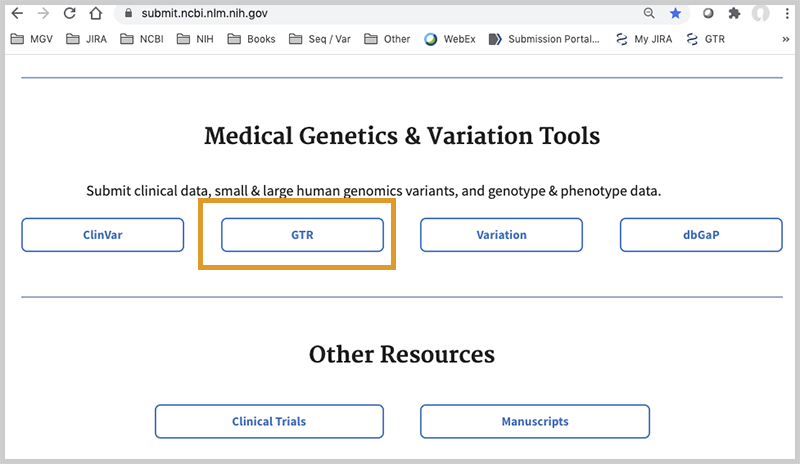
After clicking on the Home tab, you will see this page. Scroll down to the section Medical Genetics and Variation Tools and click on GTR.
Code of Conduct
When your login is successful, you will be presented with the GTR Code of Conduct. You must read and click I agree to be able to register in the GTR. This screen will not be presented again after you have accepted it. After successfully completing the login procedures and agreeing to the Code of Conduct, you will be able to enter data.
Maintain the integrity of your Submission Portal account and the data for which you are responsible
Sharing of login credentials by multiple individuals is strongly discouraged. Each individual that chooses to submit data to GTR must first read and agree to the Code of Conduct. Individuals that share their login credentials with another person may be compromising the integrity of their submissions.
The Submission Portal includes a feature that enables multiple individuals from an organization to submit data in a function called Groups. Please take advantage of the Groups functions to manage permissions for members of your laboratory to manage data, instead of sharing account information.
How to register a laboratory in the GTR
OMB NO: 0925-0651
EXPIRATION DATE: 01/31/2025
Instructions on this page assume you have logged into the GTR submission site, and are ready to add information about your laboratory.
You will start from the home page for your GTR submissions. Click on Submit a new lab.
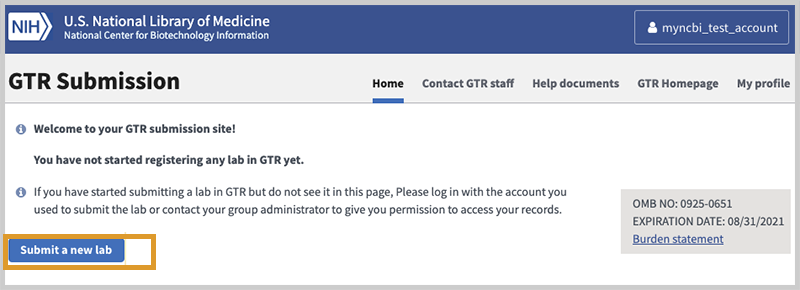
Complete your data as summarized below. Following submission, the lab will display on the public site within 24-48 hours. GTR staff will reach out to new registrants with a brief questionnaire. Once the lab is approved, you will be able to submit your tests. Be sure to enter tests that are currently in scope.
The Laboratory Record
To enter or update information into a laboratory record, you will be presented with a sequence of forms in which to submit data. Each page has a distinct tab.

In the table below, tab names in the left column link to the details about how to submit the data for the fields in that section.
| Lab information | Names, contact information, services, and more |
| Personnel | Staff members to be displayed or contacted |
| Licensure and accreditations | CLIA, State licenses, and more |
| Default parameters | Default values you want to set to appear on each test you offer |
| Review & Submit | Review your entry, preview how it will appear on the web site, and submit |
Progressing through the Laboratory Record
At the bottom of each page is the button Save & Continue. If your entries on that page validate, you will be taken to the next tab.
Your progress through the submission process is marked by the tab color. For a new lab submission, if you have not 'touched' a tab yet, text and tab color remain grey. The tab you are currently processing is shown enlarged. If you have touched a tab (blue text), you can go back to it at any time to update information (Remember to click on Save & Continue before leaving the page.). So in the tabs image above, the Lab information has been saved and you are currently on the Personnel data tab.
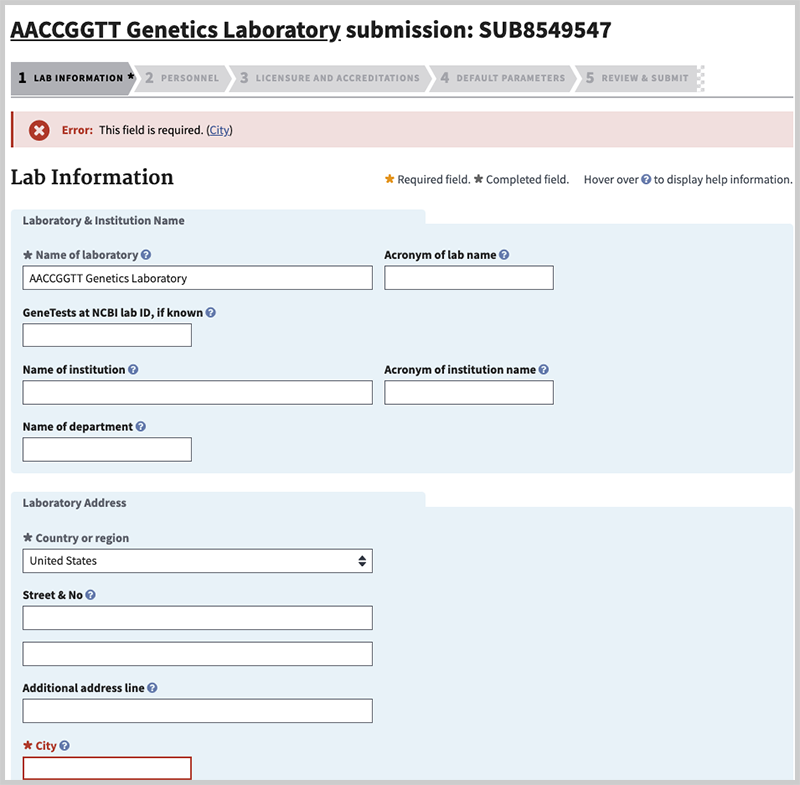
Validation errors
If there is a problem with how you completed the value for a field, you will remain on the same tab. Information will be provided to indicate what content needs to be corrected, and the type of error. In the case below, the name of the city (a required field) was omitted. If at any time you are unsure as to what information to provide in a field you can hover over the help icon (![]() ) to display help information, or review the help page for the tab you are on.
) to display help information, or review the help page for the tab you are on.
How to register a clinical test in the GTR
OMB NO: 0925-0651
EXPIRATION DATE: 01/31/2025
Instructions on this page assume you have logged into the GTR submission site, have registered your laboratory, and are now ready to submit information about a clinical genetic test.
On the home page for your GTR submissions, under 'Tests in this lab', click on 'Submit tests'. To submit a test using the submission wizard, go to the section Submit a single test. The default selection is for a clinical genetic test. Click 'Add a new test'.
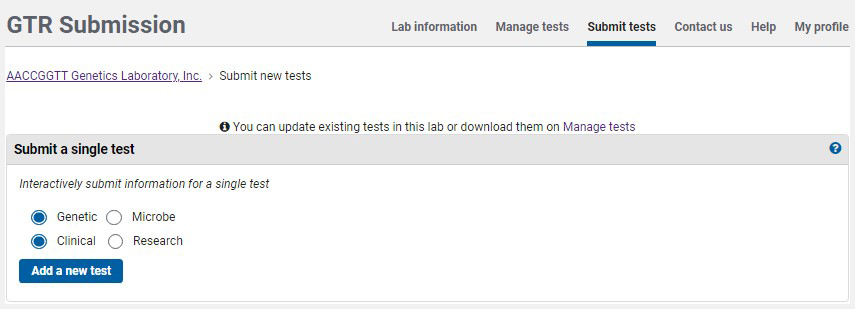
You will be presented with a sequence of pages in which to submit data. Each page has a distinct tab, as seen in the image below.

At the bottom of each page is the button Save & Continue. If your entries on that page validate, you will be taken to the next tab. If there is ever a problem with how you completed a value, you will remain on the same tab. Information will be provided to indicate what content needs to be corrected, and the type of error. If at any time you are unsure as to what information to provide in a field you can hover over the help icon (![]() ) to display help information, or review the help page for the tab you are on.
) to display help information, or review the help page for the tab you are on.
Your progress through the submission process is marked as follows. If you have not 'touched' a tab yet, text and tab color remain grey. The tab you are currently processing is shown enlarged. If you have touched a tab (blue text), you can go back to it at any time to update information (Remember click on Save & Continue before leaving the page.). So in the example above, the submitter is currently on Ordering.
The table below summarizes the content included under each tab. The names in the left column are linked to the details about how to submit the data elements in that section.
| Basics | Type of test, names licenses, etc. |
| Ordering | How to order |
| Indication | Condition(s) for which the test is appropriate |
| Methodology | Describe the methods used and what is measured (the test targets) |
| Interpretation | Describe how test results are interpreted |
| Performance | Where the test is performed, clinical validity, clinical utility, and more |
| Review & Submit | Review your data and submit |